Abstract
Toxoplasma gondii is a globally distributed parasite pathogen that infects virtually all warm-blooded animals. A hallmark of immunity to acute infection is the production of gamma interferon (IFN-γ) and interleukin-12 (IL-12), followed by a protective T cell response that is critical for parasite control. Naïve T cell activation requires both T-cell receptor (TCR) stimulation and the engagement of costimulatory receptors. Because of their important function in activating T cells, the expression of costimulatory ligands is believed to be under tight control. The molecular mechanisms governing their induction during microbial stimulation, however, are not well understood. We found that all three strains of T. gondii (types I, II, and III) upregulated the expression of B7-2, but not B7-1, on the surface of mouse bone marrow-derived macrophages. Additionally, intraperitoneal infection of mice with green fluorescent protein (GFP)-expressing parasites resulted in enhanced B7-2 levels specifically on infected, GFP+ CD11b+ cells. B7-2 induction occurred at the transcript level, required active parasite invasion, and was not dependent on MyD88 or TRIF. Functional assays demonstrated that T. gondii-infected macrophages stimulated naïve T cell proliferation in a B7-2-dependent manner. Genome-wide transcriptional analysis comparing infected and uninfected macrophages revealed the activation of mitogen-activated protein kinase (MAPK) signaling in infected cells. Using specific inhibitors against MAPKs, we determined that parasite-induced B7-2 is dependent on Jun N-terminal protein kinase (JNK) but not extracellular signal-regulated kinase (ERK) or p38 signaling. We also observed that T. gondii-induced B7-2 expression on human peripheral blood monocytes is dependent on JNK signaling, indicating that a common mechanism of B7-2 regulation by T. gondii may exist in both humans and mice.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular parasite that infects ∼30% of the human population worldwide (41). In healthy individuals, infection with T. gondii is typically mild or asymptomatic, due to a robust, protective immune response that effectively controls the acute infection (30). The parasite, however, has evolved numerous mechanisms for modulating host immunity, which likely contribute to its ability to establish a chronic, persistent infection in tissues of the infected host. Moreover, primary infection or reactivation of latent infection in immunocompromised individuals, including transplant recipients, AIDS patients, and the developing fetus, can result in life-threatening disease (20, 21, 36). The disease outcomes associated with T. gondii infection in immune-deficient hosts underscore the complex relationship between the parasite and the host immune response.
Immunity to T. gondii is initiated rapidly upon infection and is characterized by the production of interleukin-12 (IL-12) and gamma interferon (IFN-γ) and a robust cell-mediated response (58). In particular, T lymphocytes can mediate resistance to infection: the adoptive transfer of T cells from immunized mice into naïve recipients provides protection against a lethal challenge (59). CD4+ and CD8+ T lymphocytes are critical for controlling both acute infection and reactivation of chronic infection, through the production of IFN-γ (14, 15). In addition, patients lacking T cells develop clinical toxoplasmosis (21). The initiation of T cell-mediated immunity, therefore, is a critical step in host control of the parasite.
T cell activation requires two signals: (i) the interaction of the T cell receptor (TCR) with its cognate peptide and major histocompatibility complex (MHC) and (ii) the engagement of costimulatory molecules. CD28 functions as the prototypical costimulatory molecule on T cells and interacts with its ligands, the B7 family members B7-1 (CD80) and B7-2 (CD86) on antigen-presenting cells (APCs). On naïve T cells, ligation of the TCR in the absence of costimulation results in T cell anergy (22), underscoring the importance of costimulatory engagement for initiating T cell activation. Both B7-1 and B7-2 can promote T cell proliferation and the production of IL-2 (12, 49). Once T cells become activated, their responses are attenuated by the engagement of the inhibitory receptor cytotoxic T lymphocyte antigen 4 (CTLA-4) on the T cell with B7-1 or B7-2 on the APC (63, 64). In this manner, B7-1 and B7-2 contribute to both the activation and the decline of T cell responses.
As major regulators of T cell activation, the expression levels of B7-1 and B7-2 are tightly controlled. Since a naïve T cell may encounter self MHC and peptide in the periphery, the additional requirement of costimulation is believed to help discriminate between self and nonself. Identifying the mechanisms that regulate these costimulatory ligands during immune activation, therefore, is a critical step for understanding the basis for initiating protective immunity. Several studies have examined the expression of costimulatory ligands induced in monocytes, macrophages, and dendritic cells (DCs) during T. gondii infection. In the mouse, T. gondii infection upregulated the expression of B7-2 but not B7-1 in macrophages (11) and DCs (38). In a study comparing costimulatory ligand expression on T. gondii-infected peritoneal macrophages from BALB/c and BALB/b mice, B7-2 was specifically upregulated on BALB/c macrophages (11). Studies showed that the infection of human monocytes (54) and dendritic cells (55) with T. gondii resulted in the rapid upregulation of B7-1 and B7-2. More recently, a study of T. gondii-infected human monocytes, the monocyte cell line Thp-1, and human macrophages did not report a difference in either B7-1 or B7-2 expression (53). The reasons for this lack of effect are not clear. As for the parasite factors that contribute to costimulatory ligand expression, T. gondii-derived heat shock protein 70 (TgHSP70) was found to induce the maturation of human monocyte-derived DCs and mouse bone marrow-derived DCs, including B7-1 and B7-2 upregulation, through Toll-like receptor 4 (TLR4)-mediated signaling (2, 24).
Collectively, these data demonstrate that the expression of costimulatory ligands is affected by T. gondii infection, but the molecular basis for this regulation has not been well defined. We sought to determine the signaling pathways that are induced in T. gondii-infected macrophages and monocytes and that specifically affect the expression of costimulatory molecules, in an effort to better understand mechanisms of parasite immune modulation and regulation of critical immune ligands. We found that T. gondii specifically upregulated the expression of B7-2 in infected mouse macrophages and human monocytes and in CD11b+ peritoneal exudate cells following intraperitoneal (i.p.) infection. B7-2 upregulation was independent of parasite genotype but required active parasite invasion. The signaling adaptors MyD88 and TRIF were not involved, but an analysis of microarray data comparing infected and uninfected macrophages revealed a possible role for mitogen-activated protein kinase (MAPK) signaling. Using pharmacological inhibitors, we found that T. gondii-induced B7-2 expression in mouse macrophages and human monocytes was dependent on activation of the MAPK c-Jun N-terminal protein kinase (JNK). In addition, naïve T cell proliferation in response to T. gondii-infected macrophages was dependent on parasite-induced B7-2 expression.
MATERIALS AND METHODS
Host cell culture.
Human foreskin fibroblasts (HFF) were cultured in D-10% medium: Dulbecco's modified Eagle's medium (DMEM; Thermo Scientific, Logan, UT) supplemented with 10% heat-inactivated fetal calf serum (FCS; HyClone, Logan, UT), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Mouse bone marrow-derived macrophages (BMdM) from C57BL/6 mice were generated as previously described (16) and cultured in D-10% medium supplemented with 10% macrophage colony-stimulating factor (M-CSF) for 6 to 7 days before T. gondii infection assays. Femurs from MyD88−/− (1) and TRIFLps2/Lps2 (17) mice were kindly provided by Anthony DeFranco (UCSF). For the IFN-γ experiments in Fig. 1, macrophages were treated with 100 U/ml of recombinant mouse IFN-γ (rmIFN-γ) (eBioscience, San Diego, CA) for 24 h before harvest and staining for flow cytometry.
