Abstract
Poor recovery of cryopreserved human embryonic stem (hES) cells and induced pluripotent stem (iPS) cells is a significant impediment to progress with pluripotent stem cells. In this study, we demonstrate that Y-27632, a specific inhibitor of Rho kinase (ROCK) activity, significantly enhances recovery of hES cells from cryopreserved stocks when cultured with or without a growth inactivated feeder layer. Furthermore, treatment with the ROCK inhibitor for several days increased the number of colonies and colony size of hES cells compared to shorter exposures. Remarkably, hES cells that had formed relatively few colonies five days after thawing exhibited rapid growth upon addition of Y-27632. Additionally, we determined that Y-27632 significantly improves the recovery of cryopreserved human iPS cells and their growth upon subculture. Thus, Y-27632 provides a means to “kick-start” slow-growing human pluripotent stem cells, especially after being thawed from frozen stocks. Together, these results argue that Y-27632 is a useful tool in overcoming obstacles to studies involving the cultivation of both hES cells and human iPS cells.
Keywords: H9 hES cells, iPS cells, NT2/D1 cells, Y-27632, Fasudil, mTeSR1 serum-free medium
INTRODUCTION
Human pluripotent stem cells have the potential to be an important source of virtually any cell type for basic research, drug development, and clinical cell therapies. Several technical issues currently constrain the utility of human embryonic stem (hES) cells for research and clinical applications. In this regard, the ability to recover hES cells from frozen stocks can be difficult, slow, and inefficient even in experienced hands. Although several studies have described improvements in the process of cryopreserving hES cells (Fujioka et al., 2004; Ware et al., 2005; Yang et al., 2006), it is widely recognized that only a very small fraction of the frozen cell population is recovered. Moreover, re-establishment of cultures from frozen stocks using commonly used methods is often slow enough that the growth-inactivated feeder layer begins to deteriorate. In contrast to single cell methods, methods of freezing hES cells in clusters can be effective at generating high survival rates after cryopreservation, but can be labor intensive (Zhou et al., 2004; Ji et al., 2004; Suemori et al., 2006; Katkov et al., 2006). Consequently, improvements in the culture of hES cells would enhance the study and, possibly, the therapeutic use of these cells, especially when an inefficient cryopreservation method is employed. Recently, Watanabe et al.(Watanabe et al., 2007) reported that inhibition of Rho-associated coiled coil kinase (ROCK) activity promotes the survival of dissociated hES cells, and greatly improves the clonal growth of hES cells without affecting their ability to form teratomas consisting of cells derived from each of the three embryonic germ layers. This suggested that use of a ROCK inhibitor may significantly minimize the difficulties in recovering cryopreserved pluripotent human stem cells.
ROCK activity is involved in a variety of cellular functions. It plays a central role in regulating the phosphorylation of myosin light chain and a variety of other kinases and cytoskeletal binding proteins (Riento and Ridley, 2003). Its role in cytoskeletal contraction and rearrangement makes it essential to many fundamental cellular processes (reviewed in (Riento and Ridley, 2003; Burridge and Wennerberg, 2004) including apoptosis, migration, cytokinesis, proliferation (Pirone et al., 2006), and differentiation. Much of what is known about ROCK activity has been discovered using ROCK inhibitors such as Y-27632. However, many questions about ROCK inhibition in embryonic stem (ES) cells remain. In this study we address three questions. Can ROCK inhibition enhance the recovery of hES cells, human induced pluripotent stem (iPS) cells, and human embryonal carcinoma (EC) cells from frozen stocks and augment their growth after subculture? What is the optimal dose and timing for the addition of ROCK inhibitors to maximize their effect? Can ROCK inhibition improve the recovery of cryopreserved hES cells when cultured directly in a serum-free medium in the absence of a growth inactivated feeder layer?
