Abstract
OBJECTIVE: To assess the prevalence, clinical presentations, and neuroimaging abnormalities in a series of patients treated for eclampsia at Mayo Clinic in Rochester, MN.
PATIENTS AND METHODS: We reviewed the records of all pregnant patients diagnosed as having eclampsia at Mayo Clinic in Rochester, MN, between January 1, 2001, and December 31, 2008. All patients who underwent neuroimaging were identified, and all studies were reviewed by an independent neuroradiologist. Comparisons were made between groups who did and did not undergo imaging to identify differentiating clinical or laboratory variables.
RESULTS: Thirteen cases of eclampsia were found, with neuroimaging studies available for 7: magnetic resonance imaging (n=6) and computed tomography (n=1). All 7 patients developed eclamptic seizures, and 2 of 7 patients had severe hypertension, with recorded systolic blood pressures exceeding 180 mm Hg. Neuroimaging showed characteristic changes of posterior reversible encephalopathy syndrome (PRES) in all patients. Follow-up imaging showed resolution in 2 of 3 patients; 1 patient had residual neuroimaging abnormalities.
CONCLUSION: Our results suggest that the clinical syndrome of eclampsia is associated with an anatomical substrate that is recognizable by neuroimaging as PRES. The levels of blood pressure elevation are lower than those reported in cases of PRES because of hypertensive encephalopathy. Further studies are needed to determine whether more aggressive blood pressure control and early neuroimaging may have a role in the management of these patients.
ADC = apparent diffusion coefficient; DBP = diastolic blood pressure; DWI = diffusion-weighted imaging; MRI = magnetic resonance imaging; PRES = posterior reversible encephalopathy syndrome; SBP = systolic blood pressure.
Preeclampsia is a pregnancy-specific disorder clinically characterized by hypertension (blood pressure ≥140/90 mm Hg) and proteinuria (≥300 mg in a 24-hour urine collection) occurring after 20 weeks of gestation in a previously normotensive patient.1 Preeclampsia and its variants affect approximately 5% of pregnancies and remain leading causes of both maternal and fetal morbidity and mortality world-wide.2 The incidence of progression to the convulsive form (ie, eclampsia) occurs in approximately 0.5% of patients with mild preeclampsia and 2% to 3% of those with severe preeclampsia, as defined by a systolic blood pressure (SBP) of 160 mm Hg or greater, a diastolic blood pressure (DBP) of 100 mm Hg or greater, nephrotic-range proteinuria (>3.5 g/24-hour urine), renal function impairment, thrombocytopenia, and/or evidence of microangiopathic hemolytic anemia, hepatocellular injury, pulmonary edema, and neurologic disturbances.3 The incidence of eclampsia in developed countries averages 1 in 2000 to 3000 deliveries.4,5 Seizure activity can manifest as 1 or more generalized convulsions with or without coma.
In 1992, Douglas and Redman6 prospectively studied all cases of eclampsia in the United Kingdom. They identified 383 confirmed cases of eclampsia and described the occurrence of 1 or more of the following antecedent symptoms within hours before the onset of an eclamptic seizure: prodromal headache, visual disturbance (scotomata, amaurosis fugax, blurred vision, diplopia, homonymous hemianopsia), and epigastric pain. The relationship between the level of blood pressure and seizure onset, although considered relevant by most, remains controversial.
Posterior reversible encephalopathy syndrome (PRES) is a clinically recognizable entity that presents with neurologic signs and symptoms (headache, altered consciousness, visual abnormalities, and seizures) in conjunction with the unique neuroimaging findings of vasogenic edema involving the posterior circulation. An association between eclampsia and PRES was first described by Hinchey et al7 in 1996. In this initial series, 3 of 15 patients with PRES had eclampsia, with other etiologies including hypertensive encephalopathy and immunosuppressive medications. Although this study established a clear association between eclampsia and PRES, few clinical studies followed to further document and support these associations.8-10
Our study aimed to assess the prevalence and clinical presentation, along with the distribution and extent of neuroimaging abnormalities, among patients treated for eclampsia at Mayo Clinic in Rochester, MN, between 2001 and 2008. In addition, we reviewed their follow-up neuroimaging studies, when available, for evidence of persistent brain damage.
