Abstract
OBJECTIVE: To determine the combined effects of body mass index (BMI) and body fat (BF) on prognosis in coronary heart disease (CHD) to better understand the obesity paradox.
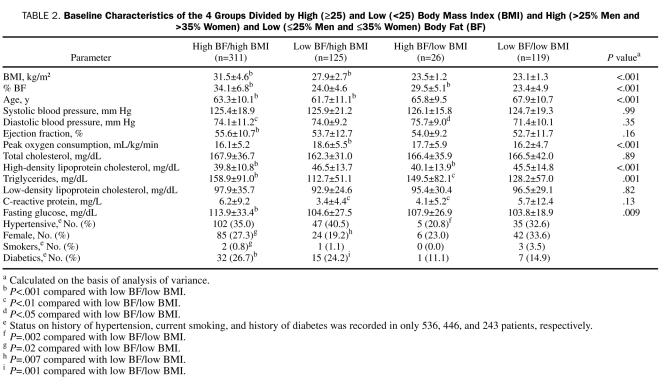
PATIENTS AND METHODS: We studied 581 patients with CHD between January 1, 2000, and July 31, 2005, who were divided into low (<25) and high BMI (≥25), as well as low (≤25% men and ≤35% women) and high BF (>25% in men and >35% in women). Four groups were analyzed by total mortality during the 3-year follow-up by National Death Index: low BF/low BMI (n=119), high BF/low BMI (n=26), low BF/high BMI (n=125), and high BF/high BMI (n=311).
RESULTS: During the 3-year follow-up, mortality was highest in the low BF/low BMI group (11%), which was significantly (P<.001) higher than that in the other 3 groups (3.9%, 3.2%, and 2.6%, respectively); using the high BF/high BMI group as a reference, the low BF/low BMI group had a 4.24-fold increase in mortality (confidence interval [CI], 1.76-10.23; P=.001). In multivariate logistic regression for mortality, when entered individually, both high BMI (odds ratio [OR], 0.79; CI, 0.69-0.90) and high BF (OR, 0.89; CI, 0.82-0.95) as continuous variables were independent predictors of better survival, whereas low BMI (OR, 3.60; CI, 1.37-9.47) and low BF (OR, 3.52; CI, 1.34-9.23) as categorical variables were independent predictors of higher mortality.
CONCLUSION: Although both low BF and low BMI are independent predictors of mortality in patients with CHD, only patients with combined low BF/low BMI appear to be at particularly high risk of mortality during follow-up. Studies are needed to determine optimal body composition in the secondary prevention of CHD.
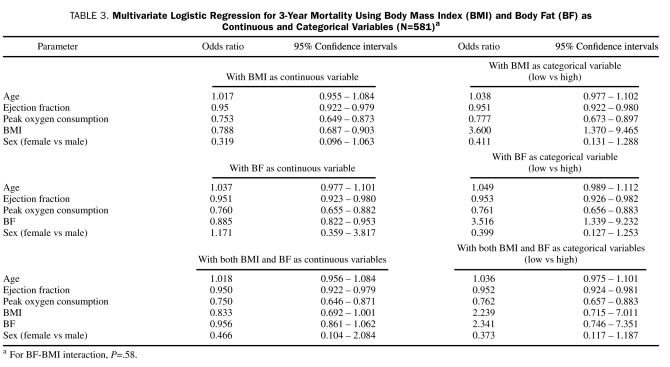
BF = body fat; BMI = body mass index; CHD = coronary heart disease; CI = confidence interval; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; CV = cardiovascular; EF = ejection fraction; HDL-C = high-density lipoprotein cholesterol; HF = heart failure; HR = hazard ratio; HTN = hypertension; LVH = left ventricular hypertrophy; OR = odds ratio; peak 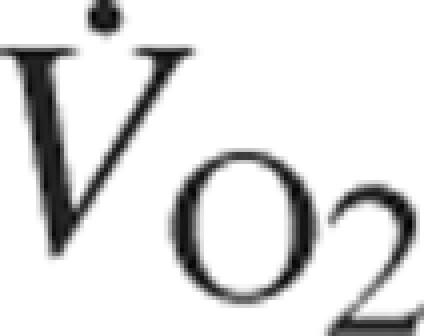 = peak oxygen consumption; T2DM = type 2 diabetes mellitus
= peak oxygen consumption; T2DM = type 2 diabetes mellitus
Despite the well-known adverse affects of obesity on almost all aspects of coronary heart disease (CHD) and CHD risk factors, including hypertension (HTN), plasma lipids, inflammation, glucose abnormalities, insulin resistance, metabolic syndrome and type 2 diabetes mellitus (T2DM), as well as left ventricular hypertrophy (LVH), many studies of cohorts with established cardiovascular (CV) disease, including heart failure (HF), HTN, as well as CHD, have demonstrated an inverse relationship between obesity, generally determined by body mass index (BMI [calculated as the weight in kilograms divided by the height in meters squared]), on subsequent mortality, referred to as the obesity paradox.1,2 The obesity paradox has also been demonstrated in non-CV studies that included patients with advanced renal disease and the elderly.3,4 Many large studies of cohorts with CHD have demonstrated this obesity paradox,5-7 which has also been demonstrated in a large meta-analysis by Romero-Corral et al8 from Mayo Clinic, who analyzed 40 cohort studies totaling more than 250,000 patients with CHD grouped according to BMI.