RESULTS
ROCK inhibitors significantly improve recovery of hES cells from cryopreserved stocks
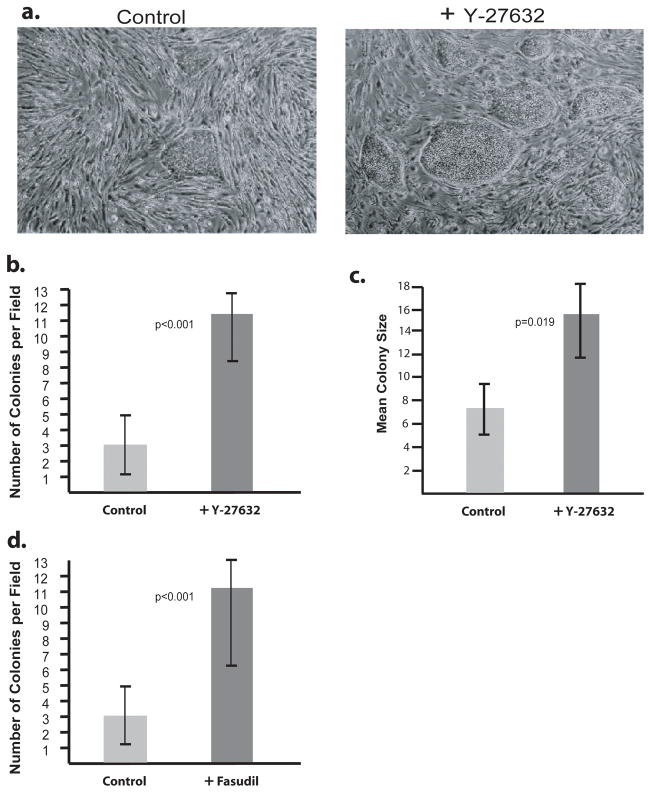
Previous work has shown that the addition of the ROCK inhibitor Y-27632 can improve the cloning efficiency and survival upon dissociation of KhES-1 hES cells without altering their karyotype or pluripotency (Watanabe et al., 2007). To address whether ROCK inhibition can similarly aid in overcoming the difficulties in recovering hES cells from frozen stocks, H9 hES cells were thawed and grown on Matrigel coated tissue culture plastic with a mouse embryonic fibroblast (MEF) feeder layer in medium with or without 10 μM Y-27632. The cells were exposed to ROCK inhibition continuously for four days before being photographed (Figure 1A). Cells treated with the ROCK inhibitor exhibited nearly a four-fold increase in the number of colonies (p< 0.001) with normal hES cell morphology (Figure 1A and B). Additionally, each ROCK inhibitor-treated colony was, on average, twice the size of the untreated counterparts (p = 0.019) (Figure 1A and C). Together, the combined increase in the number and size of colonies represents approximately an eight-fold enhancement in the number of cells recovered from frozen stocks of hES cells. These effects were not specific to Y-27632, as another ROCK specific inhibitor, Fasudil, also improves the recovery of frozen hES cells with nearly identical increases in colony size and number (Figure 1D). Thus, the use of specific ROCK inhibition not only offers a significant improvement in the regrowth of cryopreserved hES cells, but also substantially increases the likelihood that the freshly thawed hES cells will expand sufficiently prior to detachment of the feeder layer.
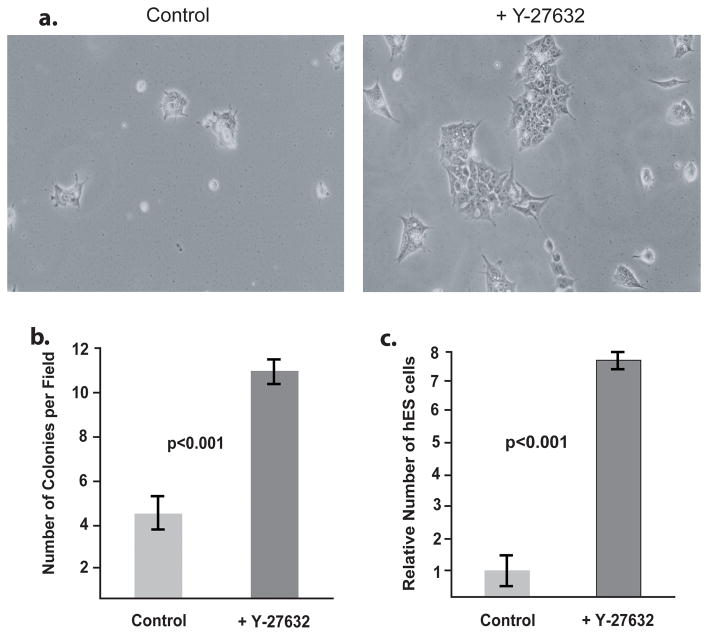
Figure 1. ROCK Inhibitor increases H9 hES cell growth upon thawing.
H9 hES cells were thawed onto a MEF feeder layer and grown 4 days in the presence or absence of Y-27632. A) Photomicrographs of H9 hES colonies on a background of MEFs. Left: Untreated. Right: Treated with 4 days of Y-27632. B) The number of H9 colonies per 40x microscope field was counted in 10 random fields and compared. C) The size of each colony in 10 random 40x fields was measured and the mean size calculated. D) H9 cells were grown with or without Fasudil for 4 days and colony numbers were determined. Error bars: ± Standard Deviation.
The effects of ROCK inhibition are reversible and enhance growth after subculture
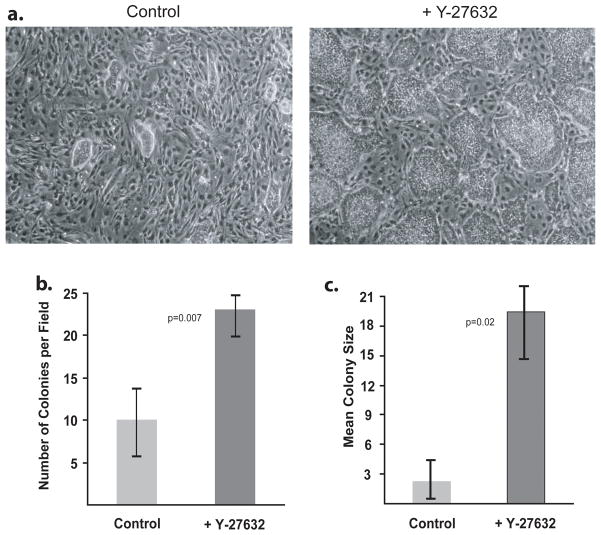
To determine the utility of ROCK inhibitors for improving hES cell yield after subculturing, H9 hES cells, which had previously been exposed to Y-27632 treatment for five days, were subcultured as single cells using Accutase (Bajpai et al., 2008) into medium containing or lacking Y-27632. This enabled us to address whether continuous ROCK inhibition creates long-term alterations in the growth characteristics of hES cells. After four days of continuous exposure to the appropriate treatment medium, the cells were photomicrographed (Figure 2A). H9 hES cells with prior exposure to Y-27632 reverted to the lower colony forming capacity of untreated cells when Y-27632 was withdrawn. Additionally, cells with prior continuous ROCK inhibitor exposure retained the ability to form nearly three times as many colonies when subcultured into medium containing Y-27632 (p = 0.007), and those colonies were on average more than six times as large as untreated cells (p = 0.02) (Figure 2B and C). These results were also recapitulated at different concentrations of Y-27632 (Figure 3A and B). These data demonstrate that ROCK inhibition can dramatically improve hES cell growth and cell yield upon subculture.
