Abstract
The discovery of the Th17 lineage in 2005 triggered a major change in how immunity to infectious diseases is viewed. Fungal infections, in particular, have long been a relatively understudied area of investigation in terms of the host immune response. Candida albicans is a commensal yeast that colonizes mucosal sites and skin. In healthy individuals it is non-pathogenic, but in conditions of immune deficiency, this organism can cause a variety of infections associated with considerable morbidity. Candida can also cause disseminated infections that have a high mortality rate and are a major clinical problem in hospital settings. Although immunity to Candida albicans was long considered to be mediated by Th1 cells, new data in both rodent models and in humans have revealed an essential role for the Th17 lineage, and in particular its signature cytokine IL-17.
Keywords: IL-17, Th17, Candida albicans, fungal infections, cytokine
Introduction
Th17 cells and IL-17: a brief history
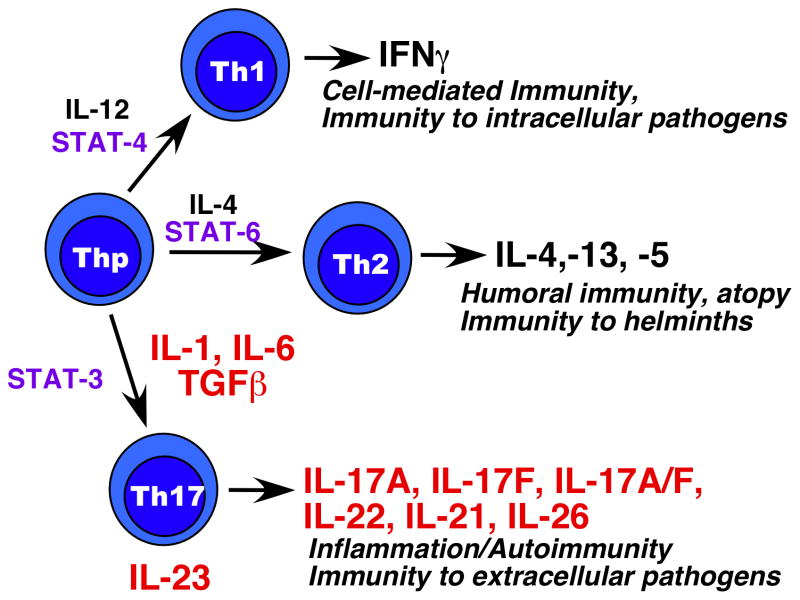
Effector CD4+ T helper cells (Th) coordinate immune responses through the production of cytokines, which in turn are instrumental in shaping the nature of the response that is generated. In 1986 it was recognized that there are at least two distinct categories of Th cells that induced different types of immune responses, termed “Th1” and Th2” (Fig. 1) (1). In contrast to generation of the antigen-specific TCR, these subsets are not pre-committed, but rather are generated depending on the microenvironment. Over time, it became clear that naïve Th cells exposed to IL-12 progressed along the Th1 differentiation pathway leading to secretion of IFNγ, a process dependent on the transcription factors STAT-4 and T-bet. Conversely, IL-4 and the transcription factors STAT-6 and GATA-3 trigger a differentiation program leading to Th2 generation, characterized by secretion of IL-4. These pathways are mutually inhibitory and self-reinforcing.
Figure 1. Diagram of major Th effector subsets.
T cell precursors (Thp) can be induced to differentiate into different subsets depending on the cytokine environment. IL-12 drives development of Th1 cells through STAT-4 (and other transcription factors, not shown). IL-4 promotes development of Th2 cells through STAT6, and the combination of IL-1/IL-6/TGFβ induce Th17 cell differentiation through STAT3. The signature cytokines of each lineage are shown (note, IL-26 is only expressed in humans).
In 2005, a third subset was identified that was clearly neither Th1 nor Th2. This Th lineage could be generated in the absence of either STAT-6 or STAT-4, its development is inhibited by IFNγ and IL-4, and results in T cells that produce IL-17 (also known as IL-17A) and IL-17F (and later, IL-22) as their signature cytokines (Fig. 1) (2, 3). These IL-17-producing T cells are generated in response to signals from TGFβ, IL-6 and IL-1β, and require the transcription factors STAT3, RORγt and RORα (reviewed in (4–6)). Dubbed “Th17,” the discovery of these cells resolved many of the discrepancies that were extant in the field, detailed in many prior review articles (7–9). Th17 cells also produce additional cytokines, including IL-17F, IL-22, IL-21, IL-26 (in humans), and at least some cells can co-produce IFNγ (4). Although usually depicted as being separate lineages, there is considerable plasticity and interconversion that can occur among subsets, which is an area of active exploration in the field (10).