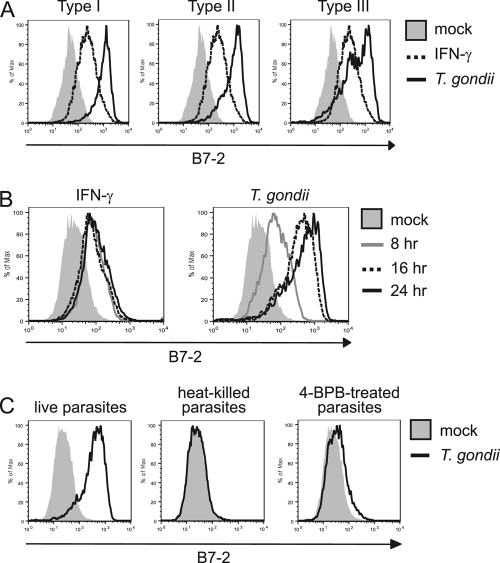
Fig. 1.
Expression of costimulatory molecules during T. gondii infection in vitro and in vivo. (A) BMdM were infected with T. gondii (type I, RH strain) or treated with IFN-γ and stained with a control Ig, anti-B7-1, or anti-B7-2 monoclonal antibody at 24 hpi. (B) BMdM were infected as in panel A but were stained with a control human Ig or a CTLA-4-Ig fusion protein at 24 hpi. (C) C57BL/6 mice were infected i.p. with T. gondii. Peritoneal exudate cells (PECs) were harvested at 3 days postinfection, and the cells were analyzed for B7-2 expression by flow cytometry.
Human monocytes were isolated from peripheral blood mononuclear cells (PBMCs) using counterflow elutriation, as previously described (4). The monocyte populations were >90% pure, as determined by CD11b+ CD14+ CD3− staining and flow cytometry. Freshly isolated monocytes were used immediately for infection experiments.
Parasite strains and infections.
The following T. gondii strains were used for infections: type I strains RHΔhpt and RHgfpluc (46), type II strain Prugniaud A7 (29), and type III strain CΔLuc123 (this was the CEP strain described in reference 51, which was transfected with cDNAs encoding green fluorescent protein [GFP] and click beetle luciferase; these type III parasites were generously provided by Jon Boyle, University of Pittsburgh). The RHgfpluc, Prugniaud A7, and CΔLuc123 strains of T. gondii constitutively express GFP. T. gondii tachyzoites were maintained by serial passage in confluent monolayers of HFF grown in D-10% medium at 37°C with 5% CO2. Mouse macrophage infections were performed as previously described (33). Briefly, BMdM were infected with syringe-lysed, washed parasites at a multiplicity of infection (MOI) of 2, or the medium was replaced for the mock-treated controls. At 2 h postinfection (hpi), the cells were washed with 1× phosphate-buffered saline (PBS) and the medium was replaced with fresh D-10%. At 8, 16, or 24 hpi, the cells were scraped, resuspended, and prepared for antibody staining and flow cytometry. Human monocyte infections were performed as described above for mouse macrophages, except that the cells were not washed at 2 hpi.
In vivo infection.
Female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were infected with syringe-lysed, washed RHgfpluc parasites (105 parasites/mouse) in sterile PBS by intraperitoneal (i.p.) injection. Mock-infected mice received an i.p. injection of PBS (200 μl/mouse). Mice were monitored daily and sacrificed at 3 days postinfection. Peritoneal exudate cells were collected by peritoneal lavage, and red blood cells were lysed prior to staining and analysis of cells by flow cytometry (described below). In vivo experiments were performed three times with similar results. A representative experiment is shown. All research involving mice was carried out in compliance with the Institutional Animal Care and Use Committee at UCI.
Flow cytometry.
Peritoneal exudate cells, BMdM, and human monocytes that were infected with T. gondii or treated with IFN-γ were resuspended in fluorescence-activated cell sorting (FACS) wash (PBS with 2% FCS) containing anti-Fc receptor antibody (for mouse, clone 2.4G2 [BD Biosciences, San Jose, CA]; for human, human Fc receptor binding inhibitor [eBioscience, San Diego, CA]) and incubated on ice for 10 min. The cells were pelleted by centrifugation and resuspended in FACS wash containing the following biotinylated primary antibodies: control Ig, anti-B7-1 (clone 16-10A1), or anti-B7-2 (mouse, clone GL1; human, clone IT2.2; eBioscience, San Diego, CA). The cells were stained with primary antibodies on ice for 30 min and washed. BMdM and human monocytes were then resuspended in FACS wash containing streptavidin-phycoerythrin (PE), while peritoneal lavage samples were resuspended in FACS wash containing streptavidin-PE-Cy5- and anti-CD11b-PE (clone M1/70)-conjugated antibodies. Samples were incubated on ice for 15 min and washed. After the final wash, the infected cells were fixed with 4% paraformaldehyde. The cells were analyzed by flow cytometry using a FACSCalibur cytometer with CellQuest software (BD Biosciences, San Jose, CA) for acquisition and FlowJo software (Treestar, Ashland, OR) for analysis. The primary and secondary antibodies were purchased from BD Biosciences (San Jose, CA), except where indicated. For the mock-treated cells, the histograms depict the total cell population. Since GFP-expressing parasites were used for the flow cytometry experiments, the histograms for the infected cells depict those cells that fell within the GFP+ gate. All the flow cytometry experiments were performed more than three times. Representative experiments are shown.
Q-PCR.
BMdM from C57BL/6 mice, MyD88−/−, or TRIFLps2/Lps2 mice were infected as described above. At 24 hpi, RNA was harvested using the RNeasy kit (Qiagen, Germantown, MD) and was treated with DNase I (Invitrogen, Carlsbad, CA). cDNA was generated and used as template in real-time quantitative reverse transcription-PCR (Q-PCR) with primers specific for mouse B7-2: GCCCATTTACAAAGGCTCAA (sense) and TGTTCCTGTCAAAGCTCGTG (antisense). Primers for mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used for normalization: GCATGGCCTTCCGTGTTC (sense) and GATGTCATCATACTTGGCAGGTTT (antisense). Q-PCR was performed in triplicate using a Bio-Rad iCycler and SYBR green detection (Bio-Rad, Hercules, CA).
The data from the Q-PCR were analyzed using the threshold cycle (ΔΔCT) method (32). The values obtained for B7-2 expression were normalized to those of GAPDH, and the data are expressed as a ratio of mRNA levels. Error bars reflect the standard deviation from triplicate samples. In all Q-PCR assays, the cDNA generated in a reaction in the absence of reverse transcriptase (−RT) was used as a control to detect contaminating genomic DNA. No amplification was observed in the −RT samples or in samples containing water in the place of DNA template.
Microarrays and data analysis.
Confluent monolayers of BMdM were mock treated or infected with RHΔhpt parasites, as described above, and total RNA was harvested at 6 hpi using TRIzol (Invitrogen, Carlsbad, CA). The experiment was performed as biological triplicates. cDNA was synthesized and labeled using the Affymetrix 3′ IVT One-Cycle target labeling kit, according to the manufacturer's instructions. Hybridization to Affymetrix mouse 430 2.0 GeneChip oligonucleotide arrays was performed by the Stanford Protein and Nucleic Acid Facility (Stanford, CA). Background adjustment, quantile normalization, and summarization were performed using the Robust Multichip Average algorithm implemented in Agilent GeneSpring GX v11.0 (Agilent Technologies, Santa Clara, CA). The data set of 45,101 probe sets, representing 21,635 unique genes, was analyzed using the volcano plot analysis pipeline in GeneSpring. A two-class unpaired test with Benjamini-Hochberg multiple-testing correction was performed to determine differences in expression between mock and infected samples that were statistically significant (≤1% false discovery rate [FDR]). The data set was then further filtered to select probe sets in which there was a greater-than-5-fold difference in mock versus infected cells. These probe sets are represented visually in a volcano scatter plot in red. MultiExperiment Viewer (www.tm4.org/mev) was used to generate the heat maps of the filtered data set and to perform hierarchical clustering. Euclidean distance was used for the distance metric, with average linkage clustering.
