Abstract
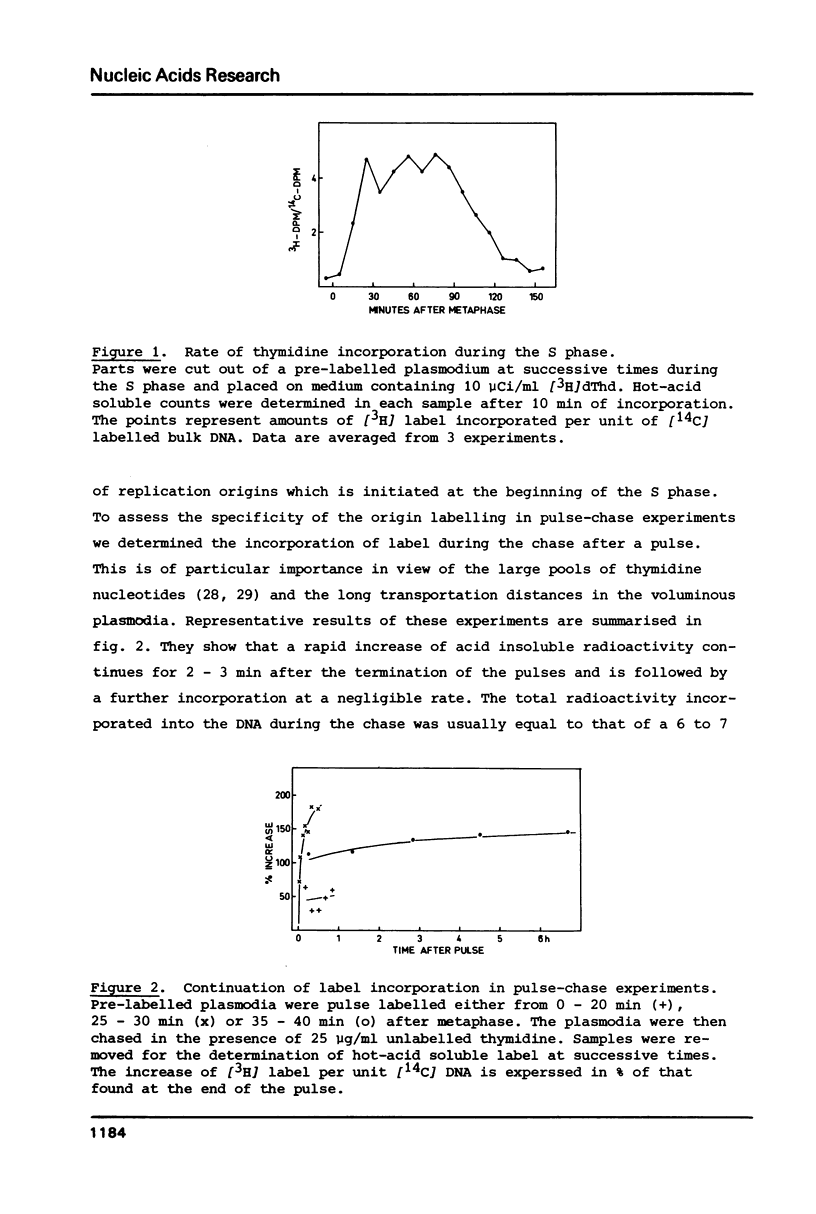
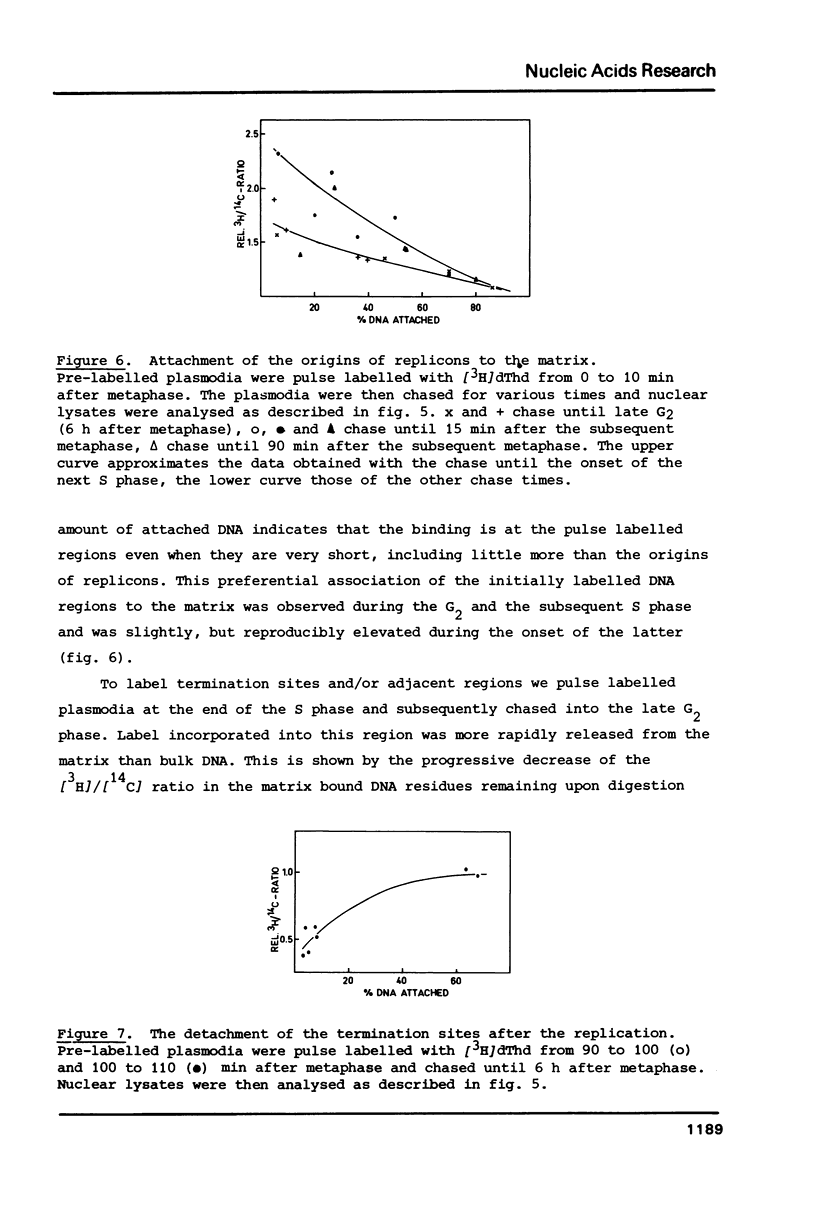
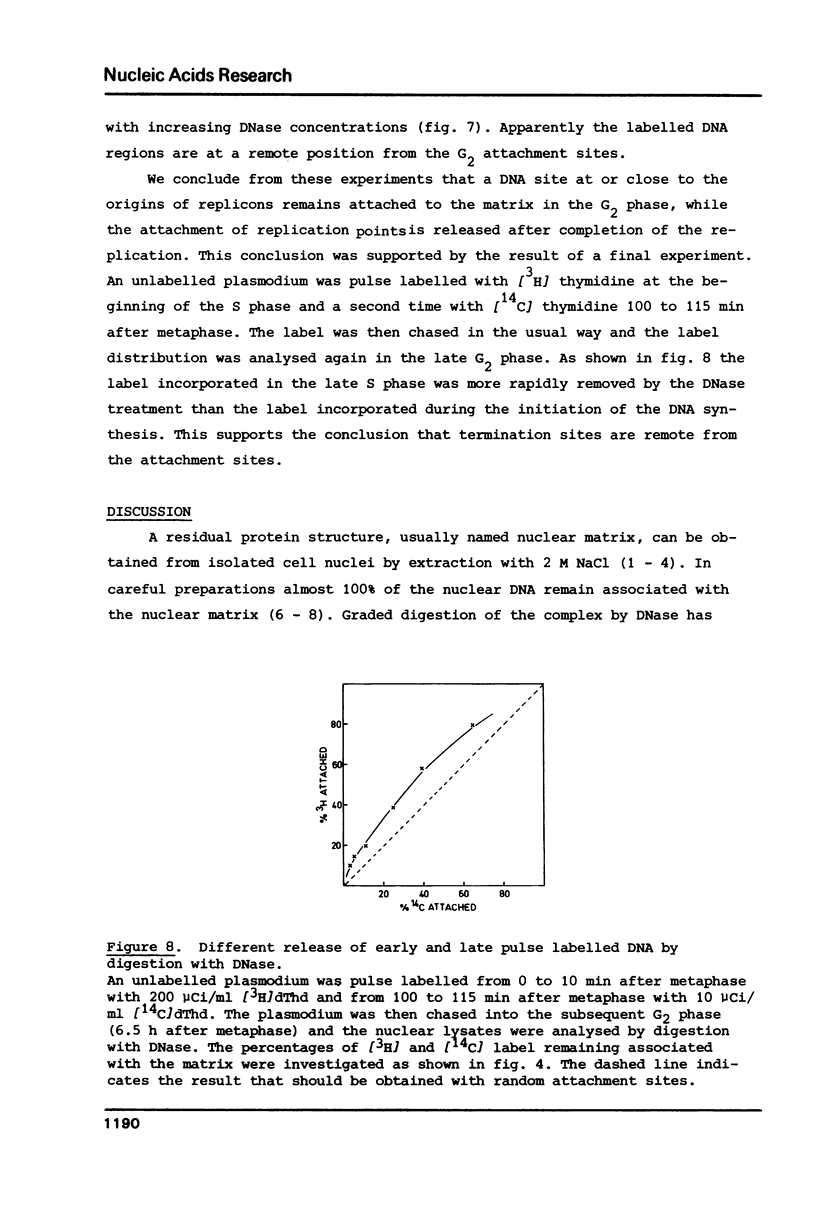
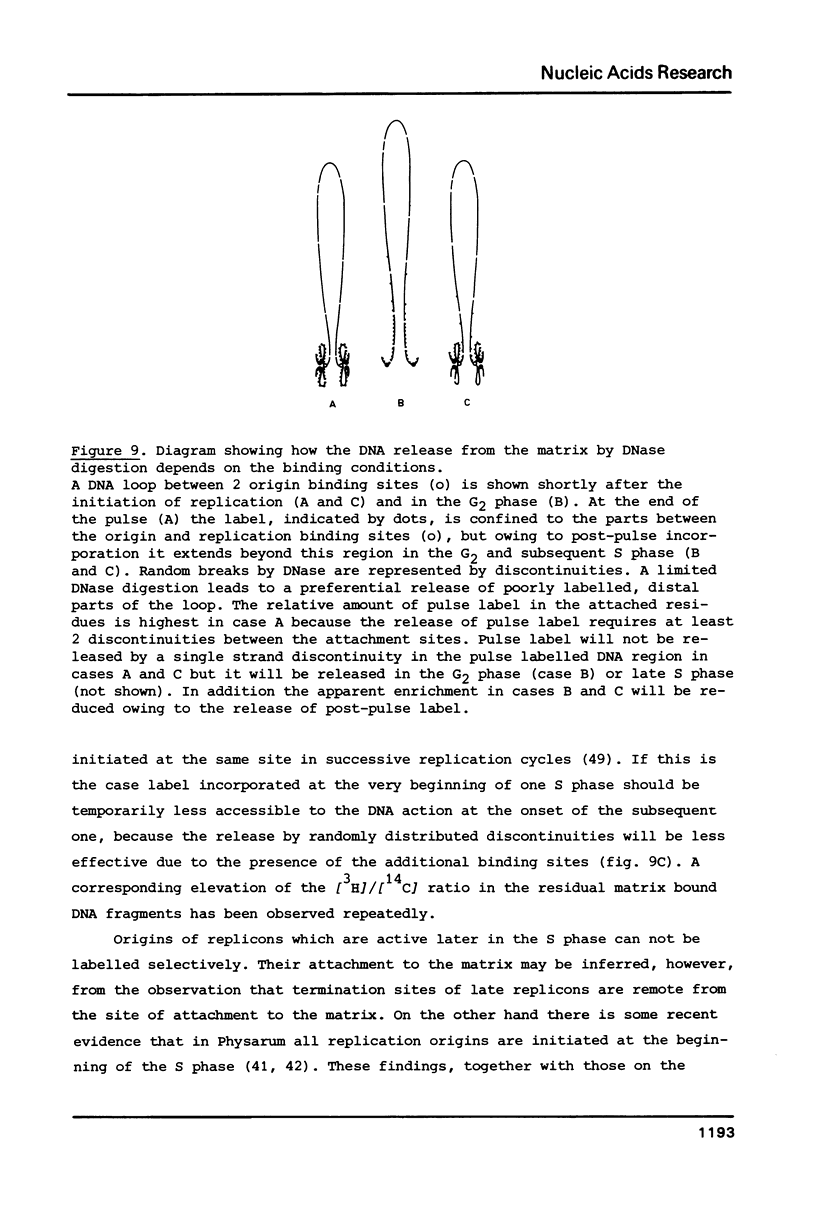
We have investigated the attachment of the DNA to the nuclear matrix during the division cycle of the plasmodial slime mold Physarum polycephalum. The DNA of plasmodia was pulse labelled at different times during the S phase and the label distribution was studied by graded DNase digestion of the matrix-DNA complexes prepared from nuclei isolated by extraction with 2 M NaCl. Pulse labelled DNA was preferentially recovered from the matrix bound residual DNA at any time of the S phase. Label incorporated at the onset of the S phase remained preferentially associated with the matrix during the G2 phase and the subsequent S phase. The occurrence of the pulse label in the matrix associated DNA regions was transiently elevated at the onset of the subsequent S phase. Label incorporated at the end of the S phase was located at DNA regions which, in the G2 phase, were preferentially released from the matrix by DNase treatment. From the results and previously reported data on the distribution of attachment sites