New findings have identified several other types of effector Th cells with different profiles of cytokines such as Th9 cells and T follicular helper cells (TFH), among others (11, 12). Although this has led to inevitable complexity and some confusion, it is hardly surprising that nuanced immune responses require complex responses. In addition to CD4+ effector populations, there are subsets of CD8+ T cytotoxic cells characterized by differential cytokine production, such as Tc1, Tc2, Tc17 (13). Collectively, these cells orchestrate immune responses in ways that are still poorly defined and that differ by immunological setting, and probably also by the genetic makeup of the host.
Although IL-17 was initially thought to be produced almost exclusively by T cells (14), it has lately become appreciated that this cytokine is also made by a variety of innate cell types. These include γδ-T cells, NKT cells, lymphoid tissue inducer (LTi) cells, macrophages, and possibly DCs and neutrophils (15). Whereas induction of IL-17 from T cells requires signaling through the antigen specific TCR (16), induction of IL-17 from these other cell types is antigen-independent.
The functional roles and regulation of the three main Th subsets are still under intense investigation, but it is now clear that their major activities are as follows: Th1 cells are the primary mediators providing immunity against intracellular pathogens (viruses, intracellular bacteria), Th2 cells mediate allergic responses and immunity to helminths, and Th17 cells mediate pathology in autoimmunity and resistance to a variety of extracellular pathogens (bacteria, some parasites and fungi) (5, 11). The focus of this review will be on Th17 cells and IL-17, and immune responses to fungal infections caused by the yeast Candida albicans.
IL-17 and candidiasis
Candida albicans is a commensal fungus in humans, found mainly in the oropharynx, skin and vaginal mucosa. In healthy individuals, it causes no pathology. Oropharyngeal candidiasis (OPC, thrush) is an AIDS-defining illness, afflicting >90% of individuals with HIV. OPC is also common in patients on chemotherapy, individuals at the extremes of age, and other immunodeficient settings. OPC is associated with considerable morbidity, and in infants can result in failure to thrive. Candida also causes chronic mucocutaneous (CMC) infections of skin and fingernails (onychomycosis), as well as recurrent vaginal infections (17). The most severe form of candidiaisis is disseminated candidemia, which is the fourth most common nosocomial infection, and is associated with a high mortality rate (averaging ~40%). Strikingly, immunity to Candida appears to differ based on the anatomic compartment. Whereas more the majority of HIV+ individuals experience OPC at least once, vaginal candidiasis is far less common, and disseminated candidemia is not a risk in these patients (17). Similarly, patients with congenital immunodeficiencies that are prone to CMC and OPC (see below, Table 1) do not generally experience systemic infections (17). This makes Candida albicans an ideal model organism with which to understand compartment-specific immunity.
Table 1.
Human genetic diseases that cause candidiasis.
| Disease | Gene Product | Nature of mutation | Mechanism related to IL-17 | Reference |
|---|---|---|---|---|
| Hyper-IgE Syndrome/Jobs Syndrome (AD)a | STAT3 (signal transducer and activator of transcription) | DNb | Failure to signal through IL-6, IL-21, IL-22, IL-23; severely reduced Th17 cell numbers | (61–63) |
| Hyper-IgE Syndrome (AR)c | Tyk2 (Janus tyrosine kinase) | LOFd | Failure to signal through Type I IFNs and IL-23; reduced Th17 numbers | (64, 65) |
| IL-17R-deficiency | IL-17RA | LOF | Failure to signal in response to IL-17A, IL-17F, IL-17A/F (possibly IL-25?) | (66) |
| IL-17F-deficiency | IL-17F | DN | Reduced signaling in response to IL-17A, IL-17A/F, IL-17F | (66) |
| Dectin-1-deficiency | Dectin-1 | LOF | Loss of responsiveness to Candida, reduced IL-6 and Th17 cell frequency | (70) |
| CARD9-deficiency | CARD9 | LOF | Loss of signaling through Dectins, TCR, Mincle; reduced Th17 differentiation | (71) |
| APS-1/APECEDe | Aire (autoimmune regulator) | LOF | Loss of central tolerance, Abs against IL-17A, IL-17F, IL-22 | (68, 69) |
AD, autosomal dominant,
dominant negative;
autosomal recessive;
loss of function;
autoimmune polyendocrine syndrome I
The most common murine model of candidiasis entails injection of conidial yeast forms of Candida into the tail vein (18). Dissemination occurs to all major organs, predominantly liver, and is fatal in high doses. Even before Th17 cells were recognized, IL-17RA-deficient mice were shown to be more susceptible to disseminated Candida infections than WT mice, which could be ameliorated by administration of IL-17A. This high susceptibility was associated with neutrophil defects (19, 20).
