Abstract
The limited efficacy of monocyte-derived dendritic cell (mo-DC)-based vaccines is primarily attributed to the reduced mo-DC migratory capacity. One undefined aspect is the initial binding of mo-DCs to endothelial cells and vascular selectins. In this study, we investigated the role and modulation of the selectin binding determinant sialyl Lewisx (sLex) in selectin-dependent mo-DC binding. Our data reveal that sLex is required for maximal binding of mo-DCs to tumor necrosis factor (TNF)-α-activated endothelial cells under static conditions, as evidenced by the use of sialidase. Sialidase treatment also abrogated mo-DC cell tethering to immobilized, purified P-, L- or E-selectin under flow. The requirement of sLex-dependent binding of mo-DC to selectins was further substantiated by using sLex free sugar and anti-sLex antibody, which significantly suppressed mo-DC-selectin binding. P-selectin glycoprotein ligand-1 is required for mo-DC binding to both P- and L-selectin, but it is dispensable for E-selectin recognition. Interestingly, the extent of mo-DC tethering was maximal on P-selectin, followed by E- and L- selectin. Accordingly, L-selectin mediated faster mo-DC rolling than E- or P-selectin. Interferon (IFN)-γ induces a significant increase in mo-DC surface sLex expression, which is probably due to the enhanced synthesis of C2GnT-I. These findings may contribute to improving mo-DC-based vaccination protocols.
Keywords: monocyte-derived dendritic cells, selectin, adhesion, shear flow, IFN-γ
INTRODUCTION
Dendritic cells (DCs) play a pivotal role in triggering and regulating immune responses due to their ability to uptake, process and present antigens to T lymphocytes [1]. DCs have been exploited in adoptive immune therapy to treat human malignancies and infectious diseases [2]. The most common approach involves the use of DCs derived in vitro from monocytes (mo-DCs), which after being loaded with tumour antigens, grant protective and therapeutic actions in cancer patients. Pro-inflammatory cytokines are often used to induce mo-DC maturation and promote efficient mo-DC interaction with T lymphocytes. However, the basic biology of mo-DCs remains uncovered, and vaccines based on mo-DCs still display a limited efficacy. One major bottleneck is the reduced migratory capacity of mo-DCs relative to the highly mobile natural DCs [3]. The clinical efficacy of these mo-DC-based vaccines lies in the accomplishment of two important steps: i) exit from the bloodstream to the tissue space and ii) subsequent homing to the draining lymph nodes, where antigen-specific T-cell stimulation takes place. Although the tethering of mo-DCs to the endothelial cell surface can regulate the extent of mo-DC homing, this step has not been carefully investigated so far.
It is well established that selectins mediate the initial tethering and rolling of leukocytes along the endothelial cell surface, followed by a cascade of molecular events which culminates in the leukocyte extravasation and migration into inflamed tissue [4]. Selectins (E-, P- and L-selectin) recognize the tetrasaccharide sialyl Lewisx (sLex; NeuAc α2,3 Gal β1,4 [Fuc α1,3] GlcNAc-R; Fig. 1A), a terminal component of glycans attached to glycoproteins and glycolipids on most circulating immune cells and some endothelial cells. sLex biosynthesis requires the sequential action of different glycosyltransferases (Fig. 1A) [5]. In the case of the frequent sLex in core 2 O-glycans, the branch is initiated by core 2 β 1,6-N-acetylglucosaminyltransferase I (C2GnT-I), followed by the alternate action of β 1,4-galactosyltransferase I (β 4GalT-I) and β 1,3-N-acetylglucosaminyltransferase (β3GlcNAcT), forming polylactosamine chains. Elongation of core 2 branches is terminated by the addition of sialic acid by α 1,3-sialyltransferases (ST3Gal) followed by the addition of fucoses by α 1,3-fucosyltransferases (FucT) [6]. The role of sLex as selectin ligand, whilst extensively studied in leukocytes [5], has not been fully examined in mo-DCs. Regarding other subtypes, peripheral blood DCs express sLex and adhere to activated endothelial cells, under static conditions, via a selectin-dependent mechanism [7]. CD34+-derived DCs bear an epitope similar to sLex, the cutaneous lymphocyte associated antigen (CLA), almost exclusively on P-selectin glycoprotein ligand-1 (PSGL-1) and bind efficiently to immobilized E- and P-selectin under both static and flow conditions [8]. More recently, Julien et al. [9] reported that immature mo-DCs express sLex on PSGL-1 but this determinant is lost upon mo-DCs maturation induced by tumor necrosis factor-alpha (TNF-α) in conjunction with prostaglandin (PG)E2. Nevertheless, it remains to be determined whether sLex expression is functionally relevant to mo-DCs adhesion to selectins.
