Abstract
BACKGROUND
Hyperandrogenemia is associated with several clinical disorders in which both reproductive dysfunction and metabolic changes may coexist [i.e. polycystic ovary syndrome (PCOS), obesity and congenital adrenal hyperplasia]. Moreover, there is growing evidence that the elevated levels of circulating androgens in obese girls may lead to an increased neuroendocrine drive to the reproductive axis, similar to that associated with PCOS.
METHODS
To test whether androgen exposure in the childhood and adolescent period could lead to pubertal alterations in LH secretory patterns, female rhesus monkeys received subcutaneous testosterone implants prepubertally beginning at 1 year of age, maintaining a 3.7-fold increase (P = 0.001) in circulating testosterone levels over cholesterol-implant controls (n = 6/group) into the post-pubertal period. In early adulthood, pulsatile secretion of LH was measured over 12 h during the early follicular phase of a menstrual cycle, and responsiveness of the pituitary to gonadotrophin-releasing hormone was determined. In addition, ultrasounds were performed to assess ovarian morphology and glucose tolerance testing was performed to assess insulin sensitivity.
RESULTS
The timing of menarche was similar between groups. Testosterone-treated animals had a significantly greater LH pulse frequency during the early follicular phase compared with controls (P = 0.039) when measured at 5 years of age. There was a larger LH response to GnRH when testosterone-treated animals were 4 years of age (P = 0.042), but not when the animals were 5 years old (P = 0.57). No differences were seen in insulin sensitivity or ovarian morphology, and the groups showed similar rates of ovulation in early adulthood.
CONCLUSIONS
Exposure to increased levels of androgens over the course of pubertal development appears to trigger physiological changes in the neural drive to the reproductive axis that resemble those of obese hyperandrogenemic girls in early adulthood and are characteristic of PCOS.
Keywords: polycystic ovary syndrome, LH, testosterone, hyperandrogenism, puberty
Introduction
Polycystic ovary syndrome (PCOS) is a common reproductive disorder affecting 4–8% of reproductive-aged women worldwide (Knochenhauer et al., 1998; Asuncion et al., 2000; Azziz et al., 2004). Symptoms include evidence of hyperandrogenism, polycystic ovaries and oligomenorrhea or amenorrhea (Knochenhauer et al., 1998; McCartney et al., 2006; Blank et al., 2008). Typically, pelvic ultrasonography reveals that women with PCOS have increased ovarian size, as well as greater numbers of antral follicles compared with healthy women (Chen et al., 2008; Shah et al., 2010). There is also an increased incidence of obesity and insulin insensitivity in women with PCOS (Legro et al., 1999, 2001; Ovalle and Azziz, 2002). Obesity reportedly aggravates PCOS symptomology (Dunaif et al., 1989; Legro, 2000), and weight loss may help women with PCOS restore metabolic and reproductive function (Pasquali et al., 1989; Hoeger, 2008). Characteristic neuroendocrine changes often seen in patients with PCOS include increased frequency of pulsatile LH secretion by the pituitary, increased pituitary responsiveness to GnRH, and decreased sensitivity of the hypothalamus to progesterone negative feedback (Rebar et al., 1976; Pastor et al., 1998; Marshall and Eagleson, 1999).
Interestingly, obese girls are often hyperandrogenemic even in early puberty, and the hyperandrogenemia (HA) is associated with a rapid progression from pubertal to adult LH secretory patterns (Apter et al., 1994; McCartney et al., 2009). In addition, in girls with HA from other causes, such as congenital adrenal hyperplasia (CAH) and premature pubarche, there is an increased incidence of elevated LH secretion and irregular menstrual cycles (Levin et al., 1991; Holmes-Walker et al., 1995). In animal studies, early exposure to elevated androgens in the prenatal period has been associated with an increased neural drive to the reproductive axis. In both rats and sheep, HA in the prenatal period leads to an increased frequency of LH pulses and higher levels of circulating LH (Sharma et al., 2002; Foecking et al., 2005; Savabieasfahani et al., 2005). Similarly, previous studies in female monkeys have shown that excess androgen exposure during fetal development can lead to later HA, irregular or absent menstrual cycles, elevated LH levels and polycystic ovaries as adults (Abbott et al., 1998, 2005, 2008). However, whether later elevation of androgen levels in childhood through adolescence could lead to similar abnormalities in the neuroendocrine drive to the reproductive axis has not been examined.
