Abstract
Objective
To evaluate and compare efficacy of 20, 40, and 60-minute mental practice (MP) sessions on affected upper extremity impairment and functional limitation.
Design
Randomized controlled study with multiple baseline design.
Subjects
29 subjects with chronic stroke and exhibiting stable, mild hemiparesis.
Interventions
Subjects were administered 30-minute rehabilitative sessions occurring 3 days/week for 10 weeks, and emphasizing affected upper extremity use during valued activities. Directly after these sessions, randomly selected subjects were administered audiotaped MP for 20, 40, or 60 minutes. Subjects assigned to a control group received the same therapy as MP groups, and an audiotaped sham intervention directly after therapy sessions.
Main Outcome Measures
The Fugl-Meyer (FM) and Action Research Arm Test.
Results
No pre-existing differences were found between groups on any demographic variable or movement scale. On the FM, MP duration significantly predicted pretesting to POST change (p = .05), with increasing duration related to larger FM score increases (5.4 point score increase for the 60 minute duration group). On the ARAT, a non-significant trend was seen (p = 0.78), favoring the 20 minute dosing condition (4.5 point increase). Importantly, regardless of dosing condition, subjects administered MP exhibited markedly larger score changes on both the FM and ARAT than subjects not receiving MP.
Conclusions
60 minutes of MP appears to most significantly reduce affected arm impairment. However, no clear change pattern was seen in affected arm functional limitation according to MP duration. Results suggest that a stroke rehabilitative regimen augmented by MP renders a greater functional impact than therapy only.
Following stroke, repetitive, task specific, practice (RTP) incorporating the affected extremity causes expansion of cortical areas representing that extremity, and correlative functional changes (for a review, see ref 1). Consequently, several contemporary rehabilitative approaches emphasize RTP incorporating the affected upper extremity (UE). However, many of these techniques are constrained by requiring particular equipment to administer,2,3 by high therapy durations,4 and/or by their invasive nature.5 Additionally, little information is available on the daily duration at which these interventions render the largest affected UE motor changes.6,7
Mental practice (MP) overcomes the above limitations, in that it is a non-invasive, easily implemented technique in which physical skills are cognitively rehearsed. The addition of MP to RTP has been shown to significantly increase affected UE use,8 kinematics,9 and function in subacute10,11 and chronic stroke patients,12,13,14 including in a recent, randomized controlled trial.15 Recent data also show that cortical organizations are primarily responsible for affected UE motor changes observed after MP use.16 However, like other RTP-based approaches, the optimal daily MP duration remains unknown and has varied in UE studies (e.g., 60 minutes;17 30 minutes12–15). Such information is fundamental to effective clinical implementation of this promising approach.
As a next step in this line of research, the purpose of the current study was to evaluate and compare the efficacy of 20, 40, and 60 minute MP durations on several affected UE movement parameters. Specifically, subjects were administered 30-minute, outpatient RTP sessions emphasizing motor learning-based, functional use of their affected UE's. Directly following these sessions, they received 20, 40, or 60-minute MP interventions emphasizing mental rehearsal of movements that they had physically practiced. Our study hypotheses were: (a) subjects administered a higher MP duration of 60 minutes would exhibit significantly larger affected arm impairment reductions (as measured by the Fugl-Meyer; our primary study outcome measure) than subjects receiving MP durations of 20 or 40 minutes; and (b) subjects administered a higher MP duration of 60 minutes would exhibit significantly larger affected arm functional limitation reductions (as measured by the Action Research Arm Test) than subjects receiving MP durations of 20 or 40 minutes. We also hypothesized that subjects administered MP + RTP at any duration would exhibit larger motor changes than a group of subjects administered RTP only without MP.