PATIENTS AND METHODS
With the approval of the Mayo Clinic Institutional Review Board, which waived the need for informed consent, we electronically reviewed the records of all obstetric patients seeking care at Mayo Clinic in Rochester, MN, between January 1, 2001, and December 31, 2008, for the diagnosis of eclampsia. Of these patients, 13 had a confirmed diagnosis of eclampsia based on International Classification of Diseases, Ninth Revision codes and the presence of previously published and widely accepted clinical criteria of hypertension, proteinuria, and seizure activity not attributable to other causes.1 Findings for 3 of the patients had been previously reported.11 Of the 13 patients, 7 patients had undergone neuroimaging subsequent to the diagnosis of eclampsia; specifically, 6 underwent magnetic resonance imaging (MRI) of the brain, and 1 underwent computed tomography of the brain. Three patients had follow-up neuroimaging between 14 and 216 days after their initial studies. All studies were reviewed by an independent neuroradiologist, who had not made the previous radiologic diagnoses of PRES. The patterns of changes as well as evidence of permanent neurologic abnormalities were noted.
To identify the demographic and clinical characteristics that may have led to the decision to order neuroimaging studies, the subgroup of eclamptic patients who underwent imaging (n=7) was compared with the subset of patients who also developed eclampsia but did not undergo brain imaging (n=6). Statistical procedures included the Wilcoxon rank sum test for continuous variables. P<.05 was prespecified as being statistically significant.
RESULTS
Of the 17,317 women who gave birth during the study period, 13 (0.075%) had a diagnosis of eclampsia. Reviews of the medical records confirmed the diagnosis of eclampsia by identifying its characteristic clinical findings: hypertension, proteinuria, and seizure activity.1 All patients who underwent imaging had neurologic abnormalities and accompanying radiologic findings of PRES (Table 1; Figure, A). Contrary to the initial report,7 and consistent with a more recent study,10 these lesions were not predominantly present in the posterior cerebrum but rather involved other areas of the brain (Table 1). In addition, PRES and eclamptic seizures occurred at an SBP of less than 180 mm Hg in the 5 of 7 patients who underwent imaging (Table 1). Similarly, eclamptic seizures developed at a peak SBP of less than 180 mm Hg in the 4 of 6 patients who did not undergo neuroimaging (Table 2). All patients except one underwent imaging within 48 hours of seizure onset; the remaining patient was evaluated 4 days after the event.
TABLE 1.
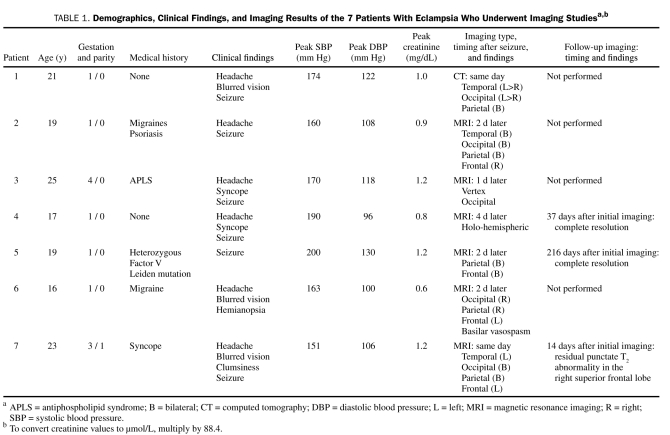
Demographics, Clinical Findings, and Imaging Results of the 7 Patients With Eclampsia Who Underwent Imaging Studiesa,b
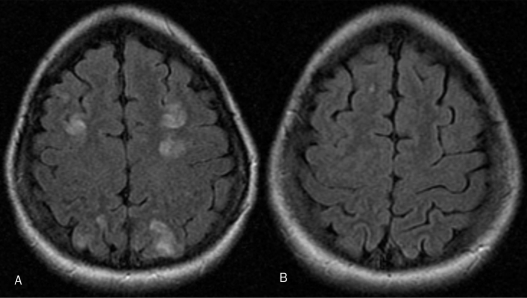
FIGURE.