Figure 2. Effects of ROCK inhibitor on H9 hES cells upon subculture.
H9 hES cells with prior exposure to Y-27632 were subcultured and grown for 4 days with or without ROCK inhibitor. A) Subcultured H9 hES cells. Left: Untreated. Right: Treated with Y-27632. B) The number of H9 colonies per 40x microscope field was counted in 10 random fields. C) The surface area of each colony in 10 random 40x fields was measured and the mean size calculated. Error bars: ± Standard Deviation.
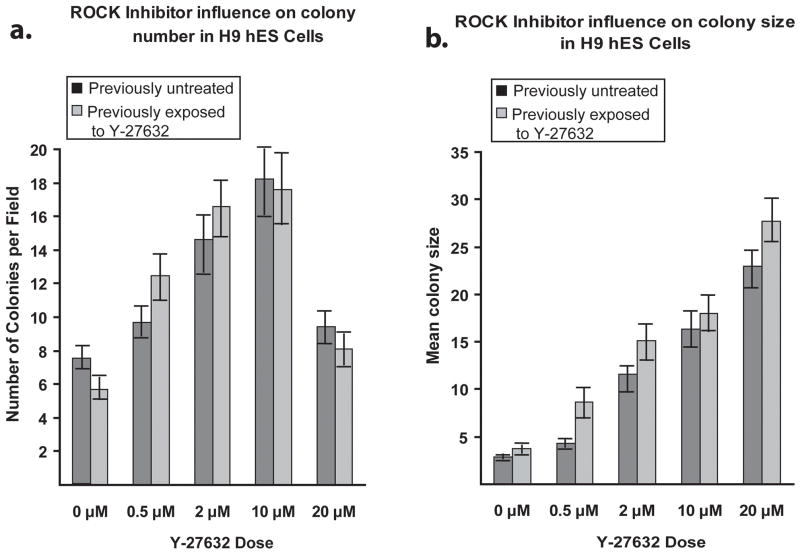
Figure 3. Dose response curve of the ROCK inhibitor Y-27632.
Cells were grown for 4 days in complete knockout media, with or without ROCK inhibitor, and subcultured into 6-well dishes for a dose curve of 0, 0.5, 2.0, 10.0, or 20 μM ROCK inhibitor for four days. 10 random 40x microscope fields were photographed. A) Comparisons of the mean number of colonies per field. B) Comparisons of the mean colony size. Error bars: ± Standard Deviation.
Adding ROCK inhibitor five days after thawing stimulates colony growth
Previous studies (Coleman et al., 2001; Croft et al., 2005; Koyanagi et al., 2008; Coleman and Olson, 2002; Olson, 2008; Minambres et al., 2006; Frisch and Screaton, 2001) suggested that the mechanism by which Y-27632 affects hES cells is to block apoptosis of suspended cells or cells at low density. This leads to the prediction that for ROCK inhibition to be effective, it should be administered early to prevent apoptosis of the density or suspension-stressed cells. To test this prediction, cryopreserved hES cells were thawed into medium lacking Y-27632. After five days, few visible colonies had formed. At that time, Y-27632 was added to the medium in half of the culture dishes for 24 hrs, leading to the rapid appearance of significantly more colonies in the treated population (p = 0.04) (Figure 4A). Thus, improvements of freshly thawed hES cells can be obtained even when Y-27632 is added days after the cryopreserved cells are recovered from frozen stocks.
Figure 4. Effects of adding Y-27632 after hES colonies are established.
A) H9 hES cells were thawed onto MEFs in medium lacking ROCK inhibitor and allowed to grow for 5 days before adding 10 μM Y-27632 to half the flasks. The number of hES colonies in 10 random 40X microscope fields was counted and compared. B) H9 hES cells were subcultured into T25 flasks and allowed to grow for 5 days before adding 10 μM Y-27632 for an additional 3 days. Photomicrographs were taken of 10 random fields from each flask. Error bars: ± Standard Deviation.
Although we determined that Y-27632 can strongly stimulate the growth of colonies five days after thawing hES cells (when the colonies were less than 10–20 cells in size), adding Y-27632 five days after passage had no discernible effect on the number of colonies (Figure 4B) or colony size (data not shown) when added to colonies that had undergone two passages after thawing and had a size of ~30 or more cells. This suggests that recently thawed cells which have attached to a matrix or feeder layer but which have not formed sizable colonies are undergoing stress alleviated by Y-27632, while cells in well-established colonies garner little benefit from the inhibition of ROCK signaling.
