Abstract
Brucella abortus is a facultative intracellular bacterial pathogen that causes abortion in domestic animals and undulant fever in humans. Recent studies have revealed that Toll-like receptor (TLR)-initiated immune response to Brucella spp. depends on myeloid differentiation factor 88 (MyD88) signaling. Therefore, we decided to study the role of the interleukin-1 receptor-associated kinase 4 (IRAK-4) in host innate immune response against B. abortus. After Brucella infection, it was shown that the number of CFU in IRAK-4−/− mice was high compared to that in IRAK-4+/− animals only at 1 week postinfection. At 3 and 6 weeks postinfection, IRAK-4−/− mice were able to control the infection similarly to heterozygous animals. Furthermore, the type 1 cytokine profile was evaluated. IRAK-4−/− mice showed lower production of systemic interleukin-12 (IL-12) and gamma interferon (IFN-γ). Additionally, a reduced percentage of CD4+ and CD8+ T cells expressing IFN-γ was observed compared to IRAK-4+/−. Further, the production of IL-12 and tumor necrosis factor alpha (TNF-α) by macrophages and dendritic cells from IRAK-4−/− mice was abolished at 24 h after stimulation with B. abortus. To investigate the role of IRAK-4 in mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways, macrophages were stimulated with B. abortus, and the signaling components were analyzed by protein phosphorylation. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 and p38 as well as p65 NF-κB phosphorylation was profoundly impaired in IRAK-4−/− and MyD88−/− macrophages activated by Brucella. In summary, the results shown in this study demonstrated that IRAK-4 is critical to trigger the initial immune response against B. abortus but not at later phases of infection.
INTRODUCTION
Brucella abortus is a Gram-negative bacterium which is pathogenic to humans and animals (8). The establishment of infection depends on an effective entrance of this bacterium through the nasal, oral, and/or conjunctival mucosa. After entering into the host cells, Brucella has the ability to infect and multiply in phagocytic and nonphagocytic cells (10, 15). However, macrophages are considered the main cells of Brucella residence in the host (3). The immune response against Brucella infection involves many molecules and cells to trigger a Th1 immune response and activation of CD8+ T cells (12, 26, 27). Furthermore, gamma interferon (IFN-γ) is essential for host control of Brucella infection (24, 32, 41).
The innate immune response against Brucella abortus infection begins with the recognition of molecular structures related to this pathogen by receptors such as Toll-like receptors (TLRs) (28). TLRs are transmembrane receptors that recognize pathogen-associated molecular patterns (PAMPs) and lead to the expression of proinflammatory cytokine-related genes (2, 36). Upon activation, TLRs associate with the myeloid differentiation factor 88 (MyD88), an adaptor molecule of all TLRs, except TLR3, recruiting interleukin-1 (IL-1) receptor-associated kinase (IRAK) proteins and tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), subsequently (1). Once interacted with MyD88, IRAK is activated by phosphorylation dependent on TAK1 (transforming growth factor-β-activated kinase) and then associates with TRAF6, leading to the activation of two distinct signaling pathways, and finally to the activation of JNK (Janus kinase) and nuclear factor κB (NF-κB) signaling pathways (1). Activated TAK1 phosphorylates IKK-β (IκB kinase) and mitogen-activated protein kinase (MAPK) kinase 6 (MKK6), culminating in the degradation of IκB, leaving the NF-κB to be translocated to the nucleus and to activate the transcription of multiple genes of proinflammatory cytokines such as IL-12 and TNF-α (29). Four members of the IRAK protein family have been identified so far: IRAK-1, IRAK-2, IRAK-3 (also known as IRAK-M), and IRAK-4 (5, 25, 40, 19). Among the members of this protein family, IRAK-4 and IRAK-1 are recruited to the TLR receptor complex through interaction with MyD88 (14, 20). However, only the involvement of IRAK-4 in the signal transduction pathway dependent on MyD88 has been demonstrated to be essential for the signaling mediated by the IL-1 receptor and TLRs (33). Furthermore, the response via the IL-18 receptor is also dependent on IRAK-4 (34).
Brucella infection induces an inflammatory response through the expression of several genes involved in inflammation (31). Some in vitro and in vivo studies have shown the involvement of TLR2, TLR4, and TLR9 in the recognition of Brucella and the induction of inflammatory response (4, 11, 13, 22, 39). Moreover, our group and others have demonstrated that MyD88 is essential for host control of Brucella infection in vivo and the induction of proinflammatory cytokines (7, 22, 39). Taking into account the critical role of MyD88 during Brucella infection, we decided to study the role of IRAK-4 by using knockout (KO) mice. In this study, we observed that the initial control of Brucella infection in vivo is IRAK-4 dependent. However, differently from MyD88 KO mice, animals deficient in IRAK-4 are as resistant as heterozygous control mice (IRAK-4+/−) at later stages of Brucella infection. Furthermore, IRAK-4 plays a crucial role in Brucella-induced IL-12 and TNF-α production by macrophages and dendritic cells (DCs) in vitro. Finally, to determine the role of IRAK-4-mediated signaling in macrophage activation by Brucella, we analyzed the phosphorylation of extracellular signal-regulated kinase 1 (ERK1), ERK2, and p38 as well as p65 NF-κB. The results obtained here indicated that IRAK-4 and MyD88 are required for cell signaling induced by Brucella activation of macrophages.
This is the first report demonstrating the role of IRAK-4 in vivo during an intracellular Gram-negative bacterial infection. In summary, IRAK-4 signaling during Brucella infection corroborates with the importance of TLR-MyD88 effective response involved in this disease.
MATERIALS AND METHODS
Mice.
