Abstract
OBJECTIVES
Quantify the prevalence, measure the severity, and describe treatment patterns in patients who present to medical clinics in Texas with community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) skin and soft-tissue infections (SSTI).
METHODS
Ten primary care clinics participated in this prospective, community-based study. Clinicians consented patients and collected clinical information, pictures, and wound swabs; data were processed centrally. MRSASelect™ was used for identification. Susceptibilities were determined via Etest®.
RESULTS
Overall, 73/119 (61%) patients presenting with SSTIs meeting eligibility requirements had CA-MRSA. Among these, 49% were male, 79% were Hispanic, and 30% had diabetes. Half (56%) of the lesions were ≥ 5 cm in diameter. Most patients had abscesses (82%) and many reported pain scores of ≥ 7/10 (67%). Many presented with erythema (85%) or drainage (56%). Most received incision and drainage (I&D) plus an antibiotic (64%). Antibiotic monotherapy was frequently prescribed: trimethoprim-sulfamethoxazole (TMP-SMX) (78%), clindamycin (4%), doxycycline (2%), and mupirocin (2%). The rest received TMP-SMX in combination with other antibiotics. TMP-SMX was frequently administered as one double-strength tablet twice daily. Isolates were 93% susceptible to clindamycin and 100% susceptible to TMP-SMX, doxycycline, vancomycin, and linezolid.
CONCLUSIONS
We report a predominance of CA-MRSA SSTIs, favorable antibiotic susceptibilities, and frequent use of TMP-SMX in primary care clinics.
Introduction
Skin and soft tissue infections (SSTIs) affect millions of individuals annually in the United States. SSTI incidence has tripled over the last 15 years in ambulatory settings, such as hospital-affiliated outpatient clinics, emergency departments, and community practices.1–3 Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) accounts for the bulk of this rise and now constitutes the leading cause of SSTIs.4 Studies examining CA-MRSA SSTIs are abdundant,4–15 but few have investigated patients managed in community settings, such as, primary care, family medicine, or general internal medicine clinics.16–18 The purpose of this study was to quantify the prevalence, measure the severity, and describe treatment patterns for patients who presented with CA-MRSA SSTIs to community-based, outpatient medical clinics.
Methods
Study Design, Setting, and Population
This investigation was a multisite, prospective, community-based, observational study. The South Texas Ambulatory Research Network (STARNet), a practice-based research network (PBRN) encompassing urban, suburban, and rural primary care clinics in South Texas, was the site for this research. Ten family practice and internal medicine clinics, spanning four Texas counties (Bexar, Comal, Travis, and Williamson) and 3,000 square miles, participated in this study; these data represent cases collected between October 01, 2009 and September 30, 2010. Patients were eligible for study enrollment if they were 18 years of age or older and presented to one of the participating clinics: (1) with a SSTI, (2) if the managing physician suspected MRSA, and (3) if a wound culture was planned. Patients were ineligible if they were pregnant, decisionally impaired, or incarcerated. The University of Texas Health Science Center Institutional Review Board (IRB) granted approval for all participating sites.
Data Collection, Processing, and Analysis
Centralized research personnel provided clinics with English and Spanish informed consent forms, a digital camera, and individually labeled plastic bags, each containing a patient data card, 15 cm plastic ruler (Accu-Ruler®, Macon, GA), and a rayon-tipped wound swab (Bactiswab®, Remel, Lenexa, KS). Modified Stuart’s Media (transport media) was encapsulated by a crushable ampoule located at the base of each swab casing, near the swab tip. STARNet clinicians consented patients, recorded demographic and clinical information, measured the size of the lesion, obtained a wound culture, crushed the ampoule to release transport media onto the swab tip, and captured digital pictures of the infection. Clinical information included patient gender, race (Black, White, Other), ethnicity (Hispanic, Non-Hispanic), diabetes history, health-related work history, skin infection history, height, weight, pain score (1 to 10, with 10 being worst) at the time of physical examination, health insurance category (e.g., private, Medicare/Medicaid, none/self-pay), infection characteristics (e.g., location, duration, size, deepest tunnel depth, erythema, smell, ulceration, drainage, abscess, satellites), incision and drainage procedures received, antibiotics prescribed, and plans for follow-up.
