Abstract
Background
Heart failure (HF) is a clinical syndrome characterized by signs and symptoms involving multiple organ systems. Longitudinal data demonstrating that asymptomatic cardiac dysfunction precedes overt HF are scarce, and the contribution of non-cardiac dysfunction to HF progression is unclear. We hypothesized that subclinical cardiac and non-cardiac organ dysfunction would accelerate the manifestation of HF.
Methods and Results
We studied 1038 participants of the Framingham Heart Study original cohort (mean age 76±5 years; 39% men) with routine assessment of left ventricular (LV) systolic and diastolic function. Major non-cardiac organ systems were assessed using serum creatinine (renal), serum albumin (hepatic), ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) (pulmonary), hemoglobin concentration (hematologic/oxygen carrying capacity) and white blood cell count (systemic inflammation). On follow-up (mean 11 years), there were 248 incident HF events (146 in women). Adjusting for established HF risk factors, antecedent LV systolic (hazards ratio 2.33; 95%CI, 1.43–3.78) and diastolic (hazards ratio 1.32; 95%CI, 1.01-1.71) dysfunction were associated with increased HF risk. Adjusting for cardiac dysfunction, higher serum creatinine, lower FEV1:FVC ratios and lower hemoglobin concentrations were associated with increased HF risk (all P<0.05); serum albumin and white blood cell count were not. Subclinical dysfunction in each non-cardiac organ system was associated with a 30% increased risk of HF (P=0.013).
Conclusions
Antecedent cardiac and non-cardiac organ dysfunction are associated with increased incidence of HF, supporting the notion that HF is a progressive syndrome and underscoring the importance of non-cardiac factors in its occurrence.
Keywords: Heart failure, Epidemiology, Echocardiography, Risk factors
INTRODUCTION
Heart failure (HF) is a clinical syndrome characterized by a constellation of signs and symptoms involving multiple organ systems such as the heart (classic ‘pump failure’), the lungs (dyspnea), the kidneys (salt and water retention) and the liver (congestion). Current HF guidelines1, 2 emphasize the importance of asymptomatic cardiac dysfunction as a preceding stage in the progression to clinically overt HF. Cross-sectional studies have demonstrated the presence of asymptomatic systolic or diastolic LV dysfunction in the community in individuals at risk of, but without HF,3 and an even higher prevalence of these abnormalities in patients with overt HF.4 However, to demonstrate a prospective association between these structural precursors and future HF, longitudinal studies are needed.
Importantly, the current HF staging system1 does not specifically acknowledge the potential association of non-cardiac dysfunction with the occurrence of HF. Since the syndrome of HF involves multiple organ systems, even mild functional derangement of a non-cardiac organ system may accelerate the manifestation of overt HF, particularly when other organ systems are also involved. Indeed, emerging evidence suggests that subclinical renal impairment,5 hypoalbuminemia,6, 7 decline in pulmonary function,8, 9 anemia10 and systemic inflammation11 may all contribute to HF progression. Of note, the prevalence of non-cardiac co-morbidities is high among patients with overt HF, and these co-morbidities are major determinants of mortality after the onset of HF.12, 13
The relations of antecedent cardiac and non-cardiac dysfunction (i.e. present before onset of overt HF) to the incidence of HF have not been studied comprehensively in the community. In a previous report,14 we described the prevalence and prognosis of asymptomatic LV systolic dysfunction in the community but that investigation did not examine the association of LV diastolic dysfunction or of non-cardiac major organ system dysfunction with the risk of HF. Accordingly, we aimed to prospectively determine the association of cardiac and non-cardiac dysfunction with the incidence of HF among older adults without HF in the community. To achieve this, we harnessed the unique availability of longitudinal data and routine surveillance in the Framingham Heart Study. We hypothesized that subclinical dysfunction in both cardiac and non-cardiac organ systems would accelerate the manifestation of HF. Further recognizing potential mechanistic differences between HF with reduced ejection fraction (HFREF) versus HF with preserved ejection fraction (HFPEF), we also hypothesized that the types of antecedent subclinical organ system dysfunction may differ according to the type of incident HF (HFREF versus HFPEF).
METHODS
Participants
The Framingham Heart Study is a longitudinal community-based cohort study that began in 1948.15 The original cohort has been under continuous surveillance and participants are examined at the Heart Study clinic approximately every 2 years. In the present investigation, we included participants attending the 20th biennial examination with routine assessment by Doppler echocardiography, but without prevalent HF (Supplementary Figure 1). Since our focus was on subclinical organ dysfunction, we excluded participants with overt organ dysfunction such as those with overt renal failure (defined as a serum creatinine>2 mg/dL [176.8 μmol/l]; N=12). All participants provided written informed consent and the study protocol was approved by the Institutional Review Board of the Boston University Medical Center.
