Abstract
Yersinia pseudotuberculosis is a Gram-negative bacterial pathogen. Virulence in Y. pseudotuberculosis requires the plasmid-encoded Ysc type III secretion system (T3SS), which functions to translocate a set of effectors called Yops into infected host cells. The effectors function to antagonize phagocytosis (e.g., YopH) or to induce apoptosis (YopJ) in macrophages infected with Y. pseudotuberculosis. Additionally, when antiphagocytosis is incomplete and Y. pseudotuberculosis is internalized by macrophages, the bacterium can survive in phagosomes. Previous studies have shown that delivery of effectors into host cells occurs efficiently when Yersinia is extracellular. However, it is not clear whether the T3SS can be utilized by intracellular Y. pseudotuberculosis to translocate Yops. This possibility was investigated here using Y. pseudotuberculosis strains that express YopJ or YopH under the control of an inducible promoter. Bone marrow-derived murine macrophages were infected with these strains under conditions that prevented the survival of extracellular bacteria. Effector translocation was detected by measuring apoptosis or the activities of Yop-β-lactamase fusion proteins. Results showed that macrophages underwent apoptosis when YopJ expression was induced prior to phagocytosis, confirming that delivery of this effector prior to or during uptake is sufficient to cause cell death. However, macrophages also underwent apoptosis when YopJ was ectopically expressed after phagocytosis; furthermore, expression of the translocator YopB from intracellular bacteria also resulted in increased cell death. Analysis by microscopy showed that translocation of ectopically expressed YopH- or YopJ-β-lactamase fusions could be correlated with the presence of viable Y. pseudotuberculosis in macrophages. Collectively, our results suggest that the Ysc T3SS of Y. pseudotuberculosis can function within macrophage phagosomes to translocate Yops into the host cytosol.
INTRODUCTION
The three species of Yersinia that are pathogenic for humans are the genetically related Yersinia pseudotuberculosis and Y. pestis and the more distantly related Y. enterocolitica (1, 50). Y. pestis is the causative agent of plague. Similar to Y. enterocolitica, Y. pseudotuberculosis is an enteropathogen that commonly causes terminal ileitis and mesenteric adenitis in humans. In rodents such as guinea pigs and laboratory strains of mice, Y. pseudotuberculosis disseminates from the intestinal tract to organs such as spleen and liver and causes systemic plague-like disease. The virulence of these pathogenic Yersinia species depends on a plasmid-encoded type III secretion system (T3SS) (11). T3SSs are also found in a number of other Gram-negative pathogens, including both intracellular and extracellular pathogens, where they play key roles in promoting bacterial pathogenesis (16, 19).
The T3SS of Yersinia is comprised of a Ysc injectisome, a tip complex formed by the LcrV protein, and translocators YopB and YopD, which are implicated in the formation of a translocon in the host cell plasma membrane (16, 28, 47). Yersinia utilizes the T3SS to deliver a set of seven effector proteins called Yops into eukaryotic cells upon bacterium-host cell interaction (8, 11). The seven Yop effectors are YopE, YopH, YopT, YopM, YopK, YopJ, and YpkA; the last two are also referred to as YopP and YopO in Y. enterocolitica (11). After translocation into the cytosol of eukaryotic cells, these Yops modulate eukaryotic signaling pathways to counteract innate and adaptive immune responses to the pathogen (11, 47).
YopE, YopH, YopT, and YpkA function together to disturb the actin cytoskeleton, resulting in inhibition of phagocytosis. YopH is a tyrosine phosphatase that dephosphorylates multiple focal adhesion proteins (4, 7, 18, 35). YopE, YopT, and YpkA disrupt either the activity or the regulation of the Rho family of small GTPases (47, 49).
YopJ has acetyltransferase activity (25, 29). YopJ acetylates Ser and Thr residues in mitogen-activated protein (MAP) kinase kinases (MEKs) and the inhibitor κB kinase β (IKKβ) to inactivate these key signaling molecules, resulting in the suppression of multiple MAP kinase and nuclear factor κB (NF-κB) pathways (25, 29). YopJ inhibits the production of tumor necrosis factor alpha (TNF-α) in macrophages infected with Yersinia (32). In addition, activation of MAP kinases and NF-κB is important to counteract a Toll-like receptor 4 (TLR4)-dependent apoptotic pathway of macrophages induced during infection with Gram-negative pathogens. Therefore, YopJ promotes apoptosis of macrophages during infection (21, 26, 53, 55).
Y. pseudotuberculosis is considered to be primarily an extracellular pathogen in vivo. For example, in mice experimentally infected with Y. pseudotuberculosis, the majority of bacteria were found to exist extracellularly in tissues, including the liver (45). However, Y. pseudotuberculosis can survive in macrophages in vitro (17, 36, 54), and the ability of Y. pseudotuberculosis to survive in macrophages is important for virulence (17). Furthermore, a recent study that employed an ex vivo gentamicin protection assay determined that ∼5% of viable Y. pseudotuberculosis bacteria were intracellular in the spleens of mice at days 3 to 4 postinfection (3). These findings suggest that although only a minority of Y. pseudotuberculosis bacteria are located intracellularly at any given time in vivo, these intracellular bacteria could play an important role in pathogenesis and/or host responses.
In macrophage infection assays carried out in vitro, the T3SS is only moderately effective at reducing phagocytosis, since ∼50% of Y. pseudotuberculosis bacteria that come into contact with macrophages are internalized (39, 40, 54). It is possible to study the function of the T3SS in the intracellular population of Y. pseudotuberculosis by the use of gentamicin to eliminate viable bacteria outside macrophages. Using this approach in recent studies, we showed that the presence of a functional T3SS decreases the survival of Y. pseudotuberculosis internalized into murine macrophage phagosomes (41, 54). Evidence was obtained that the T3SS promotes calcium influx, activating synaptotagmin VII to increase lysosome fusion with phagosomes, thereby decreasing survival of Y. pseudotuberculosis in macrophages (41). Analysis of macrophage survival following phagocytosis of Y. pseudotuberculosis showed that the intracellular bacteria could kill the host cell in a TLR4- and YopJ-dependent mechanism (54). Furthermore, production of proinflammatory cytokine TNF-α was also inhibited in a YopJ- dependent manner (54). Although it was assumed that YopJ translocated during initial contact or phagocytosis can inhibit TNF-α and kill Y. pseudotuberculosis in macrophages (41, 54), it cannot be ruled out that the T3SS continued to translocate YopJ within phagosomes. Furthermore, because de novo protein synthesis by the intracellular bacteria was required for apoptosis (54), it remained possible that continuous production of the T3SS and translocation of YopJ within phagosomes were important for Y. pseudotuberculosis to kill the host cell. This possibility was examined here using Y. pseudotuberculosis strains in which expression of Yop effectors or a translocator could be turned on before or after phagocytosis by macrophages.
MATERIALS AND METHODS
Bacterial strains.
