Abstract
We have previously reported that C57BL/6 mice vaccinated with a live, attenuated mutant of Coccidioides posadasii, referred to as the ΔT vaccine, are fully protected against pulmonary coccidioidomycosis. This model was used here to explore the nature of vaccine immunity during the initial 2-week period after intranasal challenge. Elevated neutrophil and eosinophil infiltration into the lungs of nonvaccinated mice contrasted with markedly reduced recruitment of these cells in vaccinated animals. The numbers of lung-infiltrated macrophages and dendritic cells showed a progressive increase in vaccinated mice and corresponded with reduction of the lung infection. Concentrations of selected inflammatory cytokines and chemokines were initially higher in lung homogenates of vaccinated mice but then generally decreased at 14 days postchallenge in correlation with containment of the organism and apparent dampening of the inflammation of host tissue. Profiles of cytokines detected in lung homogenates of ΔT-vaccinated mice were indicative of a mixed T helper 1 (Th1)-, Th2-, and Th17-type immune response, a conclusion which was supported by detection of lung infiltration of activated T cells with the respective CD4+ gamma interferon (IFN-γ)+, CD4+ interleukin-5 (IL-5)+, and CD4+ IL-17A+ phenotypes. While Th1 and Th2 immunity was separately dispensed of by genetic manipulation without loss of ΔT vaccine-mediated protection, loss of functional Th17 cells resulted in increased susceptibility to infection in immunized mice. Characterization of the early events of protective immunity to Coccidioides infection in vaccinated mice contributes to the identification of surrogates of immune defense and provides potential insights into the design of immunotherapeutic protocols for treatment of coccidioidomycosis.
INTRODUCTION
Coccidioides posadasii and Coccidioides immitis are the etiologic agents of a mild to potentially life-threatening respiratory disease known as coccidioidomycosis or San Joaquin Valley fever. In spite of the genetic diversity between the two species revealed by comparative genomic sequence analyses (36), laboratory studies have failed to show any significant difference in their virulence in mice. Coccidioides is considered both a primary and an opportunistic pathogen, since coccidioidal infections occur in immunocompetent as well as immunocompromised individuals (12). Disease onset typically results from inhalation of dry, air-dispersed spores (arthroconidia) released by the soilborne saprobic phase of the pathogen. An estimated 40% of individuals exposed to this microbe in regions of the southwestern United States where it is endemic develop symptomatic disease, which can manifest as an acute or progressive pneumonia with formation of pulmonary nodules and cavities, extrapulmonary nonmeningeal mycosis, or coccidioidal meningitis. The last of these is the most severe complication and commonly requires aggressive therapy (29). An additional clinical concern related to this mycosis is that latent coccidioidal infections can reactivate in solid-organ recipients, and these patients often require lifelong antifungal prophylaxis (19). Escalation of the cost of antifungal treatment of coccidioidomycosis argues for methods to prevent and better control the disease (11).
Inhaled spores of Coccidioides become hydrated and undergo isotropic growth to form spherule initials (also called round cells; 20 to 40 μm diameter) (5). These parasitic cells presumably first come into contact with epithelial cells and macrophages in the respiratory tract of the host. Little is known about the host response during the first few days after the microbial insult. Investigations of murine primary macrophage interactions with spores and spherule initials have indicated that under in vitro conditions the phagocytes are unable to efficiently kill the parasitic cells (14). The results of recent investigations of host-pathogen interactions cast doubt on whether the oxidative burst is required for phagocytic killing of Coccidioides in vitro (15, 28) and suggest that other, still undefined mechanisms of innate immunity are involved in the protective response to this fungal pathogen. The contents of mature spherules convert into a multitude of endospores, which are ultimately released from the maternal cells and can disseminate hematogenously from original sites of infection.
Histopathological examinations of infected lungs of nonvaccinated mice at 1 to 2 weeks postchallenge have revealed large numbers of neutrophils adjacent to mature spherules that have ruptured and released their endospores (43). We have proposed that neutrophils respond to the contents of these parasitic cells in a chemotaxis-like fashion; the more spherules in the lungs, the more neutrophils are present (17). This intense inflammatory response at infection sites may contribute to lung tissue damage which could exacerbate the course of disease. The majority of nonvaccinated, Coccidioides-infected C57BL/6 mice become moribund by 14 days, and all die within 3 weeks after challenge. On the other hand, vaccination of this same mouse strain with a live, attenuated mutant of C. posadasii generated by a triple-gene knockout procedure (ΔT vaccine) resulted in survival of 100% of the animals to at least 75 days after intranasal challenge with a potentially lethal suspension of viable spores (43). The survivors mounted a robust, T-cell mediated immune response to the respiratory infection, developed well-differentiated pulmonary granulomas, showed no evidence of inflammatory damage, and exhibited near clearance of the organism from lung tissue with minimal dissemination of the pathogen to extrapulmonary sites (43). Although sterilizing immunity was not achieved, the presence of residual granulomas and a benign outcome of infection in the vaccinated host constitute an acceptable goal for a vaccine against this respiratory disease. This murine model of coccidioidomycosis supports the general paradigm for granulomatous diseases: activated T lymphocytes secrete cytokines, which activate macrophages, inducing the formation of granulomas that lead to the killing or containment of the pathogen (21).
Unfortunately, the mouse model of pulmonary coccidioidomycosis is not the ideal simulation of this respiratory disease in humans. An intranasal insult of naïve C57BL/6 mice with 60 to 80 Coccidioides spores consistently manifests as an acute, disseminated infection. In contrast, a typical symptomatic, primary respiratory infection in humans initially presents as a comparatively slowly developing granulomatous disease that either resolves spontaneously or progresses to the disseminated form of the mycosis. However, we propose that investigations of the differences in patterns of innate and T-cell-mediated immune responses to Coccidioides infection between vaccinated and nonvaccinated mice during early stages of this respiratory disease can provide valuable insight into mechanisms of protection against this respiratory pathogen. The results of these studies in turn could potentially aid in the development of novel strategies for immunotherapy and contribute to the design of a human vaccine against coccidioidomycosis. In this paper, we examine the nature of protective immunity to lung infection with Coccidioides during the first 2 weeks postchallenge in C57BL/6 mice which were immunized with the live, attenuated vaccine strain.
MATERIALS AND METHODS
Fungal strains, growth conditions, and spore preparation.
The virulent fungal strain used to challenge mice in this study was a clinical isolate of C. posadasii (C735). A previously described, genetically engineered mutant (Δcts2/ard1/cts3) derived from this parental strain (43) was employed as a live, attenuated vaccine and is designated ΔT. Both strains were cultured on GYE growth medium (1% glucose, 0.5% yeast extract, 1.5% agar) for 3 to 4 weeks at 30°C to generate a confluent layer of spores on the agar surface. Petri plates were flooded with endotoxin-free saline, and spores were harvested into plastic centrifuge tubes containing sterile glass beads (5-mm diameter). Hyphal elements were disrupted by hand shaking, and the spore suspensions were filtered through a layer of nylon fiber to remove the hyphal fragments. Spore concentrates of the parental or mutant strain were obtained by centrifugation (5,000 × g), and the cell pellets were washed twice with saline and stored at 4°C. All culturing and preparatory procedures which involved live cells of C. posadasii were conducted in a biosafety level 3 (BSL3) laboratory certified by the Centers for Disease Control and Prevention and located at the University of Texas at San Antonio.
Mouse strains.
Inbred, female C57BL/6 mice were obtained from the National Cancer Institute/Charles River Laboratory (Wilmington, MA). Breeder pairs of interleukin-17A (IL-17A)-deficient (IL-17a−/−) and IL-17 receptor A-deficient (IL-17ra−/−) mice were provided by Jay Kolls at the University of Pittsburgh and by Amgen, Inc. (Thousand Oaks, CA), respectively. Gamma interferon receptor (IFN-γr)-deficient (IFN-γr−/−) mice and IL-4r−/− mice were gifts from Bernard Arulanandam at the University of Texas at San Antonio. The IL-17a−/−, IL-17ra−/−, and IFN-γr−/− mice were genetically engineered on a C57BL/6 background, while the IL-4r−/− mice were generated on a BALB/c background. All strains of mice had an average weight of 20 to 25 g when used for the reported experiments, and only female mice were employed. All mice were housed in a pathogen-free animal facility at the University of Texas at San Antonio and were handled according to guidelines approved by the Institutional Animal Care and Use Committee. The mice were relocated to the CDC-certified animal BSL3 (ABSL3) laboratory before vaccination and challenge with live Coccidioides spores.