Inhibitor assays.
BMdM were cultured in serum-free medium for 6 h prior to infection and then treated with dimethyl sulfoxide (DMSO) as a vehicle control, or with the following MAPK inhibitors, beginning at 2 h prior to infection: 10 μM PD-98059 (extracellular signal-regulated kinase [ERK]), 10 μM SP600125 (JNK), or 1 μM SB203580 (p38). These concentrations were selected based on their specificity, as published in the literature, and on dose-response experiments that we performed to select the lowest concentration that showed specific effects on the target (data not shown). The cells were harvested at 15 min postinfection (mpi) for the generation of lysates for Western blotting or at 24 hpi for antibody staining and flow cytometry. All the inhibitors were purchased from Enzo Life Sciences (Plymouth Meeting, PA) and resuspended in DMSO.
Western blotting.
BMdM were treated with MAPK inhibitors and infected, as described above. At 15 mpi, the cells were washed with ice-cold 1× PBS, and cell lysates were generated by the addition of 2× Laemmli sample buffer containing 10% β-mercaptoethanol. The lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) for immunoblotting. The membranes were blotted with antibodies against β-actin or against phosphorylated JNK, p38, ERK, or c-Jun. Horseradish peroxidase (HRP)-conjugated secondary antibodies were used. All Western blotting antibodies were obtained from Cell Signaling (Danvers, MA).
T cell proliferation assay.
BMdM were infected with type I parasites, as described above. The cells were then resuspended in PBS with 2% FCS (FACS wash) containing anti-Fc receptor antibody (clone 2.4G2; BD Biosciences, San Jose, CA) and incubated on ice for 10 min. Cells were washed and resuspended in FACS wash containing control Ig or neutralizing anti-B7-2 antibody (clone GL1; BD Biosciences, San Jose, CA), incubated on ice for 30 min, and then washed. Cells were fixed in 2% paraformaldehyde and then washed a total of 3 times in FACS wash to remove any residual paraformaldehyde. BMdM were resuspended in RPMI 1640 medium (Thermo Scientific, Logan, UT) supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 55 μM β-mercaptoethanol, 5 mM HEPES, 100 μM nonessential amino acids, and 1 mM sodium pyruvate.
Splenic T cells from C57BL/6 mice were enriched by negative selection using LS columns and biotin-conjugated antibodies against MHC class II, Ter-119, CD11b, CD11c, CD19, CD45R, CD49b, and CD105, according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Briefly, splenocytes were prepared by mechanically separating spleens through a cell strainer (40 μm) and lysing red blood cells using ACK lysis buffer (Invitrogen, Carlsbad, CA). Splenocytes were then incubated with the biotin-conjugated antibody cocktail followed by incubation with magnetic MicroBead-conjugated anti-biotin antibodies. The cell suspension was then passed through a LS column in the presence of a strong magnetic field, and enriched T cells were eluted. The purity of enriched T cells was >90% as determined by flow cytometry.
Total enriched splenic T cells were labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE) in PBS containing 0.05% bovine serum albumin for 15 min at 37°C. Cells were washed twice in medium containing 10% FCS. CFSE-labeled T cells (0.5 × 106 cells/well) were placed in wells of a 96-well plate containing 1 μg/ml of plate-bound anti-CD3 antibody (clone 17A2; BioLegend, San Diego, CA) and in the presence of infected or uninfected BMdM coated with control Ig or anti-CD86 antibodies for 4 days. The cells were then harvested and examined by flow cytometry for CFSE dilution. This experiment was performed three times with similar results. A representative experiment is shown.
RESULTS
T. gondii induces B7-2 but not B7-1 on infected macrophages.
Macrophages function in immunity to infection by producing cytokines and antimicrobial factors and by serving as professional antigen-presenting cells to T cells. Immature macrophages in tissues are highly phagocytic cells that are specialized for the uptake and processing of foreign particles. Upon exposure to pathogen-associated molecular patterns, macrophages upregulate the expression of MHC proteins and the costimulatory ligands B7-1 and B7-2 on the cell surface. By infecting BMdM with T. gondii and examining costimulatory ligand expression at 24 h postinfection (hpi), we observed that B7-2, but not B7-1, was highly induced on the surface of infected cells compared with mock-treated cells (Fig. 1A). We also compared the effect of T. gondii on B7-1 and B7-2 expression to that of IFN-γ treatment. Interestingly, T. gondii infection consistently induced B7-2 to higher levels than did IFN-γ treatment. We then investigated whether infected CD11b+ (Mac-1) cells in vivo upregulated B7-2 expression. Peritoneal exudate cells were harvested 3 days after infection of mice with type I strain parasites. By using parasites expressing GFP, we were able to examine B7-2 expression on the infected and the uninfected CD11b+ cells in the population. Analysis by flow cytometry revealed the highest level of B7-2 expression in the infected, GFP+ cell population (Fig. 1C). These data recapitulated the high induction of B7-2 observed specifically in infected cells in vitro (see Fig. 3B, C57BL/6). We also examined T. gondii infection of mouse peritoneal macrophages and bone marrow-derived dendritic cells, and B7-2 was upregulated by in vitro T. gondii infection in both of these cell types (data not shown). These data indicate that the regulation of B7-2 expression by T. gondii infection occurs both in vitro and in vivo,and may be similar in different types of professional antigen-presenting cells.
Fig. 3.
Examination of MyD88 and TRIF in T. gondii-induced B7-2 expression. (A) BMdM from C57BL/6, MyD88−/−, or TRIFLps2/Lps2 mice were infected with T. gondii for 24 h, and RNA was harvested for cDNA synthesis and analysis by Q-PCR for B7-2 transcript levels. For each condition, the expression of B7-2 relative to that of GAPDH is shown. Error bars reflect the standard deviations of triplicate samples. (B) BMdM were infected as in panel A and harvested for the analysis of B7-2 expression by flow cytometry. For each dot plot, the mean fluorescence intensity (MFI) of B7-2 expression and the percentage of cells within the gated, GFP+ population are shown.
The biological function of B7-2 expression depends on its ability to successfully interact with its cognate receptor, CD28 or CTLA-4, on T cells. To evaluate whether B7-2 induced by T. gondii is capable of interacting with its receptor, we utilized a CTLA-4-Ig fusion protein, consisting of the extracellular domain of CTLA-4 fused to the Fc domain of human IgG1. BMdM were mock treated, treated with IFN-γ as a positive control, or infected with type I parasites. The cells were stained with a control human IgG or the CTLA-4-Ig fusion protein and examined by flow cytometry at 24 hpi. Similar to the results observed with the anti-B7-2 monoclonal antibody, we found that CTLA-4-Ig staining of T. gondii-infected cells was elevated compared to that of mock-treated cells or IFN-γ-treated cells (Fig. 1B). Parasite-induced B7-2, therefore, appears to be biologically functional, since it is capable of binding to its cognate receptor.
There are three dominant strains of T. gondii (types I, II, and III), which differ in their global distribution, prevalence, virulence in mice, and, perhaps, associated morbidity in humans (18, 51, 60). Since recent studies have demonstrated that these strains also vary dramatically in their effects on immune cell activity and function (50, 52), we sought to determine whether they differentially affected B7-2 upregulation. BMdM were mock infected or infected with type I, II, or III parasites and examined by flow cytometry at 24 hpi. All three strains induced B7-2 expression (Fig. 2A), suggesting that the mechanism of B7-2 upregulation during infection is likely to be conserved among these three clonal lineages of T. gondii.
