Abstract
How and when the known genetic risk allele, apolipoprotein E-ε4 (APOEε4), confers risk to Alzheimer’s disease has yet to be determined. We studied older adults and found that APOEε4 carriers had greater neural activation in the medial frontal and parahippocampal gyrus during a memory task (cluster-corrected p<.01). When compared to a group of younger adults, interactive effects of age and APOEε4 were found in the inferior frontal—anterior temporal region, one of the first areas to develop amyloid plaques in patients with Alzheimer’s disease, and, in the posterior cingulate, one of the earliest areas to show decreased cerebral metabolism in Alzheimer’s disease. Thus, abnormally high activation in fronto-temporal areas are present in both younger and older APOEε4 carriers confronted with a working memory task when compared to non-APOEε4 carriers. This effect, however, appears to diminish with age.
Keywords: fMRI, APOE, Alzheimer’s disease, Working memory, Compensation
Introduction
Neuroimaging methods have allowed investigators to test healthy individuals for brain changes that underlie and precede the development of mild cognitive impairment (MCI) and Alzheimer’s disease. For example, structural magnetic resonance imaging (MRI) studies have noted an increased rate of hippocampal/medial temporal lobe volume loss in healthy individuals at increased risk for the development of late-onset Alzheimer’s disease by virtue of the presence of the apolipoprotein E-ε4 allele (APOEε4) (Jak et al. 2007; Wishart et al. 2006a; Cohen et al. 2001). Additional abnormalities have been found in APOEε4 carriers using metabolic and molecular probes and positron emission tomography (PET) (Cohen et al. 2003, 2006; Reiman et al. 2005; Scarmeas et al. 2004, 2005), and in the intensity and scope of regional brain activation responses to memory tasks as determined with functional MRI (fMRI) (Bartres-Faz et al. 2007; Borghesani et al. 2007; Wishart et al. 2006a; Bookheimer et al. 2000). Recently, we evaluated brain activation in APOEε4 carriers (APOEε4+) considerably younger than previously assessed, i.e., in their third decade of life (Filbey et al. 2006). Using two neuroimaging methods (i.e. fMRI and magnetoencephalography or MEG), we found greater neural activity in APOEε4+ individuals in the medial frontal gyrus (BA 10) and anterior cingulate gyrus using a visual working memory task. These results were in accord with what has been reported in older APOEε4+ individuals (Burggren et al. 2002; Wishart et al. 2006b). The findings from all of the above work are most parsimoniously interpreted to suggest that neural changes (1) are associated with the APOEε4 allele, a gene that confers risk for Alzheimer’s disease, (2) are measurable using neuroimaging techniques, and (3) are present in both younger and older healthy carriers of the APOEε4 allele. However, the effects of age and the APOEε4 genotype on neuronal function has not yet been directly determined. The aim of this study was to compare the effects of the APOEε4 allele on fMRI activation in older healthy adults with that previously determined in younger subjects. By using the same working memory task and combining the data sets from the older and younger subjects we could examine for possible interactions between age and APOEε4 and, thereby, establish whether our previous fMRI findings represent a stable trait marker. Based on the existing literature of increased neural activation in older APOEε4 adults interpreted as support for compensatory mechanisms (Han et al. 2007), our own findings in younger adults (Filbey et al. 2006), and aging studies that suggest that age-associated memory decline is due to changes in both the prefrontal executive functioning system and the medial temporal lobe memory system (Buckner 2004), we expected to find significantly greater neural responses in prefrontal and medial temporal lobe areas of older APOEε4 carriers compared to older APOEε4 non-carriers (APOEε4−). However, because neural activation generally decreases with age (for a review see (Cabeza 2000)), we expected that the widely reported compensatory mechanisms of older APOEε4 carriers would be found to be of a smaller magnitude and less effective than those in the younger, previously reported, APOEε4 carriers, particularly in the medial temporal lobe memory system—an area affected by the aging process. The current study tested for such an effect by combining the data from our previous study with our new data and evaluating the statistical significance of the age × APOEε4 interaction. We predicted a significant interaction effect in memory areas within the medial temporal lobe regions.
Methods
This protocol was approved by the National Institute of Mental Health Institutional Review Board.
Participants
All participants provided written consents according to the Declaration of Helsinki, were right-handed and were screened for history of head injury, psychiatric illness or substance abuse. All participants were evaluated during a brief inpatient stay at the NIH Clinical Center. The evaluation included a thorough medical screening, neuro-cognitive profiling, neuroimaging, APOE genotyping, collection of biological samples (cerebrospinal fluid, blood, and plasma), and behavioral observations. Medical evaluations consisted of a physical examination, electrocardiogram, and routine blood tests to eliminate other known contributors to memory and general cognitive impairment. Routine magnetic resonance imaging (MRI) or computer tomography (CT) scan was obtained and blood tests assessed venereal disease research laboratory (VDRL), complete blood count (CBC), vitamin B12 level, thyroid function, and APOE genotype. Individuals were excluded from participation if they had psychiatric or neurological disorders including current or past history of substance abuse, or other medical disorders that might alter cognition or influence brain activation patterns, e.g., significant cardiovascular disease including a history of myocardial infarction, coronary artery stent procedures or bypass surgeries. Subjects were also excluded if they were receiving medications that might have cognitive side effects or possibly influence brain activation patterns.
Both older and the younger participants were assessed and scanned concurrently. In both age groups, APOEε4+ individuals were matched with APOEε4− individuals in order to provide equal sample sizes across the groups.
-
Older group
Thirty-six healthy adults (age range = 50–75, mean education in years = 17.35±2.37) gave informed consent to take part in this study. Healthy cognitive function was assessed using the Mini Mental State Examination (mean MMSE = 29.65±.66). Of these, 18 were carriers of the APOEε4 allele (oAPOEε4+; 14 ε3/ε4 and 4 ε4/ε4; 8 males, mean age = 59.6±6.3) and 18 were without the APOEε4 allele (oAPOEε4−; 3 ε2/ε3 and 15 ε3/ε3; 9 males, mean age = 59.9±6.0). Due to technical problems, response data were not available on two older participants (one from each APOEε4 group) and were excluded from further analyses. The two genotype groups did not differ in age or gender.