Vaccination, challenge, and evaluations of fungal burden and survival.
Mice (females, 8 weeks old) were immunized subcutaneously in the abdominal region with 5.0 × 104 viable spores of the ΔT vaccine strain suspended in 100 μl sterile saline, followed 14 days later with a vaccination boost of 2.5 × 104 live spores as previously reported (43). Nonvaccinated, control mice were immunized with saline alone using the same protocol as described above. At 4 weeks after completion of the vaccination protocol, mice were challenged by intranasal instillation with 60 to 80 viable spores of the virulent strain of C. posadasii (isolate C735) suspended in 35 μl saline (43). The fungal burdens in lungs and spleen were determined at 2 to 14 days postchallenge by plating serial dilutions of separate organ homogenates on GYE agar containing 50 μg/ml chloramphenicol as previously described (43). The number of CFU was expressed on a log scale and either reported for individual mice or presented as a box plot for each group of 12 to 15 animals as previously reported (43). Survival studies of vaccinated versus nonvaccinated mice were conducted over 45 days postchallenge as previously reported (43).
Pulmonary leukocyte isolation.
The lungs of individual vaccinated or nonvaccinated mice sacrificed at 2 to 14 days postchallenge (4 mice per time point) were carefully excised, mashed with the back of a sterile 3-ml syringe plunger, and passed through a cell strainer (70-μm-diameter pore size) into a petri dish (60 by 15 mm) containing 5 ml of RPMI 1640 medium (HyClone, Logan, UT) plus 1% heat-inactivated fetal bovine serum (FBS) (HyClone). The dispersed tissue and cells of each mouse were washed with an additional 3 ml RPMI 1640 plus 1% FBS and centrifuged (500 × g) for 10 min without use of the centrifuge brake. The supernatants were carefully aspirated, and each pellet was resuspended and incubated for 3 min in 3 ml of ACK buffer (Lonza, Inc., Walkersville, MD) for lysis of erythrocytes. Two volumes of RPMI 1640 plus 1% FBS were added to the mixture, which was then filtered through a second cell strainer (40-μm-diameter pore size) The total leukocytes were centrifuged as described above and resuspended in 1 ml of RPMI 1640 medium containing 10% heat-inactivated FBS for subsequent assays. The number of live host cells obtained from each lung sample was visualized by the trypan blue exclusion test and quantified using a hemocytometer.
Quantification of innate cell types in Coccidioides-infected lungs.
Standard methodology was employed for direct monoclonal antibody (MAb) labeling and enumeration of selected pulmonary innate immune cells by fluorescence-activated cell sorting using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ) as previously reported (15). Data were acquired with CellQuest Pro software (BD Biosciences) and analyzed using a FlowJo software package (Tree Star, Inc., Ashland, OR). Fluorochrome-labeled MAbs used in this study included anti-CD11b (clone M1/70), anti-CD45 (clone 30-F11), anti-Ly6G (clone IA8), anti-CD11c (clone HL3), anti-SiglecF (clone E50-2440), and anti-Mac3 (clone M3/84) obtained from BD Biosciences. The total number of pulmonary leukocytes in each lung sample was quantified by multiplying the total number of hemocytometer-determined viable cell counts by the percentage of CD45+ cells determined by flow cytometry. The gating strategies for enumerating neutrophils, eosinophils, tissue macrophages, alveolar macrophages, and dendritic cells were Ly6G+ CD11b+ CD11c−, SiglecF+ CD11c−, Mac3+ CD11c−, intermediate level of CD11b (CD11bM) CD11c+, and high level of CD11b (CD11bH) CD11c+, respectively (15). The absolute numbers of each subpopulation of the selected innate cell type in lungs of nonvaccinated and ΔT-vaccinated mice prior to Coccidioides challenge and subsequently at 2- to 14-day intervals postchallenge were determined by multiplying the percentage of each gated population by the total number of viable pulmonary leukocytes derived from hemocytometer counts as described above.
Assays of concentrations of selected cytokines and chemokines in lung homogenates.
Concentrations of selected cytokines and chemokines in supernatants of lung homogenates were compared between nonvaccinated and ΔT-vaccinated mice sacrificed at 7 and 11 days postchallenge (4 animals per group). Lung homogenates of individual mice were prepared as previously described (15), supernatants were obtained by centrifugation (8,000 × g at 4°C for 10 min), and samples were stored at −80°C until ready for analysis. Normal, untreated mice were included for determinations of baseline amounts of each cytokine in their lung homogenates. Assays of cytokine and chemokine concentrations were conducted using a Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA) as previously reported (15), except for concentrations of the IL-22 cytokine, which were determined using a mouse IL-22 Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D, Minneapolis, MN) as recommended by the manufacturer. Assays of samples from individual mice were performed in triplicate.
Assessment of percentages of activated CD4+ and CD8+ T cells in infected lungs.
Pulmonary leukocytes isolated as described above were incubated with selected cocktails of MAbs to determine relative percentages of activated CD4+ and CD8+ T cells in lungs of nonvaccinated or vaccinated mice at 5, 7, 9, 11, and 14 days postchallenge. A cocktail which contained either fluorochrome-labeled anti-CD3 (clone 17A2) and anti-CD4 (clone RM4-5) or anti-CD3 and anti-CD8α (clone 53-6,7) was used for determination of absolute numbers of CD3+ CD4+ CD8α− cells (i.e., total CD4+ T cells) and CD3+ CD4− CD8α+ cells (i.e., total CD8+ T cells), respectively. The absolute number of each subpopulation of pulmonary leukocytes was deduced by multiplying the percentage of each gated population by the total number of pulmonary leukocytes derived from hemocytometer counts as described above. A second pair of cocktails of fluorochrome-labeled MAbs contained anti-CD45, anti-CD4, or anti-CD8α plus anti-CD44 (clone 1M7) and was used to examine absolute numbers of activated CD4+ and CD8+ T cells. The expression level of CD44, an adhesion molecule that binds to hyaluronic acid, is elevated in activated T cells and was used as an activation marker (41). The gating strategies for activated CD4+ and CD8α+ T cells were CD45+ CD4+ CD8α− CD44+ and CD45+ CD4− CD8α+ CD44+, respectively. The percentages of activated T cells within the total CD4+ and CD8α+ T-cell subpopulations were presented as the means ± standard errors of the means (SEM) for 4 animals per group of nonvaccinated or ΔT-vaccinated mice at each indicated time postchallenge.
Assays of selected cytokines produced in vitro by immune CD4+ T cells.
Spleens of nonvaccinated and ΔT-vaccinated mice infected as described above by the intranasal route were harvested (4 animals per group), separately pooled, and macerated as reported previously (44). Animals were sacrificed prior to challenge (day 0) or at 5 days after challenge with 60 to 80 spores of the virulent strain of C. posadasii as described above. Isolation of CD4+ T cells from spleen cell suspensions was conducted using a mouse CD4+ T-cell isolation kit (Miltenyi Biotec Inc., Auburn, CA). Splenocytes obtained from age- and gender-matched C57BL/6 naïve mice were used as antigen-presenting cells (APCs). These were irradiated (3,000 rads) using an RS-2000 irradiator (Rad Source Tech. Inc., Alpharetta, GA) as reported previously (39). CD4+ T cells (2.5 × 106) and APCs (2.5 × 106) were cocultured either in the presence of Coccidioides T27K antigen (40 μg/ml medium) (46) or in medium alone and transferred to wells of a 24-well plate containing 1 ml of RPMI 1640 medium plus 10% (vol/vol) heat-inactivated FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin. The T27K antigen is a multicomponent, soluble derivative of C. posadasii parasitic cell homogenates and has been shown to have protective activity against pulmonary coccidioidomycosis in mice (1). Supernatants of the stimulated immune T cells were collected after 48 h of incubation. Opt-EIA mouse cytokine kits (Pharmingen, San Diego, CA) were used for ELISAs of selected cytokines as reported previously (43). ELISAs of samples from individual mice were performed in triplicate. Standard curves were generated using purified, recombinant cytokines supplied by the manufacturers of the kits and were used to calculate the amounts of specific cytokines in each sample.