Magnetic resonance imaging of the head for a 23-year-old woman (gravida 3, para 1) who delivered at 40 weeks gestation after an uneventful pregnancy. Four days after delivery, she developed a dull headache, followed 5 days later by blurred vision and clumsiness. She had a seizure at home, and on admission, was found to be hypertensive, with a blood pressure of 158/86 mm Hg and an estimated urine protein of 349 mg/24 h. Findings on magnetic resonance imaging of the head (A) were consistent with posterior reversible encephalopathy syndrome (PRES). Blood pressure was controlled, and the patient was discharged. Two weeks after initial imaging, follow-up magnetic resonance imaging (B) revealed near-complete resolution of the changes of PRES, with a residual punctate abnormality in the right superior frontal lobe. She continued to have a dull headache. Her systolic blood pressure returned to the low 100s, and her headache subsequently resolved.
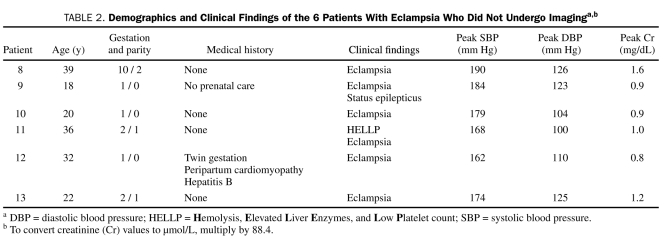
TABLE 2.
Demographics and Clinical Findings of the 6 Patients With Eclampsia Who Did Not Undergo Imaginga,b
We did not observe statistically significant differences in maternal demographics or diagnostic laboratory findings between patients who underwent imaging and those who did not (Table 3). However, 5 of 7 patients who underwent imaging had the onset of seizures between 1 and 9 days after delivery (median, 5 days). Conversely, only 1 of the patients who did not undergo imaging experienced the onset of seizure activity after delivery.
TABLE 3.
Comparison of Eclamptic Patients With and Without Imaginga,b
Before the onset of seizures, only 2 women were receiving antihypertensive medication (1 in each group). On the day of the seizure, 4 of 7 women who had undergone imaging, and 3 of 6 who had not, received antihypertensive medication. All women received antihypertensive medication by 1 day after the seizure.
Of the patients, 3 underwent follow-up imaging between 2 and 30 weeks postpartum; 2 had resolution of T2 white matter alterations, whereas 1 patient had a residual punctate abnormality in the right superior frontal region (Table 1).
DISCUSSION
Our results suggest a strong association between eclampsia and characteristic neuroradiologic PRES findings in a small series of patients who were treated at Mayo Clinic in Rochester, MN. Radiologic evidence of PRES was present in all 7 patients who developed eclamptic seizures and underwent neuroimaging studies. Because symptoms of PRES in nonpregnant patients are similar to the prodromal symptoms of eclampsia, we postulate that cerebral edema and the neuroimaging abnormalities characteristic of PRES may precede the onset of eclamptic seizures. Patients in our study appeared to develop PRES at a lower mean peak SBP (173 mm Hg) than 113 previously reported patients who were treated for PRES at our institution between 1999 and 2009: acute hypertension was present in 86% of these patients, with a mean peak SBP of 191 mm Hg.11 Of note, 3 patients who are reported in this study were included in that comprehensive series report, but clinical and radiologic findings in pregnant patients were neither separately analyzed nor contrasted to nonpregnant patients. Peak DBP was not significantly different between these groups. In the series by Hinchey et al,7 for nonpregnant patients who developed hypertensive encephalopathy and PRES (n=4), the highest SBP was documented between 180 and 200 mm Hg. For patients with eclampsia (n=3), the highest SBP ranged between 150 and 170 mm Hg, and for those treated with immunosuppressive therapy (n=7), the peak SBP varied widely and was recorded at less than 180 mm Hg in 5 of 7 patients. Posterior reversible encephalopathy syndrome in these 2 groups, compared with patients with hypertensive encephalopathy from the same report, occurred at lower blood pressure levels, conceivably due to the confounding effects of endothelial dysfunction in patients with eclampsia, and to the direct toxic effects and vasoconstriction in patients treated with calcineurin inhibitors (cyclosporine and tacrolimus).
