Abstract
BACKGROUND
Previous research suggests that maternal exposure to acute stress has a negative impact on the duration of pregnancy, and that this effect may vary by the time of exposure. It has also been proposed that stress exposure reduces the ratio of male-to-female births. To date, no study has jointly examined both outcomes, although they may be strongly related. Using population-level data with no selectivity, we jointly study the sex-specific effect of stress on the duration of pregnancy and the observed sex ratio among pregnant women exposed to a major earthquake in Chile.
METHODS
In a quasi-experimental design, women exposed to the earthquake in different months of gestation were compared with women pregnant 1 year earlier. Estimates from a comparison group of pregnant women living in areas not affected by the earthquake were also examined to rule out confounding trends. Regression models were used to measure the impact of earthquake exposure on gestational age and preterm birth by sex across month of gestation. A counterfactual simulation was implemented to assess the effect of the earthquake on the secondary sex ratio accounting for the differential impact of stress on gestational age by sex.
RESULTS
Earthquake exposure in Months 2 and 3 of gestation resulted in a significant decline in gestational age and increase in preterm delivery. Effects varied by sex, and were much larger for female than male pregnancies. Among females, the probability of preterm birth increased by 0.038 [95% confidence interval (CI): 0.005, 0.072] in Month 2 and by 0.039 (95% CI: 0.002, 0.075) in Month 3. Comparable increases for males were insignificant at the conventional P < 0.05 level. After accounting for the sex-specific impact on gestational age, a decline in the male-to-female ratio in Month 3 of exposure was detected [−0.058 (95% CI: −0.113, −0.003)].
CONCLUSIONS
Maternal exposure to an exogenous stressor early but not late in the pregnancy affects gestational age and the probability of preterm birth. This effect is much stronger in females than males. Stress exposure in early pregnancy may also contribute to a decline in the ratio of male-to-female live births in exposed cohorts.
Keywords: maternal stress, natural disaster, gestational age, preterm birth, secondary sex ratio
Introduction
Prenatal exposure to severe physical or mental stress may affect the development and life-long health of the offspring. Effects include reduced birthweight, shorter length of gestation, increased risk of metabolic, cardiac and psychiatric disease, and shortened lifespan (Barbosa, 2000; Reynolds et al., 2007; Seckl and Holmes, 2007; Xiong et al., 2008; Tamashiro and Moran, 2010; Tegethoff et al., 2010). The effect of severe maternal stress likely depends on the timing of exposure, as it may alter time-specific developmental processes (Glynn et al., 2001; Malaspina et al., 2008; Class et al., 2011; Torche, 2011). Furthermore, the consequence of acute stress may vary according to the offspring's sex (Brown et al., 1995; Franzek et al., 2008; Malaspina et al., 2008). The study of how effects of stress differ across periods of gestation and by sex has been limited, however, by the lack of information about gestational age and by small sample sizes that preclude detailed analysis.
A separate strand of the literature suggests that exposure to prenatal stress at specific times in gestation may decrease the ratio of male-to-female live births or secondary sex ratio (SSR) (Hansen et al., 1999; Catalano et al., 2006, 2009; Bruckner and Catalano, 2007). Drawing on evolutionary biology, this literature suggests that pregnant females, when facing severe stressors, preferentially abort frail male fetuses.
To the extent that acute maternal stress affects both the SSR and birth outcomes of offspring in a way that varies by sex, these processes should be examined jointly. We analyzed population-based, prospectively collected data to determine if women exposed to a severe earthquake in Chile at different months of gestation had shorter pregnancies and a higher likelihood of delivering preterm (before 37 completed weeks of gestation), and whether these outcomes differed by sex. We also evaluated whether the ratio of male-to-female births declined according to the timing of the acute stressor, after accounting for any sex-specific effect on gestational age. Information on gestational age at the time of earthquake exposure for a large population with no selectivity enabled us to determine the risk for specific birth outcomes by gestational month of exposure, so as to capture the developmental periods in which exposure to an ambient stressor may be most detrimental, for either sex.
We examined a strong earthquake as an exogenous source of acute stress. The ‘Tarapaca’ earthquake struck the northernmost region of Chile on 13 June 2005. It registered an intensity of 7.9 on the moment-magnitude scale; which is classified as ‘disastrous’. The areas most affected were the Chilean cities of Iquique and Alto Hospicio, and the surrounding towns, with a population of ∼272 000. In spite of its violence, the earthquake's toll in terms of lives and property damage was small: 11 people died, 130 were injured, 180 residences were destroyed and 0.035% of the population had to temporarily relocate to shelters. This limited damage was the result of seismic preparedness and low population density. Economic consequences were more noticeable. The regional index of economic activity dropped by 20.7% compared with the same quarter 1 year before, and recovery did not begin until early 2006. There was a small increase in the unemployment rate in the June–August quarter from 11 to 12% compared with the same quarter a year earlier, which compares with a change from 11 to 10% in the rest of the country. The most heavily damaged areas were rural villages, which accounted for no more than 8% of the population in the affected region. Displacement was therefore reduced (EERI, 2005). Limited spillovers support the hypothesis that the earthquake affected those exposed in utero primarily through the stress and anxiety it induced among pregnant women.
Evidence indicates that earthquakes are a major source of physiological and psychological stress, as signaled by health indicators such as increase in acute cardiac events and strokes (Leor et al., 1996; Dimsdale, 2008), changes in brain function (Lui et al., 2009) and population reports of high levels of distress and anxiety immediately following the disaster (Siegel, 2000). Even though Chile is a country where earthquakes are expected due to geography, it is impossible to predict when or where an earthquake will occur, which ensures the random allocation of exposure to the natural disaster within the Chilean population.
Materials and Methods
Study outcomes
We examined the effect of earthquake exposure during pregnancy on gestational age measured in weeks, and on the probability of preterm delivery (<37 completed weeks of gestation). We included this dichotomous formulation of the dependent variable because preterm birth identifies infants most at risk for mortality, morbidity and long-term developmental problems (Kline et al., 1989), and is used to target interventions to promote children's well-being (Brooks-Gunn et al., 1994; Bale et al., 2003). We also examined the SSR among infants exposed to acute maternal stress in each month of gestation.
