Abstract
We demonstrate that 2-month-old female B10.T(6R) mice are highly resistant to systemic infection with the KIM5 strain of Yersinia pestis and that B10.T(6R) mice become susceptible to Y. pestis infection by the age of 5 months. In this study, young (2-month-old) and middle-aged (5- to 12-month-old) B10.T(6R) mice were infected with equal CFU counts of Y. pestis. The 50% lethal dose (LD50) for young B10.T(6R) mice was ∼1.4 × 104 CFU, while middle-aged B10.T(6R) mice exhibited an LD50 of ∼60 CFU. Elevated bacterial burdens were found in the spleens of middle-aged mice at 24 and 60 h and in the livers at 60 h postinfection. Immune cell infiltration was greater in the livers of resistant young mice than in those of middle-aged mice and mice of the susceptible C57BL/6N strain. Unlike susceptible mice, young B10.T(6R) mice did not develop necrotic lesions throughout the liver. Instead, livers from young B10.T(6R) mice contained granuloma-like structures. Immunohistochemical staining of liver sections from these mice at 60 h postinfection revealed that the majority of immune cells present in these structures were neutrophils. These findings suggest that resistance to plague in B10.T(6R) mice correlates with early formation of neutrophilic lesions in the liver.
INTRODUCTION
Yersinia pestis is the etiological agent of plague. Plague has resulted in multiple pandemics and the loss of millions of lives (40). Unlike other pathogenic species of Yersinia, Y. pestis causes an acute systemic infection. In a susceptible host, there is an overall lack of inflammation at sites of bacterial replication early in infection (3, 36). A comparative study of Yersinia pseudotuberculosis and Y. pestis demonstrated a lack of neutrophil infiltration in the draining lymph nodes of Y. pestis-infected mice. In contrast, there were increased levels of neutrophils in the lymph nodes of mice infected with the chronic-disease-causing species Y. pseudotuberculosis (17). At later time points, Y. pestis can be found systemically and colonizing the visceral organs. This distribution coincides with a large influx of cellular infiltrates, tissue necrosis, and increased levels of proinflammatory cytokines (36).
Several laboratories (2, 8, 41, 42) have reported different mouse strains intrinsically resistant to plague. Resistance does not appear to be conferred by one specific strain or genetic background. Multiple substrains of inbred 129 mice are highly resistant to infections with the KIM5 strain of Y. pestis (8, 41). Compared to susceptible C57BL/6 mice infected with Y. pestis (50% lethal dose [LD50], 20 to 50 CFU [34]), 129S2/SV.Hsd mice had a much higher LD50 (2 × 106 CFU), and cellular infiltrates, described as predominantly polymorphonuclear leukocytes (PMNs), were observed in the livers of resistant mice at 96 h postinfection (8). The livers of susceptible C57BL/6 mice showed signs of immune cell depletion and coagulative necrosis (8). Resistance to plague in another substrain of 129 mice was mapped to a region of DNA near the interleukin 10 (IL-10) gene, and this resistance could be bred into the susceptible C57BL/6 strain (41). Most BALB/c strains of mice are susceptible to plague infection. However, BALB/cJ mice are also highly resistant to plague (42). This resistance was mapped to a region that coincided with the major histocompatibility complex on chromosome 17. The plague resistance of BALB/cJ mice could also be bred into C57BL/6 mice (42). Plague resistance has also been seen in another mouse species. The SEG/Pas strain of Mus spretus is highly resistant to pigmentation-positive Y. pestis (2). Multiple alleles that contributed to resistance in SEG/Pas mice were discovered (2). The mean times of death of SEG/Pas mice and C57BL/6 mice were similar (2). Blanchet et al. suggested that the mechanism of resistance related to an early response to infection (2).
Cellular infiltrates in the liver without necrosis are also present in mice vaccinated against plague (31). Mice vaccinated with a protein A-LcrV (PAV) fusion or treated with passive antibodies to PAV exhibited granuloma-like lesions in their livers. Granuloma-like lesion formation was also present when C57BL/6 mice were infected with an attenuated YopM− strain of Y. pestis (20). Again, these lesions were described as consisting predominantly of PMNs (20). The data observed for both resistant and vaccinated mice suggest that the formation of immune cell lesions in the liver without the presence of necrosis coincides with protection against plague.
This study demonstrates that another strain of mice, B10.T(6R), is intrinsically resistant to infection with Y. pestis. Interestingly, resistance to Y. pestis is demonstrated to disappear in B10.T(6R) mice by the age of 5 months. Granuloma-like lesions were found in the livers of B10.T(6R) mice at early time points in infection, and these lesions consisted mostly of neutrophils. There were also marked differences in the size and abundance of these lesions between young and middle-aged B10.T(6R) mice, suggesting that the ability to form larger granulomatous lesions is necessary for the immune system to contain Y. pestis. Accompanying the pathological changes seen were alterations in IL-6 and chemokine levels.
MATERIALS AND METHODS
Mice.