Research personnel retrieved the data cards, wound swabs, and pictures from the clinics and returned these materials to a central laboratory for processing. Wound swabs were plated directly onto pre-filled Tryptic Soy agar (TSA) plates (Hardy Diagnostics, Santa Maria, CA) and incubated for 18 to 24 hours at 35 to 37°C; then sub-cultured. If growth did not occur after three attempts, “no growth” was recorded. Stock solutions were prepared and stored at −20°C.
Frozen isolates were later thawed, plated, and sub-cultured. Isolates were then plated onto MRSASelect™ chromogenic agar (Bio-Rad Laboratories, Hercules, CA) plates for identification and isolation of MRSA; incubation occurred for 18 to 28 hours at 35 to 37°C, protected from light. MRSA appeared as small pink colonies.
MRSA-positive isolates were subjected to antibiotic susceptibility testing against trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, clindamycin, linezolid, and vancomycin via Etest® (AB Biodisk, Solna, Sweden) on Mueller-Hinton agar (MHA) plates (Hardy Diagnostics). MIC50, MIC90, and percent susceptible were calculated using Clinical and Laboratory Standards Institute (CLSI) breakpoints for S. aureus.19 Double-disk diffusion tests (D-tests) were performed for each MRSA isolate to evaluate the presence of inducible clindamycin resistance. Isolates with positive D-tests were reported as resistant to clindamycin. The following American Type Culture Collection (ATCC) staphylococcal strains (Remel, Lenexa, KS) were used as quality controls: ATCC 43300, ATCC 25923, and ATCC 29213.
Patients with complete clinical data and positive MRSA cultures were further evaluated. Descriptive statistics were used to characterize this population. JMP 8.0 statistical software (SAS Institute, Cary, NC) was used for all statistical analyses. Severity of illness was approximated through the application of Food and Drug Administration (FDA) criteria.20,21 The FDA defines “complicated” SSTIs as those, (1) involving deeper soft tissues, (2) requiring surgical intervention, (3) involving ulcers, burns or wounds, (4) presenting as a major abscess, (5) located near the rectal area, or (6) complicated by an underlying medical condition, (including diabetes mellitus). Recently, the FDA standardized definitions for acute bacterial skin and skin structure infections (ABSSSIs); 21 “major abscess” affects a body surface area equal to or greater than 75 cm2, whereas “minor abscess” affects an area less than 5 cm from the peripheral margin of the abscess. We considered abscesses that were neither “major” nor “minor” to be “moderate”. SSTIs were classified as “moderate or complicated” if either of the following were present: (1) lesion ≥ 5 cm, or (2) history of diabetes mellitus.
Results
A total of 123 cases were collected from 10 primary care clinics over a 12-month period. Three patients lacked clinical information and one did not have a wound culture; therefore, 119/123 (97%) patients met study eligibilty criteria. Of the 119 remaining cases, 73 (61%) cultures were MRSA positive, 30 (25%) grew organisms other than MRSA, and 16 (14%) did not grow. Table 1 depicts patient demographics for patient with (n=73) and without (n=46) MRSA.
Table 1.
