Abstract
The frontal lobes in the brain are a component of the cerebral system that supports goal-directed behaviour. However, their functional organization remains controversial. Recent studies have reported rostro-caudal distinctions in frontal cortex activity based on the abstractness of action representations. In addition, some have proposed that these differences reflect a hierarchical organization, whereby anterior frontal regions influence processing by posterior frontal regions during the realization of abstract action goals as motor acts. However, few have considered whether the anatomy and physiology of the frontal lobes support such a scheme. To address this gap, this Review surveys anatomical, neuroimaging, electrophysiological and developmental findings, and considers the question: could the organization of the frontal cortex be hierarchical?
Defining the functional organization of the frontal lobes remains a significant challenge for cognitive neuroscience. The frontal cortex (FIG. 1) has long been associated with higher cognitive functions and cognitive control1–8. Moreover, it is commonly assumed that spatially distinct portions of the prefrontal cortex (PFC) support qualitatively distinct cognitive control functions9–11. However, despite the frontal cortex having been the focus of a considerable amount of research, it has not been easy to distinguish separate frontal regions in terms of their functional properties. Although neuropsychological studies of patients with frontal lobe lesions have demonstrated large-scale distinctions in function between each hemisphere, and between lateral and medial PFC12, they have revealed few examples of double dissociations for tasks or cognitive functions5 (but see REF. 13). Moreover, many neuroimaging studies report frontal activation across a diverse range of tasks and paradigms14. Likewise, single-unit recording experiments have revealed that frontal neurons are highly flexible, capable of changing their tuning properties as task demands change15.
Figure 1. Cytoarchitectonic divisions of the human and monkey frontal lobe.
Rostral and caudal axes are labelled and the numbers represent the update by Petrides and Pandya120 of the Brodmann151 and Walker cytoarchitecture maps. Several investigators have created maps of the frontal cortex based on morphological criteria such as the gross characteristics of cells, the arrangement of these cells in cortical layers, and gross characteristics of myelin in the cortex (for examples see REFS 152,153). However, area boundaries differ significantly between maps. For example, in the lateral prefrontal cortex, Brodmann’s area 9 is extensive and area 46 does not exist152, whereas Walker includes area 46 and a much more restricted area 9 (REF. 153). Another issue concerns the correspondence between maps of monkey and human cortex. Indeed, a comparison between Brodmann’s monkey and human cortex maps reveals seemingly significant differences; for example, the human map includes area 46, but the monkey map does not151,152. Likewise, in Walker’s map of the monkey cortex, area 46 is far more extensive than that depicted in Brodmann’s map of the human cortex. To resolve these discrepancies, Petrides and Pandya10,120,121 performed an extensive comparison of the architecture of the frontal cortex between monkeys and humans. This revealed that both in monkeys and humans, areas 9 and 46 are in the mid-dorsolateral sector of the lateral prefrontal cortex. Also, in both species, area 9 lies dorsal to area 46. However, in humans, area 9 encircles area 46 caudally, which is not the case in monkeys. This caudal portion of area 9 in humans is similar to the caudal portion of area 46 in monkeys. This led Petrides and Pandya to create a new label, area 9/46, which they divided into a dorsal portion (area 9/46d) and a ventral portion (area 9/46v). The nomenclature they put forth in their maps is used in this Review. Figure is reproduced, with permission, from REF. 10 © (2002) Wiley-Blackwell.
However, recent experiments have provided initial support for a functional organizing principle along the rostro-caudal axis of the frontal lobes that is based on a hierarchy of action control16–19. To understand why actions might be processed hierarchically by the brain, consider the act of making a sandwich. The overall goal (‘make a sandwich’) is abstract in that it does not specify what particular sequence of movements is necessary to make the sandwich given one’s current situation. But this abstract goal can be broken down into more specific subgoals, like slicing bread or spreading mayonnaise, and each of these subgoals can be further broken down into more specific sub-subgoals, and so forth. One can continue to decompose the task until it is expressed as a unique series of motor actions. Unlike the abstract goal of making a sandwich, these final motor acts are highly specific. Thus, a traditional idea is that control of action may involve selecting and maintaining goals at multiple levels of abstraction, from general task goals at higher levels (‘make a sandwich’) to concrete motor responses at the lowest levels20–24. A more contentious hypothesis to emerge from cognitive neuroscience is that this hierarchy of action control is instantiated along a rostro-caudal gradient in the frontal cortex, whereby neurons in more-anterior regions of the frontal lobe process abstract action goals (like deciding to make a sandwich), and those in more-posterior regions process more-concrete information about action that is closer to the actual motor output7,16,18,19,25–43. However, it should be noted that abstraction has been defined somewhat differently across these experiments25,27 (BOX 1).
Box 1. Different types of abstraction of frontal lobe representations.
Studies that have distinguished regional differences along the rostro-caudal axis of the frontal lobe based on abstraction have used different definitions of abstraction (see REFS 25,27). These definitions are not mutually exclusive, but it is important to review their characteristics.
Domain generality
A domain-general neuron can encode information across input domains. For example, if a neuron fires to any cue that indicates a leftward joystick movement, regardless of whether the response-relevant dimension of the cue is its location on the screen (spatial domain) or its object identity (object domain; for example, is the cue a picture of an animal versus a tool?), then this is a domain-general neuron.
Relational integration
A first order of relational complexity could involve assigning a property to an item (“What is the colour?”). A second order might involve drawing relations between concrete properties (“Do the colours match?”). A third order could involve evaluating relations among relations, such as verifying the analogy ‘pilot is to airplane as captain is to ship’. As increasing numbers of independent dimensions of stimuli are related to one another in order to determine a response, a relation becomes more abstract2,30,31,43,50,95,97,135,136.
Temporal abstraction
In the example of making a sandwich, the goal ‘make a sandwich’ is relevant for longer than the goal ‘slice bread’. In general, as action goals become more abstract, the timescale of action that they govern increases. Thus, timescale is an important variable in a system that maintains action goals at different levels of abstraction. The extent to which goals (like making a sandwich) are maintained over longer temporal gaps — whereas more concrete goals (like slicing bread and spreading mayonnaise) are being updated — is referred to as temporal abstraction.
Policy abstraction
The goal ‘make a sandwich’ is an abstraction over more specific subgoals, like slicing bread versus heating up a tortilla wrap, depending on the context of the sandwich in question. Policy is the relationship between the state of a system, an action, and an anticipated outcome. Policy abstraction refers to the degree to which a given goal representation forms a generalization over lower-level goal representations27,137–139. In this sense, policy abstraction relates directly to rule complexity. A simple rule linking a stimulus and a response is a first order policy. For example, learning that a red cue means ‘press button 1’ and a blue cue means ‘press button 2’ would be a first order policy. However, assume one also learns that a square means ‘press 1’ and a circle means ‘press 2’. What does one press in response to a red circle? In this case, an additional rule is necessary to determine whether the colour or shape rules are relevant in the current context. Such a meta-rule, linking the context to a relevant set of first order rules, would be a second order policy. Adding additional contingencies will progressively result in more abstract policy.
Moreover, some researchers have suggested that frontal regions along the rostro-caudal axis interact with one another hierarchically17,19,35,37; they propose that there is a dominance relationship whereby higher, more-anterior regions influence processing in lower, more-posterior regions to a greater extent than vice versa. If such a processing hierarchy indeed exists, it would be reasonable to assume that systematic rostro-caudal patterns would be evident in the anatomy and physiology of the frontal cortex.
This Review surveys functional and anatomical studies for differences along the rostro-caudal axis of the frontal lobes. First, we evaluate neural recording, neuropsychological and functional imaging data that support a rostro-caudal gradient of function, focusing on possible regional differences in the response to different levels of abstraction. Then we consider whether the rostro-caudal anatomy of the frontal lobes, including its intrinsic connectivity, also supports this gradient. Finally, we consider how these data support and/or constrain the hypothesis that the rostro-caudal functional organization is hierarchical, and discuss what evidence would be required to demonstrate this hierarchical architecture.
