Abstract
Gamma interferon (IFN-γ) induces expression of the tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenase (IDO1) in human epithelial cells, the permissive cells for the obligate intracellular bacterium Chlamydia trachomatis. IDO1 depletes tryptophan by catabolizing it to kynurenine with consequences for C. trachomatis, which is a tryptophan auxotroph. In vitro studies reveal that tryptophan depletion can result in the formation of persistent (viable but noncultivable) chlamydial forms. Here, we tested the effects of the IDO1 inhibitor, levo-1-methyl-tryptophan (L-1MT), on IFN-γ-induced C. trachomatis persistence. We found that addition of 0.2 mM L-1MT to IFN-γ-exposed infected HeLa cell cultures restricted IDO1 activity at the mid-stage (20 h postinfection [hpi]) of the chlamydial developmental cycle. This delayed tryptophan depletion until the late stage (38 hpi) of the cycle. Parallel morphological and gene expression studies indicated a consequence of the delay was a block in the induction of C. trachomatis persistence by IFN-γ. Furthermore, L-1MT addition allowed C. trachomatis to undergo secondary differentiation, albeit with limited productive multiplication of the bacterium. IFN-γ-induced persistent infections in epithelial cells have been previously reported to be more resistant to doxycycline than normal productive infections in vitro. Pertinent to this observation, we found that L-1MT significantly improved the efficacy of doxycycline in clearing persistent C. trachomatis forms. It has been postulated that persistent forms of C. trachomatis may contribute to chronic chlamydial disease. Our findings suggest that IDO1 inhibitors such as L-1MT might provide a novel means to investigate, and potentially target, persistent chlamydial forms, particularly in conjunction with conventional therapeutics.
INTRODUCTION
Chlamydia trachomatis is an obligate intracellular bacterium that has a tropism for the columnar epithelial cells of the conjunctiva (serovars A to C) and the urogenital tract (serovars D to K) (42). Most C. trachomatis infections are asymptomatic and therefore remain undetected (36). While many individuals eventually clear infection, clearance can take several months to several years (25, 26). If left untreated, C. trachomatis infections can progress to chronic inflammatory disease. Chronic ocular infection can result in trachoma, one of the leading causes of preventable blindness (43, 55). C. trachomatis is also the major bacterial sexually transmitted agent worldwide, commonly infecting the male urethra and female endocervix (42). Bacteria can ascend from the endocervix into the endometrium and fallopian tubes; chronic infection at these sites is associated with pelvic inflammatory disease (PID) and salpingitis, potentially resulting in tubal infertility or ectopic pregnancy (reviewed in reference 13). The pathological sequelae observed during chronic C. trachomatis infections have been proposed to result from the induction of a sustained inflammatory response to persistent bacteria (43). “Chlamydial persistence” in vitro has been described as an alternative phase of the chlamydial developmental cycle in which bacteria enter a morphologically distinct viable but noncultivable growth stage that can result in a long-term relationship with their host cells (8). Indirect evidence that C. trachomatis could persist in vivo in this altered growth state arises from studies describing the detection of chlamydial antigen and nucleic acid in tissues in the absence of cultivability (22, 35) and documentation of recurrent chlamydial disease when reinfection was unlikely (18, 54).
The C. trachomatis developmental cycle is biphasic, usually characterized by alternating infectious elementary body (EB) and noninfectious reticulate body (RB) stages. EBs infect epithelial cells and then differentiate into RBs within a host-derived vacuole termed an “inclusion” (28). After RB multiplication by binary fission, RBs redifferentiate into EBs and egress from infected epithelial cells to infect neighboring cells (reviewed in reference 1). However, this developmental cycle is disrupted under stressful growth conditions in vitro, such as those imposed by gamma interferon (IFN-γ) (1, 10), antibiotics (17, 23), nutrient deprivation, herpes simplex virus (HSV) coinfection (57), and extracellular adenosine (37). Under these conditions, bacteria survive by entering into a persistent mode of growth that is typified by the formation of large, morphologically aberrant RBs (ARBs) in which bacterial chromosomes continue to divide, but binary fission and differentiation to EB are inhibited. Removal of the stress generally results in reversal of these changes and differentiation into infectious EBs (9).
The in vitro model of IFN-γ-induced persistence and reactivation of C. trachomatis has been particularly well studied (5, 6, 10). In human epithelial cells, IFN-γ induces the expression of the enzyme indoleamine-2,3 dioxygenase (IDO1), which catabolizes tryptophan to kynurenine (20, 53). Such depletion interferes with the growth of C. trachomatis, which is a tryptophan auxotroph (14, 16). Continuous exposure to IFN-γ at inhibitory concentrations results in the eradication of the infection. However, exposure to subinhibitory IFN-γ concentrations, which may be a likely scenario in vivo, results in the development of persistent forms (6). The ability to develop into a persistent form may be a key mechanism by which C. trachomatis survives and establishes long-term infections in the human host (21). Persistent forms may also be associated with the pathology observed during chronic C. trachomatis infection (6–8) and may underlie recurrent disease in the absence of documented reinfection (18, 54). Hence, strategies to block development of persistent forms and/or to eradicate established persistent forms could likely benefit the host.
The IDO inhibitor 1-methyl-dl-tryptophan (1-MT) has previously been demonstrated to inhibit IDO-mediated tryptophan catabolism in C. pneumoniae-infected cells (33, 34). Levo-methyl-tryptophan (L-1MT) is a specific IDO1 inhibitor that can accumulate to steady-state levels in vivo that are sufficient to inhibit this enzyme (51). Nontoxic IDO inhibitors, similar to L-1MT, have already entered clinical trials as potential immunotherapeutic agents for the treatment of cancer (http://clinicaltrials.gov/ct2/show/NCT00739609), because they trigger antitumor immunity, partially by improving T-cell function (38), and act synergistically with conventional chemotherapy (29). In this in vitro study, we investigated the effects of L-1MT on C. trachomatis persistence. The results reported here indicate that L-1MT blocks IFN-γ-induced persistence of C. trachomatis and reactivates C. trachomatis from established persistent growth. Significantly, L-1MT limited the production of infectious bacteria under each of the experimental conditions and improved the efficacy of doxycycline in eradicating persistent bacterial forms. In addition to providing a novel mechanism to target IFN-γ-induced doxycycline-resistant persistent forms, L-1MT can be useful to further our understanding of the IFN-γ–tryptophan–IDO1 axis, the ability of C. trachomatis to persist, and its consequences to the host.
MATERIALS AND METHODS
C. trachomatis infections and cell culture conditions.
HeLa 229 cells (hereafter, “HeLa cells”) were obtained from the ATCC and routinely propagated using antibiotic-free complete RPMI 1640 (cRPMI) culture medium, consisting of RPMI 1640 (Invitrogen) supplemented with 200 mM glutamine (Invitrogen), 0.25% (wt/vol) glucose (Sigma), and 10% fetal calf serum (Invitrogen). Human AB serum (Sigma) was used in place of fetal calf serum (FCS) in performing the described experiments (RP-10). Cells were seeded in 6- or 12-well culture plates in RP-10 and cultivated for 24 to 36 h before infection. C. trachomatis serovar D (D/UW-3/Cx) was used to infect HeLa cells at a multiplicity of infection (MOI) of 2 in all experiments (15). Infections were performed by adding 1 ml/well of C. trachomatis serovar D diluted in SPG (10 mM sodium phosphate [pH 7.2], 0.25 M glucose, 5 mM l-glutamic acid) to 6-well culture plates, which were centrifuged at 400 × g for 40 min at 37°C. Immediately after infection, SPG was removed and replaced with RP-10 alone, 10 ng/ml (200 U/ml) IFN-γ (Peprotech), L-1MT (Sigma), or L-1MT plus 10 ng/ml IFN-γ. In some cases, 40 μg/ml tryptophan was added to cultures with L-1MT in the presence or absence of IFN-γ. L-1MT was used at 0.05 to 1.0 mM, with the majority of experiments conducted with 0.2 mM. The concentration used in this study was based on preliminary titration experiments and published utilization of L-1MT in cancer studies. The concentration of IFN-γ that was chosen induced persistent forms of C. trachomatis, as defined by a noncultivable culture with small inclusions that contained few, enlarged RBs and that could be reactivated within 24 h upon removal of IFN-γ and replacement with fresh RP-10 (10). All cultures were harvested at 38 h postinfection (hpi) unless otherwise stated. Trypsin-EDTA solution (Invitrogen) was used to detach the cells from the culture plates, and harvested cells were washed once with phosphate-buffered saline (PBS) before proceeding to the appropriate experiments. For experiments in which recoverable inclusion-forming units (IFU) were quantified, cell culture supernatants were spun at 16,000 × g to collect released EBs and cell lysates were prepared by scraping cells from the culture plates. All pellets from culture supernatants and cell lysates were resuspended in SPG and frozen at −80°C until ready to titrate on fresh monolayers of HeLa cells. Before use, samples were thawed in a 37°C water bath and sonicated for 90 s. A fresh monolayer of confluent HeLa cells was infected with 1:5 to 1:1,000 dilutions of cell lysates, and the recoverable IFU/ml from each treatment were calculated as previously described (47).
qPCRs.
