Abstract
Mucosal immune responses to fungal infection range from T helper type 2 (Th2) cell-directed allergic inflammation to Th1-predominant neutrophilic inflammation, but the mechanisms directing these divergent mucosal immune outcomes and the role of T cells in host defense against mucosal fungal infections are not known. Here we examined the mouse mucosal immune responses to 12 filamentous environmental fungal species over a broad range of exposure doses and determined the requirement of T cells for host defense. For all tested fungi, low-grade conidium exposures induced Th2- and eosinophil-predominant allergic lung disease, whereas higher exposures led to rapid conversion to neutrophil- and Th1 cell-predominant inflammation, a phenomenon we term immune phenotype switching. All fungal exposure doses were further linked to the secretion of interleukin-17A (IL-17A). Fungal infections with Curvularia lunata and Aspergillus fumigatus were typically confined to the airway during allergic inflammation but became locally invasive and disseminated to the brain at higher conidium challenge doses, in association with predominant Th1 responses. Fungal dissemination occurred at relatively low challenge doses with the conidia of Aspergillus fumigatus administered to recombinase activating gene 1 (Rag-1)-deficient mice, which lack B and T cells, but B cell-deficient μMT mice and T helper cell-reconstituted Rag-1-deficient mice were comparable to wild-type mice in preventing fungal dissemination. Our findings demonstrate that Th2 cell-predominant allergic responses followed by immune phenotype switching and fungal dissemination are highly predictable outcomes with progressive fungal infectious burdens and that T helper cell responses are protective against lethal fungal dissemination.
INTRODUCTION
The pathogenic filamentous fungi comprise numerous sporulating, saprophytic organisms that may be significant causes of human disease under select circumstances. The most common of these potential pathogens derive from relatively few genera, including Aspergillus, Penicillium, Curvularia, Coccidioides, and others. Fungus-related determinants of pathogenicity are not fully understood but include proximity to human work and living spaces, ease of entry into the body, most often involving inhalation of spore-containing aerosols, and production of a variety of virulence factors that allow adherence to and invasion of host cells, competition for limiting nutrients, and resistance against sophisticated host immune responses (58).
Filamentous fungi are linked to multiple disease syndromes involving the airways. In addition to asymptomatic colonization of the upper and lower respiratory tracts, fungi are strongly linked to noninvasive disease processes in which organism growth is confined to the airway epithelial surface in association with allergic fungal rhinosinusitis (AFRS) (33), allergic bronchopulmonary aspergillosis (ABPA) (48), and aspergillomas (fungus balls). Additional syndromes in which fungi be-come locally invasive in the airway epithelium and submucosa, such as chronic necrotizing aspergillosis and advanced ABPA, are more clearly linked to the fungal infection itself (59). The most lethal fungal syndromes involve locally progressive and invasive disease leading to distant metastatic infection (59). Fungi can spread hematogenously from the lung to any organ, but the brain is especially susceptible to disease and, when involved, portends an exceptionally poor outcome (5). A diagnosis of ABPA or AFRS further carries a very strong risk of having concomitant allergic asthma, and antifungal antibiotics have recently been shown to have significant utility in the treatment of both asthma and ABPA with fungal sensitization (15, 16, 33, 41, 48). Thus, fungal mucosal infection potentially underlies a spectrum of airway disorders ranging from the rare to the exceptionally common, with important implications for the medical management of these conditions.
Effective immunity against filamentous fungi is therefore essential, but critical immune components of host defense remain incompletely defined. Studies of immunocompromised human subjects and experimental animals have established that neutrophils are essential for preventing fungal dissemination from the lung and for resolving this devastating complication when it occurs (19, 42, 55). Macrophages are further capable of phagocytosing smaller fungal forms (conidia and small hyphae) and killing them through the oxidative burst and other mechanisms (53, 56). The NADPH oxidase system that is operative in most phagocytic immune cells is especially important to fungal killing, especially that of filamentous forms (7, 38). B cells and antibodies may play a further role in antifungal immunity by binding to hyphae and activating complement, which contributes to organism phagocytosis and lysis (27–29, 35, 51, 60).
Fungal airway infections are also linked to a spectrum of T helper (Th) cell responses, including Th1, Th2, and Th17 cell activation (4, 8, 26, 39, 45). We previously demonstrated that the conidia of the ubiquitous fungus Aspergillus niger, given in relatively small amounts, trigger a highly polarized Th2-predominant allergic response in mice that provokes canonical features of asthma, including airway hyperreactivity, robust airway eosinophilia, and goblet cell metaplasia with mucus hypersecretion (45). We further demonstrated that in addition to macrophages and neutrophils, eosinophils also have potent antifungal properties. Thus, diverse innate immune cells are indispensable for host defense against invasive fungal disease. Current studies further suggest that Th2 cytokines, especially interleukin-4 (IL-4) and IL-10, are linked to loss of control of fungal infections, in part because they impair type 1 immunity, which is thought to be essential for control of fungal disease (10–12). Conversely, the type 1 cytokines gamma interferon (IFN-γ) and IL-12 have been shown to improve outcomes of experimental invasive and disseminated lung disease due to Aspergillus fumigatus (11, 12). Thus, a paradox of mucosal immunity against fungal infection is that, at least at some exposure doses, allergic inflammation is a typical response, yet such responses appear to be maladaptive. Moreover, a clear understanding of the roles of T helper cells and B cells in fungal lung disease, irrespective of effector phenotype, remains unknown.
In this study, we determined the mouse cellular immune response to a broad array of common fungi found in the homes of children in Houston, TX, and determined how this immune response changed with increasing fungal infectious burdens. We conducted further experiments to understand the specific requirement of T and B cells in host defense against the most serious complication of airway fungal infection, dissemination to the brain.
MATERIALS AND METHODS
Mice and humans.
C57BL/6J, recombinase activating gene 1-deficient (Rag1−/−) (37), and μMT−/− (25) mice were purchased from Jackson Laboratories. All mice were bred and housed at the American Association for Accreditation of Laboratory Animal Care-accredited vivarium at Baylor College of Medicine under specific-pathogen-free conditions. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and followed federal guidelines. Human studies were conducted according to Institutional Review Board protocols established at Baylor College of Medicine and the University of Texas Health Sciences Center at Houston and followed all federal guidelines. All human subjects provided written consent to participate in these studies.
House dust collection and fungal isolation.
Household dust is currently being collected from the homes of both asthmatic and healthy children as part of a case-control study and is stored at −20°C after collection. Approximately 0.5 to 2 g of dust was wetted with ∼3 ml/g endotoxin-free phosphate-buffered saline (PBS). The suspension was passed though a 45-μm mesh (BD Biosciences, San Jose, CA) to remove large debris. Samples were centrifuged at 10,000 rpm for 5 min, and the supernatant was removed. The insoluble material from the PBS extract was suspended in ∼2 ml PBS/g dust. Aliquots of 2, 10, and 100 μl were plated on Sabouraud's medium (Becton Dickinson and Co., Sparks, MD) supplemented with 50 μg/ml ampicillin and were incubated at 37°C for up to 5 days. Pure fungal species were subcloned and identified by standard morphology methods (Microcheck, Northfield, VT).