Patient Demographics
| Characteristic | Overall (n=119) | MRSA (n=73) | Non-MRSA (n=46) |
|---|---|---|---|
| Gender, % | |||
| Male | 61 (51%) | 36 (49%) | 25 (54%) |
| Female | 58 (49%) | 37 (51%) | 21 (46%) |
| Race*, % | |||
| Black | 8 (8%) | 4 (7%) | 4 (11%) |
| White | 70 (74%) | 42 (71%) | 28 (76%) |
| Other | 20 (21%) | 13 (22%) | 7 (19%) |
| Ethnicity†, % | |||
| Hispanic | 90 (82%) | 55 (79%) | 35 (88%) |
| Non-Hispanic | 20 (18%) | 15 (21%) | 5 (12%) |
| BMI‡ (kg/m2), median (IQR§) | 31 (25–37) | 30 (25–36) | 31 (23–38) |
| Risk factors, % | |||
| Diabetes | 31 (26%) | 22 (30%) | 9 (20%) |
| Provides healthcare to others | 5 (4%) | 2 (3%) | 3 (7%) |
| SSTI within past 12 months | 52 (44%) | 28 (38%) | 24 (52%) |
| Received antibiotics for SSTI within past 12 months | 22 (18%) | 14 (19%) | 8 (17%) |
| MRSA SSTI within past 12 months | 10 (8%) | 5 (7%) | 5 (11%) |
| SSTI within past 90 days | 19 (16%) | 11 (15%) | 8 (17%) |
Race missing for 15 MRSA positive and 9 MRSA negative patients
Ethnicity missing for 3 MRSA positive and 6 MRSA negative patients
BMI missing for 37 MRSA positive and 18 MRSA negative patients
IQR, interquartile range
Study Cohort (n=119)
Overall, 49% of patients were female, 74% were white, 8% were Black, and 21% were “other.” Data for ethnicity was available for 110 patients; 90 (82%) were Hispanic. Patients had a median (interquartile range) body mass index of 31 (25–37) kg/m2, 26% had diabetes, and 4% provided healthcare to others. Almost one-half of patients (44%) reported they had a SSTI in the past 12 months, 18% reported receiving antibiotics for an SSTI in the past 12 months, and 8% reported they had a MRSA SSTI in the past 12 months. Finally, 16% of patients reported a SSTI in the last 90 days.
MRSA Cohort (n=73)
Among the 73 MRSA cases, 51% of patients were female, 71% were white, 7% were Black, and 22% were “other.” Data for ethnicity was available for 70 patients; 55 (79%) were Hispanic. Patients had a median (interquartile range) body mass index of 30 (25–36) kg/m2, 30% had diabetes, and 3% provided healthcare to others. More than one-third of patients (38%) reported they had a SSTI in the past 12 months, 19% reported receiving antibiotics for an SSTI in the past 12 months, and 7% reported they had a MRSA SSTI in the past 12 months. Finally, 15% of patients reported a SSTI in the last 90 days.
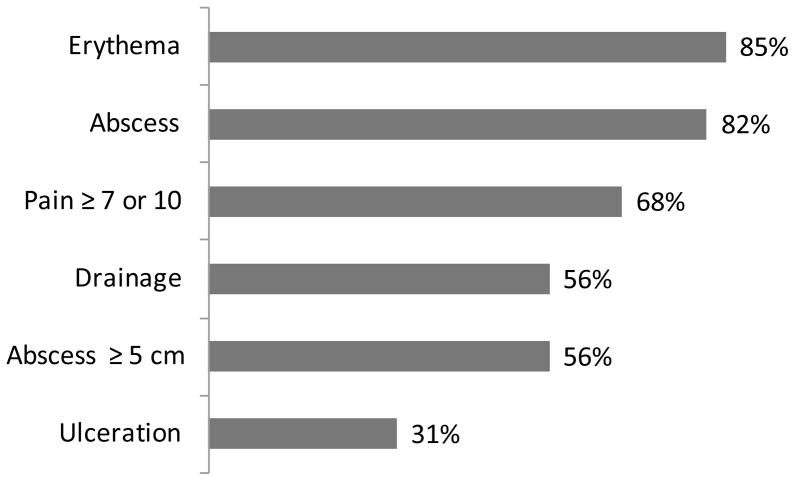
For the 70 MRSA patients in which lesion measurements were available, more than half (56%) of the skin lesions were ≥ 5 cm in diameter, with the smallest measuring 0.5 cm X 0.25 cm and the largest 14 cm X 13 cm. Data describing wound characterisitics were available for 70 MRSA patients (Figure 1).
Figure 1.