-
Younger group
Data on 16 volunteers in their third decade of life (age range = 19–32 years, mean education in years =16.73± 1.75) were re-analyzed for this study in order to investigate AGE x APOE genotype interactions (Filbey et al. 2006). Healthy cognitive function was assessed using the Mini Mental State Examination (MMSE) (mean MMSE = 30± 0). Of the younger group, eight had at least one copy of the APOE-ε4 allele (yAPOEε4+; 7 ε3/ε4 and 1 ε2/ε4; 4 males, mean age = 26±3.5) and eight did not have the APOE-ε4 allele (yAPOEε4−; 4 ε3/ε3 and 4 ε2/ε3; 4 males, mean age = 25±4.3). Due to technical problems, response data were not available on two younger participants (one from each APOEε4 group) and were excluded from further analyses. The two groups did not significantly differ in age or gender.
The older and younger groups did not differ in education and MMSE.
Working memory task
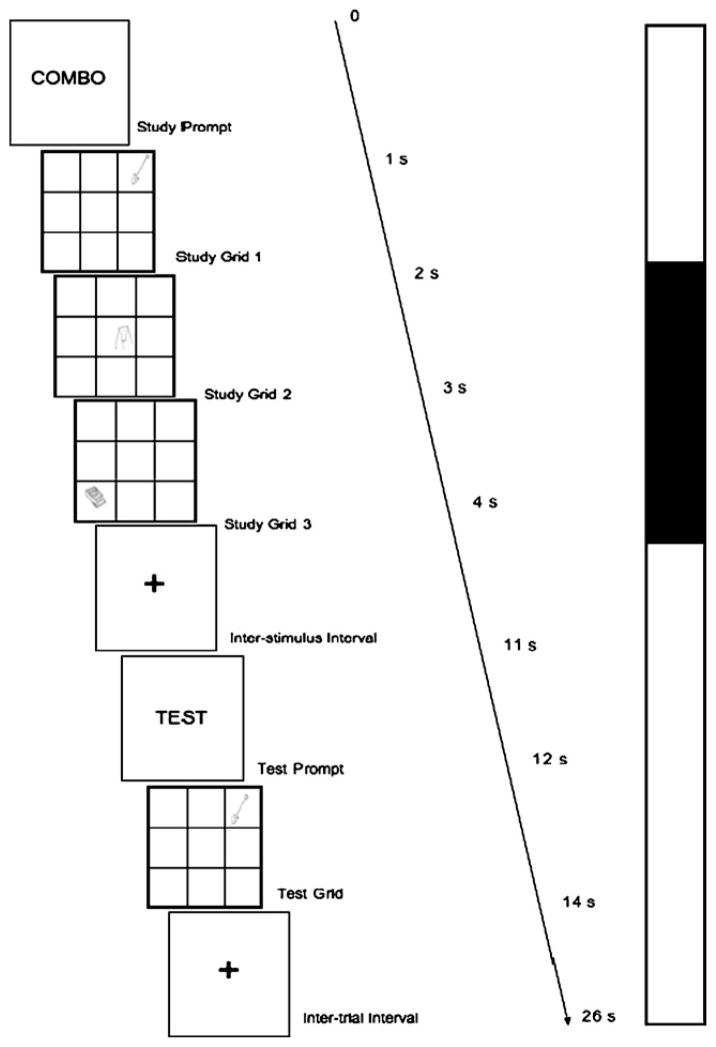
Working memory deficits in Alzheimer’s disease are well established in the literature and have recently also been reported in healthy individuals with the APOEε4 genotype (Greenwood et al. 2005; Rosen et al. 2002). In this study, we asked subjects to perform the same visual working memory task that was performed by a previously reported group of younger subjects (Filbey et al. 2006) and previously described by Mitchell et al. (2000a). Within a single trial, three study presentations (i.e., 9-square grid with an object in one of the grids) were sequentially presented followed by a delay leading to a single probe (i.e., one 9-square grid with an object in one of the grids) (see Fig. 1). The participants were asked to remember both the objects and their locations from each of the three study presentations and to respond by button pressing as to whether the probe contained the same object in the same location during any of the three preceding study presentations. We were interested in the neural activations during the encoding phase for both objects and locations of the trials. The task was given in two runs containing 12 trials each (total run time with 16 s saturation scan pre- and post-run: 5 m 46 s, 164 volumes). Of note, there were also runs where either the object or the location of the object was the target. For this report, we focused only on trials where both objects and their locations were targets. Prior to their scan, the participants were given instructions along with a practice test. All of the participants had an accuracy rate of over 80% and were included in the analyses.
Fig. 1.
Schematic of a single fMRI trial. For a single trial, sequential presentations of three study stimuli were followed by a delay leading to a single probe to which the subjects were asked to respond. Each study stimulus and probe consisted of a nine-square grid with a single black and white line drawing (Snodgrass and Vanderwart 1980) in one of the squares. The participant’s goal during each trial was to remember both the figures and their corresponding locations, and, was, therefore, instructed explicitly to remember the objects and their locations combined during the study presentations and to respond whether the same object was presented in the same location during any of the study presentations. They were asked to use their right index finger to press the button for ‘yes’ and right middle finger for ‘no’. In each of the 26-second trials, volumes 2 and 3 were coded (shaded area in the time series) as the memory encoding state
The task was programmed using PsyScope Software (Cohen et al. 1993). Responses were recorded using a Lumitouch button box (Lightwave Medical Industries, Ltd., Burnaby, CA). The participants’ accuracy rate and response times were recorded, and we tested difference between groups using SPSS Statistical Software vs. 11 (www.spss.com).
fMRI data acquisition
BOLD images were collected using a 3T GE Signa scanner (GE Medical Systems, Milwaukee, WI) using a gradient echo, echoplanar sequence (TR = 2,000 ms, TE = 30 ms, Flip angle = 90, FOV = 24 cm, matrix size = 64×64, slice thickness = 5 mm, number of slices = 25). A high-resolution structural image was collected at the end of the EPI data acquisition using a magnetization prepared rapid acquisition by gradient echo (MP-RAGE) sequence (TI/TE/TR/FA = 725/2.928/7.6/6°, FOV = 22 cm, partition thickness = 1.2 mm, 256×256, in-plane voxel size = .859375). During data acquisition, a foam pillow was used for head restraint. The task was projected on to a screen, which participants viewed using a mirror system. MRI-compatible corrective lenses were provided when necessary.