Definition of cardiac dysfunction
Established HF risk factors modeled as covariates included age, sex, body mass index, systolic blood pressure, hypertension treatment, cholesterol, diabetes mellitus, prior myocardial infarction, and valvular heart disease. LV systolic dysfunction was assessed by echocardiographic ejection fraction estimated visually.14 LV diastolic dysfunction was defined on the basis of LV filling pattern as any abnormal relaxation, pseudonormal filling or restrictive filling. Abnormal relaxation (mitral E/A<0.5, deceleration time>280 ms) or restrictive filling (mitral E/A>2.0, deceleration time<120 ms) was classified based on mitral inflow patterns.16 In the absence of tissue Doppler imaging, pseudonormal LV filling was distinguished from normal LV diastolic function by the presence of any of the following: left atrial size ≥ sex-specific 80th percentile, LV mass ≥ sex-specific 80th percentile, or any atrial fibrillation. These criteria closely parallel recommendations from the European Society of Cardiology (ESC) for the diagnosis of HFPEF,17 and use the upper sex-specific quintiles of left atrial size and LV mass to characterize atrial enlargement and LV hypertrophy,18 respectively, in our elderly cohort. Both LV systolic and diastolic dysfunction were modeled as binary variables (presence versus absence).
Definition of non-cardiac major organ system dysfunction
We evaluated non-cardiac major organ systems that could accelerate the manifestations of HF (dyspnea, fluid retention/pedal edema, and exertional fatigue). Participants underwent routine spirometry and phlebotomy. Measurement variables used to define non-cardiac function included:5-7, 10, 11
Renal system: serum creatinine.
Hepatic system: serum albumin concentration.
Pulmonary system: ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) expressed as % predicted for age and sex.
Hematologic system/oxygen carrying capacity: hemoglobin concentration.
Systemic inflammation: white blood cell count.
These non-cardiac function variables were modeled as both continuous and as binary variables (see below). The sample size for analyses of non-cardiac dysfunction variables was smaller (n=676) than the overall sample with available echocardiographic measures of LV systolic and diastolic function (n=1038; Supplementary Figure 1).
Outcome
Participants have been under ongoing, routine surveillance for incident HF since the baseline examination in 1987-1990. HF was defined as satisfying the previously published Framingham criteria19 (presence of 2 major, or of one major plus two minor criteria) and adjudicated by a panel of three experienced investigators. The ejection fraction closest to the date of the HF event was used to categorize HF into HFPEF (EF>45%) or HFREF (EF≤45%).20 Measurements of ejection fraction performed at HF onset, during HF hospitalization, or within 1 year of HF onset in the absence of intervening myocardial infarction were eligible.20
Statistical analyses
We used Cox proportional hazards regression models to assess the relationship of cardiac and non-cardiac dysfunction variables to the incidence of HF after confirming that the assumption of proportionality of hazards was met. Covariates eligible for the multivariable model included LV systolic and diastolic dysfunction, continuous measures of non-cardiac function (defined above), as well as established HF risk factors (defined above).
For each non-cardiac function variable (including those found to be not significant), we initially examined generalized additive models with penalized splines to assess potential non-linearity of the association.21 None of the associations were found to be non-linear. Therefore, we proceeded to model linear associations in Cox models. In the absence of any non-linearity of the associations, we also used a priori cut-points based on the lower 25th or upper 75th percentile of each continuous variable in order to create binary variables defining organ “dysfunction” for incorporation into a risk score. The risk score for non-cardiac dysfunction was then calculated for each participant by allocating 1 point for each affected non-cardiac organ system that was significantly associated with the risk of HF. This scoring system approach assumes that the hazards posed by dysfunction in each of the non-cardiac systems are similar (weighted the same), and offers a simple, practical score that may be meaningfully applied in clinical settings. The association of the non-cardiac risk score with incident HF was then plotted using Kaplan-Meier curves, and assessed using Cox proportional hazards modeling adjusting for established HF risk factors (noted above) and cardiac dysfunction variables.
Finally, analyses were repeated separately for incident HFREF as the outcome (and censoring cases of HFPEF at the time of that event), or incident HFPEF as the outcome (and censoring cases of HFREF at the time of that event). Cox proportional hazards regression was used, in which variables entered into the model included LV systolic and diastolic dysfunction, measures of non-cardiac function, as well as established HF risk factors noted above. Valvular heart disease was excluded in the analysis for HFPEF consistent with current diagnostic criteria,17 and included as a covariate in the analysis for HFREF.