A rostro-caudal axis processing gradient
Neurons in the frontal cortex possess a number of properties that are useful for cognitive control6,44. Specifically, they can integrate multiple sources and domains of input45,46, sustain information in the face of distraction47, acquire and represent action rules8,48,49, and change their tuning properties depending on whether a stimulus is task relevant or not15. Although all neurons in areas that lie anterior to the motor cortex possess these properties to varying degrees, there is evidence for regional differences in their instantiation, particularly with respect to domain generality (see Supplementary information S1 (box)) and rule representation. These putative functional differences are currently the primary evidence for theories regarding a rostro-caudal hierarchical architecture to the frontal cortex. In the following section, we focus on the evidence for and against a gradient in ‘rule abstraction’ along the rostro-caudal axis of the frontal lobe.
Abstract rule representations in frontal cortex
The frontal cortex is often proposed to support learning, storage and/or retrieval of rules for behaviour. An action rule specifies the relationship between a condition of the system (one of the simplest being an encounter with a perceptual stimulus), an action and an anticipated outcome2,8,48,49. For example, a conditional stimulus–response association — such as a saccade to the left in response to a high tone — would be a simple rule that specifies a concrete motor function. However, rules can also be more abstract in that they do not specify a particular action, but a class of action rules. Thus, in the above example, a more abstract rule might specify whether the saccade direction is cued by a tone or by a colour, without specifying the direction itself. As described later, growing evidence suggests that when rules are more abstract, the execution of these rules depends on more-anterior portions of the frontal cortex17,19,25,29,40,50.
The caudal frontal cortex (specifically the dorsal premotor (PMd) corresponding to area 6, see FIG. 1) contains neurons that are associated with and, as lesions studies have shown, necessary for the execution of simple action rules42,51–58. PMd neurons increase their firing to response-relevant cues during the course of training, and this increase mirrors behavioural learning curves56. Lesioning the PMd following training results in a loss of the ability to select responses on the basis of previously learnt rules51,59–62.
Neurons in the more-rostrally located lateral PFC (that is, area 45, area 46 and area 9/46) (FIG. 1) also demonstrate concrete rule selectivity45,48,63–69. However, lesioning the mid-dorsolateral PFC after rule learning does not impair performance on simple response-selection tasks. Rather, in non-human primates PFC lesions prevent the acquisition of new rules62,70. Moreover, cue-specific PFC firing decreases as the rules are acquired. Functional MRI (fMRI) studies in humans have shown a similar pattern in PFC activation during simple rule learning71,72.
The distinction between PFC and PMd activity is often interpreted as dissociating the learning from the execution of rules, but it could also be explained in terms of differences in the capacity of PFC and PMd to execute abstract versus concrete action rules. Specifically, during the course of learning, monkeys often adopt abstract strategies, like shifting to a new response after receiving negative feedback but sticking to the same response after positive feedback. Animals that adopt this ‘win-stay, lose-shift’ strategy acquire arbitrary associations between a cue and a response faster than animals that do not. Notably, such a strategy does not specify what response the animal should make to a particular stimulus, but rather indicates a class of rules by which a response should be selected given the most recent response, cue and feedback. Thus, one interpretation of the dissociation between PFC and PMd activity during rule learning is that both areas execute rules, but that these rules differ in their level of abstraction. The PMd executes the specific arbitrary association between a stimulus and a response, whereas the PFC executes higher-order strategies, such as the win-stay, lose-shift strategy. This strategy becomes obsolete once the arbitrary associations have been learnt; thus, PFC lesions have little effect after learning. Consistent with this interpretation, lateral PFC neurons fire selectively to win-stay, lose-shift strategies (regardless of the specific response that is selected)73, and lesions of the lateral PFC prevent the application of such strategies70.
PFC neurons generally fire in response to more-abstract action rules. For example, PFC neurons fire selectively based on stimulus identity and location when these features are response relevant, but their firing is less likely to depend on which motor response is associated with those cues than that of PMd neurons53,74. Likewise, during the performance of sequential actions (such as making a familiar sequence of joystick movements in response to a visual cue or sequence of cues), the activity of neurons in pre-PMd (area 8) and caudal portions of PFC (area 9/46) during the delay period (that is, the time between stimulus and response) is tuned for the full sequence of responses to be performed, rather than for the identity of the first response. For example, an individual cell in caudal PFC will fire in response to a cue indicating that a sequence of ‘push-push-pull-pull’ movements must be made but not in response to a cue indicating the sequence ‘push-pull-push-pull’, even though both sequences start with a push movement75–78. Moreover, PFC cells (those in area 9/46) can encode a category of sequences, rather than a specific sequence. For example, these cells will fire in advance of the push-push-pull-pull and pull-pull-push-push sequences even though these differ in terms of their specific movements77. Thus, PFC neurons can maintain an abstract sequential movement plan, rather than individual movements.
However, under certain circumstances, the PMd can also represent abstract rules69. Monkeys learnt to respond depending on whether a presented stimulus did or did not match a target stimulus. This response rule is abstract because the response is determined based on the relationship (match–non-match) between two stimuli and can be generalized to new items. PMd firing was selective for the match–non-match rule and this rule-selectivity was stronger in PMd than in PFC69. Two potential explanations have been given to reconcile this result with those reported in the broader literature on abstraction and PMd69,79. First, extensive training on the task could have reduced control demands and permitted transfer of the rule to PMd from PFC through automation. Second, although the task involved making generalizations about the perceptual input, or context, of the response, it also involved a simple mapping to a concrete response rather than to a class of actions. Nevertheless, the PFC might not be required to represent all types of abstraction but only particularly abstract classes or courses of action.
Data from human studies, mostly using fMRI, have been broadly consistent with the distinctions in the degree of abstraction during action decisions between PMd (area 6), pre-PMd (areas 6 and 8) and lateral PFC (area 9/46) that have been suggested by studies on non-human primates. Simple conditional-motor and response-selection tasks activate PMd and pre-PMd (area 8)74,80–85, but activation in PMd and pre-PMd can be distinguished on the basis of their respective association with motor execution or preparation versus attentional or memory processing during movement selection74,82,86. At a more abstract level, switching between two tasks87–90 or making a response based on an abstract rule91 is associated with activation in more-anterior lateral PFC regions (area 45 and area 9/46) along the middle and inferior frontal gyrus.
At the most anterior extent of the PFC, the frontopolar cortex (FPC) has been consistently associated with ‘meta-level’ control processing43,92. specifically, the human FPC is activated when one has to coordinate two simultaneously ongoing tasks by generating and maintaining subgoals93,94, when one must relate the outcomes of different simple perceptual or semantic comparison problems95–97, when one must monitor the results of episodic-memory retrieval with respect to task goals98–100, and when one must determine when to shift attention from ‘external’ information (for example, a perceptual stimulus) to ‘internal’ information (for example, information held in working memory)101. Also consistent with the hypothesis that the FPC can maintain and monitor a higher-order task goal (such as ‘make a sandwich’) while lower-order processes are ongoing, FPC activity has been shown to follow a more sustained, lower-frequency pattern of signal change relative to more-posterior frontal regions100,102,103. Hence, the FPC is associated with higher-order control functions that supervise or integrate ongoing processing in other, lower-order cognitive or motor processes43,92.
To summarize, across a range of studies, there are relative distinctions between PMd, pre-PMd, mid-lateral PFC and FPC in the degree of abstraction of the action representations that they process. But do these distinctions together form a gradient?