Data analyses
Behavioral data were analyzed using a 2 (younger, older) × 2 (APOEε4+, APOEε4−) ANOVA in SPSS.
fMRI data analyses were performed while blind to the group membership of the subjects. fMRI data were processed and analyzed using Analysis of Functional NeuroImages (AFNI) (Cox 1996). To allow signal stabilization, the first 2 volumes were excluded from the analysis. Volume registration was performed by using the EPI volume closest in time to the anatomical image as base point (i.e., 160th volume). All participants had head movement <2 mm and were included in the analyses. Smoothing was performed using Gaussian kernel full-width half maximum (FWHM) = 5 mm. Similar to prior studies utilizing this task (Mitchell et al. 2000b) (Filbey et al. 2005, 2006), study grids 2 and 3 (the second volume of each trial) were considered the encoding phase of the task (see Fig. 1). Of note, the first volume of the trial was not included as part of the encoding phase because at TR of 2, it encompassed the study prompt in addition to study grid 1. While not including study grid 1 may have decreased power, we felt that it was necessary in order to minimize contamination from the study prompt screen during which the participants may be cognitively preparing for the task, rather than strictly encoding. Individual subject data were normalized voxel-wise by dividing the mean signal of each voxel so that regression coefficients could be interpreted as percent signal change relative to the mean at each voxel. All trials were included in the analyses. Regressors were created by convolving the stimulus timing files with a standard gamma-variate impulse response function (Cohen 1997). Linear regression analysis was performed using the regressors plus a baseline and a linear drift term. Each regression coefficient reflects the percent signal change estimated for the stimulus, and the corresponding t-statistic indicates the significance of the activation associated with the stimulus. Regression coefficient (beta weight) maps of each individual brain were converted to standard Talairach space.
To extend our previous findings in the younger participants to our older participants we tested for the effects of APOEε4 in older participants only using t-tests. To determine the impact of both age and APOEε4 on neural function during working memory we tested for main effects of AGE (younger, older) and GENOTYPE (APOEε4+, APOEε4−) and for the interaction effects between AGE and GENOTYPE by performing a 2×2 between-subjects (AGE × GENOTYPE) ANOVA.
Thresholded activation maps are overlaid onto each individual’s MPRAGE scan for display purposes. We corrected the multiple comparison problem through Alpha-Sim, an AFNI program with a correction approach at cluster level, through Monte Carlo simulations based on an algorithm laid out in Forman et al. (1995). Documentation for this program can also be found here: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. Statistical significance was based on a combined requirement of individual voxel significance and cluster size. The threshold for an appropriate corrected π=0.01 was determined through Monte Carlo simulations (Forman et al. 1995) with connectivity radius of 7.3 mm, spatial smoothing of FWHM = 5 mm) as an individual voxel significance of 0.05 and a minimum cluster size of 76 voxels.
Results
Behavioral task performance analyses showed no significant main effects or interaction for percent hits, suggesting that all of the groups did not differ in accuracy. There was, however, a main effect of age on reaction time such that the older group had slower reaction times compared to the younger participants (F(2,23)=27, p<.01). There was no significant age × genotype interaction for reaction time (see Table 1). While the older group appeared to be slower in responding to the cues, we did not believe that this would impact the neural response associated with memory encoding as accuracy would. Therefore, there were no covariates in the subsequent analyses.
Table 1.
Behavioral task performance in the participants. Mean and standard deviations of percent correct response and reaction time to correct responses are summarized below for each age and genotype group. Of note, due to technical problems, response data were not available on two older participants (one from each APOEε4 group) and two younger participants (one from each APOEε4 group). ANOVA analyses showed no significant interactions between age and genotype groups for either variable
| APOEε4+
|
APOEε4−
|
F, p | |||
|---|---|---|---|---|---|
| Older (N=17) | Younger (N=7) | Older (N=17) | Younger (N=7) | ||
| % hits (SD) | 91 (.09) | 97 (4) | 91 (.08) | 95 (7) | F(2,23)=.4 p=.53 |
| Hit RT in ms (SD) | 1374.77 (178) | 1018.06 (195) | 1336.44 (138) | 1021.7 (235) | F(2,23)=.15 p=.7 |
APOEε4 effects in older subjects
As a follow-up to our previous findings of APOEε4 effects in the younger subjects (reported here (Filbey et al. 2006)), we examined whether the same effects are present in older individuals.
Behavioral data analysis of percentage of correct responses and total response time of correct responses revealed no significant differences between oAPOEε4− and oAPOEε4+ subjects during their task performance (see Table 1).
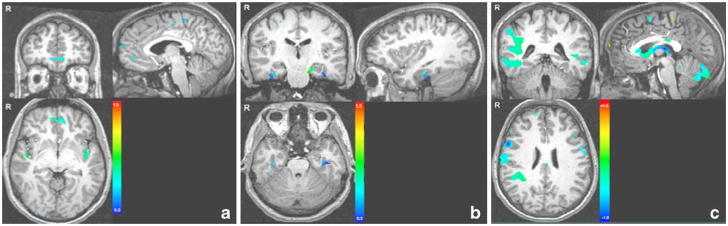
As expected, fMRI results showed that the older oAPOEε4+ group had greater activation in regions in the medial prefrontal cortex (mPFC), the superior temporal gyrus, pre- and post-central gyri and parahippocampal gyrus compared to the older oAPOEε4− individuals during encoding (cluster-corrected p<.01). However, whereas there were no areas of the brain with greater activation in younger oAPOEε4− subjects compared to younger oAPOEε4 + subjects, older oAPOEε4− had greater activation than older oAPOEε4+ individuals in a widespread network consisting of lateral frontal regions, the basal ganglia, cingulate cortex, parietal and temporal structures (cluster-corrected p<.01) (see Table 2 and Fig. 2).
Table 2.