Wound Characteristics for Patients with CA-MRSA SSTIs, n=73
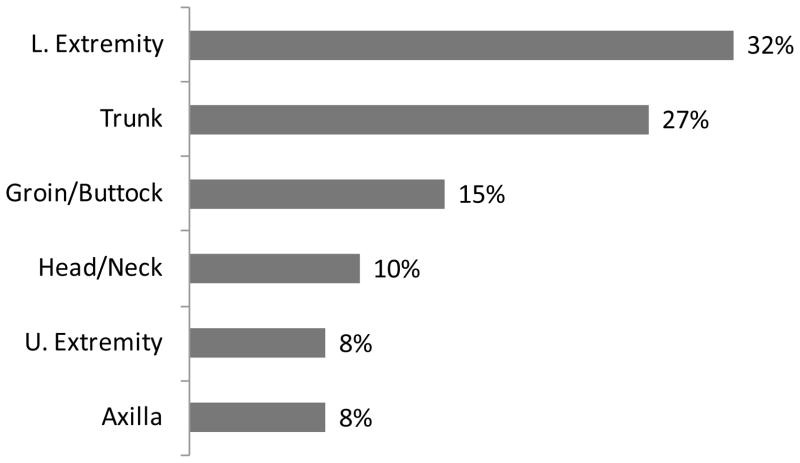
Among these, most (82%) had abscesses; 56% of the abscesses were ≥ 5 cm in diameter. Many MRSA patients presented with erythema (85%), drainage (56%), or ulceration (31%). Pain scores were recorded for 58 patients; the majority (68%) of these experienced pain scores of seven or higher (out of ten, with ten being worst) at the time of physical examination. All patients were infected at only one anatomic site (Figure 2). The lower extremities (32%) and torso (27%) were the most common sites where lesions were found, followed by the groin and gluteal regions (15%), head and neck (10%), upper extremities (8%), and axillary area (8%).
Figure 2.
Infected Body Sites for Patients with CA-MRSA SSTIs, n=73
Collectively, 66% of patients with MRSA SSTIs had moderate or complicated infections, defined by the presence of either a lesion ≥ 5 cm or a history of diabetes (Figure 3).
Figure 3. Infection Severity for Patients with CA-MRSA SSTIs, n=70*.
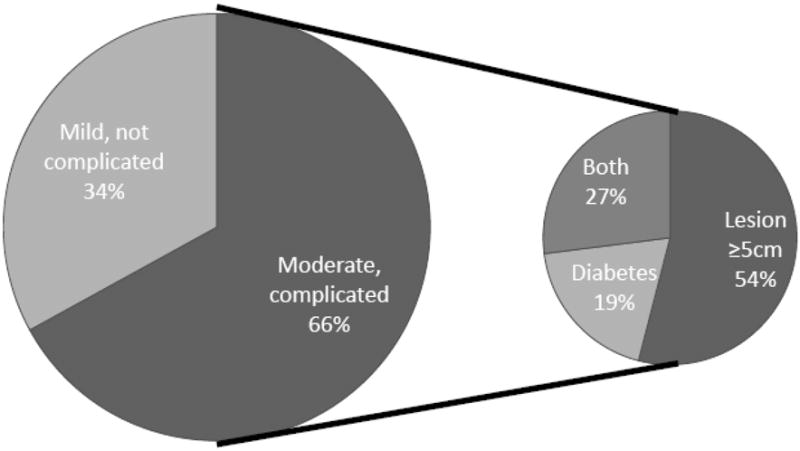
* Moderate or complicated infections were defined by the presence of either a lesion ≥ 5 cm or a patient history of diabetes. Lesion size was missing for 3 patients.