The high incidence of OPC in HIV/AIDS patients implicates CD4+ T cells in immunity. Accordingly, for many years, Th1 cells were considered to be the dominant effector cells involved in host defense. This was in part because IFNγ is highly expressed at thrush lesions, and studies in mice indicated that Th2-deficient mice (IL-4−/−) mice are resistant to experimental OPC, whereas IL-12p40−/− mice are sensitive. However, IFNγ−/− mice are resistant to OPC (21). An possible explanation for this apparent paradox began with the discovery that the IL-12p40 subunit, a constituent of the IL-12 heterodimer and required for Th1 development, is shared with IL-23 (Fig 1) (22). IL-23 is required for the activity of Th17 cells and their maintenance in vivo (15). Therefore, these findings could be better explained if Th17 cells rather than Th1 cells – which are both absent in IL-12p40−/− mice – are required for immunity to mucosal candidiasis.
Accordingly, using a quantitative mouse model of OPC (23), our group showed that IL-12p35−/− mice (lacking IL-12 and Th1 cells but retaining IL-23 and Th17 cells, Fig. 1) are resistant to thrush, although these mice show delayed clearance of Candida (24, 25). Moreover, IL-23−/− mice, IL-17 receptor-deficient mice and RORγt−/− mice are highly sensitive to thrush (Refs. (25, 26); ACP and NHS, unpublished data). Strikingly, Treg cells also promote immunity to OPC by virtue of enhancing Th17 responses, further verifying the importance of this cytokine (27). Therefore, IL-23 and IL-17 signaling appear to be vital for immunity to Candida, at least in a mouse model of oral candidiasis. Somewhat surprisingly, IL-22−/− mice did not show high susceptibility to OPC in this model, despite reduced levels of IL-22 in human CMC patients (28) and the generally high responsiveness of epithelial cells to IL-22 (25, 29). Similar results were found in studies of skin candidiasis in mice (30). Moreover, an experimental vaccine targeting Candida depends on intact Th17 responses for efficacy in mice (31), which is also true in other fungal infection vaccine settings as well (32). Intriguingly, Candida albicans appears to try to counteract the effects of IL-17 by secreting an as-yet unidentified factor that skews the immune response away from a Th17-biased response (33). Accordingly, IL-17 and IL-17 receptor signaling appear to be critical for immunity to oral and skin forms of fungal infections in murine models.
Although the predominance of data in mice argues in favor of a protective role for IL-17, collateral damage due to the inflammatory effects of IL-17 may also enhance disease. In a gastric model of mucosal candidiasis, IL-17 appears to cause more harm than good, whereas IL-22 and IFNγ serve as tissue-protective cytokines (34–36). Mouse models of vaginal candidiasis have been problematic, but so far no data have been reported indicating a role for IL-17 in this form of candidiasis (37, 38). Thus, immunity to Candida varies by mucosal site.
The major mechanisms of IL-17-mediated immunity appear to be through activation of neutrophils and regulation of anti-microbial peptides (AMPs) (24, 25). It has long been recognized that IL-17 is a potent regulator of the neutrophil response. However, IL-17 does not appear to act directly on neutrophils. Rather, activation of epithelial or mesenchymal cells via IL-17 signaling leads to enhancement of ICAM-1, CXC chemokines (IL-8, CXCL1/Groα, CXCL5, CXCL2) and G-CSF, which together promote recruitment, chemotaxis and expansion of the neutrophil lineage. In a variety of mouse models, IL-17-deficiency results in severe neutrophil deficits (39, 40). In addition, IL-17 and IL-22 are potent regulators of AMP expression. In skin, IL-22 and IL-17 cooperatively enhance expression of AMPs such as S100A7, S100A8 and S100A9 as well as β-defensins (41). In the OPC model, susceptibility is tightly linked to PMN defects but also defects in AMPs such as S100A8/9 proteins, β-defensins and salivary histatins (25, 42, 43).