Heavily sporulated plate cultures of pure, identified fungi were washed repeatedly with PBS. Harvested conidium (mold spore) suspensions were passed through 45-μm nylon mesh to remove hyphae. Conidia were washed twice with PBS by centrifugation (10,000 × g, 5 min, 4°C), and pellets were suspended in PBS. Conidial concentrations were determined using a hemacytometer and adjusted to 8 × 107 conidia/ml, and aliquots were stored in liquid nitrogen. Conidium viability after freezing was confirmed by determining CFU by plating serial dilutions on Sabouraud's medium. All experiments were performed identically, using conidia from isolates of Absidia cylindrospora, Absidia spinosa, Aspergillus flavus, Aspergillus fumigatus, Aspergillus silvaticus, Aspergillus versicolor, Chromellosporium ollare, Curvularia lunata, Paecilomyces variotii, Penicillium oxalicum, Scopulariopsis brevicaulis, and Trichoderma harzianum. For some experiments, conidia were fixed as described by Aimanianda et al. for the production of nonviable A. fumigatus conidia (3). Viable conidia were suspended in 2.5% paraformaldehyde in PBS overnight at 4°C and then washed once with 0.1 M ammonium chloride in PBS and three times in sterile PBS, followed by reconstitution in PBS for enumeration. Complete conidium inactivation was confirmed by plating on Sabouraud's medium.
Intranasal (i.n.) challenge.
Conidia were diluted in PBS to achieve the indicated number of conidia/50-μl volume. Mice were deeply anesthetized with isoflurane, and droplets containing PBS or conidia in PBS were applied to the nares until a total of 50 μl was inhaled, as described previously (24).
Quantitation of allergic lung disease.
Airway hyperreactivity was determined by assessing the increase in respiratory system resistance (RRS) to 5 increasing intravenous acetylcholine chloride (Ach) doses, measured by whole body, semi-invasive plethysmography (43). Alternatively, airway mechanics data were expressed as the provocative concentration of Ach required to induce a 200% increase in RRS from baseline values (PC200), as described previously (13, 43). Bronchoalveolar lavage fluid (BALF) was collected to quantify airway eosinophils, neutrophils, and macrophages and secreted cytokines. Enzyme-linked immunosorbent spot (ELISpot) analysis was used to detect total lung IL-4- and IFN-γ-secreting cells. Lungs were fixed with 10% formalin and stained with hematoxylin and eosin, periodic acid-Schiff stain, or Gomori methenamine silver for histopathologic analysis. All studies were performed 24 h after the final i.n. conidium challenge as previously described (45).
Brain dissemination assay.
Brains were removed from mice by starting from the back of the skull to avoid disturbing the nasal passages, using sterilized instruments in a laminar flow hood. Brains were spread directly onto Sabouraud's ampicillin plates, and the plates were sealed with Parafilm and incubated at 37°C for up to 2 weeks. CFU were enumerated and species confirmed as described above.
T cell adoptive transfer.
C57BL/6 mice were challenged i.n. weekly with 1 × 106 A. fumigatus conidia for 2 weeks to induce fungus-reactive T cells. On day 15, lungs and spleens were removed from 5 mice and CD4+ T cells were isolated by magnet-assisted cell sorting (MACS; Miltenyi) followed by fluorescence-activated cell sorting (FACS) to achieve >99% pure CD3+ CD4+ CD19− T cells. Naïve Rag1−/− mice were injected intraperitoneally with 1.5 × 106 highly purified CD4+ T cells. One day after sham or T cell reconstitution of Rag1−/− mice, animals were challenged i.n. with 1 × 106 A. fumigatus conidia daily for 18 days. Allergic disease endpoints and brain dissemination were determined on day 19.
Cytokine analysis.
The indicated cytokines were measured in BALF by using mouse cytokine/chemokine panel I and II kits (Millipore) on a Luminex-based multianalyte platform (Bio-Plex; Bio-Rad, Hercules, CA) according to the manufacturer's instrument protocols.
Statistical analysis.
Data are presented as means ± standard errors of the means. Significant differences relative to PBS-challenged mice or appropriate controls (see Fig. 7) are expressed by P values of ≤0.05, as measured by 2-way analysis of variance (ANOVA) with Bonferroni's multiple-comparison test.
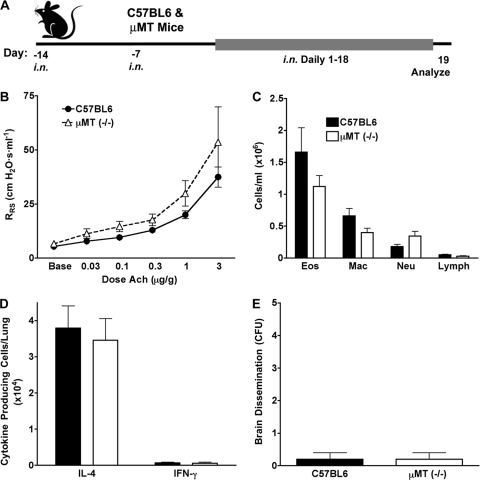
Fig. 7.
B cells are not required to prevent fungal dissemination from the airway. (A) C57BL/6 and μMT mice were challenged i.n. with 1 × 106 A. fumigatus conidia for 2 weeks to allow the adaptive immune response to develop and then were challenged daily for 18 days with 2 × 106 conidia/day. (B) Airway hyperreactivity. (C) BALF differential analysis. (D) ELISpot analysis of IL-4- and IFN-γ-secreting lung cells. (E) Mean numbers of A. fumigatus colonies cultured from brains at the indicated challenge doses. There were no significant differences between the two groups for any of the data. Data are from one of two comparable experiments (n = 5 mice per group).
RESULTS
Diverse house dust fungi induce allergic lung disease.
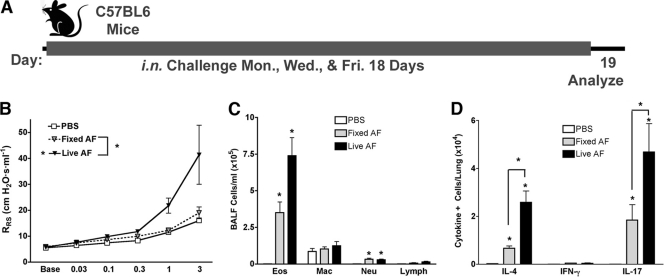
A. niger conidia administered at relatively low exposure doses consistently induce a highly polarized Th2 response, allergic inflammation, and asthma-like disease in mice (45). This observation appears inconsistent with the prior results of Aimanianda et al., who reported that dormant conidia of a closely related organism, A. fumigatus, elicited little or no inflammation when administered to cultured dendritic cells and mice in vivo (3). However, exposure to A. fumigatus conidia in these studies was limited to brief periods, and the conidia used were inactivated by pretreatment with paraformaldehyde. To further explore the inflammatory nature of fungal conidia, we applied the methodology used by Aimanianda et al. to our allergic lung disease model and compared airway physiologic and inflammatory responses to substantially reduced doses of live and paraformaldehyde-fixed A. fumigatus conidia administered over 18 days (Fig. 1). Consistent with our prior findings with A. niger, the live conidia of A. fumigatus elicited robust airway hyperreactivity as determined by increases in RRS dose-response curves, predominant lung eosinophilia, and elevated lung IL-4 responses as determined by ELISpot assay. We also noted the recruitment of a large number of lung IL-17A-secreting cells. In contrast, fixed, nonviable conidia failed to elicit airway hyperreactivity but promoted significant lung IL-4, IL-17A, and eosinophil responses, although they were reduced compared to responses to live conidia. Thus, in contrast to the findings of Aimanianda et al., nonviable conidia are proinflammatory when given to mice for prolonged periods, but only viable conidia are capable of inducing robust Th2 responses and the complete allergic lung disease phenotype.