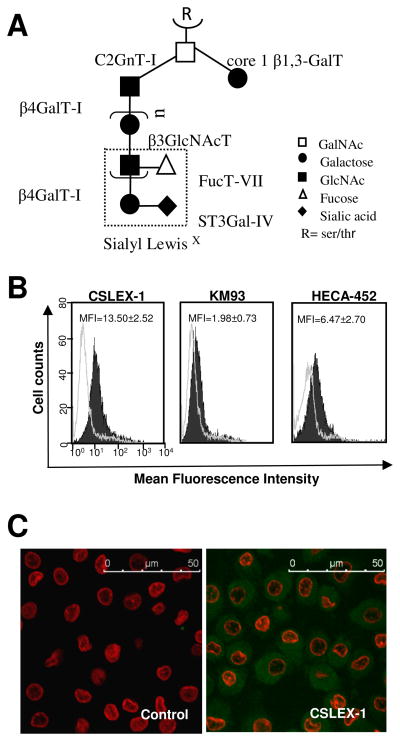
Fig. 1.
sLex expression in mo-DCs. A: simplified biosynthetic pathway of sLex core 2 decorated O-glycans, with the involved glycosyltransferases represented. B: Flow cytometry histograms of sLex expression in mo-DCs from a representative donor. Mo-DCs were stained with CSLEX-1, KM93 or HECA-452 (filled histograms) or with secondary antibody only (open histograms). The mean fluorescence intensity (MFI) ± SE of sLex labeling with CSLEX-1 (n=9 independent experiments), KM93 (n=3) or HECA-452 (n=3) antibodies is shown. C: Confocal laser-scanning microscopy of mo-DCs stained with secondary antibody only (control) or CSLEX-1 primary antibody.
In the present study, we have demonstrated the functional role of sLex surface expression by mo-DCs in selectin-dependent binding under flow. We further investigated the ability of different maturation stimuli to modulate sLex expression, and showed that interferon-gamma (IFN-γ) induces a significant increase in the sLex surface expression, which is likely regulated by increased synthesis of C2GnT-I.
Materials and Methods
Reagents
Fluorescently-conjugated or unlabeled human anti-CD14 (M5E2), anti-CD83 (HB15e), anti-CD31 (L133.1), anti-CD45 (HI30), anti-sLex (CSLEX-1), anti-E-selectin (68-5H11), anti-P-selectin (AK4) and anti-PSGL-1 (KPL1) monoclonal antibodies (mAb), and rat anti-sLex IgM (HECA-452) mAb were purchased from BD Biosciences (San Jose, CA). The mouse anti-sLex IgM (KM93) mAb was from Calbiochem (La Jolla, CA). Blocking anti-E-selectin (CL2/6) mAb was purchased from AbDSerotec (Oxford, UK). Anti-HLA-DR (L243) was from Immunostep (Salamanca, Spain) and the anti-BDCA-1 (AD5-8E7) from Miltenyi Biotec (Bergisch Gladbach, Germany). The anti-CCR7 (150503) mAb, the P- and E-selectin-IgG Fc chimeras (consisting of the extracellular domain of the human P-selectin and E-selectin, respectively, linked to the human IgG-Fc) and the human cytokines TNF-α, IFN-γ, interleukin (IL)-6, IL-1β, IL-4 and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) were purchased from R&D Systems (Minneapolis, MN). All other reagents were from Sigma (St. Louis, MO, USA) unless otherwise stated.
Cell isolation and culture
Monocytes were isolated from peripheral blood mononuclear cells by positive selection using anti-CD14 coated magnetic beads (Miltenyi Biotech) and cultured with IL-4 and GM-CSF [10]. After 5 days, mo-DC maturation was induced by lipopolysaccharide (LPS) (1 μg/mL), IL-1β (1000 U/mL), IFN-γ (1000 U/mL), TNF-α (1000 U/mL), IL-6 (10 ng/mL) or PGE2 (10 μg/mL) or IL-1β, IL-6, PGE2 and TNF-α mixture (IIPT) and on day 7 cells were collected. Differentiation and maturation of mo-DCs was confirmed by antibody staining and flow cytometry analysis. Human umbilical vein endothelial cells (HUVECs) were isolated by collagenase treatment as described in [11, 12].
Flow cytometry
Monocyte purity, mo-DC differentiation and maturation status were monitored by staining cells with fluorescently-conjugated anti-CD14, BDCA-1, HLA-DR, CCR7 and CD83 mAbs. sLex expression on mo-DCs was evaluated by indirect immunofluorescence using CSLEX-1, KM93 or HECA-452 as primary mAbs.