Although BMI is the most frequently used method to assess overweightness/obesity, especially in large epidemiologic studies, this method has been criticized because BMI does not always reflect true body fatness.1,2,9-14 Some investigators have theorized that at least part of the inconsistent relationship between obesity and major CV disease events, including mortality, may be due to the inaccurate diagnosis of obesity by the BMI assessment and that defining obesity by other methods, including waist circumference, waist/hip ratio, as well as percent body fat (BF) may be more accurate.2,9-13 We have recently demonstrated this obesity paradox in a cohort of CHD patients using both BMI and BF determinations.14
To our knowledge, no prior studies have determined the independent effects of both BMI and BF on mortality in a cohort of CHD patients. Therefore, in the current evaluation, we determined the combined and independent impact of both BMI and BF on mortality in a cohort with stable CHD.
PATIENTS AND METHODS
We retrospectively reviewed the case records of 581 consecutive patients with stable CHD who were referred for potential entry into formal cardiac rehabilitation programs between January 1, 2000, and July 31, 2005, and who had baseline anthropometric, lipid, and clinical data, as we have previously described.14 Patients were divided into low (<25) and high (≥25) BMI. Elevated BF has been defined as greater than 25% in men and greater than 35% in women,15,16 so patients were also divided into low (≤25% men and ≤35% women) and high BF (>25% men and >35% women). On the basis of this approach, 4 groups were analyzed: low BF/low BMI (n=119), high BF/low BMI (n=26), low BF/high BMI (n=125), and high BF/high BMI (n=311).
Baseline Assessment
At baseline, fasting plasma lipids, glucose, and high-sensitive C-reactive protein (CRP), as well as anthropometric profile (height, weight, BMI, and percent BF) were assessed. Body fat was assessed by the sum of the skinfold method using the average of 3 skinfolds—chest, thigh, and abdomen in men; thigh, triceps, and suprailiac in women, as previously described.14,17 All measurements were made in the early morning before exercise testing. Symptom-limited cardiopulmonary exercise testing was performed (as previously described in detail) to assess peak oxygen consumption (peak 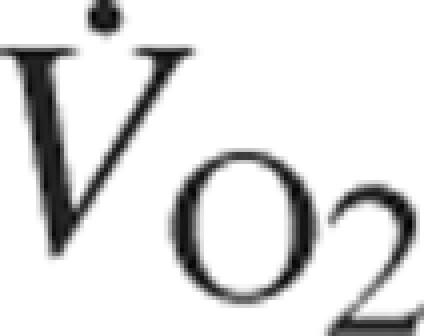 ).18,19 Prevalence of HTN, current smoking, diabetes, and chronic obstructive pulmonary disease (COPD) was recorded in 536, 446, 243, and 578 patients, respectively. Patients were followed up for an average of more than 3 years (mean ± SD, 1257±528 days; range, 231-2149 days) to determine all-cause mortality (but not cause-specific mortality) assessed by the National Death Index.
).18,19 Prevalence of HTN, current smoking, diabetes, and chronic obstructive pulmonary disease (COPD) was recorded in 536, 446, 243, and 578 patients, respectively. Patients were followed up for an average of more than 3 years (mean ± SD, 1257±528 days; range, 231-2149 days) to determine all-cause mortality (but not cause-specific mortality) assessed by the National Death Index.
Statistical Analyses
SAS 9.0 computer software (SAS Institute, Cary, NC) was used for statistical analysis. Means ± 1 SD or proportions for baseline risk factors were reported, and data for the 4 groups were compared with analysis of variance and χ2 analysis. Additionally, because mortality appeared to differ in the group with low BF/low BMI, nonpaired t tests were performed for baseline characteristics between this group and each of the other 3 groups. Kaplain-Meier survival curves were constructed to assess survival by both high and low BMI and high and low BF (using these data, a proportional hazards regression model was tested that included the BMI – BF term), as well as in the 4 distinct body composition groups. Death hazard ratios (HRs) were obtained in the 4 groups using the high BF/high BMI as the reference group. Multivariate logistic regression and Cox regression were performed to predict mortality using age, sex, ejection fraction (EF), peak 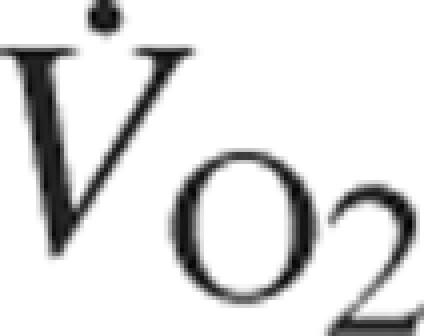 , and BMI and BF, individually and together, using these as continuous and categorical (high vs low) variables. Additionally, the interaction between BMI and BF was assessed with multivariate analysis. These analyses were also performed with and without 27 patients classified as having COPD, as well as with COPD as a categorical variable in the multivariate analyses.
, and BMI and BF, individually and together, using these as continuous and categorical (high vs low) variables. Additionally, the interaction between BMI and BF was assessed with multivariate analysis. These analyses were also performed with and without 27 patients classified as having COPD, as well as with COPD as a categorical variable in the multivariate analyses.