IRAK-4 genetically deficient mice (IRAK-4−/−) and IRAK-4-heterozygous animals (IRAK-4+/−) were gifted by Alan Sher (NIH, Bethesda, MD) and Andre Bafica (UFSC, Brazil). MyD88 genetically deficient mice (MyD88−/−) were provided by S. Akira (Osaka University, Osaka, Japan). The wild-type strain C57BL/6 mice were purchased from the Federal University of Minas Gerais (UFMG, Belo Horizonte, Brazil). The animals were maintained at UFMG and used at 6 to 8 weeks of age.
Bacteria.
Brucella abortus strain S2308 was obtained from our laboratory collection (38). Strain S2308 was grown in Brucella broth (BB) liquid medium (Difco) at 37°C under constant agitation. After 3 days of growth, the bacterial culture was centrifuged and the pellet was resuspended in 0.15 M phosphate-buffered saline (PBS), pH 7.4 (2.8 mM Na2PO4, 7.2 mM Na2HPO4, 0.14 M NaCl). Aliquots of these cultures were serially diluted and plated on BB medium containing 1.5% bacteriological agar. After incubation for 72 h at 37°C, bacterial numbers were determined by counting CFU.
B. abortus infection.
Five mice from each group (IRAK-4−/− and IRAK-4+/−) were infected intraperitoneally with 1 × 106 CFU of B. abortus virulent strain S2308. These mice were sacrificed at 1, 3, and 6 weeks after infection. The spleen harvested from each animal was macerated in 10 ml of saline (NaCl 0.8%) and used for counting of CFU and splenocyte culturing. For CFU determination, spleen cells were serially diluted and were plated in duplicate on BB agar. After 3 days of incubation at 37°C, the number of CFU was determined. Results were expressed as the mean log CFU of each group. The results are representative of three independent experiments. Blood samples were collected from IRAK-4−/− and IRAK-4+/− mice at 1, 3, and 6 weeks postinfection to determine IL-12 and IFN-γ levels by enzyme-linked immunosorbent assay (ELISA) using the Duoset kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Measurement of IFN-γ into splenocyte culture supernatants.
Spleen cells from IRAK-4−/− and IRAK-4+/− mice were treated with ACK buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2) to lyse red blood cells. After that, the cells were washed with saline (NaCl, 0.8%) and suspended in RPMI 1640 (Gibco, Carlsbad, CA) supplemented with 2 mM l-glutamine, 25 mM HEPES, 10% heat-inactivated fetal bovine serum (FBS; Gibco, Carlsbad, CA), penicillin G sodium (100 U/ml), and streptomycin sulfate (100 μg/ml). To determine cytokine concentration by ELISA, 1 × 106 spleen cells were plated per well in a 96-well tissue culture-treated dish. Splenocytes were stimulated with B. abortus S2308 (multiplicity of infection [MOI], 100:1), heat-killed B. abortus (HKBa; MOI, 100:1), Pam3CSK4 (1 μg/ml; InvivoGen, San Diego, CA), CpG ODN1826 (1 μg/ml; InvivoGen), or concanavalin A (5 μg/ml; Sigma-Aldrich, St. Louis, MO). Unstimulated cells were used as the negative control. Spleen cells were incubated at 37°C in 5% CO2 for 72 h, after which supernatants were harvested for measuring IFN-γ levels. IFN-γ was measured into cell supernatants by ELISA using the Duoset kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Flow cytometry analysis.
To determine the percentage of CD4+ or CD8+ T cells producing IFN-γ in IRAK-4−/− compared to IRAK-4+/− mice, flow cytometry analysis was performed and 1 × 106 spleen cells were plated per well in a 96-well tissue culture-treated dish. Cells were stimulated with HKBa (MOI, 100:1) or concanavalin A (5 μg/ml; Sigma). Spleen cells were incubated overnight at 37°C in 5% CO2 for 40 h, after which 1 μg/well of brefeldin A (Sigma-Aldrich) was added and the cells were incubated for an additional 4 h. Then, cells were incubated with FcBlock anti-mouse CD16/32 (clone 93; eBiosciences, San Diego, CA) in fluorescence-activated cell sorter (FACS) buffer (0.15 M PBS, 0.25% bovine serum albumin [BSA], 1 mM NaN3) for 20 min and were stained for surface markers at 4°C for 20 min with the appropriate monoclonal antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-mouse-CD4 (clone GK1.5; eBiosciences, San Diego, CA) or Cy5-conjugated anti-mouse-CD8 (clone 53-6.7; eBiosciences, San Diego, CA). The cells were washed with PBS-BSA and fixed with 4% formaldehyde for 30 min. After that, cells were permeabilized with a 0.5% saponin solution in PBS and stained for intracellular IFN-γ with phycoerythrin (PE)-conjugated anti-mouse-IFN-γ (clone XMG1.2; BD Pharmingen, San Diego, CA). Finally, samples were washed as mentioned before and resuspended in PBS-BSA. Flow cytometry analysis was performed using a FACSCalibur instrument (Becton Dickinson, San Diego, CA); 70,000 events were collected and the data were analyzed considering the percentage of IFN-γ-producing cells within the CD4+ or CD8+ T-lymphocyte population by using FlowJo software (Tree Star, Ashland, OR).
Generation and in vitro stimulation of BMDMs and BMDCs.