Fig. 1.
Comparison of live versus fixed A. fumigatus (AF) conidia. (A) Mice were challenged intranasally with PBS or 4 × 105 A. fumigatus conidia, either fully viable (live) or inactivated by fixation with formaldehyde (fixed), on Monday, Wednesday, and Friday for 18 days and then analyzed 24 h later. (B) Airway hyperreactivity as determined by significant increases in RRS values above PBS challenge values in response to increasing doses of Ach. (C) Absolute numbers of eosinophils, neutrophils, and macrophages from BALF. (D) Total lung IL-4-, IFN-γ-, and IL-17A-secreting cells. Data are from 1 of 2 comparable experiments (n = 5 per group). All responses are statistically different from those of PBS-challenged mice (P < 0.05) (*). Statistical differences between fixed and live conidia are indicated by brackets.
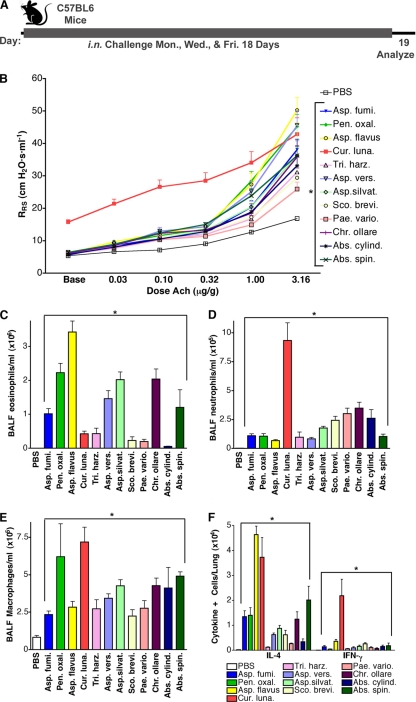
To further determine if allergic lung disease is generally induced by viable fungal conidia, naïve mice were challenged i.n. with the conidia of 12 distinct fungal species over 18 days and then assessed for typical disease manifestations (Fig. 2A). All fungal species induced airway hyperreactivity and, with the exceptions of P. variotii and S. brevicaulis, decreased PC200 values (another measurement of airway hyperreactivity [see Materials and Methods]) compared to vehicle-challenged mice (Fig. 2B; Table 1). The magnitude of airway hyperreactivity was variable; A. flavus, A. versicolor, and P. oxalicum induced the most robust responses, while P. variotii, S. brevicaulis, and T. harzianum induced the weakest airway hyperreactivity. Inhalation of A. flavus and P. oxalicum conidia elicited the strongest allergic responses, as indicated by the robust airway eosinophilia relative to other BALF cells (Fig. 2C to E), and the most lung IL-4 production (Fig. 2F), whereas P. variotii, S. brevicaulis, and T. harzianum again elicited the weakest of these responses.
Fig. 2.
Allergic lung disease induced by diverse fungi. (A) Mice were challenged according to the indicated schedule with PBS or 4 × 105 conidia of each of 12 fungal species. (B) Airway hyperreactivity as assessed by significant increases in RRS values above PBS challenge values in response to increasing doses of Ach. (C to E) Absolute numbers of eosinophils, neutrophils, and macrophages from BALF. (F) Total lung IL-4- and IFN-γ-secreting cells. Data are from 1 of 2 comparable experiments (n = 5 per group). All responses except for numbers of IFN-γ-secreting cells are statistically different from those of PBS-challenged mice (P < 0.05), as indicated by brackets or asterisks.
Table 1.
PC200 values for conidium-challenged mice
| Treatment | PC200 value (mean ± SEM) | P value vs PBS groupa |
|---|---|---|
| PBS | 2.55 ± 0.35 | 1.0000 |
| Absidia cylindrospora | 1.30 ± 0.43 | 0.0491 |
| Absidia spinosa | 0.72 ± 0.20 | 0.0007 |
| Aspergillus flavus | 0.66 ± 0.31 | 0.0005 |
| Aspergillus fumigatus | 0.96 ± 0.10 | 0.0016 |
| Aspergillus silvaticus | 0.88 ± 0.08 | 0.0012 |
| Aspergillus versicolor | 0.72 ± 0.10 | 0.0006 |
| Chromellosporium ollare | 0.99 ± 0.05 | 0.0019 |
| Curvularia lunata | NDb | |
| Paecilomyces variotii | 1.62 ± 0.29 | 0.0627 |
| Penicillium oxalicum | 0.58 ± 0.11 | 0.0004 |
| Scopulariopsis brevicaulis | 1.97 ± 0.29 | 0.3657 |
| Trichoderma harzianum | 1.40 ± 0.33 | 0.0350 |
By t test.
ND, not determinable.
C. lunata was notable for the exceptional responses it induced relative to all other fungi. Airway hyperreactivity induced by C. lunata could not technically be determined at this infective dose because the baseline RRS of C. lunata-infected mice differed significantly from that of other fungus-infected and naïve mice (15.7 ± 2.1 versus 5.4 ± 0.5 cm H2O s−1 ml−1; P < 0.01). Moreover, unlike the case for all other fungi, the predominant inflammatory cell types elicited in BALF from C. lunata-infected mice were neutrophils and macrophages (Fig. 2D and E), and larger numbers of IFN-γ-secreting cells were detected in the lungs than those for other fungus-infected mice under the same conditions (Fig. 2F). Thus, repeated inhalation of conidia of 12 fungi consistently elicited Th2-predominant mucosal inflammation, with the exception of C. lunata, which produced a mixed syndrome consisting of allergic and nonallergic cytokines and cells.
Allergenic potential of conidia varies with dose and fungal species.
Based on their relative importance to human disease and relative potency to elicit allergic inflammation as shown in Fig. 2, we chose four fungi, A. fumigatus (Fig. 3), A. flavus (see Fig. S1A, C, and F in the supplemental material), C. lunata (Fig. 3C, E, and G), and P. oxalicum (see Fig. S1C, E, and G), for dose-dependent analyses. Results for a fifth fungus, A. niger, have been reported separately (45). Because no studies reliably estimate actual conidial inhalational exposures for humans and other species, we administered a 2-log range (2 × 103 to 2 × 105) of conidia per mouse i.n. daily.
Fig. 3.
