Abstract
During infection, Streptococcus pneumoniae exists mainly in sessile biofilms rather than in planktonic form, except during sepsis. The capacity to form biofilms is believed to be important for nasopharyngeal colonization as well as disease pathogenesis, but relatively little is known about the regulation of this process. Here, we investigated the effect of exogenous iron [Fe(III)] as well as the role of luxS (encoding S-ribosylhomocysteine lyase) on biofilm formation by S. pneumoniae D39. Fe(III) strongly enhanced biofilm formation at concentrations of ≥50 μM, while Fe(III) chelation with deferoxamine was inhibitory. Importantly, Fe(III) also upregulated the expression of luxS in wild-type D39. A luxS-deficient mutant (D39luxS) failed to form a biofilm, even with Fe(III) supplementation, whereas a derivative overexpressing luxS (D39luxS+) exhibited enhanced biofilm formation capacity and could form a biofilm without added Fe(III). D39luxS exhibited reduced expression of the major Fe(III) transporter PiuA, and the cellular [Fe(III)] was significantly lower than that in D39; in contrast, D39luxS+ had a significantly higher cellular [Fe(III)] than the wild type. The release of extracellular DNA, which is an important component of the biofilm matrix, also was directly related to luxS expression. Similarly, genetic competence, as measured by transformation frequency as well as the expression of competence genes comD, comX, comW, cglA, and dltA and the murein hydrolase cbpD, which is associated with fratricide-dependent DNA release, all were directly related to luxS expression levels and were further upregulated by Fe(III). Moreover, mutagenesis of cbpD blocked biofilm formation. We propose that competence, fratricide, and biofilm formation are closely linked in pneumococci, and that luxS is a central regulator of these processes. We also propose that the stimulatory effects of Fe(III) on all of these parameters are due to the upregulation of luxS expression, and that LuxS provides for a positive Fe(III)-dependent amplification loop by increasing iron uptake.
INTRODUCTION
Streptococcus pneumoniae is a Gram-positive bacterium that is responsible for two million deaths annually (15). It is the major cause of otitis media, pneumonia, meningitis, and sepsis in young children and the elderly (18, 24, 27). During the first months of life the human nasopharynx is colonized by S. pneumoniae, where it can persist as part of the commensal flora. Progression to other sites of the human body results in disease. Like many other bacterial pathogens, pneumococcal colonization and disease often is associated with biofilm formation (13, 22). Biofilm formation is a complex and multifactorial process that involves several steps. The first step involves the adherence of the bacterial cells to the host surface; subsequently, adherent cells form a multilayer biofilm covered by an extracellular matrix. This matrix protects the bacteria from antibiotics as well as from attack by the host immune system. The final stage of biofilm development is characterized by the detachment of single cells or multicellular clusters that can disseminate and infect secondary sites (14).
S. pneumoniae biofilm formation has been shown to be influenced by a variety of factors, including colony opacity phase variation (1, 46), sugar availability (45), and the quorum-sensing (QS) signal generated by the competence-stimulating peptide (36, 44). The involvement of QS signaling in the regulation of the formation of biofilm communities is a common theme among a variety of bacterial species, and it plays a key role in coordinating the spatial disposition of cells, as well as in aggregation and exopolysaccharide formation (4, 41). The most common and widespread QS signal in both Gram-positive and Gram-negative bacteria is autoinducer-2 (AI-2), which is synthesized by the enzyme S-ribosylhomocysteine lyase (LuxS) (43). Although the central role of AI-2 in biofilm development and virulence has been established for a number of species (29, 34, 37, 42), the mechanism behind the activation or regulation of LuxS is unknown.
Another important factor for biofilm development by pathogenic bacteria is iron availability. Iron is an essential element for almost all cells due to its role as a cofactor in many enzymes, particularly those of central metabolism and respiration. The various strategies for iron acquisition by bacterial pathogens within a host environment have been well documented (3). In Pseudomonas aeruginosa, the prototype organism for biofilm formation by bacterial pathogens, increased iron levels play a key role in the persistence of infection in the lungs of cystic fibrosis patients (2, 6). The upregulation of biofilm formation by this metal also has been demonstrated for Pseudomonas fluorescens (5) and Escherichia coli (50). However, interestingly, the opposite is true for Acinetobacter baumannii (17), Streptococcus mutans (51), and staphylococci (26), where biofilm formation is significantly enhanced under low iron conditions.
In the present study, we have investigated of the roles of iron and LuxS in S. pneumoniae biofilm formation and genetic competence, demonstrating interrelated effects on both parameters.
MATERIALS AND METHODS
Strains and growth conditions.
S. pneumoniae strains used in this study are listed in Table 1. Cells were grown in a casein-based semisynthetic liquid medium (C+Y) (31) or on Columbia agar plates supplemented with 5% horse blood at 37°C in a CO2-enriched atmosphere. Bacterial stocks were prepared from mid-log-phase cultures and stored at −80°C in 10% glycerol. For overnight cultures, pneumococcal mutant strains were grown in the presence of 0.2 μg/ml erythromycin.
Table 1.