For quantitative PCRs (qPCRs), cultured cells were collected, suspended in RNA stabilization solution (RNAlater; Ambion), and stored at −80°C until used. Cell samples suspended in RNAlater were diluted with an equal volume of PBS and centrifuged at 2,800 × g for 20 min. The supernatants were discarded and the isolation of total RNA from cell pellets was carried out using the RNeasy minikit (Qiagen) following the manufacturer's instructions. After determination of the RNA concentration in each sample based on optical density at 260 nm using a NanoDrop 1000 spectrophotometer (Thermo Scientific), 2 μg total RNA was reverse transcribed into cDNA. Random hexonucleotide primer and Superscript III (Invitrogen) were used in the reverse transcription reaction.
qPCR for IDO1.
Real-time quantitative PCR (qPCR) analysis was performed on the ABI Prism 7500 system (Applied Biosystems) to semiquantitatively evaluate the gene expression of IDO1. A mixture containing sample cDNA (40 ng), specific primer sets (4 pmol), and PCR premix (Power SYBR green PCR master mix; Applied Biosystems) was added to each well in a 96-well plate. All PCRs were carried out in triplicate. The expression of β-actin was evaluated in each sample for the normalization of total RNA levels among samples. The primer sequences used were IDO1 (NM_002164) sense 5′-AGA AGT GGG CTT TGC TCT GC-3′ and antisense 5′-TGG CAA GAC CTT ACG GAC ATC TC-3′ and β-actin (NM_001101.2) sense 5′-CAT GTA CGT TGC TAT CCA GGC-3′ and antisense 5′-CTC CTT AAT GTC ACG CAC GAT-3′. The PCR cycle protocol consisted of 50°C for 2 min (UDG incubation), 95°C for 2 min (denaturation), and 45 cycles of 95°C for 15 s and 60°C for 60 s, followed by melting curve analysis. The PCR product inserted into the pCRII-TOPO plasmid vector was subcloned using a TOPO TA cloning kit (Invitrogen). Its nucleotide sequence was confirmed using standard sequence analysis.
qPCR for chlamydial genes.
For bacterial trancription analyses, primer/probe sets were designed for the C. trachomatis euo, groEL1, trypB, omcB, and hctA genes using Primer Express software (Applied Biosystems). β-Actin quantification was carried out using TaqMan β-actin assay control reagents (Applied Biosystems). Standard curves were generated using purified chromosomal template DNA at concentrations ranging from 10 to 0.001 ng/well (data not shown). Bacterial genome copy numbers were quantified (Universal PCR system; Applied Biosystems) using DNA preparations of a portion of each sample. Assays were performed (One-Step RT-PCR master mix reagent; Applied Biosystems) using RNA preparations from samples exposed to different culture conditions. Copy numbers were calculated and normalized to genome copy numbers (7500 sequence detector; ABI Prism). The primer/probe sets used are listed in Table 1.
Table 1.
Sequences of the primer and probe sets used for qPCR for chlamydial genes
| Chlamydial gene | Sequence of: |
||
|---|---|---|---|
| Forward primer | Reverse primer | Probe | |
| euo | ATCTGTCTACTTCCGAAACGTGAA | CGCATTGGTGCTATGAAAGGA | CCCAAGCAGATCCCCGACGCT |
| groEL1 | AGGACGGCCGGATTCAG | AAGACGCTTTGGTTCTAATCTACGA | TGTAAAACAGGAAGGAAATCTTTGATCCCAGAA |
| trpB | CACTCCATTTCCGCTGGA | GCTCGTCCTGACTCATGCATT | CTTCAGTTGGGCCAGATCATGCCG |
| omcB | CTCTAACACCCCGCTAGCAAA | TCATCAGACGAGCAGTGACGAT | AACTCGCCACACTAGTCACCGCGAA |
| hctA | CTTTACGATATACCTTCGCGATCTT | GTATCCAACAAAATTTGCTTAAAGCA | ACTCTTTGTGCTGCGGCTTTATTTCCTTTTT |
Immunoblotting for IDO1.
For immunoblot analyses of IDO1 expression, mock-infected cells and C. trachomatis-infected cells were counted using a Coulter counter (Beckman) and pelleted by centrifugation at 200 × g. The cell pellets were suspended in sample buffer (100 mM Tris, 10% [wt/vol] SDS, 10% [vol/vol] glycerol, 5% [vol/vol] β-mercaptoethanol, 1% bromophenol blue) at 5 × 104 cells/μl. Cells were then lysed by ultrasonication for 10 short pulses. SDS-PAGE and immunoblotting were performed using a standard protocol. IDO1 protein was visualized using 2 μg/ml anti-IDO1 mouse primary monoclonal antibody (Millipore) followed by an anti-mouse secondary antibody conjugated to horseradish peroxidase (HRP). β-Actin was used as a loading control using 40 ng/ml anti-β-actin polyclonal antibody (Abcam) followed by an anti-rabbit secondary antibody conjugated to HRP. The detection of immunoblots was performed using a conventional chemiluminescence method as described earlier (44).
Tryptophan and kynurenine measurement by reverse-phase HPLC.
The levels of tryptophan and kynurenine in cell culture supernatants were assessed at 8, 20, and 38 h postinfection by reverse-phase high-performance liquid chromatography (HPLC). To prepare samples for HPLC, a 200-μl aliquot of each cell culture supernatant was added to 800 μl HPLC-grade methanol and vortexed to mix. The mixture was incubated for 15 min at −20°C and centrifuged at 16,300 × g for 15 min at 4°C. The resulting supernatants were stored at −80°C until ready to use.
The HPLC system consisted of a Dionex Ultimate 3000 (Sunnyvale, CA) with a photodiode array UV detector. Chromatographic separation was achieved using a 150- by 4.6-mm (inner diameter) C18 reverse-phase column (particle size, 3 μm; ACCLAIM 120 C-18). For tryptophan measurement, the column was equilibrated at a flow rate of 0.5 ml/min with 0.1 M sodium acetate buffer (pH 5.0) and then eluted with a linear gradient consisting of methanol (100%) and acetonitrile (80%). For kynurenine determination, the column was eluted with a linear gradient consisting of methanol (100%) and acetonitrile (80%). The absorbance of the column effluent was monitored at 280 and 365 nm for tryptophan and kynurenine, respectively. The peaks of tryptophan or kynurenine were identified by comparison with the retention times of standard compounds (Sigma), and quantification was based on the ratios of the peak areas of compound. The limit of quantification for tryptophan and kynurenine is 0.2 μM.
L-1MT toxicity study.
Cellular toxicity of L-1MT was assessed by culturing HeLa cells in medium alone or with 0.2 mM L-1MT or 1 mM L-1MT for 48 h. As a positive control, a HeLa cell culture was exposed to 1 mM H2O2 for 2 h to induce apoptosis or cell death. Following incubation of the HeLa cell cultures, the culture supernatants were harvested and placed in 15-ml conical tubes. Adherent cells were then washed with PBS, detached using 0.25% trypsin-EDTA solution (Sigma), and neutralized by the addition of cRPMI. Detached cells were then added to the corresponding culture supernatant, and the cells were centrifuged at 350 × g at 10°C for 5 min to retrieve cell pellets. Staining using an annexin V-fluorescein isothiocyanate (FITC) conjugate and propidium iodide (PI) was performed following the manufacturer's protocol (annexin V-FITC detection kit; Calbiochem).
Immunofluorescent staining and deconvolution microscopy.
HeLa cells were cultivated on type 1 coverslips in 12-well plates for 24 to 36 h and then infected with C. trachomatis as described. At 38 to 72 hpi, the cells were fixed with 4% paraformadehyde and then permeabilized using 0.5% saponin (Sigma). To prevent nonspecific staining, the cells were blocked with Background Sniper blocking reagent. (Biocare Medical). Meriflour anti-chlamydial lipopolysaccharide (LPS) conjugated to fluorescein isothiocyanate (FITC) was used to stain C. trachomatis. Nucleic acid was counterstained with DAPI (4′,6-diamidino-2-phenylindole) (Molecular Probes). Stained cells were mounted with Prolong Gold antifade reagent (Invitrogen) and viewed under a fluorescence microscope. Images were acquired at 40× and 63× magnifications on an inverted Zeiss AxioVision AX10 microscope with an AxioCam MR camera. Thirteen 200-nm Z-sections were deconvolved using a constrained iterative Fourier transform with 30 to 40 iterations to obtain the final images. Point spread functions were calculated as described previously (44).
Ultrastructural analyses.
C. trachomatis-infected HeLa cells grown in RP-10 alone, RP-10 with 0.2 mM L-1MT, RP-10 with 10 ng/ml IFN-γ, or RP-10 with 10 ng/ml IFN-γ and 0.2 mM L-1MT were harvested at 38 hpi and 48 hpi. The cells were pelleted by centrifugation (200 × g) and washed twice by resuspension in 100 mM phosphate-buffered saline (PBS), pH 7.2. Cells were then fixed with 2.5% glutaraldehyde–2% parafomaldehyde and stored at 4°C until ready for use. Fixed cells were processed for transmission electron microscopy, as previously described (6). Electron micrographs were evaluated using ImageJ (2), as described here. At least 10 separate micrographs were evaluated for each experimental condition. After appropriately setting the scale, the freehand selection tool was used to determine the cross-sectional area of each inclusion. Images were thresholded prior to using the particle analysis function to enumerate the number of EBs per cross-sectional inclusion area. For consistency, the same threshold value (100) was used for all micrographs, and EBs were sized as between 0.02 and 0.18 μm2, with a circularity of 0.45 to 1.0. Heterogeneity in EB size arises because not every EB within the cross-sectional area is within the same plane. These values were chosen after tests using subtractive masking with several images to ensure that no RBs were selected using these parameters. Subtractive masking was also used to enumerate the number of RBs per inclusion. Aberrant inclusions were independently counted by two individuals.