The Y. pseudotuberculosis strains and plasmids used in this study are listed in Table 1. The strain ΔyopB (Table 1) was created by inactivating yopB in strain IP2666 as described previously (42). To construct pYopB2, the plasmid pYopB containing yopB in the backbone of pMMB67HE that has been described before (42) was digested with restriction enzymes HindIII and NdeI to remove the T7 Shine-Dalgarno sequence in front of the coding sequence of yopB. Religation after treatment with T4 DNA polymerase resulted in pYopB2. To construct plasmids expressing Yop-TEM-1 β-lactamase (bla) translational fusions under the control of the tac promoter, DNA fragments containing coding sequences for YopH or YopJC172A with C-terminal M45 epitope tags were amplified by PCR with primer 67NF (5′-ACAGG AAACA GAATT AATTA AGCTT G-3′) and primer M45R (5′-CAGCG TTTCT GGGTG CGTCT CTGTC TCAAA AGGAG G-3′) from plasmid pYopH (Table 1) or pYopJC172A (Table 1), respectively. The coding region of TEM-1 β-lactamase was amplified by PCR using primer BlaF (5′-TTTGA GACAG AGACG CACCC AGAAA CGCTG GTG-3′) and primer BlaR (5′-CAAAA CAGCC AAGCT TACCA ATGCT TAATC AGTGA GG-3′) from plasmid pYopJ (Table 1). The resulting DNA fragments were ligated to create a Yop-M45-TEM-1 translational fusion and inserted into the plasmid pMMB207 (Table 1) between EcoRI and HindIII sites using an In-Fusion system (Clontech). The resulting plasmids were confirmed by restriction digestion analysis, and fusion protein expression was verified by immunoblotting analysis (see below). The plasmid pmCherry (Table 1) was constructed using the same strategy used to construct p207mCherry (37). Plasmids were introduced into Y. pseudotuberculosis strains through conjugation with Escherichia coli as described previously (36). Ampicillin (Amp; 100 μg/ml) and/or chloramphenical (Cm; 25 μg/ml) was included in Luria-Bertani broth (LB) to maintain selection for pMMB67EH (Ampr)- or pMMB207 (Cmr)-derived plasmid in E. coli or Y. pseudotuberculosis.
Table 1.
Bacterial strains and plasmid used
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Yersinia strains | ||
| IP2666 | Serogroup O:3, pYV positive | 44 |
| ΔyopJ | IP2666 pYV ΔyopJ1-867; alternative name is IP26 | 22 |
| ΔyopB | IP2666 pYV yopB40 (stop codon and frameshift); alternative name is IP40 | This study |
| ysc | IP2666 pYV ysc::Tn5; alternative name is IP71; contains same pYV ysc::Tn5 as in previously published strain YP71 | 32, 41 |
| Plasmids | ||
| pVector | pMMB67EH, contains lacIq gene and tac promoter, Ampr | 15 |
| pMMB207 | Contains lacIq gene and tac promoter, Cmr | 27 |
| pYopH | pMMB67HE encoding YopHM45 under the control of the tac promoter, Ampr | 5 |
| pYopJ | pMMB67HE encoding YopJM45 under the control of the tac promoter, Ampr; alternative name is pLP17 | 33 |
| pYopJC172A | pMMB67HE encoding catalytic inactive YopJC172A M45 under the control of the tac promoter, Ampr | 31 |
| pYopB2 | pMMB67HE encoding YopB, Ampr | This study |
| pYopH-bla | pMMB207 encoding a YopH-TEM-1 β-lactamase fusion protein under the control of the tac promoter, Cmr | This study |
| pYopJC172A-bla | pMMB207 encoding a YopJC172A-TME-1 β-lactamase fusion protein under the control of the tac promoter, Cmr | This study |
| pmCherry | pMMB67EH encoding mCherry under the control of the tac promoter, Ampr | This study |
Macrophage cultures and infection conditions.
Bone marrow-derived macrophages (BMDMs) were obtained from female C57BL/6 mice (Jackson Laboratory) as described previously (52). Twenty-four hours before infection, the cells were seeded at a density of 1.5 × 105 cells/well in 24-well tissue culture plates in 1 ml Dulbecco modified Eagle medium containing 15% L-cell conditioned medium, 10% heat-inactivated fetal bovine serum (FBS; Gibco), 1 mM pyruvate, and 2 mM glutamate.
For infection, overnight Y. pseudotuberculosis cultures grown at 26°C in LB with appropriate antibiotics were diluted to an optical density at 600 nm of 0.1 in LB supplemented with 2.5 mM calcium chloride. The cultures were shaken at 37°C for 2 h. Subsequently, bacteria were washed once, resuspended in prewarmed Hanks balanced salt solution (HBSS), and diluted into fresh cell culture medium as described above to infect macrophages at a multiplicity of infection (MOI) of 10, unless otherwise indicated. After centrifugation for 5 min at 200 × g to bring the bacteria into contact with the macrophages, an incubation was performed at 37°C for 15 min; therefore, a total of 20 min was allowed for the cells to take up the bacteria. Next, the cells were incubated in cell culture medium containing gentamicin (Gm; 8 μg/ml) for 1 h to kill extracellular bacteria. Then, the wells were changed into medium containing Gm (4.5 μg/ml) until the indicated times. When indicated, isopropyl-β-d-thiogalactopyranoside (IPTG; to 0.1 mM) was included in the LB or cell culture medium to induce expression of genes under the control of tac promoters. Congo red-magnesium oxalate plating was done according to an established method (38) to determine the presence of the virulence plasmid in colonies of Y. pseudotuberculosis recovered from lysates of infected macrophages.
Propidium iodide (PI) and annexin V-FLUOS staining.
BMDMs (1.5 × 105 cells/well) in the 24-well plates were infected for 6 h. The cells were washed with phosphate-buffered saline and processed to stain by use of an annexin V-FLUOS staining kit (Roche) according to the manufacturer's instructions. The live cells were examined by epifluorescence microscopy using a Zeiss Axioplan2 microscope with a ×40 objective. Phase-contrast, green fluorescent, and red fluorescent images were captured, sequentially pseudocolored using a black-and-white Spot camera (Diagnostic Instruments, Inc.), and assembled using Adobe Photoshop software.
Immunofluorescence microscopy to detect intracellular β-lactamase activity.
Four hours after infection of BMDMs seeded in wells of a 96-well plate, CCF2- acetoxymethyl (CCF2-AM) substrate (Invitrogen) was prepared according to the manufacturer's instructions and loaded directly into the medium as a 6× solution. Cells were incubated for 1 h at room temperature prior to observation with a Zeiss Axiovert 100 microscope using a ×20 or ×32 lens. To detect the cleavage of CCF2, a blue filter set with a D470/40 filter for excitation and an OG515 filter for emission (Chroma Technology Corp.) was used. Sequential images of phase, green/blue, and red (mCherry), where applicable, were captured with a true-color Spot camera (Diagnostic Instruments, Inc.) and assembled in Adobe Photoshop software. BMDMs containing red bacteria were counted in pictures taken from one random field per experiment per infection strain and scored for green or blue fluorescence or the absence of fluorescence. On average, 50 cells were counted in each field. Alternatively, BMDMs with blue fluorescence from cells infected with either ΔyopJ/YopH-bla or ΔyopJ/YopJC172A-bla were counted for the presence or absence of red bacteria, and totals of 163 and 20 such cells were counted, respectively.
Western blot analysis.
To detect expression of YopJ in Y. pseudotuberculosis, bacterial lysates were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and analyzed by immunoblotting with antibodies against YopJ (clone C2 and D3) and DnaK (clone 8E2/2; Stressgen) as described before (54). The secondary antibody used was IRDye800-conjugated anti-mouse IgG (Rockland). The membrane was then scanned with an Odyssey VI scanner (LI-COR Biosciences).