All analyses were performed using SAS and a two-sided P value of <0.05 was used to indicate statistical significance. All authors had full access to the data and take responsibility for the integrity of the data.
RESULTS
Baseline characteristics
The study sample consisted of 1038 elderly participants (Table 1). More than three-quarters of the sample was hypertensive and about half was on antihypertensive treatment. The prevalence of asymptomatic LV systolic and diastolic dysfunction were 5% and 36%, respectively, consistent with other community-based studies.3 The distributions of measures of non-cardiac function were within the ranges expected for elderly individuals in the general population.5
Table 1.
Baseline characteristics
| Characteristics | |
|---|---|
| N | 1038 |
| Age, yrs | 76±5 |
| Men, no.,(%) | 409 (39) |
| Cardiovascular risk factors | |
| Body mass index, kg/m2 | 26.6±4.5 |
| Systolic blood pressure, mmHg | 147±22 |
| Hypertension, no., (%) | 799 (77) |
| Hypertension treatment, no., (%) | 551 (53) |
| Total cholesterol:HDL ratio | 4.85±1.65 |
| Diabetes mellitus, no., (%) | 104 (10) |
| Myocardial infarction, no, (%) | 96 (9) |
| Valve disease, no., (%) | 41 (4) |
| Cardiac function | |
| LV systolic dysfunction, no., (%) | 57 (5) |
| LV diastolic dysfunction, *no., (%) | 372 (36) |
| Non-cardiac function | |
| Serum creatinine, mg/dl | 0.93±0.23 |
| Serum albumin, g/dl | 4.23±0.36 |
| FEV1:FVC ratio, % predicted | 96.9±10.9 |
| Hemoglobin concentration, g/dl | 14.0±1.5 |
| White blood cell count, ×109/l | 6.8±2.3 |
Values are mean±SD unless otherwise stated. Serum creatinine concentration can be converted to μmol/l by multiplying the value in mg/dl by 88.4.
HDL, high-density lipoprotein; LV, left ventricular; EF, ejection fraction; FEV1:FVC, ratio of forced expiratory volume in 1 second to forced vital capacity expressed as % predicted for age and sex
Diastolic dysfunction included any abnormal relaxation (mitral E/A<0.5, deceleration time>280 ms); pseudonormal LV filling (distinguished from normal LV diastolic function by the presence of any of the following: left atrial size ≥ sex-specific 80th percentile [4.8 cm in men, 4.4 cm in women], LV mass ≥ sex-specific 80th percentile [158.4 g/m2 in men, 141.9 g/m2 in women], or any atrial fibrillation); or restrictive filling (mitral E/A>2.0, deceleration time<120 ms).
Cardiac dysfunction as a risk factor for incident HF
Over a mean follow-up of 11 years, there were 248 incident first HF events (146 in women; 119 HFREF, 101 HFPEF, EF was not available for 28 HF events). In multivariable models adjusting for established HF risk factors (age, sex, body mass index, systolic blood pressure, hypertension treatment, cholesterol, diabetes mellitus, prior myocardial infarction, and valvular heart disease), both LV systolic dysfunction and LV diastolic dysfunction were associated with increased risk of incident HF (Table 2).
Table 2.