Evidence for a functional gradient along the dorsal rostro-caudal axis
The strongest evidence to date for a functional gradient along the rostro-caudal axis of the frontal lobes comes from human fMRI studies in which the level of abstraction of response selection was progressively varied in each individual participant. One ground-breaking study demonstrated that as the contextual information required to select a response progressively became more abstract and remained relevant over a longer temporal interval, fMRI activation moved from PMd to area 44 to the mid-dorsolateral PFC (area 46; FIG. 2a)19. Another study demonstrated that as conditions were added to an action rule (so that a more abstract action decision constituted a generalization over more lower-order action decisions (that is, policy abstraction; see BOX 1)), PMd (area 6), pre-PMD (area 8), mid-dorsolateral PFC (area 9/46) and FPC (area 10) were successively activated16 (FIG. 2b). A third study demonstrated that as individual finger-press responses, short sequences of responses or a sequence of response sequences were initiated (that is, more abstract response chunks), activation was evident in PMd, area 44 and the mid-ventrolateral area (area 45), respectively18. Thus, the consistent pattern across these experiments suggests a rostro-caudal gradient in which caudal PFC regions are engaged for concrete action decisions that are closer in time and more directly related to choosing a specific motor response, and anterior PFC regions seem to guide behaviour over longer lags and at more abstract levels of action contingency.
Figure 2. Functional gradients along the rostro-caudal axis.
a | Summary of experimental findings from functional MRI (fMRI) studies and monkey electrophyiology along the rostro-caudal gradient. Across studies, there seems to be a trend for more-rostral regions to support more-abstract action rules. Anatomical locations of effects are approximate. b | Comparison of results from two fMRI studies showing functional gradients along the rostro-caudal axis of the human frontal lobe. Explicitly testing a rostro-caudal functional gradient, Koechlin et al.19 (shown in blue) and Badre et al.16 (shown in red) demonstrated highly convergent activation along a dorsal gradient from dorsal premotor cortex (PMd) (a,b; ~BA 6) to prePMd (c,d; ~BA 8) to mid-dorsolateral prefrontal cortex (PFC) (e,f; ~BA 9/46) to frontopolar cortex (g; ~BA 10), as cognitive control was required at progressively abstract levels. Although different forms of abstraction were tested — temporal abstraction in Koechlin et al.19 and policy abstraction in Badre et al.16 (see BOX 1) — the activation patterns observed in these two experiments were highly convergent. c | Race et al.113 aimed to locate a gradient of abstraction along the ventrolateral PFC by assessing repetition priming (that is, a reduction in signal change) at the stimulus (semantic), task (decision) and response levels. They showed that areas 44 and 8 (shown in orange) in caudal ventrolateral PFC showed repetition priming at the response level, that is, the signal change in these areas diminished when a motor response was repeated, regardless of the stimulus or decision associated with it. Area 45 (shown in blue) revealed repetition priming at the decision level even if the subsequent response differed. And area 47 (shown in red) demonstrated priming when the same item was encountered (semantic priming), regardless of the subsequent decision or response. These priming effects indicate a rostral-to-caudal gradient of decreasing abstraction. Part b is reproduced, with permission, from REF. 16 © (2007) MIT Press. Part c is reproduced, with permission, from REF. 113 © (2009) MIT press.
Interestingly, a recent experiment has provided evidence for interactions between parallel rostro-caudal gradients along lateral and medial frontal cortex as more-abstract motivational factors are taken into consideration during cognitive control104. specifically, the pre-supplementary motor area (in the medial frontal cortex) and the pre-PMd and/or caudal PFC (approximately areas 44 and 45; in the lateral frontal cortex) were associated with cues that indicated individual trials as having high or low stakes. By contrast, area 9/46 (in the lateral frontal cortex) and the dorsal anterior cingulate cortex (in the medial frontal cortex) were associated with cues indicating a general low or high stakes environment regardless of the status of a particular trial. Intriguingly, effective connectivity analysis indicated that the activation in lateral PFC could be explained by the activation in medial PFC, suggesting that motivational factors computed by medial frontal regions may upregulate neural activity in lateral frontal regions that support control at different levels of abstraction.
Evidence for a functional gradient along the ventral rostro-caudal axis
The studies described above focused primarily on dorsolateral frontal gradients (FIG. 2a). However, there is also evidence for a rostro-caudal gradient of abstraction along the ventrolateral frontal cortex105. Activation in rostral ventrolateral PFC (areas 47 and 45) has been distinguished from that in caudal ventrolateral PFC (areas 45 and 44) based on semantic (abstract) versus phonological (closer to an articulated response, that is, a motor response) processing106–110. Further, multiple lines of evidence suggest that anterior (area 47) and mid-ventrolateral PFC (area 45) are dissociable based on the degree of specificity at which retrieval from memory is conducted16,111,112, going from abstract semantic retrieval to post-retrieval decision processing.
A recent repetition priming experiment has provided more direct evidence of a ventrolateral PFC gradient of abstraction113. Participants were presented with pictures of everyday objects and required to make one of two semantic decisions — judgment on size (“Is the object bigger than a 13-inch box?”) or composition (“Is the object made of an organic substance?”) — and then indicate their choice with a finger-press response. The items were subsequently repeated and subjects were asked to make either the same judgment or a different judgment. Thus, this experiment permitted independent estimation of repetition priming at the item (semantic), task (decision) and response levels. Consistent with a rostro-caudal gradient of item processing along ventrolateral PFC, these three levels were associated with priming effects in rostral ventrolateral PFC (area 47), mid-ventrolateral PFC (area 45) and pre-PMv (areas 44 and 6)/PMd (area 6), respectively (FIG. 2c). Hence, a gradient of abstraction seems evident along ventrolateral PFC. Given the rostro-caudal connectivity of the frontal cortex (see later), the existence of two distinct functional pathways, both emanating from the FPC (area 10), seems reasonable. However, what distinguishes this ventral gradient from the dorsal gradient discussed previously remains an open question. One speculative hypothesis is that the ventral frontal gradient could be involved in the processing of context, retrieving or selecting relevant information that is being processed by ventral-pathway perceptual and memory systems, in order to comprehend the environment in a goal-relevant way. By contrast, the dorsal-frontal gradient could be more directly involved in the planning and execution of actions. However, whatever theoretical account will ultimately emerge to explain the difference between dorsal and ventral rostro-caudal gradients, it will probably derive partially from functional distinctions between dorsal and ventral frontal cortex9,32,114–116.
Are the functional gradients hierarchical?
Beyond a gradient of abstraction, do neurons in rostral regions influence the activity of neurons in caudal regions more than caudal regions influence rostral regions? Although this hypothesis is both contentious and preliminary, there is some evidence for a rostral-to-caudal flow of control. One study19 used structural equation modelling to show that activation in anterior frontal regions accounted for variance in activation in caudal regions but not vice versa. Another line of experiments117,118 demonstrated that the functional connectivity between a domain-general, anterior PFC/FPC region and domain-sensitive regions in caudal frontal cortex changed depending on whether a spatial or verbal working memory task was being performed. Moreover, patients with rostral PFC damage showed intact activation in the domain-specific caudal frontal regions, but functional connectivity in the task-related networks was disturbed relative to controls119. Thus, the influence of rostral PFC on caudal PFC may help the coordination of lower-order regional networks.
Crucially, however, these results do not provide strong evidence for a processing hierarchy. In particular, the asymmetry and direction of influence that a hierarchical gradient would predict have only recently been directly tested17. And, indeed, it is unlikely that rostral frontal regions pass control through interposed frontal regions in order to influence motor cortex in all cases and for all tasks. For example, at least one result demonstrated that lesions in the monkey periarcuate region, including areas 6 and 8, impair performance on various conditional motor tasks, but do not impair performance in a task that engages the more-rostral mid-dorsolateral PFC42. Thus, although there is evidence for rostro-caudal differences in the degree of abstraction during control tasks, additional evidence, including lesion and anatomical evidence, is needed to support a direct dominance relationship from rostral to caudal PFC.
Anatomy along the rostro-caudal axis
Clues as to the rostro-caudal organization of the frontal lobe can be derived from its anatomical organization35 and its development (BOX 2). As mentioned earlier, hierarchical processing architectures are characterized by a dominance relationship from higher to lower levels. Notably, such a dominance relationship should not be confused with serial stages of processing or a direct temporal flow of processing: in a hierarchical architecture, goals at different levels of abstraction could be represented in parallel in different areas and could be updated dynamically depending on control demands at each level of abstraction. Thus, a dominance hierarchy should be reflected in a rostral-to-caudal influence of processing in the frontal cortex and a characteristic pattern of cortico-cortical connections linking regions from the FPC to the motor cortex. Similarly, projections to and from the primary and unimodal association cortex outside of the frontal lobe should reflect differing degrees of abstraction along the purported rostro-caudal gradient: higher frontal cortex regions should receive more integrated, cross-domain sensory input than lower regions. Here, we consider the extent to which the anatomical evidence supports such an organization.