To detect secretion of Yop-TEM-1 fusion proteins or native Yop proteins, Y. pseudotuberculosis cultures were grown under low-Ca2+ conditions as described previously (52). Briefly, overnight cultures grown at 28°C in LB were diluted to an optical density at 600 nm of 0.1 into LB supplemented with 20 mM magnesium chloride and 20 mM sodium oxalate. IPTG was included at 0.1 mM when indicated. Then, after shaking at 28°C for 1 h and 37°C for 4 h, Yops secreted into the growth media were precipitated, resolved using 10% SDS-PAGE, and detected by staining with GelCode blue (Pierce) according to the manufacturer's instructions. Alternatively, after electrophoresis, Yops were transferred to nitrocellulose membranes for immunoblotting analysis as described before (55). The primary antibodies used were M45 monoclonal antibody (30) or murine monoclonal antibody to β-lactamase (QED Bioscience Inc.).
LDH release.
The lactate dehydrogenase (LDH) content in the supernatant collected from tissue culture plate wells containing uninfected or infected BMDMs was measured in triplicate with the CytoTox 96 nonradioactive cytotoxicity assay (Promega) following the manufacturer's instructions. Total LDH was determined from macrophages from separate uninfected wells that had been lysed by a freeze-thaw cycle. The percentage of LDH released was calculated with the formula 100 × (LDHreleased/LDHtotal).
Statistical analysis.
Statistical analysis was performed with Prism (version 4.0; Graphpad) software. The tests used are as indicated in the figure legends or main text. A P value of less than 0.05 was considered significant.
RESULTS
Ectopic expression of YopJ induces apoptosis in macrophages containing Y. pseudotuberculosis.
To test the possibility that YopJ synthesized by Y. pseudotuberculosis in phagosomes could trigger macrophage apoptosis, the strain ΔyopJ/pYopJ (Table 1) was constructed. This strain contains a deletion of yopJ on the virulence plasmid pYV and carries a low-copy-number expression vector (pMMB67EH, Ampr) in which the wild-type yopJ open reading frame appended by an M45 epitope tag is placed under the control of the tac promoter, allowing regulation by IPTG. A control strain carrying the empty vector (ΔyopJ/pVector; Table 1) was also constructed. Since YopJ does not interfere with phagocytosis or intracellular replication of Y. pseudotuberculosis (54), these processes will not be affected by the presence or absence of YopJ in infected macrophages. To demonstrate regulated expression of YopJ by IPTG in ΔyopJ/pYopJ, immunoblotting analysis was carried out using total bacterial lysates and a mixture of monoclonal antibodies specific for YopJ. YopJ protein was detected when ΔyopJ/pYopJ was grown in LB containing 2.5 mM CaCl2 at 37°C for 2 h in the presence of IPTG but not in the absence of IPTG (Fig. 1; compare lane 2 and lane 3). When IPTG was removed and the bacteria were incubated in tissue culture medium for 20 min, the steady-state level of YopJ protein decreased (Fig. 1, lane 4). In comparison, the parental wild-type strain IP2666 also expressed undetectable YopJ when grown in LB containing 2.5 mM CaCl2 at 37°C (Fig. 1, lane 5) but demonstrated robust YopJ expression when grown in LB containing a low Ca2+ concentration (Fig. 1, lane 6), which was considered to mimic the growth condition when the bacterium is in contact with a eukaryotic cell.
Fig. 1.
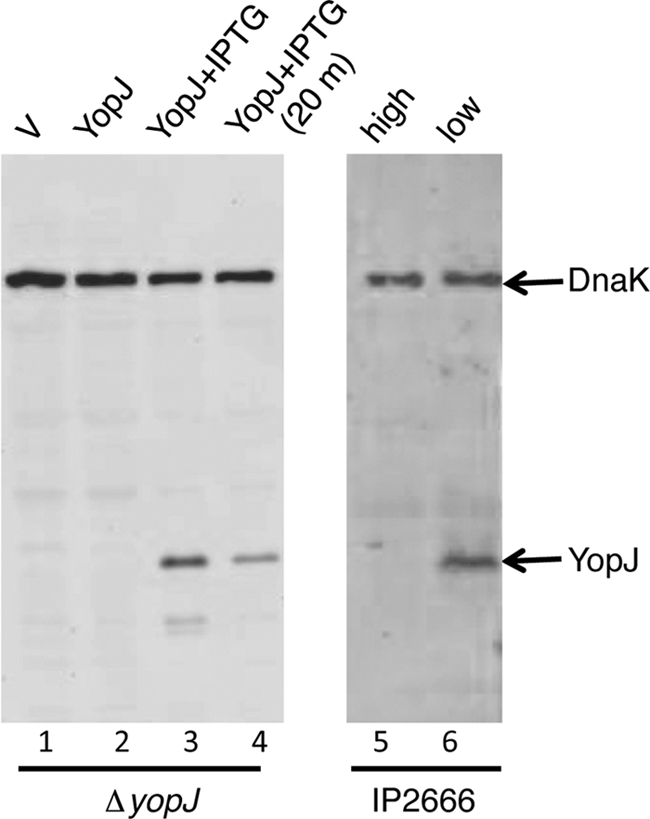
Ectopic YopJ expression in Y. pseudotuberculosis in response to IPTG induction or removal. Y. pseudotuberculosis ΔyopJ stains carrying pVector (V) or pYopJ (YopJ) were cultured at 37°C for 2 h in LB containing 2.5 mM CaCl2 either without IPTG (lanes 1 and 2) or with IPTG (lanes 3 and 4). Bacterial lysates were analyzed by immunoblotting with antibodies to YopJ and DnaK. In lane 4, a sample of the same bacteria analyzed in lane 3 was incubated in tissue culture medium for an additional 20 min at 37°C without IPTG before lysis to evaluate the stability of the YopJ protein. Alternatively, the parental wild-type strain IP2666 was cultured at 37°C for 2 h in LB containing 2.5 mM CaCl2 (lane 5, high) or cultured in LB containing 20 mM sodium oxalate and 20 mM magnesium chloride at 28°C for 1 h and then 37°C for 2 h (lane 6, low) before immunoblotting analysis.
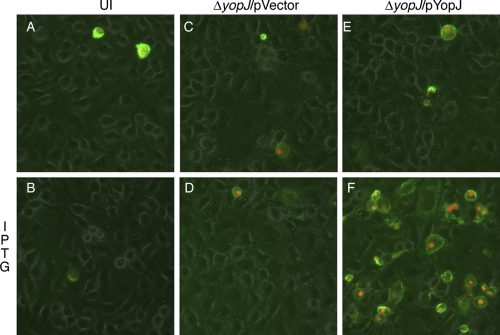
BMDMs in duplicate wells were left uninfected or infected for 20 min with ΔyopJ/pYopJ or ΔyopJ/pVector grown in LB containing 2.5 mM CaCl2 at 37°C in the absence of IPTG. After the BMDMs had been treated with 8 μg/ml Gm for 1 h, IPTG was added to half of the wells for an additional 5 h. Apoptosis was measured by PI and annexin V staining assay (see Materials and Methods). Annexin V staining (apoptosis) or annexin V and PI staining (late apoptosis or necrosis) above the background level was observed only among the BMDMs infected with ΔyopJ/pYopJ that were treated with IPTG and not among the cells similarly infected but not treated with IPTG (compare Fig. 2E and F) or among the BMDMs left uninfected (Fig. 2A and B) or infected with ΔyopJ/pVector (Fig. 2C and D). To quantify cell death under these conditions, LDH release assays were performed at 21 h postinfection. The amount of LDH released from the BMDMs infected with ΔyopJ/pYopJ and treated with IPTG (32%; lane 3 in Fig. 3) was significantly higher (P < 0.01) than the amount released from macrophages infected with the same strain yet not treated with IPTG (7%; lane 2 in Fig. 3). Together, these results suggested that YopJ synthesized by intracellular Y. pseudotuberculosis was able to promote macrophage apoptosis.