Fig. 2.
Characterization of B7-2 induction in T. gondii-infected macrophages. (A) BMdM were infected with type I, II, or III parasites and examined for B7-2 expression at 24 hpi. (B) B7-2 expression was analyzed on T. gondii-infected or IFN-γ-treated BMdM at 8, 16, and 24 h posttreatment. (C) Live, heat-killed, or 4-BPB-treated type I parasites were used for infection of BMdM, and B7-2 expression was examined 24 h later.
To determine the molecular mechanism by which the parasite induces B7-2 expression, we performed a series of experiments to understand the timing and requirements for this phenotype. A kinetic analysis would indicate whether B7-2 is induced rapidly after host cell invasion or once the parasite has established itself in the host cell and has begun to replicate. These data would help to define the parasite factor(s) that is responsible for this effect. BMdM were infected with type I strain parasites and harvested for B7-2 staining and flow cytometry at 8, 16, and 24 hpi. B7-2 expression began to increase as early as 8 hpi and was almost fully induced by 16 hpi (Fig. 2B), suggesting that early sensing of the parasite by the macrophage or the introduction of parasite products shortly after invasion contributed to B7-2 upregulation. Interestingly, at 8 h, induction of B7-2 was similar between IFN-γ treatment and T. gondii infection, but this represented the maximum induction for IFN-γ, whereas the infected cells showed further induction up to 24 h. These data indicate that the pathways that are activated by IFN-γ receptor signaling and T. gondii infection to induce B7-2 expression are likely to differ.
To further characterize the factors required for T. gondii-induced B7-2 upregulation, we investigated whether this effect required active invasion of the host cell by live parasites, or if macrophage phagocytosis of parasites would also induce B7-2 expression. The results from these studies would help to distinguish whether host cell sensing of the parasite or an active parasite mechanism is responsible for this phenotype. Live or heat-inactivated T. gondii parasites were added to macrophages, and the cells were examined for B7-2 expression by flow cytometry at 24 hpi. We observed that only live parasites induced B7-2 expression on the surface of infected macrophages (Fig. 2C), indicating that the manner in which the parasites entered the macrophages dictated the outcome of B7-2 induction. To rule out the possibility that heat killing the parasites impaired host cell recognition of T. gondii, we pretreated the parasites with a phospholipase inhibitor called 4-bromophenacyl bromide (4-BPB). Parasites treated with this inhibitor can attach to host cells but cannot actively invade (47), so they are phagocytosed by the macrophages (data not shown). As shown in Fig. 2C, 4-BPB pretreatment prevented T. gondii induction of B7-2. These data suggest that B7-2 upregulation is likely due to a mechanism stimulated by actively invading parasites.
MyD88 and TRIF are not involved in T. gondii induction of B7-2 expression.
A burgeoning field of research has focused on the processes whereby mammalian cells sense the presence of foreign pathogens and initiate innate immune responses. The Toll-like receptors (TLRs) have emerged as a large family of receptors that recognize microbial products, ranging from nucleic acids to peptidoglycan and proteins (25). Several TLRs are involved in the innate recognition of T. gondii (68). TLR2 and TLR4 recognize T. gondii glycophosphatidylinositol (9), and TLR11 recognizes a parasite actin-binding protein called profilin (69). TLR9-deficient mice are acutely susceptible to oral infection with T. gondii (40), indicating that this TLR is important in parasite host defense. In addition, mice lacking UNC93B1, which associates with TLR3, TLR7, and TLR9 and allows for their proper activation, are acutely susceptible to T. gondii infection (39). Given the involvement of TLRs in T. gondii recognition and the ability of TLR signaling to upregulate B7-2 in other systems (37), we examined their role in T. gondii induction of B7-2. TLRs associate with adaptor proteins to transduce signals into the host cell and induce inflammatory responses. MyD88 is the adaptor protein used for signaling downstream of all the TLRs, except TLR3, which uses the adaptor protein TRIF (67). To examine TLR signaling in B7-2 upregulation, macrophages deficient in MyD88 or TRIF were infected with T. gondii and examined for B7-2 mRNA levels by Q-PCR and for B7-2 cell surface protein levels by flow cytometry. T. gondii infection of BMdM from C57BL/6 mice resulted in a 9.6-fold increase in B7-2 transcript levels compared to mock-treated cells, which is consistent with the increase in mean fluorescence intensity of B7-2 on the surface of infected cells (Fig. 3B). Similarly, the infection of MyD88- and TRIF-deficient macrophages with T. gondii resulted in a 10-fold and 9.7-fold upregulation in B7-2 mRNA, respectively (Fig. 3A). The expression level of B7-2 on the surface of MyD88−/− and TRIFLps2/Lps2 macrophages after T. gondii infection was also comparable to the levels observed on infected C57BL/6 macrophages (Fig. 3B). These data indicate that T. gondii induces B7-2 at the transcript level and that TLR signaling does not appear to be involved in this induction.
Microarray analysis reveals induction of genes downstream of MAPK signaling pathways.
Since B7-2 expression was elevated at the level of mRNA in T. gondii-infected cells (Fig. 3A) but did not require MyD88 or TRIF, we performed genome-wide transcriptional profiling of T. gondii-infected macrophages, in an attempt to identify nodes of regulation of B7-2. BMdM were mock treated or infected with the type I (RH) strain of T. gondii, and total RNA was harvested at 6 hpi. This time point was selected because T. gondii induction of B7-2 on the cell surface occurred within 8 hpi (Fig. 2B), and we reasoned that gene expression changes that induced this effect would be most apparent very early after infection. Furthermore, the transcriptional profiling of cells within a short time period postinfection would be more likely to reveal those transcriptional effects that occurred due to the initial infection, rather than a secondary stress response of the host cell. Labeled cRNA was hybridized to Affymetrix mouse 430 2.0 gene expression arrays to compare genes that showed increased or decreased mRNA in T. gondii-infected versus mock-treated cells. These arrays contain 45,101 probe set identifications (IDs) corresponding to 21,635 unique known and putative genes. After standard normalization steps, a two-class unpaired t test with Benjamini-Hochberg correction was carried out to identify differentially expressed genes: 8,127 probe sets, representing 7,243 genes, were significantly different (FDR ≤ 1%) between mock- and T. gondii-infected cells. A second filter for genes that changed more than 5-fold by infection yielded 193 probe sets, representing 186 unique genes. The probe sets that met these two filtering criteria are expressed in the volcano scatter plot in red (Fig. 4A) and in the heat map (Fig. 4B). The three left columns of the heat map depict the gene expression data from mock-treated cells, and the three right columns depict those of T. gondii-infected cells. The filtered data set included two probe sets for B7-2 (CD86), which were induced 5.42-fold and 5.32-fold during infection (Fig. 4B, arrowheads).
Fig. 4.
Genome-wide transcriptional profiling of T. gondii-infected macrophages. BMdM were mock treated or infected with T. gondii (RH strain), and RNA was harvested at 6 hpi. cDNA was synthesized and labeled for hybridization on Affymetrix mouse 430 2.0 microarrays. (A) Volcano plot of gene expression data. To identify genes significantly different in expression between mock-treated and infected cells, a two-class unpaired t test with Benjamini-Hochberg FDR correction was implemented and average fold change values were calculated. Each point represents an individual probe. Green lines reflect the threshold criteria for significance (FDR, ≤1%; fold change, >5); probes meeting both these criteria are plotted in red. (B) Heat map of genes identified as significantly different between mock-treated (three left columns) and infected (three right columns) samples. The log2 gene expression values were median centered, and hierarchical clustering was performed using Euclidean distance and average linkage clustering.