Box 2. Development of the frontal lobes along the rostro-caudal axis.
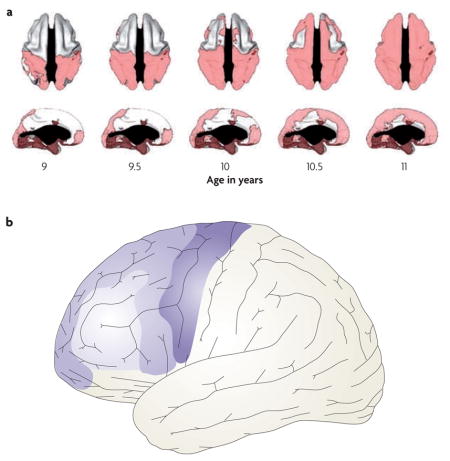
Changes in myelination and in synaptic density continue beyond infancy well into adulthood140–142, and are the primary markers of brain development in children. Important differences along the rostro-caudal axis of the frontal lobe can be seen during grey matter development throughout childhood and early adolescence. With some lamina-dependent variation143, synaptic density throughout the neocortex and subcortical structures increases sharply initially, followed by a slow decline, resulting in a skewed inverted U-shaped pattern of change in cortical thickness140,144–147. However, the ages at which grey matter changes progress differ substantially between brain regions148. Of particular interest, longitudinal data suggest that in the frontal lobes grey matter maturation progresses slowly from caudal to rostral regions, that is, from motor cortex to dorsal premotor (PMd) to area 9/46 during early childhood through to adolescence143,149.
The frontopolar cortex (area 10) seems to diverge from a linear caudal-to-rostral developmental trajectory143,149. A recent longitudinal study143 reported that lateral and medial frontopolar cortical areas mature early, around the same time as the supplementary and premotor cortices (areas 6/8). This is followed by maturation of the interposed lateral prefrontal cortex regions (areas 9/46, 47 and45); see part a of the figure, which shows the age at which different areas reach their peak cortical thickness (pink). Thus, the overall pattern of development is not a linear progression from caudal to rostral, but rather comprises a centrifugal pattern with the most rostral and caudal frontal regions developing first (see see part b of the figure, in which early maturing areas are indicated by darker colours).
This developmental pattern is significant for models of frontal lobe function, particularly those that seek to understand how a hierarchical architecture might develop in the frontal cortex (for examples see REFS 26,27,150). In general, because different frontal regions mature at different rates, the state of each region (as defined by its inputs) is distinct during synaptic pruning. This temporal differentiation in maturation could provide a basis for functional differences along the rostro-caudal axis. However, the centrifugal pattern, and consequently the early maturation of the ‘top’ and ‘bottom’ extremes of the hierarchy, means that the development of area 9/46 may be shaped by both higher-order and lower-order influence. The significance of such maturation in lateral prefrontal cortex remains to be understood. Part a of the figure is reproduced, with permission, from REF. 143 © (2008) Society for Neuroscience.
To consider the anatomical organization of the frontal cortex, one must be precise regarding the boundaries of areas that are proposed to be anatomically distinct. Here, we use the nomenclature put forth by Petrides and Pandya10,120,121 (FIG. 1). A key modification that Petrides and Pandya have made to the original cortex maps of Brodmann and Walker is the inclusion of a new area, that they refer to as area 9/46, and its division into dorsal (area 9/46d) and ventral (area 9/46v) portions (FIG. 1). In this scheme, the fMRI evidence for a dorsal rostro-caudal frontal gradient16,19 has identified several anatomically distinct lateral PFC regions along the rostral-caudal axis: rostral PFC (area 10), mid-dorsolateral PFC (areas 46 and 9/46), pre-PMd and PMd (area 6/8). Thus, we consider the anatomy of each of these regions in terms of their intrinsic organization and connectivity.
Intrinsic organization
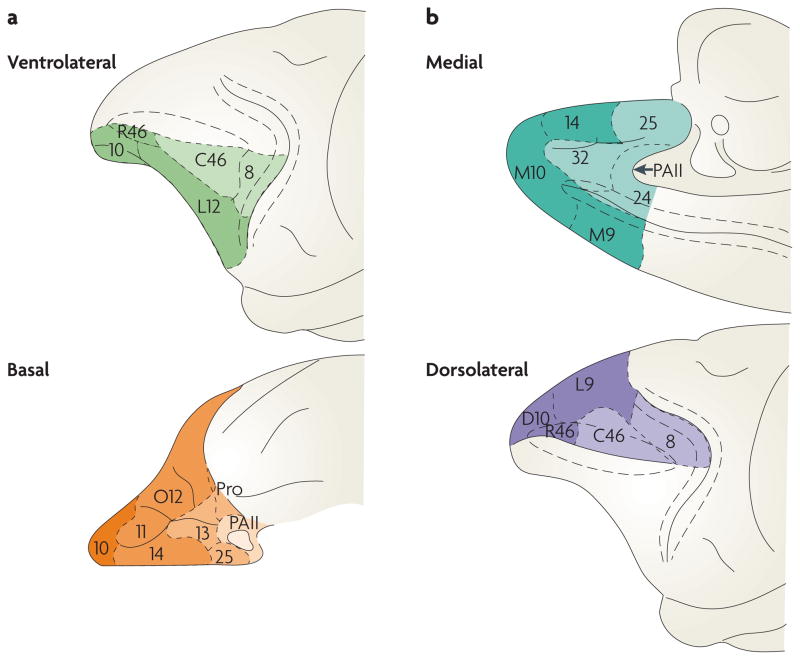
Barbas and Pandya122 proposed that the difficulty with defining PFC areas can be obviated if the cerebral cortical architecture is considered in the theoretical framework of cortical laminar organization proposed by Sanides123. In this framework, the cerebral cortex evolved from two primordial parts: the hippocampal archicortex (on the mediodorsal line) and the olfactory paleocortex (on the basoventral line). Thus, the frontal cortex could be viewed as a set of areas showing gradual changes in architectonic organization in these two major cortical lines. Careful study of the PFC anatomy in rhesus monkeys led to several important observations124. First, areas differ in their degree of differentiation, and progressively more differentiated areas are more laminated (that is, cells are organized into cortical layers). In the basoventral line, increased differentiation proceeds from caudal orbitofrontal cortex (areas 25, 13, 14 and 12) to area 10, to rostral area 46, to caudal area 46 and finally to ventral area 8 (FIG. 3). In the mediodorsal line, increased differentiation proceeds from ventromedial prefrontal cortex (areas 24, 25, 32 and 14) to medial areas 9 and 10, to lateral areas 9 and 10, to rostral 46, to caudal area 46 and finally to dorsal area 8 (FIG. 3). Relevant to this review, rostral PFC areas 10, 9 and 46 are less differentiated than more-caudal PFC areas 46 (later renamed area 9/46) and 8.
Figure 3. Architectonic stages of the prefrontal cortex.
Diagrams showing the architectonic stages in the basoventral (a) and mediodorsal (b) prefrontal cortices. Each of these two broad cortical regions shows a gradient of laminar organization (for example, differentiation) from the most anterior to more-posterior portions of the frontal cortex. This axis of differentiation proceeds in a direction from the least-differentiated (anterior frontal regions such as area 10 and rostral area 46) to the most-differentiated cortex (posterior frontal regions such as caudal areas 46 and 8). Figure is modified, with permission, from REF. 124 © (1989) Wiley.