Fig. 2.
Macrophage death resulting from intracellularly induced YopJ protein. BMDMs were either left uninfected (UI) (A and B) or infected with ΔyopJ containing pVector (C and D) or pYopJ (E or F) for 20 min. After treatment with 8 μg/ml of Gm for 1 h, IPTG was included in the medium during the last 5 h of infection for half of the wells (B, D, and F). Cell death was detected by fluorescence microscopy following staining with propidium iodide (red) and annexin V conjugated with fluorescein (green). Images show the overlays of phase, and red and green signals are presented. Results shown are representative from three independent experiments.
Fig. 3.
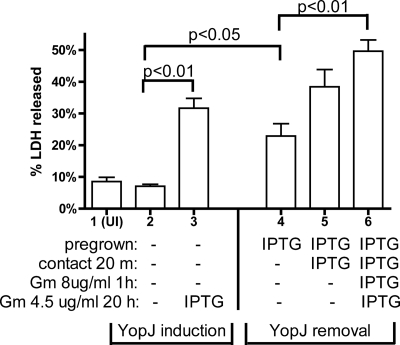
Effect of timing of YopJ expression on release of LDH from infected BMDMs. BMDMs were left uninfected (UI) or infected with ΔyopJ/pYopJ. As indicated in Materials and Methods, to carry out infection, overnight bacterial cultures were shaken in LB containing 2.5 mM CaCl2 for 2 h at 37°C (pregrown). Then, the washed bacteria were applied in fresh tissue culture medium to the BMDMs to incubate for 20 min (contact 20 m), before Gm was added to the mixture with additional medium to a final concentration of 8 μg/ml and incubation was continued for an additional 1 h (Gm 8 μg/ml 1 h). Finally, fresh medium containing Gm at 4.5 μg/ml was added to the wells and incubation was continued for an additional 20 h (Gm 4.5 μg/ml 20 h) before the tissue culture medium was analyzed for release of LDH. Inclusion of IPTG during different stages of the experiment was as indicated by IPTG in the description at the bottom, while the absence of IPTG is indicated by minus signs. Data shown are the means and SEMs of three experiments. P values were determined by one-way analysis of variance followed by Bonferroni's comparison, and P values of less than 0.05 are indicated.
A YopJ removal infection experiment was performed to determine if continuous YopJ expression by intracellular Y. pseudotuberculosis was required for macrophage apoptosis. Strain ΔyopJ/pYopJ was grown in the presence of IPTG and used to infect BMDMs. Then, IPTG was removed from the cultures at the time of infection or 20 min after the initial contact between the BMDMs and the bacteria or was maintained throughout. Cell death was quantified by measuring LDH release at 21 h postinfection. When IPTG was removed at the time of infection, the level of LDH released (23%; lane 4 of Fig. 3) was significantly higher (P < 0.05) than the level of LDH released from BMDMs infected with ΔyopJ/pYopJ grown in the absence of IPTG (7%; lane 2 of Fig. 3). This result indicated that continuous YopJ synthesis from intracellular bacteria was not required to induce macrophage apoptosis. However, and interestingly, if IPTG was removed 20 min after the initial contact or maintained throughout the infection, even higher levels of LDH were released (38% and 50%, respectively; lanes 5 and 6 of Fig. 3), and in the latter case, this difference was significant (P < 0.01) (Fig. 2). Therefore, although continuous synthesis of YopJ by intracellular Y. pseudotuberculosis was not required for macrophage apoptosis, YopJ synthesized by intracellular bacteria contributed to higher levels of cell death.
Apoptosis induced by ectopic expression of YopJ in Y. pseudotuberculosis in macrophages requires a functional T3SS.
The above-described results suggested that YopJ could be translocated across the phagosomal membrane by Y. pseudotuberculosis in macrophages. To investigate whether the process required the Ysc T3SS encoded on the virulence plasmid, the pYopJ vector was introduced into the Y. pseudotuberculosis ysc mutant (Table 1), which does not assemble an injectisome, or the ΔyopB mutant (Table 1), which is defective for translocation. The resulting strains were compared to strain ΔyopJ/pYopJ for the ability to induce death of BMDMs following ectopic expression of YopJ after the extracellular bacteria were killed with Gm for 1 h. Cell death was assessed by measuring LDH release after 24 h of infection. As shown in Fig. 4A, ΔyopB/pYopJ and ysc/pYopJ were defective in inducing BMDM apoptosis. To determine if the catalytic activity of YopJ was required for cell death, a vector encoding yopJ(C172A) (pYopJC172A; Table 1) was introduced into ΔyopJ. Following infection with ΔyopJ/pYopJC172A, ectopic expression of YopJC172A from intracellular bacteria failed to increase apoptosis in BMDMs (Fig. 4A). These results indicated that a functional Ysc T3SS and acetyltransferase activity were required for macrophage apoptosis to occur following ectopic expression of YopJ in intracellular Y. pseudotuberculosis.
Fig. 4.
Requirement for YopJ, YopB, and the function of the T3SS in BMDM death induced by intracellular Y. pseudotuberculosis. BMDMs were left uninfected (UI) or infected with the indicated Y. pseudotuberculosis strains carrying pYopJ, pYopJC172A, or pYopB2. (A) YopJ expression from intracellular bacteria was either not induced (white bars) or induced with IPTG (checked bars) after 1 h treatment with 8 μg/ml Gm. (B) YopB expression from intracellular ΔyopB/pYopB2 bacteria was not induced (no IPTG) or induced with IPTG (IPTG) after extracellular bacteria were killed with 8 μg/ml Gm for 1 h. The percentage of LDH released into the medium was determined at 24 h postinfection. Data shown are means and SEMs from three independent experiments. P values were determined by two-way analysis of variance, followed by Bonferroni's multiple-comparison test (A) or the Mann-Whitney test (B). *, significant difference (P < 0.05) compared to all the other treatments.
Next we tested whether endogenously expressed YopJ was sufficient to mediate macrophage death when injected from the intracellular location. BMDMs were left uninfected or infected with the ΔyopB strain, which expresses YopB under the control of IPTG (ΔyopB/pYopB2). After the extracellular bacteria were killed with Gm for 1 h, IPTG was added to half of the wells. Cell death was analyzed 24 h later by LDH release. Induction of YopB expression from intracellular bacteria resulted in elevated levels of LDH release (Fig. 4B; P < 0.001). This result indicated that endogenously expressed YopJ translocated from intracellular bacteria was sufficient to induce death of the infected BMDMs.
Exposure of bacteria to Gm before uptake is sufficient to prevent death of the BMDMs infected with parental wild-type Y. pseudotuberculosis.
