Abstract
The human pathogen Neisseria gonorrhoeae recruits and interacts extensively with polymorphonuclear leukocytes (PMNs) during infection. N. gonorrhoeae is able to survive the bactericidal activity of these innate immune cells and can actively modulate PMN functions in vitro. PMNs are short-lived cells which readily undergo apoptosis, and thus the effect of N. gonorrhoeae infection on PMN survival has implications for whether PMNs might serve as an important site of bacterial replication during infection. We developed and validated an HL-60 myeloid leukemia cell culture model for PMN infection and used both these cells and primary PMNs to show that N. gonorrhoeae infection alone does not induce apoptosis and furthermore that N. gonorrhoeae can inhibit both spontaneous apoptosis and apoptosis induced by the intrinsic and extrinsic apoptosis inducers staurosporine (STS) and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), respectively. N. gonorrhoeae infection also results in the activation of NF-κB signaling in neutrophils and induces secretion of an identical profile of proinflammatory cytokines and chemokines in both HL-60 cells and primary PMNs. Our data show that the HL-60 cell line can be used to effectively model N. gonorrhoeae-PMN interactions and that N. gonorrhoeae actively inhibits apoptosis induced by multiple stimuli to prolong PMN survival and potentially facilitate bacterial survival, replication, and transmission.
INTRODUCTION
Polymorphonuclear leukocytes (PMNs) are professional phagocytic cells which often serve as the first line of host defense in response to invasive microorganisms. Phagocytosis of microbes by PMNs results in the activation of bactericidal mechanisms such as generation of an oxidative burst and production of reactive oxygen species, as well as the release of microbicidal products contained within intracellular granules (7, 31). These terminally differentiated cells have a short life span in vivo, becoming senescent and undergoing spontaneous apoptosis after 1 to 2 days in the peripheral circulation in the absence of an inflammatory stimulus (1). Apoptosis is also triggered by microbial phagocytosis, which contributes to the resolution of inflammation (42, 43).
The Gram-negative bacterium Neisseria gonorrhoeae is the sole causative agent of the sexually transmitted infection gonorrhea. Uncomplicated gonococcal infections remain localized to the urogenital tract, where N. gonorrhoeae interacts with and infects the cells of the reproductive mucosal epithelium. In addition to cervical and urethral epithelial cells, N. gonorrhoeae also encounters and interacts intimately with large numbers of PMNs that are recruited to the site of infection in response to the release of chemokines, such as interleukin-8 (IL-8), from the infected mucosa (11, 24, 33, 60). Examination of urethral exudates associated with symptomatic gonococcal infection has revealed that many of the PMNs at the site of infection contain multiple ingested gonococci, and live, culturable bacteria can be isolated from these exudates (20, 22, 35, 56, 72). This observation suggests that N. gonorrhoeae has evolved mechanisms to survive the potent antimicrobial activities of these innate immune effector cells and that PMNs may be an important niche for bacterial survival and/or replication. While N. gonorrhoeae is susceptible to PMN-mediated killing (8, 25, 41, 61, 66, 73), extensive evidence also exists to show that N. gonorrhoeae can survive and replicate within PMNs subsequent to bacterial uptake (2, 9, 10, 14, 64, 67, 72). The fact that N. gonorrhoeae survives and expands within PMNs raises the question of whether the organism has additionally evolved mechanisms to actively prolong the survival of these normally short-lived cells to either maintain a replicative niche or affect transmission to new hosts.
N. gonorrhoeae has been shown to exert both pro- and antiapoptotic effects on a diversity of cell types, including epithelial cells and lymphocytes. Gonococcal infection has also been shown to exert a delaying effect on spontaneous PMN apoptosis during ex vivo infection (68). While the mechanisms by which N. gonorrhoeae manipulates apoptotic signaling in epithelial cells and lymphocytes have been extensively studied (6, 17, 27, 36, 40, 48, 50, 51), whether bacterial infection acts on the same signaling pathways in the context of PMNs is less well defined. Several other microorganisms have been shown to influence PMN survival in either a pro- or antiapoptotic manner; in particular, pathogens such as Anaplasma phagocytophilum and Chlamydia trachomatis, which reside and replicate within PMNs, have evolved mechanisms to actively delay PMN apoptosis in order to allow sufficient time to complete their replicative cycles (29, 71). Given the extensive interaction between N. gonorrhoeae and PMNs during infection, we set out to further delineate the effects of N. gonorrhoeae on PMN survival.
As part of this study, we first developed and validated a cell culture model for N. gonorrhoeae-PMN interactions using HL-60 myeloid leukemia cells differentiated down the granulocytic pathway. We used both these cells and primary PMNs to demonstrate that N. gonorrhoeae exerts an antiapoptotic effect in neutrophils. We show that N. gonorrhoeae inhibits spontaneous PMN apoptosis, in addition to apoptotic signaling induced by both intrinsic and extrinsic stimuli. N. gonorrhoeae also induces secretion of proinflammatory cytokines and chemokines from infected cells, suggesting that PMNs themselves may be a significant source of these immune modulators. Our results provide a basis for future studies of the effects of N. gonorrhoeae infection on PMN survival and give further insight into the nature of the N. gonorrhoeae-PMN interactions which likely play a critical role in the pathogenesis of infection.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Piliated derivatives of N. gonorrhoeae strain FA1090 were used for all bacterial infections. For the majority of HL-60 cell infections, piliated FA1090 expressing the 1-81-S2 pilin variant (65) and expressing the Opa proteins A, B/D, and F was used. An isolate of N. gonorrhoeae strain FA1090 in which all 11 opa genes were deleted and an opa-deleted strain where opaB was reintroduced at a second site in the chromosome were provided by J. Cannon (University of North Carolina) (28). N. gonorrhoeae was cultured on gonococcal medium base agar (GCB) (Difco) plus Kellogg's supplements (38) and was typically grown at 37°C with 5% CO2 for 18 to 20 h. Prior to HL-60 cell infections, bacteria were suspended in gonococcal liquid medium containing Kellogg's supplements and 0.042% Na2HCO3 at an optical density at 550 nm (OD550) of 0.16 and grown at 37°C to mid-logarithmic phase. For PMN infections, exponentially growing N. gonorrhoeae was obtained using a previously described protocol (16). For experiments with killed bacteria, N. gonorrhoeae was heat killed at 65°C for 30 min. Staphylococcus aureus strain ATCC 25923 was grown in Luria-Burtani broth overnight and then diluted to 1:100 in fresh broth and grown for 3 to 4 h at 37°C. S. aureus was opsonized in 10% normal human serum for 30 min at 37°C prior to infection.
HL-60 cell culture and differentiation.
The HL-60 cell line was obtained from the American Type Culture Collection (CCL-240). Undifferentiated HL-60 cells were cultured in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (MediaTech), which was supplemented with 2 mM glutamine (MediaTech) (R10 medium), and were kept at concentrations between 2 × 105 and 1 × 106 per ml. Differentiation was achieved by diluting the cells to no more than 4.5 × 105 cells per ml, followed by treatment with 0.7% dimethylformamide (Sigma) in culture medium for a period of 4 to 6 days. Differentiation was assessed by examining cellular proliferation via manual counting, by cell surface staining using a phycoerythrin (PE)-conjugated antibody directed against CD11b (clone ICRF44; BD Biosciences), by determining their ability to reduce nitroblue tetrazolium (Sigma) following stimulation with phorbol myristate acetate (PMA) (Sigma), their ability to generate reactive oxygen species by luminol-dependent chemiluminescence (see below), and the ability of adherent cells to kill Staphylococcus aureus (ATCC 25923) that had been opsonized with 10% normal human serum.
PMN donors and isolation.
Heparinized venous blood was obtained from consented healthy volunteers in accordance with a protocol approved by Northwestern University's Institutional Review Board. Dextran-sedimented cells were purified on a Ficoll-Hypaque gradient as previously described (69), and purified PMNs were resuspended in Dulbecco's phosphate-buffered saline (PBS) (without calcium and magnesium; Mediatech) containing 0.1% dextrose and kept on ice until use. PMN preparations contained >95% PMNs as assessed morphologically by phase-contrast microscopy and were >99% viable as assessed by trypan blue exclusion.
Bacterial survival assays.
Synchronized HL-60 cell infections were carried out as follows. HL-60 cells at day 6 postdifferentiation were adhered to acid-washed glass coverslips that were precoated with 0.1% poly-l-lysine (Sigma) and washed with PBSG (PBS supplemented with 0.9 mM CaCl2, 0.5 mM MgCl2, and 7.5 mM glucose) by seeding 1 × 106 cells into 24-well tissue culture-treated plates containing treated glass coverslips in a volume of 0.4 ml PBSG, centrifuging the plates at 1,500 rpm for 5 min, and then allowing the cells to adhere at 37°C and 5% CO2 for 30 min. The adherent HL-60 cells were then chilled on ice and infected with ∼106 CFU of piliated N. gonorrhoeae strain FA1090 in a volume of 0.1 ml in PBSG. The plates were centrifuged for 5 min at 1,500 rpm at 10°C. The cells were washed once, the medium was replaced with 1 ml PBSG, and infections were carried out at 37°C and 5% CO2. At various time points postinfection, the cells were lysed in 1% saponin in PBS before serial dilution and plating onto GCB agar plates to determine viable bacterial counts.
LDCL.