Y-27632 exerts significant effects when used for periods greater than 12 hrs
The surprising impact of Y-27632 on hES cells after five days in culture led us to address the optimal timing and concentration of ROCK inhibitor to administer to maximize the benefits for hES cell culture. H9 hES cells were subcultured into medium containing increasing concentrations of Y-27632. The number of hES cell colonies and the surface area of each colony were measured after four days of growth. The optimal dose for maximizing the number of hES colonies was 10 μM (Figure 3A). This is in agreement with previous studies showing that 10 μM Y-27632 was sufficient to inhibit 80–100% of ROCK’s kinase activity and cytoskeletal phenotypes (Narumiya et al., 2000). While treatment with 20 μM Y-27632 increased the average colony size in area (Figure 3B), the number of distinct hES cell colonies decreased as adjacent colonies coalesced (Figure 3A). Consequently, when both colony size and number of colonies were considered, there was no obvious benefit to increasing the concentration of ROCK inhibitor beyond 10 μM.
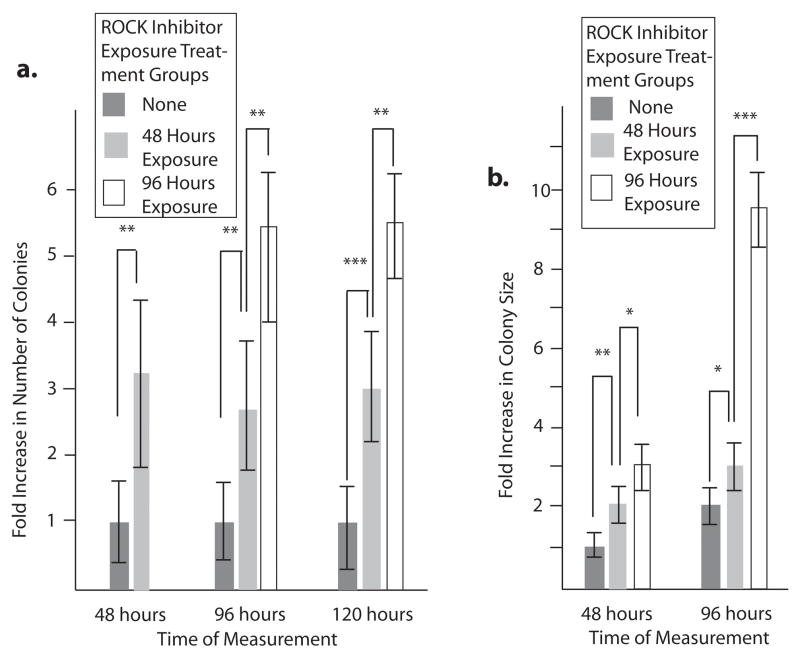
Watanabe et al. found that the use of Y-27632 beyond twelve hrs after passage increased hES cell colony size by a small but significant amount, but did not affect the number of colonies (Watanabe et al., 2007). To determine whether the longer exposure times we used were beneficial or unnecessary, we measured the colony formation and colony size effects of Y-27632 in the medium for different lengths of time from the point of passage. One group of cells was never exposed to Y-27632, another was exposed to the inhibitor for 48 hours after passage, and a third group for 96 hours. Colony size and the number of colonies were measured for each group at 48, 96, and 120 hours after passage. As before, the initial exposure to Y-27632 upon passage resulted in a significant increase in both the number of hES cell colonies that form and mean colony size. However, the presence of ROCK inhibitor in the medium for an additional 48 hrs doubled again the number of colonies and tripled the size of those colonies (Figure 5A and B). Together, these results argue that to achieve the maximum hES cell number benefit from Y-27632, treatments at 10 μM for longer duration could be used.
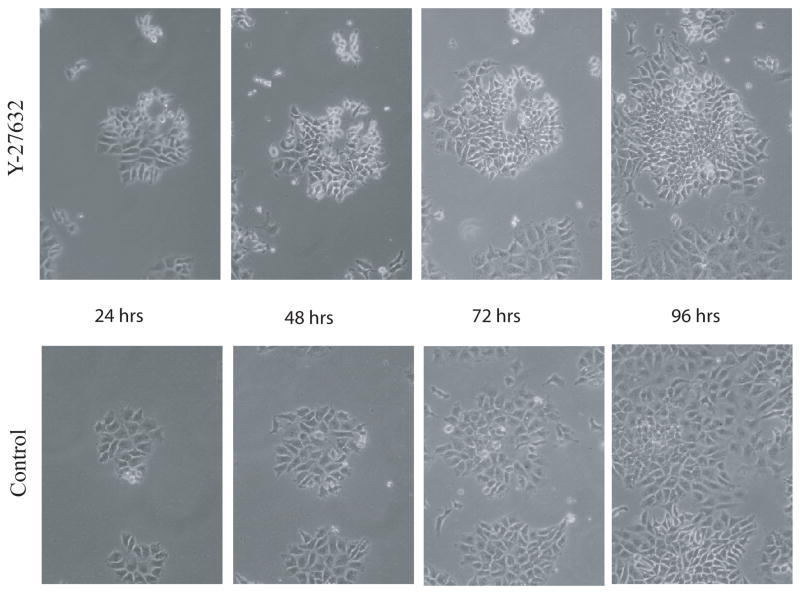
Figure 5. Duration of exposure to Y-27632 impacts hES cell colony formation and growth.
H9 hES cells were subcultured and treated with Y-27632 for varying lengths of time. 10 random 40x microscope fields for each treatment were photographed at different time points. A) The number of colonies per microscope field was counted at 48, 96, and 120 hrs and compared to the number of untreated colonies, which was set to 1 at the 48 hr time point. B) The mean size of colonies was measured at 48 and 96 hrs and compared to the size of untreated cells at the 48 hr time point, which was set to 1. Error bars: ± Standard Deviation. * = no significant difference. ** = p < 0.05. *** = p < 0.001.