We did not observe statistically significant differences in maternal demographics or diagnostic laboratory findings between patients who underwent brain imaging and those who did not. However, given the small patient number in each group, our study may have been underpowered to effectively assess these differences. A comparative study of PRES in pregnant vs nonpregnant patients with various PRES etiologies (hypertensive crisis, chemotherapy, vasculitis) demonstrated differences with respect to age, medical history, and occurrence of headaches (87.5% vs 30.8%, respectively); no differences in symptoms, imaging, and outcomes were noted.12 The study concluded that, despite different triggering events, PRES in pregnant and nonpregnant patients is the same disease entity.
Several clinical studies have indicated that eclamptic seizure activity may occur in patients with minimal elevations in blood pressure. This has fueled the ongoing debate as to the role of hypertension, if any, in the pathogenesis of eclamptic seizures. It is possible that blood pressure alone is not the exclusive cause, and that endothelial dysfunction, which is a hallmark of preeclampsia, is also a contributing factor. Alternatively, pregnancy itself may decrease the threshold at which an elevation in blood pressure may lead to cerebral hyperperfusion and brain edema.13 Taken together, these data raise an important question, which relates to the current treatment guidelines from the 2000 National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy.1 These guidelines advise the institution of antihypertensive therapy for a DBP of 105 to 110 mm Hg or higher for patients with sudden escalating hypertension or imminent or frank eclampsia. For adolescent patients with a recent DBP of less than 75 mm Hg, these guidelines suggest treatment of persistent DBP of 100 mm Hg or greater. The elevation in SBP at which treatment is indicated, however, has not been defined. A retrospective study of 28 patients who had a stroke in association with severe preeclampsia and eclampsia called for a paradigm shift and recommended treatment with antihypertensive therapy for these patients when the SBP reaches or exceeds 155 to 160 mm Hg.14 Of note, blood pressure measurements before the stroke were available in 24 patients from this group, and the SBP was 160 mm Hg or greater in 23 (95.5%) of these patients, and 155 mm Hg or greater in all 28 patients. In contrast, the DBP was 105 mm Hg or greater in only 5 (20.8%) of the 24 patients with blood pressure measurements before the stroke. On the basis of our findings of PRES and eclamptic seizures at a peak SBP of less than 180 mm Hg in 5 of 7 patients, we support these recommendations. Appropriate reduction in blood pressure may prevent progression from vasogenic to cytotoxic edema and cerebral infarction and the resultant permanent neurologic deficits. Because abrupt decreases in blood pressure may adversely affect uteroplacental perfusion and fetal status, treatment of hypertension should mandate close maternal blood pressure and fetal monitoring.
