Abstract
The mechanisms by which animals adapt to an ever-changing environment have long fascinated scientists. Different forces, conveying information regarding various aspects of the internal and external environment, interact with each other to modulate behavioral arousal. These forces can act in concert or, at times, in opposite directions. These signals eventually converge and are integrated to influence a common arousal pathway, which, depending on all the information received from the environment, supports the activation of the most appropriate behavioral response. In this review we propose that the ventromedial hypothalamic nucleus (VMN) is part of the circuitry that controls food anticipation. It is the first nucleus activated when there is a change in the time of food availability, silencing of VMN ghrelin receptors decrease food anticipatory activity (FAA), and although lesions of the VMN do not abolish FAA, parts of the response are often altered. In proposing this model, it is not our intention to exclude parallel, redundant and possibly interacting, pathways that may ultimately communicate with, or work in concert with, the proposed network; but rather to describe the neuroanatomical requirements for this circuit, and illustrate how the ventromedial nucleus of the hypothalamus is strategically placed and connected to mediate this complex behavioral adaptation.
Keywords: ventromedial hypothalamus, food anticipatory activity, generalized arousal, locomotor activity
Introduction
Generalized arousal is thought of as a primitive, elementary function of the hindbrain reticular formation that is essential for the activation of any behavior (Pfaff, 2005). It synergizes with specific forms of arousal, such as those arising from sexual needs, thirst, fear and anger. Here we consider the role of hunger-stimulated arousal under experimental circumstances in which this force for arousal is set up against a normal circadian rhythm of arousal; that is, forcing the animal to be active for feeding during the light period, a time when circadian timing mechanisms normally mandate that a nocturnal animal be resting.
Living in an environment where resources constantly fluctuate, be it on a daily, monthly, or annual basis, has provided certain challenges to terrestrial organisms. Homeostatic mechanisms have evolved that detect these changes and elicit compensatory rebounds to these challenges. Certain set-points are pre-determined and the body has antagonistic pathways that finely coordinate its responses to maintain them as close to this optimal value as possible, at all times. These challenges include hunger, thirst and sleep deprivation, to name a few. In addition to these homeostatically regulated variables, our responses are also driven by a circadian component, a rhythm that oscillates with a periodicity of approximately 24 h. How and where these forces interact and become integrated to elicit the most appropriate behavioral response remains poorly understood.
In terms of sleep physiology, it is well understood that there is a circadian (process C) and a homeostatic (process S) component that together determine the timing and amounts of sleep. The influence of each of these forces can be experimentally manipulated and Alexander Borbély and colleagues in Zurich have developed sophisticated mathematical models to predict sleep responses to variations in these two parameters (Borbély, 1982).
The circadian pacemaker is known to reside in the suprachiasmatic nuclei (SCN), located in the anterior hypothalamus. Lesioning these nuclei abolishes the consolidated periods of sleep and arousal, without affecting total sleep time (Ibuka et al., 1980). In addition, SCN-lesioned animals exhibit uncompromised responses to sleep deprivation, suggesting that the two processes (S and C) are independent of each other, and furthermore, that the brain site where homeostatic sleep mechanisms reside is not within the SCN (Mistlberger et al., 1983; Tobler et al., 1983).
Like sleep, to date, no single brain region has been identified that is sufficient and necessary to generate food anticipatory activity. That is, destruction of any one nucleus, of the likely targets already identify by neuronal activation studies, has failed to completely or permanently abolish all food anticipatory behaviors. The dorsomedial nucleus of the hypothalamus (DMH) has attracted a fair amount of interest and speculation as the potential location for where the food entrainable oscillator may reside. The data supporting this theory come from the studies of Saper et al. showing that DMH-lesioned mice fail to anticipate a timed meal (Gooley et al., 2006) and this group has also recently shown that restoration of Bmal1 signaling in the DMH using a viral vector is capable of rescuing FAA in this normally compromised transgenic model (Bmal1 knockout) (Fuller et al., 2008). Collectively these findings would strongly support the hypothesis that indeed the location for the food entrainable oscillator is the DMH and that it relies on well-characterized transcription and translational circadian loops; however, numerous attempts by several independent laboratories have failed to replicate these findings (Landry et al., 2006; Landry et al., 2007; Mistlberger et al., 2008; Moriya et al., 2009; Pendergast et al., 2009; Storch & Weitz, 2009) (for review see Mistlberger et al., 2009).
The search for the location of the food-entrainable oscillator (FEO) continues to this day. Since FAA involves activity, eating, learning/conditioning, reward/reinforcement it was thought to lie within the central nervous system. Recently, FEOs have also been sought in peripheral organs such as the liver, kidney, heart, pancreas and stomach (Comperatore & Stephan, 1990; Davidson & Stephan, 1998; Damiola et al., 2000; Yamazaki et al., 2000; Davidson et al., 2003). Interestingly, Davidson et al. have shown that peripheral tissues (e.g. liver, colon, stomach) express circadian genes products in a 24-h rhythmic cycle, and when food availability is restricted to daytime periods in nocturnal rodents, the phase of peripheral clock gene expression is set to the new feeding time (Damiola et al., 2000; Yamazaki et al., 2000; Davidson et al., 2003). However, the phase of Per1 clock remained nocturnal during subsequent food deprivation and during a 2-meal food restriction paradigm, which is not consistent with a circadian pacemaker (Davidson et al., 2003).
Redundant pathways control food anticipation
Given their critical role for survival, it is likely that redundant pathways have evolved to safeguard proper responses to changes in these variables, both in terms of detecting these changes, as well as executing the most appropriate behavioral response. This likely explains the inability of small localized brain lesions to abolish a behavioral response to sleep deprivation or hunger (timed meal). Although, if the lesion affects part(s) of the food anticipatory response, or if the region is activated at the time of food anticipation, the area is likely part of this redundant circuit.
We hypothesize that the increased arousal to a homeostatic challenge, such as food, is likely mediated by interactions among multiple brain networks, ultimately converging on common neuronal outputs that execute the most appropriate behavioral response to that particular challenge. As such, deficiencies in communication or, in extreme cases, total loss of one component of this system will not have catastrophic results for the organism. Knowing the neuroanatomical substrates that comprise the inputs (humoral), the integration of signals and neuroanatomical outputs (motor) of FAA is essential to understand the regulation of this circuit. One way of studying homeostatically driven mechanisms is by identifying neuronal groups that are selectively activated in response to stimulation of the homeostatic drive, e.g. sleep deprivation, hunger, thirst. This approach has, in fact, been extensively used and has yielded very fruitful studies correlating homeostatic need, e.g. following sleep deprivation, with selective activation of particular neuronal populations, e.g. the ventrolateral preoptic nucleus (Sherin et al., 1996). This knowledge proved critical in establishing a network of interacting brain pathways that are hypothesized to ultimately determine vigilance state. This approach has also been used to study increases in motor activity in anticipation of food (Angeles-Castellanos et al., 2004; Mendoza et al., 2005; Meynard et al., 2005; Johnstone et al., 2006; Angeles-Castellanos et al., 2007; Escobar et al., 2007; Ribeiro et al., 2007; Poulin & Timofeeva, 2008). Many brain regions have been shown to become activated accompanying feeding times, both before, during feeding or after feeding, when animals are subjected to either timed restrictive feeding, hypocaloric or hypoproteic diets (Feillet et al., 2006). Our focus here is on the neuroanatomical structures likely involved in modifying behavior in response to these changes in the timing of resource availability, where in the brain these signals are first perceived and on the neurochemical substrates that likely communicate these signals to the brain.