Confocal laser scanning microscopy
Mo-DCs were allowed to adhere to concanavalin-A coated cover glasses, fixed with 3.7% of paraformaldehyde and permeabilized with 0.1% TritonX. After blocking with 1% BSA, cells were stained using anti-sLex mAb CSLEX-1, followed by a biotinylated anti-mouse IgM antibody and PE-labelled streptavidin. The cell nuclei were stained with 1 μM TO-PRO-3 dye (Molecular Probes, Leiden, Netherlands). Images were acquired with a Leica TCS SP2 AOBS confocal microscope (Leica Microsystem, Mannheim, GmbH).
Isolation of RNA and Real-Time RT-PCR
Expression of glycosyltransferase genes was analyzed by real-time PCR [13–15] using TaqmanFast Universal PCR Master Mix, primers and Taqman probes provided by Applied Biosystems.
Adhesion of mo-DCs to HUVEC monolayers
HUVECs were cultured to confluency in 48-well flat bottom plates, and stimulated with TNF-α (10 ng/mL) for 4h at 37°C to obtain maximal levels of E-selectin expression [16]. Mo-DCs were overlaid, in duplicate wells, onto HUVEC monolayers at 5:1 (mo-DC:HUVEC) ratio and allowed to adhere for 30 min at 37°C. HUVEC and adherent mo-DCs were detached from the plate with 0.05% trypsin-EDTA solution. Assessment to the expression of specific cell markers by flow cytometry analysis allowed the characterization of adherent mo-DC cells (HLA-DR+) and HUVEC (CD31+) in the mixture.
Flow-based adhesion assay
Octadecyltrichlorosilane treated glass slides were first incubated with anti-human IgG Fc and then with the appropriate P-, L- or E-selectin IgG Fc chimera, and blocked with 1% BSA, as described in [19]. Mo-DCs (1×106 cells/mL) suspended in D-PBS buffer containing Ca2+/Mg2+ and 0.1% BSA were perfused over immobilized P-, L- or E-selectin-coated slides [20] at 1 dyn/cm2 using a microfluidic channel affixed to the selectin-coated slides. In select experiments, the selectin-coated slides were incubated with 20 μg/mL of a function-blocking anti-P- or E-selectin mAb or with 1 mM of sLex sugar. In other experiments, mo-DCs were incubated with 40 μg/mL of an anti-sLex antibody (CSLEX-1) or treated with sialidase [17, 18], before perfusion over selectin-coated slides. The extent of adhesion was quantified by enumerating the total number of tethering events in a single ×10 field of view during a 3 min period. Average rolling velocities were computed as the distance traveled by the centroid of the translating cell divided by the time interval at the given wall shear stress.
Results and Discussion
sLex is expressed by human mo-DCs
In view of the critical involvement of sLex-decorated glycoproteins and glycolipids [21] in the initial tethering and rolling of immune cells along the endothelial cell surface, we investigated the expression of sLex on the surface of mo-DCs by flow cytometry. Fig. 1B reveals that mo-DCs from all donors display moderate sLex expression, as assessed by the use of three different anti-sLex mAbs: CSLEX-1, KM93 and HECA-452. Despite the minor donor variability, the staining intensity detected using the CSLEX-1 mAb was consistently higher than that of the KM93 and HECA-452 antibodies. The different intensities of surface immunostaining observed with the three distinct mAbs is attributed to intrinsic differences in the antibody specificities with respect to the extent of polylactosamine chain as reported by others [9]. The flow cytometry results were confirmed by confocal-laser scanning microscopy visualization of CSLEX-1 staining, which showed that sLex is distributed uniformly throughout each mo-DC surface and cytoplasm (Fig. 1C).
The adhesion of mo-DCs to activated endothelium under static conditions requires sialylated structures
The observed sLex surface expression by mo-DCs prompted us to assess their ability to adhere to cytokine-activated endothelial cells under static conditions, and the potential contribution of sLex:selectin interaction to this process. Because treatment of HUVECs with TNF-α induces maximal E-selectin expression within 4h of stimulation, static adhesion assays were performed by incubating mo-DCs with TNF-α-activated (4h) HUVECs. After 30 min of co-incubation, 43.8±5.9% of mo-DCs remained adherent to the TNF-α-treated endothelial cells (Table 1). In contrast, only 25.5±5.8% of mo-DCs adhered to untreated (No TNF-α) HUVECs. To evaluate the potential involvement of sialylated structures in mo-DC binding to TNF-α-activated endothelium, mo-DCs were treated with sialidase (0.2 U/mL), which cleaves terminal sialic acid residues from the cell surface, prior to their incubation with HUVECs. This intervention resulted in a significant decrease in the extent of mo-DCs (33.3±8.4%) adherent to activated endothelium (Table 1). However, addition of the anti-sLex blocking mAb, CSLEX-1, or soluble sLex sugar to the medium did not alter the extent of mo-DC adhesion to HUVECs relative to appropriate controls (Table 1). Interestingly, similar observations were previously reported using blood DCs [7]. Use of an anti-E-selectin mAb failed to inhibit the extent of adhesion under static conditions (Table 1). The lack of an inhibitory effect by the anti-E-selectin mAb may be attributed to the engagement of other adhesion molecules in this process such as β2-integrin binding to endothelial ICAM-1.