S. pneumoniae strains and primers used in this study
| Strain or primer | Description or sequence (5′-3′) | Source or reference |
|---|---|---|
| Strain | ||
| D39 | Capsular serotype 2 | NCTC7466 |
| D39luxS | luxS insertion-duplication mutant (Eryr) | 42 |
| D39luxS+ | D39 carrying pALT2:luxS | This study |
| D39lytA | lytA insertion-duplication mutant (Eryr) | 8 |
| D39cbpD | cbpD deletion-replacement mutant (Specr) | This study |
| Primer | ||
| luxS-pAL2-F | ACAGGAGGACTCTCTATGTCAAAAGAAGTTATTGTCGAAG | |
| luxS-pAL2-R | GCGCGAATTCTTAAATCACATGACGTTCAAAGGC | |
| pAL2-F | GGATCCGAAAATTTGTTTGC | |
| pAL2-R | CATAGAGAGTCCTCCTGT | |
| 16S F | GGTGAGTAACGCGTAGGTAA | |
| 16S R | ACGATCCGAAAACCTTCTTC | |
| luxS-RT-F | CCCTATGTTCGCTTGATTGGGG | |
| luxS-RT-R | AGTCAATCATGCCGTCAATGCG | |
| cbpD-RT-F | ACCGACGATTGGTTCCATTA | |
| cbpD-RT-R | CCAACACTGCCACTATCCAA | |
| RHrtcomDF | GGTTCGTATCATGAGCGTTT | |
| RHrtcomDR | CCTGAAGGAGTCATCGTCAT | |
| RHrtcomXF | AAGGCATGCTCTGCTTACAT | |
| RHrtcomXR | TCTACGCTTCTGACTTTCCTG | |
| RHrtcomWF | GGTGATAATTTTGACTGGGAAC | |
| RHrtcomWR | AACCCCGATTCATTACCAT | |
| RHrtcglAF | TCAGTTGCAGTTGAACGAAG | |
| RHrtcglAR | CTGTCGCACCTGTCAAACTA | |
| RHrtcoiAF | AGTCCCTTGCCTCAGAAAGT | |
| RHrtcoiAR | GCCCATGTTTTGACTGAAAT | |
| cbpD1 | ATGACTACTATGAACGTGGT | |
| cbpD2 | TATGTATTCATATATATCCTCCTCGCGATGATTGCCTTCACC | |
| cbpD3 | AAATAACAGATTGAAGAAGGTATAATAATACGACGGTGGATGGC | |
| cbpD4 | AACCTGCTAACTGCCAGAT | |
| J253 | GAGGAGGATATATTTGAATACATACG | |
| J254 | TTATAATTTTTTTAATCTGTTATTTAAATAGTTTATAGTTA |
Aggregation assay.
The aggregation assay was performed by growing S. pneumoniae D39 and its derivatives in C+Y medium at 37°C in a 10-ml tube in the presence of increasing amounts of Fe(III) nitrate (10, 50, 100, and 200 μM). When the growing cultures reached an A600 of 0.2, samples were placed in a rack and examined macroscopically. All assays were performed at least four times.
Biofilm assays.
Static biofilm assay was performed by growing bacteria in 24- or 96-well flat-bottom polystyrene plates (Sarstedt). S. pneumoniae cells grown to 1 × 105 CFU/ml (mid-exponential phase) were inoculated 1:10 into 1:3-diluted C+Y medium. When required, C+Y medium was supplemented with 10, 50, 100, and 200 μM Fe(III), Fe(II), hemin, or deferoxamine mesylate salt. A combination of 50 μM Fe(III) and 10 μM deferoxamine was chosen after testing for biofilm formation and growth effects. When specified, Fe(III) was added at the beginning of the experiments, and pneumococci were incubated for 6 h before the addition of deferoxamine. The chelator compound was tested for the inhibition of biofilm formation after 2, 4, and 16 h of incubation. Alternatively, deferoxamine was added at the beginning of the experiment. The plates were incubated at 37°C in a CO2-enriched atmosphere for 4, 6, and 24 h. For the microscopic examination of biofilms, wells were washed three times with PBS, desiccated at 50°C, stained with 1% crystal violet for 30 min, and photographed under transmitted light using an AMG Evos microscope with a 60× objective. For the quantitation of biofilm density, crystal violet-stained biofilms in 96-well plates were dissolved in 70% ethanol (125 μl per well), and the absorbance at 590 nm was measured in a Spectromax M2 spectrophotometer (Molecular Devices). Alternatively, to quantify the bacteria attached to the plastic substratum, unstained plates were washed three times and wells were filled with 1 ml of fresh C+Y medium, sealed, floated on a Transonic 460/H sonicating water bath, and sonicated for 3 s at 35 KHz. These dispersed biofilm-derived bacterial suspensions, as well as samples of planktonic cells taken at various time points, were serially diluted and plated onto blood agar. For the complementation assay, 1 ml of D39luxS+ culture was used. After 6 h of incubation in C+Y medium, the supernatants were passed through a 0.22-μm filter, inoculated into D39luxS cultures, and incubated overnight. The cultures then were plated onto blood agar as previously described.
Overexpression of luxS in S. pneumoniae.
The overexpression of luxS in S. pneumoniae was achieved by cloning pneumococcal luxS into a derivative of the Streptococcus-Escherichia coli shuttle vector pVA838, such that expression was under the control of a constitutive promoter of the S. pneumoniae aminopterin resistance operon (ami). Beard and coworkers (7) constructed the vector pAL2 from pVA838, which contains the ami promoter fused to the 5′ end of the lux operon of Photorhabdus luminescens. This construct was used to generate a stable bioluminescence phenotype in S. pneumoniae. To express pneumococcal luxS using this construct, the P. luminescens lux operon was first removed from pAL2 by digestion with EcoRI, and S. pneumoniae luxS subsequently was cloned as follows. luxS was amplified by PCR using the primers luxS-pAL2-F and luxS-pAL2-R (Table 1). The reverse primer contains an EcoRI restriction site for cloning, while the forward primer contains an 18-bp tag homologous to the 3′ end of the ami promoter sequence. The amplification of the ami promoter sequence by PCR from pAL2 using primers pAL2-F and pAL2-R generated a 150-bp product which also contains an EcoRI restriction site and a ribosome binding site 3′ of the promoter element. This product was fused to the 5′ end of the luxS open reading frame (ORF) using splice overlap extension PCR, and the final product was digested with EcoRI and ligated with the EcoRI-digested pAL2. E. coli XL-10 transformants were selected on LB agar containing 500 μg/ml erythromycin, and the presence of the insert in the correct orientation was confirmed by PCR and sequencing. Competent S. pneumoniae D39 cells were transformed with pAL2:luxS, and the overexpression of luxS in this strain (designated D39luxS+) relative to that of wild-type (WT) D39 was confirmed using real-time reverse transcription-PCR (RT-PCR) with primers luxS-RT-F and luxS-RT-R (Table 1).
eDNA quantification.