it can be concluded that origins of replicons or DNA sites very close to them are attached to the matrix during the entire nuclear cycle. The data further indicate that initiations of DNA replication occur at the same origins in successive S phases. Replicating DNA is bound to the matrix, in addition, by the replication fork or a region close to it. This binding is loosened after completion of the replication.
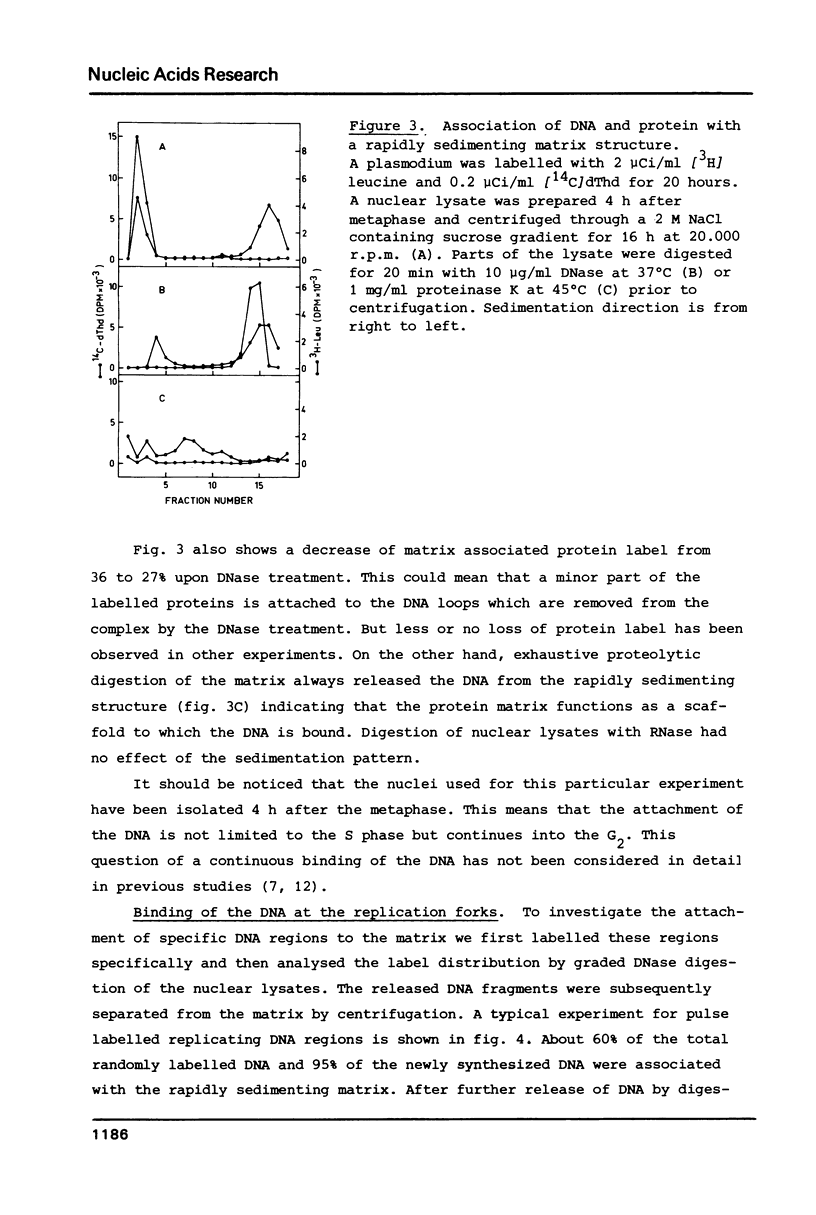
Full text
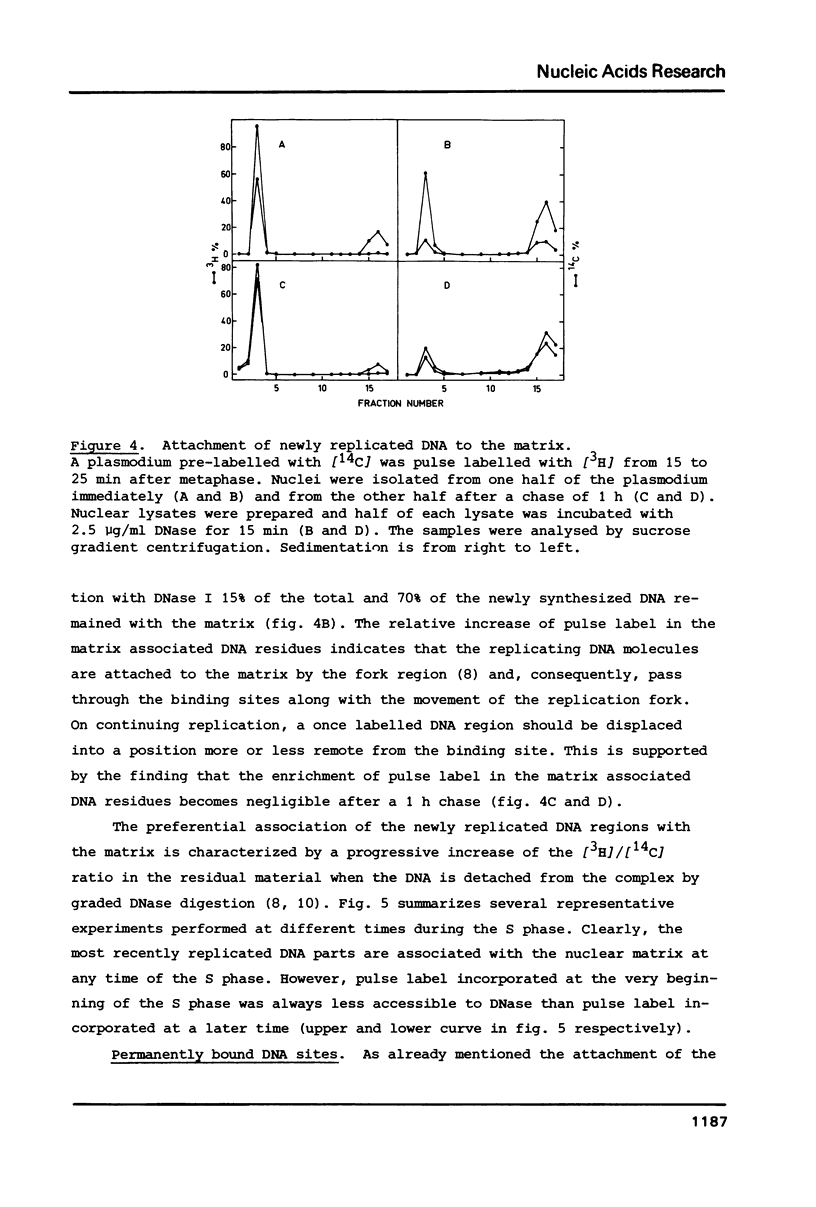
PDF



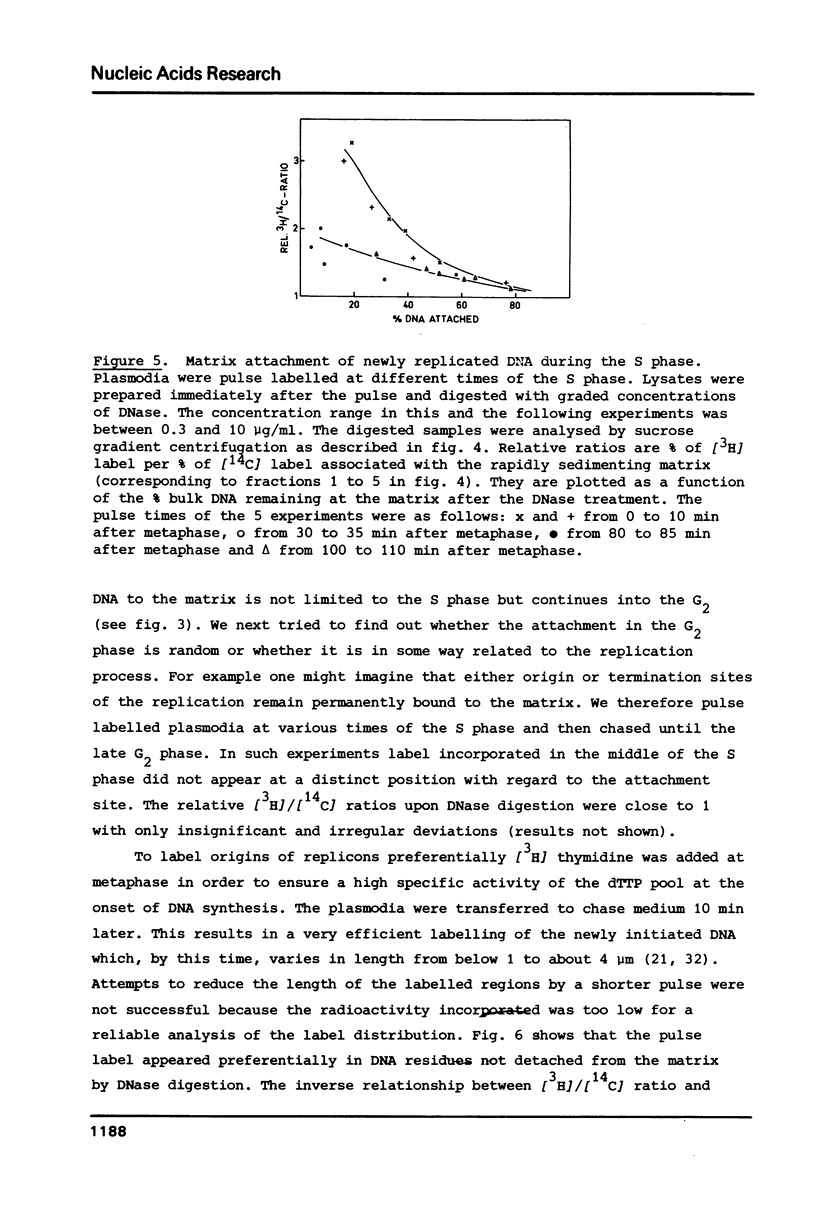




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaldi F., Buongiorno-Nardelli M., Carnevali F., Leoni L., Mariotti D., Pomponi M. Replicon origins in Chinese hamster cell DNA. II. Reproducibility. Exp Cell Res. 1973 Jul;80(1):79–87. doi: 10.1016/0014-4827(73)90277-2. [DOI] [PubMed] [Google Scholar]
- Beach D., Piper M., Shall S. Isolation of newly-initiated DNA from the early S phase of the synchronous eukaryote, Physarum polycephalum. Exp Cell Res. 1980 Sep;129(1):211–221. doi: 10.1016/0014-4827(80)90344-4. [DOI] [PubMed] [Google Scholar]