Subjects and Methods
Volunteers were recruited using advertisements placed in local rehabilitative therapy clinics, stroke support groups, and in hospitals. A research team member screened volunteers using the following inclusion criteria, derived from previous MP research: (1) 10° of active flexion in the affected wrist, as well as 2 digits in the more affected hand; (2) stroke experienced > 12 months prior to study enrollment; (3) a score ≥ 70 on the Modified Mini Mental Status Examination (MMSE);18 (4) age > 18 < 75; (5) only have experienced one stroke; (6) discharged from all forms of physical rehabilitation. Exclusion criteria were: (1) excessive spasticity in the affected UE, as defined as a score of ≥ 3 in the affected elbow, wrist, or fingers as determined by the Modified Ashworth Spasticity Scale;19 (2) excessive pain in the affected UE, as measured by a score ≥ 5 on a 10-point visual analog scale; (3) participating in any experimental rehabilitation or drug studies; (4) history of a parietal stroke (because some data suggest that ability to estimate manual motor performance through mental practice is disturbed after parietal lobe damage).20
Assessments
The upper extremity section of the Fugl-Meyer Scale (FM)21 was our primary outcome measure. FM items are organized from having the subject attempt proximal movements (e.g., shoulder abduction, internal rotation) to distal movements (e.g., mass grasp; pincer grasp). Data arise from a 3-point ordinal scale (0=cannot perform; 2=can perform fully) applied to each item, and the items are summed to provide a maximum score of 66. The FM has been shown to have high test-retest reliability (total=.98–.99; subtests=.87–1.00), interrater reliability, and construct validity.22,23
To measure affected arm limitation, we administered the Action Research Arm Test, (ARAT):24 a 19-item test divided into four categories (grasp, grip, pinch, and gross movement), with 16 of the nineteen ARAT items measuring distal regions of the arm (e.g., pinching a ball bearing or marble between the thumb and each finger of the affected hand), with each item graded on a 4-point ordinal scale (0=can perform no part of the test; 1=performs test partially; 2=completes test but takes abnormally long time or has great difficulty; 3=performs test normally) for a total possible score of 57. For this test, subjects were seated in a comfortable chair with a straight back, while the ARAT items that they had to grasp were placed on an adjustable table in front of them. Table height was adjusted according to the needs of each subject. The ARAT has high intrarater (r = .99) and retest (r = .98) reliability and validity,25,26 all in stroke-induced hemiparesis.
The above measures were chosen because of their successful use in previous MP studies,10–16 and their responsivity to motor changes in chronic stroke.27
Design, Testing, and Randomization
A single-blinded, multiple baseline, randomized controlled design was applied. After screening and signing consent forms approved by the local institutional review board, the FM and ARAT were administered by a single rater, on 2 occasions, occurring one week apart (PRE-1; PRE-2). Following PRE-2, subjects were randomized to one of 4 groups using a computer-generated random numbers table overseen by a study coordinator. When a subject completed pretesting, the study coordinator was contacted. On the first scheduled day of treatment the randomization assignment was revlealed. The four possible randomization assignments were: (a) 20 minutes MP (MP 20”); (b) 40 minutes of MP (MP 40”); (c) 60 minutes of MP (MP 60”); or (d) a group receiving RTP only (RTP only).
Interventions
Before the study began, therapists underwent extensive inservicing using uniform procedures implemented in pilot work, and detailed in a manual of procedures that had been developed for this study. The purpose of this training was to assure that RTP (described below) was consistent across therapists. Training included group review of pertinent stroke, MP, and exercise training literature, hours of cross-validation and videotaping of therapists' responses to various clinical presentations and patient goals, and periodic inservices.
Repetitive, Task Specific Training (RTP)
Beginning one week after the final testing session, each subject received individualized, ½ hour, RTP sessions, occurring three times per week, for 10 weeks. All therapy was administered by the same therapists in the same fashion and environment.
Via RTP pilot work, the team had identified a battery of familiar activities of daily living (ADLs) that many stroke patients want to relearn. Five of these ADLs were included in this study (Table 1). The ADLs were chosen because: (a) they were used in previous MP studies; (b) individual movements comprising these compound skills transfer positively to performance of other ADLs that stroke patients often desire to relearn; (c) The ADLs can be graded according to subjects' abilities to be easier or more challenging, and progressed to be increasingly difficult; and (d) The ADLs can be further progressed by having patients stand and perform them using an adjustable height bed table, perform them outside of their centers of gravity (i.e., move the items to the side of the patient), etc.