Extracellular DNA (eDNA) release during growth was measured essentially as described by Kreth et al. (30). Briefly, S. pneumoniae D39 and its derivatives were plated on blood agar and incubated at 37°C in 95% air and 5% CO2 overnight. A single colony of each strain then was used to inoculate 5 ml of C+Y broth and incubated at 37°C in a CO2-enriched atmosphere. The cultures were grown until the mid-logarithmic phase (A590 = 0.2) in C+Y medium and used to inoculate the microtiter plates for biofilm formation assays. Before the assays, the growth rates of all strains were determined, and they were essentially similar. At 6 h and 1 day after incubation in 24-well flat-bottom polystyrene plates, cells were collected and centrifuged for 2 min at 16,000 × g at 4°C. Supernatants were transferred to new tubes containing 0.5 ml TE buffer (10 mM Tris-HCl, 0.5 mM EDTA, pH 7.8)-saturated phenol-chloroform-isoamyl alcohol (25:24:1), and the mixture was vortexed for 30 s. To precipitate DNA, the mixture was centrifuged at 4°C for 5 min at 16,000 × g. The aqueous phase (0.8 ml) was removed and mixed with 80 μl of 3 M sodium acetate, pH 5.2, and 500 μl of 100% propan-2-ol. The mixture then was centrifuged for 10 min at 16,000 × g, the supernatant was decanted, and the precipitated sample was air dried and suspended in 25 μl of deionized nuclease-free H2O (Fisher Scientific, Fair Lawn, NJ). Total DNA from 1 ml of cell suspension was extracted in the same way as that described for eDNA. The total amounts of DNA and extracellular DNA were quantitated using a Nanodrop 1000 spectrophotometer (Thermo Scientific).
Quantitative Western blot analysis of cell lysates.
After biofilm growth, cells were harvested as described above and lysed in 0.1% sodium deoxycholate at 37°C for 30 min. Total protein concentration was measured using a Nanodrop 1000 spectrophotometer (Thermo Scientific). Samples then were resuspended in 2× LUG (5% β-mercaptoethanol, 62.5 mM Tris-HCl, 2% sodium dodecyl sulfate, 10% glycerol, and 0.05% bromophenol blue; pH 6.8), boiled for 5 min, and separated using SDS-PAGE. After electroblotting onto nitrocellulose membranes, samples were probed with polyclonal mouse anti-PiuA (12) by standard protocols. Novex Sharp prestained protein ladder (Invitrogen) was used as a molecular size marker. A 1:15,000 dilution of IRDye800-labeled anti-mouse IgG (Rockland, Gilbertsville, PA) was used as a secondary antibody. All antibody dilutions were made in casein blocking solution (LiCor, Lincoln, NE). Signal intensities were analyzed using the Odyssey infrared imaging system (LiCor).
ICP-MS analysis.
To perform inductively coupled plasma-mass spectrometry (ICP-MS) analysis, strains were grown in 10 ml of C+Y medium supplemented, when required, with 100 μM Fe(III) or 100 μM deferoxamine. Cultures were incubated at 37°C, and growth was measured at regular intervals until an A600 of 0.3; the experiment was performed in triplicate. Cells were harvested by centrifugation and washed three times in phosphate-buffered saline (PBS), with or without 2.5 mM EDTA (to chelate extracellular metal ions). The dry weight of the cell pellet also was determined. The pellet then was dissolved in 3.5% HNO3 and heated at 95°C for 30 min. The insoluble material was removed by centrifugation at 14,000 × g for 20 min. The supernatant was collected and diluted in 3.5% HNO3 for the analysis of metal content on an XSeries II (Thermo-Fisher) apparatus.
Competence efficiency assay.
Competence-induced transformation was performed using the protocol described by Pozzi et al. (39), with some modifications. Briefly, bacterial cultures were growth in C+Y medium with or without 50 μM Fe(III) nitrate until an A600 of 0.15. At this point, cultures were diluted 1:100 in competence CAT medium and incubated at 37°C for 10 min with 150 ng/ml CSP-1 (Chiron Technologies, Australia) to induce competence. After the addition of purified chromosomal DNA from S. pneumoniae DP1617, cells were incubated for 2 h at 37°C. Transformation efficiency was determined by plating on blood agar with or without 100 μg/ml spectinomycin.
Quantitative real-time RT-PCR.
Differences in levels of gene expression were assayed by one-step relative quantitative real-time RT-PCR in a Roche LC480 real-time cycler essentially as described previously (32). The specific primers used for the various genes are listed in Table 1 and were used at a final concentration of 200 nM per reaction. As an internal control, primers specific for 16S rRNA were employed. Amplification data were analyzed using the comparative critical threshold (2−ΔΔCT) method (33).
Mutagenesis of cbpD.
The cbpD gene was deleted from S. pneumoniae D39 and replaced with a spectinomycin cartridge by transformation with a linear DNA fragment constructed by overlap extension PCR using primers cbpD1, cbpD2, cbpD3, cbpD4, J53, and J54 (Table 1), essentially as described previously (46).
RESULTS
Iron concentration influences S. pneumoniae aggregation and biofilm development.
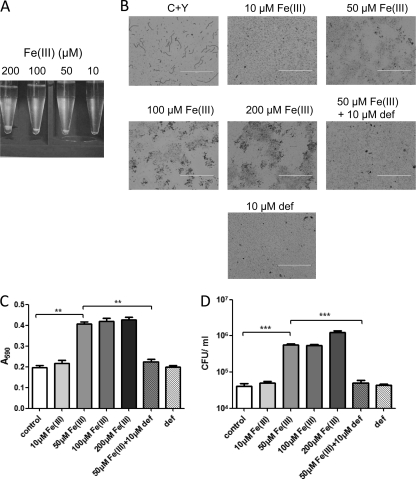
To examine the effect of iron on pneumococcal biofilm development, strain D39 was grown in a semisynthetic medium (C+Y) with the addition of Fe(III) nitrate at concentrations from 10 to 200 μM. Given that cellular aggregation is intimately associated with biofilm development, a simple aggregation assay was performed first (see Materials and Methods). Macroscopic examination indicated that pneumococcal aggregation is directly proportional to Fe(III) concentration (Fig. 1A). Pneumococcal growth rates were not significantly different in the presence of these Fe(III) concentrations (data not shown). To determine the effect of Fe(III) supplementation on the interaction of pneumococci with a solid surface, cells were grown under the same conditions in 24-well tissue culture plates and observed microscopically at 6 and 24 h. Cells supplemented with Fe(III) attached to the surface in a dense layer covered with extracellular matrix even after just 6 h of incubation (Fig. 1B). A statistically significant increase in biofilm density was observed at Fe(III) concentrations of ≥50 μM, as judged by both the A590 of crystal violet-stained biofilms (P < 0.01) (Fig. 1C) and viable counts of dispersed, unstained biofilms (P < 0.001) (Fig. 1D). Conversely, pneumococci that did not receive iron supplementation did not form a biofilm, and at 10 μM Fe(III), density was only marginally above the baseline (Fig. 1B, C, and D). These results demonstrate that S. pneumoniae requires iron for efficient biofilm development.