- Bekers A. G., Gijzen H. J., Taalman R. D., Wanka F. Ultrastructure of the nuclear matrix from Physarum polycephalum during the mitotic cycle. J Ultrastruct Res. 1981 Jun;75(3):352–362. doi: 10.1016/s0022-5320(81)80091-3. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear protein matrix: association with newly synthesized DNA. Science. 1975 Jul 25;189(4199):291–293. doi: 10.1126/science.1145202. [DOI] [PubMed] [Google Scholar]
- Bersier D., Braun R. Pools of deoxyribonucleoside triphosphates in the mitotic cycle of Physarum. Biochim Biophys Acta. 1974 Apr 10;340(4):463–471. doi: 10.1016/0005-2787(74)90067-7. [DOI] [PubMed] [Google Scholar]
- Braun R., Evans T. E. Replication of nuclear satellite and mitochondrial DNA in the mitotic cycle of Physarum. Biochim Biophys Acta. 1969 Jun 17;182(2):511–522. doi: 10.1016/0005-2787(69)90203-2. [DOI] [PubMed] [Google Scholar]
- Braun R., Mittermayer C., Rusch H. P. Ribonucleic acid synthesis in vivo in the synchronously dividing Physarum polycephalum studied by cell fractionation. Biochim Biophys Acta. 1966 Mar 21;114(3):527–535. doi: 10.1016/0005-2787(66)90101-8. [DOI] [PubMed] [Google Scholar]
- Braun R., Wili H. Time sequence of DNA replication in Physarum. Biochim Biophys Acta. 1969 Jan 21;174(1):246–252. doi: 10.1016/0005-2787(69)90248-2. [DOI] [PubMed] [Google Scholar]