RESULTS
Baseline Characteristics
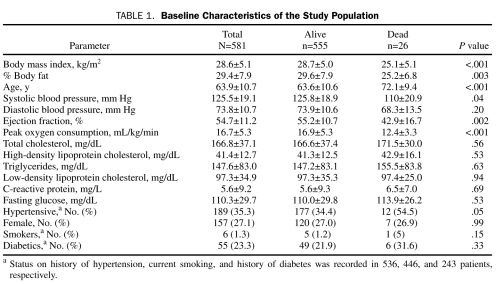
Baseline characteristics for the study population in Table 1 show that the mean BMI (28.6±5.1) was in the overweight range and that the mean BF (29.4±7.9%) was relatively high. Compared with those who died, survivors had significantly higher BMI, BF, systolic blood pressure, EF, and peak 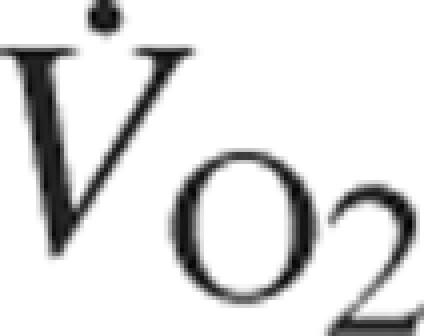 , and they had significantly lower age and prevalence of HTN. Although BMI and BF were highly correlated (r=0.60; P<.001), there was considerable variability in this relationship; the correlations were better when comparing males (r= 0.67; P<.001) and females (r=0.75; P<.001) separately, and even better when comparing similar ages and sex (data not shown). The baseline characteristics of the 4 distinct body composition groups are described in Table 2, with the groups showing significant differences in BMI and BF (by study design), but also in age, peak
, and they had significantly lower age and prevalence of HTN. Although BMI and BF were highly correlated (r=0.60; P<.001), there was considerable variability in this relationship; the correlations were better when comparing males (r= 0.67; P<.001) and females (r=0.75; P<.001) separately, and even better when comparing similar ages and sex (data not shown). The baseline characteristics of the 4 distinct body composition groups are described in Table 2, with the groups showing significant differences in BMI and BF (by study design), but also in age, peak 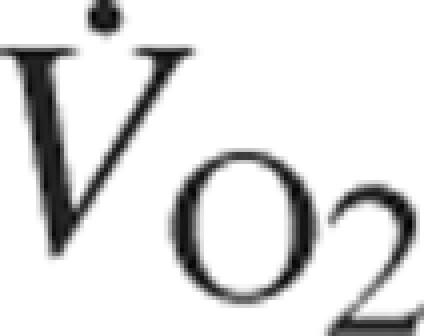 , high-density lipoprotein cholesterol (HDL-C), triglycerides, and fasting glucose. Patients with low BF/low BMI have a higher prevalence of HTN than those with high BF/low BMI (P=.02) and slightly higher current smoking than those with high BF/high BMI (P=.02) as well as lower peak
, high-density lipoprotein cholesterol (HDL-C), triglycerides, and fasting glucose. Patients with low BF/low BMI have a higher prevalence of HTN than those with high BF/low BMI (P=.02) and slightly higher current smoking than those with high BF/high BMI (P=.02) as well as lower peak 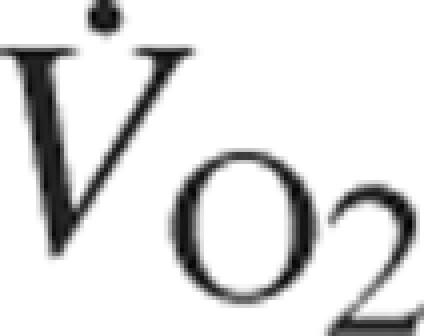 (P<.001) than the low BF/high BMI group and slightly higher mean age than the 2 high BMI groups (both P<.001). However, those with low BF/low BMI also have profiles often associated with lower CV risk, including higher levels of HDL-C (both P<.001) and lower triglycerides (P<.001 and P<.01, respectively) than the 2 high BF groups, lower glucose than the high BF/high BMI group (P<.001), and lower prevalence of diabetes than the 2 high BMI groups (P<.001 and P=.001, respectively).
(P<.001) than the low BF/high BMI group and slightly higher mean age than the 2 high BMI groups (both P<.001). However, those with low BF/low BMI also have profiles often associated with lower CV risk, including higher levels of HDL-C (both P<.001) and lower triglycerides (P<.001 and P<.01, respectively) than the 2 high BF groups, lower glucose than the high BF/high BMI group (P<.001), and lower prevalence of diabetes than the 2 high BMI groups (P<.001 and P=.001, respectively).
TABLE 1.
Baseline Characteristics of the Study Population
TABLE 2.