The ventromedial hypothalamic nucleus as part of the FAA circuitry
We found that the VMN is the only brain region, of 16 brain nuclei reported to mediate changes in arousal, to become active when FAA is first manifested in response to food restriction at a unfavorable circadian time, compared to animals anticipating food delivered during the normal active (dark) period (Ribeiro et al., 2007). Essentially, we entrained mice to anticipate a restricted meal whose timing coincided with lights off. After the animals were well entrained to the dark onset feeding time, we shifted the food presentation time of half of the mice to the middle of the light period, thus setting up these two forces for arousal – the new feeding time and the circadian light/dark cycle - against each other and monitored running wheel activity (Ribeiro et al., 2007). The day after increased running wheel activity was first observed preceding the shifted food presentation time, matched pairs of animals (a shifted and a non-shifted control) were sacrificed at the same time during the anticipatory period and the level of neuronal activation - as judged by the expression of c-FOS protein - was compared between the animal expecting a meal at dark onset (non-shifted group) and the animal expecting a meal in the middle of the light period (shifted group) (Ribeiro et al., 2007). Of the brain regions examined, only the VMN had increased c-FOS expression in the shifted animals (Ribeiro et al., 2007). This is not to say that had longer periods passed from when the FAA paradigm was imposed, other brain regions might not likewise have become activated. For instance, other neuronal activation studies have looked at c-FOS activation under various feeding schedules once FAA is robustly expressed (between 10 days and 3 weeks) (Angeles-Castellanos et al., 2004; Mendoza et al., 2005; Meynard et al., 2005; Angeles-Castellanos et al., 2007; Johnstone et al., 2006; Poulin & Timofeeva, 2008), protocols which are very different from our study that aimed to identify the very first brain region to sense the changes in food availability time (hence we sacrificed the mice as close to the development of the behavioral response as possible). Likewise, we examined neuronal activation just before food presentation (0–1h before food presentation), it is therefore possible that other brain regions had been activated earlier and/or later than the VMN, even at the early stage of the development of FAA.
Other studies also suggest a role for the VMN in the circuitry controlling food anticipation. Although lesion experiments have demonstrated that no brain region alone, including the VMN, is required for the maintenance of FAA, lesions of the VMN did abolish anticipatory activity and corticosterone rhythms in 3 studies (Krieger, 1980; Inouye, 1982; Saito et al., 1982) and in other studies attenuated FAA (Mistlberger & Rechtschaffen, 1985) and corticosterone rhythms (Honma et al., 1987) in the first weeks after surgery, although the rhythms reappeared later. VMN disruption with colchicine has also been shown to disrupt restricted feeding-induced corticosterone rhythm (Choi et al., 1998). Silencing ghrelin receptors within the VMH also decreases the amount of FAA (see below). The VMN is the only basomedial hypothalamic nucleus to be activated 2 hrs after arousal from hibernation in jerboas (El Ouezzani et al., 1999) and is essential to allow shifting of locomotor activity during restricted feeding (Challet et al., 1997). It is also important to point out that in these experiments, or in the theory proposed in this review, it is not possible to ascertain whether the VMN contains food entrainable oscillators but rather that it is part of a brain circuitry involved in food anticipation.
Feeding-related information is perceived by a combination of peripheral and central sensing mechanisms. In the periphery, gastrointestinal hormones are released by the enteric endocrine system, in response to either satiety (e.g. cholecystokinin, leptin) or hunger (e.g. ghrelin), establishing a finely-tuned homeostatic regulatory loop. Food anticipatory signals are likely communicated to the central nervous system by humoral factors since disrupting vagal or spinal afferent communication does not disrupt the ability of animals to anticipate food (Comperatore & Stephan, 1990; Davidson & Stephan, 1998, Davidson et al., 2003), or these could be redundant pathways. We next examine the inputs and outputs that may underlie the mechanisms by which the VMN is involved in food anticipation.
Humoral Inputs to VMN
Even though the VMN has long been considered the “satiety center” of the brain, it is now known that it is a heterogeneous nucleus and activation or inhibition of neuronal subpopulations can either stimulate or inhibit feeding.
Energy balance and body weight are under the fine control of a variety of factors, produced by the gastrointestinal tract, which communicate to the brain via neural (Obici, 2009) and endocrine pathways (Grün & Blumberg, 2009). These molecules act on neurons in the arcuate nucleus, which is composed of two major neuronal subtypes involved in the regulation of food intake. Some arcuate neurons stimulate feeding and contain neuropeptide Y (NYP) and agouti-related peptide (AGRP), while others suppress feeding and contain proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript. Arcuate neurons are responsive to peripheral hormonal signals that can activate or inhibit these opposing neuronal subtypes. Interestingly, POMC neurons have been shown to receive strong excitatory inputs from the ventromedial VMN, whereas NPY neurons receive only inhibitory inputs and predominantly from other arcuate neurons (Sternson et al., 2005). Under conditions of starvation, the amount of excitatory input to POMC neurons is diminished, establishing a way for VMN neurons to control the activity of arcuate neurons and eventually increase food intake (Sternson et al., 2005). The levels of circulating hormones (satiety-promoting: e.g. leptin and other postprandial gut peptides, hunger-promoting: e.g. ghrelin and PYY) influence the activity of these subpopulations of arcuate and VMN neurons, that will increase or decrease feeding according to short- and long-term signals of nutritional status.
The VMN is comprised of glucose-sensing neurons (Oomura et al., 1964; Ono et al., 1982; Ashford et al., 1990; Mobbs et al., 2001; Song & Routh, 2005), and VMN neurons have the ability to respond to a variety of humoral factors, including those that inhibit food intake: leptin (Dhillon et al., 2006; Canabal et al., 2007), cholecystokinin (Barnes et al., 1991), insulin (Canabal et al., 2007), alpha-melanocyte-stimulating hormone (Fu & van den Pol, 2008), histamine (Zhou et al., 2007), glucagon-like peptide-1 (Sanz et al., 2007), polypeptide YY (Huang et al., 2008), estrogens (Zhou et al., 2007), serotonin (Hikiji et al., 2004); and factors that promote food intake: ghrelin (Chen et al., 2005; Yanagida et al., 2008), NPY (Davidowa et al., 2002) and AGRP (Fu et al., 2008), orexins (Muroya et al., 2004), dopamine (Davidowa et al., 2002) and norepinephrine (Lee et al., 2007).
We hypothesize that, together, these properties of VMN neurons enable the brain to sense the body’s metabolic state, and by using its many projections to arousal-promoting regions, the CNS can then mount the most appropriate behavioral response (favoring either energy conservation or energy dissipation, depending on the situation).