Intracellular cytokine staining.
Isolated pulmonary leukocytes (0.5 × 106 cells/ml) from nonvaccinated or ΔT-vaccinated mice were stimulated for 4 h with anti-CD3ε (clone 145-2C11; 0.1 μg/ml) and anti-CD28 (clone 37.51; 1 μg/ml) in the presence of Golgi-Stop (BD Biosciences) to halt the transport of cytokines from the cells. The pulmonary cells were then blocked with Fc-Block and washed before incubation with fluorochrome-conjugated anti-CD4 or anti-CD8α. The cells were then formalin fixed and permeabilized with a working solution of Cytofix/Cytoperm buffer (BD Biosciences) at 4°C for 20 min as recommended by the manufacturer. Permeabilized cells were subsequently stained with a cocktail of fluorochrome-conjugated anti-IFN-γ (clone XMG1.2), anti-IL-5 (clone TRFK5), or anti-IL-17A (clone TC11-18H10) for 30 min at 4°C. The leukocytes were gated on CD4+ CD8α− or CD4− CD8α+ cells, and cytokine expression in each gate was analyzed. The absolute numbers of specific cytokine-producing CD4+ and CD8+ cells relative to total lung-infiltrated leukocytes per lung at 5, 7, 9, 11, and 14 days postchallenge were calculated by multiplying the percentage of each gated population by the total number of viable pulmonary leukocytes determined by hemocytometer counts as described above. The percentages of specific cytokine-producing CD4+ T cells per lung at 7, 9, and 11 days postchallenge relative to total activated CD4+ T cells which had infiltrated the lung were determined from data analysis using the FlowJo software package.
Statistical analyses.
The Mann-Whitney U test was used to compare the absolute cell numbers and percentages of each subset of pulmonary leukocytes of nonvaccinated versus ΔT-vaccinated mice at selected times postchallenge and for comparison of cytokine/chemokine concentrations in lung homogenates as previously described (43). The Wilcoxon rank test was used to compare differences in the median CFU values for statistical significance. Survival data were analyzed by the Kaplan-Meier method using log rank analysis to compare survival curves as reported previously (15). A P value of <0.05 was considered statistically significant.
RESULTS
Differences between early Coccidioides lung infection and dissemination in nonvaccinated and vaccinated mice.
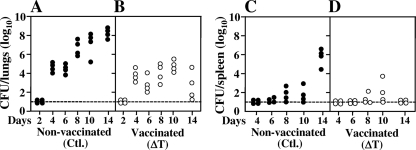
The first generation of the parasitic cycle of Coccidioides occurs by conversion of arthroconidia into spherule initials. The latter continue to grow isotropically to form large coenocytes (spherules, typically >80 μm diameter) and then differentiate internally to form a multitude of endospores (>100) which are released upon rupture of the maternal spherules. Endospores undergo isotropic growth and develop into the next generation of parasitic cells (5). The time required for arthroconidia to differentiate into endosporulating spherules in vivo is estimated to be between 48 and 72 h after intranasal challenge. Nonvaccinated mice showed a near-linear increase in the number of CFU in lung homogenates over the 2-week period postchallenge, with a major increase in fungal burden occurring between 2 and 4 days (Fig. 1 A). Dissemination of the pathogen to the spleen from initial sites of lung infection in nonvaccinated mice was detected at 7 to 8 days after intranasal inoculation, and the fungal burden in the spleen subsequently increased sharply at 14 days in correlation with the progressive infection of lung tissue (Fig. 1C). All nonvaccinated mice reached the early moribund stage by 14 days postchallenge. Lung homogenates of the ΔT-vaccinated mice, on the other hand, showed fewer CFU at 8 days than those of the nonimmunized animals, and the fungal burden of the protected mice remained significantly lower between 8 and 14 days postchallenge than in the nonprotected animals (Fig. 1A and B). By 14 days the fungal burden in lungs of the immunized mice had begun to clear. Minimal dissemination of the pathogen from lungs to spleen was observed in the vaccinated animals between 4 and 10 days, and no CFU were detected in the spleen at 14 days postchallenge (Fig. 1D).
Fig. 1.
Fungal burdens detected in lung and spleen homogenates at 2- to 14-day intervals after intranasal challenge with a potentially lethal inoculum of Coccidioides spores (C735 isolate). Two groups of C57BL/6 mice (24 animals each) either were vaccinated with a live, attenuated strain of C. posadasii previously shown to protect against pulmonary infection (ΔT vaccine) or were immunized with saline (nonvaccinated controls [Ctl.]). The data presented are representative of three separate experiments.
Nonvaccinated and vaccinated mice showed differences in lung infiltration of host innate immune cells.
Flow cytometry was employed to compare the inventories of neutrophils, macrophages, dendritic cells, and eosinophils which had infiltrated Coccidioides-infected lungs of nonvaccinated and vaccinated C57BL/6 mice during the initial 2-week period after challenge. A statistically significant difference in the size of the neutrophil population in vaccinated compared to nonvaccinated mice was first detected on day 7 (Fig. 2 A). The absolute numbers of neutrophils were subsequently sustained at moderate levels in vaccinated mice throughout the 14-day period postchallenge. In contrast, a dramatic increase in the size of the neutrophil subpopulation occurred in nonvaccinated mice during days 8 to 11, when the fungal cells had disseminated to extrapulmonary organs (Fig. 1C). The subsequent decrease in lung-infiltrated neutrophils in the nonvaccinated mice at 14 days corresponded with histopathological evidence of necrosis of infected lung tissue (43). The numbers of tissue and alveolar macrophages were consistently higher in vaccinated than in nonvaccinated mice after 5 and 7 days, respectively (Fig. 2B and C). A linear increase in the numbers of alveolar macrophages was observed in vaccinated mice between 8 and 14 days, while a sharp decrease in the numbers of these phagocytes in lungs of nonvaccinated mice was detected during this same interval postchallenge (Fig. 2C), which correlated with the period when proliferation and metastasis of the pathogen had occurred (Fig. 1C). Both vaccinated and nonvaccinated mice exhibited recruitment of dendritic cells and eosinophils to the lungs between 6 and 11 days postchallenge (Fig. 2D and E). However, during the following 3 days the number of eosinophils recruited into the lungs of nonvaccinated mice showed a major increase in contrast, to the case for ΔT-vaccinated mice, which revealed little change in the size of the eosinophil subpopulation (Fig. 2E).
Fig. 2.
Infiltration of selected innate immune cell types into lungs of vaccinated or nonvaccinated Coccidioides-infected C57BL/6 mice sacrificed prior to challenge (day 0) or at intervals of 2 to 14 days postchallenge (4 animals per time point). Absolute numbers of host cells in preparations of lung homogenates were determined by cytofluorometry using a BD FACSCalibur flow cytometer and FlowJo software. The results are presented as mean values ± SEM for each group of mice at each time point postchallenge. The asterisks indicate significantly higher numbers of innate immune cell numbers in vaccinated than in nonvaccinated mice, while the dagger indicates a higher number in nonvaccinated mice (P < 0.05). The data shown are representative of two independent experiments.
Modulation of proinflammatory cytokine and chemokine concentrations during progressive lung infection correlates with changes in innate and T helper cell responses.