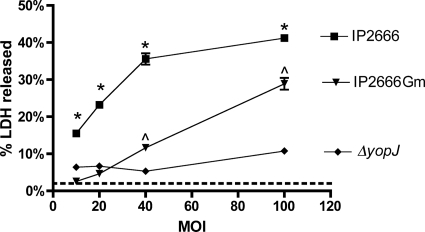
The above-described results indicated that when YopJ was ectopically expressed by IPTG induction from intracellular bacteria, the endogenously expressed T3SS was sufficient to mediate its translocation; on the other hand, when YopB was ectopically expressed through IPTG induction from intracellular bacteria, the endogenously expressed YopJ was also translocated. However, the above conclusions assume that any intracellular bacteria that may escape the macrophage would be unable to reinfect or mediate YopJ translocation due to the bactericidal action of Gm. To test this, the parental wild-type Y. pseudotuberculosis IP2666 strain was used to infect BMDMs at an MOI of 10 in medium already containing Gm at 8 μg/ml. For controls, death of the BMDMs infected with either wild-type strain IP2666 or the ΔyopJ mutant was determined under our standard intracellular infection conditions (MOI of 10 with addition of Gm at 8 μg/ml after 20 min of infection for 1 h and then incubation with Gm at 4.5 μg/ml for another 23 h). As expected from published results obtained with serogroup I Y. pseudotuberculosis strain 32777 (54), the IP2666 strain used in this study also caused cell death under the standard intracellular infection condition (Fig. 5, IP2666). Release of LDH was significantly lower in BMDMs infected with the ΔyopJ strain (Fig. 5, ΔyopJ). When IP2666 was used to infect BMDMs in medium already containing Gm (Fig. 5, IP2666 Gm), the amount of LDH released was not significantly different from that released from macrophages left uninfected or infected with ΔyopJ. Similar results were obtained when the MOI was increased to 20 (Fig. 5). With a higher MOI of either 40 or 100, infection with IP2666 in the presence of Gm did result in significant LDH release compared to the negative control (ΔyopJ) (Fig. 5). Importantly, the level of LDH released when Gm was always present was significantly lower than that under the standard intracellular infection condition regardless of the MOI (Fig. 5). Given the fact that an MOI of 10 was our standard intracellular infection condition, this result indicated that even though escape of the intracellular bacteria and reinfection could occur, they are far from sufficient to explain the level of BMDM death observed.
Fig. 5.
Release of LDH from BMDMs infected with IP2666 or ΔyopJ at different MOIs. As indicated, BMDMs were infected with either parental wild-type strain IP2666 or strain ΔyopJ at an MOI of 10, 20, 40, or 100 as described in the legend to Fig. 2 with 20 min of contact time and 1 h of incubation with 8 μg/ml of Gm followed by 23 h of incubation with 4.5 μg/ml of Gm. Alternatively, IP2666 in 0.5 ml of tissue culture medium was added to one well of BMDMs in 0.5 ml of medium containing 16 μg/ml of Gm at the indicated MOI (IP2666Gm) and incubated for the remaining time. The amount of LDH released was determined at the end of the incubation. Results shown are from one representative experiment of three. The level of LDH released from cells left uninfected is indicated with a dotted line. P values were determined by two-way analysis of variance and Bonferroni posttests where indicated. *, P < 0.05 for comparison of the values from IP2666 and either IP2666Gm or ΔyopJ of the same MOI; ^, P < 0.05 for comparison of the values between IP2666Gm and ΔyopJ.
Detection of ectopically expressed Yop-β-lactamase fusion proteins translocated from Y. pseudotuberculosis into macrophages.
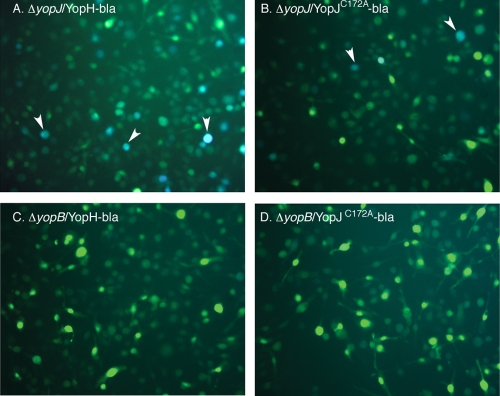
The above-described results assessed translocation of YopJ from intracellular Y. pseudotuberculosis indirectly through measuring the death of infected BMDMs. To allow detection of Yop translocation at the single-cell level, plasmids were constructed to encode translational fusions between a Yop and the mature domain of TEM-1 β-lactamase (Yop-bla). Detection of cytosolic β-lactamase activity would indicate translocation of the respective effector (10). The activity of β-lactamase was detected with the substrate CCF2-AM. This reagent is cell permeant and is trapped inside cells after hydrolysis of its ester functionalities. Thus, the CCF2-AM-loaded cells will fluoresce green. Upon cleavage by β-lactamase, the product emits blue fluorescence due to the disruption of resonance energy transfer (56). Since the wild-type YopJ protein causes death of BMDMs, which could interfere with detection of fluorescence, the catalytically inactive YopJC172A protein was fused to TEM-1 (pYopJC172A-bla; Table 1). In addition, a plasmid encoding YopH-bla (pYopH-bla; Table 1) was constructed to examine whether Yops other than YopJ could be translocated from Y. pseudotuberculosis in macrophages. These plasmids were conjugated into ΔyopJ and ΔyopB. The resulting strains and the parent ΔyopJ strain were cultured under low-Ca2+ conditions at 37°C to stimulate secretion of Yops by the T3SS, and proteins secreted into culture supernatants were analyzed by gel electrophoresis and immunoblotting (see Materials and Methods). Secretion of the Yop-bla fusion proteins into the growth medium was detected from both ΔyopJ and ΔyopB strains (Fig. 6). Next, BMDMs were infected with these strains grown in the absence of IPTG. As before, IPTG was added after 1 h treatment with Gm to induce expression of the Yop-bla fusion proteins from intracellular Y. pseudotuberculosis. Three hours later, the infected BMDMs were loaded with CCF2-AM. After an additional hour, the infected BMDMs were analyzed by two-color fluorescence microscopy. As shown in Fig. 7, blue fluorescent BMDMs indicating the presence of β-lactamase activity were detected after infection with the translocation-competent ΔyopJ derivatives but not with the translocation-defective ΔyopB strains. Furthermore, consistent with the possibility that YopH is translocated at higher levels, more BMDMs showed blue fluorescence when infected with strains producing YopH-bla than strains producing YopJC172A-bla (compare the numbers of blue cells in Fig. 7A and B). This result provided evidence that Y. pseudotuberculosis is capable of translocating ectopically expressed YopJ-bla and YopH-bla fusion proteins from inside macrophages.
Fig. 6.

Secretion of Yop-bla fusion proteins from Y. pseudotuberculosis strains. Y. pseudotuberculosis ΔyopJ and ΔyopB strains carrying either pYopH-bla (YopH-bla), pYopJC172A-bla (YopJ-bla), or no vector were grown in LB containing 20 mM MgCl2, 20 mM sodium oxalate, and IPTG for 4 h at 37°C. Secreted proteins were analyzed by SDS-PAGE and GelCode protein stain (A) or Western blotting (WB) for the M45 epitope (B) or β-lactamase (C). The positions of the protein size markers (in kilodaltons) are indicated on the left, and the locations of the secreted proteins are indicated on the right.
Fig. 7.