- Buckler-White A. J., Humphrey G. W., Pigiet V. Association of polyoma T antigen and DNA with the nuclear matrix from lytically infected 3T6 cells. Cell. 1980 Nov;22(1 Pt 1):37–46. doi: 10.1016/0092-8674(80)90152-x. [DOI] [PubMed] [Google Scholar]
- Buongiorno-Nardelli M., Micheli G., Carri M. T., Marilley M. A relationship between replicon size and supercoiled loop domains in the eukaryotic genome. Nature. 1982 Jul 1;298(5869):100–102. doi: 10.1038/298100a0. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Mapping sequences in loops of nuclear DNA by their progressive detachment from the nuclear cage. Nucleic Acids Res. 1980 Jul 11;8(13):2895–2906. doi: 10.1093/nar/8.13.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel P. A., Mullenders L. H., Wanka F. Analysis of the attachment of replicating DNA to a nuclear matrix in mammalian interphase nuclei. Nucleic Acids Res. 1979 Jan;6(1):219–230. doi: 10.1093/nar/6.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingman C. W. Bidirectional chromosome replication: some topological considerations. J Theor Biol. 1974 Jan;43(1):187–195. doi: 10.1016/s0022-5193(74)80052-4. [DOI] [PubMed] [Google Scholar]