Foci of significantly different regions of activation the genotype contrast in the older individuals (p<0.01; t= 2.0). Clusters of activation are listed in descending order of volume (i.e., cluster size). Localization is given based on the maximum intensity voxel. Coordinates are given in Talairach mm
| Volume | Localization | BA | x | y | z | % change |
|---|---|---|---|---|---|---|
| oAPOE-ε4+ > oAPOE-ε4− | ||||||
| 698 | L superior temporal gyrus | 21 | −41 | −4 | −7 | 0.15 |
| 413 | R medial frontal gyrus | 10 | 4 | 51 | −4 | 0.14 |
| 197 | R parahippocampal gyrus | 36 | 34 | −19 | −21 | 0.08 |
| 190 | R pre-central gyrus | 6 | 31 | −12 | 66 | 0.14 |
| 190 | L paracentral lobule | 6 | 0 | −30 | 60 | 0.07 |
| 111 | L medial frontal gyrus | 10 | −3 | 63 | 15 | 0.09 |
| 109 | R precuneus | 19 | 8 | −83 | 47 | 0.14 |
| 105 | R posterior central gyrus | 2 | −34 | −24 | 26 | 0.05 |
| oAPOE-ε4− > oAPOE-ε4+ | ||||||
| 67672 | R pyramis | – | 25 | −68 | −29 | 0.26 |
| 26744 | R thalamus | – | 3 | −19 | 12 | 0.27 |
| 10379 | R inferior frontal gyrus | 9 | 56 | 11 | 26 | 0.26 |
| 1777 | R mid-frontal gyrus | 6 | 36 | 3 | 53 | 0.12 |
| 1240 | L superior parietal lobule | 7 | −31 | −58 | 50 | 0.14 |
| 1227 | R superior parietal lobule | 7 | 17 | −63 | 60 | 0.16 |
| 616 | R superior frontal gyrus | 6 | 15 | −4 | 70 | 0.17 |
| 562 | R post-central gyrus | 3 | 50 | −19 | 55 | 0.10 |
| 550 | L inferior parietal lobe | 40 | −31 | −42 | 35 | 0.10 |
| 459 | L supramarginal gyrus | 40 | −60 | −50 | 30 | 0.06 |
| 381 | L cingulate gyrus | 23 | 0 | −24 | 26 | 0.08 |
| 301 | R middle temporal gyrus | 39 | 56 | −65 | 18 | 0.06 |
| 284 | R superior frontal gyrus | 9 | 8 | 59 | 29 | 0.12 |
| 137 | L middle temporal gyrus | 39 | −49 | −59 | 8 | 0.09 |
| 126 | R superior frontal gyrus | 10 | 40 | 49 | 23 | 0.18 |
Percent signal change values (% change)
BA Brodmann area; L left, R right; oAPOEε4 + older APOEε4 allele positive subjects; oAPOEε4− older APOEε4 allele negative subjects
Fig. 2.
The effects of APOEε4 in older individuals. Comparisons of individuals with and without the APOEε4 allele showed significantly greater BOLD response in (a) the right and left medial frontal and anterior cingulate gyri and the (b) right and left parahippocampal gyri during memory encoding, and significantly decreased BOLD response in (c) fronto-temporoparietal and limbic areas (cluster-corrected p< 0.01). The color scale represents percent signal change. Right hemispheric activations are shown on the left side of the images
Main effects of APOEε4 and age on brain activation
Combining the older and younger groups we were able to test by ANOVA the statistical significance of these apparent differences in genotype brain activation effects in the older and younger groups as well as testing for genotype effects that were consistent across both age groups. Significant main effects of APOEε4 were found in this combined group (cluster-corrected p<.01) with greater activation in the left precuneus, left superior frontal gyrus, left fusiform gyrus, right culmen and right lingual gyrus found in the APOEε4+ group. There were also main effects of age where greater activation was found in the younger groups (i.e., combined younger APOEε4+ and younger APOEε4−subjects), irrespective of genotype, in primarily right-lateralized areas of the superior temporal gyrus, putamen, BA20, cerebellum, temporal gyrus, medial frontal gyrus and precentral gyrus, but with a few left-hemispheric greater activations found in the middle temporal gyrus, thalamus and medial frontal gyrus (cluster-corrected p<.01) (Table 3).
Table 3.
Main effects of APOE and age (p<0.01; t=2.0). Clusters of activation for the effects of APOE and age are listed in descending order of volume (i.e., cluster size). Localization is given based on the maximum intensity voxel. Coordinates are given in Talairach mm
| Volume | Localization | BA | x | y | z | % change |
|---|---|---|---|---|---|---|
| Main effect of APOE (combined younger and older subjects) | ||||||
| 164710 | L precuneus | 19 | −21 | −82 | 39 | 0.66 |
| 792 | L brainstem, midbrain | – | −6 | −30 | −8 | 0.28 |
| 182 | L superior frontal gyrus | 6 | −6 | 23 | 61 | 0.20 |
| 180 | L fusiform gyrus | 36 | −33 | −40 | −11 | 0.12 |
| 162 | R culmen | – | 3 | −55 | 1 | 0.37 |
| 108 | R lingual gyrus | 19 | 15 | −61 | 1 | 0.16 |
| Main effect of AGE (combined APOEε4− and APOEε4+ subjects) | ||||||
| 195577 | R inferior frontal gyrus/superior temporal gyrus | 13 | 36 | 9 | −16 | 0.77 |