Table 1.
Contribution of sLex to mo-DC interaction with HUVECs under static conditions
| Assay condition | % of Interacting Cells | P Value |
|---|---|---|
| control | 43.8±5.9 | — |
| sialidase treatment | 33.3±8.4 | 0.020 |
| anti-sLex | 42.0±3.0 | 0.250 |
| sugar sLex | 37.7±9.9 | 0.395 |
| anti-E-selectin | 37.0±9.1 | 0.228 |
| No Ca2+/Mg2+ | 11.5±0.5 | 0.005 |
| No TNF-α | 25.5±5.8 | 0.020 |
Mo-DCs were overlaid on TNF-α-stimulated HUVECs and incubated for 30 min at 37°C. Values represent the percentage of interacting mo-DCs with HUVECs determined for the following conditions: no treatment (control), sialidase treatment of mo-DCs [17, 18], pre-incubation of mo-DCs with an anti-sLex antibody or in the presence of sLex sugar. In select experiments, HUVECs were pre-incubated with an anti-E-selectin antibody prior to the addition of mo-DCs. Other control experiments were performed using PBS lacking Ca2+/Mg2+ or non-TNF-α-stimulated HUVECs. Data represent the mean ± SE of n=2–5 experiments.
Mo-DCs tether and roll on purified P- E- and L-selectin under flow conditions
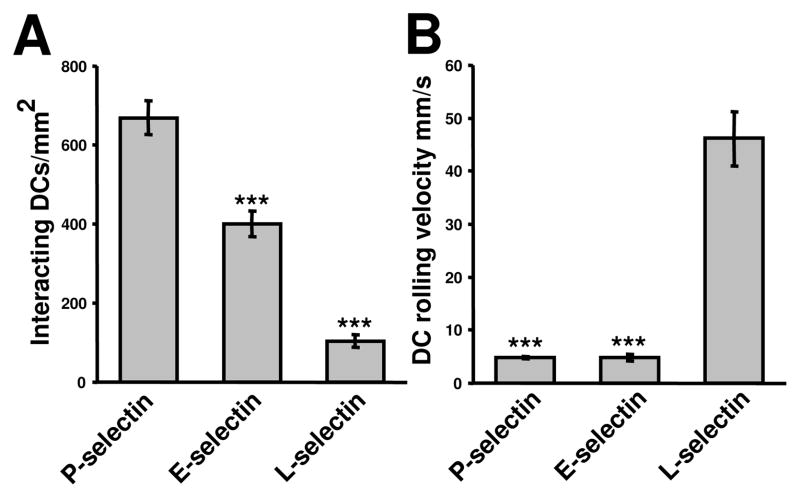
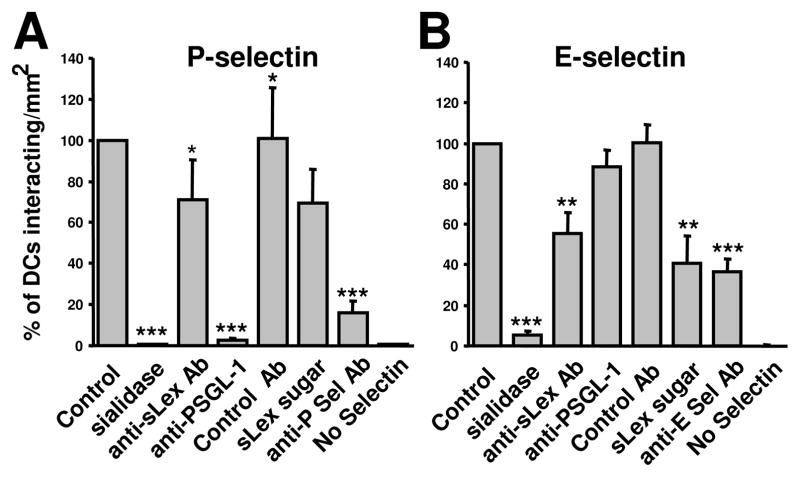
To eliminate the potential contribution of other adhesion molecules, such as ICAM-1, flow-based adhesion experiments were performed by perfusing mo-DCs over purified selectins under controlled kinematic conditions. As shown in Fig. 2, mo-DCs tethered and rolled on P, E- or L-selectin-coated surfaces at a wall shear stress of 1 dyn/cm2. The extent of mo-DC tethering was maximal on purified P-selectin-, intermediate on E-selectin-, and low on L-selectin-coated surfaces (Fig. 2A). In agreement with previous work using neutrophils [22], L-selectin mediated faster mo-DC rolling than E- or P-selectin (Fig. 2B). Treatment of mo-DCs with sialidase nearly abrogated their binding to P-selectin (Fig. 3A), E-selectin (Fig. 3B) and L-selectin (data not shown) under flow. We next evaluated the potential effects the anti-sLex blocking antibody, CSLEX-1 (40 μg/mL), and the sugar sLex (1 mM) itself on mo-DC binding to purified selectins. Interestingly, addition of either the anti-sLex antibody or the sugar to the perfusion medium was equally effective in decreasing the extent of mo-DC tethering to P-selectin by ~30% relative to untreated control cells or cells perfused in the presence of an isotype control antibody (Fig. 3A). The inhibitory effects of these agents were more pronounced on E-selectin-dependent binding under flow, as evidenced by a >50% reduction in the extent of mo-DC binding relative to controls (Fig. 3B). Use of an anti-P-selectin or