B10.T(6R) mice (originally obtained as a generous gift from Chella David, Mayo Clinic and College of Medicine, Rochester, MN) were bred in laminar flow containment and were maintained in a clean conventional area within the Center for Biological Research (CBR) at the University of North Dakota (UND). Inbred C57BL/6N (B6) mice were purchased from Harlan Laboratories (Madison, WI). B10.T(6R) and B6 mice 2 months of age (referred to here as “young”) and B10.T(6R) mice 5 to 12 months of age (referred to here as “middle-aged”) were used for Y. pestis LD50 determinations. B10.T(6R) mice 7 to 9 months of age (middle-aged) were used for histology and cytokine analysis. B6 mice bred at the UND retain susceptibility to Y. pestis KIM5 (data not shown). All animal studies were approved by the University of North Dakota IACUC.
Bacterial challenge.
Mice were infected with Y. pestis as described previously (26). Briefly, the challenge inoculum of the KIM5 strain (pPCP1, pCD1, pMT1; Pgm− [43]) was grown overnight in heart infusion broth (HIB; Difco Laboratories, Sparks, MD) at 26°C with shaking, subcultured to an optical density at 620 nm (OD620) of approximately 0.1, and incubated at 26°C with shaking to an OD620 of ∼1.0. Bacteria were centrifuged at 3,200 × g for 5 min, washed twice, and resuspended in sterile phosphate-buffered saline (PBS). All strains of mice were infected intravenously (i.v.) via the retro-orbital sinus with Y. pestis suspended in PBS. The infectious dose was determined by plating serial dilutions of Y. pestis on tryptose blood agar (TBA; Difco Laboratories, Sparks, MD). Challenge inocula of Y. pestis for LD50 studies were 1 × 101 to 1 × 107 CFU for B6 mice and 1 × 101 to 1 × 106 CFU for both groups of B10.T(6R) mice. Groups consisted of at least 6 mice for each mouse strain. The challenge inoculum of Y. pestis used for histological analysis and immunohistochemistry was 1 × 103 CFU.
LD50 calculation.
Mice were monitored daily from inoculation, on day 1, until day 21. The experimental dose that resulted in death for 50% of the mice infected was determined utilizing log dose probit analysis (at a 95% confidence level) (SPSS, version 17.0; IBM Corporation, Somers, NY).
Measurement of bacterial burden.
Livers and spleens were harvested from infected mice and were incubated in PBS containing 0.1% collagenase D (Roche, Indianapolis, IN) for 1 h at 37°C. The challenge inoculum of Y. pestis for the determination of bacterial burdens was 1 × 103 CFU. Organs were homogenized by passage through a BD Falcon 70-μm-pore-size nylon mesh cell strainer. Homogenates were centrifuged at 3,200 × g for 5 min. Pellets were resuspended in PBS containing 0.1% Triton X-100 to lyse eukaryotic cells. Bacterial burdens were determined by plating serial dilutions on Y. pestis selective medium agar (1). Statistical analysis was performed using GraphPad Prism, version 5.0d (GraphPad Software, Inc., La Jolla, CA).
Histology and immunohistochemistry.
Livers and spleens were harvested from infected mice at 24 h, 60 h, and 96 h. Organs were fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin (H&E). Paraffin-embedded sections were deparaffinized in xylene, heat activated in a sodium citrate buffer (pH 6.0), blocked for 2 h in 20% fetal bovine serum (FBS), and incubated for 16 h at 4°C with a rat anti-neutrophil (α-neutrophil) antibody (NIMP-R14 [rat monoclonal IgG2b; Abcam, Cambridge, United Kingdom]). Anti-F4/80 (rat monoclonal IgG2b; Abcam) was used as an isotype control. Slides were then incubated with 0.3% H2O2 for 10 min to inhibit intrinsic tissue peroxidase activity. A biotinylated secondary antibody was applied to sections for 2 h at room temperature. Slides were incubated for 30 min with horseradish peroxidase (HRP)-conjugated streptavidin (Abcam). Diaminobenzidine (DAB) substrate (Abcam) was applied to the sections for 10 min. Sections were washed with distilled water and were counterstained with H&E. Slides were stained without the addition of the primary antibody as a negative control. Stained slides were visualized using an Olympus BX-60 indirect epifluorescence microscope. Digital images were obtained using a SPOT-RT slider digital camera (version 3.45; Diagnostic Instruments, Inc., Sterling Heights, MI) and the Spot Win imaging program (version 3.5.2; Diagnostic Instruments, Inc.).
Cytokine and chemokine responses.
For serum cytokine levels, mice were bled retro-orbitally at 24 and 72 h postinfection. Blood was pooled, collected in serum separation tubes, centrifuged for 10 min at 10,000 × g, and stored at −70°C until it was assayed. Serum cytokine/chemokine levels were assayed using a Bio-Rad Pro magnetic cytokine/chemokine suspension array assay (Bio-Rad, Hercules, CA). Statistical analysis of proinflammatory mediators in both middle-aged B10.T(6R) and B6 mice, compared to young B10.T(6R) mice, was performed in GraphPad Prism, version 5.0d (GraphPad Software), using one way analysis of variance with Tukey's multiple-comparison posttest. A P value of <0.05 was considered significant.
RESULTS
Young B10.T(6R) mice are resistant to Y. pestis infection.