| 2816 | R superior frontal gyrus | 10 | 12 | 60 | 25 | 0.28 |
| 1221 | L middle temporal gyrus | 21 | −55 | −18 | −11 | 0.14 |
| 801 | L middle temporal gyrus | 22 | −52 | −33 | 1 | 0.23 |
| 651 | R tuber/declive | – | 50 | −70 | −26 | 0.09 |
| 522 | R putamen | – | 26 | −9 | −2 | 0.18 |
| 449 | R Brodmann area 20 | 20 | 44 | −14 | −20 | 0.15 |
| 347 | R cerebellar tonsil | – | 29 | −46 | −41 | 0.28 |
| 216 | R transverse temporal gyrus | 41 | 47 | −27 | 13 | 0.21 |
| 186 | R medial frontal gyrus | 10 | 9 | 57 | 7 | 0.36 |
| 135 | R medial frontal gyrus | 9 | 9 | 44 | 28 | 0.23 |
| 81 | L thalamus | – | −12 | −27 | 1 | 0.13 |
| 81 | R precentral gyrus | 4 | 47 | −9 | 25 | 0.11 |
Percent signal change values (% change)
BA Brodmann area; L left, R right
Age × APOEε4 interactions on brain activation
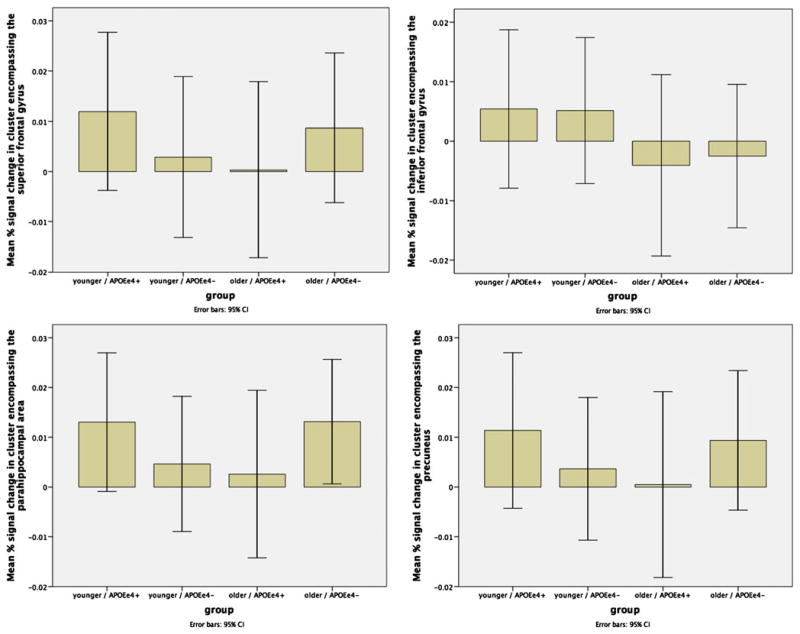
As expected, our analyses indicated that there was a significant interaction between age and APOEε4 genotype in several clusters. The largest cluster with a peak in the right inferior frontal gyrus encompassed right insula, right claustrum, right parahippocampal gyrus, right thalamus and right pulvinar. There was also a cluster with a peak in the left precuneus that encompassed the right posterior cingulate. A cluster with peak in the left medial geniculum body was also found, which included the right medial geniculum, and right superior temporal gyrus. Other clusters included peaks in the right superior frontal gyrus, the left culmen, and the left medial frontal gyrus (cluster-corrected p<.01) (see Fig. 3; peaks are listed in Table 4). To further explore the specific directionality of these interactions, we plotted the mean beta values for each significant cluster of activation against group (Fig. 4). These plots showed that the BOLD activation during working memory declines with increasing age and even more so in individuals possessing the APOEε4 allele.
Fig. 3.
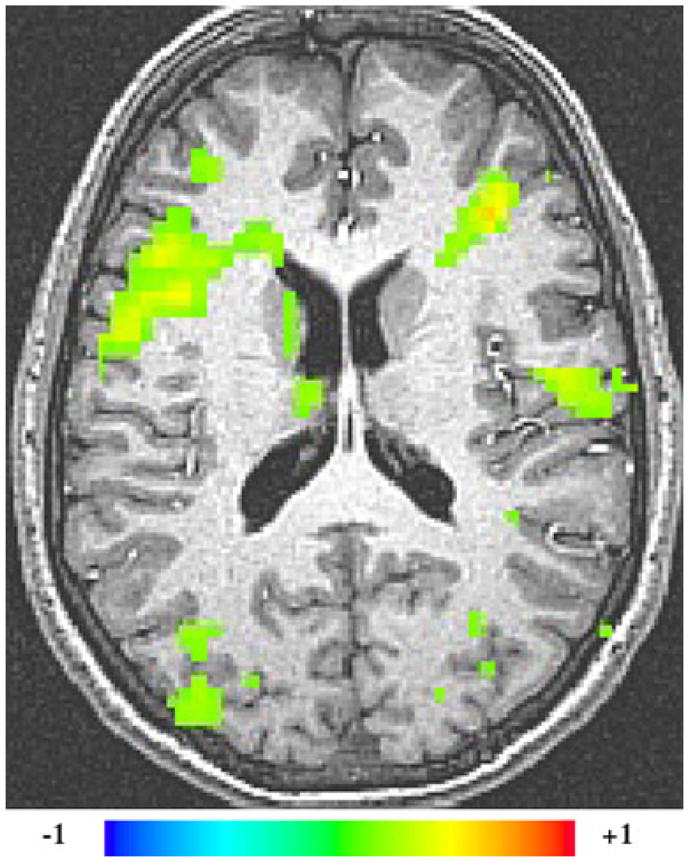
AGE × APOEε4 interaction (cluster-corrected p<0.01). The greatest area of interaction between age and APOEε4 genotype was found in the right inferior frontal, inferior temporal and superior temporal gyri that encompasses several areas including the superior temporal gyrus, inferior frontal gyrus, insula, claustrum and parahippocampal gyrus. Right hemispheric activations are shown on the left side of the images. The colorgraph represents % signal change
Table 4.
Areas of significant interaction between APOE and age (p<0.01; t=2.0). Clusters of activation are listed in descending order of volume. Localization is given based on the maximum intensity voxel. Coordinates are given in Talairach mm
| Volume | Localization | BA | x | y | z | % change |
|---|---|---|---|---|---|---|
| 33597 | R inferior frontal gyrus | 13 | 36 | 9 | −14 | 0.67 |
| 20272 | L precuneus | 7 | −3 | −43 | 49 | 0.21 |
| 11943 | L medial geniculum body | – | −18 | −24 | −2 | 0.13 |
| 7143 | R superior frontal gyrus | 8 | 21 | 29 | 46 | 0.20 |
| 6177 | L culmen | – | −9 | −43 | −8 | 0.23 |
| 5883 | L medial frontal gyrus | 8 | −3 | 35 | 40 | 0.17 |
Percent signal change values (% change)
BA Brodmann area; L left, R right
Fig. 4.
Bar graphs of mean activation per region of interest illustrating that the neural activation is greatest in the younger APOε4 carriers and least in the older APOEε4 carriers
Discussion
In this study, we found that the APOEε4 allele, which confers risk to Alzheimer’s disease, is associated with changes in neural activation in older adults during the encoding phase of a working memory task. The changes in neural activation were present despite healthy cognitive function. The areas of increased activation found in the older subjects possessing the APOEε4 allele are similar to those we previously reported for younger adults using the same paradigm (Filbey et al. 2006). The greater activation in the medial frontal gyrus of older APOEε4 allele carriers provides further support for the growing body of literature that illustrates enhanced activation in frontal areas in those at-risk for Alzheimer’s disease either as a result of genotype or on the basis of a family history of Alzheimer’s (Han et al. 2007; Bassett et al. 2006; Bookheimer et al. 2000; Fleisher et al. 2005). In addition to increases in frontal lobe response to memory tasks, others have also reported increased activity in temporal areas in APOEε4 individuals during memory tasks (Bondi et al. 2005; Han et al. 2006; Dickerson et al. 2005).