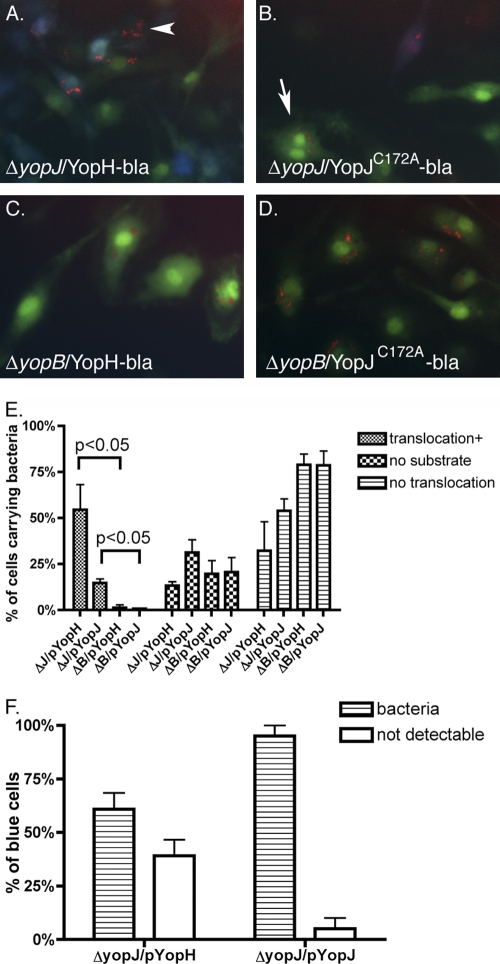
Detection of translocated Yop-bla fusion protein expressed from intracellular Y. pseudotuberculosis. BMDMs were infected with ΔyopJ or ΔyopB strains carrying pYopH-bla (YopH-bla) or pYopJC172A-bla (YopJ-bla). After 1 h of treatment with 8 μg/ml Gm, IPTG was added and included during the last 3 h of infection. BMDMs were then incubated with CCF2-AM for 1 h. Green and blue images were captured by fluorescence microscopy. Arrowheads indicate some blue cells that contain translocated Yop-bla fusion proteins.
Translocation of ectopically expressed Yop-β-lactamase fusion proteins can be correlated with the presence of Y. pseudotuberculosis in macrophages.
We next determined if detection of translocated Yop-bla fusion proteins in BMDMs could be correlated with the presence of viable intracellular Y. pseudotuberculosis. To facilitate the detection of intracellular bacteria, a low-copy-number plasmid encoding the monomeric red fluorescent protein mCherry under the control of the tac promoter was constructed from pMMB207 (pmCherry, Ampr; Table 1). The ΔyopJ and ΔyopB strains encoding the Yop-bla fusion proteins were transformed with pmCherry. The presence of different selectable markers on the pMMB67EH (Ampr)- and pMMB207 (Cmr)-derived plasmids allowed maintenance of both vectors in Y. pseudotuberculosis. BMDMs were infected with these strains, and as described before, after 1 h of treatment with Gm to kill extracellular bacteria, IPTG was included with a lower concentration of Gm for an additional 4 h to induce de novo expression of both the Yop-bla fusion protein and mCherry. Then, substrate CCF2-AM was loaded on the BMDMs for 1 h before analysis by three-color fluorescence microscopy. As shown in Fig. 8, it was possible to identify BMDMs that contained intracellular bacteria (red), were loaded with CCF2-AM (green), and had received translocated Yop-bla fusion protein (blue). While intracellular bacteria could be detected under all conditions, only BMDMs infected with the ΔyopJ strains exhibited blue fluorescence, confirming that the ΔyopB strains were defective for translocation of Yop-bla fusion proteins (Fig. 8A to D). Images of random fields of macrophages from several independent experiments were analyzed to identify BMDMs that contained intracellular bacteria and to score those cells for the presence of loaded CCF2-AM and translocated Yop-bla fusion protein. As summarized in Fig. 8E, a population of bacterium-containing BMDMs (between 15% and 38%, depending on the condition) lacked green fluorescence (for example, see the arrowhead in Fig. 8A), indicating that these cells were not loaded with the CCF2-AM substrate. Among the bacterium-containing BMDMs, the percentages that exhibited blue fluorescence indicative of Yop-bla translocation were significantly higher following infection with the ΔyopJ strains than the ΔyopB strains (Fig. 8E). In addition, as shown in Fig. 8E, a greater percentage of bacterium-containing BMDMs fluoresced blue when they were infected with the ΔyopJ strain encoding YopH-bla than when they were infected with YopJC172A-bla (54% versus 15%, respectively). This result indicated that more YopH-bla than YopJC172A-bla is translocated.
Fig. 8.
Detection of BMDMs containing translocated Yop-bla fusion proteins and intracellular Y. pseudotuberculosis. BMDMs were infected with ΔyopJ or ΔyopB strains carrying pmCherry and pYopH-bla (YopH-bla) or pYopJC172A-bla (YopJ-bla), as indicated. IPTG induction and substrate loading were carried out as described in the legend to Fig. 7. Overlaid red, green, and blue images were captured sequentially by fluorescence microscopy. (A to D) Representative images from three independent experiments are shown. (E) The percentage of cells that carried mCherry-positive (red) bacteria that were also positive for translocation (blue, translocation+) or negative for substrate loading (neither green nor blue, no substrate) or the percentage of cells in which translocation was not detectable (green, no translocation) was determined by scoring the cells in multiple images. (F) The percentage of blue cells that were positive (bacteria) or negative (not detectable) for red intracellular bacteria was determined by scoring the cells in multiple images. Results shown are the means and SEMs of three to four independent experiments. P values of less than 0.05 by the Mann-Whitney test are indicated.
Substantial percentages of BMDMs containing ΔyopJ/YopH-bla or ΔyopJ/YopJC172A-bla that were loaded with substrate did not fluoresce blue (32% and 55% in Fig. 8E, respectively; for example, see the arrow in Fig. 8B). To preclude the possibility that these bacteria were defective for translocation because they lost their virulence plasmid during the short time of infection, 100 colonies recovered from cells that were infected for 24 h were tested for the maintenance of the virulence plasmid (see Materials and Methods). The results indicated that all colonies still contained the virulence plasmid (data not shown).
It was noticed that some BMDMs that fluoresced blue did not contain detectable bacteria. To quantify these cells, images of random fields from the above-described experiments were analyzed to identify BMDMs that fluoresced blue and to score those cells for the presence of intracellular bacteria. As summarized in Fig. 8F, in BMDMs infected with ΔyopJ/pYopH-bla, 61% of the blue cells contained bacteria. In contrast, in BMDMs infected with ΔyopJ/pYopJC172A-bla, 95% of the blue cells contained bacteria. Overall, the finding that the majority of blue BMDMs contain bacteria argues that the Yop-bla fusion proteins are being translocated from intracellular Y. pseudotuberculosis.
DISCUSSION
Here we provide the first evidence that intracellular Y. pseudotuberculosis is able to deliver Yops into the macrophage cell cytosol utilizing the Ysc T3SS encoded on the virulence plasmid. The key to our approach was the use of an inducible system that allowed the expression of a translocator or effectors to be turned on after Y. pseudotuberculosis was internalized by macrophages. In addition, two sensitive enzymatic reaction-based methods were utilized to detect effector translocation. First, taking the advantage that translocated YopJ induces death of infected macrophages, YopJ expression was ectopically induced after extracellular bacteria were killed with Gm. As indicated before, YopJ does not interfere with phagocytosis or the survival of the intracellular Y. pseudotuberculosis, so cell death resulting from YopJ activity could be directly correlated to its translocation. As shown in Fig. 2 to 4, induction of YopJ expression in intracellular Y. pseudotuberculosis indeed resulted in macrophage death. Alternatively, when the expression of translocator YopB was induced ectopically from intracellular bacteria, increased death of the BMDMs was also observed (Fig. 4B). As cell death resulted from reciprocal usage of either native or ectopically expressed YopJ or YopB, the results are highly consistent with translocation events occurring from intracellular bacteria. Second, it was possible to detect the translocation of β-lactamase fused to either YopJC172A or YopH under the same infection conditions (Fig. 7 and 8), indicating that the Ysc T3SS of intracellular Y. pseudotuberculosis can mediate translocation of multiple ectopically expressed Yop effectors. More YopH-bla than YopJC172A-bla appeared to be translocated (Fig. 7 and Fig. 8E), possibly because YopH has a dedicated chaperone (SycH) that may facilitate its translocation (48).