Study population
We analyzed the file of Chilean birth certificates for 2004–2006 produced by the Chilean Ministry of Health; there are over 200 000 births a year. Birth records include information on gestational age at delivery, sex, weight and height of the newborn, type of delivery (single, multiple) and medical attention. They also include information on maternal age, education, marital status, parity and county of regular residence. Chile is divided into 350 counties which have on average an area of 816 square miles and a population of 45 000. As a result, the county level provides a precise indicator of geographical location. We merged maternal county of residence during pregnancy with information about the intensity of the earthquake across counties provided by the Chilean National Emergency Office (ONEMI) to produce a measure of exposure to the earthquake. Measures of exposure to the natural disaster include two dimensions: time and geography.
Time dimension of exposure
We defined the exposed period as all Chilean births that were in utero when the earthquake occurred. An unexposed period is also defined, comprising births conceived over the same 9 calendar months, but 12 months earlier. Gestational age was obtained from the birth record, as reported by the health-care provider during prenatal care visits on the basis of the last menstrual period information, or early-pregnancy sonogram. The information is considered reliable for women with at least one prenatal care visit. On the basis of a large probability survey, we estimated these to be 87.9% of pregnant Chilean women. For women who did not have a prenatal care visit during their pregnancy, the attending professional at the time of delivery estimated gestational age based on an interview with the mother (99.8% of deliveries are attended by a professional in Chile). This estimation procedure reduces the proportion of missing data for gestational age, which was only 0.13% in our sample. The probable cost of reducing missing data for the gestational age variable is lower reliability. However, reduced reliability would only result in bias if there was a systematic association between under- or over-estimation of gestational age during earthquake exposure, a very unlikely occurrence. In the most likely scenario of random mis-estimation, measurement error in gestational age would not induce bias.
Information on gestational age and date of birth was used to determine the date of conception. On the basis of this date, births in the exposed period conceived during weeks 39–42 of 2004 were coded as exposed in the ninth month of gestation. Conception dates for the next 8 months of exposure were as follows: eighth month: week (w) 43–w47 2004, seventh month: w48–w51 2004, sixth month: w52 2004–w4 2005, fifth month: w5 2005–w8 2005, fourth month: w9 2005–w13 2005, third month: w14–w17 2005, second month: w18–w21 2005, first month: w22–w25 2005. Births selected into the unexposed period include those conceived 1 year earlier, i.e. from Week 39, 2003 to Week 25, 2004. These definitions ensure that exposed and unexposed periods do not overlap.
Geographical dimension of exposure
The Mercalli scale was used to quantify the intensity of the earthquake across counties. The scale evaluates the effect of the earthquake on the earth's surface, humans, objects of nature and man-made structures. It is a 12-point ordinal scale with the following categories: instrumental (I), feeble (II), slight (III), moderate (IV), rather strong (V), strong (VI), very strong (VII), destructive (VIII), ruinous (IX), disastrous (X), very disastrous (XI), catastrophic (XII), with this last category identifying complete destruction. The Mercalli scale is a preferred metric of the earthquake as felt by the population (Scawthorn, 2003).
According to Mercalli, the intensity of the Tarapaca earthquake varied from I (instrumental) to IX (ruinous) across counties of the country, with most of the population barely feeling the quake. Counties with Mercalli intensity ‘very strong’ to ‘ruinous’ (VII–IX) were defined as the affected region, because the lower bound, ‘very strong’ defines a threshold at which the earthquake is felt by the entire population and damage starts occurring (Ramirez and Peek-Asa, 2005). Seven counties in the northernmost region of Chile comprised the affected region. We defined also an unaffected region by taking a random sample of births, the same number as those born in the affected region, from areas of the country where the intensity was III or less, i.e. areas where the earthquake was virtually unfelt. Selection of a random sample of equal size as the affected region ensures the same statistical power for the analysis among the affected and unaffected regions.
Statistical method
We assessed the differences in each dependent variable attributable to earthquake exposure using linear regression models separately for the affected and unaffected regions. For each region, we distinguished exposed and unexposed periods by means of an indicator variable for the unexposed period. We further divided the exposed period into months of exposure by means of a set of monthly dummies. This resulted in a total of 10 indicator variables—1 for the unexposed period, 9 for each month of the exposed period. We excluded the indicator for the unexposed period and used it as reference category in all models. As a result, the constant term captures the mean of the dependent variable of interest for births that occurred during the unexposed period. The coefficients associated with the remaining nine indicators capture the mean difference in each dependent variable between births exposed to the earthquake at different months of gestation and births in the unexposed period. We report P-values and associated 95% confidence intervals (CIs), based on robust standard errors to account for the built-in heteroskedasticity in linear models for dichotomous outcomes.
We ran these regression models separately for the affected and unaffected regions. The rationale of this double analysis was to rule out the influence of any temporal trend that may involve both affected and unaffected regions, for example, season of birth, the business cycle or any confounding event that may have affected all births in the country and which co-occurred with the earthquake. This amounts to a ‘difference-in-differences’ approach (Cameron and Trivedi, 2005). If we find significant differences in the outcomes of interest between the exposed and unexposed period in the affected region, but no differences in the unaffected region, this result will provide strong evidence for an effect of earthquake exposure on birth outcomes that cannot be attributed to confounding factors. All models included controls for maternal age (<19 years old, 20–29, 30–34, 35 or older), maternal education (primary, secondary and post-secondary schooling), parity (1 birth, 2–3, 4 or more) and urban residence (urban, rural). We stratified the samples by sex to assess differential impact of exposure among male and female births. Table I displays descriptive statistics across the four groups defined by the cross-classification of time exposure (exposed period/unexposed period) and geography (affected region/unaffected region). Table I shows that the demographic and socioeconomic characteristics of women giving birth in the unaffected region are similar to their counterparts in the affected region, providing an adequate comparison group. Furthermore, given the high centralization of the Chilean health-care system, differences across regions in terms of the organization of health-care provision during pregnancy and delivery or programs for pregnant women are not a concern.
Table I.