Luminol-dependent chemiluminescence (LDCL) assays were carried out in a total volume of 0.2 ml PBSG in black-bottom 96-well plates (Nunc). HL-60 cells at day 6 postdifferentiation were resuspended in PBSG at a concentration of 4 × 107 cells/ml, and 106 cells per well were seeded in the presence of 100 μM luminol. The cells were then stimulated with either 100 ng/ml PMA, N. gonorrhoeae that had been grown overnight in liquid medium and subjected to two rounds of dilution and growth in rich culture medium and resuspended in PBSG, or S. aureus ATCC 25923 that had been grown in liquid medium and then opsonized with 10% normal human serum in PBSG. Following stimulation, LDCL was measured every 2 min over a total period of 60 min using a SpectraMax M5 plate reader (Molecular Devices) at a temperature of 37°C.
Immunofluorescence.
Differentiated HL-60 cells at day 4 postdifferentiation were adhered to acid-washed, 0.1% poly-l-lysine-treated coverslips in 0.4 ml PBSG in 24-well tissue culture-treated plates as described above. Piliated N. gonorrhoeae strain FA1090 was resuspended in PBSG at a concentration of ∼2 × 108 CFU/ml, and 0.1 ml was used to infect the HL-60 cells (multiplicity of infection [MOI] = ∼20). After the plates were centrifuged at 1,500 rpm for 5 min, the infection was allowed to proceed for 60 min at 37°C and 5% CO2, after which the cells were fixed with 4% paraformaldehyde in PBS. Extracellular bacteria were visualized by incubating fixed nonpermeabilized cells with a polyclonal anti-N. gonorrhoeae antibody (Biodesign) (15), followed by washing with PBS and staining using an Alexa Fluor 647-conjugated goat anti-rabbit secondary antibody (Jackson). The cells were then refixed and permeabilized with 0.2% saponin, and intracellular bacteria were detected by incubation with the same anti-N. gonorrhoeae antibody and staining with a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody. The coverslips were mounted on slides with Fluoromount-G containing 2.5 mg/ml n-propyl gallate, and the cells were visualized with an LSM 510 confocal microscope (Zeiss).
DNA fragmentation assays.
A total of 1 × 106 HL-60 cells at day 4 postdifferentiation or PMNs freshly isolated from venous blood were seeded in 24-well plates in R10 medium. The cells were infected with piliated strain FA1090 at various MOIs as needed. Exponentially growing bacteria cultured in rich medium were used for infections, and the infected plates were then centrifuged at 1,500 rpm for 5 min. Infections were allowed to proceed for 3 to 4 h, and then the cells were treated with either dimethyl sulfoxide (DMSO), 1 μM staurosporine (Sigma), or 100 ng/ml tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (R&D Systems) for a further 18 to 20 h overnight. The cells were then washed with PBS, fixed in ice-cold 70% ethanol for 10 min at 4°C, washed twice more with PBS, and then stained with 0.1 ml PBS containing 0.5 mg/ml RNase A and 50 μg/ml propidium iodide (Sigma) for 30 min at room temperature in the dark. Genomic DNA content was then assessed by flow cytometry (FACSCalibur, BD Biosciences).
Caspase-3 activity assays.
HL-60 cells and primary PMNs were infected with strain FA1090 in R10 medium at various MOIs as appropriate, after which the plates were centrifuged at 1,500 rpm for 5 min to promote bacterium-cell contact. Infections were allowed to proceed for 2 to 3 h, after which the cells were treated with DMSO, 1 μM STS, or 100 ng/ml TRAIL for a further 3 to 4 h. Cell lysates were harvested by washing the cells with PBS and then subjecting them to lysis with 50 μl cell lysis buffer (BD Pharmingen), followed by a 30-min incubation on ice. The cell lysates were stored at −80°C before being used for caspase-3 assays. To measure caspase-3 activity in the cell lysates, 5 μl reconstituted caspase-3 substrate at a concentration of 1.0 mg/ml (Ac-DEVD-AMC; BD Pharmingen) was incubated with 0.2 ml HEPES buffer (20 mM HEPES [pH 7.5], 10% glycerol, 2 mM dithiothreitol [DTT]) and 25 μl cell lysates for 60 min at 37°C. 7-Amino-4-methylcoumarin (AMC) fluorescence was then measured using an excitation wavelength of 380 nm and an emission wavelength of 440 nm, using a SpectraMax M5 plate reader (Molecular Devices). Fluorescence levels were normalized to total cellular protein levels as measured by a bicinchoninic acid (BCA) protein assay (Pierce).
Cellular fractionation.
A total of 5 × 106 HL-60 cells at day 4 postdifferentiation were infected with strain FA1090 as described above. To assess cytochrome c translocation, infections were allowed to proceed for 2 to 3 h, after which the cells were treated with 1 μM STS for a further 3 h. The cells were washed with PBS and then subjected to subcellular fractionation as described in reference 74. Briefly, the plasma membrane was lysed with ice-cold digitonin buffer (200 μg/ml digitonin, 80 mM KCl, and 2 mM EDTA in PBS) for 5 min on ice to allow release of cytoplasmic contents. The cells were centrifuged, and supernatants (cytoplasmic fraction) were removed into fresh tubes. The cell pellets (mitochondrial/nuclear/membrane fraction) were then lysed with total cell lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 2 mM EDTA, 0.2% Triton X-100, 0.3% NP-40, and Halt protease inhibitor cocktail [Pierce]) for 10 min on ice and centrifuged for 10 min, after which the supernatants (mitochondrial/nuclear/membrane proteins) were collected. To assess NF-κB activation, HL-60 cells were infected with strain FA1090, and cells were harvested at various time points and subjected to cellular fractionation to separate nuclear and cytoplasmic fractions using the NE-PER nuclear and cytoplasmic extraction kit (Pierce).
Western blotting.
Either cells (2 × 105) (caspase-3 activation) or 3 to 10 μg cellular extracts (NF-κB activation, cytochrome c translocation) in SDS sample buffer containing β-mercaptoethanol were loaded on 10% or 15% SDS-polyacrylamide gels, and proteins were separated by electrophoresis and transferred to a polyvinylidene fluoride membrane in 10 mM CAPS (N-cyclohexo-3-aminopropanesulfonic acid) buffer with 10% methanol at pH 11 for 1 h. After transfer, membranes were blocked in Tris-buffered saline containing 0.1% Tween 20 and 5% nonfat dry milk for 1 h and then probed overnight with primary antibodies. The membranes were then washed with Tris-buffered saline with 0.1% Tween 20, incubated with secondary antibodies conjugated to horseradish peroxidase (1:2,000; Chemicon) for 30 to 45 min, and then washed again with Tris-buffered saline containing 0.1% Tween 20. Blots were developed with the ECL Plus Western Blotting detection reagent (Amersham, GE Healthcare), and bands were visualized using a ChemiDoc XRS molecular imager (Bio-Rad). Primary antibodies used were anti-caspase-3 (3G2, 1:1,000; Cell Signaling), anti-NF-κB p65 (C-20, 1:200; Santa Cruz), anti-IκBα (C-21, 1:100; Santa Cruz), anti-cytochrome c (7H8, 1:100; Santa Cruz), α-β-tubulin (9F3, 1:1,000; Cell Signaling), and anti-histone H3 (clone A3S, 1:2,000; Millipore).
ELISA.
Primary PMNs and differentiated HL-60 cells were seeded into 24-well tissue culture plates at a concentration of 106 cells/ml in R10 medium and were either left uninfected or infected with N. gonorrhoeae strain FA1090 at an MOI of 100. The plates were centrifuged at 1,500 rpm for 5 min to promote bacterium-cell contact, and infections were carried out at 37°C with 5% CO2. After 4 h of treatment/infection, the cells were centrifuged and the supernatants were harvested and stored at −80°C. The concentrations of cytokines and chemokines in the supernatants were assessed by enzyme-linked immunosorbent assay (ELISA) (single-analyte ELISArray kits; SA Biosciences). The assays were carried out according to the manufacturer's specifications, using a volume of 50 μl supernatant.
RESULTS
N. gonorrhoeae survives and replicates in association with HL-60 cells that have been differentiated down the granulocytic pathway.
Since PMNs are short-lived cells in vivo and ex vivo and display a great deal of variability, we first set out to develop and establish the viability of a cell culture model for N. gonorrhoeae-PMN interactions using the HL-60 myeloid leukemia cell line. This cell line was one of the first long-term human myeloid leukemic suspension cell cultures to be established (12) and has been used extensively as a model for granulocytic cells following differentiation (26, 32). HL-60 cells have been used previously to examine N. gonorrhoeae binding and uptake (3, 21, 59), but their suitability as a model for N. gonorrhoeae-PMN interactions has not been rigorously examined. We used dimethylformamide (DMF) at a concentration of 0.7% to differentiate HL-60 cells into granulocyte-like cells which ceased proliferation, expressed high levels of cell-surface CD11b, exhibited bactericidal activity against opsonized Staphylococcus aureus, and were able to mount an oxidative burst in response to both mitogenic and bacterial stimuli (data not shown).
To determine whether differentiated HL-60 cells could be useful for modeling the N. gonorrhoeae-PMN interaction, the survival of N. gonorrhoeae in association with infected HL-60 cells was examined. Differentiated, adherent HL-60 cells on glass coverslips were infected with N. gonorrhoeae at a multiplicity of infection (MOI) of 1, and at various times postinfection, the cells were lysed and viable bacteria remaining in association with the cells were quantified (Fig. 1A). Whereas N. gonorrhoeae incubated in infection medium alone did not expand significantly throughout the course of infection, N. gonorrhoeae incubated in the presence of HL-60 cells expanded an average of 5.6-fold. Over several independent assays, the extent of N. gonorrhoeae replication in the context of HL-60 cells was observed to be between 3- and 6-fold over a 3-h time course.