Current literature supports the existence of multiple memory systems that includes frontal, parietal and temporal regions (Ranganath and D’Esposito 2005). Initial work on working memory focused primarily on the prefrontal cortex, but more recently, the role of the parahippocampal gyrus during working memory processes have been elucidated. For instance it has been suggested that the parahippocampal area underlies working memory for novel stimuli (without prior representation unlike letters or numbers) (Hasselmo and Stern 2006; Stern et al. 2001). Our findings of greater medial frontal gyrus and parahippocampal activity in older APOE ε4+ individuals are congruent with these reports.
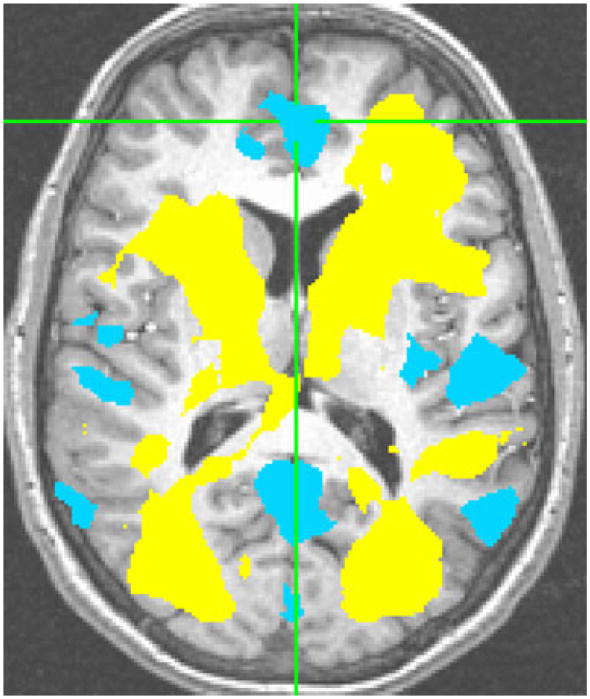
While these new results are consistent with the hypothesis of increased activation in brain areas involved in the encoding step of working memory (i.e., prefrontal and temporal regions) in older subjects at-risk for AD, the findings must be interpreted in the context of additional cortical areas in which activation was significantly less (see Fig. 5). In contrast to younger subjects, older APOEε4+ individuals had attenuated BOLD responses in several structures that included areas in the parietal cortex and posterior cingulate. The anatomical location of these areas are congruent with previous reports of hypometabolism in resting APOEε4+ individuals (Reiman et al. 1996), MCI patients (Nestor et al. 2003a, b) and those with a positive family history of Alzheimer’s disease (Johnson et al. 2007) as well as in patients in early stages of Alzheimer’s disease. Hypofunction in these same areas might be expected given the findings of resting hypometabolism leading some investigators to speculate as to whether this is the underlying mechanism for the early memory problems seen in Alzheimer’s disease (Nestoret al. 2003b). Hypofunction in the precuneus/posterior cingulate area, an area that is part of the default network, has also been found with respect to decreases in task-induced deactivations in subjects at risk for Alzheimer’s disease (Pihlajamaki and Sperling 2009; Persson et al. 2008).
Fig. 5.
Activation and deactivation in the older participants; activated areas in yellow and deactivated areas in blue during working memory
Our current findings of enhanced activity in task-related areas (i.e., areas previously reported to be activated during working memory tasks such as the PFC and temporal cortex) in conjunction with attenuation of activation in other regions are consistent with previous proposals that increased regional activations in older subjects at risk for AD reflect compensatory mechanisms (Han et al. 2006, 2007; Scarmeas and Stern 2005; Bondi et al. 2005). In the group of APOEε4 allele carriers, the mPFC, a central region for executive function and cognitive control, demonstrated enhanced activation in response to the memory task while other areas showed widespread reduction of activity. These findings suggest that the compensatory response may involve enhanced activation of task-relevant areas plus recruitment of additional resources implicated for other processes, such as executive function. The notion of compensatory processes is further supported by the lack of behavior task performance difference between the two genotype groups. Compensation theories posit that increased regional BOLD responses are the result of greater brain activity required to maintain normal task performance; thereby, enabling those with neural dysregulation associated with the APOEε4 allele to function at the same level as their non-APOEε4 counterparts (Wishart et al. 2006b; Bookheimer et al. 2000). It is also possible that greater activation in these areas indicate non-selective recruitment of regions that may not be relevant for the task at hand Logan et al. 2002; Tisserand and Jolles 2003). Nevertheless, because our data are cross-sectional and, therefore, cannot distinguish among those subjects who will develop MCI and AD, we can speculate from the AGE × GENOTYPE findings that for APOE-ε4+ subjects who may develop MCI and AD, this dysregulation, compensation or otherwise, is only temporary.
While the area illustrated in Fig. 3 demonstrates that both genotype groups have a decrement in activation with age with the decrement not as severe in the APOE4-group, the other areas (i.e., superior frontal gyrus, parahippocampal gyrus and precuneus) and potentially others not illustrated demonstrate a more profound difference. It is as if the APOE4+ group demonstrates a shift in phase such that the APOE4+ group undergoes this activation change (increased activation, possibly compensatory in nature) with age earlier (3rd and 4th decade) than the APOE4−group. The APOE4− group then undergoes an “APOE4+ like” change by their 6th and 7th decade of life with the likelihood that if we were to measure even older APOE4− subjects we would begin to see an “APOE4+ like” decrement. The largest areas of statistically significant AGE × GENOTYPE interaction was in the inferior frontal which included the insula, parahippocampal gyrus, thalamus and claustrum regions. In line with the notion of compensation, our findings of attenuated response in these areas with increased age in APOEε4+ individuals may reflect a failure to compensate. This finding is particularly noteworthy as the inferior frontal cortex and anterior temporal lobes are frequently the first areas that develop Alzheimer’s disease pathology in the form of amyloid plaque depositions as described in Stage A of Braak and Braak’s staging of Alzheimer’s disease neuropathological changes (Braak and Braak 1991). This is in contrast to the first areas of tau involvement (neurofibrillary tangles), which are in the medial temporal lobes. The AGE × APOEε4 effect in the insula may partially reflect loss of tissue in this region, as widening of the Sylvian fissure is often an early sign of cortical atrophy with aging and in neurodegenerative disease.