Doxycycline and L-1MT treatment and assessment of recoverable IFU.
HeLa cells were seeded in 12-well plates 24 h before infection. Cells were then infected with serovar D and cultivated in RP-10 with 10 ng/ml IFN-γ for 38 h to induce persistence. In the initial experiments, persistent cultures were exposed to doxycycline concentrations ranging from 0.05 to 50 μg/ml. In these experiments, it was observed that exposure to doxcycline concentrations above 20 μg/ml for 48 h was toxic to HeLa cells, consistent with previous observations with other mammalian cell lines (19, 41). Doxycycline, at a concentration of 1 μg/ml, was used alone or in combination with 0.2 mM L-1MT in the experiments reported here. To assess the recoverable IFU, treated cells were reactivated by replacing the medium with RP-10 with 40 μg/ml tryptophan and 1 μg/ml cycloheximide for 48 h. Culture media in C. trachomatis-infected HeLa cell cultures were replaced with SPG, and the cells were scraped from the culture plates. The cell scrapings were frozen at −80°C until ready to use, and the number of recoverable IFU was determined as described earlier.
Statistical analyses.
Statistical analyses were based on data from three to five experiments. Student's t test (50) was utilized to determine differences between control and experimental conditions.
RESULTS
IFN-γ induces IDO1 expression in HeLa 229 cells.
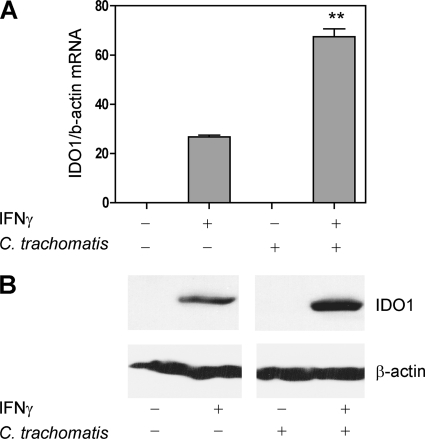
Exposure of HeLa cells to IFN-γ is well documented to induce IDO1 expression (53). Therefore, HeLa cells were exposed to 10 ng/ml IFN-γ and the level of IDO1 induction in C. trachomatis serovar D infection was assessed. Under these conditions, infection rates of 40 to 50% were detected at 38 hpi; pertinently, cycloheximide, an inhibitor of translation routinely used to prevent the proliferation of mammalian cells, was not added to the HeLa cultures in these experiments to prevent inhibition of IDO1 expression (5). IDO1 mRNA expression was first determined in C. trachomatis-infected HeLa cells and matched mock-infected control cultures cultivated under different conditions using quantitative real-time PCR. We observed that 10 ng/ml of IFN-γ induced IDO1 mRNA by approximately 30-fold in uninfected HeLa cells (Fig. 1 A). Strikingly, induction was significantly more pronounced (∼70-fold) in C. trachomatis-infected HeLa cells that were treated with IFN-γ (P < 0.001). The increased induction did not result from C. trachomatis infection alone because IDO1 induction was not observed in those C. trachomatis-infected cells not exposed to IFN-γ. As anticipated, the IDO1 inhibitor L-1MT (0.2 mM) did not affect IDO1 mRNA expression in either mock- or C. trachomatis-infected cells exposed to IFN-γ (data not shown).
Fig. 1.
IDO1 expression in IFN-γ-treated and C. trachomatis-infected HeLa 229 cells. Cells were mock infected by centrifugation with SPG alone or infected with C. trachomatis serovar D and cultivated in the absence or presence of 10 ng/ml IFN-γ for 38 h. Infection was performed in the absence of cycloheximide, at an MOI of 2, to achieve 40 to 50% infection at 38 hpi. (A) HeLa cell IDO1 mRNA expression at 38 hpi was assessed by real-time PCR. IDO1 expression was normalized to β-actin, and results are presented as fold induction relative to control (uninfected, no IFN-γ) HeLa cells. The data presented are the means and standard deviations from 3 separate experiments. The asterisks indicate that IDO1 was induced to a significantly higher level by IFN-γ exposure of C. trachomatis-infected HeLa cells relative to uninfected cells (P < 0.001). (B) Representative immunoblot of HeLa cell lysates probed with an anti-IDO1 monoclonal antibody. IDO1 migrated at ∼42 kDa relative to molecular mass standards. β-Actin was used as a loading control, and all proteins migrated with molecular masses similar to those predicted.
The levels of IDO1 mRNA were corroborated with IDO1 protein levels using immunoblot analysis (Fig. 1B). Consistent with the mRNA analysis, exposure to IFN-γ resulted in detectable levels of IDO1 protein in mock-infected HeLa cells, with higher levels of expression observed when C. trachomatis-infected cells were exposed to IFN-γ. Together these observations indicate that C. trachomatis infection of HeLa cells potentiates IDO1 induction by IFN-γ. While not detected by our immunoblot, we note that it is likely that HeLa cells not exposed to IFN-γ express low baseline levels of IDO1 protein. This is because addition of L-1MT to control HeLa cells resulted in a small increase in tryptophan levels as measured by HPLC (Fig. 2 A) (data not shown).
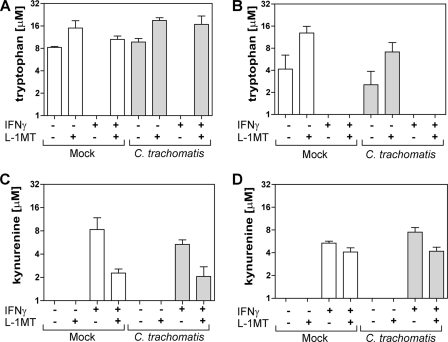
Fig. 2.
Partial inhibition of IDO1-mediated tryptophan catabolism to kynurenine by L-1MT during C. trachomatis infection of HeLa 229 cells. Tryptophan and kynurenine concentrations in culture supernatants. (A) Tryptophan at 20 hpi; (B) tryptophan at 38 hpi; (C) kynurenine at 20 hpi; (D) kynurenine at 38 hpi. Concentrations of tryptophan and kynurenine were measured by reverse-phase HPLC at 20 and 38 hpi in cell culture supernatants from mock-infected and C. trachomatis-infected HeLa cells cultivated in the presence and absence of 10 ng/ml IFN-γ, 0.2 mM L-1MT, or both. The data presented are the means and standard deviations from 3 separate experiments.
IDO1-mediated tryptophan catabolism is inhibited at 20 h postinfection by the addition of L-1MT.
IDO catabolizes tryptophan via the kynurenine pathway (46, 53). Therefore, tryptophan and kynurenine levels in cell culture supernatants can be used to determine the induction of IDO1 activity by IFN-γ. We examined tryptophan and kyurenine levels in mock-infected and C. trachomatis-infected HeLa cells under the following conditions: (i) in medium alone, (ii) with addition of 0.2 mM L-1MT, (iii) with addition of 10 ng/ml IFN-γ, or (iv) with addition of both 10 ng/ml IFN-γ and 0.2 mM L-1MT. Because one developmental cycle of C. trachomatis serovar D is typically completed in 48 h, tryptophan catabolism was measured in culture supernatants at early (8 hpi), middle (20 hpi), and mid-late (38 hpi) stages of chlamydial development. No significant difference in tryptophan levels was observed between the different culture conditions at 8 hpi (data not shown). At 20 hpi, tryptophan fell below the level of detection (0.2 μM) in cell cultures exposed to IFN-γ alone, but not in 0.2 mM L-1MT-supplemented IFN-γ-exposed cultures (Fig. 2A). These results indicate that L-1MT inhibited IFN-γ-induced IDO1 activity at the mid-stage (20 hpi) of C. trachomatis development. At 38 hpi, tryptophan levels in all culture supernatants from IFN-γ-exposed HeLa cells were below the 0.2 μM detection limit, in contrast to C. trachomatis-infected cells treated with IFN-γ plus L-1MT, which retained 0.26 μM tryptophan. This suggests that >99% of tryptophan in the culture media was either utilized or catabolized in all cultures exposed to IFN-γ at this later stage of C. trachomatis development (Fig. 2B). Furthermore, at 20 and 38 hpi, kynurenine was produced in all HeLa cell cultures that were exposed to 10 ng/ml IFN-γ (Fig. 2C and D). Because it was confirmed that kynurenine was absent in unused culture media under the four conditions evaluated, its presence in culture supernatants indicates that IFN-γ-induced IDO1 activity resulted in kynurenine production, as reported previously (32, 52, 58). We also noted that significant differences in kynurenine production could only be observed after a shorter period (20 hpi) of IFN-γ exposure, at which time kynurenine is unlikely to have been catabolized into downstream metabolites.
L-1MT has no toxic effects on HeLa 229 cells and does not inhibit C. trachomatis growth.