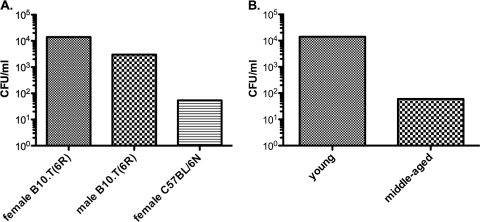
Groups of young B10.T(6R) and B6 mice were infected with increasing doses of Y. pestis KIM5. Young female B10.T(6R) mice displayed an LD50 of 1.4 × 104 CFU. Age-matched male B10.T(6R) mice were slightly less resistant to Y. pestis infection. The LD50 was 3.0 × 103 CFU in males, approximately 4-fold lower than that for female B10.T(6R) mice. This result is consistent with the observation that inbred female mice are more resistant to Y. pestis than male mice (27). Susceptible B6 mice demonstrated a lower LD50 than did B10.T(6R) mice. The calculated LD50 for Y. pestis-infected B6 mice was 54 CFU (Fig. 1A). The mean times of death were similar for all strains (4 to 5 days [data not shown]). These data demonstrate that B10.T(6R) mice are more resistant to plague than susceptible B6 mice and that resistance in B10.T(6R) mice is gender biased, with females displaying more resistance than males. B6 mice bred at UND retain susceptibility to Y. pestis KIM5 (data not shown).
Fig. 1.
Age-associated differences in the LD50s of mice infected with Y. pestis. (A) Young (2-month-old) female B10.T(6R) mice, male B10.T(6R) mice, and B6 mice were infected i.v. via the retro-orbital sinus with increasing doses of Y. pestis KIM5. After 21 days of monitoring, the LD50 was calculated using log dose probit analysis (at a 95% confidence level). (B) LD50s of middle-aged (5- to 12-month-old) female B10.T(6R) mice. Data represent the results of eight experiments for young male and female B10.T(6R) mice and two separate experiments for age-matched female C57BL/6N mice and middle-aged B10.T(6R) mice (n=6/dose group/experiment).
Rapid decrease in resistance to infection in middle-aged B10.T(6R) mice.
Susceptibility to plague increases with age in humans (28). To determine whether the resistance observed in young B10.T(6R) mice was also affected by age, groups of female B10.T(6R) mice were infected with 1 × 101 to 1 × 106 CFU of Y. pestis KIM5. Surprisingly, the LD50 of middle-aged B10.T(6R) mice decreased to approximately 60 CFU (Fig. 1B). Resistance was completely abolished in female B10.T(6R) mice. These data support a novel mouse model of the plague, where middle-aged B10.T(6R) mice exhibited a disease phenotype different from that of young B10.T(6R) mice.
Increased bacterial burdens in middle-aged B10.T(6R) mice.
Y. pestis infection eventually leads to a systemic distribution of Y. pestis bacilli throughout the visceral organs (3). Other resistant mice exhibit decreased amounts of Y. pestis in the liver and spleen (8). We assessed bacterial burdens in the spleens and livers of young versus middle-aged B10.T(6R) mice. At 24 h postinfection, young B10.T(6R) mice had 1.1 × 102 ± 22 (average ± standard deviation [SD]) CFU per spleen, while middle-aged mice had an average of 1.9 × 103 ± 2.4 × 103 CFU per spleen (Fig. 2A). There was not a significant difference based on age in bacterial burdens in livers at 24 h (Fig. 2C). However, by 60 h, both the spleens and livers of young B10.T(6R) mice contained significantly fewer bacteria (2.6 × 104 ± 5.8 × 104 and 1.2 × 104 ± 8.1 × 103, respectively) than middle-aged mice (2.2 × 106 ± 3.3 × 106 and 2.0 × 105 ± 2.4 × 105, respectively) (Fig. 2B and D). No bacteria were detected in either the spleens or livers harvested from young B10.T(6R) mice at 14 day postinfection (data not shown). These data suggest that young B10.T(6R) mice are able to induce a successful immune response to Y. pestis early in infection and perhaps begin to clear the bacteria from visceral organs.
Fig. 2.
Bacterial burdens in the spleens and livers of infected mice. (A and B) Levels of Y. pestis at 24 and 60 h in the spleens of young and middle-aged female B10.T(6R) mice challenged with 1 × 103 CFU of Y. pestis KIM5. At both time points, there was a statistically significant difference in bacterial burdens in the spleen. (At 24 h, 5 mice per group were used; *, P < 0.05. At 60 h, 9 young and 10 middle-aged B10.T(6R) mice were used; ***, P < 0.001 [by the Mann-Whitney test]. Data were pooled from two independent experiments.) Dotted horizontal lines indicate the lower limit of detection. (C and D) Bacterial burdens in the livers of young and middle-aged female B10.T(6R) mice at 24 and 60 h postinfection. There was no significant difference in bacterial burdens in the liver at 24 h, but a significant difference was observed at 60 h. (At 24 h, there were 5 mice per group; NS, no significant difference. At 60 h, 9 young and 10 middle-aged B10.T(6R) mice were used; **, P < 0.005 [by the Mann-Whitney test]. Data were pooled from two independent experiments.)
Immune cell infiltrates in the livers of young B10.T(6R) mice.