Table 1.
Sequences on each tape, and where tape should be used
| Tape Number: | Functional Task Described: | When Administered: |
|---|---|---|
| 1 | Reaching for and grasping a cup or object | Weeks 1,2 |
| 2 | Turning a page in a book | Weeks 3,4 |
| 3 | Proper use of a writing utensil | Weeks 5,6 |
| 4 | Proper use of an eating utensil | Weeks 7,8 |
| 5 | Using a hairbrush or comb | Weeks 9,10 |
Each ADL listed in Table 1 was practiced for 2 weeks. Each 2-week increment allowed time for the participant to identify the muscles that needed to be used, and undergo trial and error on each task. While use of modalities (e.g., electrical stimulation) was disallowed, therapists were given the option to use 5–10 minutes prior to each session to perform range of motion or other exercises to help with performance of the ADLs in Table 1, as needed. Therapists maintained a treatment card for each patient, so that researchers could monitor compliance with the protocol. Therapists were blinded to group assignment in that they were unaware of whether a particular subject was going to receive one of the MP interventions (and at which duration), or the time-matched sham intervention.
The above RTP therapy program, sometimes called “standard task training of the affected limb,” was consistent with the suggestions and methods of previous MP work. RTP, as structured herein, also had the advantage of repeating tasks with continuous feedback from the therapist with little formal time necessary for review of immediate-past performance, and is an accurate analog for actual therapy provided in most clinics, as it involves motor learning of functional activities performed continuously for a period of 15 to 30 minutes.28
Mental Practice (MP)
After their ½ hour RTP sessions, subjects randomly assigned to one of the three MP + RTP groups listened to an audiotaped MP intervention in a private room adjacent to the therapy area. The tapes consisted of 5, opening minutes of relaxation, asking patients to imagine themselves in a warm, relaxing place (e.g., a beach), and asking them to contract and relax their muscles (i.e., progressive relaxation). This portion of the tapes was followed by suggestions for internal, cognitive polysensory (i.e., using both kinesthetic and visual cues) images29 related to using the affected arm in one of 5 functional tasks shown in Table 1, which had been practiced during the preceding RTP sessions. The tape concluded with 5 minutes of refocusing into the room.
While all aspects of RTP were held constant, the duration of the MP tape varied according to group assignment. One group listened to a tape lasting 20 minutes, one group listened to a tape lasting 40 minutes, and one group listened to a tape lasting 60 minutes. Within each of these audiotapes, the opening and closing relaxation sequences were held constant. Only the portion during which subjects cognitively rehearsed the RTP was varied on tapes, by group.
Sham/control
A fourth group also received ½ hour RTP sessions that were matched in content, duration, and location to other subjects. However, directly after their therapy sessions, subjects in the sham/control condition listened to 20-minute “sham” tapes. To maintain interest, these tapes alternated relaxation exercises, information on stroke prevention, information on stroke, and exercises for the affected leg. Previous work suggested that exposure to such audiotapes would not have a treatment effect;15 rather, the goal of these tapes was to provide additional contact time in the room with an audiotape, to maintain consistency with subjects in the MP groups.
Posttesting
After 10 weeks, all subjects returned to the laboratory (POST), where they were again administered all instruments by an examiner blinded to group assignment. He was blinded in that he did not know to which group subjects were assigned, or if they even received an intervention.
Statistical Methods
Pre-treatment FM and ARAT scores were assessed twice and the mean of the two assessments was treated as the baseline score for each measure. Demographic variables and the baseline assessments of the outcome variables were compared across the four groups treating the groups as nominal categories (3 df). Continuous measures were compared using one-way ANOVAs or non-parametric Kruskal-Wallis tests as needed, while categorical or dichotomous variables were compared using exact tests.