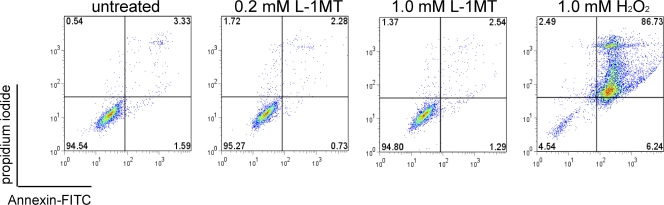
To assess the toxicity of L-1MT on host cells, HeLa cell cultures were exposed to 0.2 mM or 1 mM L-1MT for 48 h and assessed for apoptosis or necrosis by double staining using annexin V and propidium iodide (PI). Flow cytometric analyses demonstrated that exposure of HeLa cells to 0.2 mM and 1 mM L-1MT (i.e., five times more than the dosage used in this study) allowed the recovery of ∼95% viable cells, which is comparable to the untreated negative control (Fig. 3). In contrast, HeLa cells exposed to 1 mM H2O2 displayed both apoptosis and necrosis (Fig. 3).
Fig. 3.
Flow cytometric analyses of L-1MT toxicity on HeLa 229 cells. Double staining using FITC-conjugated annexin V and propidium iodide was used to profile control HeLa cells or cells exposed to 0.2 mM or 1.0 mM L-1MT. The levels of apoptosis or necrosis detected under these conditions did not differ from control conditions. Treatment with 1.0 mM H2O2 was used as a positive control to confirm that the reagents detected apoptotic and necrotic cells.
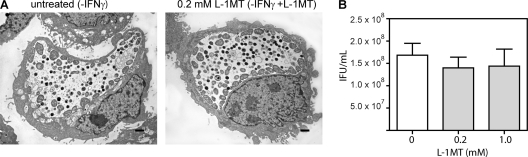
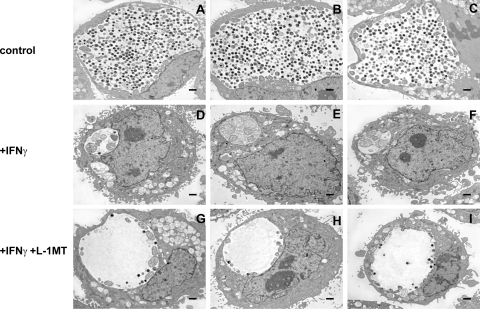
We also demonstrated that L-1MT alone does not alter the normal development of C. trachomatis, as shown by the similar ultrastructural morphology of the inclusions formed in the presence of 0.2 mM L-1MT and the untreated control (Fig. 4 A). In addition, neither 0.2 mM nor 1 mM L-1MT decreased recoverable IFU from C. trachomatis-infected cells at 48 hpi (Fig. 4B).
Fig. 4.
L-1MT does not affect the normal developmental cycle of C. trachomatis. The developmental growth of C. trachomatis in the absence or presence of 0.2 mM L-1MT was evaluated by ultrastructural analyses (A) and IFU recovery (B). Infected cells were harvested at 38 hpi for the ultrastructural comparison between untreated control and 0.2 mM L-1MT-treated infected cells. IFU were quantified as previously described from cells scraped at 48 hpi.
L-1MT blocks IFN-γ-induced C. trachomatis persistence.
IFN-γ can induce C. trachomatis to enter into a persistent mode of growth (40). Inclusions formed during IFN-γ-mediated persistence are small and contain aberrant RBs (ARBs) that display a distinct gene expression profile. IFN-γ-induced IDO expression has been demonstrated to restrict C. trachomatis growth, which is attributed to tryptophan depletion because it is reversed by addition of tryptophan. In the experiments described below, the temporal inhibition of IDO1 activity by 0.2 mM L-1MT was observed to block IFN-γ-induced C. trachomatis persistence. We assessed the capacity of 0.2 mM L-1MT to inhibit the effects of IFN-γ using quantitative analyses of inclusion images obtained by electron microscopy and through gene expression studies.
To determine the effect of L-1MT on C. trachomatis growth at the ultrastructural level, a morphological assessment of C. trachomatis inclusions formed under different growth conditions was performed at 48 hpi and quantitatively evaluated using ImageJ software (Table 2). The mean inclusion cross-sectional area of control cultures was 120.9 μm2, with a mean of 146 EBs per inclusion (Fig. 5 A to C). This is characteristic of normal development. Treatment with IFN-γ (Fig. 5D to F) significantly decreased the average cross-sectional area of inclusions to 18.8 μm2 (P < 0.0001), with a mean of less than one EB, and eight aberrant RBs per inclusion. Relative to treatment with IFN-γ alone, exposure to both IFN-γ and L-1MT (Fig. 4G to I) significantly increased the average inclusion area to 46.7 μm2, decreased the mean number of aberrant forms to less than one per inclusion (P < 0.0001), and increased average EB numbers to 13/inclusion (P < 0.0001). To evaluate the possibility that there were condition-specific differences in the number of EBs and RBs per inclusion, alterations in inclusion size and the numbers of EBs, RBs, and aberrant forms per cross-sectional area were also calculated (Table 3). The results of these analyses corroborate the pattern of findings presented in Table 2 and eliminate the possibility that decreased numbers of EBs and RBs resulted from a proportional decrease in the size of the inclusion. Thus, ultrastructural analyses indicate that addition of L-1MT to C. trachomatis-infected cells induced the formation of inclusions with unique morphological characteristics that are distinct from persistent inclusions.
Table 2.
Partial reversion by L-1MT of the effect of IFN-γ on inclusion size and contents as shown by ultrastructural analyses
| Expt | Area (μm2)a | No. ofb: |
||
|---|---|---|---|---|
| EBs/ inclusion | RBs/ inclusion | ARBs/inclusion | ||
| Control | 120.9 ± 40c | 146 ± 82c | 33.2 ± 18.2c | 0.1 ± 0.3c |
| +IFN-γ | 18.8 ± 8.6 | 0.5 ± 0.8 | 0.5 ± 0.8 | 7.6 ± 3.1 |
| +IFN-γ, +L-1MT | 46.7 ± 27.9d | 13.4 ± 8.3d | 8.6 ± 6.3d | 0.9 ± 1.4d |
Values represent the mean ± standard deviation inclusion cross-sectional area in 10 randomly selected electron micrographs (EMs).
Values represent the mean ± standard deviation numbers of elementary bodies (EBs), reticulate bodies (RBs), and aberrant reticulate bodies (ARBs) observed in 10 randomly selected EMs. EBs were counted using ImageJ as described in Materials and Methods. The distinction between RBs and ARBs was scored independently by two individuals.
Values under control conditions were determined to be significantly different from the other conditions using Student's t test (P < 0.0001).
Values observed in the presence of IFN-γ and L-1MT were significantly different from those observed in the presence of IFN-γ alone (Student's t test, P < 0.0001).
Fig. 5.
Treatment with L-1MT results in a unique morphological characteristic of C. trachomatis inclusions. Electron micrographs of 3 representative inclusions from C. trachomatis grown in medium alone (control) (A to C), medium with 10 ng/ml IFN-γ (D to F), and medium with 10 ng/ml IFN-γ together with 0.2 mM L-1MT (G to I). The scale bars represent 0.5 μm. The cells were harvested at 48 hpi and processed for transmission electron microscopy. Inclusions that were formed in culture with 10 ng/ml IFN-γ plus 0.2 mM L-1MT formed significantly less EBs and RBs than those that were grown in medium alone.
Table 3.
The reduced number of EBs/μm2 of inclusion area induced by IFN-γ exposure is not reversed by L-1MT
| Expt | No. ofa: |
||
|---|---|---|---|
| EBs/μm2 | RBs/μm2 | ARBs/μm2 | |
| Control | 1.18 ± 0.45 | 0.29 ± 0.14 | 0.001 ± 0.002 |
| +IFN-γ | 0.03 ± 0.06 | 0.02 ± 0.03 | 0.412 ± 0.412 |
| +IFN-γ, +L-1MT | 0.33 ± 0.17 | 0.21 ± 0.16 | 0.034 ± 0.059 |
The values shown represent means ± standard deviations from the same 10 samples analyzed in Table 1.
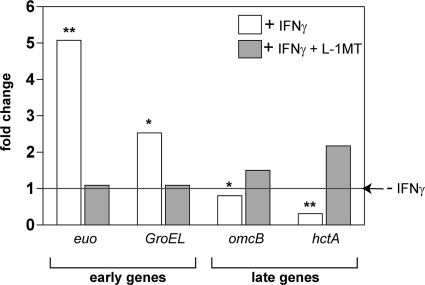
To further confirm at the molecular level whether L-1MT treatment blocks the induction of persistence, we performed gene expression analyses of two early chlamydial genes (euo and groEL) and two late chlamydial genes (omcB and hctA). A comparison of the gene expression pattern of C. trachomatis grown in L-1MT-treated-culture with that of persistent forms relative to the normal growth control is shown in Fig. 6. Consistent with previous data (6, 10), euo expression and groEL expression were upregulated during IFN-γ-induced persistence compared to normal infection (Fig. 6). euo and groEL genes are typically expressed early during chlamydial development. Because we did not observe an upregulation of euo or groEL in the culture treated with L-1MT, this indicates that these genes are differentially regulated in IFN-γ-induced persistence and IFN-γ–L-1MT exposure. This finding is supported by the observation that in contrast to persistent C. trachomatis cultures, infected cultures grown under conditions that included L-1MT upregulated the late genes omcB and hctA. These genes encode structural and regulatory proteins required for the transition of RBs back into EBs (10, 45). These data demonstrate that unlike persistent growth, C. trachomatis development under conditions with L-1MT allows for normal RB transformation to EBs. Based on the expression of selected early and late genes, the C. trachomatis growth pattern exhibited in the presence of both IFN-γ and L-1MT is distinct from C. trachomatis persistence, displayed in the presence of IFN-γ alone. Together, these data indicate that the L-1MT treatment could block the induction of C. trachomatis persistence by IFN-γ. Intriguingly, exposure to L-1MT resulted in a unique development pattern, as indicated both by ultrastructural studies and by gene expression analyses.