Allergic lung disease conidium dose-response relationships. (A) Mice were challenged every day for 18 days with PBS or increasing A. fumigatus (B, D, and F) or C. lunata (C, E, and G) conidium doses. (B and C) Airway hyperreactivity. (D and E) BALF differential analysis of eosinophils (Eos), macrophages (Mac), neutrophils (Neu), and lymphocytes (Lymph). (F and G) ELISpot analyses of IL-4- and IFN-γ-secreting lung cells. Data are from one of two comparable experiments (n = 3 mice per group). Statistical differences from PBS-challenged mice (P < 0.05) are indicated by brackets or asterisks.
Dose-dependent increases in allergic inflammation and the asthma phenotype with increasing conidial exposure were apparent with A. fumigatus, A. flavus, and P. oxalicum. Similar dose dependency was also observed for A. niger (45). The lowest dose of conidia that resulted in a significant increase in RRS varied between 2 × 103 and 5 × 103 conidia/dose (2 × 103 for A. fumigatus, 5 × 103 for A. flavus, 2 × 103 for C. lunata, and 5 × 103 for P. oxalicum) (Fig. 3B and C; see Fig. S1A and B in the supplemental material). Because A. fumigatus and C. lunata elicited airway hyperreactivity at the minimum tested conidium dose, it is likely that the lowest exposure eliciting airway hyperreactivity with these organisms is lower than 2 × 103/day. In contrast to airway hyperreactivity, regardless of fungal species, even the smallest conidial exposures (2 × 103/day) significantly induced airway eosinophil recruitment and predominant lung IL-4-secreting cells (Fig. 3D to G; see Fig. S1C to F).
The mucosal immune response and airway hyperreactivity elicited by increasing doses of C. lunata conidia differed substantially from those elicited by other conidia. Unlike the rigorous dose-responsive increases in RRS that were observed with A. fumigatus (Fig. 3B), consistent findings were not observed with C. lunata, which instead induced unpredictable airway physiological changes at different infective doses (Fig. 3C). At the highest dose of 2 × 105 conidia/day, C. lunata produced substantially elevated baseline RRS values and relatively large numbers of IFN-γ-producing cells. Moreover, increasing doses of C. lunata induced dose-dependent increases in neutrophilia and decreases in eosinophilia (Fig. 3E). C. lunata was also the only fungus for which an inverse relationship between airway eosinophil recruitment and conidium dose was observed (Fig. 3E). Similarly, only C. lunata induced significant numbers of lung IFN-γ-secreting cells, which exceeded total lung IL-4-secreting cells at the highest dose (Fig. 3G). Thus, at lower inhaled doses, the conidia of all fungi were intrinsically allergenic. Dose-dependent increases in allergic disease were strictly observed with increasing A. fumigatus, A. flavus, and P. oxalicum doses, as well as with A. niger (45). In contrast, C. lunata induced a highly variable response in which robust allergic disease observed at low conidium doses diminished and was ultimately replaced at higher doses by a fundamentally distinct inflammatory process that favored lung IFN-γ, not IL-4, responses.
C. lunata induces invasive disease.
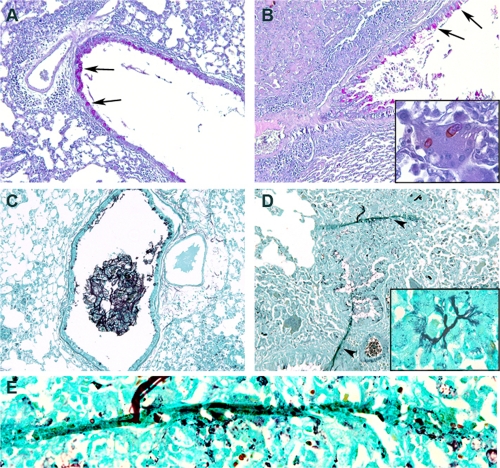
The preceding findings suggested that high doses of C. lunata conidia induce a fundamentally distinct mucosal immune response relative to those to other fungi at high conidium exposure levels. Consistent with this, whereas infection with most fungal species was well tolerated even at the highest doses (no evident change in behavior, appearance, or weight), C. lunata infection induced profound emaciation, dishevelment, and significant weight loss (27.6% weight loss induced by C. lunata compared to PBS [14.9 g ± 1.3 g versus 20.6 g ± 1.1 g; P < 0.01]) beginning at 2 × 105 conidia/dose. We further examined lungs of mice exposed to C. lunata and other selected fungi at this discriminatory dose. P. oxalicum and the Aspergillus sp. fungi induced typical allergic lung disease pathology that included goblet cell metaplasia and peribronchovascular, eosinophil-predominant inflammation (Fig. 4A and data not shown). These lung findings were similar regardless of the fungus used to induce the allergic disease (data not shown). In contrast, C. lunata induced a diffuse alveolar and interstitial pneumonia marked by the presence of multinucleate giant cells and poorly formed granulomas (Fig. 4B). Silver staining of lung tissue revealed rare hyphae of P. oxalicum and Aspergillus spp. confined strictly to airway lumens (Fig. 4C and data not shown). In contrast, hyphae of C. lunata were observed extending deep into lung parenchyma (Fig. 4D and E). Given the lung-invasive nature of C. lunata, we considered the possibility that this fungus could disseminate hematogenously to other tissues. Indeed, C. lunata was detected in the brains of 4 of 7 mice challenged with 4 × 105 conidia every other day for 16 days, whereas A. niger dissemination was not detectable (0 of 5 mouse brains) under otherwise identical conidium challenge conditions.
Fig. 4.
Noninvasive and invasive fungal lung disease. Mice were challenged daily with 200 × 103 conidia of P. oxalicum (A and C) or C. lunata (B, D, and E), and lung sections were stained for viewing by light microscopy. (A and B) Periodic acid-Schiff stain. The arrows indicate metaplastic epithelial goblet cells. (Inset) Multinucleate giant cell with phagocytosed conidia. (C to E) Gomori methenamine silver stain. (C) Hyphae, mucus, and airway inflammatory cell impaction within a medium-size airway without evidence of fungal invasion. (D) Hyphae invading lung parenchyma (arrowheads). (Inset) Parenchymal hyphae. Magnification, ×1,000.