Macrophages were derived from bone marrow (BM) of C57BL/6, MyD88−/−, IRAK-4+/−, and IRAK-4−/− mice as previously described (6). Briefly, BM cells were removed from the femurs and tibias of the animals and cultured in Dulbecco's modified Eagle medium (DMEM; Gibco, Carlsbad, CA) containing 10% FBS (HyClone, Logan, UT), 1% HEPES, and 10% L929 cell-conditioned medium (LCCM) as the source of macrophage colony-stimulating factor (M-CSF) in 24-well plates (5 × 105 cells/well). After 4 days, 100 μl/well of LCCM was added. At day 7, the medium was renewed. At day 10 of culture, when the cells had completely differentiated into macrophages, the medium was harvested, and we added supplemented DMEM (500 μl/well) containing B. abortus S2308 (MOI, 1,000:1), HKBa (MOI, 1,000:1), Pam3CSK4 (1 μg/ml; InvivoGen, San Diego, CA), CpG ODN1826 (1 μg/ml, InvivoGen), Brucella L-Omp19 lipoprotein (5 μg/ml; gift of Guillermo Giambartolomei), Brucella DNA (1 μg/ml), or Escherichia coli lipopolysaccharide (LPS) (1 μg/ml; Sigma, St. Louis, MO). DCs were derived from the femurs and the tibias of IRAK-4+/− and IRAK-4−/− mice according to the work of Macedo et al. (22). Briefly, BM cells were cultured in DMEM (Gibco, Carlsbad, CA) containing 10% FBS (HyClone, Logan, UT), 100 U/ml of penicillin, and 100 μg/ml of streptomycin plus 20 ng/ml of murine recombinant granulocyte-monocyte colony-stimulating factor (rGM-CSF). Petri dishes containing 1 × 107 cells were incubated at 37°C, under an atmosphere of 5% CO2. At day 3 of incubation, a further 5 ml of fresh complete medium containing GM-CSF was added, and on days 6 and 8, 3 ml of medium was removed from the culture and replaced with fresh supplemented medium containing GM-CSF. At day 10, nonadherent cells were harvested and seeded in round-bottom 96-well culture plates (3 × 105 cells/well). Stimulation of the BM dendritic cells (BMDCs) followed the same protocol described above for the bone marrow-derived macrophages (BMDMs), but the stimuli were added in 200 μl of DMEM/well. Culture supernatants of BMDMs and BMDCs were collected after 24 h of stimulation and assayed for the concentrations of IL-12 and TNF-α by ELISA (R&D Systems) according to the manufacturer's instructions.
Western blot analysis.
For Western blot analysis, BMDMs of C57BL/6, MyD88−/−, IRAK-4+/−, and IRAK-4−/− mice were used. BMDMs were differentiated in 6-well plates (2 × 106 cells/well). At day 10 of culture, the medium was harvested, DMEM was added, and the cells were maintained for 12 h. After 12 h, the medium was harvested and DMEM (1 ml/well) containing B. abortus S2308 (MOI, 1,000:1) or E. coli LPS (1 μg/ml; Sigma, St. Louis, MO) as the control was added to the culture. After 0, 10, 30, or 60 min of stimulation, BMDMs were lysed with a cytoplasmic lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 50 mM NaF, 10 mM β-glycerophosphate, 0.1 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate) and supplemented with 1:100 protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The lysates were then clarified by centrifugation at 13,000 × g for 10 min at 4°C, and the protein concentrations of the whole-cell lysates were measured using a bicinchoninic acid (BCA) protein assay (Bio-Rad Laboratories, Inc.). Lysates were subjected to electrophoresis on 10% SDS-PAGE gels followed by Western blotting according to standard techniques. Monoclonal antibodies used for Western blotting to phosphorylated ERK1/2 (anti-phospho-Erk1/2, Thr202/Tyr204), anti-phospho-p38 (Thr180/Tyr182), anti-phospho-NF-κB (p65, Ser536), and anti-β-actin (13E5) were purchased from Cell Signaling Technology Inc. (Danvers, MA). Immunoreactive bands were visualized using Luminol chemiluminescent horseradish peroxidase (HRP) substrate (Millipore) and analyzed in Storm System 860 (Amersham Biosciences). The analyses of densitometry were performed using software KODAK version 1D-3.5.
Statistical analysis.
The results of this study were analyzed using Student's t test with GraphPad Prism 4 (GraphPad Software, Inc). The level of significance in the analysis was P < 0.05.
RESULTS
IRAK-4−/− mice are more susceptible to B. abortus at early stages of infection.
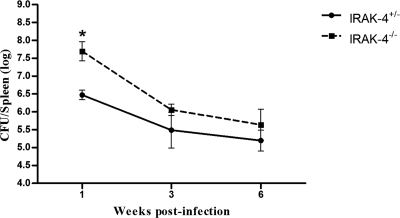
To investigate the role of the IRAK-4 molecule during B. abortus infection, IRAK-4+/− and IRAK-4−/− mice were infected with 1 × 106 CFU of B. abortus strain S2308, and the number of bacteria in mouse spleens was monitored by CFU counting. Surprisingly, IRAK-4 showed to be important for the control of Brucella only at the first week postinfection, as demonstrated in Fig. 1. Bacterial load recovery was 1.22 logs higher in the IRAK-4−/− mice than in the IRAK-4+/− animals. At 3 and 6 weeks after infection, the numbers of Brucella in spleens of IRAK-4−/− mice did not differ significantly from those for IRAK-4+/− animals. These results indicate that IRAK-4 is involved in innate immune responses to Brucella at early stages of bacterial infection.
Fig. 1.
IRAK-4−/− mice are more susceptible to B. abortus at the first week postinfection. IRAK-4−/− and IRAK-4+/− mice were infected, and the numbers of bacteria in the spleens were analyzed by counting CFU at 1, 3, and 6 weeks after infection. Data are expressed as mean ± standard deviation (SD) for five animals per time point. These results are representative of three independent experiments. A significant difference in relation to IRAK-4+/− is denoted by an asterisk (P < 0.05).
IRAK-4 is critical to induce a specific type 1 immune response to B. abortus.