Fig. 6.
C. trachomatis cultures that were exposed to IFN-γ exhibited a unique gene expression pattern with the addition of 0.2 mM L-1MT. Data are expressed as fold change compared to the no-IFN-γ control (medium alone), which was designated a value of 1 (horizontal line). White bars represent gene expression of persistent forms grown in the presence of 10 ng/ml IFN-γ, and gray bars represent gene expression of C. trachomatis in cultures treated with 10 ng/ml IFN-γ and 0.2 mM L-1MT. Statistical significance of the difference in gene expression levels between persistent (+IFN-γ) and IFN-γ- and L-1MT-treated C. trachomatis were evaluated using Student's t test. Comparisons of gene expressions between the two conditions reveal significant differences in the expression level of the indicated gene. *, P < 0.05; **, P < 0.01.
L-1MT treatment results in the limited replication of C. trachomatis.
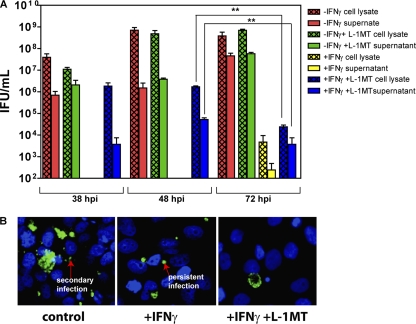
Preliminary studies indicated that L-1MT addition to IFN-γ-treated cells did not reduce the number of C. trachomatis inclusions formed at 38 hpi (data not shown). This is consistent with a study by Pantoja et al. with the C. pneumoniae infection model using 1MT, which is a mix of both L-1MT and dextro-1-methyl tryptophan (D-1MT). The distinct growth pattern of bacteria formed in IFN-γ–L-1MT-exposed cultures prompted us to further evaluate the effect of adding L-1MT to IFN-γ-exposed-cells on chlamydial development. This was achieved by assessing IFUs that could be recovered from cell lysates and cell culture supernatants at 38, 48, and 72 hpi (Fig. 7).
Fig. 7.
L-1MT limits the recovery of infectious elementary bodies from IFN-γ-treated C. trachomatis-infected cell cultures. (A) IFU counts from C. trachomatis-infected HeLa cells cultivated in (i) medium alone (control), (ii) medium supplemented with 0.2 mM L-1MT, (iii) medium supplemented with 10 ng/ml IFN-γ, or (iv) medium supplemented with both 10 ng/ml IFN-γ and 0.2 mM L-1MT. IFU were measured at 38, 48, and 72 hpi. At each time interval, culture supernatants were spun at 16,000 × g and resuspended in SPG. Monolayers of infected HeLa cells were scraped with SPG. Both supernatants and cell lysates were frozen and sonicated before titration on fresh HeLa cells to determine IFU/ml. (B) C. trachomatis growth pattern at 72 hpi. Direct immunofluorescence images were obtained by staining cells fixed on coverslips at 72 hpi using anti-chlamydial LPS conjugated to FITC. Nuclei were counterstained with DAPI.
Characteristic of normal development, the IFU in cell lysates and culture supernatants from control cultures and cultures exposed to L-1MT alone increased over the course of 72 hpi (Fig. 7A). Consistent with the persistent growth of bacteria, cell lysates and culture supernatants from C. trachomatis-infected HeLa cultures exposed to IFN-γ alone did not yield detectable IFU up to 48 hpi and yielded significantly lower IFU at 72 hpi compared to all other culture conditions. In IFN-γ–L-1MT-exposed C. trachomatis-infected HeLa cell cultures, we observed limited IFU from cell lysates and cell culture supernatants collected at 48 hpi, and this further decreased significantly at 72 hpi (Fig. 7A). Furthermore, in contrast to the control, immunofluorescent staining for chlamydia in IFN-γ–L-1MT-exposed cultures revealed little evidence of secondary infections at 72 hpi (Fig. 7B). We also did not observe evidence of persistent forms of C. trachomatis in IFN-γ–L-1MT-exposed cultures (Fig. 7B).
Together these data indicate that addition of L-1MT to IFN-γ-treated cultures blocked the induction of persistence; interestingly, this occurs via a mechanism that severely restricts recoverable IFU.
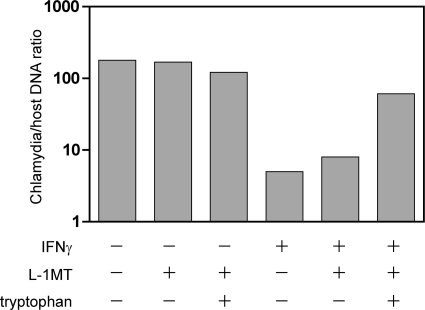
Further support that L-1MT limits the productive multiplication of C. trachomatis exposed to IFN-γ was obtained from data demonstrating that the ratio of chlamydia to host DNA was not significantly increased by the addition of L-1MT (Fig. 8). In contrast, addition of tryptophan to IFN-γ–L-1MT-exposed cells resulted in more than a log-fold increase in the relative chlamydial genome copy number (Fig. 8). These data suggest that unlike tryptophan, which has been demonstrated to induce the reactivation of C. trachomatis from persistent forms to normal productive development, L-1MT does not support the productive multiplication of the bacterium.
Fig. 8.
L-1MT does not alleviate the decrease in chlamydial genome copy numbers in IFN-γ-exposed cultures. Mock-infected and C. trachomatis-infected cells were exposed to 0.2 mM L-1MT or 0.2 mM L-1MT supplemented with 40 μg/ml tryptophan in the presence or absence of IFN-γ for 38 h. Chlamydial genome copy number was quantified by qPCR and normalized to host genome. Here we show that the addition of L-1MT did not significantly increase the chlamydial genome copy number, but addition of exogenous tryptophan was able to increase chlamydial multiplication in the presence of 10 ng/ml IFN-γ and 0.2 mM L-1MT.
Effects of exogenous tryptophan on IFN-γ–L-1MT-exposed C. trachomatis-infected HeLa cells.
To further investigate the effects of the addition of exogenous tryptophan on C. trachomatis-infected L-1MT-treated HeLa cells, qPCR was used to quantify trpB gene expression in cultures with L-1MT or L-1MT supplemented with tryptophan, in the absence or presence of IFN-γ. Our results indicate that the addition of L-1MT to IFN-γ-exposed cells decreased trpB gene expression compared to IFN-γ alone (Fig. 9). However, infected cultures treated with IFN-γ and L-1MT maintained elevated trpB expression compared to untreated (no-IFN-γ) control cultures, indicating that L-1MT by itself does not inhibit trpB expression. We interpret this finding to indicate that even during L-1MT exposure, C. trachomatis continues to respond to tryptophan depletion in the host cell (3, 59). Consistent with this interpretation, a striking decrease in trpB expression was observed when tryptophan was added to cultures treated with IFN-γ and L-1MT. Therefore, exogenous tryptophan can repress the trpB gene expression in the presence of L-1MT (Fig. 9), indicating that L-1MT likely does not interfere with tryptophan transport into the chlamydial inclusion.
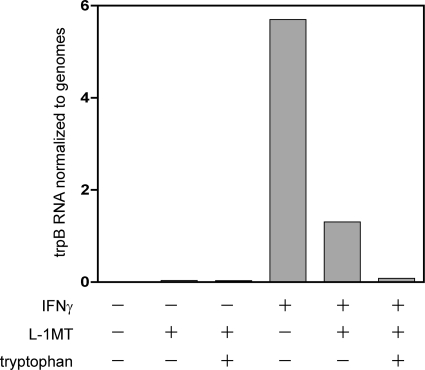
Fig. 9.
Addition of exogenous tryptophan reduced trpB gene expression in IFN-γ- and L-1MT-exposed C. trachomatis-infected HeLa cells. Mock-infected and C. trachomatis-infected HeLa cells were exposed to 0.2 mM L-1MT or 0.2 mM L-1MT and 40 μg/ml tryptophan in the presence or absence of 10 ng/ml IFN-γ. The expression of the trypB gene was quantified and is normalized to chlamydial genome copy number. An elevated level of trpB was observed in cultures exposed to IFN-γ alone. Although still elevated compared to the level in the no-IFN-γ controls, a 4-fold decrease in trpB was observed in cell cultures exposed to IFN-γ plus L-1MT compared to IFN-γ alone. Addition of 40 μg/ml exogenous tryptophan decreased trypB expression to a level comparable to that in the no-IFN-γ controls.
Next, we examined whether the addition of tryptophan raised the recoverable IFU in IFN-γ-exposed cells in the presence of L-1MT. Here we found that the addition of tryptophan not only rescued the persistent growth phenotype, but it could also countermand the decrease in recoverable IFU that results from exposure to L-1MT (Fig. 10). These data in conjunction with our other findings reveal that L-1MT's effect on chlamydial growth primarily arises from its ability to modulate tryptophan levels in cells infected by C. trachomatis and consequently to the bacterium.