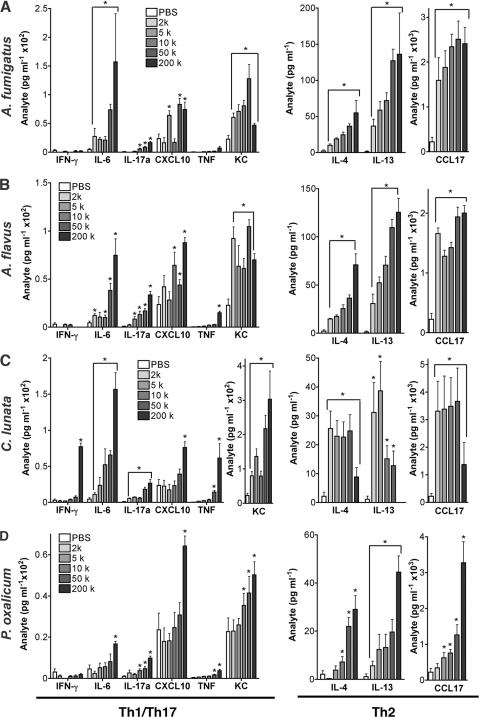
ELISpot analysis (Fig. 3G) suggested that the immune response to C. lunata changed fundamentally at higher conidium exposures. To confirm this, we extensively analyzed cytokines from BALF of conidium-challenged mice (Fig. 5). Representative noninvasive fungi (A. fumigatus, A. flavus, and P. oxalicum) (Fig. 5A, B, and D) elicited a dose-dependent increase in type 2 cytokines (IL-4 and IL-13) and chemokines (CCL17), with few or no detectable type 1 cytokines (IFN-γ and tumor necrosis factor [TNF]). The type 1 chemokines CXCL10 and KC were also strongly induced. In contrast, C. lunata (Fig. 5C) induced diminishing type 2 cytokine and chemokine responses at higher exposures and was the only fungus to induce large increases in type 1 cytokines, again only at higher exposures. All conidia induced dose-dependent increases in airway IL-6, a growth factor for Th17 cells (30), and IL-17A, the canonical type 17 cytokine (40), but the magnitudes of these changes did not vary with fungal invasiveness. Thus, at the conidium challenge doses tested, C. lunata was the only fungus to exhibit lung invasion and distant dissemination, and such escape from the airway lumen was marked by a change in immune phenotype from predominantly type 2 to type 1.
Fig. 5.
Concentrations of indicated Th1/Th17 and Th2 cytokines and chemokines as assessed in bronchoalveolar lavage fluid collected from mice challenged with the indicated conidium doses daily for 18 days. Samples were collected on day 19. (A) A. fumigatus; (B) A. flavus; (C) C. lunata; (D) P. oxalicum. Data are from one of two comparable experiments (n = 5 mice per group). Statistical differences from PBS-challenged mice (P < 0.05) are indicated by brackets or asterisks.
Mucosal immune phenotype switching and fungal invasion/dissemination are features of overwhelming fungal infection.
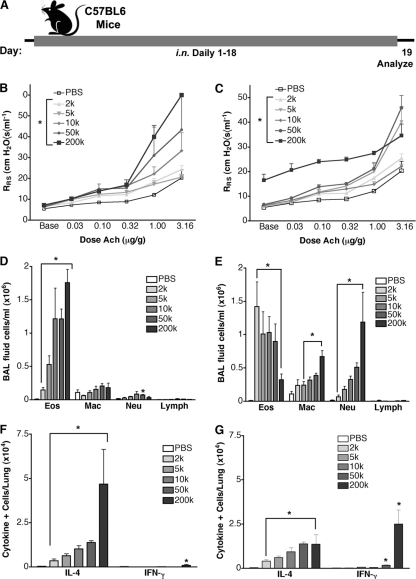
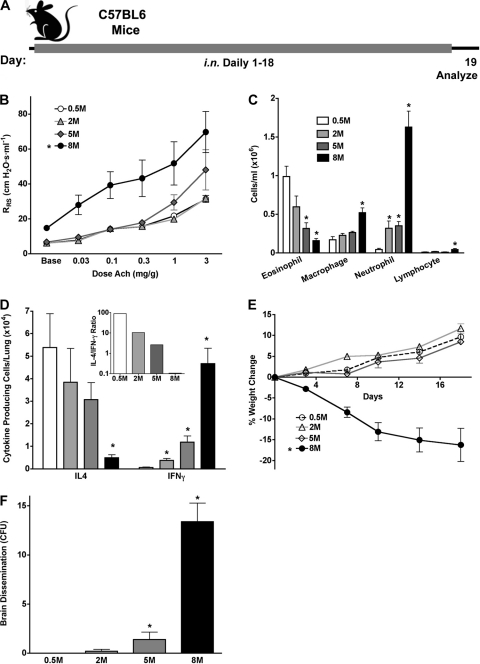
Because of the diverse mucosal immune responses that we observed with distinct fungi, we sought to determine whether the C. lunata conidium dose-dependent change in immune phenotype from predominantly type 2 to type 1, a phenomenon that we term “immune phenotype switching,” is unique to this organism. We therefore inoculated mice with increasing doses of the conidia of A. fumigatus (Fig. 6A), a typical filamentous fungus that induces a predominant Th2 immune phenotype at daily doses of 200 × 103 conidia (Fig. 3F and 5A). We found that increasing the daily exposure to 5 × 105 to 5 × 106 conidia induced further increases in airway hyperreactivity, but BALF eosinophils and lung IL-4 responses were reduced compared to those with low-dose conidium challenge, while IFN-γ responses increased markedly (Fig. 6B to D). At the highest dose (8 × 106), A. fumigatus conidium-challenged mice displayed elevated baseline airway resistance and diminishing BALF eosinophils and lung IL-4-secreting cells, with concomitant increases in BALF neutrophils and lung IFN-γ-secreting cells. Immune phenotype switching was especially striking, with the lung IL-4/IFN-γ ratio decreasing >1,000-fold over a 16-fold change in conidium dose (Fig. 6D, inset). Moreover, at this conidium dose, mice began to lose weight, became disheveled and ataxic, and demonstrated both lung-invasive disease similar to that seen with C. lunata (Fig. 4) and brain dissemination (Fig. 6E and F and data not shown). These findings for higher A. fumigatus conidium challenge doses precisely mirror those observed at the 200 × 103 daily dose of C. lunata conidia and reveal that mucosal immune phenotype switching and systemic dissemination of fungi from the airway lumen are general manifestations of overwhelming airway fungal infection.
Fig. 6.
Immune phenotype switching and disseminated disease with A. fumigatus. (A) Mice were challenged daily for 18 days with PBS or increasing A. fumigatus conidium doses, as indicated. (B) Airway hyperreactivity. (C) BALF differential analysis. (D) ELISpot analysis of IL-4- and IFN-γ-secreting lung cells. (E) Percent weight change during the challenge period. (F) Mean numbers of A. fumigatus colonies cultured from brains at the indicated challenge doses. Data are from one of two comparable experiments (n = 5 mice per group). Statistical differences from mice challenged with 0.5 × 106 conidia (P < 0.05) are indicated by brackets or asterisks.
T helper cells, but not B cells, are required to contain airway fungal infection.
In contrast to prior observations (10–12), the preceding studies suggested that mucosal allergic, Th2-predominant immune responses may promote containment and clearance of environmental fungi under conditions of continuous inhalational challenge. To test this hypothesis, we initially determined the relative importance of B cells for control of airway fungal infection by using B cell-deficient μMT mice compared to genotype-matched wild-type animals (Fig. 7 A). For these studies, mice were challenged with conidia for a longer period to maximize antibody production in wild-type animals and therefore maximize our ability to identify antibody-mediated effects on fungal infection control. Nonetheless, identical to our prior studies of the same mice challenged with a noninfectious allergen derived from A. fumigatus (14), B cell deficiency had no significant effect on allergic disease endpoints (Fig. 7B to D), nor were B cells required to preclude fungal dissemination to the brain (Fig. 7E), suggesting that T helper cells are the principal adaptive immune cells preventing this lethal outcome.