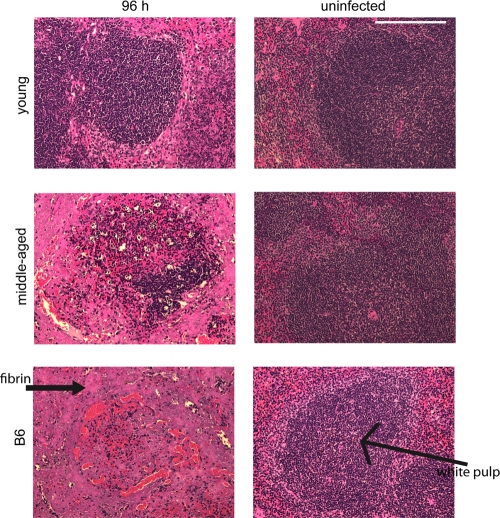
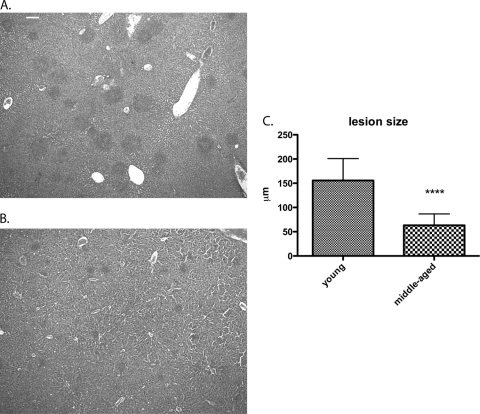
Differences in pathology and immune cell recruitment in the livers and spleens were observed among young female B10.T(6R) mice, middle-aged female B10.T(6R) mice, and B6 mice. There were few to no signs of necrosis in the spleens of both young and middle-aged mice at early time points. However, at 96 h postinfection, there was a significant difference. The spleens of young B10.T(6R) mice still contained intact white pulp and red pulp regions with little to no cellular debris or fibrin deposition (Fig. 3). The spleens of middle-aged B10.T(6R) mice and B6 mice exhibited drastic structural changes, most notably a complete depletion of the white pulp regions. This was more evident in B6 mice, where the spleen consisted mostly of cellular debris and there was fibrin deposition throughout the red and white pulp regions. At 24 h postinfection, both young and middle-aged B10.T(6R) mice had small groups of tightly formed immune cell lesions dispersed throughout the liver (data not shown). By 60 h, there were visible differences in the sizes and numbers of these immune cell lesions. Young B10.T(6R) mice exhibited substantially larger immune cell lesions in the liver (Fig. 4). By 96 h postinfection, the difference in immune cell lesion size was statistically significant (Fig. 5). The mean lesion size (diameter) for young B10.T(6R) mice was 155 μm ± 45 μm, while middle-aged mice had lesions with a mean size of 63.3 μm ± 23 μm (30 lesions/age group were measured). These lesions consisted of intact, tightly formed immune cell infiltrates without extensive signs of necrosis. The livers from Y. pestis-infected B6 mice contained coagulative focal lesions with an absence of immune cell infiltrates. To determine the types of cells present in the lesions of young B10.T(6R) mice, livers were immunohistochemically stained. Staining of the liver sections revealed that the majority of immune cells present were neutrophils (Fig. 6). Staining for F4/80 served as an isotype control for NIMP-R14 staining (Fig. 7). These experiments demonstrated the presence of neutrophils (Fig. 6) but not macrophages (Fig. 7) in the lesions. This result is consistent with those of other studies suggesting the presence of neutrophils in the livers of resistant mice infected with Y. pestis (8) and mice infected with an attenuated strain of Y. pestis (20). Additionally, at later time points, a second, unidentified cell type was seen infiltrating into the lesions (Fig. 7). To begin examining possible mechanisms of resistance, the coagulative lesions from the livers of B6 mice were stained with an anti-neutrophil antibody (Fig. 8). Surprisingly, the coagulative necrotic lesions also stained positive for neutrophils, suggesting that both resistant and susceptible mice recruit neutrophils to sites of inflammation during Y. pestis infection but that the neutrophils are not intact within the lesions of susceptible B6 mice.
Fig. 3.
Structural integrity of spleens following Y. pestis infection. Mice were infected with ∼1,000 CFU of Y. pestis KIM5, and spleens from young B10.T(6R) mice, middle-aged B10.T(6R) mice, and B6 mice were harvested 96 h postinfection, sectioned, and stained with H&E. Images of infected (left) and uninfected (right) spleens from each individual mouse group (4 mice/group) are shown. Each image represents the findings of two independent experiments. Magnification, ×200. Bar, 0.1 mm.
Fig. 4.
Cellular infiltrates and structural integrity of livers following Y. pestis infection. Mice were infected with ∼1,000 CFU of Y. pestis KIM5, and livers were harvested at 60 h and 96 h and were stained with H&E. At 96 h, a significant difference in the size of immune cell lesions between young and middle-aged mice is visually apparent. Necrotic lesions are found throughout the livers of B6 mice. Four mice per group were used, and each image represents the results of two independent experiments. Magnification, ×200. Bar, 0.1 mm.
Fig. 5.
Quantification of liver immune cell lesions in young and middle-aged B10(T6R) mice. Livers from young (A) and middle-aged (7- to 9-month-old) (B) B10.T(6R) mice were harvested 96 h postinfection, sectioned, and stained with H&E. The difference in lesion size between the sections is visible. (C) Thirty lesions from each strain were measured (diameter) and compared using an unpaired two-tailed t test. Error bars represent standard deviations. Immune cell lesions were significantly larger in young B10.T(6R) mice (P < 0.0001). Magnification, ×40. Bar, 0.1 mm.