Fig. 1.
N. gonorrhoeae (Ngo) is able to survive and replicate in association with HL-60 cells. (A) Differentiated HL-60 cells were infected with piliated strain FA1090 at an MOI of 1. Viable CFU/ml were enumerated at various times postinfection, and the data are represented as the means ± SD for 3 independent replicates and are representative of 3 independent experiments. The growth of bacteria in infection medium alone was also determined. (B) N. gonorrhoeae bacteria associated with HL-60 cells are both intracellular and extracellular. Differentiated HL-60 cells were infected at an MOI of 24 for 1 h, after which the cells were fixed and intracellular and extracellular bacteria were differentiated based on accessibility of an N. gonorrhoeae-specific antibody before and after cellular permeabilization. Extracellular bacteria appear red and yellow, and intracellular bacteria appear green.
To examine whether the HL-60 cells were binding to and internalizing the bacteria, the association of N. gonorrhoeae with infected cells was monitored by differential immunofluorescence of intra- versus extracellular bacteria, such that extracellular bacteria appear red and yellow and intracellular bacteria appear green (Fig. 1B). After 60 min of infection, there were both extracellular and intracellular bacteria associated with the cells. These results showing the internalization and replication of N. gonorrhoeae in association with differentiated HL-60 cells recapitulate previous observations using primary PMNs, though we did not observe significant levels of bacterial killing by the infected cells (14, 67).
N. gonorrhoeae actively inhibits the oxidative burst in HL-60 cells.
To determine whether N. gonorrhoeae could inhibit the oxidative burst generated in HL-60 cells as has been reported for primary PMNs (16), differentiated cells were infected with live, exponentially growing N. gonorrhoeae in the presence of luminol and stimulated with phorbol 12-myristate 13-acetate (PMA) to induce the production of reactive oxygen species (ROS). Luminol-dependent chemiluminescence (LDCL) was then measured over a 1-h time period. Unstimulated HL-60 cells did not induce detectable luminescence, whereas PMA treatment induced a significant oxidative burst (Fig. 2A). Infection with live N. gonorrhoeae alone at a multiplicity of infection of ∼200 did not induce ROS production (Fig. 2C, live Ngo) and resulted in the reduction of ROS production generated by PMA stimulation (Fig. 2A, PMA + Ngo). In order to determine whether N. gonorrhoeae infection was also able to suppress the oxidative burst induced by S. aureus, differentiated HL-60 cells were simultaneously infected with N. gonorrhoeae and opsonized S. aureus, and N. gonorrhoeae was also able to suppress ROS production induced by this bacterial stimulus (Fig. 2B). Treating HL-60 cells with the formylated peptide f-Met-Leu-Phe at concentrations up to 100 μM did not induce ROS production in differentiated HL-60 cells in either the presence or absence of 50 ng/ml IL-8 (data not shown). This observation may be due to the fact that these differentiated HL-60 cells do not express detectable amounts of formyl peptide receptor on their cell surface (data not shown).
Fig. 2.
N. gonorrhoeae actively inhibits the oxidative burst in HL-60 cells. Production of ROS was measured by LDCL over a period of 60 min. RLU, relative light units. (A) N. gonorrhoeae (Ngo) inhibits the oxidative burst induced by PMA. Differentiated HL-60 cells were either left unstimulated, were stimulated with 100 ng/ml PMA, were infected with piliated strain FA1090 at an MOI of 100, or were simultaneously infected and stimulated with PMA. The data are representative of 5 independent experiments. (B) N. gonorrhoeae inhibits the oxidative burst induced by opsonized S. aureus. Differentiated HL-60 cells were either left unstimulated or were infected with opsonized S. aureus at an MOI of 132 or coinfected with S. aureus and N. gonorrhoeae strain FA1090 at an MOI of 65. ROS production was measured over 60 min. Data are representative of 5 independent experiments. (C) Live bacteria are required to inhibit ROS production. HL-60 cells were treated with PMA and infected with either live, exponentially growing N. gonorrhoeae at an MOI of 108 or heat-killed bacteria (MOI < 1.7 × 10−6). The data are representative of 3 independent experiments. (D) ROS inhibition in HL-60 cells is not dependent on Opa expression. HL-60 cells were treated with PMA and infected with either piliated FA1090 bacteria that were Opa− or an Opa−strain with OpaB expressed from an ectopic site at an MOI of 100 or 40, respectively. The data represent 4 independent experiments. (E) Inhibition of PMA-induced ROS production is not dependent on phagocytosis. HL-60 cells were pretreated with 10 μg/ml cytochalasin D for 5 to 10 min prior to addition of PMA and/or bacteria (MOI = 90). The data are representative of 4 independent experiments.
The suppression of the oxidative burst was dependent on the presence of live bacteria, since heat-killed N. gonorrhoeae was unable to suppress PMA-induced ROS production (Fig. 2C) and was not dependent upon Opa expression by N. gonorrhoeae, since both piliated Opa+ and piliated Opa− bacteria were able to suppress ROS production to equivalent degrees (Fig. 2D). Infection of HL-60 cells with the same piliated Opa− and Opa+ isolates alone did not induce a significant respiratory burst (data not shown). These observations are in contrast to those found with primary PMNs, where Opa expression has been associated with ROS production (16, 19, 30, 54). Our failure to observe significant ROS induction in differentiated HL-60 cells stimulated with Opa-expressing bacteria is likely due to the fact that our HL-60 cells do not express significant amounts of carcinoembryonic antigen-related cellular adhesion molecule (CEACAM) receptors on their cell surface as evaluated by flow cytometry using an antibody detecting CEACAM1, CEACAM3, CEACAM6, and CEA (data not shown). To determine whether bacterial uptake was required for the suppression of ROS production, HL-60 cells were pretreated with cytochalasin D to suppress phagocytosis. Treating HL-60 cells with cytochalasin D inhibited ROS production induced by S. aureus infection (data not shown) but had no effect on either the PMA-induced oxidative burst or the ability of N. gonorrhoeae to suppress the oxidative burst induced by PMA, showing that the effect is independent of bacterial internalization (Fig. 2E).
N. gonorrhoeae infection does not induce apoptosis in HL-60 cells and inhibits apoptosis induced by staurosporine.
The survival and replication of N. gonorrhoeae in association with differentiated HL-60 cells and the suppression of the oxidative burst generated by multiple stimuli in the presence of N. gonorrhoeae suggest that HL-60 cells are an appropriate cell line with which to model the infection of primary PMNs by N. gonorrhoeae. In order to determine the effect of bacterial infection on apoptosis in HL-60 cells, differentiated cells were infected with N. gonorrhoeae for 3 h and either left untreated or treated with the apoptosis inducer staurosporine (STS) (or DMSO as a control) for a further 18 to 20 h. Genomic DNA fragmentation following infection and apoptosis induction was then measured by fixing the cells with ethanol and staining DNA with propidium iodide to measure the proportion of cells exhibiting hypodiploid DNA content (Fig. 3A). In uninfected control cells, very little DNA cleavage (3.3% of cells exhibiting hypodiploid DNA content) was observed, and N. gonorrhoeae infection did not significantly alter the extent of DNA cleavage (6.6% of cells with hypodiploid DNA content). STS treatment induced DNA cleavage in 39% of cells, and preinfection of HL-60 cells that were then treated with STS resulted in an 80% decrease in DNA fragmentation (7.9% of cells with hypodiploid DNA content in N. gonorrhoeae-infected, STS-treated cells), suggesting that N. gonorrhoeae infection inhibits STS-induced apoptotic signaling in HL-60 cells. These results were consistent over several independent experiments, where N. gonorrhoeae induced an average ∼51% reduction in the proportion of STS-treated, infected cells exhibiting hypodiploid DNA content versus uninfected cells (Fig. 3B).
Fig. 3.
N. gonorrhoeae inhibits DNA fragmentation in HL-60 cells induced by staurosporine (STS). (A) Differentiated HL-60 cells were infected with piliated, Opa-expressing strain FA1090 at an MOI of 10 and then treated with 1 μM STS (or DMSO as a control). Hypodiploid DNA content was then evaluated by flow cytometry, and the percentage of cells exhibiting hypodiploid DNA under each condition is indicated. (B) Average percentage of cells exhibiting DNA fragmentation after infection and/or treatment with 1 μM STS/DMSO. The data are averages for 5 independent experiments.
In order to rule out the possibility that the lack of DNA cleavage seen in N. gonorrhoeae-infected HL-60 cells was due to increased cytotoxicity following infection, the extent of plasma membrane damage as measured by lactate dehydrogenase (LDH) release into culture supernatants was assessed after infection. We failed to observe any consistent differences in LDH levels in the supernatants of either infected versus uninfected cells or DMSO- versus STS-treated cells (data not shown), suggesting that the lack of apoptosis induction in N. gonorrhoeae-infected, DMSO-treated cells and the decreased apoptosis in N. gonorrhoeae-infected, STS-treated cells were likely not due to necrotic lysis of the infected cells.