In a cortical line each frontal area projects to an area that is more architectonically differentiated and to one that is less differentiated. For example, rostral area 46 projects to area 10, which is less differentiated, and to area 8, which is more differentiated. However, it has been noted124,125 that areas with well-developed laminar differentiation (such as area 8 or caudal area 46) have a restricted number of connections — mostly to neighbouring regions — whereas areas that have less laminar differentiation (such as area 10) have widespread connections to other areas. Thus less-differentiated areas such as those in rostral PFC (areas 10, 9 and 46) have more diffuse projections and are well situated to be at the top of a hierarchy. By contrast, more differentiated areas such as those in caudal PFC (areas 9/46 and 8) have more intrinsic connections and are well situated to be lower in a hierarchy.
Afferent and efferent projections in the frontal cortex
When considering whether the frontal cortex is hierarchically organized, one could assess whether its anatomical organization adheres to general contiguity and asymmetry principles. Consider regions A, B and C, with region A at the top of the hierarchy and region C at the bottom. According to a contiguity principle, regions A and B, and regions B and C are contiguous and so should have reciprocal projections. For non-contiguous regions, an asymmetry principle should apply. Thus, region A can also project to region C but region C would not necessarily project back to region A. In other words, the hierarchy hypothesis predicts that there should be fewer long-range inputs from caudal regions to rostral regions and, conversely, more inputs from rostral regions to caudal regions. In the model of frontal hierarchy that we are assessing, rostral PFC (area 10) would lie at the top of the hierarchy, the lower level would be mid-dorsolateral PFC (area 9/46) and the lowest level the premotor cortex (area 6/8). Based on anatomical studies, we review the connectivity of each of these sectors below and summarize the findings in FIG. 4.
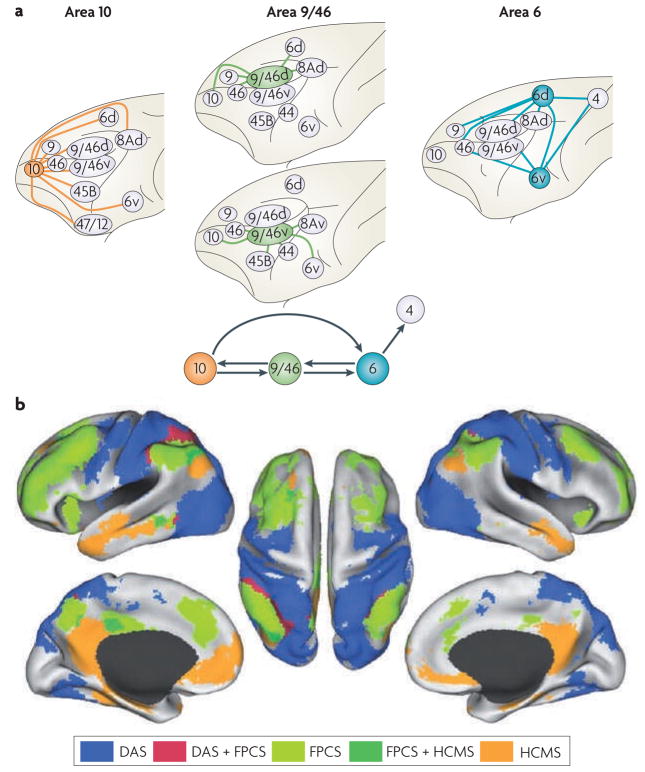
Figure 4. Rostro-caudal connectivity of the frontal cortex.
a. Intrinsic connections of the lateral prefrontal cortex (PFC; top) and a schematic summary of the connections of the principal frontal regions (area 10, shown in orange; area 9/46, shown in green; and area 6, shown in blue) that are proposed to be part of a rostro-caudal functional gradient based on functional studies (bottom). Area 4 depicts the primary motor cortex. b. Results from Vincent et al.130 showing that spontaneous activity in regions along the rostro-caudal axis of the prefrontal cortex (PFC) and in parietal and medial frontal cortex is correlated with activity in the frontopolar cortex (shown in light green). Also depicted in the figure is the spatial relationship of these regions to two other networks: the dorsal attention system (DAS) and the hippocampal-cortical memory system (HCMS), which were identified using visual motion area MT+ and the hippocampus as seeds, respectively. Importantly, these data provide evidence that the activities in regions of the frontal cortex that in other experiments were associated with control at increasing levels of abstraction are correlated with each other (and thus are part of a coherent rostro-caudal functional network, that is, the frontoparietal control system (FPCS)) but not with activity in other regions of the frontal cortex (that is, areas in the DAS and HCMS). Part b is reproduced, with permission, from REF. 130 © (2008) The American Physiological Society.
With regard to the rostral PFC, Petrides and Pandya performed a detailed analysis of the brain of a rhesus monkey126 and found that area 10 projects caudally to dorsal frontal areas 9, 46, 8 and 6; to ventral frontal areas 47/12 and 45; and to medial and orbital frontal areas 24, 25, 32, 14 and 11 (tracer injection sites included lateral, medial and orbital areas).
In the mid-dorsolateral PFC, anatomical studies of areas 9/46d and 9/46v in two rhesus monkeys127 revealed rostral projections from area 9/46d terminating in areas 9, 46 and 10. Caudal projections from area 9/46d terminated in lateral frontal areas 8Ad and 6d and medial frontal area 24. From area 9/46v, rostral projections were found terminating in areas 46 and 10, and caudal projections terminating in lateral frontal areas 8Av, 44, 45b and 6v, and in orbitofrontal areas 11 and 13.
Importantly, both area 9/46d and area 9/46v project rostrally to area 10, but their projections remain segregated (dorsal and ventral, respectively). Moreover, there are no direct connections between area 9/46v and area 9/46d. This potential independence is intriguing considering the functional evidence, discussed earlier, for distinct dorsal and ventral rostro-caudal gradients. Finally, there are no direct projections from area 9/46 to primary motor cortex; all cortico-cortical connections in the frontal cortex pass through the premotor cortex. Thus, there is no direct influence of the PFC on the primary motor cortex.
The lateral premotor cortex (area 6) is divided into a dorsal region (containing a representation of the trunk) and a ventral region (representing the neck, head and face). One study found that in rhesus monkeys, the dorsal premotor cortex (area 6d) is connected with other dorsal frontal regions and the primary motor cortex128. Specifically, the dorsal premotor cortex (area 6d) reciprocally projects to areas 46, 9, 9/46d and 8 above the principal sulcus. The ventral premotor cortex (area 6v) reciprocally projects to both dorsal and ventral PFC regions as well as to the primary motor cortex. Specifically, area 6v projects to areas 46 and 9/46v and to area 6d. Interestingly, no efferent projections from area 6 to FPC (area 10) were found.
Afferent and efferent projections outside the frontal cortex
Based on their anatomical studies, Petrides and Pandya126 made the crucial observation that area 10 (as well as area 9) does not project to the parietal cortex, the inferotemporal cortex or the visual areas of occipital cortex. Thus, they note, areas 9 and 10 do not directly interact with ventral visual pathways for object processing or dorsal visual pathways for spatial processing. By contrast, more-caudal areas of lateral PFC, such as area 9/46 (and areas 8A, 8B and 45), have massive, bidirectional projections with these areas9.
Generally consistent with this and other perspectives36, Botvinick26 constructed a connectionist model of sequential behaviour with a ladder-like architecture that is similar to this pattern of connectivity. Specifically, input and output layers communicated directly with lower layers but indirectly (through the lower layers) with higher layers. During learning, this architecture acquired more abstract representations in the higher layers. Hence, although having a structurally arrayed hierarchical organization is not a requirement for the control of complex sequential behaviour129, when such an architecture exists, the same action can be represented at different levels of abstraction.