Fig. 1.
Aggregation and biofilm formation in response to Fe(III). Pneumococcal cultures were incubated for 6 h in C+Y medium alone or in C+Y supplemented with 10, 50, 100, and 200 μM Fe(III) nitrate; cells also were grown in C+Y supplemented with 50 μM Fe(III) and 10 μM deferoxamine or with deferoxamine alone. (A) Aggregation assay showing increasing size of D39 cell pellets in Fe(III)-supplemented media. (B) Microscopic examination of crystal violet-stained biofilms (scale bar, 200 μm). Biofilm density also was quantitated by measuring the A590 of crystal violet-stained wells (C) and by performing viable counts on ultrasonically dispersed (unstained) biofilms (D), as described in Materials and Methods. Data presented are the means ± standard deviations from three independent experiments (**, P < 0.01; ***, P < 0.001; unpaired t test).
To further confirm that the phenomenon observed is iron specific, iron-induced biofilms were treated with the Fe(III) chelator deferoxamine (see Materials and Methods). Results revealed that treatment with this compound disrupts biofilm structure (Fig. 1B), significantly reducing both the A590 of stained biofilms (P < 0.01) (Fig. 1C) and the number of viable bacteria in the biofilm (P < 0.001) (Fig. 1D). However, deferoxamine did not have any effect on the growth rate of pneumococci (results not shown). Collectively, these results confirm that Fe(III) is a positive regulator of biofilm formation in S. pneumoniae.
Fe(III) specifically promotes biofilm formation.
To determine whether the phenotype observed above can be replicated using other biologically relevant forms of iron, experiments were repeated using Fe(II) sulfate and hemin. Results revealed that attachment, aggregation, and extracellular matrix production by D39 were not induced upon supplementation with either of these iron sources to the same degree as that observed with Fe(III) supplementation (data not shown). This was not due to toxicity, as hemin and Fe(II) did not impair pneumococcal growth at the concentrations used (data not shown).
LuxS is involved in pneumococcal biofilm formation.
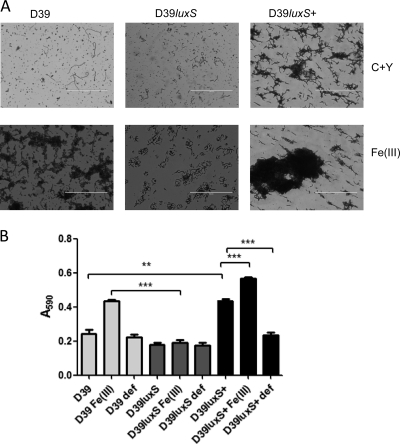
Bacteria use quorum sensing to coordinate certain behaviors, including biofilm formation, based on the local density of the bacterial population. AI-2 produced by the mononuclear iron protein S-ribosylhomocysteine lyase (LuxS) is a widespread QS signal. A luxS mutant of S. pneumoniae D39 (designated D39luxS) has previously been reported to display reduced virulence compared to that of its wild-type parent following intranasal or intraperitoneal challenge of mice (42). To determine if pneumococcal LuxS has a role in biofilm formation, as well as to examine the effect of iron on this strain, D39luxS was tested in the biofilm assay described above. Like the wild type, D39luxS formed aggregates at the bottom of 10-ml tubes in response to Fe(III) supplementation (data not shown). However, the mutant did not form a biofilm covered by extracellular matrix on the culture trays after 6 or 24 h of incubation. Microscopic examination of the tray after crystal violet staining revealed that bacteria were attached to the surface, but the extracellular matrix was absent (Fig. 2A). Total crystal violet staining (A590) also was significantly lower for D39luxS than for D39 in the presence of Fe(III) (P < 0.001) (Fig. 2B).
Fig. 2.
Biofilm formation by D39 WT, D39luxS, and D39luxS+. Cells were grown for 24 h in C+Y medium supplemented with 50 μM Fe(III). Cells were stained with crystal violet and examined microscopically (A) (scale bar, 200 μm) and quantitatively by measuring the A590 (B). Data presented are the means ± standard deviations from three independent experiments (**, P < 0.01; ***, P < 0.001; unpaired t test).
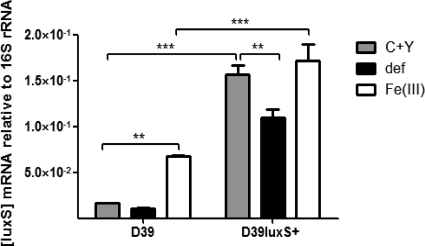
To further confirm the role of LuxS in iron-induced pneumococcal biofilm formation, luxS was overexpressed in the D39 background (designated D39luxS+), as described in Materials and Methods. To confirm overexpression, levels of luxS mRNA (relative to 16S rRNA) were measured in wild-type D39 and D39luxS+ using real-time RT-PCR (Fig. 3). As expected, luxS expression in D39luxS+ was significantly higher than that in the wild type under all conditions tested (P < 0.001). Interestingly, Fe(III) supplementation significantly increased the expression of luxS in wild-type D39, while expression in D39luxS+ was significantly lower when deferoxamine was added to the medium (P < 0.01 in both cases) (Fig. 3). This implies a direct role for Fe(III) in the regulation of luxS in S. pneumoniae.
Fig. 3.
Relative expression of luxS. The expression of luxS by D39 and D39luxS+ grown in C+Y medium, with or without 50 μM Fe(III) or 10 μM deferoxamine, was quantitated by real time RT-PCR using 16S rRNA as an internal control, as described in Materials and Methods. Data presented are the means ± standard deviations from three independent experiments (**, P < 0.01; ***, P < 0.001; unpaired t test).
Importantly, D39luxS+ was able to form a biofilm with extracellular matrix material even in medium without Fe(III) supplementation, and crystal violet staining density was significantly greater for D39luxS+ biofilms than for those of D39 (P < 0.01) (Fig. 2A and B). Moreover, the addition of Fe(III) to cultures of D39luxS+ resulted in the overproduction of extracellular matrix compared to levels for wild-type D39 grown under the same conditions, with a further significant increase in staining density (P < 0.001) (Fig. 2A and B). Similarly to the phenotype for wild-type D39, the addition of Fe(II) or hemin did not result in the stimulation of the biofilm formation of D39luxS or D39luxS+ (result not shown).
LuxS influences pneumococcal iron uptake.