Comparative Bio-Plex assays of the concentrations of selected proinflammatory cytokines and chemokines in lung homogenates were conducted at 7 and 11 days postchallenge (Table 1). These time points were chosen because they correlated with the induction of major differences in innate immunity to infection between the nonvaccinated and vaccinated mice (Fig. 2). Basal concentrations of the selected cytokines in naïve (untreated) mice were included for comparative purposes, as previously reported (44). The concentrations of three of the selected proinflammatory cytokines (IL-1α, IL-1β, and tumor necrosis factor alpha [TNF-α]) and five CC-type chemokines (CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, and CCL11/eotaxin) were significantly higher in lung homogenates of vaccinated mice than in those of nonvaccinated mice at 7 days postchallenge (Table 1). These data correlate with the early influx of neutrophils, macrophages, dendritic cells, and eosinophils into Coccidioides-infected lungs of the ΔT-vaccinated C57BL/6 mice (Fig. 2A to E). The concentrations of IL-6 showed no statistically significant difference between lung homogenates of the two groups of mice at 7 days, while at 11 days the IL-6 concentration in the lungs of nonvaccinated mice had increased by more than 2-fold and was significantly higher than that in lung homogenates of the vaccinated mice. This same trend was revealed by the changes in concentrations of granulocyte colony-stimulating factor (G-CSF). Further comparative analyses revealed that at 11 days postchallenge, the concentration of each of the selected proinflammatory cytokines in lung homogenates of vaccinated mice was significantly lower than that in the nonvaccinated mice. This correlated with a dampening of the infiltration of neutrophils and eosinophils into the lungs of vaccinated mice, while the nonvaccinated mice intensified recruitment of these innate cells. Assays of selected chemokine concentrations in lung homogenates (Table 1) also revealed decreases in concentrations of CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-β, and CCL11/eotaxin in infected lungs of vaccinated compared to nonvaccinated mice between 7 and 11 days postchallenge. This was consistent with a reduction in the degree of the tissue inflammatory response as revealed by histopathological examinations (43). Although the concentrations of CXCL1/KC (neutrophil chemoattractant) were not significantly different in the two groups of mice, they also showed the same trend as revealed by IL-6 and G-CSF. Finally, the concentration of CCL5/RANTES was maintained at a relatively high level in the lungs of vaccinated compared to nonvaccinated mice between 7 and 11 days after challenge, which correlated with an influx of macrophages (Fig. 2B and C) and T lymphocytes, as shown below.
Table 1.
Concentrations of proinflammatory cytokines and chemokines in lung homogenates of nonvaccinated and ΔT-vaccinated mice at 7 and 11 days postchallenge
| Proinflammatory cytokine or chemokine | Concn (pg/ml, mean ± SEM)a |
||||
|---|---|---|---|---|---|
| Naïve mice | 7 days postchallenge |
11 days postchallenge |
|||
| Nonvaccinated mice | ΔT-vaccinated mice | Nonvaccinated mice | ΔT-vaccinated mice | ||
| Cytokines | |||||
| IL-1α | 93.3 ± 59.3 | 1,105.6 ± 171.1 | 1,783.4 ± 279.0*b | 4,343.5 ± 976.6*c | 2,371.8 ± 975.8 |
| IL-1β | 715.0 ± 138.1 | 5,622.9 ± 1,141.1 | 9,783.0 ± 1,151.8*b | 11,830.9 ± 2,154.2*c | 11,067.5 ± 2,936.6 |
| TNF-α | 46.8 ± 9.3 | 1,755.9 ± 187.6 | 2,231.1 ± 223.1*b | 3,867.3 ± 1,225.4*c | 2,138.2 ± 441.9 |
| IL-6 | 24.1 ± 10.5 | 1,067.2 ± 422.4 | 1,817.3 ± 519.9 | 2,366.6 ± 482.9*c | 1,587.2 ± 744.7 |
| G-CSF | 44.5 ± 33.6 | 13,766.1 ± 3,035.2 | 11,773.3 ± 2,142.2 | 45,326.5 ± 9,346.0*c | 9,622.7 ± 3,941.0 |
| Chemokines | |||||
| CCL2/MCP-1 | 242.9 ± 75.2 | 4,678.7 ± 810.9 | 8,883.7 ± 1,333.4*b | 5,420.5 ± 813.6 | 4,586.5 ± 1,976.3† |
| CCL3/MIP-1α | 348.2 ± 163.0 | 6,270.9 ± 889.7 | 19,687.0 ± 3,208.2*b | 16,042.4 ± 3,444.8*c | 5,894.7 ± 2,489.1† |
| CCL4/MIP-1β | 40.7 ± 17.6 | 1,198.6 ± 258.3 | 2,977.1 ± 632.0*b | 2,299.7 ± 463.8*c | 1,600.8 ± 620.2† |
| CCL11/eotaxin | 900.5 ± 70.0 | 5,271.7 ± 417.9 | 7,189.9 ± 490.8*b | 5,704.8 ± 543.8*c | 4,842.4 ± 1,234.9† |
| CXCL1/KC | 105.9 ± 32.7 | 6,682.9 ± 1,655.3 | 7,858.7 ± 1,809.7 | 8,542.4 ± 1,856.7 | 5,477.5 ± 1,652.4 |
| CCL5/RANTES | 852.5 ± 774.3 | 1,226.5 ± 102.6 | 1,803.3 ± 380.9*b | 527.1 ± 93.0 | 1,724.7 ± 530.2*b |
*, significant difference (P < 0.05) between concentrations in lung homogenates of nonvaccinated and vaccinated mice. †, significant difference (P < 0.05) between concentrations in lung homogenates of vaccinated mice sacrificed at 7 or 11 days postchallenge.
Higher in vaccinated mice.
Higher in nonvaccinated mice.
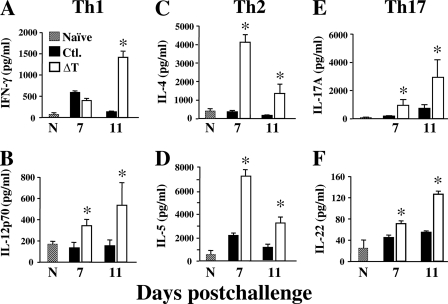
Assays of the concentrations of selected cytokines which are representative of T helper cell response to early lung infection in nonvaccinated and vaccinated mice were also conducted (Fig. 3). We compared the amounts of Th1-type cytokines (IFN-γ and IL-12p70) and representative Th2 (IL-4 and IL-5) and Th17 (IL-17A and IL-22) cytokines in lung homogenates at 7 and 11 days postchallenge. Our results revealed that the concentrations of Th1- and Th17-type cytokines in vaccinated mice increased sharply between 7 and 11 days after challenge, corresponding with early signs of pathogen clearance from the lungs of the ΔT-immunized animals, and were significantly higher than the concentrations of the same cytokines produced by nonvaccinated mice. IFN-γ concentrations in nonvaccinated mice showed a sharp decrease between 7 and 11 days, while the amounts of Th17 and other Th1-type cytokines in lungs of nonprotected animals remained relatively low and showed no significant difference between the two times postchallenge. Although the concentrations of Th2-type cytokines showed an approximately 2-fold decrease between 7 and 11 days in vaccinated mice, the amounts of IL-4 and IL-5 in lung homogenates of the vaccinated animals were significantly higher than the detected amounts of these same cytokines at both times postchallenge in nonvaccinated mice.
Fig. 3.
Determinations of cytokine concentrations in lung homogenates of infected C57BL/6 mice which were immunized either with the ΔT vaccine (open bars) or with saline (solid bars; controls [Ctl.]). Lung homogenates were obtained at 7 and 11 days postchallenge. Normal, untreated mice (N; hatched bars) were included for determinations of baseline amounts of each cytokine in their lung homogenates. Each sample was analyzed in triplicate. The asterisks indicate significantly higher concentrations of cytokines in lung homogenates of vaccinated compared to nonvaccinated mice. The results are presented as mean values ± SEM. The data presented are representative of two independent analyses.
Activated CD4+ and CD8+ T cells infiltrate infected lungs of vaccinated mice in significantly higher numbers than in nonvaccinated mice.
We determined the percentages of total activated, lung-infiltrated CD4+ and CD8+ T cells after 5 to 14 days of Coccidioides infection in nonvaccinated versus vaccinated mice (Table 2). At each selected time postchallenge, the percentages of activated CD4+ and CD8+ T cells were significantly higher in vaccinated than in nonvaccinated mice. In addition, the percentages of activated CD4+ cells present in the infected lung tissue were consistently higher than the percentages of activated CD8+ cells during the entire 9-day interval postchallenge.
Table 2.