anti-E-selectin mAb reduced mo-DC binding to the respective selectins by •85% and 65%, respectively (Fig. 3). The specificity of mo-DC tethering to selectins was demonstrated by the lack of any cell binding on bare slides (Fig. 3). Taken together, these data illustrate the role of sLex-decorated glycoconjugates in mo-DC binding to selectins. Although P-, E- and L-selectins share the ability to recognize the tetrasaccharide sLex determinant, the binding affinity of selectins for isolated monovalent sLex is very low [23]. PSGL-1 has fucose- and sialic acid-containing polylactosamine side chains, many of which terminate in sLex [24]. Although PSGL-1 is recognized by all three selectins [20], it represents the major counter-receptor only for P- and L-selectin in human leukocytes [21, 25]. In agreement with these previous observations, a function-blocking anti-PSGL-1 mAb completely abolished the binding of mo-DC to P-selectin (Fig. 3A) under flow, and reduced binding to L-selectin by ~50% (data not shown). In agreement with previous work using neutrophils [21], PSGL-1 appears to be dispensable in the tethering of mo-DCs to E-selectin under flow (Fig. 3B).
Fig. 2.
Adhesion of mo-DCs to immobilized selectins under flow. Mo-DCs were perfused over P-, E- or L- selectin immobilized surface at 1 dyn/cm2 in a microfluidic device. A: The number of interacting mo-DCs per mm2 was quantified using videomicroscopy after 3 min of cell perfusion. The data represent the mean ± SE of at least three independent experiments. B: The average mo-DC rolling velocity was calculated by videomicroscopy/digital image processing using at least 20 cells from 3 independent experiments. (***P<0.001).
Fig. 3.
Contribution of sLex to mo-DC:selectin interactions under flow. Mo-DCs were perfused over immobilized P-selectin (A) or E- selectin (B) for 3 min at 1 dyn/cm2. In select experiments, mo-DCs were treated with sialidase, or incubated with an anti-sLex or an anti-PSGL-1 or an isotype control antibody (Control) before perfusion over immobilized selectins. In other experiments, the P- or E-selectin-coated slides were incubated with an anti-P-selectin or an anti-E-selectin antibody or with the sLex sugar. Control experiments without immobilized selectins were carried out to test any non-specific binding. The number of interacting mo-DCs per mm2 with P- or E-selectin-coated surfaces was quantified for each experimental condition. Data are reported as percentage of interacting cells relative to untreated mo-DCs (control). Data represents the mean ± SE of at least three independent experiments (** P<0.01, ***P<0.001).