In this study, we tested if an elevation in androgen levels in the prepubertal and pubertal period (to levels similar to those seen in obese girls; McCartney et al., 2006) would result in alterations in pubertal LH secretory patterns that resemble those in hyperandrogenemic girls and women with PCOS. Female rhesus monkeys were exposed to low doses of testosterone beginning prepubertally (i.e. at 1 year of age) and continuing into early adulthood (i.e. at 5 years of age). We hypothesized that if a slight elevation in peripubertal testosterone leads to an increased central drive to the reproductive axis, then the testosterone-treated animals would develop at least some characteristics seen in obesity and PCOS, including a faster LH pulse frequency, higher LH responsiveness to exogenous GnRH and possibly increased numbers of small antral follicles and decreased insulin sensitivity compared with control (cholesterol-treated) animals.
Materials and Methods
Animals
Twelve 1-year-old female rhesus macaques (Macaca mulatta), weighing 1.7–2.4 kg, were obtained from the breeding corrals of the Oregon National Primate Research Center (ONPRC). They were housed in pairs in stainless steel cages (81 × 122 × 69 cm) in a temperature-controlled room (24 ± 2°C), with lights on for 12 h/day (0700–1900 h) during the first 2.5 years of the experiment. When the animals were 3.5 years of age, chronic indwelling venous catheters were implanted and the animals were then housed individually in single cages (81 × 61 × 69 cm). Monkeys were fed two meals of Purina LabDiet fiber-balanced monkey chow each day (no. 5000; Purina Mills, St. Louis, MO, USA), supplemented with fresh fruits and vegetables. Monkeys were trained to approach the front of their cage so menses could be detected daily by swabbing the vaginal area with a cotton-tipped swab. The first day of menses was designated Day 1 of a menstrual cycle. All procedures in this study were reviewed and approved by the ONPRC Institutional Animal Care and Use Committee.
Testosterone implants
Normal testosterone levels in prepubertal female rhesus macaques were determined by assaying serum from four 12-month-old female monkeys in the ONPRC colony. The average testosterone value (0.4 ng/ml) was multiplied by three to achieve the lower limit (1.2 ng/ml), and by four to achieve the upper limit (1.6 ng/ml) of target values in the testosterone-treated animals. This was based on the clinical evidence that PCOS patients have testosterone levels about 3–4 times higher than controls (Eagleson et al., 2003; Silfen et al., 2003; Moran et al., 2004). To determine the size of testosterone implant needed, monkeys from the ONPRC colony, which were not used for this study, had implants of various lengths and testosterone:cholesterol ratios placed s.c. under ketamine hydrochloride (Ketaset, 10 mg/kg i.m., Wyeth, Madison, NJ, USA) sedation. Blood samples were taken daily to determine which implants resulted in a sustained 3–4-fold increase in testosterone levels. Once an appropriate implant size was determined, all animals used in this study received either a testosterone- or cholesterol-containing (n = 6/group) implant at 1 year of age. Implants were made of Silastic tubing (Dow Corning, Midland, MI, USA) and were initially 5 mm in length with an inner and outer diameter of 0.335 and 0.465 cm, respectively. Implants were filled with cholesterol (control animals), or a testosterone/cholesterol mixture (testosterone-treated animals), with a testosterone:cholesterol ratio of 1:15 or 1:12 at the beginning of the experiment. As the animals grew, the length of the implant increased up to 1 cm and the testosterone:cholesterol ratio was increased gradually to 1:4 to maintain the desired serum testosterone levels. Both cholesterol and testosterone were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Blood collection and steroid hormone assays
To collect blood samples for tracking serum testosterone concentrations, animals were trained to jump from their cage into a portable transport box and were carried into a nearby room. The transport box door was opened and they were transferred to a specially designed cage and trained to present their leg for blood collection from the femoral vein (Hunnell et al., 2007). Weekly blood samples (2 ml each) were collected from each animal, allowed to clot at room temperature for >1 h and refrigerated overnight. Samples were then centrifuged at 1000g for 15 min at 4°C and serum was removed and stored at −20°C until assays were performed. Each week's samples were assayed for testosterone and when serum testosterone concentrations fell below the threshold of 1.2 ng/ml, the implant