Baseline Characteristics of the 4 Groups Divided by High (≥25) and Low (<25) Body Mass Index (BMI) and High (>25% Men and >35% Women) and Low (≤25% Men and ≤35% Women) Body Fat (BF)
Survival Curve
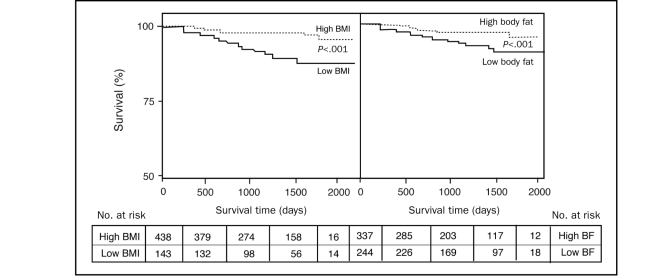
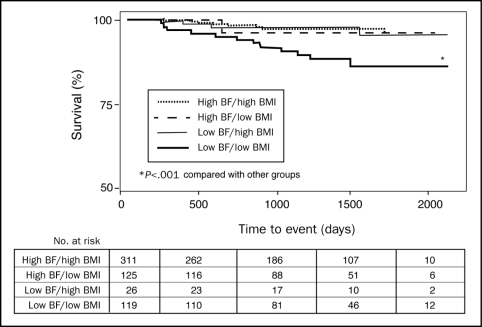
Patients with high BMI had better survival than those with low BMI (P<.001) (Figure 1). Likewise, those with high BF also had better survival than those with low BF (P<.001). We fitted a proportional hazards regression model that included a BMI-BF interaction term, and the results showed no significant interaction between BMI and BF (P=.09). When assessing survival in the 4 distinct body composition groups, patients with low BF/low BMI had higher mortality than the other 3 groups (Figure 2; P<.001). Using the high BF/high BMI as a reference, the low BF/low BMI group had a 4.24-fold increase in mortality (CI, 1.76-10.23; P=.001), whereas mortality was not significantly increased in the high BF/low BMI (HR, 1.51; confidence interval [CI], 0.19-12.06; P=.70) or low BF/high BMI groups (HR, 1.23; CI, 0.37-4.10; P=.73). Adjusting for age, EF, peak 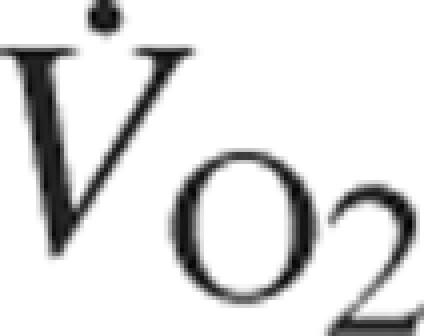 , and sex, again using the high BF/high BMI as a reference, the low BF/low BMI group had a 4.07-fold increase in mortality (CI, 1.48-11.16; P=.01), whereas mortality was not significantly increased in the high BF/low BMI (HR, 1.73; CI, 0.21-14.33; P=.61) or low BF/BMI (HR, 1.91; CI, 0.54-67.9; P=.32) groups. Eliminating the 6 patients with underweight BMI (<18.5) who had 50% mortality, those with low BF/low BMI still had higher mortality (8.9%) than did the other 3 groups (all P<.001).
, and sex, again using the high BF/high BMI as a reference, the low BF/low BMI group had a 4.07-fold increase in mortality (CI, 1.48-11.16; P=.01), whereas mortality was not significantly increased in the high BF/low BMI (HR, 1.73; CI, 0.21-14.33; P=.61) or low BF/BMI (HR, 1.91; CI, 0.54-67.9; P=.32) groups. Eliminating the 6 patients with underweight BMI (<18.5) who had 50% mortality, those with low BF/low BMI still had higher mortality (8.9%) than did the other 3 groups (all P<.001).
FIGURE 1.
Kaplain-Meier survival curves of patients divided by high (≥25; n=436) and low (<25; n=145) body mass index ([BMI] left panel) and high (>25% men and >35% women; n=337) and low (≤25% men and ≤35% women; n=244) body fat ([BF] right panel). A proportional hazard regression that included a BMI-BF interaction term showed no significant interaction between BMI and BF (P=.09).
FIGURE 2.
Kaplain-Meier survival curves of patients categorized by high and low body mass index (BMI) and high and low body fat (BF) with cut-points as in Figure 1 and categories as in Table 2.
Multivariate Predictors of Mortality
In multivariate analysis using logistic regression, both high BMI (odds ratio [OR], 0.79; CI, 0.69-0.90) and high BF (OR, 0.89; CI, 0.82-0.95) when entered into the analysis individually as continuous variables were independent predictors of lower 3-year mortality. Other independent predictors of mortality are listed in Table 3, with only higher EF and greater exercise capacity (higher peak 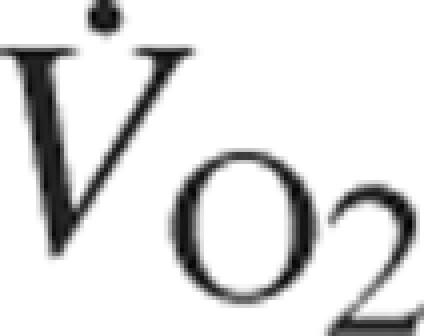 ) being independent predictors of better survival in all the models. Likewise, both low BMI (OR, 3.60; CI, 1.37-9.47) and low BF (OR, 3.52; CI, 1.34-9.23) when entered into the analysis individually as categorical values (low vs high) were also strong independent predictors of 3-year mortality. However, when both BMI and BF were entered into the model together, neither was a significant independent predictor of 3-year mortality, although low BMI as a continuous variable was nearly statistically significant (OR, 0.8; CI, 0.69-1.00). When using Cox regression analysis, both BMI (HR, 0.82; CI, 0.73-0.92; P=.001) and BF (HR, 0.90; CI, 0.84-0.96; P=.001) were significant independent predictors of mortality when entered individually as continuous variables but not when entered together (BMI: HR, 0.87; CI, 0.74-1.02; P=.09 and BF: HR, 0.95; CI, 0.87-1.04; P=.29). When using categorical variables, low BMI (HR, 2.99; CI, 1.25-7.17; P=.01) and low BF (HR, 2.93; CI, 1.20-7.18; P=.02) were independent predictors of mortality when entered individually but not when entered together (low BMI: HR, 2.00; CI, 0.72-5.58; P=.19; and low BF: HR, 2.05; CI, 0.71-5.89; P=.18).