sLex expression on mo-DCs can be modulated by maturation stimuli
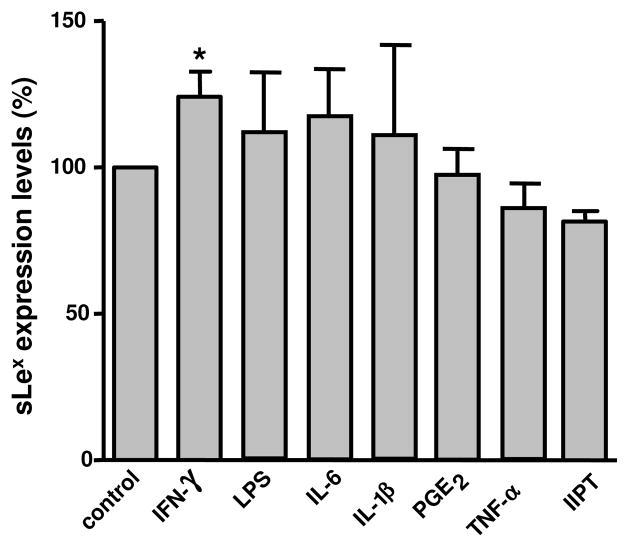
The induction of maturation is a standard protocol used in the development of mo-DC vaccines to improve their effectiveness in cancer therapy [26]. We previously reported that maturation induces significant glycosylation changes in mo-DCs [10, 27]. We therefore reasoned that sLex expression may be altered upon mo-DC maturation induced by stimuli used in the establishment of mo-DC-based vaccines [26]. To this end, we analyzed the sLex surface expression in both immature and mature mo-DCs by flow cytometry using the CSLEX-1 antibody. As shown in Fig. 4, IFN-γ significantly increased the sLex surface expression in 11 of 15 donors examined in this work. These data are consistent with previous observations showing that intradermal injection of IFN-γ leads to increased expression of sLex in a specialized type of DCs, the Langerhans cells [28]. Maturation stimuli such as LPS, IL-6, IL-1β, PGE2 and TNF-α, or a combination of IL-6, IL-1β, PGE2 and TNF-α, used in some vaccine protocols [26], failed to increase sLex expression. We confirmed the induction of maturation for all the aforementioned stimuli through the upregulation of the maturation markers HLA-DR, CD83 and CCR7 (data not shown).
Fig. 4.
Effect of maturation stimuli on sLex expression. The maturation of mo-DCs was achieved by treating mo-DCs with either IFN-γ, LPS, IL-6, IL-1β, PGE2, TNF-α alone or a cocktail mixture of IL-6, IL-1β, PGE2 plus TNF-α (IIPT). The expression of sLex was analyzed by flow cytometry using an anti-sLex antibody (CSLEX-1). The sLex expression levels are reported as percentage of CSLEX-1 staining relative to that in immature untreated mo-DCs (control). Data represent the mean ± SE of at least three independent experiments (*P<0.05).
We next aimed to determine whether the increase of sLex expression by IFN-γ was due to transcriptional changes of critical enzymes involved in sLex biosynthesis. In view of the fact that selectin ligands, such as PSGL-1, terminate in sLex on core 2 O-linked glycan chains, we investigated the following enzymes: C2GnT-I and β 4GalT-I, involved in core 2 elongation; and α 1,3-sialyltransferases, ST3Gal-IV, -VI or -III, as well as the α 1,3-fucosyltransferases, FucT-III, -IV, -VI or -VII (Fig. 1) [6, 29]. Table 2 reveals that IFN-γ stimulation induces a marked upregulation of C2GnT-I, whereas modest and non-significant increases are noted in the expression levels of and β 4GalT-I and FucT-VII. While FucT-III and -VI are not expressed (data not shown), treatment of mo-DCs with IFN-γ reduced ST3Gal-III expression. Taken together, these data suggest that IFN-γ acts at the transcriptional level, upregulating the expression of C2GnT-I glycosyltransferase, which leads to the synthesis of core 2 decorated O-glycans carrying sLex. Interestingly, others have reported that LPS-induced maturation causes a great decrease of sLex in mo-DCs, which was associated with a decrease in the C2GnT-I expression [9].
Table 2.
Effect of IFN-γ on the gene expression of glycosyltransferases involved in the sLex biosynthesis in mo-DCs
| Glycosyltransferases | mRNA expression level
|
P Value | |
|---|---|---|---|
| Non-stimulated | IFN-γ stimulated | ||
| ST3Gal-III | 0.38±0.04 | 0.15±0.03 | 0.005 |
| ST3Gal-IV | 0.14±0.04 | 0.098±0.02 | 0.351 |
| ST3Gal-VI | 3.17±0.40 | 2.89±0.39 | 0.647 |
| FucT-IV | 1.60±0.42 | 1.68±0.31 | 0.875 |
| FucT-VII | 1.92±0.68 | 2.24±0.45 | 0.699 |
| 34GalT-I | 34.38±14.99 | 37.62±8.24 | 0.859 |
| C2GnT-I | 5.55±2.49 | 10.45±2.76 | 0.035 |
The expression of relevant glycosyltransferases involved in the biosynthetic pathway of sLex core 2 decorated O-glycans (Fig. 1A) was evaluated by quantitative real-time PCR in total RNA extracted from 1×106 cells. The mRNA expression levels of the glycosyltransferases in non-stimulated and IFN-γ stimulated mo-DCs are expressed as the permillage (‰) of the expression of the endogenous controls (β-actin/GAPDH mean). Data represent the mean ± SE of at least three independent assays.