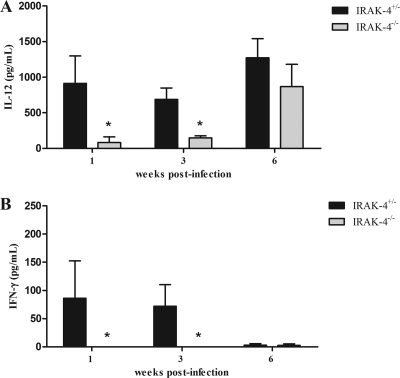
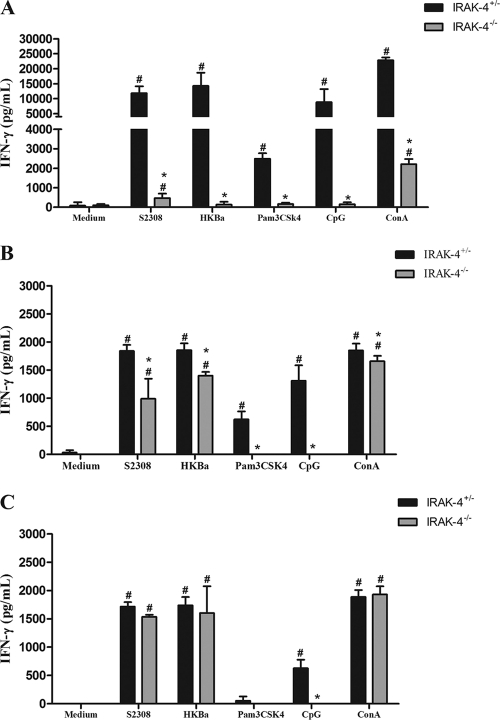
Protective immunity against infection by B. abortus is directly related to the induction of a type 1 pattern of immune response (24, 32, 41). IL-12 and IFN-γ are key cytokines involved in this type of immunity. Thus, to evaluate the role of IRAK-4 in inducing a type 1 immune response during B. abortus infection, the production of IL-12 and IFN-γ in vivo was assessed. IRAK-4+/− and IRAK-4−/− mice were infected with B. abortus and, at 1, 3, and 6 weeks after infection, serum IL-12 and IFN-γ levels were determined in these mice. IL-12 and IFN-γ production in IRAK-4−/− mice was greatly reduced at 1 and 3 weeks postinfection compared to that for IRAK-4+/− animals (Fig. 2). This result demonstrates that IRAK-4 is directly involved in IL-12 and IFN-γ synthesis during Brucella infection. Moreover, to investigate the role of IRAK-4 in IFN-γ production, splenocytes from Brucella-infected animals were stimulated with live B. abortus, HKBa, TLR2, or TLR9 agonists (Pam3CSK or CpG). After 72 h of cell culture, the supernatant was collected and the level of IFN-γ was analyzed. In this study, a dramatic reduction on IFN-γ production by IRAK-4 KO mice compared to what was seen for heterozygous cells at 1 week after infection was observed (Fig. 3A). At 3 weeks postinfection, the levels of IFN-γ produced by IRAK-4+/− and by IRAK-4−/− mice were still significantly different, even though this difference was less pronounced (Fig. 3B). However, at 6 weeks postinfection the levels of IFN-γ produced by IRAK-4−/− cells were similar to those seen for IRAK-4+/− cells (Fig. 3C). This result parallels the difference in bacterial burdens observed at 1 week postinfection in IRAK-4−/− mice and IRAK-4+/− animals. Taken together, these results suggest that the lack of IRAK-4 causes a severe defect in the induction of type 1 immune response by B. abortus during the initial phases of infection.
Fig. 2.
Serum IL-12 and IFN-γ levels after B. abortus infection. IRAK-4−/− and IRAK-4+/− mice were infected and blood samples were collected at 1, 3, and 6 weeks postinfection. IL-12 (A) and IFN-γ (B) levels were measured by ELISA. A significant difference in relation to IRAK-4+/− is denoted by an asterisk (P < 0.05).
Fig. 3.
IFN-γ production by spleen cells induced by B. abortus is partially dependent on IRAK-4. IRAK-4−/− and IRAK-4+/− mice were infected and spleens were harvested for splenocyte culture. Spleen cells were stimulated with B. abortus S2308 (MOI, 100:1), HKBa (MOI, 100:1), TLR2, or TLR9 agonists (Pam3CSK4 or CpG, 1 μg/ml of each). After 72 h, supernatants were harvested for measuring IFN-γ levels by ELISA. IFN-γ was measured at 1 (A), 3 (B), and 6 (C) weeks postinfection. These results are representative of three independent experiments. A significant difference in relation to nonstimulated cells is denoted by #, and one in relation to IRAK-4+/− mice is denoted by an asterisk (P < 0.05). ConA, concanavalin A.
Lack of IRAK-4 causes a significant reduction in proinflammatory cytokine production by macrophages and dendritic cells.
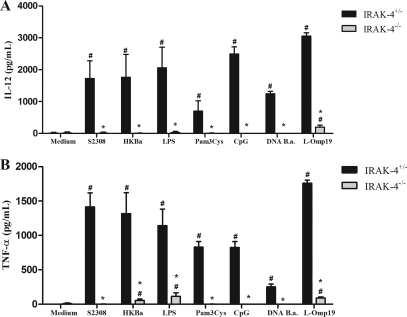
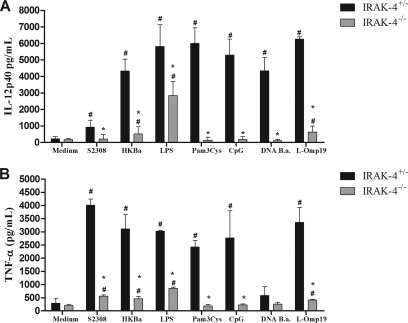
The recognition of Brucella by innate immune cells, such as macrophages and dendritic cells, results in activation and the concomitant production of proinflammatory cytokines (22). In order to evaluate the role of IRAK-4 in proinflammatory cytokine production, macrophages and dendritic cells derived from bone marrow cells of IRAK-4−/− and IRAK-4+/− mice were stimulated with B. abortus, HKBa, Brucella L-Omp19 lipoprotein, Brucella DNA, or other TLR agonists. As shown in Fig. 4, a severe deficiency in IL-12 (Fig. 4A) and TNF-α (Fig. 4B) production by macrophages from IRAK-4−/− mice activated with either Brucella or TLR agonists compared to IRAK-4+/− mouse cells was observed. Similar to what was observed for macrophages, the production of IL-12 and TNF-α by BMDCs also showed a profound defect in IRAK-4−/− mouse cells, as demonstrated in Fig. 5A and B. These results show that IRAK-4 is an important molecule involved in the signaling pathways of IL-12 and TNF-α synthesis induced by Brucella in macrophages and dendritic cells.