The first hypothesis, that POST minus pre-intervention difference scores would increase with increasing MP durations, was tested in regressions predicting the difference score from the dose (0, 20, 40, 60) in single df tests. The second hypothesis, that the three groups receiving MP combined would show more baseline to follow-up improvement on the FM and ARAT than the RTP only group, was tested using a Wilcoxon test, applied to the Post minus Pre differences in scores. All reported p-values are two-tailed and the study employed a two-tailed alpha of .05.
We wish to highlight that a statistical design in which a repeated measures ANOVA would be applied was also considered. In such a design, both baseline and post-treatment scores would be dependent variables, time would be the independent variable, and the treatment by time interaction would constitute the main assessment of the intervention. However, such a model would offer less power than above-described analyses.
Results
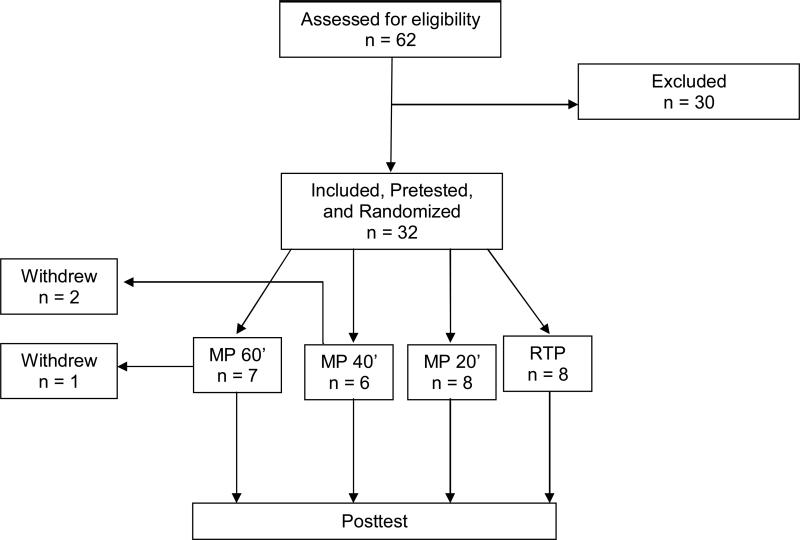
Using the above inclusion/exclusion criteria, a total of 62 volunteers were screened, with 30 subjects excluded for the following reasons: (1) insufficient active movement in the affected UE to qualify for the study (n= 20); (2) excessive spasticity (n = 7); (3) other medical comorbidities (n = 1); (4) too much motor function in the affected UE (n = 2). Thus, 32 patients were found eligible and agreed to participate. However, of the 32 subjects randomized at baseline (8 per group), two from the MP 40” group and one from the MP 60” group were lost to follow-up and removed from analyses. These 3 subjects withdrew their participation during the course of the study, due to availability and/or distance needed to travel to participate in the study. Thus, the current report discusses the outcomes of 29 patients (23 males; mean age of subjects in sample = 60.8 ± 12.3 years, age range of subjects in sample = 21 − 76 years; mean time since stroke onset for subjects in sample = 36.0 months; 23 subjects with ischemic stroke; 15 subjects with hemiparesis affecting their right arms). A flowchart depicting how subjects passed through the study is provided in Figure 1.
Figure 1.
Flow Diagram of Subjects in the Current Trial
Outcomes
Baseline means and standard deviations for Age, FM, and ARAT, for each of the four groups, are presented in Table 2. The groups did not differ significantly on these variables. The groups also did not differ significantly on ethnicity, side of infarction, affected side, stroke type, or dominant hand. There was a significant difference for gender, with all six subjects in the MP 40” group being female, and 6 of 7 in the MP 60” group being male.
Table 2.