Cardiac Dysfunction as a risk factor for incident heart failure
| Characteristic | Hazards ratio (95%CI) | P value* |
|---|---|---|
| LV systolic dysfunction | 2.33 (1.43–3.78) | <0.001 |
| LV diastolic dysfunction | 1.32 (1.01-1.71) | 0.039 |
LV, left ventricular
Adjusted for age, sex, body mass index, systolic blood pressure, hypertension treatment, cholesterol, diabetes mellitus, prior myocardial infarction, and valvular heart disease in 1038 participants (248 HF events)
Non-cardiac risk factors and major organ system dysfunction risk score for incident HF
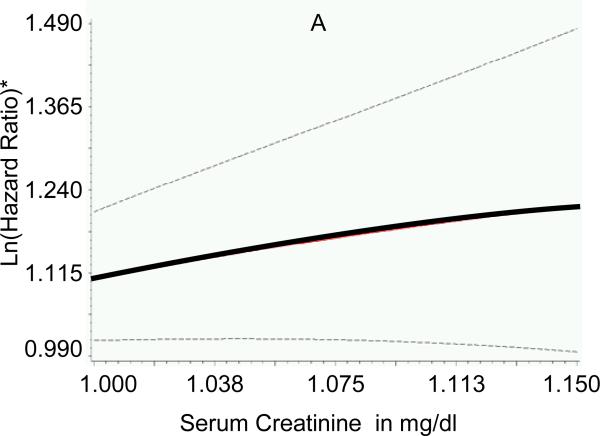
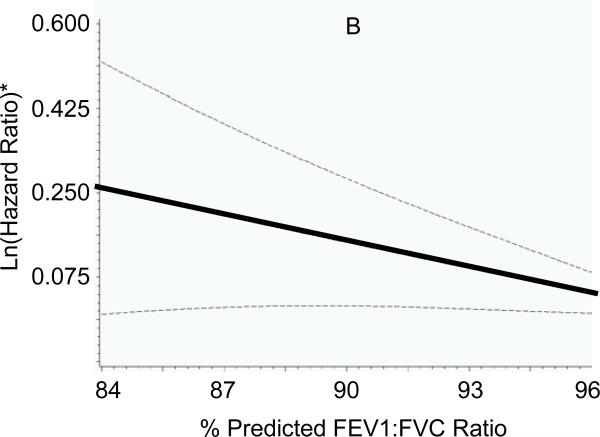
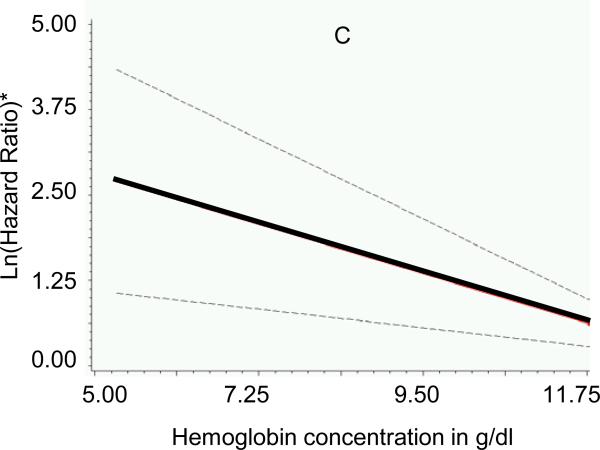
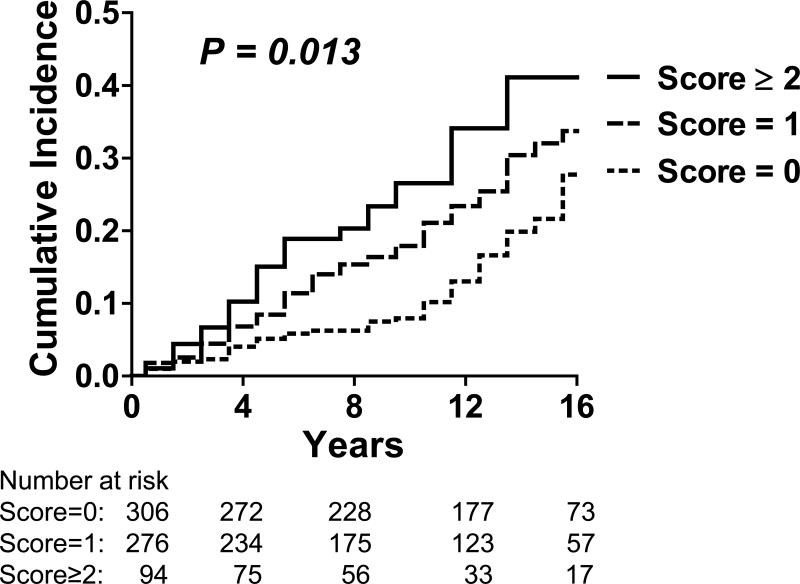
Participants (n=676; mean age 75±5 years; 42% men) without missing non-cardiac risk factor variables had similar baseline characteristics (body mass index, systolic blood pressure, diabetes) compared to those with missing variables (all P>0.05). Adjusting for established HF risk factors and presence of cardiac systolic and diastolic dysfunction, higher serum creatinine, lower FEV1:FVC ratios and lower hemoglobin concentrations were associated with increased risk of new-onset HF (Table 3). There was no association between serum albumin concentration (P=0.306) or white blood cell count (P=0.685) and incident HF. Individual Cox proportional hazards models with penalized splines for serum creatinine, FEV1:FVC ratio and hemoglobin did not reveal non-linearity for the association with HF risk (Figure 1). A risk score for non-cardiac dysfunction was calculated, therefore, using pre-determined cut-points (based on 25th or 75th percentiles of the variables in the sample, as defined above), and awarding 1 point for each affected organ system (range 0-3); regression coefficients for these variables were comparable in the multivariable models, further justifying their similar weighting in the score (Table 3). Of note, the cut-points used to define organ “dysfunction” were within the ranges frequently observed in ambulatory elderly individuals from the general population.5 Increasing non-cardiac risk score at baseline was positively associated with risk of HF (Figure 2).