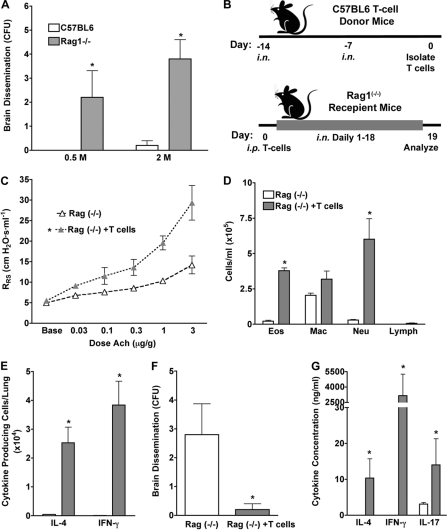
To formally test this hypothesis, we first compared the abilities of wild-type and B and T cell-deficient Rag1−/− mice to develop allergic lung disease and prevent dissemination during challenge with A. fumigatus conidia. Consistent with our previous findings obtained using other allergens (44), Rag1−/− mice were unable to manifest significant airway hyperreactivity, lung IL-4 production, and airway eosinophil responses after 18 days of fungal challenge with 400 × 103 conidia on a Monday-Wednesday-Friday schedule. Combined with the knowledge that B cell deficiency does not alter the allergic phenotype (Fig. 7), these observations suggest that T cells are primarily responsible for these allergic endpoints (see Fig. S2 in the supplemental material). To confirm this and determine the role of T cells in infection control, the same mice were challenged at two higher conidium doses (0.5 × 106 and 2 × 106 conidia given daily) over 2 weeks. In marked contrast to our analysis of μMT mice, this study revealed a profound inability to prevent fungal dissemination to the brain in the complete absence of an adaptive immune response (Fig. 8 A). We then compared Rag1−/− mice with Rag1−/− mice reconstituted selectively with T helper cells from wild-type mice that had previously been exposed to A. fumigatus conidia and then challenged the reconstituted mice with A. fumigatus conidia (Fig. 8B). As expected, conidium challenge of mice receiving wild-type, fungus-specific T cells resulted in robust allergic lung disease, including airway hyperreactivity, airway eosinophilia, and lung IL-4 secretion (Fig. 8C to E), confirming that T cells are responsible for these effects. Lung IFN-γ and neutrophil responses were also markedly enhanced in T cell-reconstituted Rag1−/− mice (Fig. 8D and E), but dissemination of infection to the brain was almost completely suppressed in T cell-reconstituted Rag1−/− mice (Fig. 8F). Cytokine analysis of BALF provided further evidence of the incomplete dominance of either Th1 or Th2 immunity and also provided evidence of Th17 activation, as indicated by increased IL-17A (Fig. 8G). In contrast, Rag1−/− mice demonstrated profoundly attenuated or undetectable airway physiological changes, eosinophilia, and lung IL-4 responses to fungal challenge and were unable to prevent dissemination of infection to the brain (Fig. 8C to F). These findings confirm that T helper cells, not B cells, are both necessary and sufficient to induce allergic lung disease in the setting of fungal airway infection and to prevent lethal central nervous system dissemination.
Fig. 8.
T helper cells are required to preclude brain dissemination of A. fumigatus. (A) C57BL/6 and Rag1−/− mice were challenged i.n. with 0.5 × 106 or 2 × 106 A. fumigatus conidia daily for 18 days, and fungal brain CFU were determined. (B) Schedule used for adoptive transfer experiment. C57BL/6 mice (T cell donors) were challenged i.n. with 1 × 106 A. fumigatus conidia, as indicated. T helper cells were then purified from lungs and spleens, and 1.5 × 106 cells were injected intraperitoneally (i.p.) into Rag1−/− mice that were then challenged i.n. daily for 18 days with 1 × 106 conidia, starting 1 day after T cell transfer. (C) Airway hyperreactivity. (D) BALF differential analysis. (E) ELISpot analysis of IL-4- and IFN-γ-secreting lung cells. (F) Mean A. fumigatus CFU cultured from brains. (G) Mean BALF cytokine values. Data are from one of two comparable experiments (n = 5 mice per group). Responses statistically different (P < 0.05) from those of challenged C57BL/6 mice (A) or Rag1−/− mice (C to G) are indicated by asterisks.
DISCUSSION
The precise environmental causes of asthma and related allergic diseases have not been established with confidence, but both human and experimental evidence increasingly links allergic airway diseases to fungal exposure and airway infection (15, 16, 45). To experimentally address the possible contribution of common environmental fungi to allergic disease, we analyzed 12 common fungal species from 8 genera, isolated from the houses of asthmatic children, for the ability to induce allergic disease in mice. Although prior studies suggested that inhaled fungal conidia do not induce immune reactions (3), our results clearly demonstrate that the ability to induce a strong Th2-mediated mucosal immune response is common, if not universal, among typical household fungi, further substantiating a possible host-pathogen environmental link not only to allergic asthma but potentially also to chronic rhinosinusitis and other allergic syndromes (31, 32, 45, 46). We further demonstrated that an inevitable consequence of having an excessive mucosal fungal burden is local tissue invasion and widespread dissemination, especially to the brain, accompanied by immune phenotype switching, thereby demonstrating for the first time that T cell immune responses to fungi exist along a continuum and are conidium dose dependent. However, fungus-specific factors are not the sole determinants of dissemination, as we have formally demonstrated for the first time that mucosal T helper cells are crucial for containing fungal infection and preventing invasion and dissemination.
Prior fungal conidium inhalation research has focused on rodent models in which typical exposures exceed 1 × 107 A. fumigatus conidia/dose (21, 36, 49, 63). These exposures surpass the minimum A. fumigatus challenge doses required to induce allergic lung disease by over 3 orders of magnitude and, where studied, typically produce invasive fungal disease and pathology devoid of allergic features. Although type 2 immunity was not reported in association with invasive disease, strong type 1 responses, which were absent in our studies, with the exception of invasive C. lunata and high doses of A. fumigatus conidia, were observed. In contrast, relatively low C. lunata and A. fumigatus conidium doses induced strictly type 2 responses and allergic lung disease. Thus, in contrast to prior studies involving very large exposure doses, we have shown that the typical response to low-grade airway mucosal exposure to fungal conidia is allergic inflammation accompanied by typical markers of Th2 cell-dependent airway obstruction, i.e., airway hyperreactivity and goblet cell metaplasia.