Effect of ROCK inhibition on hES cells in the absence of a feeder layer
In most published reports, hES cells were cultured in the presence of a growth inactivated feeder layer. However, several studies have described the growth of hES cells without a feeder layer (Xu et al., 2001; Ludwig et al., 2006a; Ludwig et al., 2006b). Therefore we sought to determine whether Y-27632 could improve the recovery of cryopreserved cells when plated without a feeder layer even when the cells had been grown on a growth-inactivated feeder layer prior to cryopreservation. For this purpose, we compared the recovery of cryopreserved BG01V/hOG hES cells cultured in mTeSR1 medium with and without Y-27632. mTeSR1 is a serum-free medium that is used in conjunction with Matrigel coated tissue culture plastic (Ludwig et al., 2006a). Although cryopreserved BG01V/hOG hES cells were recovered when thawed and cultured in mTeSR1 medium, the attachment and morphology of the cells after 48 hrs were significantly improved when the mTeSR1 medium is supplemented with Y-27632 at 10 μM (Figure 6A). In addition, when colony number and cell numbers were determined 72 hrs after the cells were thawed, we observed more than a 2-fold increase in colony number (Figure 6B) and more than a 7-fold increase in cell number (Figure 6C) when Y-27632 was added to the medium. However, we observed detached, floating, dead cells after thawing and after subculture using mTeSR1 medium both in the presence and the absence of the ROCK inhibitor. Overall, our findings argue that the recovery of hES cells is significantly improved even when cultured in hES cell growth medium that does not require a feeder layer.
Figure 6. Effect of Y-27632 on the recovery of hES cells in medium lacking a feeder layer.
Cryopreserved BG01V/hOG hES cells were thawed into mTeSR1 medium with and without 10 μM Y-27632. A) The cells with (right panel) and without (left panel) Y-27632 were photographed 72 hrs after being thawed. B) The number of colonies per microscopic field was determined at 72 hrs. Error bars: ± Standard Deviation. C) The number of cells was counted at 72 hrs and the control was set to 1.
ROCK inhibition has only modest effects on human EC cells
Human EC cells are frequently used as model systems for studying pluripotency, differentiation, and development. To determine whether these models respond to Y-27632 in a manner similar to hES cells, NT2/D1 human EC cells were thawed into medium containing or lacking Y-27632. When the human EC line NT2/D1 was thawed with Y-27632 treatment, there was no significant improvement in the number of colonies formed compared to untreated cells (p > 0.05). However, after three days of culture with the ROCK inhibitor, there were 35% (± 7%) more cells in the treated plates than in those without Y-27632 (p = 0.004), suggesting a slight improvement in human EC cell growth due to inhibition of ROCK signaling. Additionally, representative colonies were tracked for changes in morphology and cell number over three days. Colonies treated with Y-27632 were not significantly improved compared to untreated controls (Figure 7).
Figure 7. Response of human EC cells to Y-27632.
Representative colonies from Y-27632 treated (top) and untreated NT2/D1 EC cells were followed for 4 days after subculture.
Y-27632 significantly enhances the recovery and passage of human induced pluripotent stem (iPS) cells
The ability to reprogram somatic cells into a pluripotent stem cell state provides a new and valuable source of stem cells for scientific examination of both development and diseases. Because iPS cells closely resemble hES cells, and their culture and cryopreservation suffer from many of the same difficulties as hES cells, we hypothesized that use of a ROCK inhibitor would also improve the recovery from frozen stocks and passage of human iPS cells. To test this possibility, human iPS cells were thawed and grown on tissue culture plastic coated with Matrigel and a growth-inactivated MEF feeder layer in medium with or without 10 μM Y-27632. After 48 hours, the number of visible colonies was determined. Cells treated with the ROCK inhibitor exhibited colonies that were larger in size (Figure 8A) and were more than seven times more numerous (p<0.001) (Figure 8B). Human iPS cells also benefited from Y-27632 treatment upon passage. iPS cells which had been grown in the absence of the ROCK inhibitor were subcultured and passed on tissue culture plastic coated with Matrigel and a growth-inactivated MEF feeder layer in medium containing or lacking Y-27632. The number of colonies was counted after 48 hours. More than three times as many colonies had formed in the flasks treated with ROCK inhibitor (p=0.003) (Figure 8C). Thus, like hES cells, human iPS cells form significantly more colonies upon recovery from cryopreservation and dissociation for subculture when treated with Y-27632.
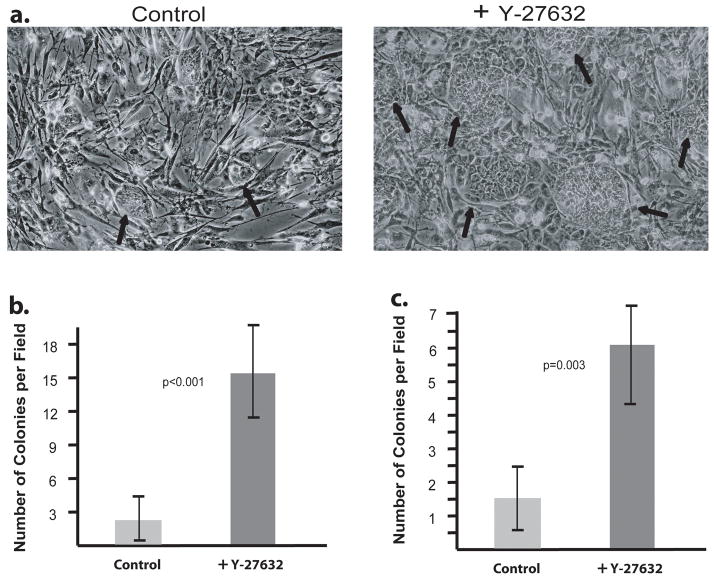
Figure 8. Effects of Y-27632 on iPS cell thawing and passaging.