While sLex:selectin interactions have been comprehensively described as essential for the initial tethering and rolling steps of different leukocyte subpopulations to the endothelial lining, this has not been demonstrated for mo-DCs. Some aspects of the relevance of sLex:selectin interactions have been previously reported for other DC subsets [8]. While all DCs share certain features, their subtypes actually represent a variety of cell types with different phenotypic traits and effector functions [1]. To the best of our knowledge, this is the first thorough in vitro study demonstrating that human mo-DCs effectively bind to P-, E- and L-selectins via a sLex-dependent pathway.
Our finding that sLex expression can be modulated upon IFN-γ-mediated maturation may have important implications in the clinical setting. IFN-γ has been more recently introduced in mo-DC vaccines protocols to treat cancer due to its potent T helper 1 (Th1) polarization and pro-inflammatory activity [30]. IFN-γ stimulates multiple mo-DC effector functions, acting as an inducer and a regulator of inflammation that can be helpful in mo-DC-based vaccination [31]. Our data suggest a new role for IFN-γ as a regulator of sLex biosynthesis with an expected impact on selectin-dependent binding and eventual extravasation of mo-DCs into tissues.
Highlights.
The role of the selectin binding determinant sLex was studied in mo-DCs.
sLex is required for maximal mo-DC binding to endothelial cells.
Mo-DCs bind to selectins via a sLex-dependent pathway.
PSGL-1 is indispensable for P- and L-, but not E-selectin recognition.
IFN-γ increases sLex expression in mo-DCs, probably by enhancing C2GnT-I synthesis.
Acknowledgments
This study was supported in part by the Fundação para a Ciência e Tecnologia, Portugal–PTDC/SAU-MII/67561/2006 and SFRH/BPD/41168/2007 (ZS) and the NIH/NCI R01 CA101135 and U54 CA143868 (KK). We thank Sérgio Dias (CEDOC/FCM, IPO, Lisbon) for kind gift of HUVEC and Dário Ligeiro (CHSUL, Lisbon) for the microscopy image acquisition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 2.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: Mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 3.Ridolfi R, Riccobon A, Galassi R, Giorgetti G, Petrini M, Fiammenghi L, Stefanelli M, Ridolfi L, Moretti A, Migliori G, Fiorentini G. Evaluation of in vivo labelled dendritic cell migration in cancer patients. J Transl Med. 2004;2:27. doi: 10.1186/1479-5876-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEver RP. Selectins: Lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 5.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho AS, Harduin-Lepers A, Magalhaes A, Machado E, Mendes N, Costa LT, Matthiesen R, Almeida R, Costa J, Reis CA. Differential expression of alpha-2,3-sialyltransferases and alpha-1,3/4-fucosyltransferases regulates the levels of sialyl lewis a and sialyl lewis x in gastrointestinal carcinoma cells. Int J Biochem Cell Biol. 2010;42:80–89. doi: 10.1016/j.biocel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas U, Larsson M, Lundblad A, Forsum U. E-selectin involvement in in vitro adhesion of blood dendritic cells to human umbilical cord endothelial cells. Scand J Immunol. 1993;38:273–278. doi: 10.1111/j.1365-3083.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 8.Kieffer JD, Fuhlbrigge RC, Armerding D, Robert C, Ferenczi K, Camphausen RT, Kupper TS. Neutrophils, monocytes, and dendritic cells express the same specialized form of PSGL-1 as do skin-homing memory T cells: Cutaneous lymphocyte antigen. Biochem Biophys Res Commun. 2001;285:577–587. doi: 10.1006/bbrc.2001.5230. [DOI] [PubMed] [Google Scholar]
- 9.Julien S, Grimshaw MJ, Sutton-Smith M, Coleman J, Morris HR, Dell A, Taylor-Papadimitriou J, Burchell JM. Sialyl-lewis(x) on P-selectin glycoprotein ligand-1 is regulated during differentiation and maturation of dendritic cells: A mechanism involving the glycosyltransferases C2GnT1 and ST3Gal I. J Immunol. 2007;179:5701–5710. doi: 10.4049/jimmunol.179.9.5701. [DOI] [PubMed] [Google Scholar]
- 10.Videira PA, Amado IF, Crespo HJ, Alguero MC, Dall’Olio F, Cabral MG, Trindade H. Surface alpha 2–3- and alpha 2–6-sialylation of human monocytes and derived dendritic cells and its influence on endocytosis. Glycoconj J. 2008;25:259–268. doi: 10.1007/s10719-007-9092-6. [DOI] [PubMed] [Google Scholar]