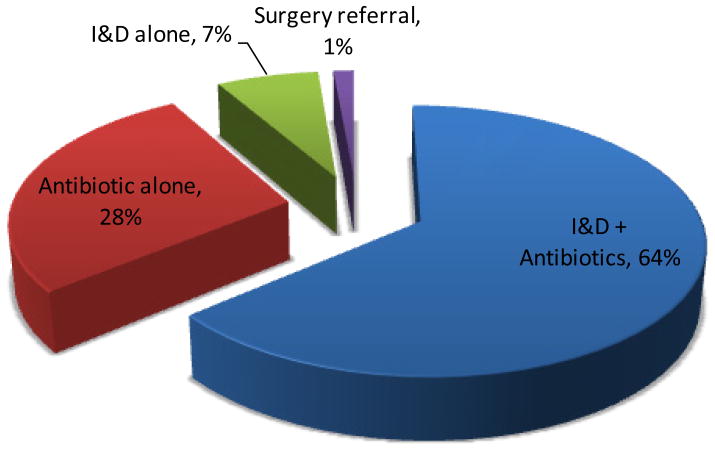
Clinicians did not document the treatment approach for one patient; therefore, the treatment information includes 72 patients (Figure 4). Most patients received incision and drainage (I&D) plus an antibiotic (64%), followed by an antibiotic alone (28%) and I&D alone (7%). The remaining patient went to surgery.
Figure 4. Treatment Approach for Patients with CA-MRSA SSTIs, n=72*.
* Treatment approach was unavailable for 1 patient
Clinicians recorded antibiotic use for 64 patients (Figure 5). The majority (86%) of these patients received antibiotic monotherapy; 78% TMP-SMX monotherapy, 4% clindamycin monotherapy, 2% doxycycline monotherapy, and 2% topical mupirocin monotherapy. The rest received TMP-SMX in combination with other antibiotics (12%) or clindamycin plus mupirocin (2%). Nearly all of the patients treated with TMP-SMX (n=53/58) received one double-strength (160/800 mg) tablet orally twice daily for 10 days.
Figure 5. Antibiotics Prescribed for Patients with CA-MRSA SSTIs, n=64*.
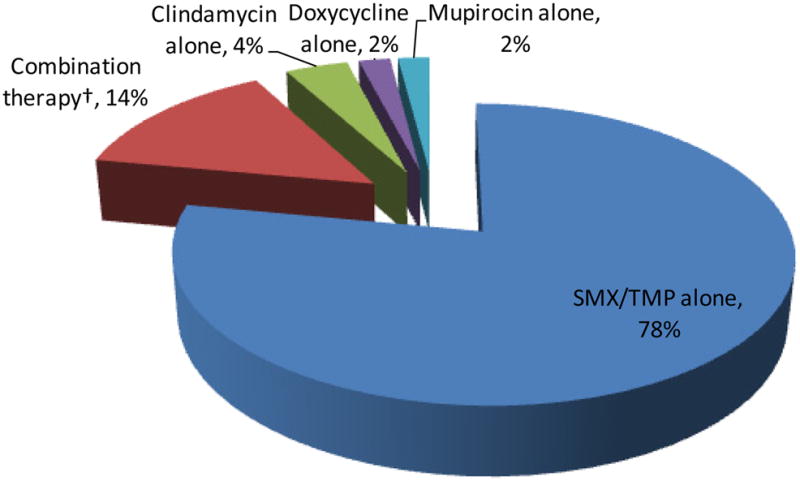
*Antibiotics were not available for 9 patients
†Combination therapy = TMP-SMX + beta-lactam (4/9), TMP-SMX + clindamycin (2/9), TMP-SMX + doxycycline (2/9), clindamycin + mupirocin (1/9)
MIC50, MIC90, and percent susceptible were determined for all 73 MRSA isolates. With the exception of clindamycin (percent susceptible = 93%), these isolates were universally susceptible to all antibiotics tested (Table 2).
Table 2.