As this article covers the role of the VMN on arousal in anticipation of food presentation, we will focus on humoral inputs that promote arousal, hunger and/or feeding. Some of the orexigenic signals are produced peripherally. Among those, ghrelin is a primary candidate in the appearance of anticipatory behavior. Ghrelin is secreted in anticipation of food and signals meal time (Drazen et al., 2006). Drazen et al. have suggested that ghrelin secretion is regulated by learned anticipation of regularly timed meals independent of the state of food deprivation making it a good candidate for “the regulation of anticipatory processes involved in food intake” (Drazen et al., 2006). Our recent studies show that transgenic animals lacking ghrelin receptors have a much reduced FAA response, compared to their littermate controls (~50% reduction in FAA), and that oxyntic cells in the stomach change their expression of circadian genes to match the time of the feeding and not the light/dark cycle (LeSauter et al., 2009). Additionally, bilateral silencing of ghrelin receptors specifically in VMN neurons using viral vector delivery of shRNA against growth hormone secretagogue 1a, likewise compromises the ability of these animals to properly anticipate a timed meal (~50% reduction in FAA) presented at either dark onset of during the middle of the light period, compared to control-injected mice (Ribeiro, LeSauter, Musatov, Arrieta-Cruz, Silver and Pfaff, unpublished observations). Together these studies provide strong evidence that ghrelin receptors in VMN neurons are involved in food anticipatory responses.
VMN neurons respond to gastric distension (Sun et al., 2006), presumably through vagal afferents (Liu et al., 2004), which suggest a neural pathway from the gut to the VMN. Ghrelin receptors are found in vagal afferents, and intravenous ghrelin administration decreases vagal electrical activity (Date et al., 2002). There are, however, conflicting results on whether or not the vagus nerve is necessary for ghrelin-induced feeding (Date et al., 2002; Date et al., 2005; Arnold et al., 2006).
Circulating gut peptides may also reach the VMN by crossing the blood-brain barrier or via circumventricular organs and act directly or indirectly on VMN neurons. The main effect of ghrelin on promoting food intake is thought to be through the NPY-AGRP neurons of the arcuate nucleus. Arcuate nucleus neurons bear growth hormone secretagogue receptors (the ligands for ghrelin) and expresses FOS following ghrelin administration (Howard et al., 1996; Guan et al., 1997; Yokote et al., 1998; Mitchell et al., 2001; Wang et al., 2002; Zigman et al., 2006). AGRP and NPY double knock-out mice do not exhibit ghrelin-induced feeding (Chen et al., 2004). The VMN contains NPY and melanocortin 4 receptor (MC4R) receptors (Bouali et al., 1995b; Lopez-Valpuesta et al., 1996; Harrold et al., 1999; Wisialowski et al., 2000; Li & Ritter, 2004; Kishi et al., 2005), suggesting that both NPY and POMC neurons project from the arcuate to the VMN. Administration of NPY and NPY agonist into the VMN increase feeding (Bouali et al., 1995b). It is thought that peripheral ghrelin activates NPY/AGRP neurons inducing the release of these orexigenic peptides in their target regions, including the VMN.
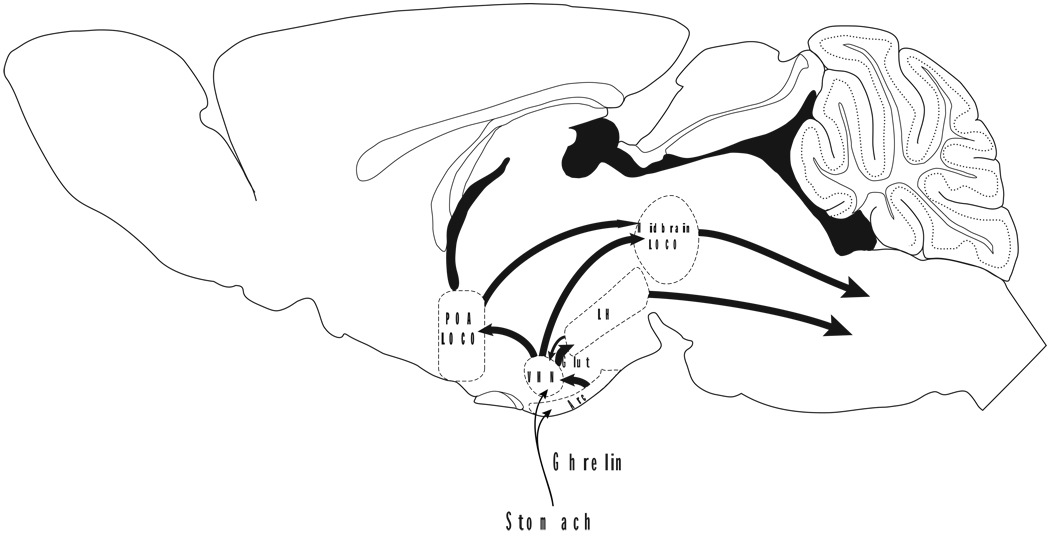
Ghrelin, also, may act directly on the VMN by passing through the blood brain barrier or by diffusion from the arcuate nucleus region (Figure 1). The VMN contains growth hormone secretagogue receptors (Howard et al., 1996; Guan et al., 1997; Yokote et al., 1998; Mitchell et al., 2001; Zigman et al., 2006), and ghrelin increases the firing rate in a large proportion (~65%) of VMN neurons in young rats (Yanagida et al., 2008). In a study differentiating glucose-sensitive from other VMN cells, ghrelin increased firing rate in 40% of non-glucose-sensitive and decreased firing rate in 82% of glucose-sensitive VMN cells, inhibiting their anorexic effects and promoting food intake. In addition, ghrelin acts in VMH through fatty acid metabolism as central inactivation of AMP-activated protein kinase in the VMH blocks the orexigenic effect of ghrelin (Lopez et al., 2008).
Figure 1.
Activation of arousal by hunger: Minimally sufficient model to explain how ventral medial hypothalamic (VMN) neurons drive food anticipatory activity. Schematic representation of humoral inputs to VMN; and neuroanatomical projections from VMN to brain regions involved in increasing generalized arousal in response to hunger (Only well-established neuroanatomical connections are depicted.) VMN: ventromedial nucleus of the hypothalamus; POA LOCO: preoptic locomotor region; LH: lateral hypothalamus (particularly orexin-containing neurons); Midbrain LOCO: midbrain locomotor region, Arc: arcuate nucleus, Glut: glutamatergic projections. Figure adapted from Paxinos and Franklin (Paxinos & Franklin, 2001).
NPY and AGRP are secreted by neural afferents from the arcuate nucleus to several hypothalamic nuclei. As stated above, there are NPY receptors in the VMN, and NPY administration in the VMN stimulates food intake (Bouali et al., 1995a). AGRP also stimulates feeding (Rossi et al., 1998). It was thought to act as a melanocortin receptor antagonist in the VMN, but this effect has been questioned (Fu & van den Pol, 2008).