) being independent predictors of better survival in all the models. Likewise, both low BMI (OR, 3.60; CI, 1.37-9.47) and low BF (OR, 3.52; CI, 1.34-9.23) when entered into the analysis individually as categorical values (low vs high) were also strong independent predictors of 3-year mortality. However, when both BMI and BF were entered into the model together, neither was a significant independent predictor of 3-year mortality, although low BMI as a continuous variable was nearly statistically significant (OR, 0.8; CI, 0.69-1.00). When using Cox regression analysis, both BMI (HR, 0.82; CI, 0.73-0.92; P=.001) and BF (HR, 0.90; CI, 0.84-0.96; P=.001) were significant independent predictors of mortality when entered individually as continuous variables but not when entered together (BMI: HR, 0.87; CI, 0.74-1.02; P=.09 and BF: HR, 0.95; CI, 0.87-1.04; P=.29). When using categorical variables, low BMI (HR, 2.99; CI, 1.25-7.17; P=.01) and low BF (HR, 2.93; CI, 1.20-7.18; P=.02) were independent predictors of mortality when entered individually but not when entered together (low BMI: HR, 2.00; CI, 0.72-5.58; P=.19; and low BF: HR, 2.05; CI, 0.71-5.89; P=.18).
TABLE 3.
Multivariate Logistic Regression for 3-Year Mortality Using Body Mass Index (BMI) and Body Fat (BF) as Continuous and Categorical Variables (N=581)a
Impact of COPD
In our cohort of 578 patients coded yes or no for COPD, 27 patients were coded as having COPD with a mortality of 18.5% compared with only 3.8% mortality in 551 not coded with COPD (P<.001). Compared with those without COPD, those with COPD had lower BMI (25.4±5.2 vs 28.7±5.1; P=.001), peak 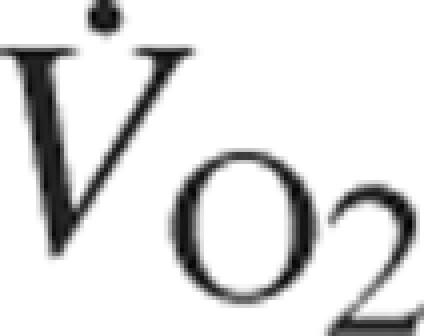 (11.8±3.1 vs 16.9±5.3 mL/kg/min; P<.001), fasting glucose (96.2±10.3 vs 110.8±30.0 mg/dL; P=.03), and diabetes (0 vs 25.1%; P=.01) and higher age (71.7±6.5 vs 63.7±10.7 years; P<.001), total cholesterol (200.7±37.5 vs 165.8±36.9 mg/dL; P<.001), HDL-C (63.1±22.8 vs 40.5±11.4 mg/dL; P<.001), low-density lipoprotein cholesterol (112.5±34.6 vs 96.2±32.7 mg/dL; P=.03), female sex (51.9 vs 25.8%; P=.003), and prevalence of HTN (65.4 vs 33%; P=.001). Those with COPD had slightly lower BF (27.3±9.0 vs 29.6±7.9%; P=.15). However, when COPD was entered into the multivariate analyses (using either logistic regression or Cox regression), COPD was not a significant independent predictor and did not have a major effect on the impact of BMI and/or BF. If all the patients with COPD were eliminated from multivariate analyses (eg, logistic regression), BMI was still a significant independent predictor (OR, 0.86; CI, 0.74-0.99), whereas BF was not significant statistically (OR, 0.94; CI, 0.86-1.03). Additionally, without the COPD patients, although mortality with the low BF/low BMI group (n=107) is lower (7.4%), it is still higher than the other groups (high BF/high BMI [n=300], 2.7%; P=.002; high BF/low BMI [n=26], 3.9%; P=.01; and low BF/high BMI [n=121], 3.3%; P=.06).
(11.8±3.1 vs 16.9±5.3 mL/kg/min; P<.001), fasting glucose (96.2±10.3 vs 110.8±30.0 mg/dL; P=.03), and diabetes (0 vs 25.1%; P=.01) and higher age (71.7±6.5 vs 63.7±10.7 years; P<.001), total cholesterol (200.7±37.5 vs 165.8±36.9 mg/dL; P<.001), HDL-C (63.1±22.8 vs 40.5±11.4 mg/dL; P<.001), low-density lipoprotein cholesterol (112.5±34.6 vs 96.2±32.7 mg/dL; P=.03), female sex (51.9 vs 25.8%; P=.003), and prevalence of HTN (65.4 vs 33%; P=.001). Those with COPD had slightly lower BF (27.3±9.0 vs 29.6±7.9%; P=.15). However, when COPD was entered into the multivariate analyses (using either logistic regression or Cox regression), COPD was not a significant independent predictor and did not have a major effect on the impact of BMI and/or BF. If all the patients with COPD were eliminated from multivariate analyses (eg, logistic regression), BMI was still a significant independent predictor (OR, 0.86; CI, 0.74-0.99), whereas BF was not significant statistically (OR, 0.94; CI, 0.86-1.03). Additionally, without the COPD patients, although mortality with the low BF/low BMI group (n=107) is lower (7.4%), it is still higher than the other groups (high BF/high BMI [n=300], 2.7%; P=.002; high BF/low BMI [n=26], 3.9%; P=.01; and low BF/high BMI [n=121], 3.3%; P=.06).
DISCUSSION
The current study has 3 main findings. First, we confirmed the obesity paradox in this population of patients with stable CHD using both BMI and BF criteria for overweightness/obesity. Second, we demonstrated that higher BMI and BF when entered individually, used as continuous or categorical variables, were independent predictors of better survival in patients with stable CHD. Third, we demonstrated that in this obesity paradox (or lean paradox as discussed subsequently), higher mortality appears to be confined to the subgroup of CHD patients with both low BMI and low BF.