Among the genes that met the two filtering criteria for being differentially affected by parasite infection, 91.4% (170 of 186) were upregulated in infected cells, compared to only 8.60% (16 of 186) that were downregulated in infected cells (Fig. 4B). These data are consistent with previously published microarray experiments performed on T. gondii-infected HFF and BMdM at later time points postinfection (3, 7, 28, 52), indicating that the host response to the parasite infection mostly involves upregulation. These transcripts are likely to be induced through a combination of host-specific sensing mechanisms and active parasite-mediated effects. Indeed, there is strong evidence for the ability of T. gondii to alter host cell transcription (3, 7, 28, 52).
Transcriptional profiling of T. gondii-induced genes by microarray enabled us to evaluate nodes of gene regulation that were induced or repressed in T. gondii-infected cells. By using Ingenuity Pathway Analysis to reveal specific canonical pathways that were differentially regulated by infection, we observed a striking enrichment for gene sets representing signaling pathways that are involved in the maturation of antigen-presenting cells. In particular, gene sets associated with NF-κB and MAPK signaling cascades, each of which is a major signaling nexus in immune cells, were most dramatically changed (data not shown). These data allowed us to investigate specific pathways that play a role in B7-2 induction during infection.
JNK is involved in T. gondii-induced B7-2 expression.
Based on the analysis of the microarray data, we chose to examine the role of MAPK signaling in B7-2 induction by using specific pharmacological inhibitors. BMdM were pretreated with inhibitors of ERK (PD-98059), JNK (SP600125), or p38 (SB203580) MAPKs and then infected with T. gondii. The cells were harvested and examined for B7-2 expression by flow cytometry. None of the inhibitors affected the level of B7-2 on uninfected cells, suggesting that these signaling molecules are not involved in the basal expression of B7-2 on unstimulated macrophages (Fig. 5A). The treatment of BMdM with the inhibitors also did not affect the ability of the parasites to infect or replicate in cells, since the percentage of infected (GFP+) cells and the number of parasite divisions were unchanged in cells that were treated with the inhibitors or treated with the DMSO control (data not shown). We also confirmed that the inhibitors did not affect the viability of the macrophages during the course of the experiment (data not shown). Treatment with the ERK and p38 inhibitors had no effect on the levels of T. gondii-induced B7-2 on the surface of infected macrophages. In contrast, treatment of macrophages with the JNK inhibitor substantially reduced T. gondii-induced B7-2 expression (Fig. 5A) in a dose-dependent fashion (data not shown).
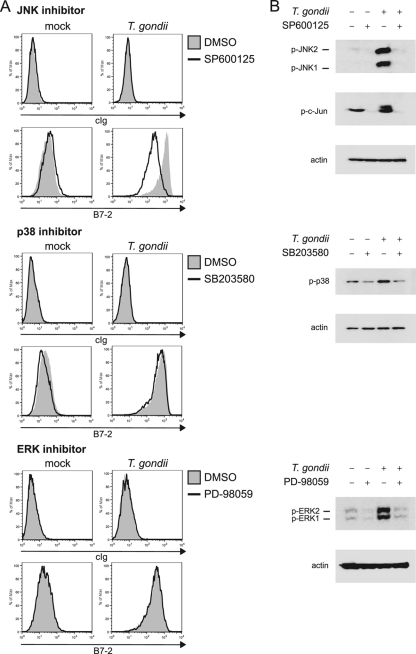
Fig. 5.
B7-2 expression after MAPK inhibitor treatment and T. gondii infection. (A) BMdM were treated with DMSO or with inhibitors of JNK (SP600125), p38 (SB203580), or ERK (PD-98059) and infected with T. gondii. The cells were harvested at 24 hpi and stained with a control Ig or with anti-B7-2 monoclonal antibody. (B) BMdM were treated and infected as described above, but lysates were generated at 15 mpi and separated by SDS-PAGE for Western blotting with antibodies against p-JNK, p-c-Jun, p-ERK, p-p38, or β-actin.
To confirm that the MAPK inhibitors functioned effectively in our assays, we performed, in parallel, Western blotting experiments on lysates from cells that were treated with the inhibitors and infected. Infection of macrophages with T. gondii resulted in rapid (within 15 min) phosphorylation of ERK, p38, and JNK, as determined by immunoblotting for the phosphorylated proteins (Fig. 5B). Pretreatment with the ERK (PD-98059), JNK (SP600125), or p38 (SB203580) inhibitor, however, blocked parasite-induced phosphorylation of the respective target proteins, indicating that these reagents functioned as predicted in our assays. In addition, we examined the inhibitory effect of SP600125 on JNK phosphorylation of its downstream target, c-Jun. The phosphorylation of c-Jun was effectively inhibited by treatment with this inhibitor (Fig. 5B). In a time course experiment, we found that JNK inhibition was sustained as late as 24 hpi (data not shown), the latest time point at which B7-2 levels were examined by flow cytometry in our assays. Based on these data, B7-2 upregulation by T. gondii seems to be dependent on signaling through JNK.
B7-2 induction in human monocytes infected with T. gondii is also dependent on JNK.
Our data have defined a role for JNK in T. gondii-induced B7-2 expression in mouse macrophages. To determine if a similar signaling apparatus is effective in human monocytes, we isolated monocytes from human peripheral blood mononuclear cells. Monocytes were infected with GFP-expressing parasites for 24 h in the presence or absence of the MAPK inhibitors used above and examined for B7-2 expression by flow cytometry. Similar to the mouse macrophages, cultured human monocytes upregulated the expression of B7-2 during infection, specifically in the infected GFP+ cells (Fig. 6). By treating cells with the MAPK inhibitors prior to infection, we determined that pretreatment with SP600125 partially inhibited parasite upregulation of B7-2 (Fig. 6). These data suggest that JNK also plays a role in the regulation of B7-2 expression in cultured human monocytes during parasite infection.
Fig. 6.

Examination of B7-2 expression on T. gondii-infected human monocytes. Human monocytes were harvested from PBMCs and treated with DMSO or with inhibitors of JNK (SP600125), p38 (SB203580), or ERK (PD-98059) before infection with T. gondii. The cells were harvested at 24 hpi and stained with a control Ig or with anti-human B7-2 monoclonal antibody.
Parasite-induced B7-2 costimulates naïve T cell proliferation in vitro.
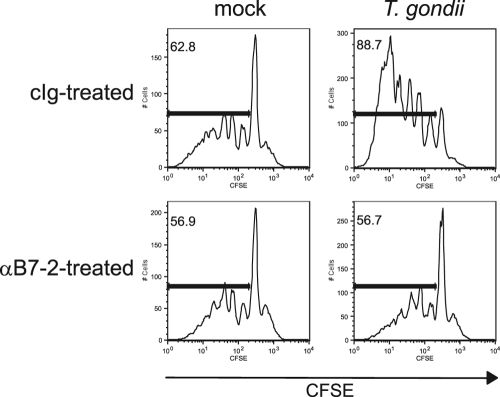
To assess the degree to which parasite-induced B7-2 contributes to costimulating T cell activation, we performed a proliferation assay in which infected BMdM were cocultured with enriched splenic T cells. To focus specifically on the role of B7-2 in costimulation, T cells were cultured with infected macrophages in the presence of plate-bound anti-CD3 to stimulate the T cells through the TCR. T cells that were cultured alone did not proliferate (data not shown). While T cell proliferation was observed in the presence of uninfected macrophages, there was a dramatic increase in the level of proliferation of T cells in the presence of T. gondii-infected macrophages (Fig. 7). This increase was reflected both in the elevated percentage of T cells that had proliferated and in an increased number of cell divisions. The cell proliferation was found to be B7-2 dependent, as blockade of B7-2 using neutralizing antibodies reduced the level of T cell proliferation to that observed during coculture with uninfected macrophages. These data indicate that the upregulation of B7-2 on infected macrophages can functionally costimulate T cell proliferation.