Human iPS cells were thawed onto a MEF feeder layer and grown for 48 hours in the presence or absence of Y-27632. A) Photomicrographs of human iPS cells on a background of MEFs. Left: Untreated. Right: Treated with Y-27632 for 48 hours. Black arrows mark iPS cell colonies. B) The number of human iPS cell colonies in ten random 100X microscope fields was counted and compared. C) Human iPS cells were subcultured onto MEFs and grown for 48 hours with or without Y-27632. The number of human iPS cell colonies in ten random 40X microscope fields was counted and compared. Error bars: ± Standard Deviation.
DISCUSSION
In this study, we demonstrate that the ROCK inhibitor Y-27632 dramatically improves the recovery and growth of cryopreserved hES cells under a wide range of culture conditions. While our studies were being completed, another group reported that Y-27632 improves the recovery of hES cells from frozen stocks (Martin-Ibanez et al., 2008). Specifically, they determined that the use of Y-27632 during the freezing step and during the recovery step improved the cloning efficiency of frozen stocks of hES cells. They also demonstrated growth in the presence of Y-27632 had no adverse effects on the karyotype of the cell, the expression of genes required for the self-renewal of hES cells, or the ability of hES cells to differentiate into cell types derived from each of the three embryonic germ layers. The results in our study confirm the utility of Y-27632 using two additional hES cell lines, including one of the most commonly used NIH-approved hES cell lines, H9. Importantly, our work extends our understanding of Y-27632 in five important ways. First, we have determined that Y-27632 improves the recovery of cryopreserved hES cells under culture conditions where a growth inactivated feeder layer is not required, as well as a culture condition where a feeder layer is needed. Second, we determined that Y-27632 does not need to be added at the time when the cells are first replated in culture to enhance growth. Third, we determined that after individual colonies of hES cells have become well established, Y-27632 has relatively little effect on their continued growth. Fourth, Y-27632 provides only a modest improvement in the recovery of cryopreserved human EC cells. Finally, Y-27632 significantly improves the recovery of cryopreserved human iPS cells. Each of these points is discussed below.
Until recently, stocks of hES cells have been maintained primarily in the presence of a growth inactivated fibroblast feeder layer. Our work, and the recent report by others, demonstrates that the use of Y-27632 under these culture conditions dramatically improves the recovery of frozen stocks of hES cells. Importantly, in this study, we have extended these findings to culture conditions where a growth inactivated feeder layer is not needed. Several years ago, a serum-free medium, mTeSR1, was developed for culturing human ES cells without a feeder layer (Ludwig et al., 2006a; Ludwig et al., 2006b). The use of this medium offers several important advantages. Besides avoiding the significant costs in time and money involved in working with a growth inactivated feeder layer, the elimination of the feeder layer greatly simplifies the biochemical analysis of hES cells, since there is no protein or RNA contribution from the cells that make up the feeder layer. Additionally, feeder-free and serum-free media culture conditions reduce key infectious disease obstacles to the clinical application of pluripotent stem cell technologies. As shown by Ludwig and colleagues, we determined that hES cells can be recovered when replated in mTeSR1 medium without a feeder layer. However, our results demonstrate that the addition of Y-27632 considerably improves the recovery of cryopreserved hES cells when replated in mTeSR1 medium. Thus, ROCK inhibitors enhance the ability of hES cells passaged as single cells to grow without feeder support, rendering them more appropriate for futher analysis and clinical application. In our studies, we thawed hES cells into mTeSR1 medium at several densities. At the lowest density where individual colonies could form (approximately 500,000 cells in a T25 flask) (Figure 6), there was a greater than 2-fold increase in colony formation, but an even larger increase (>7-fold) in cell number. Even when 5 times as many cells were thawed on a plate, the addition of Y-27632 increased cell number over a 3 day period more than 5-fold (data not shown) suggesting that ROCK inhibition is beneficial at a variety of cell densities. Thus, our results argue that the use of Y-27632 offers significant advantages for the recovery and growth of hES cells under higher density, lower density, feeder-aided, or feeder-free culture conditions. It remains to be tested whether ROCK inhibition will similarly improve the growth and survival of hES cells passaged and frozen in clusters.