On the basis of the results of neuroimaging studies in eclampsia, 2 potential pathophysiologic mechanisms underlying the development of cerebral lesions and seizures have been proposed: vasogenic and cytotoxic edema.15-17 Vasogenic edema occurs as a consequence of an abrupt elevation in blood pressure to greater than 150 mm Hg, which overcomes intrinsic myogenic vasoconstriction, leading to hyperperfusion and subsequent edema. Zeeman et al18 have shown that patients with preeclampsia or eclampsia develop significantly increased cerebral blood flow, which causes hyperperfusion with resultant vasogenic edema. In contrast, cytotoxic edema may be caused by cerebrovascular “overregulation,” which causes extreme vasospasm and infarction. Vasogenic vs cytotoxic edema can be differentiated using MRI diffusion-weighted imaging (DWI), which detects changes in water distribution in cerebral tissue. The DWI is decreased in vasogenic edema because of increased extracellular fluid but is hyperintense or “bright” in cytotoxic edema due to restricted diffusion. However, in some cases of vasogenic edema, a hyperintense signal may be present due to a “T2 shine-through” phenomenon. Further discrimination is possible by estimation of the apparent diffusion coefficient (ADC) of the involved tissue. An elevated ADC represents water molecules with increased diffusional motion (ie, vasogenic edema), whereas a decreased ADC represents restricted diffusion, which is consistent with cytotoxic edema. Published data suggest that cerebrovascular events in preeclampsia/eclampsia encompass a spectrum of severity, with reversible vasogenic edema at one extreme and irreversible cytotoxic edema and cerebral ischemia at the other. A prospective study of 27 patients with eclampsia10 reported that 25 had radiologic evidence of cerebral edema on T2-weighted imaging. Hyperintense areas were identified by DWI in 15 of these 25 patients; 12 of these 15 patients had vasogenic edema, as characterized by an increased ADC, and 6 patients had concurrent evidence of cerebral infarction. Five patients demonstrated abnormal neuroradiologic findings 6 to 8 weeks postpartum, presumably caused by gliosis in response to infarction. Similarly, in our study, 1 patient demonstrated residual neuroimaging abnormalities.
Our study raises another important question: should patients with a classical clinical presentation of eclampsia routinely undergo imaging studies, given that the results may or may not affect their treatment? Of note, current management consists of proceeding with expeditious delivery, with administration of magnesium prophylaxis for premonitory signs of eclampsia. The efficacy of magnesium sulfate in preventing eclamptic seizures may be in part related to its ability to reduce cerebral perfusion pressure in preeclamptic patients with high cerebral perfusion pressures at baseline.19 Our case series clearly demonstrates the current practice of confirming the diagnosis of eclampsia on the basis of the clinical presentation and reserving imaging for patients with atypical presentations, such as those who develop seizures after delivery. In addition, the reversibility of clinical signs and radiologic abnormalities may argue against neuroimaging of patients at risk of PRES. However, several conditions that can present during pregnancy and postpartum, including acute stroke and systemic diseases that are associated with central nervous system vasculitis (such as systemic lupus erythematosus), may mimic eclampsia. Differentiating among these conditions, which may be difficult on clinical grounds alone, may affect treatment and long-term neurologic outcomes. For example, aggressive blood pressure control is desirable in PRES, in contrast to the management recommendations for acute stroke, which permit mild to moderate hypertension. In conclusion, for patients with an uncertain diagnosis, timely imaging and a diagnosis of PRES may lead to more appropriate decisions regarding treatment of hypertension, thus preventing the possible development of permanent neurologic deficits.
Limitations of our study include its retrospective design and the fact that it represents a limited experience from a single tertiary center. In addition, 5 of 7 patients who underwent imaging experienced the onset of seizures after delivery, whereas all except 1 of the patients who did not undergo imaging experienced the onset of seizure activity before delivery. This clearly represents a selection bias, likely introduced by the fact that the differential diagnosis in patients presenting with seizures after delivery is somewhat more complex. Another selection bias stems from the fact that imaging was performed for patients with clinical signs of PRES only in the presence of seizures (ie, patients with eclampsia) and not for those without seizures (ie, patients with preeclampsia), again reflecting common clinical practice. Despite these limitations, our study suggests that the clinical syndrome of eclampsia is associated with anatomical findings recognizable by neuroimaging as PRES, which occurs at lower levels of blood pressure elevation than in the nonpregnant state. In addition, our study clearly identifies the need and sets the stage for a prospective study of clinical and neuroradiologic correlates in pregnant women with clinical signs of PRES at the time of presentation and their long-term neurologic outcomes.