was changed. Cholesterol implants were also changed regularly so that cholesterol-treated (i.e. control) animals received the same average number of implant surgeries as the testosterone-treated animals. Testosterone was measured using a radioimmunoassay (RIA) kit (DSL-4100, Diagnostic Systems Laboratories, Inc., Webster, TX, USA) by the Endocrine Services Core Laboratory at the Oregon National Primate Research Center. The sensitivity of the testosterone assay was 0.05 ng/ml and the intra- and inter-assay coefficients of variation for the assays were 2.23 and 4.00%, respectively. Blood samples were also drawn at times throughout the study to quantify serum estradiol (E2) and progesterone concentrations. Both E2 and progesterone were assayed using the Immulite 2000 platform, using methods previously published (Herod et al., 2010a). As with many validated clinical platforms, the Immulite 2000 runs three QC serum pools daily and as such, measurement variability was not assessed within the limited number of samples assayed for this study on any one day. The inter-assay coefficient of variation, reflecting variability in daily QC results over the 1.5-year period in which these assays were performed was 8.5% for E2 and 9.4% for progesterone.
Nighttime LH concentrations and LH assay
In order to measure the sleep-associated rise in LH that would be indicative of puberty (Terasawa et al., 1984; Apter et al., 1989), nocturnal blood samples were collected from each animal once a month at ∼2200 h. These samples were collected as described for the weekly daytime samples, processed in the same manner and then assayed for LH. LH was measured by RIA at the University of Pittsburgh assay core using recombinant cynomolgus monkey LH (National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA) as standards (Williams et al., 2001). The sensitivity of the LH assays was 0.1 ng/ml and the intra- and inter-assay coefficients of variation for the assays used in this study were 6.6 and 12.2%, respectively.
Catheterization
At 3.5 years of age, after all monkeys had experienced menarche, a chronic indwelling venous catheter was implanted under isoflurane anesthesia (Hospira, Lake Forest, IL, USA) as described previously (Cameron and Nosbisch, 1991). Briefly, the catheter exited in the mid-scapular region of the back and was protected by a fitted nylon jacket worn by the monkey. The jacket was connected to a flexible metal tether and swivel which allowed the monkey to have full range of motion within its cage. Silastic tubing was routed through the wall into an adjacent room where blood samples were collected and drugs infused without disturbing the monkeys or disrupting their normal activities. Previous studies established that female monkeys with chronic indwelling catheters display normal, regular menstrual cycles (Herod et al., 2010b). Catheters were kept patent with a constant infusion of physiological saline (Baxter Healthcare, Deerfield, IL, USA) containing heparin sodium (4 IU/ml) at a rate of ∼100 ml/day. Animals were sedated weekly with ketamine in order to inspect the catheter system and replace a sterile dressing covering the exit site. No ketamine was administered in the 24 h preceding any experiment. Following catheterization surgery, animals were allowed a minimum of 3 weeks to recover before any experiments were performed.
Measurement of pulsatile LH secretion
Pulsatile LH secretion was measured during the early follicular phase (D2–3) of the menstrual cycle in animals that were ovulatory and 5 years of age. Animals that were non-ovulatory still experienced periods of menstrual bleeding, and samples were conducted on D2–3 after onset of menses in these animals as well. Blood samples (0.4 ml each) were collected into sterile heparinized syringes through the remote sampling system, every 10min from 1300 to 0100 h. This interval provided samples for 6h during the light phase and 6h during the dark phase of the day-night cycle in order to detect differences between daytime and nighttime LH secretion. Immediately after collection, the samples were placed into sterile plastic tubes and centrifuged at 1000g for 15 min at 4°C. Plasma was removed and placed into plastic O-ring vials (containing 20 µl of a solution composed of equal volumes of 38% sodium citrate and 1000 IU/ml sodium heparin to prevent clotting of plasma proteins) and stored at −20°C until assays were performed. In a sterile manner, red blood cells were resuspended in saline and reinfused through the catheter system to the animal. Hematocrit was recorded at the beginning, middle and end of the experiment to ensure that it remained in the normal physiological range.