Fig. 6.
Characterization of immune cell infiltrates in the livers of young Y. pestis-infected B10.T(6R) mice. Livers were harvested at 60 h postinfection, sectioned, stained for neutrophils, and counterstained with H&E. Staining revealed tight formations of cells consisting mostly of neutrophils. Some individual neutrophils were found dispersed throughout the liver. Images represent results from two independent experiments each for four mice. Magnification, ×200 (A) and ×400 (B). Bars, 0.1 mm.
Fig. 7.
Change in the cellular composition of lesions at 96 h postinfection. Livers were harvested at 96 h postinfection, sectioned, stained for neutrophils (A) or macrophages (B), and counterstained with H&E. Magnification, ×200 (left) and ×400 (right). (A) Staining with an α-neutrophil antibody revealed a distinct cell type surrounding the perimeters of lesions that did not stain positive for NIMP-R14. (B) Immune cell lesions did not contain F4/80+ cells. Black arrows indicate individual cells positive for F4/80. Bars, 0.1 mm.
Fig. 8.
Staining of coagulative necrotic lesions in B6 mouse livers with an α-neutrophil antibody. Livers from B6 mice were harvested at 96 h postinfection, sectioned, stained for neutrophils, and counterstained with H&E. Necrotic lesions stained positive for NIMP-R14. Magnification, ×200 (A) and ×400 (B). Bars, 0.1 mm.
Analysis of IL-6 and chemokines.
To address the mechanism of resistance in young B10.T(6R) mice, cytokine (IL-6) and chemokine levels were analyzed. Resistant BALB/cJ mice exhibit lower levels of IL-6 in serum after infection with two different doses of Y. pestis KIM5 than susceptible B6 mice (42). IL-6 and chemokine levels were determined in young B10.T(6R) mice, middle-aged B10.T(6R) mice, and B6 mice. At 72 h postinfection, young B10.T(6R) mice exhibited significantly lower levels of IL-6, keratinocyte-derived chemokine (KC), monocyte chemoattractant protein 1 (MCP-1), and CCL5 (RANTES) than both middle-aged B10.T(6R) mice and B6 mice (Table 1). Further, there were dose-dependent increases in the levels of IL-6 in young and middle-aged B10.T(6R) mice infected with 1 × 103 CFU of KIM5 over those in mice infected with 1 × 102 CFU of KIM5 (Table 1). Serum IL-6 levels increased from 105 ± 6 pg/ml to 205 ± 8 pg/ml in young B10.T(6R) mice and from 517 ± 16 pg/ml to 1,207 ± 80 pg/ml in middle-aged B10.T(6R) mice. There were dose-dependent increases in serum CCL5 (RANTES) levels in all infected mice. Interestingly, the responses to infection were different for susceptible B6 mice versus middle-aged B10.T(6R) mice. Unlike middle-aged B10.T(6R) mice, B6 mice did not exhibit a drastic elevation in serum IL-6 levels with an increasing dosage of Y. pestis KIM5. This difference between B6 mice and middle-aged B10.T(6R) mice was also evident in KC and MCP-1 levels. B6 mice appeared to make less KC at a higher dosage, where middle-aged B10.T(6R) made less MCP-1 with increasing dosages of KIM5. The dose-dependent increase in IL-6 or chemokine levels observed in this model supports evidence from a sublethal Y. pseudotuberculosis infection model (45). These data also suggest that young B10.T(6R) mice may produce a more regulated serum proinflammatory response to Y. pestis than susceptible middle-aged B10.T(6R) mice or B6 mice. The difference in response observed between middle-aged B10.T(6R) mice and B6 mice may be due to strain-specific host immune response mechanisms.
Table 1.
Concentrations of IL-6 and chemokines in the sera of mice infected with Y. pestis KIM5
| Cytokine or chemokine | Y. pestis dose | Mean concn (pg/ml) ± SD in seruma |
|||||
|---|---|---|---|---|---|---|---|
| Young B10.T(6R) mice |
Middle-aged B10.T(6R) mice |
C57BL/6 mice |
|||||
| Uninfected | Infected | Uninfected | Infectedb | Uninfected | Infectedb | ||
| IL-6 | 102 | ND | 105 ± 6 | ND | 517 ± 16*** | ND | 358 ± 8*** |
| 103 | ND | 205 ± 8 | ND | 1,207 ± 80*** | ND | 355 ± 17* | |
| CCL5 | 102 | 105 ± 2 | 138 ± 5 | 92 ± 1 | 337 ± 7*** | 81 ± 2 | 226 ± 8*** |
| 103 | 119 ± 9 | 168 ± 6 | 62 ± 2 | 1,184 ± 38*** | 100 ± 1 | 999 ± 40*** | |
| KC | 102 | 65 ± 2 | 519 ± 11 | 50 ± 4 | 1,076 ± 56* | 70 ± 8 | 3,538 ± 338*** |
| 103 | 71 ± 0.5 | 998 ± 61 | 76 ± 3 | 3,309 ± 70*** | 71 ± 0.6 | 2,468 ± 167*** | |
| MCP-1 | 102 | 307 ± 40 | 2,435 ± 39 | 288 ± 12 | 8,027 ± 108*** | 323 ± 44 | 4,919 ± 258*** |
| 103 | 321 ± 45 | 2,231 ± 27 | 379 ± 2 | 6,023 ± 50*** | 310 ± 22 | 7,069 ± 290*** | |
Determined 3 days postinfection for the pooled sera of mice (n=6) infected with 1 × 102 CFU or 1 × 103 CFU of Y. pestis KIM5. Serum cytokine or chemokine concentrations were determined using a protein suspension array assay. The SD for each sample in triplicate is given. Results are from individual experiments with two infectious doses. ND, not detected.