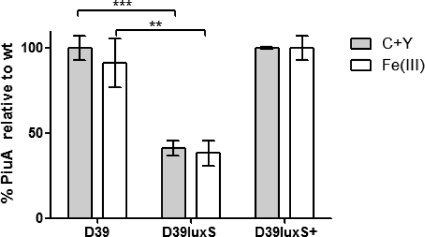
The data presented above outline the importance of LuxS in the stimulation of biofilm formation in the presence of iron. This suggests that iron uptake is influenced by LuxS activity. Three major iron uptake systems (ABC transporters) have been identified in S. pneumoniae: Pit (sp0241) (11), Piu (sp1869-72) (10), and Pia (sp1032-35) (12). The piu operon is known to be regulated by the orphan response regulator, RitR (47), and it is thought that Piu is the major iron uptake system in the pneumococcus. Therefore, the expression of PiuA in D39, D39luxS, and D39luxS+ growing in media with and without Fe(III) supplementation was determined by quantitative Western blot analysis. Cells were collected at the early exponential phase (A600 = 0.15) as well as at mid-exponential phase (A600 = 0.4). The expression of PiuA in D39luxS was 60% less than that in the wild type (P < 0.001) in the early exponential phase (Fig. 4), but this difference was not as pronounced in the mid-exponential phase (data not shown). The expression of PiuA in D39luxS+ was similar to that of the wild type. Furthermore, the addition of excess Fe(III) did not affect PiuA expression in any of the three strains tested (Fig. 4).
Fig. 4.
PiuA expression by D39, D39luxS, and D39luxS+. Pneumococci were grown in C+Y medium with or without 50 μM Fe(III) until an A600 of 0.15. PiuA protein in cell lysates was assayed by quantitative Western blotting as described in Materials and Methods. PiuA levels are expressed as a percentage of that in D39 grown in C+Y. Data presented are the means ± standard deviations from three independent experiments (**, P < 0.01; ***, P < 0.001; unpaired t test).
To confirm that these results correspond with differential iron uptake, ICP-MS was used to measure the intracellular iron content of pneumococci grown in C+Y media (see Materials and Methods). Wild-type D39 was found to contain 19.5 ± 5.6 μmol iron per g of cell dry weight, compared to 4.7 ± 1.9 μmol/g for D39luxS (P < 0.05; unpaired t test, one-tailed) and 48 ± 6.6 μmol/g for D39luxS+ (P < 0.05 relative to the wild type). Thus, there is a direct relationship between LuxS activity and iron uptake in the pneumococcus.
The stimulatory effect of Fe(III) on biofilm formation is linked to DNA release.
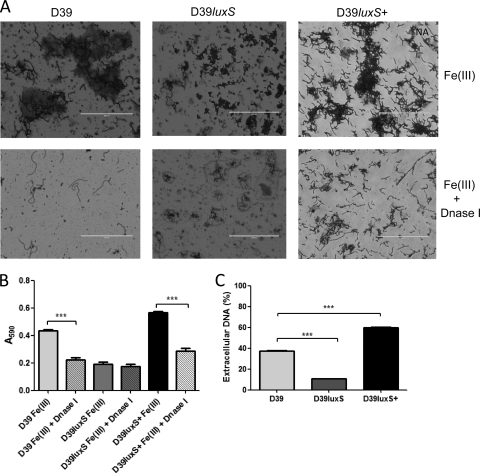
Extracellular DNA has been characterized as one of the matrix components in S. pneumoniae biofilms (35). To test if the matrix produced when pneumococci are grown at high Fe(III) concentrations included extracellular DNA, 1-day-old S. pneumoniae biofilms were treated with DNase I. For both D39 and D39luxS+ biofilms, treatment resulted in the significant breakdown of the matrix and dispersal of the attached cells, correlating with a significant decrease in crystal violet staining density (P < 0.001 in both cases) (Fig. 5A and B). However, DNase treatment had little effect on D39luxS cells attached to the surface (Fig. 5A and B). The amount of extracellular DNA also was quantified under biofilm growth conditions, as described in Materials and Methods. Extracellular DNA accounted for approximately 40% of total DNA for wild-type D39. This compared with approximately 60% extracellular DNA release for D39luxS+ but less than 10% for D39luxS (P < 0.001 relative to D39 in both cases) (Fig. 5C). Thus, LuxS expression directly correlates with DNA release.
Fig. 5.
Influence of luxS on eDNA release. D39, D39luxS, and D39luxS+ were grown in C+Y medium with 50 μM Fe(III) for 24 h. Crystal violet-stained biofilms with and without DNase I treatment for 1 h were examined microscopically (A) (scale bar, 200 μm) and quantitatively by measuring the A590 (B). (C) Percentage of total DNA released into the extracellular compartment. Data presented are the means ± standard deviations from three independent experiments (***, P < 0.001; unpaired t test).
Autolysis, mediated by LytA, might be expected to play a role in the release of DNA by S. pneumoniae. To examine this, a lytA mutant D39 strain was tested for biofilm formation in the presence of Fe(III), but its phenotype was indistinguishable from that of wild-type D39. Moreover, the addition of choline, an inhibitor of LytA activity, to the Fe(III)-supplemented medium did not reduce biofilm formation or extracellular DNA release by D39 (result not shown). Quantitative Western blot analysis also showed that there was no difference in LytA expression between D39, D39luxS, and D39luxS+ (data not shown).
Pneumococcal competence is related to LuxS-mediated Fe(III)-induced biofilm formation.