- Fink K. Fluctuations in deoxyribo- and ribonucleoside triphosphate pools during the mitotic cycle of Physarum polycephalum. Biochim Biophys Acta. 1975 Nov 18;414(1):85–89. doi: 10.1016/0005-2787(75)90127-6. [DOI] [PubMed] [Google Scholar]
- Fink K., Nygaard P. Pyrimidine metabolism in microplasmodia of Physarum polycephalum. Eur J Biochem. 1978 Sep 1;89(2):417–424. doi: 10.1111/j.1432-1033.1978.tb12544.x. [DOI] [PubMed] [Google Scholar]
- Funderud S., Andreassen R., Haugli F. DNA replication in Physarum polycephalum: UV photolysis of maturing 5-bromo-deoxyuridine substituted DNA. Nucleic Acids Res. 1978 Sep;5(9):3303–3313. doi: 10.1093/nar/5.9.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderud S., Andreassen R., Haugli F. Size distribution and maturation of newly replicated DNA through the S and G2 phases of Physarum polycephalum. Cell. 1978 Dec;15(4):1519–1526. doi: 10.1016/0092-8674(78)90074-0. [DOI] [PubMed] [Google Scholar]
- Haugli F., Andreassen R., Funderud S. DNA replication in Physarum polycephalum: electron microscopic analysis of patterns of DNA replication in the presence of cycloheximide. J Cell Biol. 1982 Oct;95(1):323–331. doi: 10.1083/jcb.95.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C. E., Gurney E. G. Minor components of the DNA of Physarum polycephalum. Cellular location and metabolism. J Cell Biol. 1969 Feb;40(2):484–496. doi: 10.1083/jcb.40.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Hunt B. F., Vogelstein B. Association of newly replicated DNA with the nuclear matrix of Physarum polycephalum. Nucleic Acids Res. 1981 Jan 24;9(2):349–363. doi: 10.1093/nar/9.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igó-Kemenes T., Zachau H. G. Domains in chromatin structure. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):109–118. doi: 10.1101/sqb.1978.042.01.012. [DOI] [PubMed] [Google Scholar]
- McCready S. J., Godwin J., Mason D. W., Brazell I. A., Cook P. R. DNA is replicated at the nuclear cage. J Cell Sci. 1980 Dec;46:365–386. doi: 10.1242/jcs.46.1.365. [DOI] [PubMed] [Google Scholar]
- Mitchelson K. R., Bekers A. G., Wanka F. Isolation of a residual protein structure from nuclei of the myxomycete Physarum polycephalum. J Cell Sci. 1979 Oct;39:247–256. doi: 10.1242/jcs.39.1.247. [DOI] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Isolation