*, P < 0.05; ***, P < 0.001. The statistical significances of the differences between young and middle-aged B10.T(6R) mice and between young B10.T(6R) mice and B6 mice were determined using one-way analysis of variance (Tukey's multiple-comparison posttest).
DISCUSSION
Y. pestis has adapted a myriad of functions to avoid and manipulate the host immune response. Y. pestis synthesizes a unique tetra-acylated lipopolysaccharide (LPS) at 37°C that acts as a Toll-like receptor 4 (TLR4) antagonist (29). TLR4-antagonist interaction would result in decreased activation of macrophages, dendritic cells, and granulocytes. The F1 protein is expressed at 37°C and forms a capsule-like structure around the bacterium. This capsule increases resistance to phagocytosis (11). Multiple effector proteins secreted by the type III secretion system (T3SS) into the host cell cytoplasm have been shown to suppress the host immune response. YopJ targets naïve macrophages for apoptosis and is dependent on TLR4 signaling (44). Interestingly, in LPS-activated macrophages, YopJ contributes to a decrease in pyroptosis-mediated release of IL-1β (25). IL-1β is an important factor in neutrophil migration (14). There has also been evidence of a YopM-mediated global depletion of natural killer (NK) cells (20). A rapid decrease in the overall numbers of NK1.1+ cells occurs in a Y. pestis-infected host. However, infection with a YopM mutant abolishes the NK cell depletion, and immune cell lesions can be found in the liver without necrosis. NK cells are an important source of gamma interferon (IFN-γ), which, in turn, is a potent activator of macrophages (23). LcrV is a protective protein associated with the tip of the T3SS needle apparatus (30) and is necessary for proper secretion of effector Yops into the host cell cytosol (33). LcrV has also been shown to induce the production of the anti-inflammatory cytokine IL-10 (32) and also forms a complex with human IFN-γ (15). Strains of Y. pestis that lack the T3SS are avirulent (18). And, more importantly, the host is able to produce a robust immune response to T3SS− Y. pestis that includes the recruitment of inflammatory cells to the sites of bacterial replication (7). Y. pestis uses these many mechanisms to develop a niche for vegetative growth within the host while avoiding a robust immune response. Normally, by the time a protective immune response can be generated against Y. pestis, it is too late to clear the bacteria, and the host succumbs rapidly to sepsis due to the overwhelming, systemic burden of the growing bacteria (4).
A resistant host is, however, able to overcome the virulence mechanisms of plague. As demonstrated in this study, B10.T(6R) mice are highly resistant to infection with Y. pestis. B10.T(6R) mice are also known to be resistant to infection with Trichuris muris (13). Resistance to Trichuris muris is associated with the H-2q haplotype of B10.T(6R) mice (13). B10.T(6R) mice are a substrain of C57BL/10 mice that differ at the H-2 locus. C57BL/10 and B6 mice are H-2b, and the resistant 129 substrains of mice are H-2b or H-2bc, whereas the resistant BALB/cJ mouse strain is H-2d. Therefore, the haplotype of the H-2 locus of resistant or susceptible mice may not correlate with resistance. However, whether resistance to Y. pestis is related to the H-2q haplotype is currently being investigated.
The kinetics of our infection model suggest that bacterial clearance begins in the spleens of resistant mice. At 24 h postinfection, there was a significant difference between the splenic bacterial burdens of resistant (young) and susceptible (middle-aged) mice. This difference was not observed in livers at this time point. However, at 60 h postinfection, both the spleens and livers of young mice contained significantly fewer bacteria. We hypothesize that in our model, the key to bacterial clearance is the recruitment of neutrophils to sites of bacterial replication early in infection. Neutrophils are resistant to YopJ-induced apoptosis (38), and human PMNs are able to successfully phagocytize and kill Y. pestis (37). Ablation of Gr+ neutrophils increases the virulence of Y. pestis in a lung infection model (24). These studies suggest the importance of neutrophils in the clearance of Y. pestis. Large immune cell lesions were observed throughout the livers of resistant mice. The vast majority of cells in these lesions consisted of neutrophils. Although similar lesions could be found in some middle-aged B10.T(6R) mice following infection, the size and number of lesions were decreased. Resistance to Y. pestis infection correlated with the presence of these neutrophil-rich lesions.