Among other areas that showed AGE × APOEε4 interaction was the left precuneus. This is of particular interest as the posterior cingulate/precuneus areas have repeatedly been reported to demonstrate very early metabolic loss in individuals with early Alzheimer’s disease, MCI, individuals with a family history of Alzheimer’s disease and in APOEε4+ individuals (Kemppainen et al. 2007; Devanand et al. 2006; Mosconi et al. 2007; Reiman et al. 2005). This finding is further supported by important PET studies using beta-amyloid marker [(11)C]Pittsburgh Compound B (PIB) that showed greatest binding in the posterior cingulate/precuneus in vivo (Rowe et al. 2007) in individuals with Alzheimer’s disease, and in APOEε4 hetero- and homozygotes of similar aged older group to that reported here (Reiman et al. 2009). Thus, the literature combined with our current findings suggests the importance of these areas in the preclinical course of neurodegeneration in Alzheimer’s disease. Our data also support age-related differential effects of APOEε4, and as such is consistent with the hypothesis recently put forth by Han and Bondi (Han and Bondi 2008).
Other significant findings included a main effect of APOEε4 in several areas including the precuneus, the area displaying the largest activation difference between the younger APOEε4 carriers and non-carriers. The significant interaction between age and genotype in the precuneus primarily reflects the loss of this differential activation effect in the older APOEε4 group. A main effect was also found in the parahippocampal region (cluster peak in the medial geniculate body), and again reflects an area that shows considerably greater differential activation in the younger APOEε4+ group compared to APOEε4− than in the older APOEε4+ and APOEε4− comparison. In our previously published data, there was no statistically significant difference in the right superior frontal gyrus between the younger APOEε4+ and APOEε4− groups (Filbey et al. 2006). However, there was greater activation in this region in the older APOEε4− group compared to the older APOEε4+ group. Again, this interaction in the right superior frontal gyrus is likely to be due to greater reduction in activation in this region with age in the APOEε4+ group relative to the APOEε4− group.
Some limitations must be taken into consideration when interpreting the present findings. First, because only 10 of our ‘older’ participants were ≥ 61, and in order to maximize our data, they were categorized with middle-aged individuals (50–60 year olds) into the ‘older’ group. As such, one would expect that effects may have been dampened by the middle-aged individuals. Regardless, our findings suggest that neural changes are robust such that they are observable even in a predominantly middle-aged sample. Future studies, however, should investigate middle- and older-aged participants separately to better elucidate the changes throughout the lifespan. Another caveat is the possible differential effects by specific genotypes (e.g., dose-dependent effects). Due to the small number of homozygotes included in the study, we chose to categorize ε4 homozygotes and heterozygotes together. Of note, it may be of concern that an individual in the younger APOEε4+ group was ε2/ε4 because the ε2 allele may be protective. However, in our analyses of the younger participants, we did not observe an outlier in the APOEε4+ group in the areas that differed between the genotype groups. Future studies, however, should look at the specific effects of the allele dose. Similarly, while our older participant groups were reasonably sized for each genotype, the numbers for our young participants were relatively small. Thus, one must interpret the interaction effects under this light. Lastly, we cannot rule out other possible explanations for these effects. Differences in neurovascular coupling or cognitive strategies between groups are possible, but are less likely explanations for the regional differences observed.
In conclusion, our findings add to the growing body of evidence that suggests the presence of pre-clinical functional neural changes in cognitively healthy individuals who, as a group, are at genetic-risk for Alzheimer’s disease. The current findings taken together with our previous findings suggest that a single APOEε4 allele is associated with some areas of increased activation during memory encoding in both younger and older subjects. It is also noteworthy that the areas where the older, but not younger, APOE-ε4+ subjects were found to have diminished activation overlap with areas (i.e, posterior superior temporal and inferior parietal areas, posterior cingulate) that show lower resting glucose metabolism reductions in subjects at risk for Alzheimer’s disease and that show the earliest reductions in patients with Alzheimer’s disease (Anderson et al. 2005). Further, interactive effects of age and APOEε4 were found with decreased activation in older APOEε4 subjects, but not younger APOEε4 subjects in the inferior frontal—anterior temporal region, one of the first areas to develop amyloid plaques in patients with Alzheimer’s disease. These data provide further support for the growing body of evidence that suggests the association between the APOEε4 allele and Alzheimer’s disease (Roses 1996, 2006; Tsai et al. 1994) and the possibility of using patterns of neural activation as an intermediate phenotype for evaluating Alzheimer’s disease risk. These findings argue that the ε4 allele is associated with compensatory or non-selective mechanisms during working memory, and that Alzheimer’s disease (and not just aging) attenuates the ability to engage that extra activation. Thus, carriers of the ε4 allele may display clinical symptoms of Alzheimer’s disease at an earlier stage of the molecular pathology because the disease reduces their capacity to engage the needed extra neural activation. To conclude, the determination of intermediate phenotypes on the path to Alzheimer’s disease is a necessity if we are to determine those at risk, develop treatments designed o prevent the development of the disease, and apply them safely, economically and expeditiously.
Acknowledgments
We are grateful to Karen Putnam for managing our blind to the subjects’ genotypes. Also, thanks to Irene Dustin, NP for providing the clinical screens and to Heather Kiefer and Kelly Slack for the data collection.
This work was supported by NIMH ZO1 MH00330-14
Contributor Information
Francesca M. Filbey, Email: ffilbey@mrn.org, Geriatric Psychiatry Branch, NIMH, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892, USA. The Mind Research Network, 1101 Yale Blvd., Albuquerque, NM 87106, USA
Gang Chen, Scientific and Statistical Computing Core, NIMH, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892, USA.
Trey Sunderland, Geriatric Psychiatry Branch, NIMH, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892, USA.