Fig. 4.
IL-12 and TNF-α production induced by B. abortus in macrophages is IRAK-4 dependent. Bone marrow from IRAK-4−/− and IRAK-4+/− mouse cells was differentiated in macrophages and stimulated with B. abortus S2308 (MOI, 100:1), HKBa (MOI, 100:1), Pam3CSK4 (1 μg/ml), CpG (1 μg/ml), Brucella L-Omp19 lipoprotein (5 μg/ml), Brucella DNA (DNA B.a.; 1 μg/ml), or E. coli LPS (1 μg/ml). Supernatants were harvested for measuring IL-12 (A) and TNF-α (B) after 24 h by ELISA. A significant difference in relation to nonstimulated cells is denoted by #, and a significant difference in relation to IRAK-4+/− mice is denoted by an asterisk (P < 0.05).
Fig. 5.
IRAK-4 deficiency affects Brucella-induced IL-12 and TNF-α production by dendritic cells. Bone marrow cells from IRAK-4−/− and IRAK-4+/− mice were differentiated in dendritic cells and stimulated with B. abortus S2308 (MOI, 100:1), HKBa (MOI, 100:1), Pam3CSK4 (1 μg/ml), CpG (1 μg/ml), Brucella L-Omp19 lipoprotein (5 μg/ml), Brucella DNA (DNA B.a.; 1 μg/ml) or E. coli LPS (1 μg/ml). Supernatants were harvested for measuring IL-12 (A) and TNF-α (B) after 24 h by ELISA. A significant difference in relation to nonstimulated cells is denoted by #, and a significant difference in relation to IRAK-4+/− mice is denoted by an asterisk (P < 0.05).
IRAK-4 deficiency accounts for reduced numbers of CD4+ and CD8+ T cells producing IFN-γ.
To determine whether IRAK-4 deficiency plays a role in the production of IFN-γ by different T-cell subsets during Brucella infection, IRAK-4+/− and IRAK-4−/− mice were infected, and the percentage of CD4+ or CD8+ T cells producing IFN-γ after restimulation with HKBa was determined. At the first week after infection, we observed that the production of IFN-γ by CD4+ or CD8+ T cells was IRAK-4 dependent. As demonstrated in Table 1, the percentages of CD4+ (4.58 ± 1.11) and CD8+ (1.59 ± 0.43) T cells producing IFN-γ from IRAK-4−/− mice were significantly reduced compared with what was seen for CD4+ (17.55 ± 4.56) and CD8+ (10.23 ± 4.25) T lymphocytes from IRAK-4+/− mice. Interestingly, at the third week postinfection, there was no significant difference in the percentages of CD4+ or CD8+ T cells expressing IFN-γ between infected IRAK-4+/− and IRAK-4−/− mice.
Table 1.
Percentages of CD4+ and CD8+ T cells producing IFN-γ in IRAK-4−/− and IRAK-4+/− mice at the first and third weeks of infection with B. abortus
| Week | T-cell type and mouse genotype | % cells producing IFN-γ after stimulation witha: |
||
|---|---|---|---|---|
| Medium | HKBa | ConAb | ||
| 1 | CD4+ | |||
| IRAK-4+/− | 3.49 ± 1.12 | 17.55 ± 4.56# | 27.98 ± 3.78# | |
| IRAK-4−/− | 3.16 ± 0.62 | 4.58 ± 1.11#* | 14.92 ± 2.17#* | |
| CD8+ | ||||
| IRAK-4+/− | 1.91 ± 0.33 | 10.23 ± 4.25# | 16.50 ± 4.67# | |
| IRAK-4−/− | 0.94 ± 0.25 | 1.59 ± 0.43#* | 8.11 ± 2.53#* | |
| 3 | CD4+ | |||
| IRAK-4+/− | 2.68 ± 0.60 | 7.19 ± 2.39# | 9.43 ± 3.05# | |
| IRAK-4−/− | 2.67 ± 1.04 | 6.21 ± 0.75# | 8.37 ± 1.73# | |
| CD8+ | ||||
| IRAK-4+/− | 0.58 ± 0.06 | 3.34 ± 0.94# | 3.91 ± 1.12# | |
| IRAK-4−/− | 1.26 ± 0.58 | 2.79 ± 0.76# | 7.96 ± 1.78#* | |
#, Statistically significant compared to nonstimulated cells. *, Statistically significant compared to IRAK-4+/− mice.
ConA, concanavalin A.
IRAK-4-mediated signaling is required for MAPK and NF-κB activation induced by B. abortus in macrophages.