Obesity Paradox
Obesity clearly has numerous adverse effects on nearly all the major CHD risk factors, including dyslipidemia, HTN, glucose abnormalities (insulin resistance, metabolic syndrome, and T2DM), inflammation (especially levels of CRP), as well as LVH, and is probably an independent CHD risk factor.1 Besides being a risk factor for CHD, obesity is associated with increased risk of the most common CV diseases, including HTN, HF, atrial fibrillation, as well as many other CV diseases, as we have reviewed previously.1,2,20 However, despite this powerful association between obesity and CHD risk factors, CHD, and most CV diseases, numerous studies have now demonstrated that in cohorts with established CV diseases, including HTN, HF, peripheral arterial disease, atrial fibrillation, as well as CHD, patients with overweightness/obesity have better clinical prognosis than do their lean counterparts,1,2,,6,8,14,21-27 which has been termed the obesity paradox.
Many studies have now demonstrated this obesity paradox in CHD cohorts,5-7 including patients with CHD with and without revascularization procedures.8 In a meta-analysis from Mayo Clinic, Romero-Corral et al8 analyzed 40 cohort studies totaling more than 250,000 patients with CHD grouped according to BMI. In an analysis of all-cause mortality, the low BMI group had by far the highest mortality, whereas the obese patients had lower risk. Overweight patients had the lowest relative risk in their adjusted analysis, whereas obese and severely obese patients had no increased risk. Recently, this obesity paradox was also described in nearly 7000 male non-HF veterans referred for stress testing.27
Use of BF Determination
Although BMI is the most common method to define overweightness/obesity, especially in major epidemiologic studies, this method has been criticized because it may not always reflect true body fatness, and BMI/body fatness may differ considerably among the various ages, sexes, and races.9-13,28 Part of the explanation for the unexpected association between BMI and outcomes in CHD and other CV diseases could be due to the poor diagnostic performance of BMI to define body fatness and lean body mass, factors that could be associated with opposing outcomes in CV diseases.1,8-14,29 In fact, the group from Mayo Clinic has previously demonstrated that BMI performs suboptimally in predicting obesity as defined by BF (>25% in men and >35% in women) in a CHD population.9 Some clinical populations have low BMI and high BF and have higher CHD prevalence than do their low BF counterparts.30 Stroke has also been associated with BF rather than with BMI.31 Nevertheless, we previously demonstrated an obesity paradox using both BMI and BF criteria in our CHD population.14
In the current study of patients with stable CHD, we also demonstrated an obesity paradox using both BMI and BF. In fact, both higher BMI and BF, when entered individually into a multivariate analysis, either as continuous or categorical variables, were associated with lower mortality. In patients with BMI of 25 or greater or BF greater than 25% in men and greater than 35% in women, 3-year mortality was reduced by 3.6-fold and 3.5-fold, respectively.
A recent study by McAuley et al32 of 12,417 veterans who were referred for exercise stress testing demonstrated that fitness altered the obesity paradox. In fact, highly fit overweight men had the lowest mortality risk, and overweight and obese men with moderate fitness had mortality rates similar to the highly fit normal-weight reference group. In the current study, however, we defined fitness precisely by cardiopulmonary exercise testing and precisely determined peak 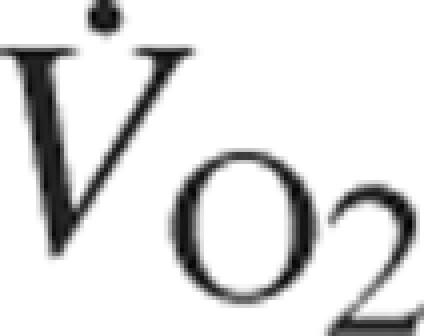 , as opposed to simply estimating fitness by speed and workload on the treadmill. Although high peak
, as opposed to simply estimating fitness by speed and workload on the treadmill. Although high peak 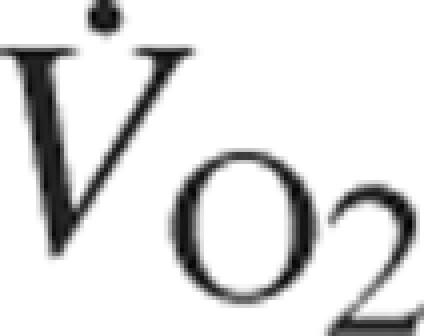 was an independent predictor of lower mortality in all our multivariate analyses, high BMI or high BF, as both continuous and categorical variables, was still independently associated with lower mortality even when fitness was considered.
was an independent predictor of lower mortality in all our multivariate analyses, high BMI or high BF, as both continuous and categorical variables, was still independently associated with lower mortality even when fitness was considered.
Obesity Paradox vs Lean Paradox
The mechanisms to explain this puzzling paradox are difficult to decipher. As in most studies, our study did not control for nonpurposeful weight loss before study entry. However, in general, patients referred for cardiac rehabilitation programs are stable from a non-CV standpoint. Also, the obesity paradox may be modified by physical wellness or other unmeasured confounding factors that link the presence of chronic diseases to outcomes.33 However, only 6 of our 581 study patients were classified as “underweight” (BMI <18.5), although this group had a 50% mortality rate. We also did not measure pulmonary function in our simple assessment for COPD; however, only a very small number of our patients were active smokers, and many studies of CHD cohorts have demonstrated that smoking cessation in patients with CHD is associated with a favorable prognosis, nearly equal to never smokers within almost 6 months of smoking cessation.34 Even in patients with peripheral arterial disease, in whom smoking may be a more powerful risk factor than for CHD, chronic lung disease did not explain the obesity paradox, and in our cohort, COPD did not appear to be an independent predictor of mortality or to impact the independent role of BMI and BF on mortality.24,34
In our study population, high mortality was confined to the population with low BF and low BMI. Although our population with high BF and low BMI was small (n=26), which makes mortality assessment difficult, the group with low BF and high BMI (n=125) also had low mortality, similar to the group with both high BF and high BMI (n=311). With only 26 patients with high BF and low BMI (referred to as obese sarcopenia), our study is not powered to fully assess this group. However, if we use a cutpoint of 30% BF in women instead of 35%, our number in this group increased to 39 patients, with a 3-year mortality of only 2.8%. Although the patient group with both low BF and low BMI had some factors that could be associated with a poor prognosis (higher age and slightly higher current smokers), it also had a low percentage of diabetes, higher HDL-C, and lower triglycerides and fasting glucose, factors that may be associated with a lower overall risk. As we have suggested previously,1,2,14 overweight and obese patients with CV diseases might not have developed these diseases, including CHD as in the current study, in the first place if weight gain had been prevented. In contrast, leaner patients, especially those with low BF and low BMI as in the current study, who developed this same disease may have a different pathophysiologic etiology, including genetic predisposition, that may lead to a poor prognosis, thus suggesting a lean paradox as opposed to an obesity paradox.