and DNA content of nuclei of Physarum polycephalum. Exp Cell Res. 1971 Jun;66(2):305–316. doi: 10.1016/0014-4827(71)90682-3. [DOI] [PubMed] [Google Scholar]
- NYGAARD O. F., GUTTES S., RUSCH H. P. Nucleic acid metabolism in a slime mold with synchronous mitosis. Biochim Biophys Acta. 1960 Feb 26;38:298–306. doi: 10.1016/0006-3002(60)91245-2. [DOI] [PubMed] [Google Scholar]
- Oppenheim A., Wahrman J. DNA-membrane association during the mitotic cycle of Physarum polycephalum. Exp Cell Res. 1973 Jun;79(2):287–294. doi: 10.1016/0014-4827(73)90447-3. [DOI] [PubMed] [Google Scholar]
- Opstelten R. J., Dijkwel P. A., Wanka F. Evidence against the specific initiation of Okazaki fragments at the internucleosomal linkers. Biochem Biophys Res Commun. 1981 Aug 14;101(3):807–813. doi: 10.1016/0006-291x(81)91822-2. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B., Coffey D. S. A fixed site of DNA replication in eucaryotic cells. Cell. 1980 Feb;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. The structure of histone-depleted metaphase chromosomes. Cell. 1977 Nov;12(3):817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Mantieva V. L., Georgiev