Table 3.
Non-cardiac risk factors and risk score for incident heart failure
| Characteristic | Hazards ratio (95% CI)* | P value* | Cut-off percentile | Cut-off value | Points awarded |
|---|---|---|---|---|---|
| Serum creatinine | 1.21 (1.01–1.45) | 0.036 | >75th percentile | > 1.05 mg/dl (>92.8 μmol/l) | 1 |
| FEV1:FVC ratio | 1.21 (1.02–1.43) | 0.029 | <25th percentile | < 91 % predicted | 1 |
| Hemoglobin concentration | 1.24 (1.09–1.40) | <0.001 | <25th percentile | < 13 g/dl | 1 |
FEV1:FVC, ratio of forced expiratory volume in 1 second to forced vital capacity
Hazards ratio are for 1SD increase in serum creatinine, 1SD decrease in FEC1:FVC ratio and 1 unit decrease in hemoglobin concentration, adjusting for age, sex, body mass index, systolic blood pressure, hypertension treatment, cholesterol, diabetes mellitus, prior myocardial infarction, valvular heart disease, and left ventricular systolic and diastolic function in 676 participants without any missing variables (170 HF events)
Figure 1. Association of measures of major non-cardiac organ system function with risk of incident heart failure.
Generalized additive models with penalized splines were used to assess the association of multivariable-adjusted hazards ratio for heart failure with (A) serum creatinine concentration, (B) ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1:FVC ratio) and (C) hemoglobin concentration. Lines indicate means (solid) and 95% confidence intervals (dotted). *Y-axes represent multivariable-adjusted Ln (hazards ratio). To obtain serum creatinine concentration in μmol/l, multiply values in mg/dl by 88.4.
Figure 2. Cumulative incidence of incident heart failure according to non-cardiac major organ system dysfunction risk score.
The non-cardiac organ system dysfunction risk score awarded 1 point for the presence of each of the following (range 0-3): serum creatinine >1.05 mg/dl (92.8 μmol/l), ratio of forced expiratory volume in 1 second to forced expiratory volume <91% predicted, hemoglobin concentration <13 g/dl. Increasing non-cardiac risk score at baseline was associated with increasing risk of incident heart failure (HF) in our community-based sample (log rank P value = 0.013).
In secondary analyses, results were similar when estimated glomerular filtration rate (eGFR by MDRD equation) was used instead of serum creatinine (hazards ratio for each 1SD decrease in eGFR was 1.24 (95% CI, 1.03-1.50; P=0.026) adjusting for established HF risk factors and presence of cardiac dysfunction). Other biomarkers of non-cardiac dysfunction (blood urea nitrogen, total bilirubin, transaminases, hematocrit, C-reactive protein measured by traditional assays [high sensitivity assays unavailable], uric acid) were also tested for their associations with incident HF in secondary analyses, and results are shown in Supplementary Table 1. These secondary analyses supported the original selection of creatinine, albumin, FEV1:FVC ratio and hemoglobin as simple, convenient and widely available biomarkers to include in the final risk score. We also performed sensitivity analyses using difference percentile cutpoints to define the risk score (tertiles instead of quartiles) and found similar results (data not shown).
Multivariable risk factors for all incident HF, HFREF and HFPEF
In multivariable modeling for all incident HF, LV systolic dysfunction, LV diastolic dysfunction and the non-cardiac risk score were each associated with incident HF, adjusting for established HF risk factors (Table 4). There was no significant interaction between non-cardiac risk score and the presence of LV systolic or diastolic dysfunction (P=0.78 and 0.84 respectively). In multivariable modeling for HFREF and HFPEF separately, antecedent LV systolic dysfunction, greater serum creatinine and lower hemoglobin concentration were associated with incident HFREF; whereas antecedent LV diastolic dysfunction and lower FEV1:FVC ratio were associated with incident HFPEF, adjusting for established HF risk factors (Table 4).
Table 4.