Study Limitations
Several potential study limitations should be emphasized. First, this is a relatively small (especially small number with high BF and low BMI) retrospective study of a select cohort referred to cardiac rehabilitation from a metropolitan area with a high prevalence of overweightness/obesity, with a relatively short follow-up. Second, we assessed BF by the sum of the skinfold method, as opposed to more sophisticated methods, such as hydrostatic weighing, air displacement plethysmography, bioelectrical impedance, and the criterion standard dual-energy x-ray absorptiometry.2,14,35 Third, we did not assess other surrogate markers of “at risk” obesity, such as waist-to-hip ratio and, especially, waist circumference. In fact, a recent study of 15,923 CHD patients from 5 studies, including the Mayo Clinic's CV Rehabilitation Database, demonstrated that central obesity was associated with mortality, including in patients with “normal” BMI (18.5-25) as well as in those with BMI of 30 or greater,2,36 and a study in patients with end-stage renal disease also suggested that the obesity paradox is not present in patients with high waist circumferences.37 However, another recent study in HF indicated that high waist circumference is an independent predictor of better event-free survival and demonstrated that the best prognosis was in HF patients with both high waist circumference and high BMI.38,39 We also did not assess peripheral BF, particularly thigh fat, which may be inversely related to all-cause mortality and may independently enhance cardiometabolic health and may help explain the obesity paradox.40-42 As previously mentioned, our study had a very small number in the group with obese sarcopenia. We also used BF cutpoints of 25% in men and 35% in women,15,16 and as we have recently discussed, these cutoffs could be in the 20% to 25% range in men and 30% to 38% range in women.43,44 Finally, we assessed total mortality, which is certainly an important end point, but did not assess other CV and CHD events.
CONCLUSION
Our data indicate that in CHD patients, an obesity paradox (or actually lean paradox) exists, suggesting that patients with higher BMI or higher BF have lower mortality than those with less obesity. However, our results indicate that only those patients with combined low BF and low BMI appeared to be at particularly high risk of mortality during follow-up. Further studies are needed to determine optimal body composition (using more sophisticated methods to assess BF such as dual-energy x-ray absorptiometry scans) in both primary and secondary CHD prevention.
Supplementary Material
Footnotes
Presented in part at the American Heart Association Scientific Sessions; November 15, 2010; Chicago, IL.
REFERENCES
- 1. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925-1932 [DOI] [PubMed] [Google Scholar]
- 2. Lavie CJ, Milani RV, Ventura HO, Romero-Corral A. Body composition and heart failure prevalence and prognosis: getting to the fat of the matter in the ``obesity paradox.'' Mayo Clin Proc. 2010;85(7):605-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Artham SM, Lavie CJ, Patel DA, Ventura HO. Obesity paradox in the elderly: is fatter really fitter? Aging Health. 2009;5(2):177-184 [Google Scholar]
- 4. Kalantar-Zadeh K, Streja E, Kovesdy CP, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85(11):991-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oreopoulos A, McAlister FA, Kalantar-Zadeh K, et al. The relationship between body mass index, treatment, and mortality in patients with established coronary artery disease: a report from APPROACH. Eur Heart J. 2009;30(21):2584-2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller TM, Lavie CJ, White CJ. Impact of obesity on the pathogenesis and prognosis of coronary heart disease. J Cardiometab Syndr. 2008;3(3):162-167 [DOI] [PubMed] [Google Scholar]
- 7. Hastie CE, Padmanabhan S, Slack R, et al. Obesity paradox in a cohort of 4880 consecutive patients undergoing percutaneous coronary intervention. Eur Heart J. 2010;31(2):222-226 [DOI] [PubMed] [Google Scholar]
- 8. Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666-678 [DOI] [PubMed] [Google Scholar]
- 9. Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. 2007;28(17):2087-2093 [DOI] [PubMed] [Google Scholar]
- 10. Romero-Corral A, Lopez-Jimenez F, Sierra-Johnson J, Somers VK. Differentiating between body fat and lean mass: how should we measure obesity? Nat Clin Pract Endocrinol Metab. 2008;4(6):322-323 [DOI] [PubMed] [Google Scholar]
- 11. Poirier P. Adiposity and cardiovascular disease: are we using the right definition of obesity? Eur Heart J. 2007;28(17):2047-2048 [DOI] [PubMed] [Google Scholar]
- 12. See R, Abdullah SM, McGuire DK, et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50(8):752-759 [DOI] [PubMed] [Google Scholar]
- 13. de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28(7):850-856 [DOI] [PubMed] [Google Scholar]
- 14. Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO. The obesity paradox, weight loss, and coronary disease. Am J Med. 2009;122(12):1106-1114 Medline [DOI] [PubMed] [Google Scholar]