Pre-intervention Demographics and Scores, By Group. Results shown as Mean (SD)
| Relaxation + PP (n=8) | 20'MP +PP (n=8) | 40'MP +PP (n=6) | 60'MP +PP (n=7) | |
|---|---|---|---|---|
| Age | 54.0 (18.5) | 66.4 (6.3) | 56.5 (9.4) | 66.0 (6.1) |
| FM score | 32.1 (12.5) | 29.1 (14.2) | 26.0 (5.1) | 30.7 (11.2) |
| ARA score | 24.1 (14.0) | 21.9 (20.0) | 17.2 (13.5) | 26.4 (15.4) |
Note: There were no significant differences (p<.05) using a one way ANOVA by groups. FM and ARA mean scores of means of 2 testings per subject, with SDs taken on those mean scores across subjects.
On the primary outcome measure (the FM), MP duration (0, 20”, 40”, and 60”) predicted pretesting to POST motor change (1 df) using an OLS regression. This trend was statistically significant (p = .05), with increasing duration related to greater FM increases, and each increase in duration corresponding to a progressively increased FM score at POST (+ 1.6 points for the control group; up to + 5.4 points for the 60 minute duration group). (Table 3).
Table 3.
Mean Patient Scores on FM and ARAT Before and After Intervention
| FM | ARAT | |||||
|---|---|---|---|---|---|---|
| PRE-1 | PRE-2 | POST | PRE-1 | PRE-2 | POST | |
| Relaxation + PP | 31.10 | 32.13 | 33.75 | 23.8 | 24.38 | 24.25 |
| 20' MP + PP | 30.86 | 32.12 | 34.2 | 24.22 | 21.22 | 26.78 |
| 40' MP + PP | 25.89 | 26.1 | 30.0 | 16.3 | 18.0 | 18.67 |
| 60' MP + PP | 28.33 | 29.0 | 34.0 | 25.17 | 24.67 | 26.83 |
On the ARAT, a dose-response effect was not observed. Specifically, the 0 minute, 40 minute, and 60 minute groups all exhibited approximately + 1.5 point increases, while the 20 minute group exhibited a + 4.5 change. ARAT differences between dosing conditions were not significant (p = 0.78).
Regardless of dosing condition, subjects administered MP exhibited larger score changes on both the FM and ARAT than subjects who did not receive MP. On the FM, subjects receiving MP exhibited a mean score change of + 4.2 (4.4); subjects not receiving MP only exhibited a mean score change of + 1.6 (1.9). This trend approached significance on the Wilcoxon test (p = 0.11). Similarly, on the ARAT, subjects receiving MP exhibited a mean score change of + 2.7 (3.9); subjects not receiving MP only exhibited a mean score change of + 0.2 (2.1). This trend also approached significance on the Wilcoxon test (p = 0.07).
Discussion
The purpose of this study was to evaluate and compare the impact of various MP durations, when administered as an adjunct to RTP for the affected UE. Secondarily, we also wished to confirm that MP, provided at any duration, was a statistically significant superior adjunct to RTP than a time-matched sham intervention. It was hypothesized that provision of greater amounts of MP (i.e., a higher daily MP duration) should provide a greater number of neural activations that simulate real practice, and render larger clinical effects, than MP administered at lower durations. Consistently, subjects administered MP at the highest duration (i.e., 60 minutes) exhibited the greatest FM gains of the three MP dosing groups. Moreover, as MP duration progressively increased, the magnitude of the treatment effect on impairment reduction was larger. Thus, the first portion of our primary study hypothesis was confirmed. However, on the ARAT, the 20 minute MP duration group exhibited the largest changes, while smaller, consistent changes were observed among the 0, 40, and 60 minute groups. Thus, the second portion of our study hypothesis – which pertained to to affected extremity functional limitation - was not confirmed.