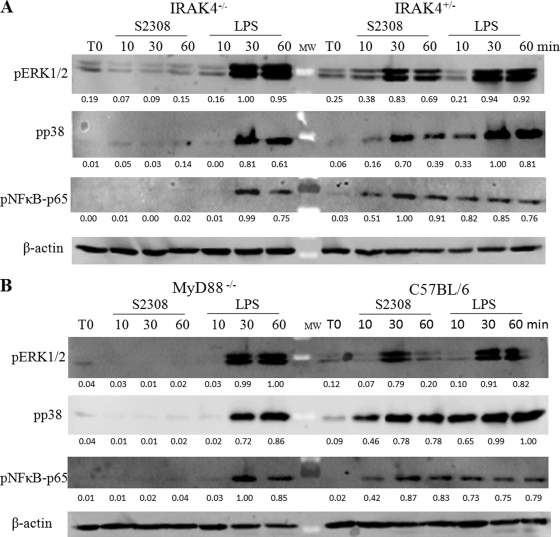
To investigate the role of IRAK-4 in MAPK and NF-κB signaling pathways induced by Brucella, macrophages were stimulated with B. abortus strain S2308 or E. coli LPS during 0, 10, 30, and 60 min of antigen stimulation. Further, the signaling components of the main MAPK and NF-κB activation pathways were analyzed by protein phosphorylation. The phosphorylation of ERK1, ERK2, and p38 as well as p65 NF-κB was profoundly impaired in IRAK-4−/− macrophages activated by Brucella but not E. coli LPS (Fig. 6A). Conversely, B. abortus induced ERK1, ERK2, and p38 as well as p65 NF-κB phosphorylation at 30 and 60 min in IRAK-4+/− mouse cells. These results indicate that Brucella activates an IRAK-4-dependent signaling pathway, leading to the activation of MAPK and NF-κB. Additionally, we investigated the role of the adaptor molecule MyD88 in this signaling pathway induced by B. abortus. Similar findings were detected in MyD88−/− macrophages compared to IRAK-4−/− cells (Fig. 6B). Regarding E. coli LPS, we observed a delayed kinetics in p38 and p65 NF-κB phosphorylation in IRAK-4−/− and MyD88−/− macrophages; this result suggests that the TLR4-mediated TRIF-dependent pathway is intact in these cells (9). Collectively, these results suggest that Brucella-mediated activation of MAPK and NF-κB pathways is mainly IRAK-4 and MyD88 dependent.
Fig. 6.
NF-κB and MAPK signaling pathways triggered by B. abortus are abrogated in IRAK−/− macrophages. Bone marrow-derived macrophages from IRAK4−/− and IRAK4−/+ mice (A) or MyD88−/− and C57BL/6 mice (B) were treated for 0, 10, 30, or 60 min with B. abortus strain S2308 (MOI, 1,000:1) or 1 μg/ml of E. coli LPS. Cell lysates were then subjected to Western blot analysis by using anti-phospho-ERK1/2, anti-phospho-p38, and anti-phospho-p65. β-Actin was used as a loading control. Values below each band indicate the quantification of band intensity relative to the loading control.
DISCUSSION
The involvement of TLRs in the recognition of Brucella has been shown previously by several investigators (4, 11, 22, 39). The recognition of pathogens by TLRs leads to an immune response that culminates in the production of the proinflammatory cytokines, amplification of the innate immune response, and activation of the adaptive immune system (2). Furthermore, IL-12 and IFN-γ are directly related to control of infection with B. abortus (24, 32, 41). In this context, a better understanding of how TLR signaling events are initiated and how they are transduced within the cell provides important information to help decipher the puzzle of host-Brucella relationship.
The first study showing the involvement of TLRs in resistance to B. abortus used mice deficient for the adaptor molecule MyD88 (13). MyD88−/− mice were unable to induce IL-12 and TNF-α when stimulated with heat-killed Brucella abortus (HKBa). Further studies about the role of MyD88 in Brucella response in vivo revealed the exacerbation of murine brucellosis in mice deficient for this adaptor molecule (7, 22, 39). Herein, we have demonstrated that IRAK-4 is necessary for Brucella host control only at the first week postinfection but not at 3 and 6 weeks after bacterial inoculation. Suzuki et al. (33) have demonstrated that IRAK-4−/− mice are highly susceptible to Staphylococcus aureus infection. All IRAK-4-deficient mice died within 10 days of S. aureus inoculation. Also, other reports with IRAK-4-deficient patients revealed that they suffered from pyogenic infections and showed impaired production of key cytokines in response to different TLR agonists (23, 30). Although these patients have a strong impairment in TLR signaling, IRAK-4-deficient subjects become resistant to bacterial infections with age, and it is likely that adaptive immunity plays a crucial role in the control of these pathogens (18).
In order to determine which factors could be involved with enhanced susceptibility to initial Brucella infection in IRAK-4−/− mice, we determined the concentrations of IL-12 and IFN-γ in serum of these animals. A compromised systemic production of IL-12 and IFN-γ in IRAK-4−/− mice was detected at the first and third weeks of infection compared to what was seen for IRAK-4+/− animals. This result correlates with augmented susceptibility of IRAK-4−/− mice at 1 week postinfection. Additionally, we also evaluated the levels of IFN-γ produced by splenocytes and examined the percentages of CD4+ and CD8+ T cells producing this cytokine. Interestingly, at 1 week postinfection we observed a dramatic difference in IFN-γ production by spleen cells from IRAK-4−/− mice compared to IRAK-4+/− animals when the cells were stimulated with live Brucella, HKBa, TLR2, or TLR9 agonists. However, at 3 weeks postinfection this difference in IFN-γ production observed between IRAK-4+/− and knockout mice was diminished. Conversely, at 6 weeks postinfection the levels of IFN-γ produced by IRAK-4−/− splenocytes to Brucella increased to levels similar to those seen for IRAK-4+/− mice. These results agree with the recovery of resistance to infection of IRAK-4−/− mice at 3 and 6 weeks postinfection as compared to IRAK-4+/− mice. Regarding the cell types involved in IFN-γ production, at 1 week postinfection we observed reduced numbers of Brucella-activated CD4+ and CD8+ T lymphocytes expressing IFN-γ in IRAK-4−/− mice compared to IRAK-4+/− mice. In contrast, at 3 weeks postinfection the percentage of these cells producing IFN-γ returned to levels similar to those observed for IRAK-4+/− animals. There are studies on the role of IRAK-4 in the signaling pathways in different cell types. Suzuki et al. (35) have demonstrated that in the absence of IRAK-4, in vivo T-cell responses were significantly impaired. Since T-cell receptor (TCR) engagement triggers the activation of two distinct transcription factors, NF-κB and NFAT (37), these pathways were investigated. This investigation demonstrated that IRAK-4−/− T cells showed a clear defect in NF-κB activation upon TCR stimulation with impairment of αCD3-dependent IκB degradation. These results suggest that IRAK-4 is directly involved in signaling for the activation of NF-κB but not NFAT in T cells. In contrast, Kawagoe et al. (17) and Lye et al. (21) have demonstrated that TCR signaling was not impaired in IRAK-4−/− mice but that the kinase activity of IRAK-4 is essential for the regulation of TLR-mediated innate immune responses. Furthermore, Suzuki et al. (34) and Kanakaraj et al. (16) have also determined that IFN-γ production by Th1 cells from IRAK-4−/− mice is severely impaired. In our study, we speculate that reduced IFN-γ production by Brucella-primed CD4+ and CD8+ T lymphocytes from IRAK-4−/− mice is probably due to the reduced production of IL-12 by macrophages and dendritic cells rather than a defect in TCR signaling, since these T cells recovered the ability to produce IFN-γ at later stages of infection at levels similar to those seen for IRAK-4+/− animals.