To evaluate whether N. gonorrhoeae infection inhibits caspase-dependent apoptosis, HL-60 cells were infected with N. gonorrhoeae for 3 h and then treated with 1 μM STS for a further 3 h, after which cell lysates were harvested and caspase-3 activity was assessed using the fluorogenic caspase-3 substrate Ac-DEVD-AMC (Fig. 4A). The data are expressed as relative fluorescence normalized to that of uninfected, control DMSO-treated cells. Infection of HL-60 cells with N. gonorrhoeae alone did not induce significant caspase-3 activity over that of uninfected controls (relative caspase-3 activity of 0.7). Treatment of HL-60 cells with 1 μM STS for 3 h increased caspase-3 activity an average of 5.6-fold over that of control DMSO-treated cells, and preinfection with N. gonorrhoeae at an MOI of 50 decreased STS-induced caspase-3 activity by an average of ∼63%. Concurrent treatment of cells with both STS and the pancaspase inhibitor z-VAD-fmk resulted in a reduction in fluorescence to control levels, suggesting that the increased fluorescence observed was due specifically to increased caspase activity in the cell lysates. The mitigating effect of N. gonorrhoeae infection on STS-induced caspase-3 activation was dose dependent, since the inhibition of caspase-3 activity became more pronounced with increasing MOIs (Fig. 4C).
Fig. 4.
N. gonorrhoeae inhibits caspase-3 activity induced by STS in HL-60 cells. (A) N. gonorrhoeae inhibits caspase-3 activity in HL-60 cell lysates. Differentiated HL-60 cells were infected with piliated strain FA1090 at an average MOI of 61 and then treated with 1 μM STS, 20 μM z-VAD-fmk, or DMSO. Caspase-3 activity was measured using the fluorogenic substrate Ac-DEVD-AMC, and data are presented as the caspase-3 activation relative to that for uninfected, DMSO-treated controls. Data are representative of at least 3 biological replicates. (B) N. gonorrhoeae inhibits procaspase-3 cleavage in STS-treated cells. Differentiated HL-60 cells were infected at an MOI of 27 and then treated with 1 μM STS. Cell lysates were harvested and subjected to Western blotting using an antibody directed against full-length procaspase-3. Data are representative of 3 independent experiments. (C) Inhibition of STS-induced caspase-3 activity by N. gonorrhoeae is dose dependent. HL-60 cells were infected with N. gonorrhoeae at increasing MOIs and then treated with STS. Data are representative of at least 3 independent experiments. (D) Inhibition of STS-induced caspase-3 activity requires live bacteria. HL-60 cells were infected with live (average MOI = 60) or heat-killed bacteria, followed by treatment with STS, after which caspase-3 activity in cell lysates was assessed. The data are representative of 4 independent experiments.
Activation of caspase-3 in response to N. gonorrhoeae infection and STS treatment was additionally evaluated by assessing cleavage of inactive procaspase-3 in infected cells. The Ac-DEVD-AMC substrate used to evaluate caspase-3 activation shows a high affinity for caspase-3 but can also bind to other caspases, such as caspase-7 (70), and thus it was important to demonstrate specific cleavage of caspase-3. HL-60 cells were preinfected with N. gonorrhoeae for 3 h and treated with STS for a further 3 h, and cell lysates were harvested for Western blotting with an antibody directed against full-length, inactive procaspase-3 (Fig. 4B). Infection of differentiated HL-60 cells with N. gonorrhoeae alone did not result in increased procaspase-3 cleavage relative to that of uninfected cells and in some experiments appeared to increase cellular levels of procaspase-3. Treatment of HL-60 cells with 1 μM STS resulted in a >90% decrease in procaspase-3 levels in cell lysates relative to those in DMSO-treated controls (relative procaspase-3 levels of 0.08 in cells treated with STS only). The cleavage of procaspase-3 induced by STS was mitigated by preinfection of cells with N. gonorrhoeae (relative procaspase-3 levels of 0.8 in N. gonorrhoeae-infected, STS-treated cells), consistent with the results observed in the caspase activity assays (Fig. 4A), and this suggests that N. gonorrhoeae infection prevents the activation of caspase-3 induced by STS. Cellular levels of the caspase-3 substrate PARP were also examined in parallel, and the extent of PARP cleavage mirrored that of caspase-3 (data not shown).
To evaluate whether the antiapoptotic activity of N. gonorrhoeae requires live bacteria, the effect of heat-killed bacteria on STS-treated HL-60 cells was examined. Cells were incubated with live or heat-killed bacteria prior to treatment with STS, and lysates were harvested for caspase-3 activity assays (Fig. 4D). Whereas infection of HL-60 cells with live bacteria decreased STS-induced caspase-3 activity by 70%, infection with heat-killed bacteria had no significant mitigating effect on caspase-3 activation, suggesting that live bacteria are required for N. gonorrhoeae to exert its antiapoptotic effects. Additionally, infection of HL-60 cells with heat-killed bacteria alone did not increase caspase-3 activity over that of uninfected controls.
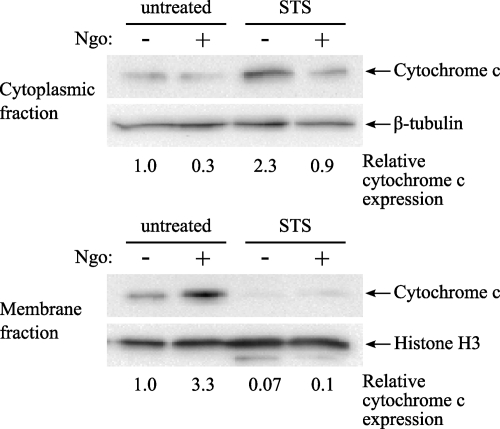
N. gonorrhoeae inhibits cytochrome c translocation to the cytosol.
Mitochondria are a central regulator of intrinsic apoptotic signaling. One of the key events in the triggering of irreversible cellular apoptosis is the loss of mitochondrial membrane potential and the release of mitochondrial factors, such as cytochrome c and apoptosis-inducing factor (AIF), into the cytosol, where the former acts to activate cellular caspases. Previous work has suggested that N. gonorrhoeae infection can affect mitochondrial function in epithelial cells and PMNs undergoing spontaneous apoptosis by inhibiting cytochrome c translocation and loss of mitochondrial membrane potential, respectively (6, 68). To determine whether N. gonorrhoeae-mediated inhibition of apoptosis in HL-60 cells involves cellular mitochondria, the extent of cytochrome c release from the mitochondrial intermembrane space into the cytoplasm was assessed after N. gonorrhoeae infection and induction of apoptosis by STS (Fig. 5). Basal levels of cytochrome c release in uninfected, untreated HL-60 cells was low, although in some experiments a low level of spontaneous translocation of cytochrome c from mitochondrial fractions to the cytosol was observed. N. gonorrhoeae infection alone did not induce cytochrome c translocation but rather inhibited spontaneous cytochrome c release when it occurred (Fig. 5) (cytosolic levels of cytochrome c were decreased 3-fold, with a corresponding 3-fold increase in mitochondrial cytochrome c levels). In contrast, treatment of HL-60 cells with STS resulted in an almost complete loss of cytochrome c from the mitochondrial fraction. Preinfection with N. gonorrhoeae prior to STS treatment resulted in decreased cytochrome c translocation (Fig. 5) (a 2.5-fold decrease in cytosolic cytochrome c in extracts from infected, STS-treated cells versus that for STS-treated cells alone), suggesting that N. gonorrhoeae can influence apoptotic signaling via inhibition of cytochrome c release from the mitochondrial intermembrane space.
Fig. 5.
N. gonorrhoeae inhibits cytochrome c translocation to the cytoplasm. Differentiated HL-60 cells were infected with N. gonorrhoeae at an MOI of 20 for 3 h and then were left untreated or were treated with 1 μM STS for a further 3 h. The cells were subjected to cellular fractionation to separate cytoplasmic proteins from membrane-associated proteins. Both cytoplasmic and membrane fractions were subjected to Western blotting using an antibody directed against cytochrome c, and antibodies directed against β-tubulin and histone H3 were used as loading controls. The results are representative of 4 independent experiments.