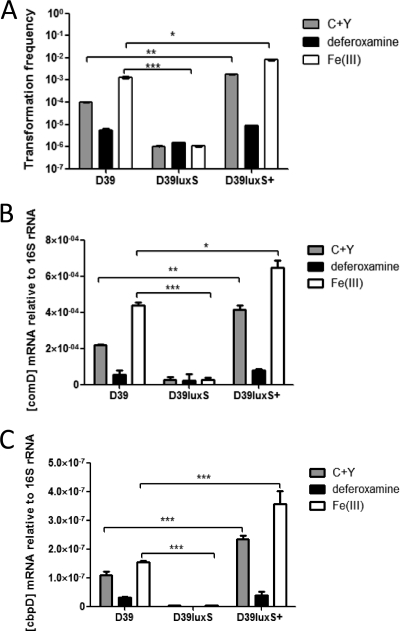
It has been reported that the genetic competence state of pneumococci can influence aggregation and biofilm formation (21, 44). Therefore, the transformation efficiency of wild-type D39, D39luxS, and D39luxS+ was measured in cells grown in the presence or absence of Fe(III) or deferoxamine (see Materials and Methods). The transformation efficiency of wild-type D39 was strongly influenced by iron availability; Fe(III) supplementation increased the transformation frequency 10-fold, whereas deferoxamine treatment had the opposite effect (Fig. 6A). The transformation frequency for D39luxS was two orders of magnitude lower than that observed for wild-type D39 (P < 0.001), and it was independent of Fe(III) availability (Fig. 6A). Conversely, D39luxS+ exhibited a >10-fold higher transformation efficiency than wild-type D39 in nonsupplemented medium (P < 0.05), and the addition of either Fe(III) or deferoxamine had effects similar to those seen in wild-type D39 (Fig. 6A). Romao et al. (40) also have reported the reduced competence of luxS mutant pneumococci in early-exponential-phase growth.
Fig. 6.
Effects of LuxS and Fe(III) on competence and competence-related gene expression. D39, D39luxS, and D39luxS+ strains were grown in C+Y medium with or without 50 μM Fe(III) or 10 μM deferoxamine. (A) Transformation efficiency was determined as described in Materials and Methods. Levels of comD (B) and cbpD (C) mRNA were assayed by real time RT-PCR using 16S rRNA as an internal control, as described in Materials and Methods. Data presented are the means ± standard deviations from three independent experiments (*, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired t test).
The genes involved in pneumococcal competence have been well characterized (20, 38). To determine if critical competence genes (comD, comX, comW, and the late competence-related genes cglA and dltA) are differentially regulated in response to LuxS activity or iron availability, levels of competence gene mRNA (relative to 16S rRNA) were measured using real-time RT-PCR. Data for comD expression are shown in Fig. 6B; essentially identical results were obtained for the other genes tested (data not shown). The difference in comD expression levels between strains and growth conditions closely paralleled the pattern of transformation efficiency seen in Fig. 6A. Thus, it is clear that iron levels influence the competence state of pneumococci in a LuxS-dependent manner.
The various effects of luxS expression on biofilm formation, DNA release, and competence could be attributable either to quorum-sensing signaling mediated by extracellular AI-2 or to the intracellular activity of the LuxS enzyme, which plays an important role in cellular metabolism. We therefore attempted to complement the phenotypic defect in D39luxS by growth in conditioned medium derived from a 6-h culture of D39luxS+, which would be expected to have significant levels of extracellular AI-2. However, the exposure of D39luxS to this conditioned medium resulted in the total lysis of the cells (result not shown). The D39luxS+ conditioned medium had a lesser lytic effect on wild-type D39 cultures. Also, conditioned medium from D39 had a lesser lytic effect on D39luxS cultures than that from D39luxS+ cultures (result not shown). Genetically competent pneumococci are able to kill noncompetent cells, triggering DNA release mediated principally by CbpD, a murein hydrolase (16, 19). We therefore measured cbpD mRNA (relative to 16S rRNA) by real-time RT-PCR in D39 versus D39luxS and D39luxS+ in C+Y medium with or without Fe(III) supplementation or deferoxamine (Fig. 6C). The expression of cbpD under the various conditions exactly paralleled that for comD described above.
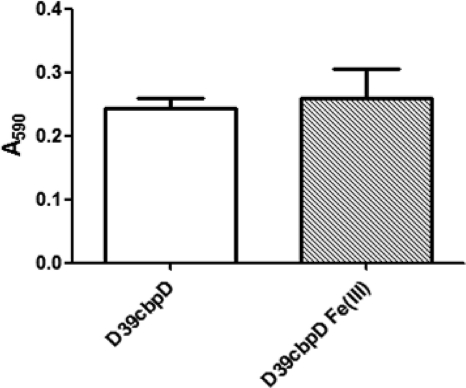
To confirm the importance of CbpD-mediated DNA release in biofilm formation, a D39 cbpD deletion mutant (D39cbpD) was constructed, as described in Materials and Methods. Unlike the wild type (see Fig. 1), D39cbpD was unable to form a biofilm in C+Y medium supplemented with 50 μM Fe(III), even after 24 h of incubation (Fig. 7).
Fig. 7.
Biofilm formation by D39cbpD. D39cbpD was incubated for 24 h in C+Y medium alone or in C+Y supplemented with 50 μM Fe(III). Biofilm density was quantitated by measuring the A590 of crystal violet-stained wells as described in Materials and Methods. Data presented are the means ± standard deviations from three independent experiments.
DISCUSSION
Homologues of luxS are ubiquitous in bacteria and encode S-ribosylhomocysteine lyase, an enzyme that produces the non-species-specific quorum-sensing molecule AI-2 as a by-product of the detoxification of S-adenosyl-l-homocysteine as part of the central activated methyl cycle. In this study, we have shown that in S. pneumoniae, the capacity to form a biofilm requires a functional luxS gene, and that the overexpression of this gene leads to a hyper-biofilm-forming phenotype. Iron, in the form of Fe(III), also stimulated biofilm formation, but this effect required luxS. Moreover, the supplementation of media with Fe(III) significantly increased the expression of luxS in wild-type D39. To our knowledge, this is the first such report for any bacterial species. Thus, effects of luxS expression and Fe(III) on biofilm formation are directly linked. In addition, the expression of PiuA, the metal-binding component of the major pneumococcal iron uptake ABC transport system, was significantly lower in D39luxS than in the wild type. This is consistent with ICP-MS analysis, which showed that the cellular [Fe] was 4-fold lower in D39luxS than in wild-type D39, while in D39luxS+ cells the [Fe] was 2.5-fold greater. As part of a separate study, we have also measured [Fe] in the nasopharynx, lungs, blood, and brain of both uninfected and S. pneumoniae-infected mice. Total Fe varied from 26 to 140 μM in uninfected mice and from 35 to 250 μM in infected mice (C. A. McDevitt, A. D. Ogunniyi, E. Valkov, M. C. Lawrence, B. Kobe, A. G. McEwan, and J. C. Paton, unpublished data). These data were used as a basis for the selection of the Fe concentration ranges tested in Fig. 1. However, no currently available analytical techniques are capable of distinguishing between Fe(II), which has no impact on biofilm formation, and Fe(III), which is highly stimulatory. Nevertheless, the ratio of Fe(III) to Fe(II) is likely to be higher under conditions of oxidative stress and, thus, might favor biofilm formation at sites of inflammation, particularly in the nasopharynx or lungs, where the partial pressure of oxygen is high compared to levels in the blood or brain.