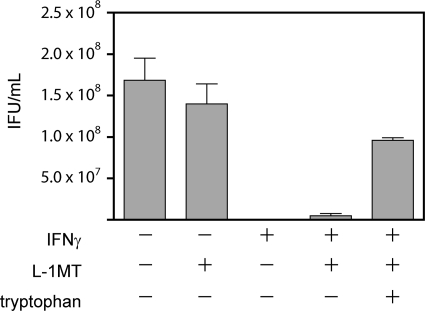
Fig. 10.
Addition of tryptophan can alleviate the limited IFU recovery in C. trachomatis-infected cultures exposed to IFN-γ and L-1MT. C. trachomatis-infected HeLa cells were cultured in the presence or absence of IFN-γ with 0.2 mM L-1MT or 0.2 mM L-1MT with 40 μg/ml exogenous tryptophan. Infected HeLa cells were scraped, frozen, thawed, and sonicated to determine the number of recoverable IFU. The decrease in IFU observed in the presence of IFN-γ was not completely alleviated by the addition of L-1MT alone, but was increased significantly with the addition of exogenous tryptophan, even in the presence of L-1MT.
L-1MT treatment can reactivate C. trachomatis from an established persistent growth mode.
C. trachomatis that have entered into an IFN-γ-induced persistent mode of growth can be reactivated by removing IFN-γ and adding exogenous tryptophan. Therefore, the ability of L-1MT to reactivate an established persistent C. trachomatis infection was examined. For these investigations, 0.2 mM L-1MT was added to IFN-γ-treated cultures at 38 hpi, after persistent forms had developed. Ultrastructural analyses clearly indicated that L-1MT could reactivate C. trachomatis from a persistent mode of growth (Fig. 11 A). A change in the morphology of persistent chlamydial forms was observed as early as 24 h post-L-1MT treatment (Fig. 11A2), and redifferentiation into EBs was observed at 48 h post-L-1MT treatment (Fig. 11A3). However, in contrast to cultures exposed to IFN-γ alone, the number of inclusions in persistent C. trachomatis cultures reactivated with L-1MT decreased significantly over time. Quantitation revealed significantly fewer inclusions at 48 h post-L-1MT treatment compared to 24 h post-L-1MT treatment (P < 0.01) (Fig. 11B). These data indicate that unlike the outcome of conventional reactivation of persistent C. trachomatis by IFN-γ removal and/or tryptophan replenishment, reactivation by adding L-1MT severely restricts bacterial multiplication.
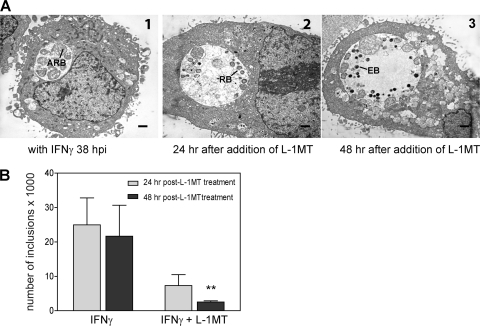
Fig. 11.
L-1MT can reactivate C. trachomatis from persistence but results in a decrease in the number of inclusions. (A1) Electron photomicrograph of inclusion at 38 hpi with IFN-γ: the inclusion has few large aberrant reticulate bodies (ARB) typical of C. trachomatis persistence. (A2) 24 h after L-1MT treatment of a persistent C. trachomatis culture, reticulate bodies (RB) start to form. (A3) 48 h after L-1MT treatment of a persistent C. trachomatis culture, few small and dense elementary bodies (EB) are formed. The scale bars are equivalent to 0.5 μm. (B) Inclusion counts of C. trachomatis-infected cultures after addition of IFN-γ or L-1MT with IFN-γ at 24 hpi (gray bar) and at 48 hpi (black bar). Over time, there was no significant decrease in the number of inclusions in cells treated with IFN-γ alone. Although L-1MT can reactivate C. trachomatis from the persistent growth mode, the number of inclusions decreased over time in cultures treated with L-1MT (**, P < 0.01). Data were pooled from 3 independent experiments.
Treatment with L-1MT sensitizes persistent C. trachomatis to doxycycline.
In a recent study by Reveneau and coworkers, IFN-γ-induced persistent forms of C. trachomatis were more refractory to doxycycline exposure than those that underwent normal development (39). As doxycycline is one of the two antibiotics used most often to treat C. trachomatis infection, we examined the effect of L-1MT on doxycycline resistance of persistent forms of C. trachomatis. Because L-1MT can reactivate C. trachomatis from persistent growth to a limited extent, we tested whether combining L-1MT with doxycycline was more effective at eradicating IFN-γ-induced persistent forms of C. trachomatis than doxycycline alone.
The experimental protocol depicted in Fig. 12 A was used to determine the number of recoverable IFU when IFN-γ-treated cultures were also exposed to L-1MT alone, doxycycline alone, or doxycycline plus L-1MT. Briefly, IFN-γ was added at the time of infection to induce persistence. At 38 hpi, persistent cultures were treated with doxycycline, L-1MT, or both for an additional 24 h. Following this, IFN-γ, doxcycline, and L-1MT were substituted for by culture medium supplemented with tryptophan and cycloheximide for 48 h, after which recoverable IFU were measured.
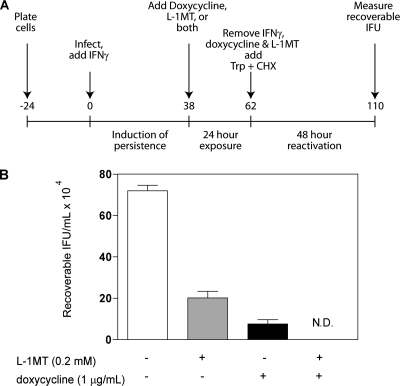
Fig. 12.
L-1MT improves the efficacy of doxycycline in clearing persistent C. trachomatis infection in HeLa cells. (A) Schematic diagram of the experiment. A monolayer of HeLa cells was infected with C. trachomatis serovar D, and IFN-γ was added at the time of infection to induce persistence. At 38 hpi, persistent cultures were treated with IFN-γ, doxycycline, L-1MT, or doxycycline with L-1MT for an additional 24 h. Following this, IFN-γ, doxycycline, and L-1MT were substituted for by culture medium supplemented with tryptophan (Trp) and cycloheximide (CHX) for 48 h, after which recoverable IFU were measured. (B) Recoverable IFU from persistent C. trachomatis cultures from untreated control cells (white bar), cells treated with 0.2 mM L-1MT (gray bar), and cells treated with 1 μg/ml doxycyline (black bar) and 1 μg/ml doxycyline with 0.2 mM L-1MT. N.D., not detectable by the assay. Doxycycline or doxycycline with L-1MTwas added at 38 hpi, when persistence had been established on C. trachomatis cultures treated with 10 ng/ml IFN-γ. Data represent means and standard deviations from four independent experiments.
Recoverable IFU were decreased by approximately 4-fold by exposure to 0.2 mM L-1MT alone and 9-fold by exposure to 1 μg/ml doxycycline alone, compared to untreated cultures (Fig. 12B). Strikingly, treatment with 0.2 mM L-1MT combined with 1 μg/ml doxycycline reduced recoverable IFU to a level below the assay's detection limit. Indeed, this combination was more effective at reducing recoverable IFU than using doxycycline alone, even at concentrations as high as 10 μg/ml (data not shown). Therefore, these data indicate that the combination L-1MT and doxycycline is more effective at clearing persistent forms of C. trachomatis than doxycycline alone.
DISCUSSION
Problems associated with persistent chlamydial growth include the possibilities that it (i) permits immune evasion (6), (ii) provides a reservoir of bacteria that can be reactivated when conditions in the infected microenvironment are permissive (9, 10), and (iii) may underlie the recalcitrance of chlamydiae to some commonly used antibiotics (11, 12, 39, 60). In this study, we explored the effects of the specific IDO1 inhibitor L-1MT on the growth and development of C. trachomatis in HeLa cells. Our results indicate that 0.2 mM L-1MT can (i) block IFN-γ-mediated C. trachomatis persistence, (ii) limit the formation of infectious EBs, and (iii) increase the susceptibility of persistent C. trachomatis forms to doxycycline treatment.
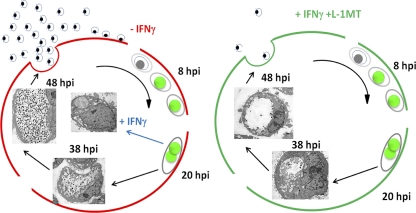
We hypothesize that the mechanism underlying the ability of L-1MT to rescue C. trachomatis from IFN-γ-mediated persistence, while limiting the productive multiplication of the bacterium, involves the temporal depletion of tryptophan (Table 4). We infer that because L-1MT prevented tryptophan depletion at the mid-stage of chlamydial development (20 hpi), C. trachomatis bypassed IFN-γ-induced persistence. However, by permitting depletion of tryptophan at the mid-late stage of the chlamydial development (38 hpi), the available tryptophan was insufficient to support normal late-stage development of bacteria. Thus, as a consequence of the addition of L-1MT to IFN-γ-exposed C. trachomatis, a unique chlamydial growth pattern was observed that is distinct from persistent forms and also characterized by a limited productive release of infectious EBs (Fig. 13). Although L-1MT is considered a stable molecule (56, 61), its half-life under the conditions we have used is unknown. Therefore, it is also possible that the reduced depletion of tryptophan at 38 hpi resulted from a decreased inhibition of IDO1 at that time.