Macrophages and DCs are key elements in innate immune responses, and recognition of Brucella components results in the production of proinflammatory cytokines by these cells (22). Herein, we investigated the involvement of IRAK-4 in Brucella-induced IL-12 and TNF-α production by macrophages and DCs. Macrophages and DCs deficient in IRAK-4 showed a blockage in the production of TNF-α and IL-12 not only when they were stimulated with live Brucella and HKBa but also when they were stimulated by TLR2 and TLR9 agonists. Additionally, our results revealed that Brucella L-Omp19 lipoprotein and Brucella DNA are important bacterial PAMPs responsible for TLR activation leading to IRAK-4 signaling. This demonstrates that IRAK-4 is a critical component involved in the production of these cytokines as result of Brucella recognition by innate immune cells and corroborates the idea of the importance of this kinase in the TLR signaling pathway. Suzuki et al. (33) also found that macrophages deficient in IRAK-4 were unable to produce inflammatory mediators in response to treatment with TLR2 agonist (peptidoglycan). Interestingly, when LPS (TLR4 agonist) was used as the stimulus, we observed partial production of IL-12 and TNF-α by DCs and TNF-α by macrophages. This observation is due to the fact that TLR4 uses an alternative signaling pathway for cytokine production that is TRIF dependent and MyD88 independent (9).
TLRs signal through IRAK-4 to mediate many cellular responses, including proinflammatory cytokine production (33). To investigate whether Brucella requires IRAK-4 to mediate intracellular signaling, we examined the phosphorylation of MAPKs (ERK1, ERK2, and p38) and the p65 subunit of NF-κB. Herein, we observed that Brucella-mediated activation of MAPK and NF-κB pathways in macrophages is IRAK-4 and MyD88 dependent. Thus, the lack of Brucella-induced signaling in macrophages may account for the low cytokine production observed in IRAK-4−/− mouse macrophages. In contrast, LPS-induced MAPK and NF-κB activation seems to be only partially regulated by IRAK-4 and MyD88 molecules (9).
Collectively, we have demonstrated that IRAK-4 is important in in vivo control of Brucella at early stages of infection and that the lack of this molecule impaired IFN-γ production by CD4+ and CD8+ T cells. Furthermore, IRAK-4 deficiency resulted in no activation of MAPK and NF-κB signaling pathways affecting IL-12 and TNF-α production by macrophages and dendritic cells. However, the mechanisms by which IRAK-4−/− mice reestablish a normal IFN-γ response and control later phases of Brucella infection remain to be explored.
ACKNOWLEDGMENTS
This work was supported by grants from CNPq, CNPq/ANPCyT (490528/2008-2), FAPEMIG, CAPES/PNPD, CNPq/MAPA, CNPq/REPENSA, INCT-Vacinas, CNPq/CONICET, and FAPEMIG (PRONEX).
Footnotes
Published ahead of print on 15 August 2011.