Our results also indicated that translocation of ectopically expressed YopJ from intracellular Y. pseudotuberculosis required the Ysc injectisome and the YopB translocon protein (Fig. 4). A chromosomally encoded T3SS, similar to the SPI-2-encoded system of Salmonella enterica, is found in Y. pseudotuberculosis (9, 36) and Y. pestis (13, 34). A distinct T3SS is encoded on the chromosome of biovar 1B strains of Y. enterocolitica, and this Ysa system has been shown to be important for translocation of YopP into macrophages (24, 51). Our results (Fig. 4A) rule out the possibility that the chromosomal T3SS in Y. pseudotuberculosis can function in the absence of the Ysc system to mediate bacterial translocation of ectopically expressed YopJ from phagosomes into the cytosol of macrophages.
Despite the detection of translocation of Yops from intracellular bacteria, it is important to reiterate that translocation of effectors through T3SS does not benefit the intracellular bacteria through promoting bacterial survival or replication. Rather, T3SS decreases survival of intracellular bacteria. As shown before, at 5 h postinfection, intracellular bacteria have been cleared from about half of the cells that harbored intracellular bacteria initially (54). The same phenomenon was seen in this study when the presence of intracellular mCherry-expressing Y. pseudotuberculosis was correlated with the translocation of a Yop-bla fusion protein. When BMDMs that were positive for the YopJ-bla fusion protein were scored for the presence of mCherry-positive Y. pseudotuberculosis, there was an excellent correlation, as 95% of the blue fluorescent macrophages contained red bacteria (Fig. 8F). In the case of BMDMs infected with the strain expressing the YopH-bla fusion protein, a majority of the blue fluorescent macrophages contained red bacteria (61%), but 39% did not (Fig. 8F). There are several explanations for the origin of the 39% of BMDMs that contain translocated YopH-bla but not bacteria. We favor the idea that more YopH-bla than YopJ-bla was translocated from intracellular bacteria, and therefore, fewer viable intracellular bacteria were needed to reach the threshold of the Yop-bla fusion protein for detection. The single cell assay also revealed that among the BMDMs containing intracellular bacteria, translocation was undetectable in a considerable population (Fig. 8B, arrow, and E). For example, in 32% of the cells, translocation of YopH-bla was undetectable at 5 h postinfection, and the value for YopJ-bla was 54% (Fig. 8E). The lack of Yop-bla translocation suggested that these bacteria probably downregulated the expression of the Ysc T3SS. Alternatively, these bacteria may not have had the opportunity to express the Ysc T3SS before the initial contact with BMDMs. This phenomenon reflected the potential heterogeneity in the expression or functional profiles of the intracellular bacteria.
Given the detection of these translocation events, it is important to emphasize that Ysc T3SS-mediated translocation is likely a continuous process. Furthermore, it is well established that translocation of effectors by the Ysc T3SS does not require internalization of Yersinia into host cells. Two previous studies concluded that Yops are not translocated by intracellular Yersinia. Rosqvist et al. showed that the ability of Y. pestis to cause cytotoxicity (an outcome of YopE translocation) was inhibited when the bacteria were internalized into HeLa cells (40). In addition, as shown by immunoblotting, the intracellular bacteria were shown to express substantially smaller amounts of Yops than Y. pestis attached to the surface of HeLa cells (40). Similarly, Cowan et al., using immunoblotting, obtained evidence that intracellular Y. pestis is unable to translocate YopH into macrophages (12). Our results agree with these findings that the intracellular bacteria are not as efficient as their extracellular counterparts in injecting the Yops into host cell cytosol. However, by employing more sensitive detection methods, we were able to detect that the Ysc T3SS remains functional for some time within the macrophage phagosomes, such that ectopically expressed effectors can be translocated from this location.
Studies show that a pool of effectors is synthesized in the bacteria prior to host cell contact and these effectors can be translocated within minutes of interaction with phagocytes (2, 6, 23). Our own results are consistent with this concept, in that the induction of YopJ in Y. pseudotuberculosis prior to infection of BMDMs is sufficient to cause apoptosis (Fig. 3). Our results also suggested the possibility that the T3SS remains functional for some time after internalization of the bacteria to mediate translocation of the native Yop effectors. Given the strong expression of the Yops after contact with a eukaryotic cell, it is conceivable that when the existing pool of Yops is large enough or the synthesis of the Yops is able to keep up with translocation, T3SS-mediated translocation of Yops should continue, despite the closure of the phagocytic cup. However, translocation of the effectors through T3SS may be regulated at discrete steps. For example, for Salmonella enterica, contact with a host cell triggered effector translocation within seconds, and translocation continued for several minutes, until the intrabacterial pool of effectors was depleted (43). For Yersinia, the kinetics of effector translocation has yet to be revealed in such detail. At least by microarray analysis, the situation of Yersinia seems to be different from that of Salmonella, in that Y. pestis inside macrophages expresses yop mRNA at detectable levels (14). Therefore, it is possible that the intracellular Yersinia may translocate some newly synthesized effectors.
So if intracellular Yersinia bacteria mediate translocation of effectors through T3SS at the cost of their very survival, why would they do this? The process is certainly detrimental to the individuals located intracellularly, but it benefits the whole population, especially the ones located extracellularly. It is possible that the role of intracellular bacteria is important especially initially during in vivo infection, when the ratio of bacteria to host cells is low. In addition, since the function of T3SS depends on contact with the host cell and phagocytes are continuously recruited to sites of bacterial presence during infection, increased levels of translocated effectors will help to thwart the host innate response, regardless of the source. Furthermore, the amount of translocated proteins from the intracellular population seems to be sparse; however, as the majority of the extracellular bacteria replicate in microcolonies that do not make direct contact with host cells (20), the small amount could become important.
Overall, the evidence presented here supports a model where translocation of the Yop effectors initiated strongly during phagocytosis; once the bacteria were internalized, translocation of Yops persisted for some bacteria, while others may replicate through restricting the expression of the T3SS. During Yersinia infection, the coexistence of both extracellular and intracellular bacteria can diverge the host immune responses, while the presence of different functional populations of intracellular bacteria can diverge such responses even further. This could be the basis of the daunting task that multiple branches of the host immune responses have to be activated for a successful vaccine against Yersinia (46).
ACKNOWLEDGMENTS
We thank Lance Palmer and Michelle Ryndak for constructing strains ΔyopJ and ΔyopB, respectively, and Mark Koeppel at Invitrogen for assistance with the usage of CCF2-AM reagent.
This work is supported by grants from the National Institutes of Health (R01-AI043389, R56-AI043389, and P01-AI055621) and the Northeast Biodefense Center (U54-AI057158-Lipkin) awarded to J.B.B.
Footnotes
Published ahead of print on 15 August 2011.