Percentages of activated versus total CD4+ and CD8+ T cells in the lungs of nonvaccinated and ΔT-vaccinated mice at 5 to 14 days postchallenge
| Day postchallenge | No. of CD4+ CD8α− CD44+ cells/total no. of CD4+ T cells × 100 (mean ± SEM)a |
No. of CD8α+ CD4− CD44+ cells/total no. of CD8+ T cells × 100 (mean ± SEM)a |
||
|---|---|---|---|---|
| Nonvaccinated mice | ΔT-vaccinated mice | Nonvaccinated mice | ΔT-vaccinated mice | |
| 5 | 38.43 ± 1.83 | 62.40 ± 2.85*† | 18.88 ± 1.75 | 49.07 ± 1.18* |
| 7 | 33.50 ± 1.95 | 49.45 ± 3.06*† | 25.56 ± 2.29 | 41.10 ± 2.50* |
| 9 | 44.40 ± 0.78 | 76.93 ± 2.75*† | 18.84 ± 3.60 | 45.30 ± 1.59* |
| 11 | 32.23 ± 1.94 | 73.68 ± 5.32*† | 16.72 ± 2.64 | 42.57 ± 3.32* |
| 14 | 45.70 ± 3.04 | 63.98 ± 2.94*† | 23.00 ± 2.17 | 44.63 ± 1.04* |
*, statistically significant difference between vaccinated and nonvaccinated mice (P <0.05); †, statistically significant difference between activated CD4+ and CD8+ T cells in ΔT-vaccinated mice (P <0.05).
ΔT vaccine-primed CD4+ T cells respond in vitro to Coccidioides antigen by production of Th1-, Th2-, and Th17-type cytokines.
We employed a recall response assay to determine whether immune CD4+ effector T cells isolated from total splenocytes at just 5 days postchallenge secrete the same set of cytokines indicative of T helper cell activation as detected in lung homogenates of ΔT-vaccinated mice. As previously pointed out, the T27K antigen used to evaluate this in vitro response has previously been used as a pathogen-specific antigen to stimulate peripheral blood monocytic cells of patients with confirmed Coccidioides infection (1). Immune CD4+ T cells isolated from spleens of vaccinated but noninfected mice (day 0) responded to the presence of T27K by production of low but detectable concentrations of IFN-γ, IL-5, and IL-17A cytokines (Fig. 4). Significantly enhanced production of these same cytokines by immune CD4+ T cells was observed at 5 days postchallenge, suggesting that clonal expansion of the Coccidioides-specific Th1, Th2, and Th17 cells had occurred. Minimal amounts of these cytokines were detected in supernatants of T27K-stimulated CD4+ T cells obtained from nonvaccinated mice on days 0 and 5 postchallenge.
Fig. 4.
Determinations of concentrations of representative Th1-, Th2-, and Th17-type cytokines detected in culture supernatants of in vitro-stimulated, immune CD4+ T cells isolated from spleens of ΔT-vaccinated C57BL/6 mice (open bars) or nonvaccinated mice (solid bars; controls [Ctl.]). In vitro stimulation was conducted using the T27K antigen. The asterisks indicate significantly higher concentrations of selected cytokines in vaccinated mice at 5 days postchallenge than at day 0 (P < 0.001), while the daggers indicate higher cytokine concentrations in vaccinated than in nonvaccinated mice (P < 0.05). Immune T cells incubated with medium alone served as a control. Each sample was analyzed in triplicate. The results are presented as mean values ± SEM.
ΔT-vaccinated and infected mice show progressive expansion of Th1 and Th17 cells but limited expansion of Th2 cells.
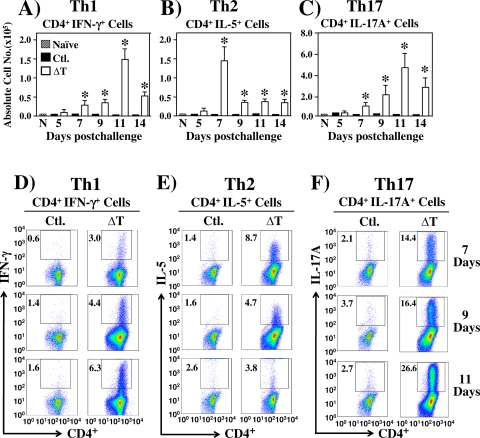
We used flow cytometry and intracellular cytokine staining methods to determine percentages of IFN-γ+-, IL-5+-, and IL-17A+-expressing subpopulations of CD4+ T cells in both the total leukocyte and total activated CD4+ T-cell populations which had infiltrated the lungs of nonvaccinated versus vaccinated mice between 5 and 14 days postchallenge (Fig. 5 A to F). The results revealed that the subset of CD4+ IFN-γ+ T cells in vaccinated/infected mice had expanded sharply between 9 and 11 days, but then dramatically decreased between 11 and 14 days after challenge (Fig. 5A and D). The subpopulation of CD4+ IL-17A+ T cells showed a relatively greater increase at 11 days than the CD4+ IFN-γ+ subpopulation, but this surge of infiltration was also followed by a decrease at 14 days postchallenge (Fig. 5C and F). On the other hand, the absolute numbers and percentages of CD4+ IL-5+ T cells in the lungs of vaccinated and infected mice peaked at 7 days and then decreased sharply between 7 and 9 days postchallenge and remained suppressed through 14 days (Fig. 5B and E). These data correlate well with our reports of the concentrations of corresponding cytokines detected in lung homogenates of vaccinated, infected mice during the same period postchallenge (Fig. 3A, D, and E). The nonvaccinated, infected mice, on the other hand, showed comparatively little expansion of the subsets of Th1, Th2, and Th17 cell types during this same period of examination. We also compared the absolute numbers of CD8α+ T cells expressing IFN-γ, IL-5, and IL-17A in vaccinated versus nonvaccinated mice (data not shown). In contrast to the case for CD4+ IFN-γ+ T cells, the number of lung-infiltrated CD8α+ IFN-γ+ T cells detected in vaccinated mice reached a maximum at 7 days and then sharply decreased between 9 and 14 days postchallenge. The absolute numbers of CD8α+ IFN-γ+ T cells in immunized mice were significantly lower than the numbers of CD4+ IFN-γ+ T cells between 5 and 14 days postchallenge. Comparatively few CD8α+ IL-5+ and CD8α+ IL-17A+ cells were detected in the ΔT-immunized mice, and minimal change was recorded between 5 and 14 days after challenge. Nonvaccinated/infected mice showed significantly lower numbers of the three phenotypes of CD4+ and CD8+ T cells than vaccinated mice throughout the period of examination.
Fig. 5.
FACS analysis of IFN-γ-, IL-5-, and IL-17A-expressing Th1, Th2, and Th17 cells, respectively, in lungs of ΔT-vaccinated compared to nonvaccinated (Ctl.) C57BL/6 mice at 5 to 14 days postchallenge. The absolute numbers (A to C) and percentages of gated, specific cytokine-producing cells per lung (insets in panels D to F) were determined by intracellular cytokine staining. Asterisks in panels A to C indicate significantly higher absolute numbers of the respective T-cell phenotypes in lungs of vaccinated compared to nonvaccinated mice. The results are presented as mean values ± SEM. The data presented are representative of three independent experiments.
IFN-γ and IL-4 receptors are not essential for protective immunity induced by the ΔT vaccine.
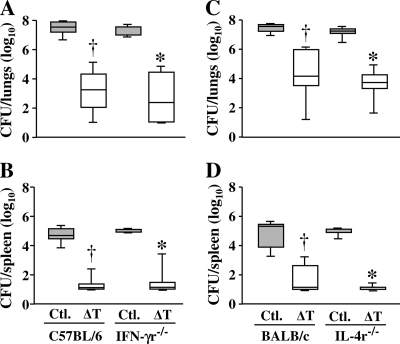
We used IFN-γ and IL-4 receptor knockout mice to determine whether the respective signal pathways play essential roles in ΔT vaccine-mediated protection (Fig. 6 A to D). Fungal burdens in the lungs and spleen were determined at 14 days postchallenge. Nonvaccinated IFN-γr−/− (C57BL/6 background) and IL-4r−/− (BALB/c background) mice were as susceptible to Coccidioides infection as the corresponding age- and gender-matched nonvaccinated wild-type strains based upon comparable CFU in their lung and spleen homogenates. In addition, the ΔT-vaccinated and challenged knockout strains and their wild-type counterparts showed comparable reductions of CFU in their lungs and spleens, indicating that loss of IFN-γ or IL-4 receptor function did not result in increased susceptibility to Coccidioides infection.
Fig. 6.