Orexins also act on the VMN and likely mediate increased arousal in response to hunger. Intracerebroventricular (i.c.v.) injection of orexins induces a rapid increase in food intake (Lubkin & Stricker-Krongrad, 1998; Sakurai et al., 1998; Edwards et al., 1999; Haynes et al., 2000; Jain et al., 2000; Sartin et al., 2001). In addition to other hypothalamic nuclei, orexin receptors are present in the VMN (Trivedi et al., 1998; Lu et al., 2000; Hervieu et al., 2001; Cluderay et al., 2002; Zhang et al., 2005). Orexin-A and -B can activate NPY and inhibit POMC neurons in the arcuate and inhibit glucose-sensitive neurons in the VMN to stimulate food intake (Muroya et al., 2004). I.c.v. injection of orexins also evokes emotion-related behavior such as stereotypy and hyperlocomotion (Ida et al., 1999; Nakamura et al., 2000).
Given their critical roles in mediating feeding and locomotor activities, several studies have examined the effects of orexins on FAA. Lack of orexin signaling has been shown to compromise the ability of animals to properly anticipate a timed meal, suggesting that orexin neurons could serve as an important efferent signal to locomotor areas (Mieda et al., 2004). On the other hand, saporin ablation of hypocretin-2 cells in the lateral hypothalamus does not abolish FAA (Mistlberger et al., 2003), orexin knockout animals, despite having reduced FAA, do have robust anticipatory rhythms of body temperature, suggesting, again, that orexins may be involved in an efferent pathway to induce locomotor activity, and the generation of FAA (Kaur et al., 2008).
There are other putative FAA signals. For example intestinal apolipoprotein A-IV is rhythmically released and the rhythm is controlled by the timing of meals, with levels increasing before meal onset. Adrenal corticosterone levels are also rhythmic and peak in anticipation of meals (Shen et al., 2005) and these levels are influenced by timing of meals (Wilkinson et al., 1979; Honma et al., 1984). During restricted feeding, corticosterone levels increase about 1 hr before food presentation. This precedes the premeal increase in apolipoprotein A-IV (Shen et al., 2005). Adrenalectomy abolishes the rhythms of both corticosterone and apolipoprotein A-IV. These effects may take place at least in part in the VMH as adrenalectomy abolishes NPY induced insulin release and reduce the levels of Y1 and Y5 receptor mRNA in the VMN (Wisialowski et al., 2000).
VMN arousal-promoting outputs
In addition to receiving massive humoral inputs conveying information about energy status of the organism, and the ability of VMN neurons to sense changes in glucose availability (Oomura et al., 1964; Ono et al., 1982; Ashford et al., 1990; Mobbs et al., 2001; Song & Routh, 2005) and modify their firing properties accordingly, the VMN must also faithfully signal this information to the appropriate brain regions to increase foraging and locomotion (Figure 1).
VMN has long been associated with feeding and the regulation of energy balance, starting from Hetherington and Ranson, who in 1940, first demonstrated that lesions at “the base of the diencephalon” (corresponding to the location of the VMN) were associated with extreme obesity in animals and humans (Hetherington & Ranson, 1940). Since then, many studies have examined the role of the VMN in energy homeostasis and collectively they have found that lesions (Hetherington & Ranson, 1940; Shimizu et al., 1987), chemical blockade (Maes, 1980) or genetic ablation (Majdic et al., 2002) of VMN neurons leads to obesity, while chemical (Shimazu & Ishikawa, 1981) or electrical (Stenger et al., 1991) stimulation of this nucleus leads to satiety and decreased food intake. Position emission tomography shows blood flow increase of the hypothalamic nuclei during hunger and a decrease after eating (Tataranni et al., 1999; Morris & Dolan, 2001). Functional magnetic resonance imaging shows decrease in signal after eating in PVN and VMN (Matsuda et al., 1999). Interestingly, Kokoeva et al. recently demonstrated that changes in energy balance are accompanied by neurogenesis in VMN (Kokoeva et al., 2005), thus illustrating the dynamic changes that can occur within hypothalamic feeding circuits depending on the metabolic state of the animal.
Morphologically, the VMN can be subdivided into 3 major cytoarchitectural areas: the ventrolateral region, a central region and the dorsomedial region; and these three areas are anatomically, neurochemically and behaviorally distinct. Each of these regions has distinct projection patterns (Saper et al., 1976; Canteras et al., 1994), and they can broadly be grouped as ascending fibers that innervate anterior targets, including medial and lateral preoptic regions, lateral septum, preoptic and thalamic periventricular nuclei, bed nucleus of the stria terminalis and the amygdala (Saper et al., 1976; Krieger et al., 1979; Canteras et al., 1994). The majority of these long projections arise from the ventrolateral VMN (Conrad & Pfaff, 1976; Saper et al., 1976; Krieger et al., 1979; Canteras et al., 1994), although some sparse dorsomedial projections to the preoptic area (POA) have also been observed (Krieger et al., 1979; Canteras et al., 1994).
Particularly relevant to the promotion of arousal and locomotion are the projections to the POA, a well-characterized brain region known to be antidromically activated along with the midbrain locomotor region in promoting locomotor behaviors (Sakuma, 1976; Swanson et al., 1987) (Figure 1).
The VMN also projects directly (Moga & Moore, 1997) or indirectly (Canteras et al., 1994; Moga & Moore, 1997) to the SCN and can allow for phase shifts in locomotor activity when homeostatic challenges are set in antiphase to environmental light/dark conditions (Challet et al., 1997). More rostrally, VMN projecting fibers extend to the cholinergic ventral and diagonal band of broca (Conrad & Pfaff, 1976; Krieger et al., 1979), which are well known to contribute to the promotion of arousal and cortical activation (Jones, 2004).
VMN neurons also send dense excitatory projections to the nearby arcuate nucleus, specifically to the POMC neurons, and this excitatory tone is decreased with fasting (Sternson et al., 2005), thus demonstrating the dynamic interplay between energy state and output strength from the VMN. Food restriction also leads to morphological changes of VMN neurons, shortening long primary dendrites of lateral projecting neurons in the ventrolateral VMN and reducing the soma area of dorsomedial VMN neurons with medially projecting long primary dendrites (Flanagan-Cato et al., 2008).
Caudal projections from VMN generally follow three major routes: one pathway travels along the medial hypothalamus projects to the ventral tegmental area, the supramammillary nucleus and mammillary complex; a second pathway travels along the periventricular region and eventually projects to the posterior hypothalamus, including the histaminergic tuberomammillary nucelus, the noradrenergic locus coeruleus and the serotinergic dorsal raphe nuclei (Saper et al., 1976; Krieger et al., 1979) [all members of the classical ascending reticular activating system originally proposed by Moruzzi and Magoon in 1949 (Moruzzi & Magoun, 1949)]; and finally, a pathway that travels along the supraoptic commissure, which eventually projects to the zona incerta, central amygdala, peripeduncular nucleus and central tegmental fields (Conrad & Pfaff, 1976; Saper et al., 1976; Krieger et al., 1979; Canteras et al., 1994).
The VMN also has extensive projections to the nearby arousal and foraging promoting orexinergic lateral hypothalamic fields (Arees & Mayer, 1967; Sutin & Eager, 1969; Millhouse, 1973) (Figure 1). These findings have recently been confirmed, and extended, and VMN does indeed send a large number of projections to all areas of lateral hypothalamus (LH), with particularly high density to the medial LH; in fact, 23% of all LH neurons receive VMN appositions (Yoshida et al., 2006). Electrical stimulation of the LH has been shown to increase locomotor activity, and these increases are not mediated via the mibrain locomotor region (Sinnamon & Stopford, 1987).