Functional connectivity in the frontal cortex
The basic anatomy reviewed here suggests that regions in the frontal cortex are not ‘fully connected’ in the sense that every region connects to every other region directly. Rather, there are constraints in frontal connectivity that follow contiguity and asymmetry principles. Specifically, adjacent regions along the rostro-caudal axis are connected to one another but do not project to more-rostral regions beyond those immediately adjacent, as in the specific case of area 6 (see FIG. 4). In addition, dorsal and ventral rostro-caudal gradients are only connected at the highest and lowest levels (areas 10 and 6, respectively), but not in the ‘middle’ (between areas 9/49d and 9/46v). But do these distinct rostro-caudal tracts form coherent functional systems, as would be required by a special rostro-caudal processing architecture such as a hierarchy?
A recent study130 used an analysis of spontaneous fluctuations in BOLD activity to characterize the functional system that includes the FPC (area 10). This technique relies on the correlations among low-frequency BOLD changes that exist in the absence of an overt task, and has been a fruitful, ‘task-free’ approach of defining ensembles of regions that form a functional system131–134.
Seeding the FPC yielded a network that included the full dorsal-frontal gradient (areas 10, 9/46, 8 and 6) (FIG. 4) and that was distinct from networks acquired using the hippocampus or visual motion area MT+ as seeds. The network also included regions of medial frontal cortex, anterior insula, the head of the caudate nucleus and anterior inferior parietal lobule. Thus, a hierarchical architecture of the frontal cortex would not necessarily mean that this system is independent from the rest of the brain or is the only source of top-down signals. Rather, it must ultimately be understood as a component of a broader functional system, such as the fronto-parietal control system130.
Conclusions and future directions
The evidence discussed in this Review supports two new insights about frontal organization. First, neurons in progressively rostral regions of the frontal cortex seem distinguished by their ability to support more-abstract representations and more-complex rules. Second, regions arrayed along dorsal and ventral rostro-caudal gradients act as coherent functional networks, along with regions of parietal and lateral temporal cortex. Is the rostro-caudal axis of the frontal lobe organized hierarchically? The results reviewed here suggest that the frontal cortex could, indeed, support such an architecture. Progressively rostral frontal lobe regions seem capable of supporting increasingly abstract neural representations and complex action rules. Such a differentiation would be essential in a hierarchical system, where ‘higher’ levels must maintain their state independent of the state of lower level processors or moment-to-moment changes in the environment. Moreover, the anatomical and functional connectivity data suggest that the rostro-caudal axis (that is, area 10 to area 9/46 to area 8 to area 6) forms a coherent functional network with longer connections, such as those from areas 10 to 6, being unidirectional. Again, such an arrangement is an important precondition for a dominance hierarchy arrayed along the frontal cortex.
Crucially, although a hierarchical organization might require both of these features of frontal organization, these features do not, by themselves, require that the organization be hierarchical; they could be consistent with other architectures as well. So, additional research is required to provide definitive evidence for a hierarchy. However, a recent study in patients with focal damage due to stroke in the frontal cortex provides, perhaps, the strongest evidence to date for an asymmetric anterior to posterior dominance relationship17. In particular, these patients were impaired at making action decisions at a level of abstraction that was dependent on whether their damage was in pre-PMd (caudal, more concrete) or mid-DLPFC (more rostral, more abstract). Moreover, they were impaired on all tasks requiring more-abstract but not less-abstract action decisions. Importantly, the sites of damage among patient groups corresponded precisely to the foci identified for these levels of abstraction in healthy controls undergoing fMRI while performing this task16. This asymmetric pattern of deficits provides evidence of a rostral-to-caudal flow of processing during action control. However, it remains crucial to test whether perturbations in neural processing, such as due to stroke, asymmetrically influence processing in intact regions that are caudal but not rostral to the site of perturbation.
Broadly, the data reviewed here suggest that there are important regional distinctions along the rostro-caudal axis of the frontal lobe and that these regions act as a coherent functional network. These repeatable empirical phenomena demand an explanation, and hierarchy is one intriguing possibility. Whether it is hierarchy or some other scheme that explains the functional organization along the rostro-caudal axis of the frontal lobe, characterizing processing architecture is fundamental to our understanding of frontal lobe function.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (MH63901 and NS40813) and the Veterans Administration Research Service.
Glossary
- Cognitive control
Also termed executive function, cognitive control allows flexible behaviour by guiding thought and action based on goals, plans and intentions
- Double dissociation
When two experimental manipulations have different effects on two dependent variables (for example, on the left and right hemisphere or on the medial and lateral prefrontal cortex)
- Domain (stimulus/input domains)
A type of information, such as spatial versus verbal versus object-related information. Domains are often associated with independent or modular input systems
- Action rule
A type of knowledge that specifies how to behave given a particular state. A stimulus-to-response mapping is a simple rule
- Semantic
Conceptual knowledge, beliefs and facts about the world. In the verbal domain, semantic refers to word meanings
- Phonology (phonological)
The sound structure of a word in terms of the smallest sound units that distinguish different words in a language
- Repetition priming
Facilitated processing of a stimulus upon repetition, which happens even following an extended delay
- Structural equation modelling
A statistical approach for testing proposed causal relationships between variables
- Seeding
A step in functional connectivity analysis whereby a region of interest is defined to which connectivity of all other regions is estimated
Footnotes
FURTHER INFORMATION
D. Badre’s homepage: http://www.cog.brown.edu/research/badrelab/index.html
M. D’Esposito’s homepage: http://bic.berkeley.edu/despolab
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- 2.Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Carter CS, et al. Anterior cingulate cortex, error detection, and the on-line monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 4.D’Esposito M, et al. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 5.Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nature Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- 6.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. This authoritative review provides a fundamental background on the cognitive control function of the PFC. It focuses on the theory that action goals are maintained as distributed patterns of activity in PFC, as a consequence of which other neural systems are biased to perform in goal-relevant ways. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- 8.Passingham RE, Rowe JB. In: Principles of Frontal Lobe Function. Stuss DT, Knight RT, editors. Oxford University Press; New York: 2002. pp. 221–232. [Google Scholar]
- 9.Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 11.Petrides M, Pandya DN. In: Principles of Frontal Lobe Function. Stuss DT, Knight RT, editors. Oxford University Press; New York: 2002. pp. 31–50. [Google Scholar]
- 12.Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirigu A, et al. Distinct frontal regions for processing sentence syntax and story grammar. Cortex. 1998;34:771–778. doi: 10.1016/s0010-9452(08)70780-9. [DOI] [PubMed] [Google Scholar]
- 14.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 15.Freedman DJ, Reisenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 16.Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 17.Badre D, Hoffman J, Cooney JW, D’Esposito M. Hierarchical cognitive control deficits following damage to the human frontal lobe. Nature Neurosci. 2009;12:515–522. doi: 10.1038/nn.2277. This study provides evidence that in patients with damage due to stroke the impairment at tasks requiring cognitive control at a level of abstraction depended on how far rostrally their lesion is located. This is also the first study to directly demonstrate rostral-to-caudal dependencies in processing, a necessary component of hierarchy in the frontal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koechlin E, Jubault T. Broca’s area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. This paper reports the first neuroimaging experiment showing a rostro-caudal gradient of activity in frontal cortex based on a systematic manipulation of abstraction across conditions. [DOI] [PubMed] [Google Scholar]