Studies of other bacteria have indicated potentially pleiotropic roles for luxS in iron acquisition. For example, in Porphyromonas gingivalis, mutation of luxS had opposite effects on the expression of the TonB-linked hemin binding proteins Tlr and HmuR (25). Similarly, mutation of luxS in Vibrio vulnificus increased the expression of the vulnibactin receptor but decreased the expression of receptors for aerobactin and heme (28). However, in contrast to the present study, the effect of the luxS mutations on actual cellular iron levels in these organisms was not determined.
Mutation of luxS also has been reported to have differential effects on biofilm formation in other bacterial species, including members of the genus Streptococcus. Several studies have reported that luxS mutants of S. mutans exhibit defects in biofilm formation; biofilms were thinner overall and adopted a loose or granular appearance compared to the smooth, confluent biofilms produced by wild-type cells (34, 49, 51). Altered biofilm microcolony architecture and/or reduced biofilm thickness also has been reported for luxS mutants of S. gordonii and S. suis (9, 48). In contrast, Huang et al. (23) reported that luxS mutants of S. mutans exhibit accelerated biofilm formation relative to that of the wild type, albeit using a methodologically distinct biofilm assay. Effects of luxS mutation on biofilm formation have not been reported previously for S. pneumoniae.
In S. pneumoniae, extracellular DNA is a major component of the macromolecular biofilm matrix produced by wild-type cells. We found that the level of extracellular DNA release by D39luxS was significantly lower than that for wild-type D39, whereas DNA release by D39luxS+ was significantly greater. Thus, the luxS-dependent capacity to form a dense biofilm might be directly attributable to LuxS-mediated DNA release. Such DNA release has been described in association with the phenomenon of competence-dependent fratricide, whereby competent pneumococci are capable of killing noncompetent pneumococci in their immediate vicinity. This releases DNA for potential uptake by the competent bacteria, facilitating genetic exchange. However, the large amounts of DNA released in such circumstances also would provide the basis of a macromolecular matrix to promote and sustain biofilm formation. The release of DNA during competence-induced fratricide is largely attributable to the actions of the murein hydrolase CbpD, and unsurprisingly, cbpD is regulated in parallel with late competence genes (16). Consistently with these findings, we have shown in the present study that the mutation of luxS has parallel inhibitory effects on the transformation frequency and expression of early and late competence genes, including cbpD, whereas the overexpression of LuxS significantly upregulates all of these parameters. Supplementation with Fe(III) significantly boosted all of these parameters in D39 and D39luxS+ but had no effect in D39luxS. We also have demonstrated that the deletion of cbpD abolishes the capacity of D39 to form a biofilm in the presence of Fe(III), underscoring the central role of fratricide-mediated DNA release in this process. Thus, we propose that competence, fratricide, and biofilm formation are closely linked in pneumococci, and that luxS is the central regulator of these processes. We also propose that the stimulatory effects of Fe(III) on all of these parameters are due to the upregulation of luxS expression, and that LuxS provides for a positive Fe(III)-dependent amplification loop by increasing iron uptake by pneumococci.
ACKNOWLEDGMENTS
This research was supported by Program Grant 565526 and Project Grant 627142 from the National Health and Medical Research Council (NHMRC) of Australia. J.C.P. is an NHMRC Australia Fellow.
Footnotes
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Allegrucci M., Sauer K. 2008. Formation of Streptococcus pneumoniae non-phase-variable colony variants is due to increased mutation frequency present under biofilm growth conditions. J. Bacteriol. 190:6330–6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson G. G., Moreau-Marquis S., Stanton B. A., O'Toole G. A. 2008. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect. Immun. 76:1423–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrews S. C., Robinson A. K., Rodriguez-Quinones F. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215–237 [DOI] [PubMed] [Google Scholar]
- 4. Bala A., Kumar R., Harjai K. 2011. Inhibition of quorum sensing in Pseudomonas aeruginosa by azithromycin and its effectiveness in urinary tract infections. J. Med. Microbiol. 60:300–306 [DOI] [PubMed] [Google Scholar]
- 5. Banin E., et al. 2008. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. U. S. A. 105:16761–16766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banin E., Vasil M. L., Greenberg E. P. 2005. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 102:11076–11081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beard S. J., Salisbury V., Lewis R. J., Sharpe J. A., MacGowan A. P. 2002. Expression of lux genes in a clinical isolate of Streptococcus pneumoniae: using bioluminescence to monitor gemifloxacin activity. Antimicrob. Agents Chemother. 46:538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berry A. M., Lock R. A., Hansman D., Paton J. C. 1989. Contribution of autolysin to the virulence of Streptococcus pneumoniae. Infect. Immun. 57:2324–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blehert D. S., Palmer R. J., Jr., Xavier J. B., Almeida J. S., Kolenbrander P. E. 2003. Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions. J. Bacteriol. 185:4851–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown J. S., Gilliland S. M., Holden D. W. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40:572–585 [DOI] [PubMed] [Google Scholar]
- 11. Brown J. S., Gilliland S. M., Ruiz-Albert J., Holden D. W. 2002. Characterization of Pit, a Streptococcus pneumoniae iron uptake ABC transporter. Infect. Immun. 70:4389–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown J. S., Ogunniyi A. D., Woodrow M. C., Holden D. W., Paton J. C. 2001. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect. Immun. 69:6702–6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coates H., et al. 2008. The role of chronic infection in children with otitis media with effusion: evidence for intracellular persistence of bacteria. Otolaryngol. Head Neck Surg. 138:778–781 [DOI] [PubMed] [Google Scholar]
- 14. Costerton J. W., Stewart P. S., Greenberg E. P. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 15. Dagan R. 2009. Pneumococcal conjugate vaccines probe studies: the solution points to the problem. Adv. Exp. Med. Biol. 634:69–77 [DOI] [PubMed] [Google Scholar]