Very dense VMN projections are likewise observed traveling up to the mesencephalic reticular formation, including the anterior ventromedial midbrain, posterodorsal midbrain, which have been previously shown to facilitate locomotor behavior in anesthetized rats (Sinnamon et al., 1987) (Figure 1).
Finally, labeled projections from the VMN have been identified as far caudal as the pontine and mesencephalic reticular formation, including gigantocellular neurons (Conrad & Pfaff, 1976; Krieger et al., 1979; Parent & Steriade, 1981; Canteras et al., 1994), which we hypothesize play a key role in the modulation of a generalized arousal state (Martin, Pavlides and Pfaff, unpublished observations).
Promotion of Arousal/Locomotion: A theory of how FAA is initiated
We propose that activation of VMN neurons initiates FAA by virtue of its efferents to three neural groups: POA locomotor region, midbrain locomotor region and lateral hypothalamic orexin neurons (via glutamatergic projections) (Figure 1).
The most well characterized brain areas for promoting locomotor behaviors in the brain are the POA and the midbrain locomotor region. Both these centers receive dense innervations from the VMN (Conrad & Pfaff, 1976; Saper et al., 1976; Krieger et al., 1979; Canteras et al., 1994). Sinnamon et al. have identified discrete locations within the POA where chemical or electrical stimulation leads to locomotor stepping in anesthetized rats (Sinnamon, 1987; Levy & Sinnamon, 1990; Sinnamon et al., 1991; Sinnamon, 1992). Within the POA, the areas most likely to induce locomotor behaviors in anesthetized rats extend from the ventral bed nucleus of the stria terminalis, the lateral part of the POA, horizontal band of the diagonal band of Broca, anterior hypothalamic area and the medial and rostral parts of the ventral pallidum (Sinnamon, 1992). Likewise, the midbrain locomotor region is composed of discrete regions where electrical stimulation is particularly effective at initiating locomotion (Mori et al., 1978), specifically the medioventral medulla, including the nucleus reticularis gigantocellularis and the nucleus reticularis ventralis that are essential for locomotion (Garcia-Rill et al., 1987).
The POA locomotor region and the midbrain locomotor region have been shown to communicate with each other; in fact, using a combination of neuroanatomical tracings and electrophysiological recordings Swanson et al. have established that the POA and the midbrain locomotor region (including the zona incerta and pedunculopontine tegmental area) exhibit bidirectional connectivity, and they also demonstrated that neurons in the POA are antidromically activated with stimulation of the midbrain locomotor region, and vice-versa (Swanson et al., 1987) (Figure 1).
Neurons within the lateral hypothalamus are also involved in locomotor behaviors, for instance animals bred for high running wheel activity have higher c-FOS-positive neurons within the lateral hypothalamus compared to animals whose wheels were locked (Rhodes et al., 2003) (Figure 1). Furthermore, electrical stimulation of the lateral hypothalamus elicited locomotor activity in rats (Sinnamon et al., 1987), central administration of orexins increases locomotor activity (Nakamura et al., 2000) and cerebrospinal levels of orexin-A is increased after forced activity (Martins et al., 2004). Furthermore, Yamanaka et al. reported that levels of orexin mRNA in lateral hypothalamic neurons are inversely correlated to blood glucose levels, leptin and food intake (Yamanaka et al., 2003). Animals that lack orexin neurons fail to increase their locomotor activity and arousal responses under fasting conditions (Yamanaka et al., 2003). VMN neurons send dense projections to LH neurons (Arees & Mayer, 1967; Sutin & Eager, 1969; Millhouse, 1973; Conrad & Pfaff, 1976; Saper et al., 1976), which are hypothesized to be excitatory, glutamatergic, in nature, that presumably activate food-seeking mechanisms.
Future directions
Given its vast numbers of humoral inputs, the ability of its neurons to sense and respond to changes in glucose levels, and its connectivity to major arousal promoting areas, we propose that the VMN is a prime candidate for the integration of feeding signals and for orchestrating the most appropriate behavioral response to constant fluctuations in resource availability.
Our previous work (Ribeiro et al., 2007) identified VMN neurons as first responders in an FAA paradigm. Silencing VMH ghrelin receptors reduces FAA. . Now we must identify the transcriptomes of those neurons, first by immunocytochemistry and then by single cell RT/PCR. For example,, using a combination of retrograde markers microinjected amongst orexin neurons and RT/PCR, we will be able to test the hypothesis that glutamatergic VMN neurons have the correct connectivity to excite orexin neurons, thus to increase the animal's arousal prior to food availability. Subsequent electron microscopy of orexin neurons following anterograde marker injections in VMN would confirm that synapses from those VMN neurons are actually made onto immunochemically-identified orexin neurons, which have previously been implicated in increasing locomotor activity during FAA (Mieda et al., 2004; Kaur et al., 2008).
Additionally, future studies aimed at investigating the possibility that VMN neurons could act as food entrainable oscillators should be undertaken. For instance, in vivo recording of VMN neurons during food anticipatory activity, both during food restriction days, as well as ad libitum and food deprivation days (typical food anticipatory protocol) – presumably these neurons should increase their firing rate during times of food anticipation, the increased firing should cease under ad libitum food, and once food is scarce the firing of these neurons should increase to match the anticipatory timing prior to ad libitum conditions. Again, a complete transcriptome analysis of these electrophysiologically-identified neurons may reveal novel signaling peptides or receptors involved in anticipating temporally restricted feedings.
In conclusion, the evidence that VMN neurons are activated when food anticipatory activity is first expressed (Ribeiro et al., 2007), that ghrelin may be a signal for food anticipation (LeSauter et al., 2009) and silencing VMN ghrelin receptors reduces FAA (results from our lab), and that different types of VMN lesions either abolish or decrease at least some food anticipation responses (Krieger, 1980; Honma et al., 1987; Inouye, 1982; Mistlberger & Rechtschaffen, 1985; Saito et al., 1982), strongly suggest that the VMN is part of a redundant circuitry controlling FAA.
Acknowledgments
This research was supported by NHLBI award HL-086018 to A.C.R., NIH award NS-37919 to Rae Silver and J. L. and NIH award HD-05751 to D.W.P.