G. P. The similarity of DNA sequences remaining bound to scaffold upon nuclease treatment of interphase nuclei and metaphase chromosomes. Nucleic Acids Res. 1979 Nov 24;7(6):1713–1735. doi: 10.1093/nar/7.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACHSENMAIER W. ZUR DNS- UND RNS-SYNTHESE IM TEILUNGSCYCLUS SYNCHRONER PLASMODIEN VON PHYSARUM POLYCEPHALUM. Biochem Z. 1964 Nov 6;340:541–547. [PubMed] [Google Scholar]
- Solao P. B., Shall S. Control of DNA replication in Physarum polycephalum. I. Specific activity of NAD pyrophosphorylase in isolated nuclei during the cell cycle. Exp Cell Res. 1971 Dec;69(2):295–300. doi: 10.1016/0014-4827(71)90227-8. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Wanka F. Decreased DNA synthesis in mammalian cells after exposure to deoxyadenosine. Exp Cell Res. 1974 Apr;85(2):409–414. doi: 10.1016/0014-4827(74)90143-8. [DOI] [PubMed] [Google Scholar]
- Wanka F., Mullenders L. H., Bekers A. G., Pennings L. J., Aelen J. M., Eygensteyn J. Association of nuclear DNA with a rapidly sedimenting structure. Biochem Biophys Res Commun. 1977 Jan 24;74(2):739–747. doi: 10.1016/0006-291x(77)90364-3. [DOI] [PubMed] [Google Scholar]