C-type lectin receptors and Candida pattern recognition
The pattern recognition receptors (PRRs) for yeast have only recently been elucidated. Although Toll-like receptors and the mannose receptor play roles in mediating immune responses to Candida (44, 45), the C-type lectin receptors (CLRs) appear to be the dominant PRRs implicated in C. albicans recognition (46, 47). These receptors are expressed on antigen presenting cells and recognize components of the C. albicans cell wall such as mannans and β-glucans. N-linked mannans, which are located in the outer portion of the cell wall, are recognized by DC-SIGN, the macrophage mannose recognition receptor (MMR) and Dectin-2 (47). β-glucans, located in the inner portion of the cell wall, are recognized by Dectin-1 and Mincle (48). Engagement of these receptors by their corresponding ligands results in the production of IL-6, TNFα, IL-23, and IL-17, which are important for Th17 development and homeostasis, or are products of the Th17 response (45, 49–51). One line of Dectin-1-knockout mice did not exhibit susceptibility to disseminated candidemia (52), but a different line did (48). Dectin-2-knockout and Mincle-knockout mice both exhibit sensitivity to candidiasis, using disseminated and oral models, respectively (53, 54). Upon oral infection of mice with Candida, there is strong upregulation of Candida-sensing CLRs, including Dectin-1, Dectin-2 and Mincle. These CLRs are upregulated normally in IL-17RA-deficient mice (25), thus placing IL-17 downstream of CLR signaling.
The cascade of events that precedes cytokine production and the induction of Th17 responses upon CLR engagement have not been described in detail. However, both Dectin-1 and Dectin-2 couple to spleen tyrosine kinase (Syk) and the adaptor molecule CARD-9 to induce production of TNFα, IL-6 and IL-23 (49, 51). In addition, studies implicate the NRLP3 inflammasome and IL-1β in inducing responses to OPC, which is also a Th17-inducing cytokine (55, 56). Clearly innate recognition of Candida is complex and mediated by a number of pattern recognition receptors, and doubtless more details on this pathway will emerge in the near future.
Candidiasis in humans with immunologic deficiencies
In addition to HIV, there are a number of rare primary genetic diseases in humans that cause susceptibility to recurrent mucosal forms of candidiasis (Table 1). The genetic mutations responsible for these syndromes can be segregated into two categories: (i) those affecting the Th17 signaling pathway, and (ii) those affecting direct recognition of C. albicans by immune cells. Of the mutations that affect the Th17/IL-17 pathway, mutations in the gene encoding STAT3 are most common, and cause autosomal-dominant Hyper IgE Syndrome (AD-HIES). AD-HIES is associated with a constellation of clinical features, particularly mucocutaneous candidiasis, eczema, recurrent Staphylococcus aureus infections, high serum IgE levels and characteristic facial and skeletal abnormalities. Although AD-HIES patients suffer from recurrent OPC, disseminated candidiasis and other fungal infections are rare in these patients (57–59). In the majority of patients with AD-HIES, dominant negative mutations in the DNA binding or SH2 domains of STAT3 lead to over 50% reduction in STAT3 activity. This reduction leads to Th17 and IL-17 deficiency due to defects in IL-6 and IL-23 signaling, which are required for induction and stabilization of the Th17 lineage (60–63).
The autosomal recessive form of HIES (AR-HIES) is also characterized by increased susceptibility to mucocutaneous candidiasis, increased IgE, severe eczematous rashes from birth and recurrent skin and sinopulmonary infections. However, these patients do not exhibit the characteristic facial or bony tissue abnormalities present in AD-HIES patients, but do have a strong propensity for recurrent cutaneous viral infections. The genetic basis for AR-HIES has not been characterized in all cases, but one patient with a documented homozygous Tyk2 mutation who presented with an AR-HIES-like syndrome has been reported (64) (Table 1). Tyk2 is a Janus kinase family member that phosphorylates several transcription factors, including STAT3. Tyk2 is downstream of IL-23 and type I interferons, explaining susceptibility to both fungal and viral pathogens. Although this report prompted further genetic examination of patients with AR-HIES, Tyk2 mutations were not found in any of the patients screened to date (65).