Abbreviations
- AGRP
agouti-related peptide
- FAA
food anticipatory activity
- FEO
food entrainable oscillator
- i.c.v
intracerebroventricular
- LH
lateral hypothamalus
- MC4R
melanocortin 4 receptor
- midbrain LOCO
midbrain locomotor regions
- POA
preoptic area
- POA LOCO
preoptic locomotor area
- NPY
neuropeptide Y
- POMC
proopiomelanocortin
- SCN
suprachiasmatic nuclei
- VMN
ventromedial nucleus of the hypothalamus
References
- Angeles-Castellanos M, Aguilar-Roblero R, Escobar C. c-Fos expression in hypothalamic nuclei of food-entrained rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R158–R165. doi: 10.1152/ajpregu.00216.2003. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144:344–355. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Arees EA, Mayer J. Anatomical connections between medial and lateral regions of the hypothalamus concerned with food intake. Science. 1967;157:1574–1575. doi: 10.1126/science.157.3796.1574. [DOI] [PubMed] [Google Scholar]
- Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990;415:479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Barnes S, Whistler HL, Hughes J, Woodruff GN, Hunter JC. Effect of cholecystokinin octapeptide on endogenous amino acid release from the rat ventromedial nucleus of the hypothalamus and striatum. J Neurochem. 1991;56:1409–1416. doi: 10.1111/j.1471-4159.1991.tb11439.x. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Bouali SM, Fournier A, St-Pierre S, Jolicoeur FB. Effects of NPY and NPY2-36 on body temperature and food intake following administration into hypothalamic nuclei. Brain Res Bull. 1995a;36:131–135. doi: 10.1016/0361-9230(94)00177-3. [DOI] [PubMed] [Google Scholar]
- Bouali SM, Fournier A, St-Pierre S, Jolicoeur FB. Influence of ambient temperature on the effects of NPY on body temperature and food intake. Pharmacol Biochem Behav. 1995b;50:473–475. doi: 10.1016/0091-3057(94)00266-l. [DOI] [PubMed] [Google Scholar]
- Canabal DD, Potian JG, Duran RG, McArdle JJ, Routh VH. Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R592–R600. doi: 10.1152/ajpregu.00207.2007. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Challet E, Pevet P, Lakhdar-Ghazal N, Malan A. Ventromedial nuclei of the hypothalamus are involved in the phase advance of temperature and activity rhythms in food-restricted rats fed during daytime. Brain Res Bull. 1997;43:209–218. doi: 10.1016/s0361-9230(96)00439-x. [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Chen X, Ge YL, Jiang ZY, Liu CQ, Depoortere I, Peeters TL. Effects of ghrelin on hypothalamic glucose responding neurons in rats. Brain Res. 2005;1055:131–136. doi: 10.1016/j.brainres.2005.06.080. [DOI] [PubMed] [Google Scholar]
- Choi S, Wong LS, Yamat C, Dallman MF. Hypothalamic ventromedial nuclei amplify circadian rhythms: do they contain a food-entrained endogenous oscillator? J Neurosci. 1998;18:3843–3852. doi: 10.1523/JNEUROSCI.18-10-03843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Comperatore CA, Stephan FK. Effects of vagotomy on entrainment of activity rhythms to food access. Physiol Behav. 1990;47:671–678. doi: 10.1016/0031-9384(90)90076-g. [DOI] [PubMed] [Google Scholar]
- Conrad LC, Pfaff DW. Efferents from medial basal forebrain and hypothalamus in the rat. II. An autoradiographic study of the anterior hypothalamus. J Comp Neurol. 1976;169:221–261. doi: 10.1002/cne.901690206. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, Nakazato M. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146:3518–3525. doi: 10.1210/en.2004-1240. [DOI] [PubMed] [Google Scholar]
- Davidowa H, Li Y, Plagemann A. Differential response to NPY of PVH and dopamine-responsive VMH neurons in overweight rats. Neuroreport. 2002;13:1523–1527. doi: 10.1097/00001756-200208270-00007. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav. 2003;2:32–39. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Stephan FK. Circadian food anticipation persists in capsaicin deafferented rats. J Biol Rhythms. 1998;13:422–429. doi: 10.1177/074873049801300507. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160:R7–R12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- El Quezzani S, Tramu G, Magoul R. Neuronal activity in the mediobasal hypothalamus of hibernating jerboas (Jaculus orientalis) Neurosci Lett. 1999;260:13–16. doi: 10.1016/s0304-3940(98)00927-6. [DOI] [PubMed] [Google Scholar]
- Escobar C, Martinez-Merlos MT, Angeles-Castellanos M, del Carmen Minana M, Buijs RM. Unpredictable feeding schedules unmask a system for daily resetting of behavioural and metabolic food entrainment. Eur J Neurosci. 2007;26:2804–2814. doi: 10.1111/j.1460-9568.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- Feillet CA, Albrecht U, Challet E. "Feeding time" for the brain: a matter of clocks. J Physiol Paris. 2006;100:252–260. doi: 10.1016/j.jphysparis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato LM, Fluharty SJ, Weinreb EB, LaBelle DR. Food restriction alters neuronal morphology in the hypothalamic ventromedial nucleus of male rats. Endocrinology. 2008;149:93–99. doi: 10.1210/en.2007-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci. 2008;28:5433–5449. doi: 10.1523/JNEUROSCI.0749-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull. 1987;18:731–738. doi: 10.1016/0361-9230(87)90208-5. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304:19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Harrold JA, Widdowson PS, Williams G. Altered energy balance causes selective changes in melanocortin-4(MC4-R), but not melanocortin-3 (MC3-R),receptors in specific hypothalamic regions: further evidence that activation of MC4-R is a physiological inhibitor of feeding. Diabetes. 1999;48:267–271. doi: 10.2337/diabetes.48.2.267. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Hetherington SW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat. Rec. 1940;78:149–172. [Google Scholar]
- Hikiji K, Inoue K, Iwasaki S, Ichihara K, Kiriike N. Local perfusion of mCPP into ventromedial hypothalamic nucleus, but not into lateral hypothalamic area and frontal cortex, inhibits food intake in rats. Psychopharmacology (Berl) 2004;174:190–196. doi: 10.1007/s00213-003-1735-0. [DOI] [PubMed] [Google Scholar]
- Honma KI, Honma S, Hiroshige T. Feeding-associated corticosterone peak in rats under various feeding cycles. Am J Physiol. 1984;246:R721–R726. doi: 10.1152/ajpregu.1984.246.5.R721. [DOI] [PubMed] [Google Scholar]
- Honma S, Honma K, Nagasaka T, Hiroshige T. The ventromedial hypothalamic nucleus is not essential for the prefeeding corticosterone peak in rats under restricted daily feeding. Physiol Behav. 1987;39:211–215. doi: 10.1016/0031-9384(87)90011-4. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Huang XF, Yu Y, Li Y, Tim S, Deng C, Wang Q. Ventromedial hypothalamic NPY Y2 receptor in the maintenance of body weight in diet-induced obesity in mice. Neurochem Res. 2008;33:1881–1888. doi: 10.1007/s11064-008-9661-5. [DOI] [PubMed] [Google Scholar]
- Ibuka N, Nihonmatsu I, Sekiguchi S. Sleep-wakefulness rhythms in mice after suprachiasmatic nucleus lesions. Waking Sleeping. 1980;4:167–173. [PubMed] [Google Scholar]
- Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821:526–529. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- Inouye ST. Ventromedial hypothalamic lesions eliminate anticipatory activities of restricted daily feeding schedules in the rat. Brain Res. 1982;250:183–187. doi: 10.1016/0006-8993(82)90967-2. [DOI] [PubMed] [Google Scholar]