Descriptive statistics.
| Exposed period |
Unexposed period |
|||
|---|---|---|---|---|
| Affected region | Unaffected region | Affected region | Unaffected region | |
| Mean/Prop. (SD) | Mean/Prop. (SD) | Mean/Prop. (SD) | Mean/Prop. (SD) | |
| Weeks of gestation | 38.74 (1.70) | 39.57 (1.83) | 38.77 (1.81) | 38.61 (1.79) |
| Proportion preterm | 0.058 (0.233) | 0.072 (0.259) | 0.057 (0.232) | 0.065 (0.246) |
| Proportion male births | 0.504 (0.500) | 0.512 (0.500) | 0.518 (0.500) | 0.514 (0.500) |
| Mother's age | ||||
| <19 | 15.1% | 15.1% | 16.0% | 15.0% |
| 20–29 | 52.5% | 45.6% | 50.5% | 47.3% |
| 30–34 | 18.9% | 22.6% | 19.7% | 21.0% |
| 35 or more | 13.6% | 16.7% | 13.7% | 16.7% |
| Mother's education | ||||
| Primary | 15.0% | 19.8% | 16.9% | 21.5% |
| Secondary | 63.3% | 58.5% | 64.4% | 58.8% |
| Tertiary | 21.7% | 21.5% | 18.7% | 19.7% |
| Parity | ||||
| 1 | 40.73% | 43.23% | 37.7% | 42.3% |
| 2–3 | 49.52% | 47.91% | 49.7% | 48.5% |
| 4 or more | 9.75% | 8.86% | 12.6% | 9.2% |
| Urban | 0.958 (0.199) | 0.879 (0.326) | 0.973 (0.161) | 0.880 (0.331) |
| n | 3447 | 3588 | 3427 | 3527 |
Demographic and socioeconomic characteristics of births in utero during the Tarapaca earthquake, in regions affected and unaffected by the earthquake.
Analysis of the SSR, counterfactual simulation
In a final step, we analyzed the effect of maternal stress on the SSR across month of exposure. For this analysis, we cannot simply evaluate the male-to-female live birth ratio across the month of exposure, because the observed SSR depends on the sex-specific effect of stress on gestational age. For example, if acute maternal stress in the first month of gestation results in the selective miscarriage of male embryos that would reduce the SSR (‘male culling’) and, at the same time, in reduced gestational age of female embryos, the combination of these two processes will mask a decline in the SSR 8 months later because both male and female births will decline 8 months after the earthquake. Male births will decline because of culling, and female births will decline because of preterm delivery.
To differentiate the effect of stress on the sex ratio from any sex-specific influence of stress on gestational age, we produced the counterfactual SSR that would have been observed at each month of exposure to the earthquake if exposure had not affected the gestational age of the births exposed. We implemented this counterfactual simulation by imputing a randomly selected value from the gestational age distribution of the entire population of births to each preterm birth in the exposed period. Note that we replaced the gestational age of each preterm birth with a randomly selected value from the overall population's gestational age distribution rather than assigning a full-term value (e.g. 40 weeks). This accounts for the fact that exposed preterm births could have been born preterm even if they had not been exposed to the earthquake (with a probability similar to that of the overall population).
Results
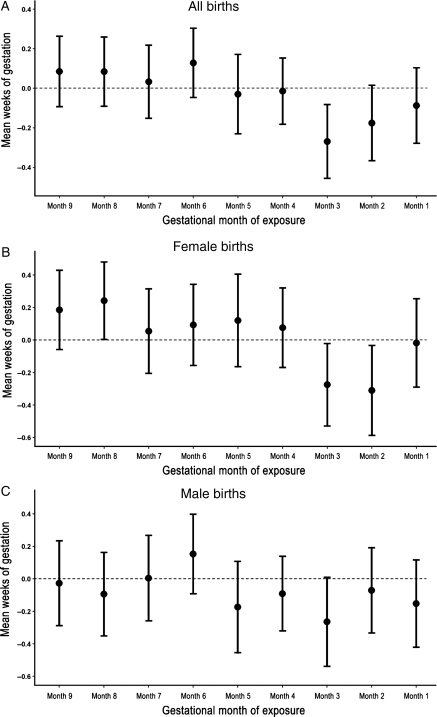
Births exposed to the earthquake in the second and third months of gestation in the affected region had significantly reduced gestational age compared with the unexposed period. Among births exposed in Month 2, there was an average decline of 0.18 weeks (CI: −0.37, 0.02), while exposure in Month 3 is related to a 0.27 week decline (CI: −0.46, −0.08) (Table II, Fig. 1A). In contrast, no change in gestational age across the month of exposure was detected in the unaffected region. Maternal stress resulted not only in reduced gestational length but also in a higher proportion of infants born preterm. Exposure in Month 3 in the affected region translated into a substantial increase in the probability of a preterm birth, which rose from 6.0 to 9.4%, an increase of 3.4% (CI: 0.9, 5.9%). Again, no change was detected in the unaffected region.
Table II.
Association between exposure to earthquake at each month of gestation and mean weeks of gestation/probability of preterm birth in affected and unaffected regions.
| Affected region |
Unaffected region |
|||||||
|---|---|---|---|---|---|---|---|---|
| Weeks gestation |
Preterm birth |
Weeks gestation |
Preterm birth |
|||||
| Coeff. (95% CI) | P-value | Coeff. (95% CI) | P-value | Coeff. (95% CI) | P-value | Coef. (95% CI) | P-value | |
| All births | ||||||||
| Exposed m9 | 0.082 (−0.099, 0.263) | 0.374 | −0.015 (−0.039, 0.009) | 0.234 | −0.009 (−0.204, 0.185) | 0.925 | 0.003 (−0.024, 0.031) | 0.814 |
| Exposed m8 | 0.081 (−0.097, 0.259) | 0.370 | 0.003 (−0.020, 0.027) | 0.790 | −0.006 (−0.180, 0.169) | 0.949 | −0.002 (−0.026, 0.023) | 0.903 |
| Exposed m7 | 0.030 (−0.158, 0.218) | 0.752 | −0.008 (−0.033, 0.017) | 0.521 | −0.136 (−0.325, 0.053) | 0.159 | 0.008 (−0.019, 0.035) | 0.553 |