The immunological responses of young B10.T(6R) mice, middle-aged B10.T(6R) mice, and B6 mice infected with Y. pestis KIM5 were compared by analyzing IL-6 and chemokine levels. Serum IL-6 and chemokine levels were significantly elevated in middle-aged B10.T(6R) and B6 mice (Table 1). Among the analytes tested, IL-6 was elevated in young B10.T(6R) mice in response to increasing dosages of KIM5. However, IL-6 levels in young B10.T(6R) mice were significantly lower than those seen in susceptible mice. IL-6 levels are also decreased in resistant BALB/cJ mice infected with KIM5 (42). At 24 h postinfection, this is the only cytokine whose levels are significantly different for BALB/cJ versus susceptible B6 mice (42). IL-6 is important in the clearance of bacterial infections (9, 22) and in the inflammatory response (21). IL-6−/− mice infected with Yersinia enterocolitica have increased bacterial burdens in visceral organs, elevated levels of proinflammatory mediators, and a decrease in neutrophil migration to the site of infection (12). The immune response to Y. enterocolitica infection in IL-6−/− mice can be restored to the levels observed in wild-type mice by the administration of exogenous recombinant IL-6 (rIL-6). Important observations in the previous study, consistent with our results, were the reduced levels of MCP-1 in wild-type mice and the fact that neutrophil recruitment was restored in IL-6−/− mice treated with rIL-6 (12). Our data, coupled with the ability of IL-6 to induce the production of IL-1 receptor antagonist (IL-1ra) and to downregulate the proinflammatory response (19, 35), substantiate the importance of IL-6 in both neutrophil recruitment and the regulation of the immune response. Young B10.T(6R) mice produced decreased levels of IL-6, and this, in turn, may have influenced the levels of proinflammatory mediators. In humans, there is a positive correlation between serum IL-6 levels and disease outcome for septic patients (5, 6, 10). In an experimental sepsis model, blockage of IL-6 increases survival in polymicrobial sepsis (39). Since the host is thought to succumb to sepsis during the late stages of Y. pestis infection (4), and since IL-6 is elevated in both clinical and experimental models, the elevated levels of IL-6 in susceptible mice in our model may be a result of plague-induced sepsis. The IL-6 levels observed in young B10.T(6R) mice may be more reflective of a controlled inflammatory response, with IL-6 playing an anti-inflammatory role by downregulating other inflammatory mediators, such as MCP-1, KC, and CCL5.
A particularly interesting finding of this study is that the resistance to Y. pestis observed in young female B10.T(6R) mice is completely abolished by the age of 5 months. Resistance is also abolished in middle-aged male B10.T(6R) mice (data not shown). We infected 10 young male and 10 middle-aged male B10.T(6R) mice with 1,000 CFU of Y. pestis KIM5. All of the middle-aged mice succumbed to infection, while the young mice survived (data not shown). The middle-aged B10.T(6R) mice did show, to a lesser degree, some level of inflammatory response (i.e., smaller neutrophilic lesions and decreased tissue necrosis) to Y. pestis infection. However, the LD50 for the middle-aged mice was similar to that for B6 mice.
It is of particular interest that this loss of resistance occurs by the age of 5 months, the equivalent of early middle age in humans. This is a much earlier time point than most other reports of age-associated changes in host responses to bacteria. For example, the well-characterized model of age-related enhanced susceptibility to Streptococcus pyogenes infection in BALB/c mice utilizes mice that are at least 20 months of age (16). Immunosenescence is most often studied in the aged, after immune changes have occurred. We believe this newly described model, in which loss of resistance occurs around the age of 5 months, will provide an excellent tool for understanding changes in resistance/susceptibility to host immune responses to Y. pestis and possibly other infectious agents as immunological changes occur. Further, we speculate that this model could potentially contribute to studies on immunosenescence.
ACKNOWLEDGMENTS
We thank Patrick Carr and Jane Dunlevy of the Department of Anatomy and Cell Biology, University of North Dakota School of Medicine and Health Sciences, for assistance with microscopy and histology.
This work was funded by the Department of Microbiology and Immunology, University of North Dakota School of Medicine and Health Sciences.
Footnotes
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Ber R., et al. 2003. Development of an improved selective agar medium for isolation of Yersinia pestis. Appl. Environ. Microbiol. 69:5787–5792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blanchet C., et al. 2011. Mus spretus SEG/Pas mice resist virulent Yersinia pestis, under multigenic control. Genes Immun. 12:23–30 [DOI] [PubMed] [Google Scholar]
- 3. Brubaker R. R. 1991. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol. Rev. 4:309–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brubaker R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calandra T., Gerain J., Heumann D., Baumgartner J. D., Glauser M. P. 1991. High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines. The Swiss-Dutch J5 Immunoglobulin Study Group. Am. J. Med. 91:23–29 [DOI] [PubMed] [Google Scholar]
- 6. Casey L. C., Balk R. A., Bone R. C. 1993. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann. Intern. Med. 119:771–778 [DOI] [PubMed] [Google Scholar]
- 7. Comer J. E., et al. 2010. Transcriptomic and innate immune responses to Yersinia pestis in the lymph node during bubonic plague. Infect. Immun. 78:5086–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Congleton Y. H., Wulff C. R., Kerschen E. J., Straley S. C. 2006. Mice naturally resistant to Yersinia pestis Δpgm strains commonly used in pathogenicity studies. Infect. Immun. 74:6501–6504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalrymple S. A., et al. 1995. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect. Immun. 63:2262–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damas P., et al. 1992. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann. Surg. 215:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du Y., Rosqvist R., Forsberg A. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 70:1453–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dube P. H., Handley S. A., Lewis J., Miller V. L. 2004. Protective role of interleukin-6 during Yersinia enterocolitica infection is mediated through the modulation of inflammatory cytokines. Infect. Immun. 72:3561–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Else K. J., Wakelin D., Wassom D. L., Hauda K. M. 1990. The influence of genes mapping within the major histocompatibility complex on resistance to Trichuris muris infections in mice. Parasitology 101(Pt. 1):61–67 [DOI] [PubMed] [Google Scholar]