We propose that the therapeutic targets for SBP be included in the guidelines regarding antihypertensive therapy in pregnancy, particularly when associated with the premonitory signs of eclampsia.1 Further research is needed to determine both the clinical utility of MRI for patients with preeclampsia and the benefit of more aggressive blood pressure control, with the intent of predicting eclampsia and optimizing neurologic outcomes, respectively. As recent epidemiologic data have indicated that preeclampsia is an independent risk factor for stroke later in life20 and that women who have had eclampsia may experience impaired cognitive functioning later in life,21 optimization of blood pressure management at the time of delivery may not only improve immediate pregnancy outcomes but may lower the future cerebrovascular impact of these disorders in affected patients.
CONCLUSION
In our retrospective study in patients with eclampsia, all those who underwent imaging displayed clinical and radiologic findings of PRES. We could find no clinical differences between patients who underwent imaging and those who did not. Several patients developed seizures without a severe elevation of blood pressure. We propose that PRES and the seizures of eclampsia are pathophysiologically related and that eclamptic patients may have seizure onset at lower blood pressures than patients with hypertensive encephalopathy and PRES. On the basis of our results and in the context of previously published work by others, we support the addition of the therapeutic targets for SBP to the guidelines for antihypertensive management of hypertension in pregnancy.
REFERENCES
- 1. National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22 [PubMed] [Google Scholar]
- 2. Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998-2005. Obstet Gynecol. 2010;116(6):1302-1309 [DOI] [PubMed] [Google Scholar]
- 3. Lindheimer MD, Taler SJ, Cunningham FG. ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich). 2009;11(4):214-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersgaard AB, Herbst A, Johansen M, et al. Eclampsia in Scandinavia: incidence, substandard care, and potentially preventable cases. Acta Obstet Gynecol Scand. 2006;85(8):929-936 [DOI] [PubMed] [Google Scholar]
- 5. Ventura SJ, Martin JA, Curtin SC, Mathews TJ, Park MM. Births: final data for 1998. Natl Vital Stat Rep. 2000;48(3):1-100 [PubMed] [Google Scholar]
- 6. Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309(6966):1395-1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500 [DOI] [PubMed] [Google Scholar]
- 8. Jürgensen JS, Nibbe L, Hoffmann KT, Niehaus L. Postpartum blindness. Lancet. 2001;358(9290):1338 [DOI] [PubMed] [Google Scholar]
- 9. Servillo G, Striano P, Striano S, et al. Posterior reversible encephalopathy syndrome (PRES) in critically ill obstetric patients. Intensive Care Med. 2003;29(12):2323-2326 [DOI] [PubMed] [Google Scholar]
- 10. Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol. 2004;190(3):714-720 [DOI] [PubMed] [Google Scholar]
- 11. Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: is there a difference between pregnant and non-pregnant patients? Eur Neurol. 2009;62(3):142-148 [DOI] [PubMed] [Google Scholar]
- 13. Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50(1):14-24 [DOI] [PubMed] [Google Scholar]
- 14. Martin JN, Jr, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105(2):246-254 [DOI] [PubMed] [Google Scholar]
- 15. Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23(6):1038-1048 [PMC free article] [PubMed] [Google Scholar]
- 16. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeeman GG, Hatab M, Twickler DM. Maternal cerebral blood flow changes in pregnancy. Am J Obstet Gynecol. 2003;189(4):968-972 [DOI] [PubMed] [Google Scholar]
- 19. Belfort M, Allred J, Dildy G. Magnesium sulfate decreases cerebral perfusion pressure in preeclampsia. Hypertens Pregnancy. 2008;27(4):315-327 [DOI] [PubMed] [Google Scholar]
- 20. Garovic VDBK, Boerwinkle E, Hunt SC, et al. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J Hypertens. 2010;28(4):826-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aukes AM, Wessel I, Dubois AM, Aarnoudse JG, Zeeman GG. Self-reported cognitive functioning in formerly eclamptic women. Am J Obstet Gynecol. 2007;197(4):365.e1-365.e6 [DOI] [PubMed] [Google Scholar]