Association of cardiac and non-cardiac dysfunction with incident heart failure
| Characteristics | Hazards ratio (95%CI)* | P value* |
|---|---|---|
| All incident HF | ||
| LV systolic dysfunction | 1.97 (1.05–3.68) | 0.034 |
| LV diastolic dysfunction | 1.40 (1.02-1.93) | 0.039 |
| Non-cardiac risk score† per 1 unit increase | 1.30 (1.06-1.60) | 0.013 |
| Incident HFREF | ||
| LV systolic dysfunction | 3.93 (1.86 – 8.30) | <0.001 |
| Serum creatinine per 1SD increase | 1.32 (1.04 – 1.69) | 0.025 |
| Hemoglobin concentration per 1unit decrease | 1.31 (1.10 – 1.55) | 0.002 |
| Incident HFPEF | ||
| LV diastolic dysfunction | 1.88 (1.13 – 3.13) | 0.016 |
| FEV1:FVC ratio per 1SD decrease | 1.38 (1.04 – 1.83) | 0.024 |
HF, heart failure; LV, left ventricular; HFREF, heart failure with reduced ejection fraction; HFPEF, heart failure with preserved ejection fraction; FEV1:FVC, ratio of forced expiratory volume in 1 second to forced vital capacity
Adjusted for age, sex, body mass index, systolic blood pressure, hypertension treatment, cholesterol, diabetes mellitus, prior myocardial infarction, and valvular heart disease (valvular heart disease excluded for HFPEF) in 676 participants without any missing variables (170 total HF events: 82 HFREF, 66 HFPEF, 22 EF unavailable).
Components of the non-cardiac risk score are described and their individual hazards ratios are shown in Table 3.
In further analyses adjusting for smoking and excluding participants with overt pulmonary dysfunction (FEV1/FVC < 60%; N=3), hypoalbuminemia (albumin < 2.5 g/dL; N=0, no participant had hypoalbuminemia) or anemia (hemoglobin < 10.5g/dL; N=7), results were essentially unchanged (data not shown). Using an EF cutpoint of 50% (instead of 45%) to distinguish HFPEF from HFREF revealed similar results (Supplementary Table 2).
DISCUSSION
Principal findings
In our prospective study of a large, community-based sample, antecedent subclinical cardiac and non-cardiac major organ system dysfunction were associated with risk of future HF. The presence of asymptomatic LV systolic and diastolic dysfunction preceded and increased the risk of incident HF by more than two-fold and more than 30%, respectively. These findings support the emphasis in current HF guidelines regarding the progressive nature of HF and importance of recognizing preceding asymptomatic cardiac dysfunction. Our data also extend the previous HF staging system by providing evidence for the association of non-cardiac dysfunction with progression to clinical HF. Adjusting for cardiac dysfunction, the presence of subclinical renal impairment, airflow limitation or anemia was each associated with 30% increased risk of incident HF. Finally, antecedent LV systolic dysfunction was associated with future HFREF, whereas antecedent LV diastolic dysfunction was associated with future HFPEF. Further, subclinical renal impairment and lower hemoglobin concentrations were associated with a higher incidence of HFREF, whereas baseline airflow obstruction was related positively to the risk of future HFPEF. The implications of these findings for the early identification of individuals at risk of HF, and potential strategies to prevent progression to overt HF, deserve further study.
Left ventricular systolic and diastolic dysfunction and risk of HF
Previous cross-sectional studies have provided evidence for the existence of asymptomatic LV dysfunction in the general community (Stage B HF in the American College of Cardiology/American Heart Association classification system),3, 22 as well as increased prevalence and severity of LV dysfunction in patients with clinical HF (Stage C HF).3, 4 While these cross-sectional data supported the proposed stages of the HF, prior studies were limited by potential reverse causality, since assessment of LV function was performed at the same point in time as the diagnosis of clinical HF. Further, cross-sectional studies may be criticized for scientific circularity of reasoning in that the presence of LV systolic or diastolic dysfunction is used to make the diagnosis HFREF or HFPEF, respectively. Prospective data are needed to resolve these issues. In the Cardiovascular Health Study,23 LV systolic and diastolic dysfunction predicted incident HF over a mean follow-up of 5.2 years. However, the relations between the type of LV dysfunction (systolic versus diastolic) and the type of HF (HFREF versus HFPEF) were not assessed. More recently, researchers from the Mayo Clinic reported a 2-year HF incidence rate of 1.9% in a selected sample of 82 patients with preclinical diastolic dysfunction.24 Patients with systolic dysfunction were not studied. Our current data are consistent with these prior data and extend previous knowledge by demonstrating that LV systolic dysfunction predicts future HFREF, whereas LV diastolic dysfunction portends HFPEF. The current data, therefore, help to fill the knowledge gap linking Stage B to Stage C in the American College of Cardiology/American Heart Association classification scheme, whether referring to HFREF or HFPEF.