Our work with Y-27632 provides several important insights into its mode of action. The first report where Y-27632 was used to improve the cloning efficiency of hES cells suggested that it may act by preventing apoptosis of detached cells as in a previous study (Watanabe et al., 2007). However, our finding that the addition of Y-27632 enhances colony formation, even five days after cryopreserved hES cells were thawed, argues that ROCK inhibition is likely to act by an additional mechanism(s) to enhance hES cell colony growth upon recovery from frozen stocks. In this connection, our findings argue that the major impact of Y-27632 on the growth of hES cells occurs when the cells are at low density. In contrast to the findings of Watanabe et al. (2007), we did detect a statistically significant increase in cell growth with continued ROCK inhibitor treatment. However, this effect was relatively small (~2-fold) compared to the early effects of Y-27632; hence, we conclude that once a colony of hES cells reaches a critical size, Y-27632 has only small effects on the growth of the cells. Our work with Y-27632 makes two other points. First, the effects of Y-27632 on H9 hES cell growth are reversible when ROCK inhibitor is removed from the culture environment. This argues that Y-27632 is not simply selecting for a subset of hES cells, and is not inducing long term changes in the colony-forming capacity of hES cells. Second, cells treated with prior continuous exposure to Y-27632 do not become desensitized to the colony forming effects of the drug when subcultured. Given the likely action of Y-27632 on ROCK kinase, it is probable that the phosphorylation effects of Y-27632 relieve some of the stresses associated with manipulation of hES cells. Thus, it would be fruitful, in future studies, to examine the hES cell pathways impacted by ROCK inhibition.
Our studies also indicate that in contrast to hES cells, Y-27632 has only a modest positive effect on the recovery of cryopreserved NT2/D1 human EC cells. Currently, it is unclear why Y-27632 has such a limited effect on NT2/D1 cells. Several factors may be responsible. NT2/D1 cells are the most commonly studied human EC cell line and, as such, they are well adapted to growth in tissue culture. Moreover, the growth requirements for hES cells and human EC cells are clearly different. Human EC cells can be cultured in standard cell culture medium supplemented with fetal bovine serum, which will not sustain the self-renewal of human ES cells.
In conclusion, it is evident that the use of Y-27632 can significantly improve the recovery of hES cells from cryopreserved stocks when widely different culture conditions are employed. Moreover, although the studies described in this study focused on hES cells, ROCK inhibitors are likely to be useful when working with other human pluripotent stem cells. In the past few years, several groups have reported the creation of iPS cells from human fibroblasts, which appear by many criteria to be remarkably similar to hES cells (Takahashi et al., 2007; Yu et al., 2007; Park et al., 2008; Lowry et al., 2008; Liao et al., 2008; Nakagawa et al., 2008). Thus, it is not surprising that ROCK inhibitors also improve the recovery of cryopreserved human iPS cells (Figure 8). Thus, as efforts are made to reprogram human somatic cells without viral vectors, the addition of ROCK inhibitors, at the right time and at the correct level, during reprogramming and subsequent culture and cryopreservation may prove to be very beneficial.
MATERIALS AND METHODS
Cell Culture conditions
MEFs were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT). When used as a feeder layer, MEFs were plated on growth factor reduced Matrigel-coated (BD Biosciences, San Diego, CA) plates in DMEM supplemented with 10% FBS and 0.1 mM β-mercaptoethanol. Prior to use as a feeder layer, they were growth inactivated by exposure to a total of 3000 rads. Where indicated, H9 hES cells (WiCell Research Institute, Madison, WI), and BG01V/hOG hES cells (Invitrogen, Carlsbad, CA #R7799-105) were cultured on growth arrested MEFs at a density of approximately 500,000 hES cells per well in six-well plates, or 1.2 × 106 hES cells per 25 cm2 culture flask. hES and human iPS cells on feeder layers were cultured in complete Knockout (KO) medium. KO medium consisted of KO DMEM (Invitrogen), 20% Knockout serum replacement (Invitrogen), 1% non-essential amino acids (100X, Invitrogen), 1% 200 mM L-glutamine (200mM, Invitrogen), 0.2% β-mercaptoethanol (55mM, Invitrogen), 1% antibiotic/antimycotic (100X, final working concentration of 8 ng/mL), and 8 ng/mL basic FGF (Sigma-Aldrich, St. Louis, MO). To test the effect of ROCK inhibition when hES cells are grown without a feeder layer, the cells were thawed directly into mTeSR1 medium obtained from StemCell Technologies (Vancouver, BC, Canada). Unless indicated otherwise, Y-27632 (Calbiochem, San Diego, CA, #688000) or Fasudil (Calbiochem, # HA1077) was added at a final concentration of 10 μM. Fresh ROCK inhibitor (Y-27632 or Fasudil) was reapplied to the media every day when the media were changed. Y-27632 is light sensitive; hence it was handled in subdued “yellow” lighting. Human iPS cells, which were obtained from Stephen Duncan (Medical College of Wisconsin, Milwaukee, WI) were derived from human foreskin fibroblasts using the Thomson protocol (Yu et al., 2007). Human iPS cells were cultured on tissue culture plastic coated with Matrigel and a growth inactivated feeder layer in complete KO medium. NT2/D1 human EC cells (Andrews, 1984) were cultured in DMEM plus 10% FBS and passaged with 0.1% trypsin (Sigma-Aldrich). All cells were incubated in a moist atmosphere of 95% air and 5% CO2.
Single cell passaging of hES cells and iPS cells with Accutase
Human ES cells were washed twice with PBS and treated with Accutase™ (Chemicon, Temecula, CA) as previously described (Bajpai et al., 2008) for 10–15 minutes in a 37°C incubator. Detached MEFs were removed by rinsing with PBS. Accutase/PBS was aspirated and 2 mL complete KO media was added to the hES cell colonies while still loosely attached. The cells were pipetted 5–8 times, dissociating them into single cells.