At conidium challenge doses that induced predominantly Th2 cell and allergic responses, no or minimal evidence of lung invasion, distant dissemination, or other morbidity (i.e., weight loss, altered behavior, etc.) was observed. However, all Rag1−/− mice exposed to comparable A. fumigatus conidium doses manifested gross dissemination of infection to the brain, with profound morbidity (Fig. 8). Thus, although Th2 cytokines are believed to impair the normally protective Th1 immune response and inhibit infection control (10–12), our data demonstrate that the Th2-based allergic response is not merely the typical murine response to low-grade airway mucosal fungal infection but also is linked to protection against fungal invasion and dissemination. Moreover, T cells alone are required adaptive immune cells, as we could demonstrate no role for B cells or antibodies in protection against fungal infection in this context. Nonetheless, even highly polarized Th2 responses against fungal infection are accompanied to some degree by both type 1 and type 17 cytokines and chemokines (Fig. 3 and 8). Future studies are therefore required to determine separately the contributions of type 1, type 2, and type 17 cytokines to optimal fungal infection control at the airway mucosa.
An apparent paradox of allergic type 2 immunity in response to fungal airway infection is that it is associated with both protective (i.e., facilitating organism removal and preventing dissemination) and detrimental (i.e., asthma-like airway obstructive disease) outcomes. Our study demonstrates, however, that allergic lung disease with airway obstruction is a far less morbid alternative to invasive/disseminated disease that is the inevitable consequence of overwhelming infection. Systemic dissemination was inevitably accompanied by brain involvement that resulted in ataxia, rapid weight loss, and, ultimately, death (data not shown). ABPA is linked to locally invasive fungal airway infection (47), which in turn may be the cause of irreversible bronchiectasis that is a characteristic feature of the severest forms of this condition (2, 23, 62). Moreover, deep cutaneous and central nervous system fungal infections have rarely been documented for subjects whose only significant medical condition was asthma (9, 17, 50, 52). We therefore postulate that the potentially protective role of the type 2 immune response, potentially in conjunction with type 1 and 17 responses, against mucosal fungal infection represents a highly specific mechanism to prevent the disastrous consequences arising from invasion of the lung parenchyma and dissemination of airway fungi.
Although our experiments have revealed the importance of T cells in preventing fungal dissemination at high infectious challenge doses, the importance of innate immune neutrophils, eosinophils, and macrophages should not be discounted. T cells are most likely of greatest relevance when the fungal infectious burden is relatively high, as demonstrated by Rag1−/− mice, which tolerated relatively low A. fumigatus infectious burdens (<0.5 × 106 conidia/day) with minimal or no morbidity (data not shown). Thus, innate immunity alone is sufficient to successfully cope with low-grade fungal airway infections that continuously arise through routine inhalational exposures, and T cells likely assume critically important protective roles only in the settings of high infectious burdens and innate immune cell dysfunction.
Patients with asthma are at risk for developing several life-threatening complications, including status asthmaticus (profound airway obstruction often requiring mechanical ventilatory assistance) (18), steroid resistance (1), ABPA (48), Churg-Strauss syndrome (allergic granulomatosis and angiitis) (22), eosinophilic pneumonia, and disseminated fungal disease. Of these conditions, only ABPA is obviously linked to airway fungal infection. However, given the nearly universal propensity of fungi to cause airway infection and allergic lung disease, as shown here, the possibility exists that all of these complications are related at least in part to underlying, preexisting fungal mucosal infection. Severe, steroid-resistant asthma is a particular concern, as the patient subset with this manifestation accounts for the majority of asthma-related health care expenditures (6). Our demonstration that control of airway fungal infection is strongly T cell associated raises further concerns regarding current asthma therapy, which is based on administration of immunosuppressive corticosteroids that suppress T cell function and that, while clearly beneficial for treatment of asthma, are also contraindicated in the setting of any infectious disease. Thus, our findings urgently call for clarification of the precise role played by fungal infection and the most appropriate use of corticosteroids and alternative therapies such as antifungal antibiotics for treatment of asthma (16).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants HL75243 and AI057696 (to D.B.C.), AI070973 (to G.L.D., S.H., F.K., and D.B.C.), and AI07495 (to P.C.P.), Southwest Center for Occupational and Environmental Health: NIOSH Pilot Project award 27333-I (to P.C.P.), and the Biology of Inflammation Center and Cullen Trust for Health Care at Baylor College of Medicine.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Adcock I. M., Ford P. A., Bhavsar P., Ahmad T., Chung K. F. 2008. Steroid resistance in asthma: mechanisms and treatment options. Curr. Allergy Asthma Rep. 8:171–178 [DOI] [PubMed] [Google Scholar]
- 2. Agarwal R., Gupta D., Aggarwal A. N., Behera D., Jindal S. K. 2006. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest 130:442–448 [DOI] [PubMed] [Google Scholar]
- 3. Aimanianda V., et al. 2009. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121 [DOI] [PubMed] [Google Scholar]
- 4. Anand R., Tiwary B. N. 2010. Th1 and Th2 cytokines in a self-healing primary pulmonary Aspergillus flavus infection in BALB/c mice. Cytokine 52:258–264 [DOI] [PubMed] [Google Scholar]
- 5. Baddley J. W., Salzman D., Pappas P. G. 2002. Fungal brain abscess in transplant recipients: epidemiologic, microbiologic, and clinical features. Clin. Transplant. 16:419–424 [DOI] [PubMed] [Google Scholar]
- 6. Beasley R. 2002. The burden of asthma with specific reference to the United States. J. Allergy Clin. Immunol. 109:S482–S489 [DOI] [PubMed] [Google Scholar]
- 7. Bjorgvinsdottir H., et al. 1997. Retroviral-mediated gene transfer of gp91phox into bone marrow cells rescues defect in host defense against Aspergillus fumigatus in murine X-linked chronic granulomatous disease. Blood 89:41–48 [PubMed] [Google Scholar]
- 8. Carney A. S., et al. 2006. Th2 immunological inflammation in allergic fungal sinusitis, nonallergic eosinophilic fungal sinusitis, and chronic rhinosinusitis. Am. J. Rhinol. 20:145–149 [PubMed] [Google Scholar]
- 9. Carter E., Boudreaux C. 2004. Fatal cerebral phaeohyphomycosis due to Curvularia lunata in an immunocompetent patient. J. Clin. Microbiol. 42:5419–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cenci E., et al. 1999. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J. Infect. Dis. 180:1957–1968 [DOI] [PubMed] [Google Scholar]
- 11. Cenci E., et al. 1998. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178:1750–1760 [DOI] [PubMed] [Google Scholar]
- 12. Cenci E., et al. 1997. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect. Immun. 65:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corry D. B., et al. 1996. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med. 183:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corry D. B., et al. 1998. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol. Med. 4:344–355 [PMC free article] [PubMed] [Google Scholar]