Recently, Puel, et al. reported the first documented human deficiency in the IL-17 cytokine family (IL-17F) and also the IL-17 receptor (IL-17RA) (66, 67). In these cases, the genetic lesion in IL-17F is an autosomal dominant negative mutation, whereas the IL-17RA deficiency is a loss of function, autosomal recessive allele (Table 1). Both present with chronic mucocutaneous candidiasis (CMC). CMC presents clinically with recurrent C. albicans infections at the mucosal surfaces, including the oral and genital tracts, and the skin. These inborn defects in IL-17, as the only immunologic defects present in these two patient populations were related to the IL-17 signaling pathway for both IL-17A and IL-17F.
Another striking correlation between IL-17 and CMC came from an unexpected study. Namely, patients with mutations in the AIRE gene, which regulates central tolerance, also suffer from CMC (66, 68, 69). Proper expression of AIRE in the thymus is essential for presentation of peripheral self antigens and therefore maintaining immune tolerance. Accordingly, mutations in AIRE result in the development of multi-system autoimmunity, known as autoimmune polyendocrine syndrome type I (APS-I). However, molecular basis of these patients’ increased susceptibility to CMC has long been obscure. A recent study by two groups showed that APS-I patients universally demonstrated high titers of auto-antibodies specific for the Th17 cytokines IL-17A, IL-17F and IL-22. These antibodies were highly specific and strongly neutralizing for IL-17 signaling, supporting a major role for IL-17 in preventing CMC in humans. Notably, such auto-antibodies were essentially undetectable in both normal controls and in patients with autoimmune disorders other than APS-I. Interestingly, antibodies against these Th17 cytokines were also found in a subset of thymoma patients, who also had CMC. Thus, naturally occurring anti-Th17 antibodies strongly predispose to infections with Candida albicans.
The other class of genetic deficiencies that are associated with increased susceptibility to C. albicans infections are mutations in pathways responsible for pattern recognition of this organism, specifically in Dectin-1 and its adaptor CARD-9 (70, 71). Mutations in Dectin-1, which specifically recognizes C. albicans cell wall β-glucan moieties, are associated with increased vulvovaginal candidiasis as well as increased risk of onchomycosis (70, 72). Patients with these mutations demonstrate not only severe, recurrent mucosal C. albicans infections, but also invasive systemic fungal infections, including candida meningitis (71). Notably, patients with both Dectin-1 and CARD-9 mutations demonstrated defects in generation of effective IL-17 responses. Specifically, patients with Dectin-1 deficiency had reduced IL-6 and IL-17 production in monocytes and macrophages challenged with either β-glucan or heat-killed C. albicans, and patients with CARD-9 deficiencies exhibited defects in Th17 differentiation. From these patient studies, it is clear that the role of PRRs are not only essential for mounting a proper immune response to C. albicans, but that these PRRs act in a cumulative manner, rather in isolation, to mount a Th17 response that protects not only mucosal sites, but also from systemic infection.
Th17 in Candida vaccines
To date, there are no effective vaccines against fungi. However, some experimental vaccines against Candida and other fungal pathogens have been shown to involve IL-17. Vaccination studies using the N-terminus of Als3p, a Candida adhesion protein, have been demonstrated to elicit both Th1 and Th17 responses when administered subcutaneously (31, 73). Interestingly, among both canonical CCR6-IL-17- Th1 cells and CCR6+IFNγ− Th17 cells, a population of CCR6+ Th1/17 cells producing both IL-17 and IFNγ were also induced by vaccination. Vaccination-induced production of these Th1, Th17 and Th1/17 cells was associated with increased survival under systemic C. albicans challenge as well as increased expression of the neutrophil chemoattractants KC (CXCL1) and MIP-1α. These results suggest that for a comprehensive vaccination approach to C. albicans, vaccines and their adjuvants must jointly stimulate both a robust Th1 and a Th17 response. Similarly, vaccination studies for several other endemic fungal infections have also been shown to involve Th17 cells (32), indicating this may be a common mechanism of immunity to fungal organisms.