Microbiologic Data for Patients with CA-MRSA SSTIs, n=73
| Antibiotic | MIC50 (μg/mL) | MIC90 (μg/mL) | % of Isolates Susceptible |
|---|---|---|---|
| TMP-SMX | 0.06 | 0.12 | 100 |
| Doxycycline | 0.06 | 0.06 | 100 |
| Clindamycin | 0.06 | 0.12 | 93 |
| Linezolid | 1.0 | 2.0 | 100 |
| Vancomycin | 2.0 | 2.0 | 100 |
Discussion
This study provides new information regarding the prevalence, severity, and treatment of CA-MRSA skin and soft tissue infections in primary care clinics. The prevalence of CA-MRSA in these 10 clinics (61%) was similar to that seen in a prior study of 11 emergency departments (59%).4 We also report a high percentage of moderate or complicated infections (66%), a low rate of clindamycin resistance (7%), and frequent use of TMP-SMX in our community.
SSTIs comprise a myriad of infections that vary greatly in presentation and disease severity. Although severity scoring systems have been developed for SSTIs, 22,23 their implementation has been elusive. Recently, a severity scoring system for cellulitis was developed at an outpatient academic tertiary-care clinic in Honolulu, Hawaii.18 Patients received 1 point for each of the following clinical characteristics: lesion size ≥ 5 cm in diameter, > 3 affected areas, concurrent ulcers or abscesses, fever, leukocytosis, and hypotension. Points were then summed to derive the severity score: mild (0–1), moderate (2–3), or severe (4–6). Unfortunately, no validated scale exists for skin abscesses and other culturable SSTIs. For the present study, FDA criteria were applied to approximate SSTI severity.
More than half of the patients in this study had lesions ≥ 5 cm. A prior study in 69 pediatric patients admitted to an emergency department found that CA-MRSA infections > 5 cm in diameter were associated with an increased need for hospitalization.5 subsequent second study supports this finding,14 while a third disagrees.10 Furthermore, 30% of our cohort had diabetes, a complicating condition implicated in FDA guidance documents; several studies do not report diabetes,4,6,11 while others report prevalence ranging from 2%10 to 36%18. Collectively, 66% of patients either had a lesion ≥ 5 cm or a history of diabetes. Our data provide evidence that patients present to primary care clinics with a wide spectrum of CA-MRSA infections, including those that meet FDA criteria for “complicated” infections.
The bulk of patients in our cohort received I&D with (64%) or without (7%) antibiotics. National guidelines emphasize the need for I&D in patients with purulent infections.24–26 Some studies suggest I&D alone might be sufficient for the management of uncomplicated SSTIs;5,7,9,11 however, other studies have found additional benefit when anti-MRSA antibiotics are administered with I&D.8,12,13,16,18 The guidelines state concurrent antibiotics are warranted when any of the following are present: severe, extensive, or rapidly progressing disease, signs and symptoms of systemic illness, comorbid conditions, immunosuppression, extremes of age, SSTI in an area difficult to drain, previous failure of I&D, or septic phlebitis;26 66% of patients from our community would warrant antibiotic use, according to these guidelines.
Concern for MRSA has caused a major shift in prescribing from beta-lactams to other antibiotics with better in vitro susceptibilities against MRSA.1 The primary care physicians in our clinics exclusively prescribed antibiotics with in vitro activity against MRSA. This is consistent with a recent survey of 207 board-certified emergency physicians in which 80% answered that they would sometimes (31%) or always (49%) prescribe an antibiotic in addition to I&D for a patient presenting with simple abscess to the emergency department; anti-MRSA antibiotics were recommended by 81% of physicians who advocated empiric antibiotics.27
Our rate of clinidamycin susceptibility was much higher than previously reported by other groups, even when accounting for inducible resistance.28 A study conducted within the 2004 LEADER surveillance network reported clindamycin resistance rates as high as 32% for U.S. outpatient MRSA isolates,29 whereas other studies have reported susceptibility rates > 90%.8,14,16 Protein synthesis inhibitors, like clindamycin and linezolid, may offer some unique advantages against CA-MRSA. Clindamycin decreases exotoxin production in vitro,30 and may inhibit Panton-Valentine Leukocidin (PVL), a potent exotoxin commonly harbored by CA-MRSA. Unlike doxycycline and TMP-SMX, clindamycin displays reliable in vitro activity against group A streptococci (GAS), a common cause of cellulitis. While the use of clindamycin is associated with justified concerns such as frequent gastrointestinal side effects, variable MRSA susceptibilty, and risks of colonization and infection with Clostrium difficile, its activity against GAS and ability to decrease exotoxin production offer potential benefits.