Comparison of box plots of CFU detected in dilution plate cultures of lung homogenates obtained from ΔT-vaccinated and nonvaccinated (Ctl.) wild-type mice versus specific cytokine receptor knockout mice (IFN-γr−/− and IL-4r−/−) at 14 days after intranasal challenge with a potentially lethal inoculum of Coccidioides spores. The boxes indicate the 25th and 75th percentiles. The horizontal lines within the boxes represent the medians. The daggers indicate statistically significant differences in CFU between vaccinated and nonvaccinated wild-type mice (C57BL/6 or BALB/c). The asterisks indicate significant differences between vaccinated and nonvaccinated knockout mice with either deletion of the IFN-γ receptor gene (C57BL/6) or deletion of the IL-4 receptor gene (BALB/c). No statistically significant difference in CFU data was found between the groups of ΔT-vaccinated and nonvaccinated wild-type C57BL/6 or BALB/c mice and their respective receptor knockout strains. The results are representative of two separate experiments.
IL-17 receptor A is essential for ΔT vaccine-mediated protection.
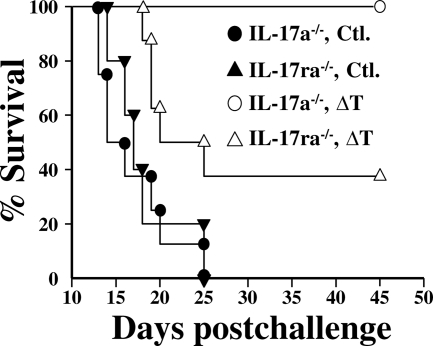
We previously reported that IL-17A knockout (IL-17a−/−) mice are still able to contain and partially clear Coccidioides from their lungs after intranasal challenge, and we suggested that this was attributable to redundancy of the family of IL-17 cytokines that respond to the infection (42). We have supported this observation here by the demonstration that 100% of the vaccinated and infected IL-17a−/− mice survived to at least 45 days postchallenge (Fig. 7). In contrast, loss of function of the IL-17 receptor A in IL-17ra−/− mice rendered them more susceptible to Coccidioides infection after immunization with the ΔT vaccine than immunized IL-17a−/− mice. Figure 7 shows that 60% of the ΔT-vaccinated and infected IL-17ra−/− mice died over the first 3 weeks postchallenge. The survivors failed to recover their original weight loss and became increasingly moribund during the period of observation. Flow cytofluorometric studies of lung-infiltrated innate immune cells in infected lungs of the ΔT-vaccinated IL-17ra−/− mice at 7 days postchallenge revealed significantly decreased recruitment of neutrophils, eosinophils, dendritic cells, and macrophages compared to that in vaccinated wild-type mice (data not shown). All nonvaccinated and infected knockout mice (IL-17a−/− and IL-17ra−/−) became moribund by 12 to 18 days after challenge and died by 25 days.
Fig. 7.
Comparison of survival plots of ΔT-vaccinated (open symbols) and nonvaccinated (solid symbols) C57BL/6 mice which have deletions of either the IL-17A gene (IL-17a−/−) or the IL-17 receptor A gene (IL-17ra−/−). Each group of mice (15 per group) was challenged with equal numbers of viable spores of C. posadasii (isolate C735). Comparisons of the survival plots of the ΔT-vaccinated IL-17ra−/− and IL-17a−/− mice and the plots of the vaccinated IL-17ra−/− and the nonvaccinated IL-17ra−/− mice revealed statistically significant differences (P = 0.01 and P = 0.001, respectively). The data are representative of two separate experiments.
DISCUSSION
Live vaccines typically generate optimal protection against infectious agents by stimulation of multiple immune effectors that target a broad spectrum of antigenic molecules of the pathogen (18). Immunization of C57BL/6 mice with a previously described live, attenuated “ΔT” vaccine strain of Coccidioides has been shown to induce protection against pulmonary coccidioidal infection based on early reduction of parasitic cell number and containment of the organism (43). Minimal dissemination of the pathogen to the spleen from sites of colonization in the lungs was observed over the initial 10-day period after intranasal inoculation, and clearance of the extrapulmonary infection had occurred by 2 weeks postchallenge. Immunity to coccidioidomycosis in ΔT-vaccinated mice was characterized by robust innate and T-cell-mediated immune responses to early stages of the disease. However, this protective response appeared to be manifested under moderated conditions managed by undefined regulatory mechanisms which effectively curtailed the host inflammatory pathology that was observed in nonvaccinated mice (43).
The host response to the initial phase of coccidioidal pneumonia is signaled by targeted neutrophil infiltration, which is a pathological hallmark of bacterial and fungal infections of the respiratory system and pivotal for host defense (2). Neutrophilic inflammation, however, can also lead to acute lung injury as a result of uncontrolled accumulation of the phagocytes combined with high concentrations of inflammatory cytokines in the infected host tissue. Dendritic cells and macrophages also infiltrate the infected lungs of C57BL/6 mice during the first 2 weeks after intranasal challenge with Coccidioides spores, although in lower numbers than neutrophils. However, they too contribute significantly to early innate immunity as a result of their phagocytic activity, production of inflammatory mediators, and efficient processing and presentation of antigenic peptides that lead to T-lymphocyte activation and induction of an adaptive immune response to infection. A wide array of host cells express numerous germ line-encoded pattern recognition receptors (PRRs) that recognize conserved pathogen-derived protein, polysaccharide, or nucleic acid motifs and activate various inflammatory and antimicrobial pathways (37). Dectin-1 and Toll-like receptor 2 (TLR-2) are two such PRRs which are expressed in response to the presence of Coccidioides spherules and have been shown to be important in immune cell activation and trafficking of phagocytes to infected lungs of C57BL/6 mice (40). Fungal cell wall-associated β-glucans are known to bind to the Dectin-1 recognition receptor, which mediates its biological effects through cooperation with TLR-2 (13). Human neutrophil recruitment and inflammation in Mycobacterium leprae infection have been reported to be coordinately regulated by activation of TLR-2 and IL-1β (23). We have shown that neutrophil infiltration into the lungs of both nonvaccinated and vaccinated mice at 7 days postchallenge occurred in the presence of proinflammatory cytokines IL-1β, IL-1α, and TNF-α. However, the concentrations of these and other proinflammatory cytokines (i.e., IL-6 and G-CSF) sharply decreased in the infected lungs of vaccinated compared to nonvaccinated mice at 11 days after challenge. This modulation of cytokine production correlated with a major reduction in the absolute number of lung-infiltrated neutrophils in vaccinated mice but with a significant increase in the numbers of tissue and alveolar macrophages in the protected animals. The concentration of CCL5/RANTES, an inflammatory chemokine known to be secreted by airway epithelial cells in response to infectious agents, was markedly elevated in the lung homogenates of immunized/infected mice. Release of this chemokine from host cells is induced by the presence of IFN-γ and TNF-α and has been shown to function as a chemoattractant for monocytes and T lymphocytes (7, 16, 30). Tissue and alveolar macrophages were recruited in significantly higher numbers to the lungs of vaccinated than to the lungs of nonvaccinated mice and presumably played roles in clearance of the organism from pulmonary tissue of the protected animals. Dendritic cells, which have been suggested to play a dominant role in antigen presentation to and activation of T lymphocytes during coccidioidomycosis (35), showed comparable levels of lung infiltration in the nonvaccinated and vaccinated mice during the initial 14 days postchallenge. On the other hand, the degrees of lung infiltration of activated T cells in the two groups of mice were markedly different. The numbers of activated CD4+ and CD8+ T cells which had infiltrated the lungs of vaccinated mice between 5 and 14 days postchallenge were significantly higher than those in the lungs of nonvaccinated animals. Although CD4+ was the dominant phenotype detected in the lung homogenates, both CD4+ and CD8+ T cells have been shown to mediate vaccine-induced protection against Coccidioides infection (10).