N. gonorrhoeae infection inhibits apoptosis induced by TRAIL.
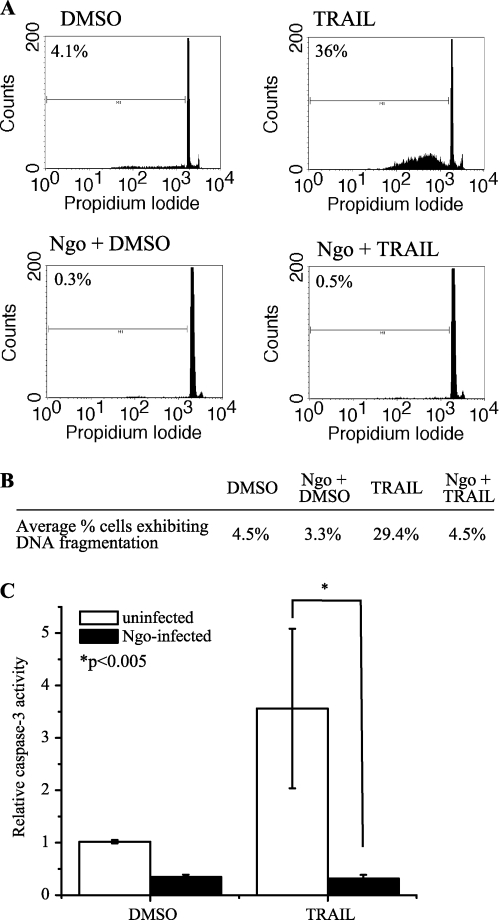
The impact of N. gonorrhoeae infection on apoptotic signaling induced by extrinsic proapoptotic stimuli was also examined. HL-60 cells were treated with recombinant human tumor necrosis factor alpha (TNF-α), Fas ligand, or TNF-related apoptosis-inducing ligand (TRAIL) to induce extrinsic apoptotic signaling, and only TRAIL was able to induce significant levels of apoptosis as measured by genomic DNA cleavage and caspase-3 activity (data not shown). To evaluate whether N. gonorrhoeae infection is able to inhibit extrinsic apoptotic signaling induced by TRAIL, differentiated HL-60 cells were infected with N. gonorrhoeae for 2 to 3 h and then were treated with 100 ng/ml TRAIL for a further 18 to 20 h. Genomic DNA fragmentation following infection and apoptosis induction was then measured by staining ethanol-fixed cells with propidium iodide to evaluate the proportion of cells exhibiting hypodiploid DNA content (Fig. 6A). TRAIL treatment resulted in induction of DNA cleavage in 36% of the cells, and N. gonorrhoeae preinfection decreased the extent of DNA cleavage such that only 0.5% of N. gonorrhoeae-infected, TRAIL-treated cells exhibited any degree of DNA fragmentation. These results were consistent over several independent experiments, and N. gonorrhoeae induced an average ∼85% reduction in the percentage of TRAIL-treated, infected cells exhibiting hypodiploid DNA content versus uninfected cells (Fig. 6B).
Fig. 6.
N. gonorrhoeae inhibits extrinsic apoptosis induced by TRAIL. (A) N. gonorrhoeae inhibits DNA fragmentation in HL-60 induced by TRAIL. Differentiated HL-60 cells were infected with piliated, Opa-expressing FA1090 at an MOI of 9 and then treated with 100 ng/ml TRAIL. DNA fragmentation was evaluated by flow cytometry, and the percentage of cells exhibiting hypodiploid DNA under each condition is indicated. (B) Average percentage of cells exhibiting DNA fragmentation after infection and/or treatment with 100 ng/ml TRAIL. The data are averages for 3 independent experiments. (C) N. gonorrhoeae infection inhibits TRAIL-induced caspase-3 activity in HL-60 cell lysates. HL-60 cells were infected with piliated strain FA1090 at an average MOI of 15 and then treated with 100 ng/ml TRAIL. Caspase-3 activity was measured using Ac-DEVD-AMC. Data are presented as the relative caspase-3 activity compared to that of uninfected, untreated controls and are representative of 6 independent biological replicates.
We also examined the ability of N. gonorrhoeae to inhibit caspase activation induced by TRAIL signaling. HL-60 cells were preinfected with N. gonorrhoeae for 2 h and then treated with 100 ng/ml TRAIL for a further 4 h, after which cell lysates were harvested and caspase-3 activity was assessed using Ac-DEVD-AMC (Fig. 6C). Treatment of HL-60 cells with 100 ng/ml TRAIL for 4 h increased caspase-3 activity an average of 3.5-fold over that for control DMSO-treated cells, and preinfection of cells with N. gonorrhoeae decreased TRAIL-induced caspase-3 activity by an average of >90%. These results suggest that N. gonorrhoeae infection inhibits extrinsic apoptotic signaling induced by TRAIL, in addition to intrinsic apoptosis induced by STS.
N. gonorrhoeae infection inhibits apoptosis in primary PMNs.
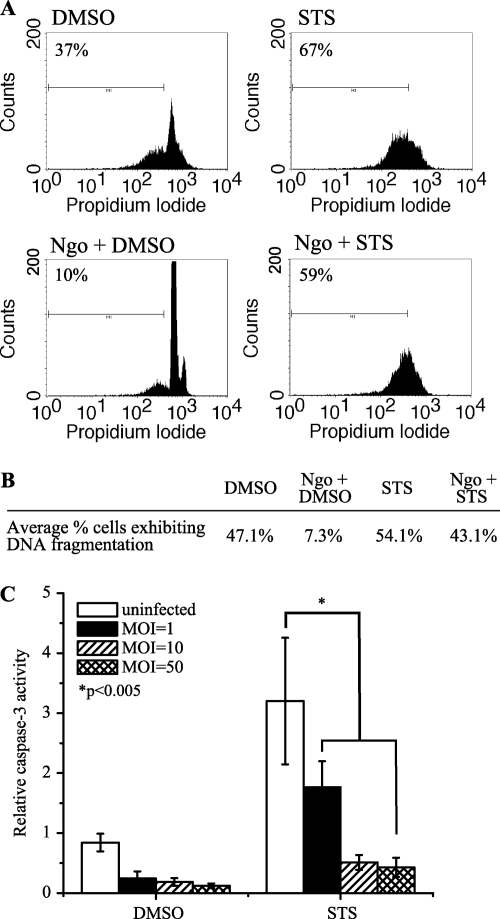
To verify that the effects of N. gonorrhoeae infection on apoptosis observed in differentiated HL-60 cells were also true of primary cells, PMNs isolated from the venous blood of healthy human donors were infected with piliated N. gonorrhoeae strain FA1090. Since HL-60 cells do not express the CEACAM receptors for bacterial Opa proteins, a piliated, Opa− FA1090 strain was utilized to minimize any potentially confounding effects of Opa expression. PMNs were infected with bacteria at increasing MOIs for 3 h and then treated with STS (or DMSO as a control) for a further 18 to 20 h, after which the cells were fixed with 70% ethanol and genomic DNA was stained with propidium iodide to examine cellular DNA content (Fig. 7A and B). Whereas DNA fragmentation that occurred as a result of spontaneous PMN apoptosis was suppressed by bacterial infection at MOIs ranging from 1 to 50 (at least a 70% reduction at all MOIs), DNA fragmentation induced by STS was not significantly affected by N. gonorrhoeae infection at MOIs up to 50.
Fig. 7.
N. gonorrhoeae infection inhibits spontaneous apoptosis in primary PMNs but does not fully inhibit STS-induced apoptosis. (A) N. gonorrhoeae infection inhibits DNA fragmentation in PMNs undergoing spontaneous apoptosis. Primary PMNs isolated from healthy human volunteers were infected at an MOI of 44 and were treated with 1 μM STS overnight. DNA fragmentation was evaluated by flow cytometry. (B) Average percentage of cells exhibiting DNA fragmentation after infection at an MOI of ∼50 and/or treatment with STS/DMSO. The data are representative of 6 independent experiments. (C) N. gonorrhoeae infection inhibits caspase-3 activity in PMNs undergoing both spontaneous and STS-induced apoptosis. Primary PMNs isolated from healthy human volunteers were infected at increasing MOIs and then treated with STS. Caspase-3 activity in cell lysates was measured using Ac-DEVD-AMC. Data are presented as the relative caspase-3 activity compared to that of uninfected, untreated controls and are representative of at least 8 independent biological replicates.
In addition to examining DNA fragmentation, caspase-3 activity in infected PMNs was also assessed at 3 h post-STS treatment. Cell lysates from PMNs infected with N. gonorrhoeae at increasing MOIs prior to STS treatment were harvested, and caspase-3 activity was determined using Ac-DEVD-AMC (Fig. 7C). Infection with N. gonorrhoeae alone resulted in a decrease in basal caspase-3 activity; at an MOI of ∼50, the caspase-3 activity in infected cells was decreased by ∼85% relative to that in uninfected controls. Treating PMNs with STS alone resulted in an ∼3-fold increase in caspase-3 activity relative to that of untreated cells, which was then diminished by the presence of N. gonorrhoeae in a dose-dependent manner, such that caspase-3 activity was decreased by ∼86% in cells that were preinfected at an average MOI of ∼50 before being treated with STS. These results suggest that N. gonorrhoeae inhibits caspase-3 activity that occurs as a result of both spontaneous and STS-induced apoptosis in primary PMNs but cannot inhibit all apoptotic signaling that occurs as a result of STS treatment.
N. gonorrhoeae induces NF-κB activation in infected cells and results in proinflammatory cytokine production by PMNs and HL-60 cells.
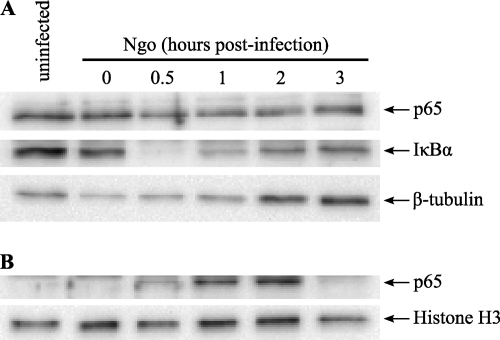
The NF-κB family of transcription factors plays a critical role in modulating cellular proinflammatory responses and also regulates genes involved in cell proliferation, adhesion, and apoptosis. N. gonorrhoeae has been shown to activate NF-κB signaling in epithelial cells (27, 55, 75), which is involved in the release of proinflammatory cytokines from infected epithelia (75), as well as expression of antiapoptotic genes (27). To examine whether N. gonorrhoeae also activates NF-κB signaling in infected neutrophils, differentiated HL-60 cells were infected with N. gonorrhoeae and the extent of IκBα degradation and NF-κB p65 translocation into the nucleus was assessed by Western blotting of nuclear and cytoplasmic extracts from infected cells (Fig. 8). IκBα degradation was observed as early as 0.5 h postinfection, which coincided temporally with a decrease in the levels of NF-κB p65 in the cytoplasm and the appearance of NF-κB p65 in the nucleus.