Clinicians from these 10 medical clinics prescribed TMP-SMX to 91% of patients that received antibiotics. This is consistent with a national study in which TMP-SMX was the most common oral anti-MRSA antibiotic prescribed for SSTIs.1 Practitioners in our clinics prescribed one double-strength tablet in all but five cases.
This study has strengths and limitations. First, the study involved non-pregnant adults from one geographic region, which may limit the generalizability. This study included only those patients for whom the clinician suspected MRSA and obtained a culture; therefore, this study possibly over estimates the burden of CA-MRSA in SSTIs that do not meet these criteria. We assumed all patients presenting with MRSA had CA-MRSA, but we did not perform molecular testing to validate this; however, the high rates of antibiotic susceptibility suggest these isolates were CA-MRSA. Our assessment of SSTI severity has not been validated, but was adapted from guidance released by the FDA. Despite these limitations, our study has unique strengths. The literature is limited regarding the investigation of CA-MRSA SSTIs in the community.16–18 Most CA-MRSA SSTI studies to date have been based in emergency departments, specialty clinics, or hospital-affiliated outpatient clinics.4–15 The multi-site, prospective design used in this study permitted collection of demographic, clinical, and microbiological data from several community-based primary care clinics in a practice-based research network.
Conclusion
Our investigation provides new data regarding the prevalence, severity, and treatment of CA-MRSA skin and soft-tissue infections in primary care clinics. Many patients presenting to medical clinics in Texas with SSTIs have CA-MRSA. These infections commonly manifest as painful, erythematous abscesses. Many are moderate disease severity or complicated by an underlying medical condition. Most patients receive incision and drainage plus anti-MRSA antibiotics. CA-MRSA isolates in this region are frequently susceptible to non-beta-lactam antibiotics. TMP-SMX-containing antibiotic regimens are frequently prescribed.
Acknowledgments
Financial Support: This work was supported by the United States National Institutes of Health in the form of a CTSA pilot project grant (UL1 RR025767) and a KL2 career development award (KL2 RR025766), both awarded to Dr. Frei. Drs. Parchman and Mortensen were supported by the South Texas Veterans Health Care System when this work was completed. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Many thanks to our South Texas Ambulatory Research Network (STARNet) colleagues: Holly Hayes, Stephanie Reyes, Marisa Rodriquez, Paula Winkler, Liem Du, Natalie Nyren, Michael Mann, Joel Peña, Guillermo Rocha, Abilio Muñoz, Stella Koretsky, Miguel Ayala, Lucina Treviño, Sylvia Treviño, Yolanda Marcos, Mitch Finnie, and Sandra Esparza.
Footnotes
Conflicting and Competing Interests: JHJ has received funds for consulting from BD Microbiology and institutional grants from Biomérieux and Pfizer. CRF has received funds for consulting and board membership from Ortho-McNeil Janssen and institutional grants from Ortho-McNeil Janssen and Pfizer. All others declared no conflicts of interest.