- 15. Denning D. W., O'Driscoll B. R., Hogaboam C. M., Bowyer P., Niven R. M. 2006. The link between fungi and severe asthma: a summary of the evidence. Eur. Respir. J. 27:615–626 [DOI] [PubMed] [Google Scholar]
- 16. Denning D. W., et al. 2009. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: the Fungal Asthma Sensitization Trial (FAST) study. Am. J. Respir. Crit. Care Med. 179:11–18 [DOI] [PubMed] [Google Scholar]
- 17. Erbagci Z. 2002. Deep dermatophytoses in association with atopy and diabetes mellitus: Majocchi's granuloma tricophyticum or dermatophytic pseudomycetoma? Mycopathologia 154:163–169 [DOI] [PubMed] [Google Scholar]
- 18. Fahy J. V., Corry D. B., Boushey H. A. 2000. Airway inflammation and remodeling in asthma. Curr. Opin. Pulm. Med. 6:15–20 [DOI] [PubMed] [Google Scholar]
- 19. Gerson S. L., et al. 1984. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann. Intern. Med. 100:345–351 [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. Hogaboam C. M., et al. 2000. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am. J. Pathol. 156:723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katzenstein A. L. 2000. Diagnostic features and differential diagnosis of Churg-Strauss syndrome in the lung. A review. Am. J. Clin. Pathol. 114:767–772 [DOI] [PubMed] [Google Scholar]
- 23. Kauffman H. F., Tomee J. F., van der Werf T. S., de Monchy J. G., Koeter G. K. 1995. Review of fungus-induced asthmatic reactions. Am. J. Respir. Crit. Care Med. 151:2109–2115 [DOI] [PubMed] [Google Scholar]
- 24. Kheradmand F., et al. 2002. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 169:5904–5911 [DOI] [PubMed] [Google Scholar]
- 25. Kitamura D., Roes J., Kuhn R., Rajewsky K. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423–426 [DOI] [PubMed] [Google Scholar]
- 26. Kobayashi T., et al. 2009. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J. Immunol. 182:2502–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kozel T. R., Wilson M. A., Farrell T. P., Levitz S. M. 1989. Activation of C3 and binding to Aspergillus fumigatus conidia and hyphae. Infect. Immun. 57:3412–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kruse D., Cole G. T. 1990. Isolation of tube precipitin antibody-reactive fractions of Coccidioides immitis. Infect. Immun. 58:169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kurup V. P., Ramasamy M., Greenberger P. A., Fink J. N. 1988. Isolation and characterization of a relevant Aspergillus fumigatus antigen with IgG- and IgE-binding activity. Int. Arch. Allergy Appl. Immunol. 86:176–182 [DOI] [PubMed] [Google Scholar]
- 30. Liang S. C., et al. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luong A., Davis L. S., Marple B. F. 2009. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am. J. Rhinol. Allergy 23:281–287 [DOI] [PubMed] [Google Scholar]
- 32. Luong A., Marple B. 2005. The role of fungi in chronic rhinosinusitis. Otolaryngol. Clin. North Am. 38:1203–1213 [DOI] [PubMed] [Google Scholar]
- 33. Luong A., Marple B. F. 2004. Allergic fungal rhinosinusitis. Curr. Allergy Asthma Rep. 4:465–470 [DOI] [PubMed] [Google Scholar]
- 34. Reference deleted.
- 35. Marx J. J., Flaherty D. K. 1976. Activation of the complement sequence by extracts of bacteria and fungi associated with hypersensitivity pneumonitis. J. Allergy Clin. Immunol. 57:328–334 [DOI] [PubMed] [Google Scholar]
- 36. Mehrad B., Strieter R. M., Standiford T. J. 1999. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162:1633–1640 [PubMed] [Google Scholar]
- 37. Mombaerts P., et al. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877 [DOI] [PubMed] [Google Scholar]
- 38. Morgenstern D. E., Gifford M. A., Li L. L., Doerschuk C. M., Dinauer M. C. 1997. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 185:207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murdock B. J., et al. 2011. Coevolution of TH1, TH2, and TH17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect. Immun. 79:125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nurieva R., et al. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448:480–483 [DOI] [PubMed] [Google Scholar]
- 41. Pasqualotto A. C., Powell G., Niven R., Denning D. W. 2009. The effects of antifungal therapy on severe asthma with fungal sensitization and allergic bronchopulmonary aspergillosis. Respirology 14:1121–1127 [DOI] [PubMed] [Google Scholar]
- 42. Polak-Wyss A. 1991. Protective effect of human granulocyte colony-stimulating factor (hG-CSF) on Cryptococcus and Aspergillus infections in normal and immunosuppressed mice. Mycoses 34:205–215 [DOI] [PubMed] [Google Scholar]
- 43. Polikepahad S., et al. 13 April 2010. A reversible, non-invasive method for airway resistance measurements and bronchoalveolar lavage fluid sampling in mice. J. Vis. Exp. doi: 10.3791/1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Porter P., et al. 2011. Respiratory tract allergic disease and atopy: experimental evidence for a fungal infectious etiology. Med. Mycol. 49(Suppl. 1):S158–S163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Porter P., et al. 2009. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2:504–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Porter P. C., Ongeri V., Luong A., Kheradmand F., Corry D. B. 2011. Seeking common pathophysiology in asthma, atopy and sinusitis. Trends Immunol. 32:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riley D. J., Mackenzie J. W., Uhlman W. E., Edelman N. H. 1975. Allergic bronchopulmonary aspergillosis: evidence of limited tissue invasion. Am. Rev. Respir. Dis. 111:232–236 [DOI] [PubMed] [Google Scholar]
- 48. Riscili B. P., Wood K. L. 2009. Noninvasive pulmonary Aspergillus infections. Clin. Chest Med. 30:315–335 [DOI] [PubMed] [Google Scholar]
- 49. Rivera A., et al. 2006. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity 25:665–675 [DOI] [PubMed] [Google Scholar]
- 50. Romano C., et al. 1997. Two cases of cutaneous phaeohyphomycosis by Alternaria alternata and Alternaria tenuissima. Mycopathologia 137:65–74 [DOI] [PubMed] [Google Scholar]
- 51. Rosas A. L., MacGill R. S., Nosanchuk J. D., Kozel T. R., Casadevall A. 2002. Activation of the alternative complement pathway by fungal melanins. Clin. Diagn. Lab. Immunol. 9:144–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanchez C., et al. 1995. Treatment of cerebral Aspergillosis with itraconazole: do high doses improve the prognosis?. Clin. Infect. Dis. 21:1485–1487 [DOI] [PubMed] [Google Scholar]
- 53. Sasada M., Johnston R. B., Jr 1980. Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J. Exp. Med. 152:85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reference deleted.
- 55. Schaffner A., Douglas H., Braude A. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Invest. 69:617–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schaffner A., Douglas H., Braude A. I., Davis C. E. 1983. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect. Immun. 42:1109–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reference deleted.
- 58. Shoham S., Levitz S. M. 2005. The immune response to fungal infections. Br. J. Haematol. 129:569–582 [DOI] [PubMed] [Google Scholar]
- 59. Soubani A. O., Chandrasekar P. H. 1988. The clinical spectrum of pulmonary aspergillosis. Chest 121:1988–1999 [DOI] [PubMed] [Google Scholar]
- 60. Sturtevant J. E., Latge J. P. 1992. Interactions between conidia of Aspergillus fumigatus and human complement component C3. Infect. Immun. 60:1913–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reference deleted.
- 62. Vlahakis N. E., Aksamit T. R. 2001. Diagnosis and treatment of allergic bronchopulmonary aspergillosis. Mayo Clin. Proc. 76:930–938 [DOI] [PubMed] [Google Scholar]
- 63. Werner J. L., et al. 2009. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182:4938–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.