| Exposed m6 | 0.125 (−0.053, 0.303) | 0.168 | −0.007 (−0.030, 0.017) | 0.574 | 0.045 (−0.128, 0.218) | 0.609 | 0.003 (−0.021, 0.027) | 0.815 |
| Exposed m5 | −0.033 (−0.236, 0.171) | 0.753 | −0.005 (−0.032, 0.022) | 0.702 | −0.025 (−0.221, 0.170) | 0.801 | −0.002 (−0.029, 0.026) | 0.907 |
| Exposed m4 | −0.017 (−0.188, 0.153) | 0.843 | −0.015 (−0.038, 0.007) | 0.180 | −0.028 (−0.198, 0.142) | 0.749 | 0.009 (−0.014, 0.033) | 0.435 |
| Exposed m3 | −0.271 (−0.460, −0.081) | 0.005 | 0.034 (0.009, 0.059) | 0.009 | −0.104 (−0.304, 0.096) | 0.306 | 0.013 (−0.015, 0.042) | 0.346 |
| Exposed m2 | −0.178 (−0.371, 0.016) | 0.072 | 0.012 (−0.014, 0.038) | 0.357 | −0.054 (−0.249, 0.141) | 0.587 | 0.023 (−0.004, 0.050) | 0.098 |
| Exposed m1 | −0.090 (−0.284, 0.104) | 0.364 | 0.019 (−0.007, 0.044) | 0.155 | 0.000 (−0.200, 0.200) | 0.997 | 0.017 (−0.011, 0.045) | 0.232 |
| Constant | 39.030 (38.768, 39.292) | 0.000 | 0.060 (0.026, 0.095) | 0.001 | 38.920 (38.751, 39.089) | 0.000 | 0.062 (0.038, 0.086) | 0.000 |
| n | 6874 | 6874 | 7115 | 7115 | ||||
| Female births | ||||||||
| Exposed m9 | 0.185 (−0.063, 0.434) | 0.143 | −0.023 (−0.056, 0.009) | 0.158 | −0.056 (−0.310, 0.198) | 0.666 | 0.021 (−0.015, 0.056) | 0.262 |
| Exposed m8 | 0.242 (−0.001, 0.485) | 0.051 | −0.015 (−0.047, 0.016) | 0.344 | −0.089 (−0.322, 0.145) | 0.457 | −0.009 (−0.042, 0.025) | 0.613 |
| Exposed m7 | 0.054 (−0.210, 0.319) | 0.686 | −0.001 (−0.036, 0.033) | 0.954 | −0.019 (−0.274, 0.236) | 0.885 | −0.009 (−0.045, 0.027) | 0.610 |
| Exposed m6 | 0.093 (−0.161, 0.347) | 0.474 | −0.005 (−0.039, 0.028) | 0.748 | 0.050 (−0.183, 0.284) | 0.674 | −0.005 (−0.038, 0.028) | 0.759 |
| Exposed m5 | 0.120 (−0.169, 0.410) | 0.416 | −0.021 (−0.058, 0.017) | 0.282 | −0.133 (−0.405, 0.139) | 0.337 | 0.016 (−0.022, 0.055) | 0.411 |
| Exposed m4 | 0.076 (−0.174, 0.325) | 0.553 | −0.017 (−0.050, 0.016) | 0.307 | −0.110 (−0.342, 0.121) | 0.349 | 0.019 (−0.014, 0.051) | 0.262 |
| Exposed m3 | −0.275 (−0.534, −0.017) | 0.037 | 0.038 (0.005, 0.072) | 0.025 | −0.064 (−0.333, 0.204) | 0.638 | 0.027 (−0.011, 0.065) | 0.164 |
| Exposed m2 | −0.310 (−0.592, −0.029) | 0.031 | 0.039 (0.002, 0.075) | 0.040 | −0.035 (−0.303, 0.232) | 0.795 | 0.022 (−0.016, 0.060) | 0.252 |
| Exposed m1 | −0.018 (−0.295, 0.258) | 0.896 | 0.010 (−0.026, 0.046) | 0.580 | 0.042 (−0.225, 0.310) | 0.756 | 0.009 (−0.029, 0.047) | 0.630 |
| Constant | 39.011 (38.625, 39.397) | 0.000 | 0.077 (0.027, 0.127) | 0.003 | 38.993 (38.766, 39.220) | 0.000 | 0.071 (0.039, 0.103) | 0.000 |
| n | 3364 | 3364 | 3466 | 3466 | ||||
| Male births | ||||||||
| Exposed m9 | −0.027 (−0.293, 0.239) | 0.842 | −0.006 (−0.042, 0.030) | 0.748 | 0.042 (−0.256, 0.339) | 0.783 | −0.016 (−0.057, 0.026) | 0.453 |
| Exposed m8 | −0.094 (−0.356, 0.167) | 0.478 | 0.023 (−0.012, 0.058) | 0.198 | 0.072 (−0.187, 0.330) | 0.588 | 0.004 (−0.032, 0.040) | 0.812 |
| Exposed m7 | 0.004 (−0.263, 0.272) | 0.975 | −0.015 (−0.051, 0.021) | 0.410 | −0.237 (−0.516, 0.041) | 0.095 | 0.023 (−0.016, 0.062) | 0.247 |
| Exposed m6 | 0.153 (−0.096, 0.402) | 0.228 | −0.008 (−0.041, 0.026) | 0.645 | 0.039 (−0.216, 0.294) | 0.765 | 0.011 (−0.025, 0.046) | 0.563 |
| Exposed m5 | −0.174 (−0.460, 0.112) | 0.232 | 0.010 (−0.029, 0.048) | 0.623 | 0.068 (−0.212, 0.348) | 0.634 | −0.018 (−0.057, 0.021) | 0.374 |
| Exposed m4 | −0.091 (−0.325, 0.143) | 0.447 | −0.015 (−0.047, 0.016) | 0.347 | 0.052 (−0.196, 0.300) | 0.680 | 0.001 (−0.034, 0.036) | 0.952 |
| Exposed m3 | −0.264 (−0.543, 0.014) | 0.063 | 0.029 (−0.009, 0.066) | 0.134 | −0.152 (−0.447, 0.144) | 0.314 | −0.000 (−0.041, 0.041) | 0.997 |
| Exposed m2 | −0.071 (−0.338, 0.196) | 0.601 | −0.010 (−0.046, 0.025) | 0.569 | −0.055 (−0.338, 0.228) | 0.702 | 0.021 (−0.018, 0.061) | 0.286 |
| Exposed m1 | −0.152 (−0.426, 0.121) | 0.275 | 0.026 (−0.011, 0.062) | 0.171 | −0.040 (−0.336, 0.256) | 0.790 | 0.023 (−0.018, 0.065) | 0.267 |
| Constant | 39.055 (38.696, 39.414) | 0.000 | 0.045 (−0.004, 0.093) | 0.071 | 38.837 (38.587, 39.086) | 0.000 | 0.055 (0.021, 0.090) | 0.002 |
| n | 3510 | 3510 | 3649 | 3649 | ||||
Estimates are linear regression coefficients, 95% CIs, and exact P-values (robust standard errors used). Dummy coded 1 for birth in unexposed period excluded and used as reference category. Estimates capture difference in mean weeks of gestation/proportion preterm births exposed to earthquake at each month of gestation and births in unexposed time period (in utero 12 months earlier). Models control for maternal age, maternal education, parity and rural residence. Exposed population is births in area where earthquake intensity was very strong to ruinous. Unexposed region is random sample of births in areas where earthquake intensity was slight or less.