Authorship: All authors met the following three critieria for authorship: (1) provided contributions to the conception and design, acquisition, or analysis of data, (2) aided in drafting or critically revising the manuscript for important intellectual content, and (3) provided approval for the final manuscript
References
- 1.Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168:1585–91. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 2.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51(3):291–8. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Taira BR, Singer AJ, Thode HC, Jr, Lee CC. National epidemiology of cutaneous abscesses: 1996 to 2005. Am J Emerg Med. 2009;27:289–92. doi: 10.1016/j.ajem.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 5.Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123–7. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 6.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 7.Paydar KZ, Hansen SL, Charlebois ED, Harris HW, Young DM. Inappropriate antibiotic use in soft tissue infections. Arch Surg. 2006;141:850–4. doi: 10.1001/archsurg.141.9.850. [DOI] [PubMed] [Google Scholar]
- 8.Ruhe JJ, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44:777–84. doi: 10.1086/511872. [DOI] [PubMed] [Google Scholar]
- 9.Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2010;55:401–7. doi: 10.1016/j.annemergmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044–8. doi: 10.1128/AAC.00377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283–7. doi: 10.1016/j.annemergmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Ruhe JJ, Menon A. Tetracyclines as an oral treatment option for patients with community onset skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(9):3298–303. doi: 10.1128/AAC.00262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szumowski JD, Cohen DE, Kanaya F, Mayer KH. Treatment and outcomes of infections by methicillin-resistant Staphylococcus aureus at an ambulatory clinic. Antimicrob Agents Chemother. 2007;51:423–8. doi: 10.1128/AAC.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frazee BW, Lynn J, Charlebois ED, Lambert L, Lowery D, Perdreau-Remington F. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45:311–20. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 15.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–17. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 16.Frei CR, Miller ML, Lewis JS, 2nd, Lawson KA, Hunter JM, Oramasionwu CU, Talbert RL. Trimethoprim-sulfamethoxazole or clindamycin for community-associated MRSA (CA-MRSA) skin infections. J Am Board Fam Med. 2010;23:714–9. doi: 10.3122/jabfm.2010.06.090270. [DOI] [PubMed] [Google Scholar]
- 17.Parchman ML, Munoz A. Risk factors for methicillin-resistant Staphylococcal aureus skin and soft tissue infections presenting in primary care: a South Texas Ambulatory Research Network (STARNet) study. J Am Board Fam Med. 2009;22:375–9. doi: 10.3122/jabfm.2009.04.090003. [DOI] [PubMed] [Google Scholar]
- 18.Khawcharoenporn T, Tice A. Empiric outpatient therapy with trimethoprim-sulfamethoxazole, cephalexin, or clindamycin for cellulitis. Am J Med. 2010;123:942–50. doi: 10.1016/j.amjmed.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 19.CLSI. CLSI approved standard M100-S17. Clinical and Laboratory Standards Institute; Wayne, PA: 2007. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 20.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) [Accessed 20 January 2011.];Guidance for industry: uncomplicated and complicated skin and skin structure infections—developing antimicrobial drugs for treatment. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf.
- 21.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) [Accessed 21 January 2011.];Draft guidance for industry: acute bacterial skin and skin structure infections—developing drugs for treatment. 2010 August; http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf.
- 22.Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA. Expert panel on managing skin and soft tissue infections. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(Suppl S1):i3–17. doi: 10.1093/jac/dkg466. [DOI] [PubMed] [Google Scholar]
- 23.Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbio. 2008;19:173–84. doi: 10.1155/2008/846453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 25.Gorwitz RJ, Jernigan DB, Powers JH Participants in the CDC Convened Experts’ Meeting on Management of MRSA in the Community. Strategies for clinical management of MRSA in the community: Summary of an experts’ meeting convened by the Centers for Disease Control and Prevention. 2006. [Accessed 10 January 2011.]; Available at http://www.cdc.gov/ncidod/dhqp/ar_mrsa_ca.html.
- 26.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 27.LoVecchio F, Perera N, Casanova L, et al. Board-certified emergency physicians’ treatment of skin and soft tissue infections in the community-acquired methicillin-resistant Staphylococcus aureus era. Am J Emerg Med. 2009;27:68–70. doi: 10.1016/j.ajem.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JS, 2nd, Jorgensen JH. Inducible clindamycin resistance in Staphylococci: should clinicians and microbiologists be concerned? Clin Infect Dis. 2005;40:280–5. doi: 10.1086/426894. [DOI] [PubMed] [Google Scholar]
- 29.Draghi DC, Sheehan DF, Hogan P, Sahm DF. Current antimicrobial resistance profiles among methicillin-resistant Staphylococcus aureus encountered in the outpatient setting. Diagn Microbiol Infect Dis. 2006;55:129–33. doi: 10.1016/j.diagmicrobio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis. 2007;195:202–11. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]