- 16. Eldholm V., Johnsborg O., Haugen K., Ohnstad H. S., Håvarstein L. S. 2009. Fratricide in Streptococcus pneumoniae: contributions and role of the cell wall hydrolases CbpD, LytA and LytC. Microbiology 155:2223–2234 [DOI] [PubMed] [Google Scholar]
- 17. Gaddy J. A., Actis L. A. 2009. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 4:273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gray B. M., Converse G. M., III, Dillon H. C., Jr 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923–933 [DOI] [PubMed] [Google Scholar]
- 19. Guiral S., Mitchell T. J., Martin B., Claverys J. P. 2005. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc. Natl. Acad. Sci. U. S. A. 102:8710–8715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Håvarstein L. S., Hakenbeck R., Gaustad P. 1997. Natural competence in the genus Streptococcus: evidence that streptococci can change phenotype by interspecies recombinational exchanges. J. Bacteriol. 179:6589–6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Håvarstein L. S., Martin B., Johnsborg O., Granadel C., Claverys J. P. 2006. New insights into the pneumococcal fratricide: relationship to clumping and identification of a novel immunity factor. Mol. Microbiol. 59:1297–1307 [DOI] [PubMed] [Google Scholar]
- 22. Hoa M., et al. 2010. Biofilms and chronic otitis media: an initial exploration into the role of biofilms in the pathogenesis of chronic otitis media. Am. J. Otolaryngol. 31:241–245 [DOI] [PubMed] [Google Scholar]
- 23. Huang Z., et al. 2009. LuxS-based quorum-sensing signaling affects biofilm formation in Streptococcus mutans. J. Mol. Microbiol. Biotechnol. 17:12–19 [DOI] [PubMed] [Google Scholar]
- 24. Ispahani P., Slack R. C., Donald F. E., Weston V. C., Rutter N. 2004. Twenty year surveillance of invasive pneumococcal disease in Nottingham: serogroups responsible and implications for immunisation. Arch. Dis. Child. 89:757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. James C. E., et al. 2006. LuxS involvement in the regulation of genes coding for hemin and iron acquisition systems in Porphyromonas gingivalis. Infect. Immun. 74:3834–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson M., Cockayne A., Morrissey J. A. 2008. Iron-regulated biofilm formation in Staphylococcus aureus Newman requires ica and the secreted protein Emp. Infect. Immun. 76:1756–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kadioglu A., Weiser J. N., Paton J. C., Andrew P. W. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288–301 [DOI] [PubMed] [Google Scholar]
- 28. Kim C. M., Shin S. H. 2011. Modulation of iron-uptake systems by a mutation of luxS encoding an autoinducer-2 synthase in Vibrio vulnificus. Biol. Pharm. Bull. 34:632–637 [DOI] [PubMed] [Google Scholar]
- 29. Kim S. Y., et al. 2003. Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol. Microbiol. 48:1647–1664 [DOI] [PubMed] [Google Scholar]
- 30. Kreth J., Vu H., Zhang Y., Herzberg M. C. 2009. Characterization of hydrogen peroxide-induced DNA release by Streptococcus sanguinis and Streptococcus gordonii. J. Bacteriol. 191:6281–6291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lacks S., Hotchkiss R. D. 1960. A study of the genetic material determining an enzyme in Pneumococcus. Biochim. Biophys. Acta 39:508–518 [DOI] [PubMed] [Google Scholar]
- 32. LeMessurier K. S., Ogunniyi A. D., Paton J. C. 2006. Differential expression of key pneumococcal virulence genes in vivo. Microbiology 152:305–311 [DOI] [PubMed] [Google Scholar]
- 33. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 34. Merritt J., Qi F., Goodman S. D., Anderson M. H., Shi W. 2003. Mutation of luxS affects biofilm formation in Streptococcus mutans. Infect. Immun. 71:1972–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moscoso M., Garcia E., Lopez R. 2006. Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J. Bacteriol. 188:7785–7795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oggioni M. R., et al. 2006. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol. Microbiol. 61:1196–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petersen F. C., Ahmed N. A., Naemi A., Scheie A. A. 2006. LuxS-mediated signalling in Streptococcus anginosus and its role in biofilm formation. Antonie Van Leeuwenhoek 90:109–121 [DOI] [PubMed] [Google Scholar]
- 38. Peterson S. N., et al. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 51:1051–1070 [DOI] [PubMed] [Google Scholar]
- 39. Pozzi G., et al. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 178:6087–6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romao S., Memmi G., Oggioni M. R., Trombe M. C. 2006. LuxS impacts on LytA-dependent autolysis and on competence in Streptococcus pneumoniae. Microbiology 152:333–341 [DOI] [PubMed] [Google Scholar]
- 41. Rybtke M. T., et al. 2011. The implication of Pseudomonas aeruginosa biofilms in infections. Inflamm. Allergy Drug Targets 10:141–157 [DOI] [PubMed] [Google Scholar]
- 42. Stroeher U. H., Paton A. W., Ogunniyi A. D., Paton J. C. 2003. Mutation of luxS of Streptococcus pneumoniae affects virulence in a mouse model. Infect. Immun. 71:3206–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Surette M. G., Bassler B. L. 1999. Regulation of autoinducer production in Salmonella typhimurium. Mol. Microbiol. 31:585–595 [DOI] [PubMed] [Google Scholar]
- 44. Trappetti C., et al. 2011. The impact of the competence quorum sensing system on Streptococcus pneumoniae biofilms varies depending on the experimental model. BMC Microbiol. 11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trappetti C., et al. 2009. Sialic acid: a preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J. Infect. Dis. 199:1497–1505 [DOI] [PubMed] [Google Scholar]
- 46. Trappetti C., Ogunniyi A. D., Oggioni M. R., Paton J. C. 2011. Extracellular matrix formation enhances the ability of Streptococcus pneumoniae to cause invasive disease. PLoS One 6:e19844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ulijasz A. T., Andes D. R., Glasner J. D., Weisblum B. 2004. Regulation of iron transport in Streptococcus pneumoniae by RitR, an orphan response regulator. J. Bacteriol. 186:8123–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y., Zhang W., Wu Z., Zhu X., Lu C. 2011. Functional analysis of luxS in Streptococcus suis reveals a key role in biofilm formation and virulence. Vet. Microbiol. doi: 10.1016/j.vetmic.2011.04.029 [DOI] [PubMed] [Google Scholar]
- 49. Wen Z. T., Burne R. A. 2004. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 186:2682–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu Y., Outten F. W. 2009. IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. J. Bacteriol. 191:1248–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoshida A., Ansai T., Takehara T., Kuramitsu H. K. 2005. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 71:2372–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]