Fig. 7.
Effect of blocking T. gondii-induced B7-2 expression on T cell proliferation. T. gondii-infected BMdM were incubated with a control Ig or an anti-B7-2 monoclonal antibody, fixed, and then cocultured with naïve, syngeneic CFSE-labeled splenic T cells in the presence of plate-bound anti-CD3. The cells were harvested after 4 days, and proliferation was analyzed as a function of CFSE dilution by flow cytometry. For each histogram plot, the marker was set to indicate cells that had undergone division. The percentage of cells within this marker is shown in the upper left corner of the plot.
DISCUSSION
The costimulatory ligands B7-1 and B7-2 provide an important function in the activation and attenuation of T cell-mediated immunity, through their engagement with CD28 and CTLA-4. As a result, their expression is thought to be tightly regulated. The signaling pathways that are involved in infection-induced B7-2 expression, however, are not well known. This study demonstrates the involvement of MAPK signaling in B7-2 upregulation by T. gondii infection. Given the key role of costimulatory signals in initiating immune responses, these data contribute to our understanding of the molecular mechanisms that underpin host immunity to infection.
The differential effect of parasite infection on B7-1 and B7-2 in mouse macrophages is intriguing. Although both accessory molecules serve as natural ligands for CD28 and CTLA-4, they have limited homology (26% sequence identity) (13). Functional studies suggest that they serve both overlapping and distinct functions, which contribute to the inherent complexity in this receptor-ligand system. B7-2 is expressed at higher levels on APCs, and its induction by external stimuli occurs more rapidly (31). B7-2 expression in T. gondii-infected cells increased at the transcript level within 6 h of infection. These data suggest that B7-1 and B7-2 are induced by different signaling mechanisms and may explain the different effects of the parasite on the expression of these proteins. Although B7-1 is typically upregulated with slower kinetics, its affinity for both CD28 and CTLA-4 is substantially higher than that of B7-2 for these receptors (8). Moreover, the affinities of both B7-1 and B7-2 for CTLA-4 are higher than those for CD28 (8). The rapid kinetics of upregulation and broad expression of B7-2 on APCs have led to the idea that it may be important for the initiation of immune responses. In contrast, B7-1 may play a more critical role in amplifying and sustaining T cell activation. Indeed, B7-1 has been shown to be a more potent stimulator of T cell activation (10). Interestingly, we and others have observed that T. gondii does upregulate the expression of B7-1 in cultured human monocytes (data not shown and reference 54). The differential induction of B7-1 and B7-2 in T. gondii-infected human and mouse cells may alter the nature of T cell responses to infection in these hosts.
The in vivo consequences of B7-2 upregulation by T. gondii remain unclear. Since CTLA-4-Ig fusion protein binding to macrophages increased after infection, parasite-induced B7-2 appears to be capable of interacting with its cognate receptor. Moreover, B7-2 upregulation on infected cells costimulated naïve T cell activation in vitro, indicating a potential biological function and role in T cell responses in vivo. Previous research has demonstrated that T. gondii inhibits macrophage antigen presentation to T cells by downregulating MHC class II and impairing the upregulation of IFN-γ-induced MHC class I expression (34, 35). As a result, ovalbumin (OVA)-specific T cells exhibited reduced proliferation when cultured with OVA peptide-pulsed, parasite-infected macrophages compared with that when cultured with uninfected macrophages (35). The likely explanation for the different effects that we have observed on T cell proliferation is that our study examined the ability of T. gondii-induced B7-2 to functionally costimulate T cells in the absence of specific antigen, since anti-CD3 was used to stimulate TCR signaling. As a result, these experiments do not rely on the expression of MHC class I or II. Given that T. gondii can alter MHC expression, the role of costimulatory molecules in vivo may vary, depending on the context in which the T cell and APC interact. To examine the importance of B7-2 upregulation in vivo will likely require the identification of the parasite factors responsible for B7-2 induction. This would enable the generation of parasites deficient in this ability and a comparison with wild-type parasites in T cell responses in vivo.
Although B7-2 was rapidly upregulated in infected cells, both as mRNA and as protein expression on the cell surface, this induction was independent of MyD88 or TRIF signaling. This suggests that B7-2 induction by T. gondii is independent of Toll-like receptor signaling. Infection of MyD88 and TRIF double-knockout macrophages would definitively demonstrate a TLR-independent mechanism of induction for B7-2 during infection, since there remains a possibility that one adaptor may compensate for the loss of the other. MyD88 has an important role in host defense against T. gondii infection, as MyD88-deficient mice are acutely susceptible to infection, compared with C57BL/6 controls. TLR11 was found to be responsible for the specific recognition of a T. gondii protein, called profilin, and induces DC production of IL-12 during in vivo infection (69). Unlike MyD88−/− mice, however, TLR11−/− mice remain resistant to T. gondii infection (69), indicating that other defense mechanisms that utilize MyD88 are involved in controlling the parasite. Although it is perhaps surprising that MyD88 signaling is not required for B7-2 upregulation by T. gondii, lipopolysaccharide (LPS)-induced DC maturation was also found to be independent of MyD88 (23). MyD88 is also a signaling adaptor downstream of the IL-1 receptor (1). T. gondii induces high levels of IL-1 during infection (19, 45); however, our experiments using MyD88−/− macrophages infected with T. gondii suggest that IL-1 receptor signaling may not contribute to B7-2 expression.
Given that B7-2 induction by T. gondii occurred predominantly in infected cells and required active invasion by live parasites, we reasoned that B7-2 may be upregulated through a mechanism involving the introduction of a secreted parasite protein during invasion. T. gondii uses its actin-myosin cytoskeletal machinery to actively invade host cells (5). A key step in parasite invasion is the secretion of proteins from the specialized secretory organelles of the parasite, namely, the rhoptries, micronemes, and dense granules. While some of these secreted proteins play a role in host cell attachment and invasion, others are translocated into the parasitophorous vacuole or the host cell cytosol (6). There is now clear evidence that secreted proteins interact with host proteins and modulate their activity (43, 66). Whether a secreted protein is involved in the induction of B7-2 transcript during infection remains to be determined, although there is certainly precedent for a rhoptry protein (ROP16) trafficking to the nucleus and altering host cell gene transcription (52). We do not believe that ROP16 is responsible for the effects observed in these studies, however, since its activity is strain specific and the induction of B7-2 occurs during infection with all three parasite strains. In addition, B7-2 transcript levels did not differ in macrophages that were infected with the parental type I (RH) strain or Δrop16 parasites (A. Shastri and J. Boothroyd, personal communication). Another possibility is that a secreted protein is sensed by the host cell through a mechanism independent of MyD88 and TRIF, leading to the upregulation of B7-2 mRNA. This possibility, which has interesting implications for parasite modulation of APC function, is currently being investigated.