Figure 1.
Difference in mean weeks of gestation (95% CI) of births exposed to earthquake compared with births in unexposed period, across gestational month of exposure. (A) All births: estimates obtained from Column 1 Table II. (B) Female births: estimates from Column 1 Table II. (C) Male births: estimates obtained from Column 1 Table II.
The effect of earthquake exposure on gestational age was much more pronounced for female than male gestations (Tables II, Fig. 1B and C). Among female births, the probability of preterm birth increased by 3.8% (CI: 0.01, 7.2%) if exposure occurred in Month 3 and 3.9% (CI: 0.2, 7.5%) if exposure occurred in Month 2. In contrast, the increase in preterm births was not statistically significant at the conventional P < 0.05 level among male births. Again, these effects emerged only in the region affected by the earthquake, without significant changes in the unaffected region. Taken together, these findings show a detrimental effect of maternal exposure to an acute stressor on gestational age only among those exposed in Months 2 and 3 of gestation. This effect was much stronger for female than male births. (In order to examine robustness of the findings to the method chosen, we replicated the analyses using a longer time series and applying methods for interrupted time series. Results are substantively identical to those reported here. Implementation of the analysis and findings are reported in the Supplementary data.)
Exposure to acute stress may also affect the observed SSR across the month of exposure. Table III shows a decline in the sex ratio for those exposed to the earthquake in the third month of gestation, which does not reach significance at the standard P < 0.05 level [−5.1% (CI: −10.5, 0.3) P = 0.07]. However, this result depends on the differential impact of earthquake exposure on the duration of pregnancy by sex, which, as we have shown, is stronger for females than for males. This sex-specific effect on the gestational length could bias the estimate of the impact of stress on the sex ratio. For example, in calculating the sex ratio, a female infant exposed to the earthquake in Month 3 and born at 36 weeks of gestation will not be compared with a male infant also exposed in Month 3 but born at term (40 weeks), but rather with a term male infant exposed in Month 2, artificially ‘inflating’ the relative number of males among those exposed in Month 3. We implemented a counterfactual simulation to address this issue. The simulation amounts to ‘displacing’ exposed births born preterm from their actual birth month to the month they would have been born had their gestational age not been shortened by the earthquake. Because, as we have shown, the effect of stress on preterm birth was stronger for females, this ‘displacement’ will affect more female than male births.
Table III.
Association between exposure to earthquake at each month of gestation and secondary sex ratio (SSR) in affected and unaffected regions.
| Affected region |
Unaffected region |
|||
|---|---|---|---|---|
| Coeff. (95% CI) | P-value | Coeff. (95% CI) | P-value | |
| Exposed m9 | −0.050 (−0.101, 0.002) | 0.058 | −0.042 (−0.096, 0.012) | 0.125 |
| Exposed m8 | −0.051 (−0.101, 0.000) | 0.050 | −0.011 (−0.060, 0.037) | 0.647 |
| Exposed m7 | −0.017 (−0.070, 0.037) | 0.541 | −0.004 (−0.056, 0.049) | 0.892 |
| Exposed m6 | 0.003 (−0.047, 0.054) | 0.895 | −0.004 (−0.052, 0.044) | 0.864 |
| Exposed m5 | −0.004 (−0.062, 0.054) | 0.890 | 0.030 (−0.024, 0.084) | 0.280 |
| Exposed m4 | 0.025 (−0.023, 0.074) | 0.305 | 0.007 (−0.040, 0.054) | 0.782 |
| Exposed m3 | −0.051 (−0.105, 0.003) | 0.066 | −0.007 (−0.062, 0.049) | 0.816 |
| Exposed m2 | 0.022 (−0.033, 0.077) | 0.436 | 0.014 (−0.040, 0.068) | 0.619 |
| Exposed m1 | −0.004 (−0.059, 0.052) | 0.901 | −0.010 (−0.065, 0.046) | 0.733 |
| Constant | 0.566 (0.492, 0.641) | 0.000 | 0.508 (0.462, 0.555) | 0.000 |
| n | 6874 | 7115 | ||
Table IV shows the results of the counterfactual simulation. It estimates the SSR across the month of exposure that would have been observed if earthquake exposure had not shortened duration of pregnancy of any birth. Simulated data suggest a decline in the SSR among those exposed in Month 3 of gestation by 5.8% (CI: −11.3, −0.3%) (Table IV). This result is consistent with a sex-specific effect of exposure to the earthquake in Month 3 of gestation: A reduction in gestational age among exposed female births is found concurrently with a relative decline in the proportion of male births.
Table IV.
Association between exposure to earthquake at each month of gestation and secondary sex ratio (SSR) among affected and unaffected regions, counterfactual distribution assuming no effect of earthquake exposure on weeks of gestation.
| Affected region |
Unaffected region |
|||
|---|---|---|---|---|
| Coeff. (95% CI) | P-value | Coeff. (95% CI) | P-value | |
| Exposed m9 | −0.050 (−0.101, 0.002) | 0.057 | −0.042 (−0.096, 0.012) | 0.125 |
| Exposed m8 | −0.051 (−0.101, 0.000) | 0.050 | −0.011 (−0.060, 0.037) | 0.647 |
| Exposed m7 | −0.017 (−0.070, 0.037) | 0.541 | −0.004 (−0.056, 0.049) | 0.892 |
| Exposed m6 | 0.002 (−0.048, 0.053) | 0.935 | −0.004 (−0.052, 0.044) | 0.862 |
| Exposed m5 | −0.003 (−0.060, 0.055) | 0.925 | 0.032 (−0.022, 0.086) | 0.242 |
| Exposed m4 | 0.020 (−0.027, 0.068) | 0.405 | 0.008 (−0.039, 0.054) | 0.744 |
| Exposed m3 | −0.058 (−0.113, −0.003) | 0.039 | −0.005 (−0.060, 0.051) | 0.867 |
| Exposed m2 | 0.032 (−0.025, 0.089) | 0.271 | 0.008 (−0.048, 0.064) | 0.773 |
| Exposed m1 | −0.002 (−0.057, 0.053) | 0.941 | −0.010 (−0.065, 0.046) | 0.733 |
| Constant | 0.566 (0.492, 0.640) | 0.000 | 0.508 (0.462, 0.555) | 0.000 |
| n | 6874 | 7115 | ||
Discussion
In our population-based analysis, the women who experienced a severe earthquake during their second and third months of pregnancy had shorter pregnancies, and were also at higher risk of delivering preterm (<37 weeks gestation). This effect was markedly sex-specific; it was weak or non-existent among males, and pronounced among females. These findings are striking because in our unexposed Chilean population, as in most populations (Zeitlin et al., 2002; Ingemarsson, 2003; Di Renzo et al., 2007; Brettell et al., 2008), males are more likely to be born preterm. Estimates from the Chilean birth registry indicate that the proportion of preterm births in the 2004–2007 period was 5.6% among females, but 6.8% among males. While acute maternal stress has been linked previously with shorter pregnancies (Barbosa, 2000; Glynn et al., 2001; Xiong et al., 2008; Lipkind et al., 2010; Zhu et al., 2010; Torche, 2011) and some research documents sex-specific changes in very low birthweight following ambient stressors (Catalano et al., 1999; Catalano et al., 2005), to our knowledge there are no other population-based analyses showing a difference by sex in effects of exposure to an acute stressor on the timing of parturition.