REFERENCES
- 1. Achtman M., et al. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. U. S. A. 96:14043–14048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersson K., et al. 1996. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol. Microbiol. 20:1057–1069 [DOI] [PubMed] [Google Scholar]
- 3. Bergman M. A., Loomis W. P., Mecsas J., Starnbach M. N., Isberg R. R. 2009. CD8(+) T cells restrict Yersinia pseudotuberculosis infection: bypass of anti-phagocytosis by targeting antigen-presenting cells. PLoS Pathog. 5:e1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black D. S., Bliska J. B. 1997. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16:2730–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black D. S., Marie-Cardine A., Schraven B., Bliska J. B. 2000. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell. Microbiol. 2:401–414 [DOI] [PubMed] [Google Scholar]
- 6. Bliska J. B., Black D. S. 1995. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63:681–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bliska J. B., Guan K. L., Dixon J. E., Falkow S. 1991. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. U. S. A. 88:1187–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brodsky I. E., et al. 2010. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7:376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chain P. S., et al. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. U. S. A. 101:13826–13831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charpentier X., Oswald E. 2004. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J. Bacteriol. 186:5486–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cornelis G. R., et al. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cowan C., Philipovskiy A. V., Wulff-Strobel C. R., Ye Z., Straley S. C. 2005. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect. Immun. 73:6127–6137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng W., et al. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 184:4601–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukuto H. S., Svetlanov A., Palmer L. E., Karzai A. W., Bliska J. B. 2010. Global gene expression profiling of Yersinia pestis replicating inside macrophages reveals the roles of a putative stress-induced operon in regulating type III secretion and intracellular cell division. Infect. Immun. 78:3700–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furste J. P., et al. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host range tacP expression vector. Gene 48:119–131 [DOI] [PubMed] [Google Scholar]
- 16. Galan J. E., Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567–573 [DOI] [PubMed] [Google Scholar]
- 17. Grabenstein J. P., Marceau M., Pujol C., Simonet M., Bliska J. B. 2004. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infect. Immun. 72:4973–4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan K., Dixon J. E. 1990. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science 249:553–556 [DOI] [PubMed] [Google Scholar]
- 19. He S. Y., Nomura K., Whittam T. S. 2004. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim. Biophys. Acta 1694:181–206 [DOI] [PubMed] [Google Scholar]
- 20. Lathem W. W., Crosby S. D., Miller V. L., Goldman W. E. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. U. S. A. 102:17786–17791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemaitre N., Sebbane F., Long D., Hinnebusch B. J. 2006. Yersinia pestis YopJ suppresses tumor necrosis factor alpha induction and contributes to apoptosis of immune cells in the lymph node but is not required for virulence in a rat model of bubonic plague. Infect. Immun. 74:5126–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lilo S., Zheng Y., Bliska J. B. 2008. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect. Immun. 76:3911–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lloyd S. A., Norman M., Rosqvist R., Wolf-Watz H. 2001. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39:520–531 [DOI] [PubMed] [Google Scholar]
- 24. Matsumoto H., Young G. M. 2006. Proteomic and functional analysis of the suite of Ysp proteins exported by the Ysa type III secretion system of Yersinia enterocolitica biovar 1B. Mol. Microbiol. 59:689–706 [DOI] [PubMed] [Google Scholar]
- 25. Mittal R., Peak-Chew S. Y., McMahon H. T. 2006. Acetylation of MEK2 and IkappaB kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc. Natl. Acad. Sci. U. S. A. 103:18574–18579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monack D. M., Mecsas J., Bouley D., Falkow S. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morales V. M., Backman A., Bagdasarian M. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39–47 [DOI] [PubMed] [Google Scholar]
- 28. Mueller C. A., Broz P., Cornelis G. R. 2008. The type III secretion system tip complex and translocon. Mol. Microbiol. 68:1085–1095 [DOI] [PubMed] [Google Scholar]
- 29. Mukherjee S., et al. 2006. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312:1211–1214 [DOI] [PubMed] [Google Scholar]
- 30. Obert S., O'Connor R. J., Schmid S., Hearing P. 1994. The adenovirus E4-6/7 protein transactivates the E2 promoter by inducing dimerization of a heteromeric E2F complex. Mol. Cell. Biol. 14:1333–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Orth K., et al. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920–1923 [DOI] [PubMed] [Google Scholar]
- 32. Palmer L. E., Hobbie S., Galan J. E., Bliska J. B. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953–965 [DOI] [PubMed] [Google Scholar]
- 33. Palmer L. E., Pancetti A. R., Greenberg S., Bliska J. B. 1999. YopJ of Yersinia spp. is sufficient to cause downregulation of multiple mitogen-activated protein kinases in eukaryotic cells. Infect. Immun. 67:708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parkhill J., et al. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523–527 [DOI] [PubMed] [Google Scholar]
- 35. Persson C., Carballeira N., Wolf-Watz H., Fallman M. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16:2307–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pujol C., Bliska J. B. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 71:5892–5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pujol C., et al. 2009. Yersinia pestis can reside in autophagosomes and avoid xenophagy in murine macrophages by preventing vacuole acidification. Infect. Immun. 77:2251–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riley G., Toma S. 1989. Detection of pathogenic Yersinia enterocolitica by using Congo red-magnesium oxalate agar medium. J. Clin. Microbiol. 27:213–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosqvist R., Bolin I., Wolf-Watz H. 1988. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect. Immun. 56:2139–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosqvist R., Forsberg A., Rimpilainen M., Bergman T., Wolf-Watz H. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defense. Mol. Microbiol. 4:657–667 [DOI] [PubMed] [Google Scholar]
- 41. Roy D., et al. 2004. A process for controlling intracellular bacterial infections induced by membrane injury. Science 304:1515–1518 [DOI] [PubMed] [Google Scholar]
- 42. Ryndak M. B., Chung H., London E., Bliska J. B. 2005. Role of predicted transmembrane domains for type III translocation, pore formation, and signaling by the Yersinia pseudotuberculosis YopB protein. Infect. Immun. 73:2433–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schlumberger M. C., et al. 2005. Real-time imaging of type III secretion: Salmonella SipA injection into host cells. Proc. Natl. Acad. Sci. U. S. A. 102:12548–12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simonet M., Falkow S. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect. Immun. 60:4414–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simonet M., Richard S., Berche P. 1990. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 58:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smiley S. T. 2008. Immune defense against pneumonic plague. Immunol. Rev. 225:256–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Viboud G. I., Bliska J. B. 2005. Yersinia outer proteins: role in modulation of host cell signalling responses and pathogenesis. Annu. Rev. Microbiol. 59:69–89 [DOI] [PubMed] [Google Scholar]
- 48. Woestyn S., Sory M. P., Boland A., Lequenne O., Cornelis G. R. 1996. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol. Microbiol. 20:1261–1271 [DOI] [PubMed] [Google Scholar]
- 49. Wong K. W., Isberg R. R. 2005. Yersinia pseudotuberculosis spatially controls activation and misregulation of host cell Rac1. PLoS Pathog. 1:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wren B. W. 2003. The yersiniae—a model genus to study the rapid evolution of bacterial pathogens. Nat. Rev. Microbiol. 1:55–64 [DOI] [PubMed] [Google Scholar]
- 51. Young B. M., Young G. M. 2002. Evidence for targeting of Yop effectors by the chromosomally encoded Ysa type III secretion system of Yersinia enterocolitica. J. Bacteriol. 184:5563–5571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Y., Bliska J. B. 2003. Role of Toll-like receptor signaling in the apoptotic response of macrophages to Yersinia infection. Infect. Immun. 71:1513–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Y., Bliska J. B. 2010. YopJ-promoted cytotoxicity and systemic colonization are associated with high levels of murine interleukin-18, gamma interferon, and neutrophils in a live vaccine model of Yersinia pseudotuberculosis infection. Infect. Immun. 78:2329–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang Y., Murtha J., Roberts M. A., Siegel R. M., Bliska J. B. 2008. Type III secretion decreases bacterial and host survival following phagocytosis of Yersinia pseudotuberculosis by macrophages. Infect. Immun. 76:4299–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Y., Ting A. T., Marcu K. B., Bliska J. B. 2005. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J. Immunol. 174:7939–7949 [DOI] [PubMed] [Google Scholar]
- 56. Zlokarnik G., et al. 1998. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science 279:84–88 [DOI] [PubMed] [Google Scholar]