- 15. AACE/ACE Obesity Task Force AACE/ACE position statement on the prevention, diagnosis, and treatment of obesity (1998 revision). Endocr Pract. 1998;4(5):297-350 http://dev.aace.com/sites/default/files/obesityguide.pdf Accessed May 17, 2011 [Google Scholar]
- 16. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89(6):2595-2600 [DOI] [PubMed] [Google Scholar]
- 17. Jackson AS, Pollock ML. Practical assessment of body composition. Physician Sport Med. 1985;13:76-90 [DOI] [PubMed] [Google Scholar]
- 18. Milani RV, Lavie CJ, Mehra MR. Cardiopulmonary exercise testing: how do we differentiate the cause of dyspnea? Circulation. 2004;110(4):e27-e31 [DOI] [PubMed] [Google Scholar]
- 19. Milani RV, Lavie CJ, Mehra MR, Ventura HO. Understanding the basics of cardiopulmonary exercise testing. Mayo Clin Proc. 2006;81(12):1603-1611 [DOI] [PubMed] [Google Scholar]
- 20. Wanahita N, Messerli FH, Bangalore S, et al. Atrial fibrillation and obesity: results of a meta-analysis. Am Heart J. 2008;155(2):310-315 [DOI] [PubMed] [Google Scholar]
- 21. Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120(10):863-870 [DOI] [PubMed] [Google Scholar]
- 22. Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91(7):891-894 [DOI] [PubMed] [Google Scholar]
- 23. Badheka AO, Rathod A, Kizilbash MA, et al. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010;123(7):646-651 [DOI] [PubMed] [Google Scholar]
- 24. Galal W, van Gestel Y, Hoeks SE, et al. The obesity paradox in patients with peripheral arterial disease. Chest. 2008;134(5):925-930 [DOI] [PubMed] [Google Scholar]
- 25. Lavie CJ, Milani RV, Ventura HO, et al. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. Am J Cardiol. 2007;100(9):1460-1464 [DOI] [PubMed] [Google Scholar]
- 26. Lavie CJ, Milani RV, Patel D, Artham SM, Ventura HO. Disparate effects of obesity and left ventricular geometry on mortality in 8,088 elderly with preserved systolic function. Postgrad Med. 2009;121(3):119-125 [DOI] [PubMed] [Google Scholar]
- 27. McAuley P, Myers J, Abella J, Froelicher V. Body mass, fitness and survival in veteran patients: another obesity paradox? Am J Med. 2007;120(6):518-524 [DOI] [PubMed] [Google Scholar]
- 28. Jackson AS, Stanforth PR, Gangnon J, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26(6):789-796 [DOI] [PubMed] [Google Scholar]
- 29. Kragelund C, Omland T. A farewell to body-mass index? Lancet. 2005;366(9497):1589-1591 [DOI] [PubMed] [Google Scholar]
- 30. Deurenberg-Yap M, Chew SK, Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes Rev. 2002;3(3):209-215 [DOI] [PubMed] [Google Scholar]
- 31. Walker SP, Rimm EB, Ascherio A, et al. Body size and fat distribution as predictors of stroke among US men. Am J Epidemiol. 1996;144(12):1143-1150 [DOI] [PubMed] [Google Scholar]
- 32. McAuley PA, Kokkinos PF, Oliveira RB, Emerson BT, Myers JN. Obesity paradox and cardiorespiratory fitness in 12,417 male veterans aged 40 to 70 years. Mayo Clin Proc. 2010;85(2):115-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ades PA, Savage PD. The obesity paradox: perception vs knowledge. Mayo Clin Proc. 2010;85(2):112-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lavie CJ, Ventura HO, Milani RV. The ``obesity paradox'': is smoking/lung disease the explanation? Chest. 2008;134(5):896-898 [DOI] [PubMed] [Google Scholar]
- 35. Oreopoulos A, Ezekowitz JA, McAlister FA, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85(7):609-617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coutinho T, Goel K, Corrêa de Sá D, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis utilizing individual subject data. J Am Coll Cardiol. 2011;57(19):1877-1886 [DOI] [PubMed] [Google Scholar]
- 37. Postorino M, Marino C, Tripepi G, Zoccali C; CREDIT (Calabria Registry of Dialysis and Transportation) Working Group Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol. 2009;53(15):1265-1272 [DOI] [PubMed] [Google Scholar]
- 38. Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17(5):374-380 [DOI] [PubMed] [Google Scholar]
- 39. Lavie CJ, Ventura HO. Weighing in on obesity and the obesity paradox in heart failure. J Card Fail. 2011;17(5):381-383 [DOI] [PubMed] [Google Scholar]
- 40. Tankó LB, Christiansen C. Can the obesity paradox be explained by the protective effects of peripheral adiposity? Arch Intern Med. 2005;165(15):1796-1797 [DOI] [PubMed] [Google Scholar]
- 41. Hietmann BL, Frederiksen P. Thigh circumference and risk of heart disease and premature death: prospective cohort study [published correction appears in BMJ. 2009;339:b3744]. BMJ. 2009;339:b3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hunter GR, Chandler-Laney PC, Brock DW. Fat distribution, aerobic fitness, blood lipids, and insulin sensitivity in African-American and European-American women. Obesity (Silver Spring). 2010;18(2):274-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lavie CJ, Milani RV, Ventura HO. De Schutter Alban. Use of body fatness cutoff points [letter reply]. Mayo Clin Proc. 2010;85(11):1057-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oreopoulos A, Lavie CJ, Snitker S, Romero-Corral A. More on body fat cutoff points. Mayo Clin Proc. 2011;86(6):584 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.