Fig. 8.
N. gonorrhoeae infection induces NF-κB nuclear translocation. (A) N. gonorrhoeae induces IκBα degradation and decreased levels of NF-κB p65 in the cytoplasm. Differentiated HL-60 cells were infected with N. gonorrhoeae at an MOI of ∼25, and cell lysates were harvested at various time postinfection and subjected to cellular fractionation to separate nuclear and cytoplasmic fractions. The cytoplasmic fractions were subjected to Western blotting using antibodies directed against p65, IκBα, and β-tubulin as a cytoplasmic marker. (B) N. gonorrhoeae induces nuclear translocation of NF-κB p65. Nuclear extracts from infected HL-60 cells were subjected to Western blotting for p65 and histone H3 as a nuclear marker. The results are representative of 3 independent experiments.
N. gonorrhoeae has been associated with the production of proinflammatory cytokines and chemokines, both during in vivo infection and upon infection of immortalized cervical, vaginal, and urethral epithelial cells (24, 33, 60, 75). PMNs treated with Neisseria meningitidis outer membrane vesicles also secrete the cytokines TNF-α and IL-1β and the chemokines IL-8, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β (46). To determine whether infected neutrophils could be a significant source of cytokines and chemokines during N. gonorrhoeae infection, primary PMNs and differentiated HL-60 cells were either left untreated, or infected with N. gonorrhoeae at an MOI of ∼100. The cells were harvested 4 h post-infection/treatment, cell-free supernatants were collected, and the concentrations of various cytokines and chemokines in the supernatants were assessed by ELISA (Table 1). Neither PMNs nor HL-60 cells produced significant amounts of IL-17 or gamma interferon (IFN-γ) in response to N. gonorrhoeae infection. However, N. gonorrhoeae infection induced both PMNs and HL-60 cells to secrete IL-1β, IL-6, IL-8, and MIP-1α, although the HL-60 cells were induced to secrete higher levels of each cytokine/chemokine than were PMNs. Stimulation of both PMNs and HL-60 cells with PMA did not induce secretion of any cytokine tested other than IL-8 (data not shown), and the levels of IL-8 secretion induced by PMA were significantly less than those induced by N. gonorrhoeae infection (2.6 ± 1.6 and 13.8 ± 10.7 ng/ml by PMNs and HL-60 cells, respectively). Additionally, infection of PMNs and HL-60 cells with opsonized S. aureus at an equivalent MOI did not induce significant cytokine production (data not shown), with the exception of IL-1β secretion from infected HL-60 cells (178 ± 131 pg/ml). Thus, HL-60 cells exhibit a cytokine secretion profile similar to that of primary PMNs in response to N. gonorrhoeae infection, and this response is distinct from that induced by other bacteria (i.e., S. aureus).
Table 1.
Cytokine secretion by PMNs and HL-60 cells in response to N. gonorrhoeae infection
| Cytokine (units) | Amt secreteda |
|||
|---|---|---|---|---|
| Uninfected PMNs | PMNs + N. gonorrhoeae | Uninfected HL-60 cells | HL-60 cells + N. gonorrhoeae | |
| IL-1β (pg/ml) | <10 | 17.7 ± 6.9 | <10 | 318.5 ± 59.7 |
| IL-6 (pg/ml) | <10 | 72.2 ± 8.5 | <10 | 272.7 ± 34.8 to 2340 ± 310 |
| IL-17 (pg/ml) | <10 | <10 | <10 | <10 |
| IFN-γ (pg/ml) | <10 | <10 | <10 | <10 |
| IL-8 (ng/ml) | 0.02 ± 0.01 | 7.9 ± 2.7 | 1.9 ± 0.9 | 85.5 ± 20.7 |
| MIP-1α (ng/ml) | <0.01 | 0.58 ± 0.11 | 0.015 ± 0.006 | 4.4 ± 0.25 to 12.6 ± 0.57 |
Cytokine levels are expressed as means ± standard deviations. The data are averages for 6 biological replicates, except for IL-6 and MIP-1α secretion from infected HL-60 cells, which is presented as two sets of triplicates to illustrate a variation in the magnitude of cytokine release in different experiments.
DISCUSSION
During infection of the human urogenital tract, N. gonorrhoeae interacts intimately and extensively with a variety of cell types, including the PMNs which are recruited in large numbers in response to bacterial infection. The presence of ingested, live bacteria in PMNs from gonococcal urethral exudates and the fact that N. gonorrhoeae is able to survive and replicate intracellularly after being ingested by PMNs (14, 20, 22, 35, 56, 67, 72) suggest that the PMN phagosome may be an important replicative niche for N. gonorrhoeae in the urogenital tract. PMN apoptosis is triggered by receptor-mediated phagocytosis (43), and other organisms that use PMNs as a replicative compartment have evolved mechanisms to inhibit apoptosis induced by bacterial uptake (29, 71); thus, whether N. gonorrhoeae actively influences the survival of these short-lived cells during infection has important implications for our understanding of N. gonorrhoeae pathogenesis. In this study, we demonstrate that N. gonorrhoeae inhibits both spontaneous PMN apoptosis and apoptosis induced by intrinsic and extrinsic stimuli. We also show that PMNs can be a significant source of proinflammatory cytokines and chemokines during infection, in addition to the epithelial cells which are thought to be responsible for initial PMN recruitment.
Numerous studies of epithelial cells and lymphocytes have shown both pro- and antiapoptotic effects of infection with both N. gonorrhoeae (6, 27, 36, 39, 40, 50, 51) and the closely related N. meningitidis (17, 48). However, PMN apoptosis during N. gonorrhoeae infection has not been examined as extensively (68), and it has not been explored at all with N. meningitidis. To facilitate our studies, we developed and characterized a cell culture model for N. gonorrhoeae-PMN interactions using HL-60 myeloid leukemia cells. While HL-60 cells differentiated down the granulocytic pathway do not appear to exhibit bactericidal activity against N. gonorrhoeae (Fig. 1), possibly due to the fact that they lack specific and gelatinase granules (37), we considered the potential utility of HL-60 cells for studying other aspects of the N. gonorrhoeae-PMN interaction. We have shown that N. gonorrhoeae bacteria are internalized by differentiated HL-60 cells, are able to replicate in association with cells, and actively inhibit ROS production in response to multiple stimuli, thus demonstrating that several other important aspects of the N. gonorrhoeae-PMN interaction can be recapitulated by these cells (16, 67).
We failed to detect any proapoptotic effects upon infection of either primary PMNs or differentiated HL-60 cells with N. gonorrhoeae at multiplicities of infection up to 100 and for infection periods of up to 20 h. In addition to a lack of apoptosis induction, spontaneous PMN apoptosis was delayed as a result of N. gonorrhoeae infection, consistent with previous studies of primary PMNs (68). We observed increased apoptosis inhibition with increasing MOIs in primary cells, in contrast to Simons et al., who reported antiapoptotic effects only at early times postinfection and at low MOIs (68). The reasons for the discrepancy are not clear but may be related to the use of different N. gonorrhoeae strains or differences in bacterial piliation and/or expression of opacity proteins. Our results also contrast with data demonstrating a proapoptotic effect of N. gonorrhoeae infection on epithelial cells (39, 40, 50, 51), but those discrepancies may reflect the vast differences between PMNs and epithelial cells.
In addition to a lack of apoptosis induction and inhibition of spontaneous PMN apoptosis, N. gonorrhoeae was able to repress STS-induced apoptotic signaling in HL-60 cells and STS-induced caspase-dependent apoptosis in primary PMNs. Again, our results are consistent with those in a subset of previous reports describing an antiapoptotic effect of pathogenic Neisseria infection in epithelial cells (6, 27, 36, 48). Inhibition of STS-induced apoptosis required live bacteria, which was consistent with results observed in epithelial cells (27) but inconsistent with observations of primary PMNs, where nonviable N. gonorrhoeae retained the ability to inhibit caspase activity during spontaneous apoptosis (68). It is possible that the inhibition of spontaneous versus induced apoptosis occurs via distinct mechanisms, one of which requires live bacteria and one of which does not. The fact that N. gonorrhoeae is able to inhibit DNA fragmentation in primary PMNs during spontaneous apoptosis but not DNA fragmentation induced by STS suggests that the two processes may occur via different pathways and that N. gonorrhoeae can inhibit one but not the other in primary cells. STS is a protein kinase C inhibitor that can induce both caspase-dependent and caspase-independent apoptosis (4), and thus the DNA fragmentation that occurs in N. gonorrhoeae-infected, STS-treated primary PMNs despite caspase-3 inhibition may be due to a caspase-independent mechanism of apoptotic signaling.