Previous research has documented an interaction between the timing of prenatal stress and the sex of the fetus in the determination of other outcomes in offspring such as the risks for schizophrenia, addictive disorder, affective psychosis and Type I Diabetes (Brown et al., 1995; Franzek et al., 2008; Malaspina et al., 2008; Virk et al., 2010). The sex-specific effects on the timing of delivery that we detected, however, likely entail different mechanisms. The placenta is thought to help set the duration of pregnancy and so may play a central role in our findings. Researchers have hypothesized that a decrease in gestational length after early prenatal stress might be triggered by a spike in maternal cortisol secondary to acute stress. Higher levels of maternal cortisol early in the pregnancy are known to stimulate placental production of corticotrophin-releasing hormone (CRH) in the early third trimester (Pike, 2005), which is in turn predictive of an increased risk for preterm birth (Majzoub et al., 1999; Glynn et al., 2001; Wadhwa et al., 2004; Sandman et al., 2006; Smith and Nicholson, 2007). While some report no significant levels of placental CRH during the first trimester (Sasaki et al., 1987), others find ample evidence for an important role for both placental and endometrial CRH in early pregnancy, especially in the early development of the placenta (Makrigiannakis et al., 2003, 2004; Choy et al., 2004; Kalantaridou et al., 2007), which will be central to setting the time of parturition later on.
Should the placenta contribute to the effects of prenatal stress on the duration of pregnancy, this could explain, at least in part, the sex-specificity of effects. First off, the placenta originates from fetal cells, which are inherently sex-specific. In addition, the epigenetic machinery that influences gene expression in the placenta may have basal differences by sex (Mueller and Bale, 2008). These mechanisms for sex-dependent effects of the placenta remain unknown, but may be related to a buffering of genes on the X chromosome. As X inactivation occurs to a much lesser extent in the placenta, increased levels of expressed X chromosome genes in female placentas may underlie different adaptations to the maternal environment (Dunn et al., 2011). Another potential pathway for differences by sex in the duration of pregnancy could involve processes in early pregnancy that result in increased maternal cortisol levels during the second trimester and in placental CRH at 31 weeks. These have been linked to decreases in infant maturation in males only (Ellman et al., 2008), suggesting that biological pathways involved in the duration of gestation have been linked to other outcomes that differ by sex. The female placenta may be more responsive to maternal stress, signaled by increased maternal levels of glucocorticoids, than the male placenta. This may result in the differential adaptation of male and female fetuses to this maternal cue (Sandman et al., 2011). More studies are needed, however, to explore if differences in the placentas of males and females may influence the relationship between prenatal stress and preterm birth.
We also studied the link between prenatal stress and the secondary sex ratio, which we ultimately found to be related to our primary finding about shorter duration of pregnancy. Studies have shown that cohorts that have experienced severe maternal stress while in utero, such as from natural disasters, terrorist attacks or mass layoffs, have lower than expected SSRs (Bruckner et al., 2010; Catalano et al., 2010). It is theorized that this is because natural selection has conserved mechanisms by which stressed pregnant females preferentially cull frail male fetuses. Male fetuses grow larger and therefore require greater investment of resources by the mother, and may also not adapt their development to a stressful intrauterine environment; females use less resources as they develop and are thought to reduce growth and demands on the mother in response to maternal stress (Sandman et al., 2011). Male newborns are also more likely to die than females (Bhaumik et al., 2004). As a result, weaker males may be culled when the mother experiences stress as a way to maximize the benefit of expending limited resources during demanding times (Catalano and Bruckner, 2006). Our findings on decreased SSR address this hypothesis. We detected a decline in the SSR by 5.8% [(CI: −11.3, −0.3%), P < 0.04) among those exposed in the third month of gestation (Table IV). Note that this finding emerges only after we account for the fact that females exposed in the third month of gestation are more likely to be born preterm, by means of a counterfactual simulation.
We also observed a decline in the SSR among those exposed to stress in Month 8 of gestation [−5.1% (CI: −0.10%, 0.00), P = 0.05], though there was no decline in the unaffected region (Table III); these findings were unchanged in the counterfactual simulation and so were not related to the duration of pregnancy (Table IV). We cannot explore whether this decrease might be linked to the preferential loss of male fetuses as was reported in studies described below or to other mechanisms as we do not have data on fetal deaths for this cohort. A comparable decline in the SSR is also observed among those exposed to the earthquake in Month 9 [−5.0% (CI: −0.10, 0.02%), P = 0.06] (Tables III and IV). However, a similar decline of 4.2% is observed in the unaffected region. Even though this decline fails to reach significance (P = 0.12), the coefficients for the affected and unaffected regions are virtually identical (P-value for the comparison test for difference = 0.80). This suggests that the decline in the SSR was similar across populations in affected and unaffected regions that were in their ninth month of gestation when the earthquake occurred. The decline could, therefore, be due to seasonal patterns or other temporal factors affecting the entire country and therefore not attributable to the natural disaster.