Summary & perspectives
In summary, studies in the last several years have radically shifted our understanding of immunity to Candida albicans, which is leading the way for studies on other fungal pathogens as well. The involvement of C-type lectin receptors and the Th17 responses have revealed a previously unappreciated pathway that appears to comprise the essential elements of protection against cutaneous candidiasis. Taken together, these studies in animal models and lessons from human primary immunodeficiencies demonstrate the crucial role of Th17 generation and specifically IL-17 signaling in the immunologic response to C. albicans. These findings have direct clinical implications extending beyond rare congenital immunodeficiency syndromes, as antibodies to IL-17 and its receptor are in clinical trials to treat psoriasis, rheumatoid arthritis and other autoimmune diseases (74), which may predispose patients to candidal infections.
Acknowledgments
SLG was supported by the National Institutes of Health grants AI89768 and AR054389. NHS is supported by AR054389.
Abbreviations
- OPC
oropharygeal candidiasis
- CMC
chronic mucocutaneous candidiaisis
- HIES
Hyper-IgE Syndrome
- APS-1
autoimmune polyendocrine syndrome-1
- AMP
antimicrobial peptide
- PRR
pattern recognition receptor
- AR
autosomal recessive
- LOF
loss of function
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 2.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature immunology. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annual review of immunology. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–518. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 6.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–33. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 7.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 8.Gor DO, Rose NR, Greenspan NS. TH1–TH2: a procrustean paradigm. Nature immunology. 2003;4:503–5. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 9.Gaffen SL, Kramer JM, Yu JJ, Shen F. The IL-17 Cytokine Family. In: Litwack G, editor. Vitamins and Hormones. London: Academic Press; 2006. pp. 255–82. [DOI] [PubMed] [Google Scholar]
- 10.Hirahara K, Ghoreschi K, Laurence A, Yang XP, Kanno Y, O’Shea JJ. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21:425–34. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–11. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–8. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrikant PA, Rao R, Li Q, Kesterson J, Eppolito C, Mischo A, et al. Regulating functional cell fates in CD8 T cells. Immunol Res. 2010;46:12–22. doi: 10.1007/s12026-009-8130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Lin X, Gaffen SL. Crucial role for nuclear factor of activated T cells (NFAT) in T cell receptor-mediated regulation of the human interleukin-17 gene. The Journal of biological chemistry. 2004;279:52762–71. doi: 10.1074/jbc.M405764200. [DOI] [PubMed] [Google Scholar]
- 17.Dongari-Bagtoglou A, Fidel P. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J Dent Res. 2005;84:966–77. doi: 10.1177/154405910508401101. [DOI] [PubMed] [Google Scholar]
- 18.Clancy C, Cheng S, Nguyen M. Animal Models of Candidiasis. In: Cihlar R, Calderone R, editors. Candida albicans: Methods and Protocols. Humana Press; 2009. pp. 65–76. [Google Scholar]
- 19.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 20.van de Veerdonk FL, Kullberg BJ, Verschueren IC, Hendriks T, van der Meer JW, Joosten LA, et al. Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock. 2010;34:407–11. doi: 10.1097/SHK.0b013e3181d67041. [DOI] [PubMed] [Google Scholar]
- 21.Farah C, Hu Y, Riminton S, Ashman R. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene targeting. Oral Microbiol Immunol. 2006;21:252–5. doi: 10.1111/j.1399-302X.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 22.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 23.Kamai Y, Kubota M, Kamai Y, Hosokawa T, Fukuoka T, Filler S. New model of oropharyngeal candidiasis in mice. Anti-microb Agents Chemo. 2001;45:3195–7. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti HR, Gaffen SL. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010;12:518–27. doi: 10.1016/j.micinf.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conti H, Shen F, Nayyar N, Stocum E, JNS, Lindemann M, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. The Journal of experimental medicine. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho A, Shen F, Conti H, Patel N, Childs E, Peterson A, et al. IL-17RC is required for immune signaling via an extended SEFIR domain in the cytoplasmic tail. J Immunol. 2010;185:1063–70. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandiyan P, Conti H, Zheng L, Peterson A, Mathern D, Hernandez-Santos N, et al. CD4+CD25+Foxp3+ regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 infection model. Immunity. 2011;34:422–34. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H, et al. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. The Journal of investigative dermatology. 2008;128:2640–5. doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- 29.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–82. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–62. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, et al. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis. 2006;194:256–60. doi: 10.1086/504691. [DOI] [PubMed] [Google Scholar]