We suspect that the above differences in FM and ARAT data trends can be traced to two factors: (a) First, the activities in which subjects participated (Table 1) placed comparatively more emphasis on gross movements (e.g., shoulder flexion; elbow extension; wrist extension; mass finger flexion and extension) that are detected by the FM. This is one of the reasons why we chose the FM as the primary outcome measure in this study, and would explain why subjects registered more noticeable FM changes in the current study. Future researchers may wish to enroll a less impaired sample, thus enabling researchers to examine the sensitivity of the ARAT, and allowing examination of whether less impaired subjects exhibit greater responses to the MP intervention herein described. (b) Secondarily, there are likely different cognitive and physical demands associated with re-learning more gross, isolated, impairment-based movements (measured by the FM) versus re-learning finer, activity based movements (measured by the ARAT). The optimal MP duration may differ, according to whether movements being targeted encompass the domains of impairment (i.e., the FM), functional limitation (i.e., the ARAT), or the ultimate goal of fully performing activities in a normalized environment. Indeed, this phenomenon has been shown valid in the speech literature (for a discussion, see ref 30), where high duration therapies have been shown to have differential effects on impairment and activity limitation than low-duration therapies. Future authors are encouraged to further examine this phenomenon, by incorporating a variety of outcome assessments targeting different functional domains in future dosing studies.
While the primary study hypothesis was only partially confirmed, the final hypothesis received some confirmation. That is, regardless of dosing condition, subjects administered both MP and RTP exhibited markedly better FM and ARAT scores than subjects who received the same RTP only. These trends approached statistical significance, and further suggest that the addition of MP increases affected arm outcomes in stroke. Subjects exhibited stable motor deficits before intervention, as evidenced by FM and ARAT scores that remained unchanged between PRE-1 and PRE-2, and by comparison of subjects' FM scores at pretesting to those reported at therapy discharge. The stability of subjects' motor deficits, and the rapid period during which MP subjects then exhibited motor changes, suggest that motor changes associated with MP were unlikely attributable to chance. Given that this study was pilot in nature, and that trends seen the final hypothesis have been corroborated in other studies, a larger MP efficacy trial seems justified to further confirm findings herein described.
Clinicians are likely to gain several clinically-relevant insights from results of the current study. First, results suggest that a RTP regimen augmented by mental practice is more efficacious than provision of RTP alone; a finding that has been corroborated in other studies.8–15 Thus, clinicians may wish to consider developing recorded audio sequences of movements that their patients commonly perform. These sequences could then be taken home or administered in patients' rooms between therapy sessions, so that patients can mentally rehearse what they physically practiced on a particular day. With this being said, the duration with which the mental practice is administered appears to be important in terms of gross, isolated movements, but to not impact fine, distally-based movements. Thus, therapists should be selective in the duration with which mental practice is administered, possibly based on the nature of the skills to be performed.
Recent data also suggest that mental practice can be used as a possible “gateway” to other therapies,31 or could be used as an adjunct to other upper extremity therapies to make them more efficacious.32 Thus, therapists will likely be able to use mental practice to either prepare patients for oher therapies, or to enhance the efficacy of already-proven regimens.
While results of this exploratory study appear promising, some study limitations should be noted. First, although groups were well-matched in terms of motor ability and demographics, the total size of each group was relatively small, which diminished power. It is hoped that larger studies examining MP efficacy will continue to refine MP dosing features. This could be accomplished by including additional, high-duration, study arms in such efficacy studies, as was recently done in a study of constraint-induced movement therapy.33 This work would be valuable, as it would provide insights into the differential responses to MP at the impairment, functional limitation, and other levels. Secondly, while MP dosing and efficacy were again verified, the sample herein described could be described as “minimally impaired.” Questions, thus, remain whether subjects exhibiting more impairment could benefit from MP, and how the cognitive and physical demands associated with MP may differ in various impairment strata. For example, it is plausible that, for patients with more impaired UEs, imagining functional use of their UE's may be less salient. Future researchers may wish to examine this issue by adding a more impaired group of subjects as a tertiary aim.