Transcriptional profiling by microarray analysis revealed nodes of regulation in infected cells, in particular the induction of MAPK signaling and the upregulation of APC maturation markers. Given the early time point used in our experiments (6 hpi), it is clear that the activation of a large number of genes by these pathways occurs within a very short time frame postinfection and that host cells can mount a swift response to the parasite. Indeed, the detection of phosphorylated forms of JNK, ERK, and p38 MAPKs within 15 mpi indicates a rapid alteration in host processes by the parasite. These data are consistent with previous reports on MAPK activation following infection (26, 44, 61). Among the downregulated transcripts in our analysis, many of the genes are negative regulators of MAPK signaling, which may be actively repressed by infection. Indeed, integrative genomic approaches have revealed the importance of secreted parasite kinases that can downregulate host genes associated with MAPK signaling (44). In this way, T. gondii may have a global effect on MAPK signaling cascades in infected cells, by inducing positive regulators and repressing negative regulators.
The role of MAPK signaling in immunity to T. gondii has largely been examined in the context of macrophage IL-12 production. In macrophages, autophosphorylation of p38 is the dominant player in activating IL-12 secretion (27). In neutrophils, IL-12 and CCL2/monocyte chemoattractant protein 1 (MCP-1) production require JNK2, as polymorphonuclear leukocytes (PMN) from JNK2−/− mice were totally deficient in the production of these cytokines (56). Neutrophil chemotaxis is also partially dependent on JNK2 (56). Using pharmacological inhibitors, we found that B7-2 upregulation in T. gondii-infected cells is dependent on JNK signaling. In contrast, IFN-γ-induced B7-2 expression was not affected by treatment with the JNK inhibitor (data not shown). JNK2−/− mice were recently infected with T. gondii and examined for their disease resistance and pathology. After oral infection, mice lacking JNK2 had a lower parasite burden and reduced pathology, compared with wild-type C57BL/6 controls (57). Decreased neutrophil recruitment to the intestinal mucosa and reduced inflammation at this site accounted for the reduced pathology in the knockout mice. These data indicate an important role for JNK2 in parasite-induced immune pathology.
To what degree is B7-2 induction during infection beneficial for parasite dissemination or for the host immune response? Costimulation is clearly important for the activation of naïve T cells, but effector T cells are less reliant on this engagement. In addition, the inducible costimulator (ICOS) pathway can mediate resistance to T. gondii in the absence of CD28 (62). Because B7-2 can bind to both an activating (CD28) and an inhibitory (CTLA-4) receptor, the functional consequences of B7-2 expression may vary dramatically, depending on the stage of infection in which the APC and T cell encounter one another. As we have shown, B7-2 induced by T. gondii is capable of activating T cells and promoting their proliferation. However, the importance of T. gondii-induced B7-2 in vivo may depend on the type of APC that the parasite infects and the type of T cell that the infected APC encounters. Based on studies of CD28−/− mice, the B7-CD28 interaction was not critical for resistance to acute infection by T. gondii but enhanced immune-mediated pathology and the severity of toxoplasmic encephalitis in the brain: CD28−/− mice had less brain pathology and longer survival than did wild-type mice (48). The possibility of interaction between infected APCs and regulatory T cells (Tregs) also has interesting implications for B7-2 function. T. gondii infection of mice has been shown to reduce the number of natural and induced Foxp3+ Tregs (42). Tregs express high levels of CTLA-4 (65), and parasite induction of B7-2 may contribute to modulating the Treg population in infected animals. As we continue to develop our understanding of the intricate relationship between the parasite and the host, it will be of great value to consider the manner in which T. gondii alters or manipulates host responses to ensure its viability and propagation.
ACKNOWLEDGEMENTS
We thank all members of the Boothroyd, Tenner, Nelson, and Morrissette labs for helpful discussion on this project. We also thank Shizuo Akira, Anthony DeFranco, and Baidong Hui for the MyD88−/− and TRIFLps2/Lps2 mouse femurs, Marie Benoit for monocyte isolation, Jose Limon for help with CFSE labeling, Jon Boyle for the CΔLuc123 parasites, and Elizabeth Zuo at the Stanford Protein and Nucleic Acid Facility for microarray hybridization and scanning.
This work was supported by NIH-MBRS training grant GM055246 (P.M.), ACS IRG-98-279-07 (M.B.L.), AHA Scientist Development grant 10SDG3140025 (M.B.L.), a Smith Stanford Graduate Fellowship (Y.-C.O.), and NIH-ROI-AI72756 and -AI21423 (J.C.B.).
Footnotes
Published ahead of print on 12 September 2011.
REFERENCES
- 1. Adachi O., et al. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 [DOI] [PubMed] [Google Scholar]
- 2. Aosai F., et al. 2006. Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via Toll-like receptor 4. Cell Stress Chaperones 11:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blader I. J., Manger I. D., Boothroyd J. C. 2001. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J. Biol. Chem. 276:24223–24231 [DOI] [PubMed] [Google Scholar]
- 4. Bobak D. A., Frank M. M., Tenner A. J. 1986. Characterization of C1q receptor expression on human phagocytic cells: effects of PDBu and fMLP. J. Immunol. 136:4604–4610 [PubMed] [Google Scholar]
- 5. Carruthers V., Boothroyd J. C. 2007. Pulling together: an integrated model of Toxoplasma cell invasion. Curr. Opin. Microbiol. 10:83–89 [DOI] [PubMed] [Google Scholar]
- 6. Carruthers V. B. 1999. Armed and dangerous: Toxoplasma gondii uses an arsenal of secretory proteins to infect host cells. Parasitol. Int. 48:1–10 [DOI] [PubMed] [Google Scholar]
- 7. Chaussabel D., et al. 2003. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102:672–681 [DOI] [PubMed] [Google Scholar]
- 8. Collins A. V., et al. 2002. The interaction properties of costimulatory molecules revisited. Immunity 17:201–210 [DOI] [PubMed] [Google Scholar]
- 9. Debierre-Grockiego F., et al. 2007. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 179:1129–1137 [DOI] [PubMed] [Google Scholar]
- 10. Fields P. E., et al. 1998. B7.1 is a quantitatively stronger costimulus than B7.2 in the activation of naive CD8+ TCR-transgenic T cells. J. Immunol. 161:5268–5275 [PubMed] [Google Scholar]
- 11. Fischer H. G., Dorfler R., Schade B., Hadding U. 1999. Differential CD86/B7-2 expression and cytokine secretion induced by Toxoplasma gondii in macrophages from resistant or susceptible BALB H-2 congenic mice. Int. Immunol. 11:341–349 [DOI] [PubMed] [Google Scholar]
- 12. Freeman G. J., et al. 1993. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J. Exp. Med. 178:2185–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freeman G. J., et al. 1993. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science 262:909–911 [DOI] [PubMed] [Google Scholar]
- 14. Gazzinelli R., Xu Y., Hieny S., Cheever A., Sher A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175–180 [PubMed] [Google Scholar]
- 15. Gazzinelli R. T., Hakim F. T., Hieny S., Shearer G. M., Sher A. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286–292 [PubMed] [Google Scholar]
- 16. Hamerman J. A., Tchao N. K., Lowell C. A., Lanier L. L. 2005. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat. Immunol. 6:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoebe K., et al. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743–748 [DOI] [PubMed] [Google Scholar]
- 18. Howe D. K., Sibley L. D. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172:1561–1566 [DOI] [PubMed] [Google Scholar]
- 19. Hunter C. A., Abrams J. S., Beaman M. H., Remington J. S. 1993. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii: importance of T-cell-independent regulation of resistance to T. gondii. Infect. Immun. 61:4038–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Israelski D. M., Remington J. S. 1993. Toxoplasmosis in patients with cancer. Clin. Infect. Dis. 17(Suppl. 2):S423–S435 [DOI] [PubMed] [Google Scholar]