- 20.Lashley KS. In: Cerebral Mechanisms in Behavior. Jeffress LA, editor. Wiley; New York: 1951. pp. 112–136. [Google Scholar]
- 21.Miller GA, Galanter E, Pribram KH. Plans and the Structure of Behavior. Holt, Rinehart and Winston, Inc; New York: 1960. [Google Scholar]
- 22.Newell A. Unified Theories of Cognition. Harvard University Press; Cambridge, Massachusetts: 1990. [Google Scholar]
- 23.Rumelhart DE, Norman DA. Simulating a skilled typist: a study of skillled cognitive-motor performance. Cogn Sci. 1982;6:1–36. [Google Scholar]
- 24.Schank RC, Abelson R. Scripts, plans, goals, and understanding. Lawrence Erlbaum Associates, Ltd; Hove, UK: 1977. [Google Scholar]
- 25.Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. This review discusses the various forms of abstraction that have been proposed to account for functional differences along the rostro-caudal axis of the frontal cortex, considering their common and potentially distinguishing characteristics. [DOI] [PubMed] [Google Scholar]
- 26.Botvinick MM. Multilevel structure in behaviour and in the brain: a model of Fuster’s hierarchy. Philos Trans R Soc Lond B Biol Sci. 2007 doi: 10.1098/rstb.2007.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botvinick MM. Hierarchical models of behavior and prefrontal function. Trends Cogn Sci. 2008;12:201–208. doi: 10.1016/j.tics.2008.02.009. This paper reviews computational accounts of hierarchical control of behaviour and their relationship to frontal lobe function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckner RL. Functional-anatomic correlates of control processes in memory. J Neurosci. 2003;23:3999–4004. doi: 10.1523/JNEUROSCI.23-10-03999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunge SA, Zelazo PD. A brain-based account of the development of rule use in childhood. Curr Dir Psychol Sci. 2006;15:118–121. [Google Scholar]
- 30.Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchal organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- 31.Christoff K, Keramatian K. In: Perspectives on Rule-Guided Behavior. Bunge SA, Wallis JD, editors. Oxford University Press; New York: 2007. [Google Scholar]
- 32.Courtney SM. Attention and cognitive control as emergent properties of information representation in working memory. Cogn Affect Behav Neurosci. 2004;4:501–516. doi: 10.3758/cabn.4.4.501. [DOI] [PubMed] [Google Scholar]
- 33.Courtney SM, Roth JK, Sala JB. In: The Cognitive Neuroscience of Working Memory. Osaka N, Logie R, D’Esposito M, editors. Oxford University Press; Oxford: 2007. pp. 369–383. [Google Scholar]
- 34.Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. Lippincott-Raven Publishers; Philadelphia, PA: 1997. This book provides an authoritative review of frontal lobe anatomy and function and articulates one of the first proposals of a hierarchical architecture of frontal lobe organization, termed the ‘perception-action cycle’. [Google Scholar]
- 36.Fuster JM. The prefrontal cortex—an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 37.Fuster JM. Upper processing stages of the perception-action cycle. Trends Cogn Sci. 2004;8:143–145. doi: 10.1016/j.tics.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Hazy TE, Frank MJ, O’Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc Lond B Biol Sci. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- 40.Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 41.O’Reilly RC, Noelle DC, Braver TS, Cohen JD. Prefrontal cortex and dynamic categorization tasks: representational organization and neuromodulatory control. Cereb Cortex. 2002;12:246–257. doi: 10.1093/cercor/12.3.246. [DOI] [PubMed] [Google Scholar]
- 42.Petrides M. In: From Monkey Brain to Human Brain: A Fyssen Foundation Symposium. Dehaene S, Duhamel J-R, Hauser MD, Rizzolatti G, editors. The MIT Press; Cambridge, Massachusetts: 2006. pp. 293–314. [Google Scholar]
- 43.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 44.Miller BT, D’Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Fuster JM, Bodner M, Kroger J. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- 46.Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276 doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- 47.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 49.Passingham RE. The Frontal Lobes and Voluntary Action. Oxford University Press; Oxford: 1993. [Google Scholar]
- 50.Christoff K, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- 51.Petrides M. In: The frontal lobes revisited. Perecman E, editor. IRBN Press; New York: 1987. pp. 91–108. [Google Scholar]
- 52.Brasted PJ, Wise SP. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci. 2004;19:721–740. doi: 10.1111/j.0953-816x.2003.03181.x. [DOI] [PubMed] [Google Scholar]
- 53.di Pellegrino G, Wise SP. Visuospatial versus visuomotor activity in the premotor and prefrontal cortex of a primate. J Neurosci. 1993;13:1227–1243. doi: 10.1523/JNEUROSCI.13-03-01227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadj-Bouziane F, Meunier M, Boussaoud D. Conditional visuo-motor learning in primates: a key role for the basal ganglia. J Physiol Paris. 2003;97:567–579. doi: 10.1016/j.jphysparis.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Lucchetti C, Bon L. Time-modulated neuronal activity in the premotor cortex of macaque monkeys. Exp Brain Res. 2001;141:254–260. doi: 10.1007/s002210100818. [DOI] [PubMed] [Google Scholar]
- 56.Mitz AR, Godschalk M, Wise SP. Learning-dependent neuronal activity in the premotor cortex: activity during the acquisition of conditional motor associations. J Neurosci. 1991;11:1855–1872. doi: 10.1523/JNEUROSCI.11-06-01855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol. 2006;95:3596–3616. doi: 10.1152/jn.01126.2005. [DOI] [PubMed] [Google Scholar]
- 58.Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Passingham RE. Premotor cortex and preparation for movement. Exp Brain Res. 1988;70:590–596. doi: 10.1007/BF00247607. [DOI] [PubMed] [Google Scholar]
- 60.Passingham RE. Premotor cortex and the retrieval of movement. Brain Behav Evol. 1989;33:189–192. doi: 10.1159/000115927. [DOI] [PubMed] [Google Scholar]
- 61.Petrides M. Deficits in non-spatial conditional associative learning after periarcuate lesions in the monkey. Behav Brain Res. 1985;16:95–101. doi: 10.1016/0166-4328(85)90085-3. [DOI] [PubMed] [Google Scholar]
- 62.Petrides M. Deficits on conditional associative-learning tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1985;23:601–614. doi: 10.1016/0028-3932(85)90062-4. [DOI] [PubMed] [Google Scholar]
- 63.Amemori K, Sawaguchi T. Rule-dependent shifting of sensorimotor representation in the primate prefrontal cortex. Eur J Neurosci. 2006;23:1895–1909. doi: 10.1111/j.1460-9568.2006.04702.x. [DOI] [PubMed] [Google Scholar]
- 64.Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol. 2000;84:451–459. doi: 10.1152/jn.2000.84.1.451. [DOI] [PubMed] [Google Scholar]
- 65.Everling S, DeSouza JF. Rule-dependent activity for prosaccades and antisaccades in the primate prefrontal cortex. J Cogn Neurosci. 2005;17:1483–1496. doi: 10.1162/0898929054985455. [DOI] [PubMed] [Google Scholar]
- 66.Muhammad R, Wallis JD, Miller EK. A comparison of abstract rules in the prefrontal cortex, premotor cortex, inferior temporal cortex, and striatum. J Cogn Neurosci. 2006;18:974–989. doi: 10.1162/jocn.2006.18.6.974. [DOI] [PubMed] [Google Scholar]
- 67.White IM, Wise SP. Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res. 1999;126:315–335. doi: 10.1007/s002210050740. [DOI] [PubMed] [Google Scholar]
- 68.Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- 69.Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol. 2003;90:1790–1806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
- 70.Bussey TJ, Wise SP, Murray EA. The role of ventral and orbital prefrontal cortex in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:971–982. doi: 10.1037//0735-7044.115.5.971. [DOI] [PubMed] [Google Scholar]
- 71.Boettiger CA, D’Esposito M. Frontal networks for learning and executing arbitrary stimulus-response associations. J Neurosci. 2005;25:2723–2732. doi: 10.1523/JNEUROSCI.3697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toni I, Krams M, Turner R, Passingham RE. The time course of changes during motor sequence learning: a whole-brain fMRI study. Neuroimage. 1998;8:50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- 73.Genovesio A, Brasted PJ, Mitz AR, Wise SP. Prefrontal cortex activity related to abstract response strategies. Neuron. 2005;47:307–320. doi: 10.1016/j.neuron.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boussaoud D. Attention versus intention in the primate premotor cortex. Neuroimage. 2001;14:S40–45. doi: 10.1006/nimg.2001.0816. [DOI] [PubMed] [Google Scholar]
- 75.Averbeck BB, Lee D. Prefrontal neural correlates of memory for sequences. J Neurosci. 2007;27:2204–2211. doi: 10.1523/JNEUROSCI.4483-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohbayashi M, Ohki K, Miyashita Y. Conversion of working memory to motor sequence in the monkey premotor cortex. Science. 2003;301:233–236. doi: 10.1126/science.1084884. [DOI] [PubMed] [Google Scholar]
- 77.Shima K, Isoda M, Mushiake H, Tanji J. Categorization of behavioural sequences in the prefrontal cortex. Nature. 2007;445:315–318. doi: 10.1038/nature05470. This papers reports an elegant experiment showing that single-unit recording in monkeys provides evidence that neurons in prefrontal cortex are tuned to abstract categories of response sequences that generalize across the specific responses that form these sequences. [DOI] [PubMed] [Google Scholar]