Other, minor study limitations included the following: (a) there was an unequal distribution among groups for gender. However, there is no literature suggesting that gender affects stroke recovery, so these differences should not diminish the findings; (b) some may argue that the exclusion rate for the study was high. However, such trends are commonly observed in clinical trials, given the focal inclusion criteria that are needed to answer the study question. The current study was no exception. In practice, we expect that MP could be used as an adjunctive strategy to many affected UE interventions, provided that subjects are able to meaningfully participate from a physical and cognitive standpoint. (c) Patients and therapists in this study fully complied with all facets of the intervention, including the rehabilitative intervention as well as the mental practice treatments (even the higher duration ones) with no problems reported. However, it should be acknowledged that volunteers for research studies are typically motivated to be compliant and participate in all facets of the study. Some stroke survivors encountered in clinical environments may not fully comply to such interventions, due to a variety of psychosocial, physical, or other factors.
Clinical Messages
When combined with repetitive task specific practice, mental practice administered for 60 minutes renders the largest reductions in affected upper extremity impairment.
There is not necessarily a mental practice duration at which affected upper extremity functional limitation is optimally diminished.
Repetitive task specific practice is more efficacious when augmented by mental practice, regardless of the duration of the mental practice.
Acknowledgements
This study was supported by a grant from the National Institutes of Health (R21 AT002110-01).
References
- 1.Nudo RJ. Plasticity. NeuroRx. 2006;3:420–7. doi: 10.1016/j.nurx.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taub E, Lum PS, Hardin P, Mark VW, Uswatte G. AutoCITE: automated delivery of CI therapy with reduced effort by therapists. Stroke. 2005 Jun;36(6):1301–4. doi: 10.1161/01.STR.0000166043.27545.e8. [DOI] [PubMed] [Google Scholar]
- 3.Volpe BT, Ferraro M, Lynch D, Christos P, Krol J, Trudell C, Krebs HI, Hogan N. Robotics and other devices in the treatment of patients recovering from stroke. Curr Neurol Neurosci Rep. 2005 Nov;5(6):465–70. doi: 10.1007/s11910-005-0035-y. [DOI] [PubMed] [Google Scholar]
- 4.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006 Nov 1;296(17):2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 5.Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2008 Feb;62(Suppl 2):853–62. doi: 10.1227/01.neu.0000316287.37618.78. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health Timing, Intensity, and Duration of Rehabilitation for Hip Fracture and Stroke Workshop Summary. National Institute of Child Health and Human Development, NIH, DHHS. 2001 [Google Scholar]
- 7.Dobkin BH. Confounders in rehabilitation trials of task-oriented training: lessons from the designs of the EXCITE and SCILT multicenter trials. Neurorehabil Neural Repair. 2007 Jan–Feb;21(1):3–13. doi: 10.1177/1545968306297329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page SJ, Levine P, Leonard AC. Effects of mental practice on affected limb use and function in chronic stroke. Arch Phys Med Rehabil. 2005 Mar;86(3):399–402. doi: 10.1016/j.apmr.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Hewitt TE, Ford K, Levine P, Page SJ. Reaching Kinematics to Measure Motor Changes After Mental Practice in Stroke. Top Stroke Rehabil. 2007 Jul–Aug;14(4):23–9. doi: 10.1310/tsr1404-23. [DOI] [PubMed] [Google Scholar]
- 10.Page SJ, Levine P, Sisto S, Johnston M. Imagery combined with physical practice for upper limb motor deficit in sub-acute stroke: A case report. Phys Ther. 2001;81(8):1455–62. doi: 10.1093/ptj/81.8.1455. [DOI] [PubMed] [Google Scholar]
- 11.Page SJ, Levine P, Sisto S, Johnston M. A randomized, efficacy and feasibility study of imagery in acute stroke. Clin Rehabil. 2001;15(3):233–40. doi: 10.1191/026921501672063235. [DOI] [PubMed] [Google Scholar]