Table 4.
Temporal depletion of tryptophan by addition of L-1MT to IFN-γ-exposed C. trachomatis-infected HeLa cell cultures
Fig. 13.
Schematic model of the proposed mechanism underlying L-1MT's ability to block IFN-γ-mediated persistence while limiting the productive multiplication of the bacterium. The red path denotes normal development, blue denotes induction of persistence, and green denotes development in the presence of IFN-γ and 0.2 mM L-1MT. L-1MT prevented tryptophan depletion at the mid-stage of chlamydial development (20 hpi), allowing C. trachomatis to bypass the IFN-γ-induced persistence. However, it allows tryptophan depletion at the mid-late stage of the chlamydial development (38 hpi); hence, the available tryptophan was insufficient to support normal late-stage development of bacteria (Table 4). Thus, addition of L-1MT to IFN-γ-exposed C. trachomatis results in a temporal tryptophan depletion supporting the unique chlamydial growth pattern that is distinct from persistent forms and also characterized by a limited productive release of infectious EBs.
Reveneau and coworkers reported that doxycycline, a first-line antibiotic commonly used to treat C. trachomatis infections, was more effective in clearing normal replicating C. trachomatis infections in vitro than those that were cultivated in the presence of IFN-γ. Their observations permitted them to conclude that IFN-γ-induced persistent forms of C. trachomatis were more resistant to this antibiotic (39). Although L-1MT blocked persistence, it also severely restricted bacterial multiplication. Therefore, we investigated whether using L-1MT in combination with doxycycline was more effective against persistent bacteria than doxycycline alone. Our data indicate that L-1MT reactivated persistent C. trachomatis ineffectually, resulting in a sharp decrease in the number of EBs observed ultrastructurally and IFUs measured biologically. This ineffectual reactivation resulted in an increased susceptibility to doxycycline. Although as yet unexplored, we propose that the increased sensitivity to doxycycline in the presence of L-1MT results from the molecular mechanism by which doxycycline functions—that is blockade of protein synthesis by binding the ribosome acceptor site and therefore inhibiting the association of aminoacyl-tRNA with the mRNA-ribosome complex. It is known that tryptophan deprivation severely decreases protein synthesis in persistent C. trachomatis forms without completely blocking genome replication (31). Therefore, even a limited alleviation of tryptophan deprivation likely provides molecular targets for doxycycline by restoring protein synthesis to some measure. Our finding that a combination of L-1MT and doxycycline is superior to the use of doxycycline alone is similar to previous suggestions that combination drug treatment of C. trachomatis can be superior to therapy with a single antibiotic (49).
Currently, there is no direct and indisputable evidence that persistence occurs in vivo due to the challenging nature of these studies. However, multiple indications from morphological (12), clinical (18, 35), and physiological (4) studies support the in vivo occurrence of a C. trachomatis persistent state. If IFN-γ-mediated persistent forms of chlamydiae exist in vivo, a potential therapeutic complication would be the apparent relative resistance of this growth form to doxycycline treatment (39). There are reports of recurrent infections in women who have been previously treated with doxycycline (11, 18). While the majority of recurrent infections likely result from reinfection, it remains possible that some arise from reactivation of small reservoirs of persistent bacteria that have not been fully eliminated by antibiotics. This possibility is supported by the study of Dean et al., who observed multiple, same-serovar recurrent C. trachomatis infections in women over a 2- to 5-year period despite antibiotic treatment (18). Since the most serious ramifications of C. trachomatis infections to human health are primarily associated with chronic or repeated infections, the observation by Reveneau and coworkers that IFN-γ-induced persistent forms of C. trachomatis are relatively resistant to doxycycline may have serious repercussions.
The implication of IFN-γ-mediated persistence in vivo has been elegantly discussed by others (7, 27), and consequently, the use of tryptophan supplementation in combination with antibiotics as a therapeutic approach has previously been proposed (48). The data presented here imply this approach would not be effective in clearing C. trachomatis infection in vivo; indeed, it may be detrimental to disease outcome because reactivation of persistent bacterial forms through tryptophan supplementation would restore efficient production of elementary bodies. Furthermore, based on reports by others (15, 27), we believe that tryptophan supplementation may naturally occur in vivo when coinfecting microorganisms like Trichomonas vaginalis could provide an indole source that might be utilized by genital serovars of C. trachomatis to synthesize tryptophan. This phenomenon has been postulated to be detrimental in controlling chronic C. trachomatis infections as it may reactivate IFN-γ-induced persistent bacterial forms that could act as bacterial reservoirs (15, 27, 39). In contrast to tryptophan supplementation, treatment of persistent forms with L-1MT results in a restricted return to the normal development cycle, with limited production of EBs. Consequently, L-1MT-treated persistent forms are more sensitive to doxycycline treatment. We hypothesize that this combination approach might be more efficacious for treatment as it would decrease the probability of escape of persistent forms from the effects of doxycycline alone. For these reasons, we propose L-1MT would be a novel and effective approach to experimentally explore, and interfere with, the survival and the long-term association of C. trachomatis with its human host.
We believe that our findings provide sufficient proof of principle to warrant further investigation of the efficacy of L-1MT in human in vitro models and nonhuman primate models of C. trachomatis infection. If the biological half-life of L-1MT at mucosal sites is found to reduce its availability in C. trachomatis-infected cells, we note that L-1MT can be administered several times per day, as reported for animal models that explore its use as a therapeutic against cancer (56, 61). Should our findings translate to improved in vivo clearance of C. trachomatis in nonhuman primate models, it would indicate that partial rescue of auxotropy in combination with therapy that targets replication is a potent mechanism to clear infection by a pathogen. Indeed, we note that auxotrophy is not unusual among intracellular pathogens, where evolution has often driven the loss of a pathogen's capacity to synthesize metabolites readily available from the host. For example, the intracellular protozoan parasite Leishmania is a tryptophan auxotroph (24), which frequently escapes therapeutic surveillance commonly imposed by antileishmanial therapy that targets replication (30). Our studies support the concept that partial restriction of tryptophan availability may also increase the efficacy of such drugs against Leishmania. Thus, our findings may provide insights into the design of new therapeutic approaches that are also more broadly applicable to many intracellular microorganisms.
ACKNOWLEDGMENTS
This work was supported by NIH grants U19AI061972 (A.J.Q. and D.J.S.) and AI095859 (A.J.Q. and A.A.A.) and by the Louisiana Vaccine Center and the South Louisiana Institute for Infectious Disease Research sponsored by the Louisiana Board of Regents. A.A.A. was also supported by NIH awards CA 112564 and AI055172.
We thank Gyanendra Singh and Sheila Greene for technical help and David Martin and Priscilla Wyrick for critical reading of the manuscript.
Footnotes
Published ahead of print on 12 September 2011.