- 78.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 79.Wallis JD. In: Neuroscience of Rule-Guided Behavior. Bunge SA, Wallis JD, editors. Oxford University Press; New York: 2008. [Google Scholar]
- 80.Kurata K, Tsuji T, Naraki S, Seino M, Abe Y. Activation of the dorsal premotor cortex and pre-supplementary motor area of humans during an auditory conditional motor task. J Neurophysiol. 2000;84:1667–1672. doi: 10.1152/jn.2000.84.3.1667. [DOI] [PubMed] [Google Scholar]
- 81.Moore CI, et al. Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol. 2000;84:558–569. doi: 10.1152/jn.2000.84.1.558. [DOI] [PubMed] [Google Scholar]
- 82.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 83.Pochon JB, et al. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb Cortex. 2001;11:260–266. doi: 10.1093/cercor/11.3.260. [DOI] [PubMed] [Google Scholar]
- 84.Schumacher EH, D’Esposito M. Neural implementation of response selection in humans as revealed by localizing effects of stimulus-response compatibility on brain activation. Hum Brain Mapp. 2002;17:193–201. doi: 10.1002/hbm.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schumacher EH, Elston PA, D’Esposito M. Neural evidence for representation-specific response selection. J Cogn Neurosci. 2003;15:1111–1121. doi: 10.1162/089892903322598085. [DOI] [PubMed] [Google Scholar]
- 86.Kennerley SW, Sakai K, Rushworth MF. Organization of action sequences and the role of the pre-SMA. J Neurophysiol. 2004;91:978–993. doi: 10.1152/jn.00651.2003. [DOI] [PubMed] [Google Scholar]
- 87.Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci USA. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- 89.Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- 90.Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: the role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- 92.Gilbert SJ, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 93.Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- 94.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 95.Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- 96.Christoff K, Ream JM, Geddes LPT, Gabrieli JDE. Evaluating self-generated information: Anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117:1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- 97.Kroger JK, et al. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- 98.Ranganath C, Johnson MK, D’Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci. 2000;20:RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ranganath C, Paller KA. Neural correlates of memory retrieval and evaluation. Brain Res Cogn Brain Res. 2000;9:209–222. doi: 10.1016/s0926-6410(99)00048-8. [DOI] [PubMed] [Google Scholar]
- 100.Velanova K, et al. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 102.Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- 103.Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- 104.Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nature Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- 105.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 106.Gold BT, Balota DA, Kirchhoff BA, Buckner RL. Common and Dissociable Activation Patterns Associated with Controlled Semantic and Phonological Processing: Evidence from fMRI Adaptation. Cereb Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- 107.Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 108.Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poldrack RA, Wagner AD. What can neuroimaging tell us about the mind? Insights from prefrontal cortex. Curr Direct Psychol Sci. 2004;13:177–181. [Google Scholar]
- 110.Poldrack RA, et al. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- 111.Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 112.Gold BT, et al. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Race EA, Shanker S, Wagner AD. Neural Priming in Human Frontal Cortex: Multiple Forms of Learning Reduce Demands on the Prefrontal Executive System. J Cogn Neurosci. 2009;21:1766–1781. doi: 10.1162/jocn.2009.21132. This fMRI study of repetition priming provides within-subject evidence for a rostro-caudal functional gradient along the ventrolateral PFC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- 115.D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- 116.Hampshire A, Duncan J, Owen AM. Selective tuning of the blood oxygenation level-dependent response during simple target detection dissociates human frontoparietal subregions. J Neurosci. 2007;27:6219–6223. doi: 10.1523/JNEUROSCI.0851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nature Neurosci. 2003;6:75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- 118.Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J Neurosci. 2006;26:1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rowe JB, et al. Is the prefrontal cortex necessary for establishing cognitive sets? J Neurosci. 2007;27:13303–13310. doi: 10.1523/JNEUROSCI.2349-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petrides M, Pandya DN. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Elsevier; Amsterdam: 1994. pp. 17–58. In this chapter, anatomists Petrides and Pandya report an extensive comparison of the architecture of the frontal cortex between monkeys and humans. [Google Scholar]
- 121.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 122.Barbas H, Pandya DN. In: Frontal lobe function and dysfunction. Levin HS, Eisenberg H, Benton AL, editors. Oxford University Press; Oxford: 1991. pp. 35–58. [Google Scholar]
- 123.Sanides F. In: The Structure and Function of the Nervous System. Bourne GH, editor. Academic Press; New York: 1972. pp. 329–453. [Google Scholar]
- 124.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. In this study, anatomists Barbas and Pandya present data from rhesus monkeys demonstrating that there is a gradient of laminar organization within the frontal cortex from the most anterior (least differentiated) to posterior portions. [DOI] [PubMed] [Google Scholar]
- 125.Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- 126.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. Using the autoradiographic method, this study reports the course and terminations of the efferent cortico–cortical connections of the rostral prefrontal region (area 10) in macaque monkeys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmahmann JDh. Fiber pathways of the brain. Oxford University Press; Oxford: 2006. [Google Scholar]
- 128.Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- 129.Botvinick M, Plaut DC. Doing without schema hierarchies: a recurrent connectionist approach to normal and impaired routine sequential action. Psychol Rev. 2004;111:395–429. doi: 10.1037/0033-295X.111.2.395. [DOI] [PubMed] [Google Scholar]
- 130.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. This study applied functional connectivity analysis to resting state fMRI data and characterized a specific fronto-parietal network that was coherent with the frontal pole (approximately area 10). In the frontal cortex, this analysis identified a dorsal rostro-caudal network that closely corresponds to the gradient of rostro-caudal regions identified in association with hierarchical control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. discussion 1097–9. [DOI] [PubMed] [Google Scholar]
- 132.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 135.Halford GS. Children’s Understanding: The Development of Mental Models. Erlbaum; Hillsdale, NJ: 1993. [Google Scholar]
- 136.Robin N, Holyoak KJ. In: The Cognitive Neurosciences. Gazzaniga MS, editor. MIT Press; Cambridge, MA: 1995. pp. 987–997. [Google Scholar]
- 137.Daw ND, Niv Y, Dayan P. In: Recent Breakthroughs in Basal Ganglia Research. Bezard E, editor. Nova Science, Inc; New York: 2006. [Google Scholar]
- 138.Dayan P. Bilinearity, rules, and prefrontal cortex. Front Comput Neurosci. 2007;1:1–14. doi: 10.3389/neuro.10.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sutton RS, Barto AG. Reinforcement Learning: An Introduction. MIT Press; Cambridge, Massachusetts: 1998. [Google Scholar]
- 140.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 141.Inder TE, Huppi PS. In vivo studies of brain development by magnetic resonance techniques. Ment Retard Dev Disabil Res Rev. 2000;6:59–67. doi: 10.1002/(SICI)1098-2779(2000)6:1<59::AID-MRDD8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 142.Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 145.Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- 146.Solomon M, Ozonoff SJ, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. Int J Dev Neurosci. 2008;26:239–247. doi: 10.1016/j.ijdevneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sowell ER, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hazy TE, Frank MJ, O’Reilly RC. Banishing the homunculus: making working memory work. Neuroscience. 2006;139:105–118. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 151.Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. VI. Mitteilung. Die Cortexgliederung des Menschen. J Psychol Neurol (Lzp) 1908;10:231–246. (in German) [Google Scholar]
- 152.Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. III. Mitteilung. Die Rindenfelder der niederen Affen. J Psychol Neurol (Lzp) 1905;4:177–226. (in German) [Google Scholar]
- 153.Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 1940;73:59–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.