- 14. Faccioli L. H., Souza G. E., Cunha F. Q., Poole S., Ferreira S. H. 1990. Recombinant interleukin-1 and tumor necrosis factor induce neutrophil migration “in vivo” by indirect mechanisms. Agents Actions 30:344–349 [DOI] [PubMed] [Google Scholar]
- 15. Gendrin C., et al. 2010. Hijacking of the pleiotropic cytokine interferon-γ by the type III secretion system of Yersinia pestis. PLoS One 5:e15242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldmann O., Lehne S., Medina E. 2010. Age-related susceptibility to Streptococcus pyogenes infection in mice: underlying immune dysfunction and strategy to enhance immunity. J. Pathol. 220:521–529 [DOI] [PubMed] [Google Scholar]
- 17. Guinet F., Avé P., Jones L., Huerre M., Carniel E. 2008. Defective innate cell response and lymph node infiltration specify Yersinia pestis infection. PLoS One 3:e1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jawetz E., Meyer K. F. 1944. Experimental infection of the chick embryo with virulent and avirulent Pasteurella pestis. Am. J. Pathol. 20:457–469 [PMC free article] [PubMed] [Google Scholar]
- 19. Jordan M., et al. 1995. Neutralization of endogenous IL-6 suppresses induction of IL-1 receptor antagonist. J. Immunol. 154:4081–4090 [PubMed] [Google Scholar]
- 20. Kerschen E. J., Cohen D. A., Kaplan A. M., Straley S. C. 2004. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 72:4589–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kopf M., et al. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339–342 [DOI] [PubMed] [Google Scholar]
- 22. Ladel C. H., et al. 1997. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect. Immun. 65:4843–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lanier L. L. 1997. Natural killer cells: from no receptors to too many. Immunity 6:371–378 [DOI] [PubMed] [Google Scholar]
- 24. Laws T. R., Davey M. S., Titball R. W., Lukaszewski R. 2010. Neutrophils are important in early control of lung infection by Yersinia pestis. Microbes Infect. 12:331–335 [DOI] [PubMed] [Google Scholar]
- 25. Lilo S., Zheng Y., Bliska J. B. 2008. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect. Immun. 76:3911–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matson J. S., Durick K. A., Bradley D. S., Nilles M. L. 2005. Immunization of mice with YscF provides protection from Yersinia pestis infections. BMC Microbiol. 5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mecsas J., et al. 2004. Evolutionary genetics: CCR5 mutation and plague protection. Nature 427:606. [DOI] [PubMed] [Google Scholar]
- 28. Migliani R., et al. 2001. Resurgence of the plague in the Ikongo district of Madagascar in 1998. 1. Epidemiological aspects in the human population. Bull. Soc. Pathol. Exot. 94:115–118(In French.) [PubMed] [Google Scholar]
- 29. Montminy S. W., et al. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7:1066–1073 [DOI] [PubMed] [Google Scholar]
- 30. Mueller C. A., et al. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674–676 [DOI] [PubMed] [Google Scholar]
- 31. Nakajima R., Brubaker R. R. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nedialkov Y. A., Motin V. L., Brubaker R. R. 1997. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 65:1196–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nilles M. L., Fields K. A., Straley S. C. 1998. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J. Bacteriol. 180:3410–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perry R. D., Fetherston J. D. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schindler R., et al. 1990. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 75:40–47 [PubMed] [Google Scholar]
- 36. Sebbane F., Gardner D., Long D., Gowen B. B., Hinnebusch B. J. 2005. Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 166:1427–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spinner J. L., Cundiff J. A., Kobayashi S. D. 2008. Yersinia pestis type III secretion system-dependent inhibition of human polymorphonuclear leukocyte function. Infect. Immun. 76:3754–3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spinner J. L., et al. 2010. Neutrophils are resistant to Yersinia YopJ/P-induced apoptosis and are protected from ROS-mediated cell death by the type III secretion system. PLoS One 5:e9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Starnes H. F., et al. 1990. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J. Immunol. 145:4185–4191 [PubMed] [Google Scholar]
- 40. Stenseth N. C., et al. 2008. Plague: past, present, and future. PLoS Med. 5:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turner J. K., Xu J. L., Tapping R. I. 2009. Substrains of 129 mice are resistant to Yersinia pestis KIM5: implications for interleukin-10-deficient mice. Infect. Immun. 77:367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Turner J. K., McAllister M. M., Xu J. L., Tapping R. I. 2008. The resistance of BALB/cJ mice to Yersinia pestis maps to the major histocompatibility complex of chromosome 17. Infect. Immun. 76:4092–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Une T., Brubaker R. R. 1984. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect. Immun. 43:895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y., Bliska J. B. 2003. Role of Toll-like receptor signaling in the apoptotic response of macrophages to Yersinia infection. Infect. Immun. 71:1513–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Y., Bliska J. B. 2011. Mathematical relationship between cytokine concentrations and pathogen levels during infection. Cytokine 53:158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]