REFERENCES
- 1. Abdelrahman Y. M., Belland R. J. 2005. The chlamydial developmental cycle. FEMS Microbiol. Rev. 29:949–959 [DOI] [PubMed] [Google Scholar]
- 2. Abramoff M. D., Magalhaes P. J., Ram S. J. 2004. Image processing with ImageJ. Biophotonics Int. 11:36–42 [Google Scholar]
- 3. Akers J. C., Tan M. 2006. Molecular mechanism of tryptophan-dependent transcriptional regulation in Chlamydia trachomatis. J. Bacteriol. 188:4236–4243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arno J. N., et al. 1990. Interferon-gamma in endocervical secretions of women infected with Chlamydia trachomatis. J. Infect. Dis. 162:1385–1389 [DOI] [PubMed] [Google Scholar]
- 5. Beatty W. L., Belanger T. A., Desai A. A., Morrison R. P., Byrne G. I. 1994. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect. Immun. 62:3705–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beatty W. L., Byrne G. I., Morrison R. P. 1993. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 90:3998–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beatty W. L., Byrne G. I., Morrison R. P. 1994. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 2:94–98 [DOI] [PubMed] [Google Scholar]
- 8. Beatty W. L., Morrison R. P., Byrne G. I. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 58:686–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beatty W. L., Morrison R. P., Byrne G. I. 1995. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect. Immun. 63:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belland R. J., et al. 2003. Transcriptome analysis of chlamydial growth during IFN-gamma-mediated persistence and reactivation. Proc. Natl. Acad. Sci. U. S. A. 100:15971–15976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhengraj A. R., Vardhan H., Srivastava P., Salhan S., Mittal A. 2010. Decreased susceptibility to azithromycin and doxycycline in clinical isolates of Chlamydia trachomatis obtained from recurrently infected female patients in India. Chemotherapy 56:371–377 [DOI] [PubMed] [Google Scholar]
- 12. Bragina E. Y., Gomberg M. A., Dmitriev G. A. 2001. Electron microscopic evidence of persistent chlamydial infection following treatment. J. Eur. Acad. Dermatol. Venereol. 15:405–409 [DOI] [PubMed] [Google Scholar]
- 13. Brunham R. C., Rey-Ladino J. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149–161 [DOI] [PubMed] [Google Scholar]
- 14. Byrne G. I., Lehmann L. K., Landry G. J. 1986. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect. Immun. 53:347–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caldwell H. D., et al. 2003. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J. Clin. Invest. 111:1757–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carlin J. M., Borden E. C., Byrne G. I. 1989. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J. Interferon Res. 9:329–337 [DOI] [PubMed] [Google Scholar]
- 17. Clark R. B., Schatzki P. F., Dalton H. P. 1982. Ultrastructural effect of penicillin and cycloheximide on Chlamydia trachomatis strain HAR-13. Med. Microbiol. Immunol. 171:151–159 [DOI] [PubMed] [Google Scholar]
- 18. Dean D., Suchland R. J., Stamm W. E. 2000. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J. Infect. Dis. 182:909–916 [DOI] [PubMed] [Google Scholar]
- 19. Ermak G., Cancasci V. J., Davies K. J. 2003. Cytotoxic effect of doxycycline and its implications for tet-on gene expression systems. Anal. Biochem. 318:152–154 [DOI] [PubMed] [Google Scholar]
- 20. Feng G. S., Taylor M. W. 1989. Interferon gamma-resistant mutants are defective in the induction of indoleamine 2,3-dioxygenase. Proc. Natl. Acad. Sci. U. S. A. 86:7144–7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hogan R. J., Mathews S. A., Mukhopadhyay S., Summersgill J. T., Timms P. 2004. Chlamydial persistence: beyond the biphasic paradigm. Infect. Immun. 72:1843–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holland S. M., et al. 1992. Demonstration of chlamydial RNA and DNA during a culture-negative state. Infect. Immun. 60:2040–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsumoto A., Manire G. P. 1970. Electron microscopic observations on the fine structure of cell walls of Chlamydia psittaci. J. Bacteriol. 104:1332–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McConville M. J., et al. 2008. Analysis of the Leismania metabolome, p. 75–106 Myler P. J., Fasel N. (ed.), Leishmania: after the genome. Caister Academic Press, Norwich, United Kingdom [Google Scholar]
- 25. Molano M., et al. 2005. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J. Infect. Dis. 191:907–916 [DOI] [PubMed] [Google Scholar]
- 26. Morre S. A., et al. 2002. The natural course of asymptomatic Chlamydia trachomatis infections: 45% clearance and no development of clinical PID after one-year follow-up. Int. J. STD AIDS 13(Suppl. 2):12–18 [DOI] [PubMed] [Google Scholar]
- 27. Morrison R. P. 2003. New insights into a persistent problem—chlamydial infections. J. Clin. Invest. 111:1647–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moulder J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muller A. J., DuHadaway J. B., Donover P. S., Sutanto-Ward E., Prendergast G. C. 2005. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 11:312–319 [DOI] [PubMed] [Google Scholar]
- 30. Ouellette M., et al. 2008. Drug resistance in Leishmania, p. 159–176 In Myler P. J., Fasel N. (ed.), Leishmania: after the genome. Caister Academic Press, Norwich, United Kingdom [Google Scholar]
- 31. Ouellette S. P., et al. 2006. Global transcriptional upregulation in the absence of increased translation in Chlamydia during IFNgamma-mediated host cell tryptophan starvation. Mol. Microbiol. 62:1387–1401 [DOI] [PubMed] [Google Scholar]
- 32. Ozaki Y., Edelstein M. P., Duch D. S. 1988. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon gamma. Proc. Natl. Acad. Sci. U. S. A. 85:1242–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pantoja L. G., Miller R. D., Ramirez J. A., Molestina R. E., Summersgill J. T. 2001. Characterization of Chlamydia pneumoniae persistence in HEp-2 cells treated with gamma interferon. Infect. Immun. 69:7927–7932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pantoja L. G., Miller R. D., Ramirez J. A., Molestina R. E., Summersgill J. T. 2000. Inhibition of Chlamydia pneumoniae replication in human aortic smooth muscle cells by gamma interferon-induced indoleamine 2,3-dioxygenase activity. Infect. Immun. 68:6478–6481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patton D. L., et al. 1994. Detection of Chlamydia trachomatis in fallopian tube tissue in women with postinfectious tubal infertility. Am. J. Obstet. Gynecol. 171:95–101 [DOI] [PubMed] [Google Scholar]
- 36. Peipert J. F. 2003. Clinical practice. Genital chlamydial infections. N. Engl. J. Med. 349:2424–2430 [DOI] [PubMed] [Google Scholar]
- 37. Pettengill M. A., Lam V. W., Ojcius D. M. 2009. The danger signal adenosine induces persistence of chlamydial infection through stimulation of A2b receptors. PLoS One 4:e8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qian F., et al. 2009. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res. 69:5498–5504 [DOI] [PubMed] [Google Scholar]
- 39. Reveneau N., Crane D. D., Fischer E., Caldwell H. D. 2005. Bactericidal activity of first-choice antibiotics against gamma interferon-induced persistent infection of human epithelial cells by Chlamydia trachomatis. Antimicrob. Agents Chemother. 49:1787–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rothermel C. D., Byrne G. I., Havell E. A. 1983. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect. Immun. 39:362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sangare L., Morisset R., Omri A., Ravaoarinoro M. 1998. Incorporation rates, stabilities, cytotoxicities and release of liposomal tetracycline and doxycycline in human serum. J. Antimicrob. Chemother. 42:831–834 [DOI] [PubMed] [Google Scholar]
- 42. Schachter J. 1978. Chlamydial infections. N. Engl. J. Med. 298:428–435 [DOI] [PubMed] [Google Scholar]
- 43. Schachter J., et al. 1988. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? J. Infect. Dis. 158:1347–1352 [DOI] [PubMed] [Google Scholar]
- 44. Sears J., Kolman J., Wahl G. M., Aiyar A. 2003. Metaphase chromosome tethering is necessary for the DNA synthesis and maintenance of oriP plasmids but is insufficient for transcription activation by Epstein-Barr nuclear antigen 1. J. Virol. 77:11767–11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaw E. I., et al. 2000. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol. Microbiol. 37:913–925 [DOI] [PubMed] [Google Scholar]
- 46. Shimizu T., Nomiyama S., Hirata F., Hayaishi O. 1978. Indoleamine 2,3-dioxygenase. Purification and some properties. J. Biol. Chem. 253:4700–4706 [PubMed] [Google Scholar]
- 47. Shirey K. A., Jung J. Y., Carlin J. M. 2006. Up-regulation of gamma interferon receptor expression due to Chlamydia-Toll-like receptor interaction does not enhance signal transducer and activator of transcription 1 signaling. Infect. Immun. 74:6877–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singla M. 2007. Role of tryptophan supplementation in the treatment of Chlamydia. Med. Hypotheses 68:278–280 [DOI] [PubMed] [Google Scholar]
- 49. Smelov V., Gorelov A., Smelova N., Krylova T. 2004. Single-drug or combined antibacterial therapy in the treatment of patients with chronic prostatitis and Chlamydia trachomatis? Int. J. Antimicrob. Agents 23(Suppl. 1):S83–S87 [DOI] [PubMed] [Google Scholar]
- 50. Student A. 1908. The probable error of a mean. Biometrika 6:1–25 [Google Scholar]
- 51. Szanto S., et al. 2007. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res. Ther. 9:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takikawa O., Kuroiwa T., Yamazaki F., Kido R. 1988. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J. Biol. Chem. 263:2041–2048 [PubMed] [Google Scholar]
- 53. Taylor M. W., Feng G. S. 1991. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 5:2516–2522 [PubMed] [Google Scholar]
- 54. Thygeson P. 1963. Epidemiologic observations on trachoma in the United States. Invest. Ophthalmol. 2:482–489 [PubMed] [Google Scholar]
- 55. Thylefors B. 1995. Global trachoma control past, present and future approaches. Rev. Int. Trach. Pathol. Ocul. Trop. Subtrop. Sante Publique 72:13–80 [PubMed] [Google Scholar]
- 56. Van den Endyne B. J., Uttenhove T. I. C., Colau D., Pilotte L., Stroobant V. 2007. Tumoral immune resistance based on tryptophan degradation by indoleamine 2,3-dioxygenase. Int. Cong. Ser. 1304:274–277 [Google Scholar]
- 57. Vanover J., et al. 2008. Herpes simplex virus co-infection-induced Chlamydia trachomatis persistence is not mediated by any known persistence inducer or anti-chlamydial pathway. Microbiology 154:971–978 [DOI] [PubMed] [Google Scholar]
- 58. Werner-Felmayer G., et al. 1989. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim. Biophys. Acta 1012:140–147 [DOI] [PubMed] [Google Scholar]
- 59. Wood H., et al. 2003. Regulation of tryptophan synthase gene expression in Chlamydia trachomatis. Mol. Microbiol. 49:1347–1359 [DOI] [PubMed] [Google Scholar]
- 60. Wyrick P. B., Knight S. T. 2004. Pre-exposure of infected human endometrial epithelial cells to penicillin in vitro renders Chlamydia trachomatis refractory to azithromycin. J. Antimicrob. Chemother. 54:79–85 [DOI] [PubMed] [Google Scholar]
- 61. Yang H. J. A combination of the metabolic enzyme inhibitor APO866 and the immune adjuvant L-1-methyl tryptophan induces additive antitumor activity. Exp. Biol. Med. (Maywood) 235:869–876 [DOI] [PubMed] [Google Scholar]