T helper type 17 cells are a distinct lineage of T lymphocytes that are activated in response to early stages of Coccidioides infection and further contribute to the recruitment of innate cells to the lungs (25). IL-17 and IL-22 are proinflammatory cytokines produced primarily by Th17 cells, and they have been shown to participate in the induction of neutrophil recruitment and tissue repair (25). Numerous innate cell types can also produce these cytokines (e.g., neutrophils, macrophages, and mucosal NK cells), which underscores their relevance to the early inflammatory response (31). We have previously reported that secretion of IL-17 and IL-22 in Coccidioides-infected lung tissue stimulates innate immune cells to increase production of IL-1β, TNF-α, and IL-6 secretion (42). Enhanced IL-6 production during initial stages of infection has been proposed to act in a positive feedback loop to further increase the differentiation of Th17 cells (32). IL-17 also influences neutrophil expansion and survival, and modulates neutrophil infiltration to infection sites via G-CSF and CXCL1/KC stimulation, together with other members of the family of CXC-type chemokines (26). The higher concentrations of both G-CSF and CXCL1/KC in lungs of nonvaccinated compared to vaccinated mice at 14 days postchallenge correlate with the persistent recruitment of neutrophils in the former, while reduced concentrations of these chemokines in immunized animals corresponds with the apparent dampening of lung inflammation. IL-17 also induces production of CCL2/MCP-1 and CCL3/MIP-1α, which are chemoattractants for monocytes, macrophages, and dendritic cells (32). Lung homogenates of vaccinated mice showed enhanced secretion of these two chemokines at 7 days followed by a reduction in concentration at 14 days postchallenge. On the other hand, a progressive increase in the concentration of these chemical signals was observed in the lungs of nonvaccinated mice. These observations and data presented in an earlier report (42) provide evidence that the Th17 signal pathway is a major contributor to both the initiation and control of the inflammatory response to Coccidioides infection.
Recent studies have suggested that eosinophils are multifunctional leukocytes involved in tissue homeostasis and innate immunity and are capable of producing immunoregulatory cytokines (22). A progressive increase in the number of lung-infiltrated eosinophils was observed in both nonvaccinated and vaccinated animals during the first 11 days after Coccidioides challenge. This was followed during the next 3 days by a major influx of these innate cells into the lungs of nonvaccinated mice versus a decrease in the lungs of ΔT-vaccinated animals. The presence of pulmonary eosinophilic microabscesses in patients infected with Coccidioides has been correlated with disseminated disease and a poor prognosis (8). IL-5 promotes the development of eosinophils from hematopoietic progenitor cells, while IL-4 and CCL11/exotaxin cooperate in the promotion of eosinophil trafficking to mucosal tissue. Eosinophils produce a wide range of proinflammatory cytokines and chemokines, although generally in smaller amounts than other leukocytes (38). The precise role of cytokines secreted by eosinophils during coccidioidomycosis development and host defense is poorly understood.
Th1-type immunity, which is characterized by activation of CD4+ T cells and secretion of IFN-γ and IL-12p70, has been shown to stimulate a protective cell-mediated immune (CMI) response to Coccidioides infection in the lungs of C57BL/6 mice (43). Experimental evidence derived from murine models of coccidioidomycosis has indicated that activation of the Th1 signal pathway induces the ability of phagocytes to kill Coccidioides at sites of host tissue invasion, while loss of IL-12 or IFN-γ secretion increases the severity of disease (6). On the other hand, Fierer and coworkers (10) have shown that CD4+ T-cell-deficient mice immunized with a live, attenuated vaccine strain of Coccidioides could still be protected against pulmonary coccidioidomycosis, and they suggested that CD8+ T cells also play a role in protection even though they are not required in the CD4+ competent host. We have revealed that CD8+ IFN-γ+ T cells had infiltrated the infected lungs by 7 days postchallenge and may act synergistically with CD4+ IFN-γ+ cells in early host defense.
Th2 immunity is promoted by IL-4 and IL-5 secretion, which induces B cells to produce antibodies, activates eosinophils, and is suggested to downregulate the host CMI response to coccidioidal infection (43). In spite of the evidence that a persistently elevated Th2 response during coccidioidomycosis is not protective, activation of the Th2 signal pathway is a consistent feature of vaccine immunity to this respiratory disease in mice (4, 43). Investigators of animal models of other fungal infections have argued that antibodies are required for Th2 and Th17 cell differentiation, collaborate with phagocytic cells and T lymphocytes to kill the pathogen, and modulate inflammation to prevent tissue damage (27, 34). Primary pulmonary coccidioidomycosis in humans is typically signaled within the first few days of infection by a marked rise in the titer of anti-Coccidioides IgM antibody, which is soon followed by an isotype switch to coccidioidal antigen-specific IgG production (33). The possibility that this initial rise in IgM production could have a positive influence on early cellular immune responses to coccidioidal infection has not yet been explored. Rapaka and coworkers (34) have proposed that IgM antibodies targeting highly conserved fungal cell wall carbohydrates (e.g., β-glucan and chitin) play a role in the binding of fungal organisms, influence early migration of dendritic cells to draining pulmonary lymph nodes, and contribute to Th2 and Th17 cell responses. During the initial 7 days after intranasal challenge with Coccidioides, we detected significantly higher concentrations of IL-12p70, IL-4, IL-5, IL-17A, and IL-22 in lung homogenates of ΔT-vaccinated mice than in those of nonimmunized animals. In addition, the concentrations of those cytokines representing activated Th2 (IL-4 and IL-5) and Th17 (IL-17A) signal pathways were substantially higher than the detected amounts of Th1-type cytokines (IFN-γ and IL-12p70) at this early time postchallenge. In support of these observations, our results of in vitro recall assays of cytokine production suggested that greater clonal expansion of immune Th2 and Th17 cells than of Th1 lymphocytes had occurred by 5 days postchallenge. Significantly higher percentages of CD4+ IL-5+ and CD4+ IL-17A+ T cells than of CD4+ IFN-γ+ T cells had also infiltrated the lungs of vaccinated, infected mice. These combined observations attest to the stimulation of a strong combined Th2 and Th17 cell response to the respiratory insult of Coccidioides spores and parasitic cells during the first 7 days after challenge. Moreover, during the next 7 days, surrogates of the level of Th2 cell activation showed a sharp decrease (both cytokine concentrations and T-cell numbers) in lung tissue of vaccinated animals, while indicators of Th1 and Th17 cell response revealed a progressive increase between 7 and 11 days. However, comparative analyses of both the cytokine and fluorescence-activated cell sorter (FACS) data at 11 days postchallenge suggest that the Th17 signal pathway plays a dominant role in the response of the vaccinated host to infection at this stage in the course of the disease. Furthermore, the essential requirement of vaccine-induced Th17 cells for protection against Coccidioides was demonstrated in mice by genetic deletions designed to dispense of Th1, Th2, or Th17 immunity. Both IFN-γ and IL-4 receptor-deficient mice could still be protected against intranasal challenge with Coccidioides by immunization with the ΔT vaccine to a degree that was equivalent to that for vaccinated wild-type mice. IL-17 receptor A knockout mice, on the other hand, could be only partially protected. These findings support our earlier conclusion that a vaccine against coccidioidomycosis should be able to induce the Th17 signal pathway if it is to be effective (42).
In summary, the results of our analyses of the initial expansion of lung-infiltrated immune cells in response to coccidioidal infection together with associated production of cytokine and chemokine signals in vaccinated mice provide a profile of the early events of protective immunity to this respiratory disease. Evidence derived from investigations of animal models of fungal infections as well as clinical studies of human mycoses indicate that IL-17-producing cells play key roles in bridging between innate and adaptive immunity (20). Although the Th1 signal pathway has been shown to be dispensable for vaccine immunity to coccidioidomycosis, Th1 and Th17 cells most likely share specific tasks in the control of fungal infection in the immunocompetent host. An intriguing possibility is that Th17 cells can be converted into Th1/Th17 cells by combined IFN-γ and IL-12 signaling (24), which indicates a synergistic role for these T helper cells in response to infection. The function of the Th2 signal pathway in defense against coccidioidomycosis is still unresolved, and research efforts are required to determine whether antibodies can contribute to early regulation of cell-mediated immunity and pathogen clearance. The consistent and well-delineated changes in cytokine/chemokine production and T-cell infiltration of infected lung tissue in vaccinated mice observed in this study imply the existence of a precisely orchestrated regulation of the T helper cell response to Coccidioides infection. The results of our examinations of the expression levels of the T-bet, GATA-3, and RORγt genes, which encode transcription factors required for the differentiation of Th1, Th2, and Th17 cells, respectively (45), during early stages of the disease will be reported in a separate publication. A modulating factor in Th1 and Th17 promotion of the inflammatory response appears to be the activation of IL-10-producing T regulatory cells, which can inhibit the synthesis of IFN-γ and TNF-α and limit the magnitude of phagocyte recruitment to infection sites (3, 9). Current studies of our murine model of vaccine immunity to coccidioidomycosis are focused on the characterization of these immune regulatory mechanisms that limit inflammatory pathology while promoting pathogen clearance.