During infection, PMNs may encounter extrinsic apoptotic ligands, such as TNF-α and TRAIL, in the urogenital tract; the presence of TNF-α has been observed during experimental urethral infection and in vaginal secretions of infected BALB/c mice, and N. gonorrhoeae induces TNF-α secretion from infected epithelial cells in vitro (11, 57, 60, 75). PMNs themselves are capable of expressing and secreting both TNF-α and TRAIL (18, 44), and thus it is possible that N. gonorrhoeae infection of PMNs could additionally elicit TNF-α and/or TRAIL expression, potentially driving an autocrine feedback loop where PMN-derived ligands result in accelerated apoptosis. Since TNF-α can have biphasic effects on neutrophil survival (53), TRAIL was utilized to examine the effects of N. gonorrhoeae on extrinsic apoptotic signaling. N. gonorrhoeae has been shown to inhibit TNF-α-induced apoptosis in epithelial cells at high MOIs (49), but the effects of N. gonorrhoeae on extrinsic apoptosis in PMNs had not been previously determined. We have shown that N. gonorrhoeae was able to inhibit signaling downstream of TRAIL in addition to the inhibition of intrinsic apoptosis induced by STS. Inhibition of TRAIL-induced signaling was assessed by examining caspase-3 activation and DNA fragmentation, which are apoptotic markers common to both intrinsic and extrinsic apoptosis pathways, and thus it remains to be determined whether N. gonorrhoeae-mediated inhibition of TRAIL-induced apoptosis occurs at a signaling step common to both intrinsic and extrinsic apoptosis or whether N. gonorrhoeae uses distinct mechanisms to inhibit multiple pathways.
Inhibition of spontaneous apoptosis, in addition to apoptosis induced by intrinsic stresses and extrinsic apoptotic ligands that PMNs may encounter during N. gonorrhoeae infection, likely serves to facilitate the survival and expansion of bacteria in association with and within these cells. We found that N. gonorrhoeae infection inhibits the release of cytochrome c from the mitochondrial intermembrane space, suggesting that N. gonorrhoeae inhibits apoptosis in PMNs by preventing mitochondrial depolarization. This effect may be mediated by the neisserial major outer membrane porin, which has been demonstrated to localize to mitochondria in infected cells and to mediate some of the pro- and antiapoptotic phenotypes effected by bacterial infection when cells are treated with purified protein (45, 47, 48, 50-52). The involvement of N. gonorrhoeae porin in modulating PMN survival during infection is under investigation.
Pro- and antiapoptotic members of the cellular Bcl-2 family of proteins play important roles in regulating mitochondrial membrane permeabilization, cytochrome c release into the cytosol, and caspase activation. Diverse Bcl-2 family proteins have been found to play roles in the proapoptotic (39, 40) and antiapoptotic (5, 6, 27, 36) phenotypes observed upon N. gonorrhoeae infection in epithelial cells, but the extent to which individual Bcl-2 proteins act to mediate PMN survival has not been determined. A major regulator of neutrophil survival is thought to be the antiapoptotic Mcl-1 protein (1), whose role in inhibiting apoptosis in N. gonorrhoeae-infected PMNs remains to be examined. We have also shown that N. gonorrhoeae induces NF-κB p65 nuclear translocation in infected neutrophils, which is a major regulator of antiapoptotic gene expression (58) and which may be involved in both upregulation of antiapoptotic factors in infected PMNs and the induction of cytokine secretion.
Since PMNs themselves can be a significant source of several cytokines and chemokines (63), we also examined the secretion of several cytokines/chemokines from infected primary PMNs and HL-60 cells. Elevated levels of proinflammatory cytokines in response to N. gonorrhoeae have been observed in the genital tract during experimental urethral infection, in uncomplicated natural infections, and in the vaginal secretions of infected mice (34, 57, 60). Recruitment of PMNs to the site of infection is thought to be mediated by the release of proinflammatory mediators by infected epithelia (11, 24, 33, 75), but whether PMNs exposed to N. gonorrhoeae are in turn stimulated to secrete additional cytokines has not been examined. IL-1β, IL-6, IL-8, and MIP-1α were secreted from both cell types in response to N. gonorrhoeae infection, and in all cases the HL-60 cells were induced to produce higher cytokine levels than primary cells. The induction of proinflammatory cytokines from PMNs in addition to epithelial cells suggests the presence of a positive feedback mechanism to recruit further numbers of PMNs to the site of infection. MIP-1α is a potent chemoattractant for monocytes, macrophages, and NK cells (62), and thus the secretion of this chemokine could play a role in the recruitment of monocytes to the infected genital tract. PMNs are capable of producing IL-17 (46a), but the lack of IL-17 secretion induced by N. gonorrhoeae suggests that PMNs are likely not a significant source of IL-17 in in vivo infection and that IL-17 production is likely mediated by T lymphocyotes (23). IL-1β and IL-8 have been shown to exert a prosurvival effect on PMNs (1); thus, in addition to recruitment of further PMNs to the site of infection, these cytokines may additionally exert autocrine effects on infected cells to prolong their life span.
PMNs are a critical component of the immune response against N. gonorrhoeae infection and may serve as a significant replicative niche for a subset of N. gonorrhoeae bacteria which are able to resist and survive their antimicrobial activities. We have developed a viable model for N. gonorrhoeae-PMN interactions using HL-60 cells and have demonstrated that N. gonorrhoeae inhibits spontaneous and induced apoptosis in these cells and primary PMNs. The active inhibition of apoptosis, in addition to the induction of cytokines that promote PMN recruitment and survival, may play an important role in facilitating N. gonorrhoeae replication and contribute to gonorrhea pathogenesis and transmission to new hosts.
ACKNOWLEDGEMENTS
Flow cytometry was performed at the Northwestern University Interdepartmental ImmunoBiology Flow Cytometry Core Facility. Imaging work was performed at the Northwestern University Cell Imaging Facility, generously supported by NCI CCSG P30 CA060553, awarded to the Robert H Lurie Comprehensive Cancer Center.
This work was supported by grants R01 AI044239, R01AI055977, and R37 AI033493 to H.S.S. A.C. was partially supported by American Heart Association grant 10POST2550017.
Footnotes
Published ahead of print on 15 August 2011.
REFERENCES
- 1. Akgul C., Moulding D. A., Edwards S. W. 2001. Molecular control of neutrophil apoptosis. FEBS Lett. 487:318–322 [DOI] [PubMed] [Google Scholar]
- 2. Apicella M. A., et al. 1996. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J. Infect. Dis. 173:636–646 [DOI] [PubMed] [Google Scholar]
- 3. Bauer F. J., Rudel T., Stein M., Meyer T. F. 1999. Mutagenesis of the Neisseria gonorrhoeae porin reduces invasion in epithelial cells and enhances phagocyte responsiveness. Mol. Microbiol. 31:903–913 [DOI] [PubMed] [Google Scholar]
- 4. Belmokhtar C. A., Hillion J., Segal-Bendirdjian E. 2001. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 20:3354–3362 [DOI] [PubMed] [Google Scholar]
- 5. Binnicker M. J., Williams R. D., Apicella M. A. 2004. Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infect. Immun. 72:6408–6417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Binnicker M. J., Williams R. D., Apicella M. A. 2003. Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cell Microbiol. 5:549–560 [DOI] [PubMed] [Google Scholar]
- 7. Borregaard N., Cowland J. B. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503–3521 [PubMed] [Google Scholar]
- 8. Buck P., Rest R. F. 1981. Effects of human neutrophil granule extracts on macromolecular synthesis in Neisseria gonorrhoeae. Infect. Immun. 33:426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casey S. G., Shafer W. M., Spitznagel J. K. 1986. Neisseria gonorrhoeae survive intraleukocytic oxygen-independent antimicrobial capacities of anaerobic and aerobic granulocytes in the presence of pyocin lethal for extracellular gonococci. Infect. Immun. 52:384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casey S. G., Veale D. R., Smith H. 1979. Demonstration of intracellular growth of gonococci in human phagocytes using spectinomycin to kill extracellular organisms. J. Gen. Microbiol. 113:395–398 [DOI] [PubMed] [Google Scholar]
- 11. Christodoulides M., et al. 2000. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Mol. Microbiol. 35(1):32–43 [DOI] [PubMed] [Google Scholar]
- 12. Collins S. J., Gallo R. C., Gallagher R. E. 1977. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature 270:347–349 [DOI] [PubMed] [Google Scholar]
- 13. Reference deleted.