- 11.Burdick MM, McCaffery JM, Kim YS, Bochner BS, Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am J Physiol Cell Physiol. 2003;284:C977–87. doi: 10.1152/ajpcell.00423.2002. [DOI] [PubMed] [Google Scholar]
- 12.Burdick M, Konstantopoulos K. Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am J Physiol Cell Physiol. 2004;287:C539–47. doi: 10.1152/ajpcell.00450.2003. [DOI] [PubMed] [Google Scholar]
- 13.Zhu F, Wang P, Kontrogianni-Konstantopoulos A, Konstantopoulos K. Prostaglandin (PG)D(2) and 15-deoxy-delta(12,14)-PGJ(2), but not PGE(2), mediate shear-induced chondrocyte apoptosis via protein kinase A-dependent regulation of polo-like kinases. Cell Death Differ. 2010;17:1325–1334. doi: 10.1038/cdd.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu F, Wang P, Lee NH, Goldring MB, Konstantopoulos K. Prolonged application of high fluid shear to chondrocytes recapitulates gene expression profiles associated with osteoarthritis. PLoS One. 2010;5:e15174. doi: 10.1371/journal.pone.0015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Videira PA, Correia M, Malagolini N, Crespo HJ, Ligeiro D, Calais FM, Trindade H, Dall’Olio F. ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer. 2009;9:357. doi: 10.1186/1471-2407-9-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: An inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 17.Thomas SN, Schnaar RL, Konstantopoulos K. Podocalyxin-like protein is an E-/L-selectin ligand on colon carcinoma cells: Comparative biochemical properties of selectin ligands in host and tumor cells. Am J Physiol Cell Physiol. 2009;296:C505–13. doi: 10.1152/ajpcell.00472.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J Biol Chem. 2008;283:15647–15655. doi: 10.1074/jbc.M800543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh M, Alves C, Tong Z, Tettey K, Konstantopoulos K, Stebe KJ. Multifunctional surfaces with discrete functionalized regions for biological applications. Langmuir. 2008;24:8134–8142. doi: 10.1021/la8006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanley WD, Wirtz D, Konstantopoulos K. Distinct kinetic and mechanical properties govern selectin-leukocyte interactions. J Cell Sci. 2004;117:2503–2511. doi: 10.1242/jcs.01088. [DOI] [PubMed] [Google Scholar]
- 21.Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, Von Seggern CE, Cotter RJ, Bochner BS, Tiemeyer M, Konstantopoulos K, Schnaar RL. E-selectin receptors on human leukocytes. Blood. 2008;112:3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri KD, Finger EB, Springer TA. The faster kinetics of L-selectin than of E-selectin and P-selectin rolling at comparable binding strength. J Immunol. 1997;158:405–413. [PubMed] [Google Scholar]
- 23.Varki A. Selectin ligands: Will the real ones please stand up? J Clin Invest. 1997;99:158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97–103. [PubMed] [Google Scholar]
- 25.Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation of P-selectin in vitro. J Clin Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee AW, Truong T, Bickham K, Fonteneau JF, Larsson M, Da Silva I, Somersan S, Thomas EK, Bhardwaj N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: Implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 27.Crespo HJ, Cabral MG, Teixeira AV, Lau JT, Trindade H, Videira PA. Effect of sialic acid loss on dendritic cell maturation. Immunology. 2009;128:e621–31. doi: 10.1111/j.1365-2567.2009.03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross EL, Barker JN, Allen MH, Chu AC, Groves RW, MacDonald DM. Langerhans’ cell expression of the selectin ligand, sialyl lewis x. Immunology. 1994;81:303–308. [PMC free article] [PubMed] [Google Scholar]
- 29.Ellies LG, Sperandio M, Underhill GH, Yousif J, Smith M, Priatel JJ, Kansas GS, Ley K, Marth JD. Sialyltransferase specificity in selectin ligand formation. Blood. 2002;100:3618–3625. doi: 10.1182/blood-2002-04-1007. [DOI] [PubMed] [Google Scholar]
- 30.Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Frasca L, Nasso M, Spensieri F, Fedele G, Palazzo R, Malavasi F, Ausiello CM. IFN-gamma arms human dendritic cells to perform multiple effector functions. J Immunol. 2008;180:1471–1481. doi: 10.4049/jimmunol.180.3.1471. [DOI] [PubMed] [Google Scholar]