- Jain MR, Horvath TL, Kalra PS, Kalra SP. Evidence that NPY Y1 receptors are involved in stimulation of feeding by orexins (hypocretins) in sated rats. Regul Pept. 2000;87:19–24. doi: 10.1016/s0167-0115(99)00102-0. [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313–321. doi: 10.1016/j.cmet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Jones BE. Paradoxical REM sleep promoting and permitting neuronal networks. Arch Ital Biol. 2004;142:379–396. [PubMed] [Google Scholar]
- Kaur S, Thankachan S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, Shiromani PJ. Entrainment of temperature and activity rhythms to restricted feeding in orexin knock out mice. Brain Res. 2008;1205:47–54. doi: 10.1016/j.brainres.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, Hollenberg AN, Friedman JM, Elmquist JK. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor. J Comp Neurol. 2005;482:217–243. doi: 10.1002/cne.20432. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Ventromedial hypothalamic lesions abolish food-shifted circadian adrenal and temperature rhythmicity. Endocrinology. 1980;106:649–654. doi: 10.1210/endo-106-3-649. [DOI] [PubMed] [Google Scholar]
- Krieger MS, Conrad LC, Pfaff DW. An autoradiographic study of the efferent connections of the ventromedial nucleus of the hypothalamus. J Comp Neurol. 1979;183:785–815. doi: 10.1002/cne.901830408. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms. 2007;22:467–478. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- Lee JG, Choi IS, Park EJ, Cho JH, Lee MG, Choi BJ, Jang IS. beta(2)-Adrenoceptor-mediated facilitation of glutamatergic transmission in rat ventromedial hypothalamic neurons. Neuroscience. 2007;144:1255–1265. doi: 10.1016/j.neuroscience.2006.10.049. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DI, Sinnamon HM. Midbrain areas required for locomotion initiated by electrical stimulation of the lateral hypothalamus in the anesthetized rat. Neuroscience. 1990;39:665–674. doi: 10.1016/0306-4522(90)90251-x. [DOI] [PubMed] [Google Scholar]
- Li AJ, Ritter S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur J Neurosci. 2004;19:2147–2154. doi: 10.1111/j.1460-9568.2004.03287.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Qiao X, Chen JD. Vagal afferent is involved in short-pulse gastric electrical stimulation in rats. Dig Dis Sci. 2004;49:729–737. doi: 10.1023/b:ddas.0000030081.91006.86. [DOI] [PubMed] [Google Scholar]
- Lopez-Valpuesta FJ, Nyce JW, Myers RD. NPY-Y1 receptor antisense injected centrally in rats causes hyperthermia and feeding. Neuroreport. 1996;7:2781–2784. doi: 10.1097/00001756-199611040-00075. [DOI] [PubMed] [Google Scholar]
- Lopez M, Lage R, Saha AK, Perez-Tilve D, Vazquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodriguez-Cuenca S, Deoliveira RM, Castaneda T, Datta R, Dong JZ, Culler M, Sleeman MW, Alvarez CV, Gallego R, Lelliott CJ, Carling D, Tschop MH, Dieguez C, Vidal-Puig A. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37:335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun. 1998;253:241–245. doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- Maes H. Time course of feeding induced by pentobarbital-injections into the rat's VMH. Physiol Behav. 1980;24:1107–1114. doi: 10.1016/0031-9384(80)90055-4. [DOI] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- Martins PJ, D'Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept. 2004;117:155–158. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes. 1999;48:1801–1806. doi: 10.2337/diabetes.48.9.1801. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Differential role of the accumbens Shell and Core subterritories in food-entrained rhythms of rats. Behav Brain Res. 2005;158:133–142. doi: 10.1016/j.bbr.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Meynard MM, Valdes JL, Recabarren M, Seron-Ferre M, Torrealba F. Specific activation of histaminergic neurons during daily feeding anticipatory behavior in rats. Behav Brain Res. 2005;158:311–319. doi: 10.1016/j.bbr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–10501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhouse OE. The organization of the ventromedial hypothalamic nucleus. Brain Res. 1973;55:71–87. [PubMed] [Google Scholar]
- Mistlberger RE, Bergmann BM, Waldenar W, Rechtschaffen A. Recovery sleep following sleep deprivation in intact and suprachiasmatic nuclei-lesioned rats. Sleep. 1983;6:217–233. doi: 10.1093/sleep/6.3.217. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Rechtschaffen A. Periodic water availability is not a potent zeitgeber for entrainment of circadian locomotor rhythms in rats. Physiol Behav. 1985;34:17–22. doi: 10.1016/0031-9384(85)90070-8. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC, Kilduff TS, Jones M. Food- and light-entrained circadian rhythms in rats with hypocretin-2-saporin ablations of the lateral hypothalamus. Brain Res. 2003;980:161–168. doi: 10.1016/s0006-8993(03)02755-0. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Yamazaki S, Pendergast JS, Landry GJ, Takumi T, Nakamura W. Comment on "Differential rescue of light- and food-entrainable circadian rhythms". Science. 2008;322:675. doi: 10.1126/science.1161284. author reply 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Buijs RM, Challet E, Escobar C, Landry GJ, Kalsbeek A, Pevet P, Shibata S. Standards of evidence in chronobiology: critical review of a report that restoration of Bmal1 expression in the dorsomedial hypothalamus is sufficient to restore circadian food anticipatory rhythms in Bmal1−/− mice. J Circadian Rhythms. 2009;7:3. doi: 10.1186/1740-3391-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell V, Bouret S, Beauvillain JC, Schilling A, Perret M, Kordon C, Epelbaum J. Comparative distribution of mRNA encoding the growth hormone secretagogue-receptor (GHS-R) in Microcebus murinus (Primate, lemurian) and rat forebrain and pituitary. J Comp Neurol. 2001;429:469–489. doi: 10.1002/1096-9861(20010115)429:3<469::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Kow LM, Yang XJ. Brain glucose-sensing mechanisms: ubiquitous silencing by aglycemia vs. hypothalamic neuroendocrine responses. Am J Physiol Endocrinol Metab. 2001;281:E649–E654. doi: 10.1152/ajpendo.2001.281.4.E649. [DOI] [PubMed] [Google Scholar]
- Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389:508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Mori S, Nishimura H, Kurakami C, Yamamura T, Aoki M. Controlled locomotion in the mesencephalic cat: distribution of facilitatory and inhibitory regions within pontine tegmentum. J Neurophysiol. 1978;41:1580–1591. doi: 10.1152/jn.1978.41.6.1580. [DOI] [PubMed] [Google Scholar]
- Morris JS, Dolan RJ. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci. 2001;21:5304–5310. doi: 10.1523/JNEUROSCI.21-14-05304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, Shibata S. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci. 2009;29:1447–1460. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neuro. 1949;1:455–473. [PubMed] [Google Scholar]
- Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M, Sakurai T, Shioda S, Yada T. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19:1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–187. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- Obici S. Minireview: Molecular targets for obesity therapy in the brain. Endocrinology. 2009;150:2512–2517. doi: 10.1210/en.2009-0409. [DOI] [PubMed] [Google Scholar]
- Ono T, Nishino H, Fukuda M, Sasaki K, Muramoto K, Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Res. 1982;232:494–499. doi: 10.1016/0006-8993(82)90295-5. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal Activities of the Ventromedial and Lateral Hypothalamic Areas of Cats. Science. 1964;143:484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- Parent A, Steriade M. Afferents from the periaqueductal gray, medial hypothalamus and medial thalamus to the midbrain reticular core. Brain Res Bull. 1981;7:411–418. doi: 10.1016/0361-9230(81)90039-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. Academic Press; 2001. [Google Scholar]