- 12.Page SJ. Imagery improves motor function in chronic stroke patients with hemiplegia: A pilot study. Occ Ther J Res. 2000;20(3):200–15. [Google Scholar]
- 13.Crosbie JH, McDonough SM, Gilmore DH, Wiggam MI. The adjunctive role of mental practice in the rehabilitation of the upper limb after hemiplegic stroke: a pilot study. Clin Rehabil. 2004 Feb;18(1):60–8. doi: 10.1191/0269215504cr702oa. [DOI] [PubMed] [Google Scholar]
- 14.Dijkerman HC, Letswaart M, Johnston M, MacWalter RS. Does motor imagery training improve hand function in chronic stroke patients? A pilot study. Clin Rehabil. 2004 Aug;18(5):538–49. doi: 10.1191/0269215504cr769oa. [DOI] [PubMed] [Google Scholar]
- 15.Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: Results of a randomized, placebo controlled trial. Stroke. 2007 Apr;38(4):1293–7. doi: 10.1161/01.STR.0000260205.67348.2b. [DOI] [PubMed] [Google Scholar]
- 16.Page SJ, Szaflarski JP, Eliassen J, Pan H, Cramer SC. Cortical Plasticity Following Motor Skill Learning During Mental Practice in Stroke. Neurorehabil Neural Repair. 2009 May;23(4):382–8. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riccio I, Iolascon G, Barillari MR, Gimigliano R, Gimigliano F. Mental practice is effective in upper limb recovery after stroke: a randomized single-blind cross-over study. Eur J Phys Rehabil Med. 2010 Mar;46(1):19–25. [PubMed] [Google Scholar]
- 18.Teng EL, Chui HC. The Modified Mini-Mental State Exam. J Clin Psychiatry. 1987;48(8):314–18. [PubMed] [Google Scholar]
- 19.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 20.Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996 Sep 13;273(5281):1564–8. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- 21.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 22.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–10. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 23.DiFabio RP, Badke RB. Relationship of sensory organization to balance function in patients with hemiplegia. Phys Ther. 1990;70(9):542–8. doi: 10.1093/ptj/70.9.542. [DOI] [PubMed] [Google Scholar]
- 24.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–92. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001 Jan;82(1):14–9. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh CL, Hsueh IP, Chiang FM, Lin PH. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing. 1998;27:107–13. doi: 10.1093/ageing/27.2.107. [DOI] [PubMed] [Google Scholar]
- 27.Van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the action research am test and the Fugl-Meyer assessment scale in chronic stroke patients. J Rehab Med. 2001;33:110–13. doi: 10.1080/165019701750165916. [DOI] [PubMed] [Google Scholar]
- 28.Latham NK, Jette DU, Coster W, Richards L, Smout RJ, James RA, Gassaway J, Horn SD. Occupational therapy activities and intervention techniques for clients with stroke in six rehabilitation hospitals. Am J Occup Ther. 2006 Jul–Aug;60(4):369–78. doi: 10.5014/ajot.60.4.369. [DOI] [PubMed] [Google Scholar]
- 29.Paivio A. Cognitive and motivational functions of imagery in human performance. J Applied Sport Sci. 1985;10(4):22–28. [PubMed] [Google Scholar]
- 30.Cherney LR, Patterson JP, Raymer A, Frymark T, Schooling T. Evidence-based systematic review: effects of intensity of treatment and constraint-induced language therapy for individuals with stroke-induced aphasia. J Speech Lang Hear Res. 2008 Oct;51(5):1282–99. doi: 10.1044/1092-4388(2008/07-0206). [DOI] [PubMed] [Google Scholar]
- 31.Page SJ, Levine P, Hill V. Mental practice as a gateway to modified constraint-induced movement therapy: a promising combination to improve function. Am J Occup Ther. 2007 May–Jun;61(3):321–7. doi: 10.5014/ajot.61.3.321. [DOI] [PubMed] [Google Scholar]
- 32.Page SJ, Levine P, Khoury JC. Modified constraint-induced therapy combined with mental practice: thinking through better motor outcomes. Stroke. 2009 Feb;40(2):551–4. doi: 10.1161/STROKEAHA.108.528760. [DOI] [PubMed] [Google Scholar]
- 33.Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL, Edwards DF. Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS): A single-center RCT. Neurology. 2009 Jul 21;73(3):195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]