Previous research using data from a Californian population found that the SSR was lower than expected in the cohort born in December 2001, 3 months after the 11th September attacks, reflecting loss of males stressed in utero during Month 6 of gestation (Catalano et al., 2005). That study also reported that the sex ratio of very low weight births was unexpectedly decreased in December 2001, while the sex ratio for fetal deaths over 20 weeks of gestation was highest in the 2 months after the attacks, suggesting that the decline in the SSR for a cohort prenatally stressed in the sixth month of gestation resulted from weaker male fetuses being preferentially aborted. A cohort in New York City was found to have a lower SSR 4 months after the September, 2001 terrorist attacks, suggesting loss of males stressed at 5 months (Catalano et al., 2006). We cannot directly evaluate the factors driving the change in the SSR among the exposed population, such as an increase in death of male fetuses, as we do not have information on fetal deaths for our study population. Nevertheless, our finding of a decline in the SSR among those exposed in the third month of pregnancy is consistent with the above hypothesis that observed declines are due to culling of male fetuses. Observed differences in the timing of stress and outcomes may be due to factors such as differences in populations, and in the type, duration and severity of stressors, and call for further investigation.
A growing body of research suggests that prenatal stress at specific times in pregnancy may result in sex-specific outcomes. By analyzing a large population without selectivity, using a fully exogenous stressor, and defining comparison groups to address the differences in the timing and in the location of the stressor, we hope to have provided strong evidence for the sex-specificity of the effect of acute maternal stress exposure in early pregnancy. In spite of these advantages, our study also has limitations. In particular, as noted above, lacking information on fetal deaths, we cannot directly evaluate the factors driving the change in the SSR among the exposed population. Also, our analytical strategy exploits the difference between affected and unaffected areas to account for confounding emerging from shared trends. If women in the unaffected area also experienced some stress as a result of the earthquake, our analysis will underestimate the effect in the affected area. This possibility is unlikely given the limited damage and spillover effect of the earthquake. If it occurred, however, it would hamper our ability to reject the null hypothesis of no effect, making our tests conservative.
We found a substantial increase in preterm delivery among female gestations, and a potential decline in the SSR among those exposed in the third month of pregnancy. Since in most populations males are more likely to be born preterm, there are clearly sex-specific mechanisms at work in both acutely stressed and unstressed populations. Our findings highlight the need for future translational research into the sex-specific pathophysiology of preterm delivery. This knowledge would provide a foundation for the development of novel interventions to reduce preterm delivery, an important predictor of infant morbidity and mortality in the USA and elsewhere (Bale et al., 2003; Mathews and MacDorman, 2007).
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
F.T. and K.K. contributed to the study design. F.T. performed the statistical analysis, with assistance from K.K. F.T. wrote the first draft of the paper. F.T. and K.K. contributed to the final draft. Both authors participated in the reporting stage and have seen and approved the final draft of the paper.
Funding
This work was supported by National Science Foundation (grant number 1023841 to F.T.), and the National Institutes of Health (grant number K08MH085807 to K.K.).
Conflict of interest
None declared.
Supplementary Material
References
- Bale JR, Stoll BJ, Lucas AO. Improving Birth Outcomes: Meeting the Challenges in the Developing World. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Barbosa GA. The association of life events to gestational age at delivery among low-income, urban, African American women. J Perinatol. 2000;20:438–442. doi: 10.1038/sj.jp.7200423. [DOI] [PubMed] [Google Scholar]
- Bhaumik U, Aitken I, Kawachi I, Ringer S, Orav J, Lieberman E. Narrowing of sex differences in infant mortality in Massachusetts. J Perinatol. 2004;24:94–99. doi: 10.1038/sj.jp.7211021. [DOI] [PubMed] [Google Scholar]
- Brettell R, Yeh PS, Impey LW. Examination of the association between male gender and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2008;141:123–126. doi: 10.1016/j.ejogrb.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, McCarton CM, Casey PH, McCormick MC, Bauer CR, Bernbaum JC, Tyson J, Swanson M, Bennett FC, Scott DT, et al. Early intervention in low-birth-weight premature infants. Results through age 5 years from the Infant Health and Development Program. JAMA. 1994;272:1257–1262. [PubMed] [Google Scholar]
- Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. Br J Psychiatry. 1995;166:601–606. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- Bruckner T, Catalano R. The sex ratio and age-specific male mortality: evidence for culling in utero. Am J Hum Biol. 2007;19:763–773. doi: 10.1002/ajhb.20636. [DOI] [PubMed] [Google Scholar]
- Bruckner TA, Catalano R, Ahern J. Male fetal loss in the U.S. following the terrorist attacks of September 11, 2001. BMC Public Health. 2010;10:273. doi: 10.1186/1471-2458-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AC, Trivedi PK. Microeconometrics: Methods and Applications. Cambridge, NY: Cambridge University Press; 2005. p. xxii. 1034 pp. [Google Scholar]
- Catalano R, Bruckner T. Secondary sex ratios and male lifespan: damaged or culled cohorts. Proc Natl Acad Sci USA. 2006;103:1639–1643. doi: 10.1073/pnas.0510567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano R, Hansen HT, Hartig T. The ecological effect of unemployment on the incidence of very low birthweight in Norway and Sweden. J Health Soc Behav. 1999;40:422–428. [PubMed] [Google Scholar]
- Catalano R, Bruckner T, Gould J, Eskenazi B, Anderson E. Sex ratios in California following the terrorist attacks of September 11, 2001. Hum Reprod. 2005;20:1221–1227. doi: 10.1093/humrep/deh763. [DOI] [PubMed] [Google Scholar]
- Catalano R, Bruckner T, Marks AR, Eskenazi B. Exogenous shocks to the human sex ratio: the case of September 11, 2001 in New York City. Hum Reprod. 2006;21:3127–3131. doi: 10.1093/humrep/del283. [DOI] [PubMed] [Google Scholar]
- Catalano R, Ahern J, Bruckner T, Anderson E, Saxton K. Gender-specific selection in utero among contemporary human birth cohorts. Paediatr Perinat Epidemiol. 2009;23:273–278. doi: 10.1111/j.1365-3016.2009.01028.x. [DOI] [PubMed] [Google Scholar]
- Catalano R, Zilko CE, Saxton KB, Bruckner T. Selection in utero: a biological response to mass layoffs. Am J Hum Biol. 2010;22:396–400. doi: 10.1002/ajhb.21011. [DOI] [PubMed] [Google Scholar]
- Choy MY, Leung TN, Lau TK. Corticotropin-releasing hormone peptide and human first-trimester placental growth. Early Hum Dev. 2004;79:77–80. doi: 10.1016/j.earlhumdev.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Class QA, Lichtenstein P, Langstrom N, D'Onofrio BM. Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: a population study of 2.6 million pregnancies. Psychosom Med. 2011;73:234–241. doi: 10.1097/PSY.0b013e31820a62ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4:19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- Dunn GA, Morgan CP, Bale TL. Sex-specificity in transgenerational epigenetic programming. Horm Behav. 2011;59:290–295. doi: 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- EERI EERI. Intensities and damage distribution in the June 2005 Tarapaca, Chile, earthquake. EERI Special Earthquake Report. 2005. 1–8.
- Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzek EJ, Sprangers N, Janssens AC, Van Duijn CM, Van De Wetering BJ. Prenatal exposure to the 1944–45 Dutch ‘hunger winter’ and addiction later in life. Addiction. 2008;103:433–438. doi: 10.1111/j.1360-0443.2007.02084.x. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Hansen D, Moller H, Olsen J. Severe periconceptional life events and the sex ratio in offspring: follow up study based on five national registers. BMJ. 1999;319:548–549. doi: 10.1136/bmj.319.7209.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingemarsson I. Gender aspects of preterm birth. BJOG. 2003;110:34–38. doi: 10.1016/s1470-0328(03)00022-3. (Suppl 20) [DOI] [PubMed] [Google Scholar]
- Kalantaridou SN, Zoumakis E, Makrigiannakis A, Godoy H, Chrousos GP. The role of corticotropin-releasing hormone in blastocyst implantation and early fetal immunotolerance. Horm Metab Res. 2007;39:474–477. doi: 10.1055/s-2007-980190. [DOI] [PubMed] [Google Scholar]
- Kline J, Stein Z, Susser M. Conception to Birth: Epidemiology of Prenatal Development. New York: Oxford University Press; 1989. [Google Scholar]
- Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334:413–419. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- Lipkind HS, Curry AE, Huynh M, Thorpe LE, Matte T. Birth outcomes among offspring of women exposed to the September 11, 2001, terrorist attacks. Obstet Gynecol. 2010;116:917–925. doi: 10.1097/AOG.0b013e3181f2f6a2. [DOI] [PubMed] [Google Scholar]
- Lui S, Huang X, Chen L, Tang H, Zhang T, Li X, Li D, Kuang W, Chan RC, Mechelli A, et al. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc Natl Acad Sci USA. 2009;106:15412–15417. doi: 10.1073/pnas.0812751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majzoub JA, McGregor JA, Lockwood CJ, Smith R, Taggart MS, Schulkin J. A central theory of preterm and term labor: putative role for corticotropin-releasing hormone. Am J Obstet Gynecol. 1999;180:S232–S241. doi: 10.1016/s0002-9378(99)70707-6. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Zoumakis E, Kalantaridou S, Chrousos G, Gravanis A. Uterine and embryonic trophoblast CRH promotes implantation and maintenance of early pregnancy. Ann N Y Acad Sci. 2003;997:85–92. doi: 10.1196/annals.1290.010. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Zoumakis E, Kalantaridou S, Chrousos G. Endometrial and placental CRH as regulators of human embryo implantation. J Reprod Immunol. 2004;62:53–59. doi: 10.1016/j.jri.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, Friedlander Y, Harlap S. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry. 2008;8:71. doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2004 period linked birth/infant death data set. Natl Vital Stat Rep. 2007;55:1–32. [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike IL. Maternal stress and fetal responses: evolutionary perspectives on preterm delivery. Am J Hum Biol. 2005;17:55–65. doi: 10.1002/ajhb.20093. [DOI] [PubMed] [Google Scholar]
- Ramirez M, Peek-Asa C. Epidemiology of traumatic injuries from earthquakes. Epidemiol Rev. 2005;27:47–55. doi: 10.1093/epirev/mxi005. [DOI] [PubMed] [Google Scholar]
- Reynolds EW, Riel-Romero RM, Bada HS. Neonatal abstinence syndrome and cerebral infarction following maternal codeine use during pregnancy. Clin Pediatr (Phila) 2007;46:639–645. doi: 10.1177/0009922807300795. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2011 doi: 10.1159/000327017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Shinkawa O, Margioris AN, Liotta AS, Sato S, Murakani O, Go M, Shimizu Y, Hanew K, Yoshinaga K. Immunoreactive corticotropin-releasing hormone in human plasma during pregnancy, labor, and delivery. J Clin Endocrinol Metab. 1987;64:224–229. doi: 10.1210/jcem-64-2-224. [DOI] [PubMed] [Google Scholar]
- Scawthorn C. Earthquakes: seismogenesis, measurement, and distribution. In: Chen W, Scawthorn C, editors. Earthquake Engineering Handbook. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Siegel J. Emotional Injury and the Northridge, California Earthquake. Nat Hazards Rev. 2000;1:204–211. [Google Scholar]
- Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Front Biosci. 2007;12:912–918. doi: 10.2741/2113. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Moran TH. Perinatal environment and its influences on metabolic programming of offspring. Physiol Behav. 2010;100:560–566. doi: 10.1016/j.physbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegethoff M, Greene N, Olsen J, Meyer AH, Meinlschmidt G. Maternal psychosocial adversity during pregnancy is associated with length of gestation and offspring size at birth: evidence from a population-based cohort study. Psychosom Med. 2010;72:419–426. doi: 10.1097/PSY.0b013e3181d2f0b0. [DOI] [PubMed] [Google Scholar]
- Torche F. The effect of maternal stress on birth outcomes: exploiting a natural experiment. Demography. 2011;48:1473–1491. doi: 10.1007/s13524-011-0054-z. [DOI] [PubMed] [Google Scholar]
- Virk J, Li J, Vestergaard M, Obel C, Lu M, Olsen J. Early life disease programming during the preconception and prenatal period: making the link between stressful life events and type-1 diabetes. PLoS One. 2010;5:e11523. doi: 10.1371/journal.pone.0011523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Xiong X, Harville EW, Mattison DR, Elkind-Hirsch K, Pridjian G, Buekens P. Exposure to Hurricane Katrina, post-traumatic stress disorder and birth outcomes. Am J Med Sci. 2008;336:111–115. doi: 10.1097/MAJ.0b013e318180f21c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin J, Saurel-Cubizolles MJ, De Mouzon J, Rivera L, Ancel PY, Blondel B, Kaminski M. Fetal sex and preterm birth: are males at greater risk? Hum Reprod. 2002;17:2762–2768. doi: 10.1093/humrep/17.10.2762. [DOI] [PubMed] [Google Scholar]
- Zhu P, Tao F, Hao J, Sun Y, Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. Am J Obstet Gynecol. 2010;203:1–8. doi: 10.1016/j.ajog.2010.02.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.