ACKNOWLEDGMENTS
Support for this work was provided by Public Health Service grants AI-071118 and AI-070891 from the National Institute of Allergy and Infectious Disease, National Institutes of Health, awarded to G.T.C. Additional support was provided by the Margaret Batts Tobin Foundation, San Antonio, TX.
We thank Natalia Castro-Lopez and Michael J. Bellecourt for their technical assistance in determinations of fungal burden in Coccidioides-infected mice.
Footnotes
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Ampel N. M., et al. 2002. In vitro whole-blood analysis of cellular immunity in patients with active coccidioidomycosis by using the antigen preparation T27K. Clin. Diagn. Lab. Immunol. 9:1039–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balamayooran G., Batra S., Fessler M. B., Happel K. I., Jeyaseelan S. 2010. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am. J. Respir. Cell Mol. Biol. 43:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belkaid Y., Tarbell K. 2009. Regulatory T cells in the control of host-microorganism interactions. Annu. Rev. Immunol. 27:551–589 [DOI] [PubMed] [Google Scholar]
- 4. Cole G. T., et al. 2004. A vaccine against coccidioidomycosis is justified and attainable. Med. Mycol. 42:189–216 [DOI] [PubMed] [Google Scholar]
- 5. Cole G. T., et al. 2006. Virulence mechanisms of Coccidioides, p. 363–391 In Heitman J., Filler S. G., Edwards J. E., Mitchell A. P. (ed.), Molecular principles of fungal pathogens. ASM Press, Washington, DC [Google Scholar]
- 6. Cox R. A., Magee D. M. 2004. Coccidioidomycosis: host response and vaccine development. Clin. Microbiol. Rev. 17:804–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Culley F. J., et al. 2006. Role of CCL5 (RANTES) in viral lung disease. J. Virol. 80:8151–8157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Echols R. M., Palmer D. L., Long G. W. 1982. Tissue eosinophilia in human coccidioidomycosis. Rev. Infect. Dis. 4:656–664 [DOI] [PubMed] [Google Scholar]
- 9. Fierer J., Walls L., Eckmann L., Yamamoto T., Kirkland T. N. 1998. Importance of IL-10 in genetic susceptibility of mice to Coccidioides immitis. Infect. Immun. 66:4397–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fierer J., Waters C., Walls L. 2006. Both CD4+ and CD8+ T cells can mediate vaccine-induced protection against Coccidioides immitis infection in mice. J. Infect. Dis. 193:1323–1331 [DOI] [PubMed] [Google Scholar]
- 11. Flaherman V. J., Hector R., Rutherford G. W. 2007. Estimating severe coccidioidomycosis in California. Emerg. Infect. Dis. 13:1087–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galgiani J. N., et al. 2005. Coccidioidomycosis. Clin. Infect. Dis. 41:1217–1223 [DOI] [PubMed] [Google Scholar]
- 13. Gantner B. N., Simmons R. M., Canavera S. J., Akira S., Underhill D. M. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzalez A., Hung C.-Y., Cole G. T. 2011. Coccidioides releases a soluble factor that suppresses nitric oxide production by murine primary macrophages. Microb. Pathog. 50:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzalez A., Hung C.-Y., Cole G. T. 2011. Nitric oxide synthase activity has limited influence on the control of Coccidioides infection in mice. Microb. Pathog. 51:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Homma T., et al. 2010. Cooperative activation of CCL5 expression by TLR3 and tumor necrosis factor-alpha or interferon-gamma through nuclear factor-kappaB or STAT-1 in airway epithelial cells. Int. Arch. Allergy Immunol. 152(Suppl. 1):9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hung C. Y., et al. 2005. A metalloproteinase of Coccidioides posadasii contributes to evasion of host detection. Infect. Immun. 73:6689–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamei A., Coutinho-Sledge Y. S., Goldberg J. B., Priebe G. P., Pier G. B. 2011. Mucosal vaccination with a multivalent, live-attenuated vaccine induces multifactorial immunity against Pseudomonas aeruginosa acute lung infection. Infect. Immun. 79:1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keckich D. W., Blair J. E., Vikram H. R., Seville M. T., Kusne S. 2011. Reactivation of coccidioidomycosis despite antifungal prophylaxis in solid organ transplant recipients. Transplantation 92:88–93 [DOI] [PubMed] [Google Scholar]
- 20. Khader S. A., Gaffen S. L., Kolls J. K. 2009. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kirkland T. N., Cole G. T. 2001. Coccidioidomycosis: pathogenesis, immune response and vaccine development, p. 365–399 In Calderone R. A., Cihlar L. C. (ed.), Fungal pathogenesis: principles and applications. Marcel Dekker, New York, NY [Google Scholar]
- 22. Kita H. 2011. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol. Rev. 242:161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee D. J., et al. 2010. Integrated pathways for neutrophil recruitment and inflammation in leprosy. J. Infect. Dis. 201:558–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lexberg M. H., et al. 2010. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur. J. Immunol. 40:3017–3027 [DOI] [PubMed] [Google Scholar]
- 25. Lin Y., Slight S., Khader S. 2010. Th17 cytokines and vaccine-induced immunity. Semin. Immunopathol. 32:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacCallum D. M., Castillo L., Brown A. J., Gow N. A. R., Odds F. C. 2009. Early-expressed chemokines predict kidney immunopathology in experimental disseminated Candida albicans infections. PLoS One 4:e6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magliani W., et al. 2005. Antibody-mediated protective immunity in fungal infections. New Microbiol. 28:299–309 [PubMed] [Google Scholar]
- 28. Margolis D. A., Viriyakosol S., Fierer J., Kirkland T. N. 2011. The role of reactive oxygen intermediates in experimental coccidioidomycois in mice. BMC Microbiol. 11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathisen G., Shelub A., Truong J., Wigen C. 2010. Coccidioidal meningitis: clinical presentation and management in the fluconazole era. Medicine 89:251–284 [DOI] [PubMed] [Google Scholar]
- 30. Matsuzaki S., et al. 2010. Lysophosphatidic acid inhibits CC chemokine ligand 5/RANTES production by blocking IRF-1-mediated gene transcription in human bronchial epithelial cells. J. Immunol. 185:4863–4872 [DOI] [PubMed] [Google Scholar]
- 31. McAleer J. P., Kolls J. K. 2011. Mechanisms controlling Th17 cytokine expression and host defense. J. Leukoc. Biol. doi:10.1189/jlb.021109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Onishi R. M., Gaffen S. L. 2010. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pappagianis D., Zimmer B. L. 1990. Serology of coccidioidomycosis. Clin. Microbiol. Rev. 3:247–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rapaka R. R., et al. 2010. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J. Exp. Med. 207:2907–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richards J. O., Ampel N. M., Galgiani J. N., Lake D. F. 2001. Dendritic cells pulsed with Coccidioides immitis lysate induce antigen-specific naive T cell activation. J. Infect. Dis. 184:1220–1224 [DOI] [PubMed] [Google Scholar]
- 36. Sharpton T. J., et al. 2009. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 19:1722–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Skeldon A., Saleh M. 2011. The inflammasomes: molecular effectors of host resistance against bacterial, viral, parasitic, and fungal infections. Front. Microbiol. 2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stone K. D., Calman P., Dean D. M. 2010. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 125:S73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tarcha E. J., Basrur V., Hung C. Y., Gardner M. J., Cole G. T. 2006. Multivalent recombinant protein vaccine against coccidioidomycosis. Infect. Immun. 74:5802–5813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Viriyakosol S., Fierer J., Brown G. D., Kirkland T. N. 2005. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect. Immun. 73:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waters W. R., et al. 2003. Expression of L-selectin (CD62L), CD44, and CD25 on activated bovine T cells. Infect. Immun. 71:317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wüthrich M., et al. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest. 121:554–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xue J., et al. 2009. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect. Immun. 77:3196–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xue J., Hung C.-Y., Yu J.-J., Cole G. T. 2005. Immune response of vaccinated and non-vaccinated mice to Coccidioides posadasii infection. Vaccine 23:3535–3544 [DOI] [PubMed] [Google Scholar]
- 45. Yang X. O., et al. 2008. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28:29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zimmermann C. R., et al. 1998. Protection against lethal murine coccidioidomycosis by a soluble vaccine from spherules. Infect. Immun. 66:2342–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]