- 14. Criss A. K., Katz B. Z., Seifert H. S. 2009. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol. 11:1074–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Criss A. K., Seifert H. S. 2006. Gonococci exit apically and basally from polarized epithelial cells and exhibit dynamic changes in type IV pili. Cell Microbiol. 8:1430–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Criss A. K., Seifert H. S. 2008. Neisseria gonorrhoeae suppresses the oxidative burst of human polymorphonuclear leukocytes. Cell Microbiol. 10:2257–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deghmane A. E., et al. 2009. Differential modulation of TNF-alpha-induced apoptosis by Neisseria meningitidis. PLoS Pathog. 5:e1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubravec D. B., Spriggs D. R., Mannick J. A., Rodrick M. L. 1990. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc. Natl. Acad. Sci. U. S. A. 87:6758–6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elkins C., Rest R. F. 1990. Monoclonal antibodies to outer membrane protein PII block interactions of Neisseria gonorrhoeae with human neutrophils. Infect. Immun. 58:1078–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evans B. A. 1977. Ultrastructural study of cervical gonorrhea. J. Infect. Dis. 136:248–255 [DOI] [PubMed] [Google Scholar]
- 21. Farrell C. F., Rest R. F. 1990. Up-regulation of human neutrophil receptors for Neisseria gonorrhoeae expressing PII outer membrane proteins. Infect. Immun. 58:2777–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farzadegan H., Roth I. L. 1975. Scanning electron microscopy and freeze-etching of gonorrhoeal urethral exudate. Br. J. Vener. Dis. 51:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feinen B., Jerse A. E., Gaffen S. L., Russell M. W. 2010. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 3:312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fichorova R. N., Desai P. J., Gibson F. C., III, Genco C. A. 2001. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect. Immun. 69:5840–5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fischer S. H., Rest R. F. 1988. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect. Immun. 56:1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fleck R. A., Romero-Steiner S., Nahm M. H. 2005. Use of HL-60 cell line to measure opsonic capacity of pneumococcal antibodies. Clin. Diagn. Lab. Immunol. 12:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Follows S. A., Murlidharan J., Massari P., Wetzler L. M., Genco C. A. 2009. Neisseria gonorrhoeae infection protects human endocervical epithelial from apoptosis via expression of host antiapoptotic proteins. Infect. Immun. 77:3602–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fulcher N. B. 2004. Ph. D. dissertation. The role of Neisseria gonorrhoeae opacity proteins in host cell interactions and pathogenesis. University of North Carolina, Chapel Hill, NC [Google Scholar]
- 29. Ge Y., Rikihisa Y. 2006. Anaplasma phagocytophilum delays spontaneous human neutrophil apoptosis by modulation of multiple apoptotic pathways. Cell Microbiol. 8:1406–1416 [DOI] [PubMed] [Google Scholar]
- 30. Gray-Owen S. D., Lorenzen D. R., Haude A., Meyer T. F., Dehio C. 1997. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol. Microbiol. 26:971–980 [DOI] [PubMed] [Google Scholar]
- 31. Hampton M. B., Kettle A. J., Winterbourn C. C. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007–3017 [PubMed] [Google Scholar]
- 32. Harris P., Ralph P. 1985. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J. Leukoc. Biol. 37:407–422 [DOI] [PubMed] [Google Scholar]
- 33. Harvey H. A., Post D. M., Apicella M. A. 2002. Immortalization of human urethral epithelial cells: a model for the study of the pathogenesis of and the inflammatory cytokine response to Neisseria gonorrhoeae infection. Infect. Immun. 70:5808–5815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hedges S. R., Sibley D. A., Mayo M. S., Hook E. W., III, Russell M. W. 1998. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 178:742–751 [DOI] [PubMed] [Google Scholar]
- 35. Hook E. W., Holmes K. K. 1985. Gonococcal infections. Ann. Intern. Med. 102:229–243 [DOI] [PubMed] [Google Scholar]
- 36. Howie H. L., Shiflett S. L., So M. 2008. Extracellular signal-regulated kinase activation by Neisseria gonorrhoeae downregulates epithelial cell proapoptotic proteins Bad and Bim. Infect. Immun. 76:2715–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnston J. J., et al. 1992. Lactoferrin gene promoter: structural integrity and nonexpression in HL60 cells. Blood 79:2998–3006 [PubMed] [Google Scholar]
- 38. Kellogg D. S., Jr., Peacock W. L., Deacon W. E., Brown L., Pirkle C. I. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kepp O., et al. 2009. Bim and Bmf synergize to induce apoptosis in Neisseria gonorrhoeae infection. PLoS Pathog. 5:e1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kepp O., Rajalingam K., Kimmig S., Rudel T. 2007. Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. EMBO J. 26:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. King G. J., Swanson J. 1978. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect. Immun. 21:575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kobayashi S. D., et al. 2003. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. U. S. A. 100:10948–10953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kobayashi S. D., Voyich J. M., Buhl C. L., Stahl R. M., DeLeo F. R. 2002. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc. Natl. Acad. Sci. U. S. A. 99:6901–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koga Y., Matsuzaki A., Suminoe A., Hattori H., Hara T. 2004. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res. 64:1037–1043 [DOI] [PubMed] [Google Scholar]
- 45. Kozjak-Pavlovic V., Ross K., Rudel T. 2008. Import of bacterial pathogenicity factors into mitochondria. Curr. Opin. Microbiol. 11:9–14 [DOI] [PubMed] [Google Scholar]
- 46. Lapinet J. A., Scapini P., Calzetti F., Perez O., Cassatella M. A. 2000. Gene expression and production of tumor necrosis factor alpha, interleukin-1beta (IL-1beta), IL-8, macrophage inflammatory protein 1alpha (MIP-1alpha), MIP-1beta, and gamma interferon-inducible protein 10 by human neutrophils stimulated with group B meningococcal outer membrane vesicles. Infect. Immun. 68:6917–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a. Li L., et al. 2010. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 120:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Massari P., Ho Y., Wetzler L. M. 2000. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl. Acad. Sci. U. S. A. 97:9070–9075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Massari P., King C. A., Ho A. Y., Wetzler L. M. 2003. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell Microbiol. 5:99–109 [DOI] [PubMed] [Google Scholar]
- 49. Morales P., et al. 2006. Infection of human fallopian tube epithelial cells with Neisseria gonorrhoeae protects cells from tumor necrosis factor alpha-induced apoptosis. Infect. Immun. 74:3643–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Muller A., et al. 2000. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 19:5332–5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muller A., et al. 1999. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 18:339–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Muller A., et al. 2002. VDAC and the bacterial porin PorB of Neisseria gonorrhoeae share mitochondrial import pathways. EMBO J. 21:1916–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murray J., et al. 1997. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood 90:2772–2783 [PubMed] [Google Scholar]
- 54. Naids F. L., Rest R. F. 1991. Stimulation of human neutrophil oxidative metabolism by nonopsonized Neisseria gonorrhoeae. Infect. Immun. 59:4383–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Naumann M., Wessler S., Bartsch C., Wieland B., Meyer T. F. 1997. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor kappaB and activator protein 1 and the induction of inflammatory cytokines. J. Exp. Med. 186:247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ovcinnikov N. M., Delektorskij V. V. 1971. Electron microscope studies of gonococci in the urethral secretions of patients with gonorrhoea. Brit. J. Vener. Dis. 47:419–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Packiam M., Veit S. J., Anderson D. J., Ingalls R. R., Jerse A. E. 2010. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect. Immun. 78:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pahl H. L. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853–6866 [DOI] [PubMed] [Google Scholar]
- 59. Pantelic M., et al. 2004. Retinoic acid treated HL60 cells express CEACAM1 (CD66a) and phagocytose Neisseria gonorrhoeae. FEMS Immunol. Med. Microbiol. 42:261–266 [DOI] [PubMed] [Google Scholar]
- 60. Ramsey K. H., et al. 1995. Inflammatory cytokines produced in response to experimental human gonorrhea. J. Infect. Dis. 172:186–191 [DOI] [PubMed] [Google Scholar]
- 61. Rest R. F. 1979. Killing of Neisseria gonorrhoeae by human polymorphonuclear neutrophil granule extracts. Infect. Immun. 25:574–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rollins B. J. 1997. Chemokines. Blood 90:909–928 [PubMed] [Google Scholar]
- 63. Scapini P., et al. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177:195–203 [DOI] [PubMed] [Google Scholar]
- 64. Seib K. L., et al. 2005. Investigation of oxidative stress defenses of Neisseria gonorrhoeae by using a human polymorphonuclear leukocyte survival assay. Infect. Immun. 73:5269–5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seifert H. S., Wright C. J., Jerse A. E., Cohen M. S., Cannon J. G. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Invest. 93:2744–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shafer W. M., Onunka V. C., Martin L. E. 1986. Antigonococcal activity of human neutrophil cathepsin G. Infect. Immun. 54:184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Simons M. P., Nauseef W. M., Apicella M. A. 2005. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect. Immun. 73:1971–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Simons M. P., Nauseef W. M., Griffith T. S., Apicella M. A. 2006. Neisseria gonorrhoeae delays the onset of apoptosis in polymorphonuclear leukocytes. Cell Microbiol. 8:1780–1790 [DOI] [PubMed] [Google Scholar]
- 69. Stohl E. A., Criss A. K., Seifert H. S. 2005. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol. Microbiol. 58:520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Talanian R. V., et al. 1997. Substrate specificities of caspase family proteases. J. Biol. Chem. 272:9677–9682 [DOI] [PubMed] [Google Scholar]
- 71. van Zandbergen G., et al. 2004. Chlamydia pneumoniae multiply in neutrophil granulocytes and delay their spontaneous apoptosis. J. Immunol. 172:1768–1776 [DOI] [PubMed] [Google Scholar]
- 72. Veale D. R., Goldner M., Penn C. W., Ward J., Smith H. 1979. The intracellular survival and growth of gonococci in human phagocytes. J. Gen. Microbiol. 113:383–393 [DOI] [PubMed] [Google Scholar]
- 73. Virji M., Heckels J. E. 1986. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J. Gen. Microbiol. 132:503–512 [DOI] [PubMed] [Google Scholar]
- 74. Waterhouse N. J., Steel R., Kluck R., Trapani J. A. 2004. Assaying cytochrome C translocation during apoptosis. Methods Mol. Biol. 284:307–313 [DOI] [PubMed] [Google Scholar]
- 75. Wessler S., Muenzner P., Meyer T. F., Naumann M. 2005. The anti-inflammatory compound curcumin inhibits Neisseria gonorrhoeae-induced NF-kappaB signaling, release of pro-inflammatory cytokines/chemokines and attenuates adhesion in late infection. Biol. Chem. 386:481–490 [DOI] [PubMed] [Google Scholar]