- 21. Israelski D. M., Remington J. S. 1993. Toxoplasmosis in the non-AIDS immunocompromised host. Curr. Clin. Top. Infect. Dis. 13:322–356 [PubMed] [Google Scholar]
- 22. Jenkins M. K., Schwartz R. H. 1987. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 165:302–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaisho T., Takeuchi O., Kawai T., Hoshino K., Akira S. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166:5688–5694 [DOI] [PubMed] [Google Scholar]
- 24. Kang H. K., et al. 2004. Toxoplasma gondii-derived heat shock protein 70 stimulates the maturation of human monocyte-derived dendritic cells. Biochem. Biophys. Res. Commun. 322:899–904 [DOI] [PubMed] [Google Scholar]
- 25. Kawai T., Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373–384 [DOI] [PubMed] [Google Scholar]
- 26. Kim L., Butcher B. A., Denkers E. Y. 2004. Toxoplasma gondii interferes with lipopolysaccharide-induced mitogen-activated protein kinase activation by mechanisms distinct from endotoxin tolerance. J. Immunol. 172:3003–3010 [DOI] [PubMed] [Google Scholar]
- 27. Kim L., et al. 2005. p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J. Immunol. 174:4178–4184 [DOI] [PubMed] [Google Scholar]
- 28. Kim S. K., Fouts A. E., Boothroyd J. C. 2007. Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J. Immunol. 178:5154–5165 [DOI] [PubMed] [Google Scholar]
- 29. Kim S. K., Karasov A., Boothroyd J. C. 2007. Bradyzoite-specific surface antigen SRS9 plays a role in maintaining Toxoplasma gondii persistence in the brain and in host control of parasite replication in the intestine. Infect. Immun. 75:1626–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krick J. A., Remington J. S. 1978. Toxoplasmosis in the adult—an overview. N. Engl. J. Med. 298:550–553 [DOI] [PubMed] [Google Scholar]
- 31. Lenschow D. J., et al. 1993. Expression and functional significance of an additional ligand for CTLA-4. Proc. Natl. Acad. Sci. U. S. A. 90:11054–11058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 33. Lodoen M. B., Gerke C., Boothroyd J. C. 2010. A highly sensitive FRET-based approach reveals secretion of the actin-binding protein toxofilin during Toxoplasma gondii infection. Cell. Microbiol. 12:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luder C. G., Lang T., Beuerle B., Gross U. 1998. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin. Exp. Immunol. 112:308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luder C. G., Walter W., Beuerle B., Maeurer M. J., Gross U. 2001. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1alpha. Eur. J. Immunol. 31:1475–1484 [DOI] [PubMed] [Google Scholar]
- 36. Luft B. J., Remington J. S. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211–222 [DOI] [PubMed] [Google Scholar]
- 37. Mazzoni A., Segal D. M. 2004. Controlling the Toll road to dendritic cell polarization. J. Leukoc. Biol. 75:721–730 [DOI] [PubMed] [Google Scholar]
- 38. McKee A. S., Dzierszinski F., Boes M., Roos D. S., Pearce E. J. 2004. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J. Immunol. 173:2632–2640 [DOI] [PubMed] [Google Scholar]
- 39. Melo M. B., et al. 2010. UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog. 6:e1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Minns L. A., et al. 2006. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J. Immunol. 176:7589–7597 [DOI] [PubMed] [Google Scholar]
- 41. Montoya J. G., Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976 [DOI] [PubMed] [Google Scholar]
- 42. Oldenhove G., et al. 2009. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31:772–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ong Y. C., Reese M. L., Boothroyd J. C. 2010. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J. Biol. Chem. 285:28731–28740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peixoto L., et al. 2010. Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe 8:208–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pelloux H., et al. 1994. Tumor necrosis factor alpha, interleukin 1 alpha, and interleukin 6 mRNA expressed by human astrocytoma cells after infection by three different strains of Toxoplasma gondii. Parasitol. Res. 80:271–276 [DOI] [PubMed] [Google Scholar]
- 46. Pernas L., Boothroyd J. C. 2010. Association of host mitochondria with the parasitophorous vacuole during Toxoplasma infection is not dependent on rhoptry proteins ROP2/8. Int. J. Parasitol. 40:1367–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ravindran S., Lodoen M. B., Verhelst S. H., Bogyo M., Boothroyd J. C. 2009. 4-Bromophenacyl bromide specifically inhibits rhoptry secretion during Toxoplasma invasion. PLoS One 4:e8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reichmann G., Villegas E. N., Craig L., Peach R., Hunter C. A. 1999. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J. Immunol. 163:3354–3362 [PubMed] [Google Scholar]
- 49. Reiser H., et al. 1992. Murine B7 antigen provides an efficient costimulatory signal for activation of murine T lymphocytes via the T-cell receptor/CD3 complex. Proc. Natl. Acad. Sci. U. S. A. 89:271–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosowski E. E., et al. 2011. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 208:195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saeij J. P., et al. 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314:1780–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saeij J. P., et al. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445:324–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seipel D., et al. 2009. Monocytes/macrophages infected with Toxoplasma gondii do not increase co-stimulatory molecules while maintaining their migratory ability. APMIS 117:672–680 [DOI] [PubMed] [Google Scholar]
- 54. Subauste C. S., de Waal Malefyt R., Fuh F. 1998. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J. Immunol. 160:1831–1840 [PubMed] [Google Scholar]
- 55. Subauste C. S., Wessendarp M. 2000. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and -independent T cell production of IFN-gamma. J. Immunol. 165:1498–1505 [DOI] [PubMed] [Google Scholar]
- 56. Sukhumavasi W., Egan C. E., Denkers E. Y. 2007. Mouse neutrophils require JNK2 MAPK for Toxoplasma gondii-induced IL-12p40 and CCL2/MCP-1 release. J. Immunol. 179:3570–3577 [DOI] [PubMed] [Google Scholar]
- 57. Sukhumavasi W., Warren A. L., Del Rio L., Denkers E. Y. 2010. Absence of mitogen-activated protein kinase family member c-Jun N-terminal kinase-2 enhances resistance to Toxoplasma gondii. Exp. Parasitol. 126:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518 [DOI] [PubMed] [Google Scholar]
- 59. Suzuki Y., Remington J. S. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943–3946 [PubMed] [Google Scholar]
- 60. Taylor G. A., et al. 2000. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc. Natl. Acad. Sci. U. S. A. 97:751–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Valere A., et al. 2003. Activation of the cellular mitogen-activated protein kinase pathways ERK, P38 and JNK during Toxoplasma gondii invasion. Parasite 10:59–64 [DOI] [PubMed] [Google Scholar]
- 62. Villegas E. N., Elloso M. M., Reichmann G., Peach R., Hunter C. A. 1999. Role of CD28 in the generation of effector and memory responses required for resistance to Toxoplasma gondii. J. Immunol. 163:3344–3353 [PubMed] [Google Scholar]
- 63. Walunas T. L., Bakker C. Y., Bluestone J. A. 1996. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 183:2541–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walunas T. L., et al. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1:405–413 [DOI] [PubMed] [Google Scholar]
- 65. Wing K., et al. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322:271–275 [DOI] [PubMed] [Google Scholar]
- 66. Yamamoto M., et al. 2009. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J. Exp. Med. 206:2747–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yamamoto M., Takeda K., Akira S. 2004. TIR domain-containing adaptors define the specificity of TLR signaling. Mol. Immunol. 40:861–868 [DOI] [PubMed] [Google Scholar]
- 68. Yarovinsky F. 2008. Toll-like receptors and their role in host resistance to Toxoplasma gondii. Immunol. Lett. 119:17–21 [DOI] [PubMed] [Google Scholar]
- 69. Yarovinsky F., et al. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626–1629 [DOI] [PubMed] [Google Scholar]