REFERENCES
- 1. Akira S. 2006. TLR signaling. Curr. Top. Microbiol. Immunol. 311:1–16 [DOI] [PubMed] [Google Scholar]
- 2. Akira S., Uematsu S., Takeuchi O. 2006. Pathogens recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- 3. Archambaud C., et al. 2010. Contrasting roles of macrophages and dendritic cells in controlling initial pulmonary Brucella infection. Eur. J. Immunol. 40:3458–3471 [DOI] [PubMed] [Google Scholar]
- 4. Campos M. A., et al. 2004. Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect. Immun. 72:176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao Z., Henzel W. J., Gao X. 1996. IRAK: a kinase associated with the interleukin-1 receptor. Science 17:1128–1131 [DOI] [PubMed] [Google Scholar]
- 6. Carvalho N. B., et al. 2011. Toll-like receptor 9 is required for full host resistance to Mycobacterium avium infection but plays no role in induction of Th1 responses. Infect. Immun. 79:1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Copin R., De Baetselier P., Carlier Y., Letesson J. J., Muraille E. 2007. MyD88-dependent activation of B220-CD11b+LY-6C+ dendritic cells during Brucella melitensis infection. J. Immunol. 178:5182–5191 [DOI] [PubMed] [Google Scholar]
- 8. Corbel M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cusson-Hermance N., Khurana S., Lee T. H., Fitzgerald K. A., Kelliher M. A. 2005. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J. Biol. Chem. 280:36560–36566 [DOI] [PubMed] [Google Scholar]
- 10. Detilleux P. G., Deyoe B. L., Cheville N. F. 1990. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 58:2320–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giambartolomei G. H., et al. 2004. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 173:4635–4642 [DOI] [PubMed] [Google Scholar]
- 12. Golding B., et al. 2001. Immunity and protection against Brucella abortus. Microbes Infect. 3:43–48 [DOI] [PubMed] [Google Scholar]
- 13. Huang L. Y., et al. 2003. Heat-killed Brucella abortus induces TNF and IL-12p40 by distinct MyD88-dependent pathways: TNF, unlike IL-12p40 secretion, is Toll-like receptor 2 dependent. J. Immunol. 171:1441–1446 [DOI] [PubMed] [Google Scholar]
- 14. Janssens S., Beyaert R. 2003. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 11:293–302 [DOI] [PubMed] [Google Scholar]
- 15. Jones S. M., Winter A. J. 1992. Survival of virulent and attenuated strains of Brucella abortus in normal and gamma interferon-activated murine peritoneal macrophages. Infect. Immun. 60:3011–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanakaraj P., et al. 1999. Defective interleukin (IL)-18-mediated natural killer and T helper cell type 1 responses in IL-1 receptor-associated kinase (IRAK)-deficient mice. J. Exp. Med. 189:1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawagoe T., et al. 2007. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J. Exp. Med. 204:1013–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ku C. L., et al. 2007. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J. Exp. Med. 204:2407–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li S., Strelow A., Fontana E. J., Wesche H. 2002. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. U. S. A. 99:5567–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lye E., Mirtsos C., Suzuki N., Suzuki S., Yeh W. C. 2004. The role of interleukin 1 receptor-associated kinase-4 (IRAK-4) kinase activity in IRAK-4-mediated signaling. J. Biol. Chem. 279:40653–40658 [DOI] [PubMed] [Google Scholar]
- 21. Lye E., Dhanji S., Calzascia T., Elford A. R., Ohashi P. S. 2008. IRAK-4 kinase activity is required for IRAK-4-dependent innate and adaptive immune responses. Eur. J. Immunol. 38:870–876 [DOI] [PubMed] [Google Scholar]
- 22. Macedo G. C., et al. 2008. Central role of MyD88-dependent dendritic cell maturation and proinflamatory cytokine production to control Brucella abortus infection. J. Immunol. 180:1080–1087 [DOI] [PubMed] [Google Scholar]
- 23. Medvedev A. E., et al. 2003. Distinct mutations in IRAK-4 confer hyporesponsiveness to lipopolysaccharide and interleukin-1 in a patient with recurrent bacterial infections. J. Exp. Med. 198:521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murphy E. A., Sathiyaseelan J., Parent M. A., Zou B., Baldwin C. L. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muzio M., Ni J., Feng P., Dixit V. M. 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278:1612–1615 [DOI] [PubMed] [Google Scholar]
- 26. Oliveira S. C., et al. 1998. The role of T cell subsets and cytokines in the regulation of intracellular bacterial infection. Braz. J. Med. Biol. Res. 31:77–84 [DOI] [PubMed] [Google Scholar]
- 27. Oliveira S. C., Splitter G. A. 1995. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur. J. Immunol. 25:2551–2557 [DOI] [PubMed] [Google Scholar]
- 28. Oliveira S. C., de Oliveira F. S., Macedo G. C., de Almeida L. A., Carvalho N. B. 2008. The role of innate immune receptors in the control of Brucella abortus infection: toll-like receptors and beyond. Microbes Infect. 10:1005–1009 [DOI] [PubMed] [Google Scholar]
- 29. O'Neill L. A. J., Bowie A. G. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat. Rev. Immunol. 7:353–364 [DOI] [PubMed] [Google Scholar]
- 30. Picard C., et al. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299:2076–2079 [DOI] [PubMed] [Google Scholar]
- 31. Roux C. M., et al. 2007. Brucella requires a functional type IV secretion system to elicit innate immune responses in mice. Cell. Microbiol. 9:1851–1869 [DOI] [PubMed] [Google Scholar]
- 32. Sathiyaseelan J., et al. 2006. Treatment of Brucella-susceptible mice with IL-12 increases primary and secondary immunity. Cell. Immunol. 243:1–9 [DOI] [PubMed] [Google Scholar]
- 33. Suzuki N., et al. 2002. Severe impairment of interleukin-1 and Toll-like receptor signaling in mice lacking IRAK-4. Nature 416:750–754 [DOI] [PubMed] [Google Scholar]
- 34. Suzuki N., et al. 2003. IL-1 receptor-associated kinase 4 is essential for IL-18-mediated NK and Th1 cell responses. J. Immunol. 170:4031–4035 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki N., et al. 2006. A critical role for the innate immune signaling molecule IRAK-4 in T cell activation. Science 311:1927–1932 [DOI] [PubMed] [Google Scholar]
- 36. Takeda K., Akira S. 2004. TLR signaling pathways. Semin. Immunol. 16:3–9 [DOI] [PubMed] [Google Scholar]
- 37. Thome M., Tschopp J. 2003. TCR-induced NF-kappaB activation: a crucial role for Carma1, Bcl10 and MALT1. Trends Immunol. 24:419–424 [DOI] [PubMed] [Google Scholar]
- 38. Trant C. G., et al. 2010. The Brucella abortus phosphoglycerate kinase mutant is highly attenuated and induces protection superior to that of vaccine strain 19 in immunocompromised and immunocompetent mice. Infect. Immun. 78:2283–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weiss D. S., Takeda K., Akira S., Zychlinsky A., Moreno E. 2005. MyD88, but not Toll-like receptors 4 and 2, is required for efficient clearance of Brucella abortus. Infect. Immun. 73:5137–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wesche H., et al. 1999. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J. Biol. Chem. 274:19403–19410 [DOI] [PubMed] [Google Scholar]
- 41. Zhan Y., Cheers C. 1993. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 61:4899–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]