- Pendergast JS, Nakamura W, Friday RC, Hatanaka F, Takumi T, Yamazaki S. Robust food anticipatory activity in BMAL1-deficient mice. PLoS One. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D. Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Harvard University Press; 2005. [Google Scholar]
- Poulin AM, Timofeeva E. The dynamics of neuronal activation during food anticipation and feeding in the brain of food-entrained rats. Brain Res. 2008;1227:128–141. doi: 10.1016/j.brainres.2008.06.039. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003;117:1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Ribeiro AC, Sawa E, Carren-LeSauter I, LeSauter J, Silver R, Pfaff DW. Two forces for arousal: Pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci U S A. 2007;104:20078–20083. doi: 10.1073/pnas.0710096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Sakuma Y. Antidromic identification of hypothalamic neurosecretory cells which participate in the anterior pituitary control (author's transl) Nippon Naibunpi Gakkai Zasshi. 1976;52:1–20. doi: 10.1507/endocrine1927.52.1_1. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92 doi: 10.1016/s0092-8674(02)09256-5. 1 page following 696. [DOI] [PubMed] [Google Scholar]
- Sanz C, Roncero I, Vazquez P, Navas MA, Blazquez E. Effects of glucose and insulin on glucokinase activity in rat hypothalamus. J Endocrinol. 2007;193:259–267. doi: 10.1677/JOE-06-0146. [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J Comp Neurol. 1976;169:409–442. doi: 10.1002/cne.901690403. [DOI] [PubMed] [Google Scholar]
- Sartin JL, Dyer C, Matteri R, Buxton D, Buonomo F, Shores M, Baker J, Osborne JA, Braden T, Steele B. Effect of intracerebroventricular orexin-B on food intake in sheep. J Anim Sci. 2001;79:1573–1577. doi: 10.2527/2001.7961573x. [DOI] [PubMed] [Google Scholar]
- Shen L, Ma LY, Qin XF, Jandacek R, Sakai R, Liu M. Diurnal changes in intestinal apolipoprotein A-IV and its relation to food intake and corticosterone in rats. American journal of physiology. 2005;288:G48–G53. doi: 10.1152/ajpgi.00064.2004. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Ishikawa K. Modulation by the hypothalamus of glucagon and insulin secretion in rabbits: studies with electrical and chemical stimulations. Endocrinology. 1981;108:605–611. doi: 10.1210/endo-108-2-605. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Oomura Y, Plata-Salaman CR, Morimoto M. Hyperphagia and obesity in rats with bilateral ibotenic acid-induced lesions of the ventromedial hypothalamic nucleus. Brain Res. 1987;416:153–156. doi: 10.1016/0006-8993(87)91508-3. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM. Glutamate and picrotoxin injections into the preoptic basal forebrain initiate locomotion in the anesthetized rat. Brain Res. 1987;400:270–277. doi: 10.1016/0006-8993(87)90626-3. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM. Microstimulation mapping of the basal forebrain in the anesthetized rat: the "preoptic locomotor region". Neuroscience. 1992;50:197–207. doi: 10.1016/0306-4522(92)90392-f. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Ginzburg RN, Kurose GA. Midbrain stimulation in the anesthetized rat: direct locomotor effects and modulation of locomotion produced by hypothalamic stimulation. Neuroscience. 1987;20:695–707. doi: 10.1016/0306-4522(87)90120-5. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Marciello M, Goerner DW. Locomotor sites mapped with low current stimulation in intact and kainic acid damaged hypothalamus of anesthetized rats. Behav Brain Res. 1991;46:49–61. doi: 10.1016/s0166-4328(05)80096-8. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Stopford CK. Locomotion elicited by lateral hypothalamic stimulation in the anesthetized rat does not require the dorsal midbrain. Brain Res. 1987;402:78–86. doi: 10.1016/0006-8993(87)91049-3. [DOI] [PubMed] [Google Scholar]
- Song Z, Routh VH. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2005;54:15–22. doi: 10.2337/diabetes.54.1.15. [DOI] [PubMed] [Google Scholar]
- Stenger J, Fournier T, Bielajew C. The effects of chronic ventromedial hypothalamic stimulation on weight gain in rats. Physiol Behav. 1991;50:1209–1213. doi: 10.1016/0031-9384(91)90584-b. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Tang M, Zhang J, Chen JD. Excitatory effects of gastric electrical stimulation on gastric distension responsive neurons in ventromedial hypothalamus (VMH) in rats. Neurosci Res. 2006;55:451–457. doi: 10.1016/j.neures.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Sutin J, Eager R. II. Anatomical substrates and electrophysiological properties of neural systems regulating food and water intake. Fiber degeneration following lesions in the hypothalamic ventromedial nucleus. Ann N Y Acad Sci. 1969;157:610–628. doi: 10.1111/j.1749-6632.1969.tb12910.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Mogenson GJ, Simerly RB, Wu M. Anatomical and electrophysiological evidence for a projection from the medial preoptic area to the 'mesencephalic and subthalamic locomotor regions' in the rat. Brain Res. 1987;405:108–122. doi: 10.1016/0006-8993(87)90995-4. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler I, Borbely AA, Groos G. The effect of sleep deprivation on sleep in rats with suprachiasmatic lesions. Neurosci Lett. 1983;42:49–54. doi: 10.1016/0304-3940(83)90420-2. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Wang G, Lee HM, Englander E, Greeley GH., Jr Ghrelin--not just another stomach hormone. Regul Pept. 2002;105:75–81. doi: 10.1016/s0167-0115(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson CW, Shinsako J, Dallman MF. Daily rhythms in adrenal responsiveness to adrenocorticotropin are determined primarily by the time of feeding in the rat. Endocrinology. 1979;104:350–359. doi: 10.1210/endo-104-2-350. [DOI] [PubMed] [Google Scholar]
- Wisialowski T, Parker R, Preston E, Sainsbury A, Kraegen E, Herzog H, Cooney G. Adrenalectomy reduces neuropeptide Y-induced insulin release and NPY receptor expression in the rat ventromedial hypothalamus. J Clin Invest. 2000;105:1253–1259. doi: 10.1172/JCI8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yanagida H, Morita T, Kim J, Yoshida K, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Effects of ghrelin on neuronal activity in the ventromedial nucleus of the hypothalamus in infantile rats: an in vitro study. Peptides. 2008;29:912–918. doi: 10.1016/j.peptides.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Yokote R, Sato M, Matsubara S, Ohye H, Niimi M, Murao K, Takahara J. Molecular cloning and gene expression of growth hormone-releasing peptide receptor in rat tissues. Peptides. 1998;19:15–20. doi: 10.1016/s0196-9781(97)00263-5. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Blache D, Vercoe PE, Adam CL, Blackberry MA, Findlay PA, Eidne KA, Martin GB. Expression of orexin receptors in the brain and peripheral tissues of the male sheep. Regul Pept. 2005;124:81–87. doi: 10.1016/j.regpep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lee AW, Devidze N, Zhang Q, Kow LM, Pfaff DW. Histamine-induced excitatory responses in mouse ventromedial hypothalamic neurons: ionic mechanisms and estrogenic regulation. J Neurophysiol. 2007;98:3143–3152. doi: 10.1152/jn.00337.2007. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]