Non-cardiac dysfunction and risk of HF
HF is a clinical syndrome characterized by a constellation of signs and symptoms involving multiple organ systems besides the heart. Thus, even mild functional derangement of a non-cardiac organ system, which in itself is not severe enough to produce symptoms, may accelerate the manifestation of overt HF particularly when other organ systems are also involved. A decline in renal function affects sodium handling and fluid homeostasis, thus increasing the propensity to manifest fluid overload.5 Pulmonary function has a direct impact on the manifestation of dyspnea. Subclinical chronic pulmonary disease is characterized by low-grade inflammation and may contribute to progression of atherosclerosis and myocardial dysfunction,8 while even mild airflow obstruction is associated with abnormal LV diastolic filling.9 Anemia affects the oxygen-carrying capacity of the blood and is an adverse marker in overt HF.10 The availability of systematic, multi-system measurements during routine surveillance in the Framingham Heart Study enabled comprehensive assessment of these varied non-cardiac organ systems in relation to incident HF in the community. Our findings regarding the association with renal impairment are consistent with the Health ABC Heart Failure Model for incident HF in the elderly.25 In contrast, we did not find a significant association with hypoalbuminemia, and this may be due to differences in study samples (larger proportion of blacks and lower baseline serum albumin in the Health ABC Study).
In aggregate, these results suggest that the manifestation of clinically overt HF may be hastened by subclinical dysfunction in multiple organ systems. This is likely to particularly affect elderly individuals who have age-related decline in multi-organ function or multiple non-cardiac co-morbidities. Recognizing the contribution of non-cardiac dysfunction to HF progression may carry important clinical implications for preventing and managing heart failure. Further studies are warranted to validate these findings in other populations, evaluate for potential effect modification by covariates such as sex, and assess the potential impact of treatment of these risk factors on the risk of future HF.
Association of non-cardiac dysfunction with HFREF versus HFPEF
The distinction between factors associated with incident HFREF versus HFPEF deserves comment. The association of renal dysfunction and anemia with the risk of HFREF are consistent with classic studies of the cardiorenal syndrome and the known prognostic impact of anemia in overt HFREF.10 Interestingly, the most prominent non-cardiac predictor of incident HFPEF was airflow obstruction. This observation is supported by large epidemiologic studies showing a high prevalence of pulmonary disease in patients with HFPEF,26, 27 the frequent co-existence of HF in patients with chronic obstructive lung disease,28 as well as a recent study in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort demonstrating an association between airflow obstruction and abnormal LV filling.9 While this also raises the question of potential misdiagnosis of HFPEF, this is unlikely given the high specificity of the Framingham criteria for HF,29 the reliability of the diagnosis as demonstrated by consistent application of the same criteria over decades and stringent adjudication of endpoints in the Heart Study, and the lack of an alternative explanation for the clinical presentation (since the extent of pulmonary impairment was not severe enough to explain symptoms). Overall, the different predictors of HFREF versus HFPEF are consistent with prior epidemiologic,20 pathophysiologic,30 molecular,31 and outcome32 data supporting the notion that HFREF and HFPEF may represent separate entities. These observations may guide future clinical trial design, particularly in HFPEF where trials have, so far, been disappointing.
Strengths and limitations
The strengths of our study include the large community-based sample, uniform measurements of function of multiple organ systems, and longitudinal follow-up with continuous surveillance for, and careful validation of, HF outcomes. Further, the use of purely clinical criteria for the diagnosis of HF19 independent of LVEF is particularly advantageous in this setting. Limitations include the lack of tissue Doppler characterization of diastolic dysfunction, and the inherent pitfalls in using echocardiographic indices of diastolic filling as indicators of diastolic dysfunction. Nonetheless, mitral Doppler indices are widely available; and our results are consistent with previous studies using more comprehensive assessment of diastolic dysfunction.3, 24 We acknowledge the observational nature of our study which precludes conclusions regarding causality, as well as the modest effect sizes for non-cardiac organ system variables and potential for residual confounding or effect modification by other factors. We did not use time-varying covariates, but speculate that organ dysfunction would worsen with aging in most individuals, leading to even stronger associations with incident HF. Our uniformly white sample limits the generalizability of our findings to other ethnicities, and independent validation in other cohorts is warranted.
Conclusions
Our prospective observations in a large, community-based sample demonstrate that antecedent subclinical cardiac and non-cardiac major organ system dysfunction are associated with increased risk of clinical HF. These findings contribute to the understanding of HF as a progressive disease syndrome, and underscore the potential importance of non-cardiac risk factors in predisposing to the manifestation of overt HF.