Cryopreservation of hES and human iPS cells by slow-cooling
Human iPS and hES cells were detached using Accutase as described above. 500 μL of detached cells and medium were mixed with 500 μL of freezing medium (growth medium with 20% DMSO added) in cryogenic vials. This mixture was then slow-cooled in a Taylor-Wharton 35HC liquid nitrogen tank using a TW-51F Handi-Freeze freezing tray. The cells in the freezing tray were placed in the neck of the liquid nitrogen tank (position six) for 30 minutes cooling to approximately −18°C; next they were placed at a lower position in the liquid nitrogen tank (position three) for 30 minutes cooling to approximately −46°C; finally, the vials were lowered to position one for 60 minutes cooling to approximately −129°C before placing the tubes in the cryogenic liquid for storage.
Recovery of cryopreserved hES and human iPS cells
Cryopreserved stocks of hES cells were recovered by partially submerging the vial of frozen cells in water at 40°C. The vial of cells was gently swirled until fully melted. Next, the contents of the vial were transferred to a 15 ml conical tube with 10 ml complete KO media and the colonies were allowed to settle by gravity to the bottom. As much as possible of this wash media was removed, and the cells were transferred onto MEFs and cultured. Human EC cells were thawed as described above and plated in DMEM plus 10% FBS.
Colony counting and photomicroscopy
Ten random fields per flask were placed on the microscope platform by one person for counting by the microscopist who could not change the selected field. Similarly, ten random fields were placed on the platform for photography, and once placed, the random field could not be changed except to focus the camera.
Colony size measurement
Photomicrographs of 40x microscope fields were printed in 10.2 by 12.7 cm format and the surface area of each colony was measured by mass.
Counting individual cells
Where individual cell numbers were counted, a Z2 Coulter Counter and Size Analyzer was used to count and calculate the number of cells per mL with size range between 8 and 24 microns.
Statistical analysis
Statistical comparisons between sets to calculate p-values were performed using the Student’s T-test function in Microsoft Excel.
Acknowledgments
The authors would like to thank Stephen Duncan for the gift of human iPS cells. We also thank Alexey Terskikh for detailed protocols on Accutase hES cell dissociation. This work was supported the Nebraska Research Initiative and the National Institutes of Health (GM-080751).
LITERATURE CITED
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells Accutase-dissociated human embryonic stem cells. Mol Reprod Dev. 2008;75:818–827. doi: 10.1002/mrd.20809. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002;9:493–504. doi: 10.1038/sj.cdd.4400987. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL, Olson MF. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol. 2005;168:245–255. doi: 10.1083/jcb.200409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Yasuchika K, Nakamura Y, Nakatsuji N, Suemori H. A simple and efficient cryopreservation method for primate embryonic stem cells. Int J Dev Biol. 2004;48:1149–1154. doi: 10.1387/ijdb.041852tf. [DOI] [PubMed] [Google Scholar]
- Ji L, de Pablo JJ, Palecek SP. Cryopreservation of adherent human embryonic stem cells. Biotechnol Bioeng. 2004;88:299–312. doi: 10.1002/bit.20243. [DOI] [PubMed] [Google Scholar]
- Katkov II, Kim MS, Bajpai R, Altman YS, Mercola M, Loring JF, Terskikh AV, Snyder EY, Levine F. Cryopreservation by slow cooling with DMSO diminished production of Oct-4 pluripotency marker in human embryonic stem cells. Cryobiology. 2006;53:194–205. doi: 10.1016/j.cryobiol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Takahashi J, Arakawa Y, Doi D, Fukuda H, Hayashi H, Narumiya S, Hashimoto N. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res. 2008;86:270–280. doi: 10.1002/jnr.21502. [DOI] [PubMed] [Google Scholar]
- Liao J, Wu Z, Wang Y, Cheng L, Cui C, Gao Y, Chen T, Rao L, Chen S, Jia N, Dai H, Xin S, Kang J, Pei G, Xiao L. Enhanced efficiency of generating induced pluripotent stem (iPS) cells from human somatic cells by a combination of six transcription factors. Cell Res. 2008 doi: 10.1038/cr.2008.51. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006a;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, Thomson JA. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006b;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Martin-Ibanez R, Unger C, Stromberg A, Baker D, Canals J, Hovatta O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008 doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- Minambres R, Guasch RM, Perez-Arago A, Guerri C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci. 2006;119:271–282. doi: 10.1242/jcs.02723. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki T, Uehata M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000;325:273–284. doi: 10.1016/s0076-6879(00)25449-9. [DOI] [PubMed] [Google Scholar]
- Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Pirone DM, Liu WF, Ruiz SA, Gao L, Raghavan S, Lemmon CA, Romer LH, Chen CS. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J Cell Biol. 2006;174:277–288. doi: 10.1083/jcb.200510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Suemori H, Yasuchika K, Hasegawa K, Fujioka T, Tsuneyoshi N, Nakatsuji N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun. 2006;345:926–932. doi: 10.1016/j.bbrc.2006.04.135. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Ware CB, Nelson AM, Blau CA. Controlled-rate freezing of human ES cells. Biotechniques. 2005;38:879–3. doi: 10.2144/05386ST01. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Yang PF, Hua TC, Wu J, Chang ZH, Tsung HC, Cao YL. Cryopreservation of human embryonic stem cells: a protocol by programmed cooling. Cryo Letters. 2006;27:361–368. [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhou CQ, Mai QY, Li T, Zhuang GL. Cryopreservation of human embryonic stem cells by vitrification. Chin Med J (Engl ) 2004;117:1050–1055. [PubMed] [Google Scholar]