- 32.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. The Journal of clinical investigation. 2011;121:554–68. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng SC, van de Veerdonk F, Smeekens S, Joosten LA, van der Meer JW, Kullberg BJ, et al. Candida albicans Dampens Host Defense by Downregulating IL-17 Production. J Immunol. 2010;185:2450–7. doi: 10.4049/jimmunol.1000756. [DOI] [PubMed] [Google Scholar]
- 34.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 35.Zelante T, Iannitti R, De Luca A, Romani L. IL-22 in antifungal immunity. Eur J Immunol. 2011;41:270–5. doi: 10.1002/eji.201041246. [DOI] [PubMed] [Google Scholar]
- 36.De Luca A, Zelante T, D’Angelo C, Zagarella S, Fallarino F, Spreca A, et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010;3:361–73. doi: 10.1038/mi.2010.22. [DOI] [PubMed] [Google Scholar]
- 37.de Repentigny L. Animal models in the analysis of Candida host-pathogen interactions. Cur Op Microbiol. 2004;7:324–9. doi: 10.1016/j.mib.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Fidel PL, Jr, Cutler JE. Prospects for development of a vaccine to prevent and control vaginal candidiasis. Curr Infect Dis Rep. 2011;13:102–7. doi: 10.1007/s11908-010-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Yu J, Gaffen SL. Interleukin-17: A novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci. 2008;13:170–7. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- 41.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. The Journal of experimental medicine. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saunus JM, Kazoullis A, Farah CS. Cellular and molecular mechanisms of resistance to oral Candida albicans infections. Front Biosci. 2008;13:5345–58. doi: 10.2741/3085. [DOI] [PubMed] [Google Scholar]
- 43.Conti H, Baker O, Freeman A, Jang W, Li R, Holland S, et al. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011 doi: 10.1038/mi.2011.5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, et al. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. The Journal of clinical investigation. 2007;117:3664–72. doi: 10.1172/JCI28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007;196:1565–71. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 48.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nature immunology. 2007;8:31–8. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leibundgut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nature immunology. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 50.Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. The Journal of biological chemistry. 2008;283:20590–9. doi: 10.1074/jbc.M709334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. The Journal of experimental medicine. 2009;206:2037–51. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nature immunology. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 53.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, et al. The Macrophage-Inducible C-Type Lectin, Mincle, Is an Essential Component of the Innate Immune Response to Candida albicans. J Immunol. 2008;180:7404–13. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 55.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–97. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–6. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 57.Freeman AF, Holland SM. The hyper-IgE syndromes. Immunology and allergy clinics of North America. 2008;28:277–91. viii. doi: 10.1016/j.iac.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freeman AF, Kleiner DE, Nadiminti H, Davis J, Quezado M, Anderson V, et al. Causes of death in hyper-IgE syndrome. J Allergy Clin Immunol. 2007;119:1234–40. doi: 10.1016/j.jaci.2006.12.666. [DOI] [PubMed] [Google Scholar]
- 59.Desai K, Huston D, Harriman G. Previously undiagnosed hyper-IgE syndrome in an adult with multiple systemic fungal infections. J Allergy Clin Immunol. 1996;98:1123–4. doi: 10.1016/s0091-6749(96)80202-8. [DOI] [PubMed] [Google Scholar]
- 60.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 61.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. The Journal of experimental medicine. 2008;205:1551–7. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC, et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122:181–7. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–55. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Woellner C, Schaffer AA, Puck JM, Renner ED, Knebel C, Holland SM, et al. The hyper IgE syndrome and mutations in TYK2. Immunity. 2007;26:535. doi: 10.1016/j.immuni.2007.05.007. author reply 6. [DOI] [PubMed] [Google Scholar]
- 66.Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22:467–74. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puel A, Cypowji S, Bustamante J, Wright J, Liu L, Lim H, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011 doi: 10.1126/science.1200439. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. The Journal of experimental medicine. 2010;207:291–7. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. The Journal of experimental medicine. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–7. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, et al. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–32. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 73.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]