Robert M. Cohen, Geriatric Psychiatry Branch, NIMH, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892, USA. Department of Psychiatry and Behavioral Neurosciences and S. Mark Taper Department of Imaging, Cedars-Sinai Medical Center, 8730 Alden Dr., Los Angeles, CA 90048, USA
References
- Anderson VC, Litvack ZN, Kaye JA. Magnetic resonance approaches to brain aging and Alzheimer disease-associated neuropathology. Topics in Magnetic Resonance Imaging. 2005;16(6):439–452. doi: 10.1097/01.rmr.0000245458.05654.d0. [DOI] [PubMed] [Google Scholar]
- Bartres-Faz D, Serra-Grabulosa JM, Sun FT, Sole-Padulles C, Rami L, Molinuevo JL, et al. Functional connectivity of the hippocampus in elderly with mild memory dysfunction carrying the APOE varepsilon4 allele. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, et al. Familial risk for Alzheimer’s disease alters fMRI activation patterns. Brain. 2006;129(Pt 5):1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. The New England Journal of Medicine. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Johnson LC, Shelton AL, Peskind ER, Aylward EH, Schellenberg GD, et al. Altered medial temporal lobe responses during visuospatial encoding in healthy APOE*4 carriers. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Small GW, Sabb FW, Bookheimer SY. Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. American Journal of Geriatric Psychiatry. 2002;10(1):44–51. [PubMed] [Google Scholar]
- Cabeza R. Handbook of functional neuroimaging of cognition. Cambridge: MIT Press; 2000. [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: a new graphic interactive environment for designing psychology experiments. Behavior Research Methods, Instruments, & Computers. 1993;25(2):257–271. [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57(12):2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Podruchny TA, Bokde AL, Carson RE, Herscovitch P, Kiesewetter DO, et al. Higher in vivo muscarinic-2 receptor distribution volumes in aging subjects with an apolipoprotein E-epsilon4 allele. Synapse. 2003;49(3):150–156. doi: 10.1002/syn.10225. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Carson RE, Filbey F, Szczepanik J, Sunderland T. Age and APOE-epsilon4 genotype influence the effect of physostigmine infusion on the in-vivo distribution volume of the muscarinic-2-receptor dependent tracer [18F]FP-TZTP. Synapse. 2006;60(1):86–92. doi: 10.1002/syn.20276. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Habeck CG, Tabert MH, Scarmeas N, Pelton GH, Moeller JR, et al. PET network abnormalities and cognitive decline in patients with mild cognitive impairment. Neuropsychopharmacology. 2006;31(6):1327–1334. doi: 10.1038/sj.npp.1300942. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Holroyd T, Carver F, Sunderland T, Cohen RM. A magnetoencephalography spatiotemporal analysis of neural activities during feature binding. NeuroReport. 2005;16(16):1747–1752. doi: 10.1097/01.wnr.0000185020.75057.19. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Slack KJ, Sunderland TP, Cohen RM. Functional magnetic resonance imaging and magnetoencephalography differences associated with APOEepsilon4 in young healthy adults. NeuroReport. 2006;17(15):1585–1590. doi: 10.1097/01.wnr.0000234745.27571.d1. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, et al. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Archives of Neurology. 2005;62(12):1881–1888. doi: 10.1001/archneur.62.12.1881. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant change in functional magnetic resonance imaging (fMri): use of a cluster size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19(2):199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Bondi MW. Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimers Dement. 2008;4(4):251–254. doi: 10.1016/j.jalz.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiology of Aging. 2007;28(2):238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends in Cognitive Sciences. 2006;10(11):487–493. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dementia and Geriatric Cognitive Disorders. 2007;23(6):382–389. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Ries ML, Hess TM, Carlsson CM, Gleason CE, Alexander AL, et al. Effect of Alzheimer disease risk on brain function during self-appraisal in healthy middle-aged adults. Archives of General Psychiatry. 2007;64(10):1163–1171. doi: 10.1001/archpsyc.64.10.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Wilson IA, Nagren K, Helin S, Bruck A, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68(19):1603–1606. doi: 10.1212/01.wnl.0000260969.94695.56. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Ding YS, Franceschi D, Wang GJ, Volkow ND, et al. Strategy for the formation of parametric images under conditions of low injected radioactivity applied to PETstudies with the irreversible monoamine oxidase A tracers [11C]clorgyline and deuterium-substituted [11C]clorgyline. Journal of Cerebral Blood Flow and Metabolism. 2002;22(11):1367–1376. doi: 10.1097/01.WCB.0000040947.67415.e1. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Research Cognitive Brain Research. 2000a;10(1–2):197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Research Cognitive Brain Research. 2000b;10(1–2):197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, et al. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(48):19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease) The European Journal of Neuroscience. 2003a;18(9):2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Annals of Neurology. 2003b;54(3):343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Sleegers K, Van Broeckhoven C, et al. Altered deactivation in individuals with genetic risk for Alzheimer’s disease. Neuropsychologia. 2008;46(6):1679–1687. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Sperling RA. Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer’s disease and atrisk older individuals. Behavioural Neurology. 2009;21(1):77–91. doi: 10.3233/BEN-2009-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Directing the mind’s eye: prefrontal, inferior and medial temporal mechanisms for visual working memory. Current Opinion in Neurobiology. 2005;15(2):175–182. doi: 10.1016/j.conb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. The New England Journal of Medicine. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen VM, Bergeson JL, Putnam K, Harwell A, Sunderland T. Working memory and apolipoprotein E: what’s the connection? Neuropsychologia. 2002;40(13):2226–2233. doi: 10.1016/s0028-3932(02)00132-x. [DOI] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annual Review of Medicine. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Roses AD. On the discovery of the genetic association of Apolipoprotein E genotypes and common late-onset Alzheimer disease. Journal of Alzheimer’s Disease. 2006;9(3 Suppl):361–366. doi: 10.3233/jad-2006-9s340. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;65(9):1514–1515. doi: 10.1212/wnl.65.9.1514-a. author reply 1514–1515. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Anderson KE, Hilton J, Park A, Habeck C, Flynn J, et al. APOE-dependent PET patterns of brain activation in Alzheimer disease. Neurology. 2004;63(5):913–915. doi: 10.1212/01.wnl.0000137274.93125.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, et al. APOE related alterations in cerebral activation even at college age. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:1440–1444. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11(4):337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39(4–5):1107–1128. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Tangalos EG, Petersen RC, Smith GE, Schaid DJ, Kokmen E, et al. Apolipoprotein E: risk factor for Alzheimer disease. American Journal of Human Genetics. 1994;54(4):643–649. [PMC free article] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, McAllister TW, Rabin LA, McDonald BC, Flashman LA, et al. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006a;67(7):1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, et al. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. The American Journal of Psychiatry. 2006b;163(9):1603–1610. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]