Supplementary Material
CLINICAL COMMENTARY.
In our prospective study of a large, community-based sample, antecedent subclinical cardiac and non-cardiac major organ system dysfunction were associated with risk of future heart failure. The presence of asymptomatic left ventricular systolic and diastolic dysfunction preceded and increased the risk of incident heart failure by more than two-fold and more than 30%, respectively. Adjusting for cardiac dysfunction, the presence of subclinical renal impairment, airflow limitation or anemia was each associated with 30% increased risk of incident heart failure. Notably, the significant risk factors differed according to the type of incident heart failure (preserved versus reduced ejection fraction). Antecedent left ventricular systolic dysfunction, subclinical renal impairment and lower hemoglobin concentrations were associated with a higher incidence of heart failure with reduced ejection fraction, whereas antecedent diastolic dysfunction and baseline airflow obstruction were related positively to the risk of future heart failure with preserved ejection fraction. This study provides longitudinal evidence for the progressive nature of heart failure as emphasized in current heart failure guidelines, and underscores the potential importance of non-cardiac risk factors in predisposing to the manifestation of overt heart failure. The implications for the early identification of individuals at risk of heart failure and potential strategies to prevent progression to overt heart failure deserve further study.
Acknowledgments
Funding sources: National Heart, Lung and Blood Institute (Contract No. NO1-HC-25195). Dr. Lee was supported by a clinician-scientist award from the Canadian Institutes of Health Research.
Footnotes
Conflict of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 2.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Levy S, Linde C, Lopez-Sendon JL, Nieminen MS, Pierard L, Remme WJ. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 3.Redfield MM, Jacobsen SJ, Burnett JC, Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith GL, Shlipak MG, Havranek EP, Foody JM, Masoudi FA, Rathore SS, Krumholz HM. Serum urea nitrogen, creatinine, and estimators of renal function: mortality in older patients with cardiovascular disease. Arch Intern Med. 2006;166:1134–1142. doi: 10.1001/archinte.166.10.1134. [DOI] [PubMed] [Google Scholar]
- 6.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008;155:883–889. doi: 10.1016/j.ahj.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 7.Arques S, Ambrosi P, Gelisse R, Luccioni R, Habib G. Hypoalbuminemia in elderly patients with acute diastolic heart failure. J Am Coll Cardiol. 2003;42:712–716. doi: 10.1016/s0735-1097(03)00758-7. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJ. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, Shahar E, Smith LJ, Watson KE. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 11.Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. JAMA. 1987;257:2318–2324. [PubMed] [Google Scholar]
- 12.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431–433. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 13.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 15.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagie A, Benjamin EJ, Galderisi M, Larson MG, Evans JC, Fuller DL, Lehman B, Levy D. Reference values for Doppler indexes of left ventricular diastolic filling in the elderly. J Am Soc Echocardiogr. 1993;6:570–576. doi: 10.1016/s0894-7317(14)80174-0. [DOI] [PubMed] [Google Scholar]
- 17.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 18.Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, Levy D. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288:1252–1259. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 19.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 20.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 22.Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, Doring A, Broeckel U, Riegger G, Schunkert H. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320–328. doi: 10.1016/s0195-668x(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 23.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 24.Correa de Sa DD, Hodge DO, Slusser JP, Redfield MM, Simari RD, Burnett JC, Chen HH. Progression of preclinical diastolic dysfunction to the development of symptoms. Heart. 96:528–532. doi: 10.1136/hrt.2009.177980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 27.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, Lammers JW, Hoes AW. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005;26:1887–1894. doi: 10.1093/eurheartj/ehi291. [DOI] [PubMed] [Google Scholar]
- 29.Mosterd A, Deckers JW, Hoes AW, Nederpel A, Smeets A, Linker DT, Grobbee DE. Classification of heart failure in population based research: an assessment of six heart failure scores. Eur J Epidemiol. 1997;13:491–502. doi: 10.1023/a:1007383914444. [DOI] [PubMed] [Google Scholar]
- 30.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 31.Borbely A, Papp Z, Edes I, Paulus WJ. Molecular determinants of heart failure with normal left ventricular ejection fraction. Pharmacol Rep. 2009;61:139–145. doi: 10.1016/s1734-1140(09)70016-7. [DOI] [PubMed] [Google Scholar]
- 32.Somaratne JB, Berry C, McMurray JJ, Poppe KK, Doughty RN, Whalley GA. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11:855–862. doi: 10.1093/eurjhf/hfp103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.