GnRH stimulation
LH responsiveness to GnRH was measured between 0900 and 1000 h on D8–10 of a menstrual cycle when the monkeys were 4 and 5 years of age. GnRH was obtained from the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA, USA), dissolved in 0.9% saline at 1 µg/100 µl and stored in 200 µl aliquots at −20°C until use. Immediately before use, saline was added to individual aliquots to bring the concentration to 1 µg/ml. GnRH (250 ng/kg, i.v.) was infused at time 0 and blood samples (0.4 ml) were collected at −15, −1, 15, 30, 60 and 90 min, as described previously (Cameron and Nosbisch, 1991). This dose was chosen so that monkeys would receive a physiological dose that caused a response, but a response that was sub-maximal to allow detection of individual differences in LH responsiveness. Samples were collected, centrifuged and stored, and then red blood cells were reinfused as described for the pulsatile LH experiments.
Ovarian ultrasound
Ovarian ultrasounds were performed on D1–3 of a menstrual cycle when the animals were 5 years of age. Ultrasounds were performed using a GE Medical Systems Voluson® 730 Expert Doppler ultrasound instrument (GE Healthcare, Waukesha, WI) with both 2D (4.5–16.5 MHz) and 4D (3.3–9.1 MHz) transabdominal probes. Methods were similar to previous studies in adult female macaques (Bishop et al., 2009). Animals were assigned random identifiers, the sonographer (C.V.B.) was blinded to animal treatment, and the follicle cohort present in each ovary and ovarian size were assessed. The 2D probe was used to orient image field to the uterus and identify the ovaries. The 4D probe was then used to generate a data file of each individual ovary which included a series of images collected in one scan through the entire ovary. Archived scans from each animal were analyzed at one time by the sonographer. Ovaries were analyzed for ovarian area (cm2), circumference (cm) and diameter (mm), number of visible antral follicles on each ovary, the mean, maximum and minimum size (cm) of the antral follicles on each ovary and the total number of antral follicles per female. Follicle counts and size of follicles were measured using previously defined methods in adult female rhesus monkeys (Bishop et al., 2009). All parameters were then decoded for comparisons between treatment groups.
DEXA scanning
Percent body fat, percent central fat, fat mass in grams and lean tissue mass were determined using dual-energy X-ray absorptiometry (DEXA) scanning. Monkeys were sedated with ketamine and positioned supine on the bed of a Hologic Dexa scanner (Discovery scanner, Hologic Inc., Bedford, MA, USA). Two to three scans were performed for each monkey in ‘infant whole body’ mode and averages were calculated for each measure. To delineate central fat mass from peripheral fat mass, fat in the trunk (including both the subcutaneous and visceral compartments) and fat in the extremities were calculated using standard methodology (Clark et al., 2005).
Glucose tolerance testing and insulin assay
Glucose tolerance testing (GTT) was performed during the early follicular phase of a menstrual cycle. For monkeys not showing regular menstrual cycles, the GTT was performed when a blood sample showed that estrogen and progesterone levels were low, indicating the absence of a dominant follicle or corpus luteum in the ovaries. Each animal was sedated initially with telazol (tiletamine hydrochloride and zolazepam hydrochloride, Fort Dodge Animal Health, Fort Dodge, IA, USA) and subsequently with ketamine to maintain sedation, and the protocol was based on that designed by Richard Bergman (1979). We performed a modified Bergman Frequent Sampling Insulin Glucose Tolerance (FSIGT) used in humans using only 12 measurements within 180 min (Steil et al., 1993) and the results of this simpler protocol had correlated with the full FSIGT. Dextrose (300 mg/kg) was infused i.v. through the catheter system and blood samples were taken from 15 min before to 3 h after the glucose infusion. Tolbutamide (5 mg/kg) was infused i.v. at 20 min in order to stimulate the pancreas to secrete more insulin. All samples were immediately assayed for glucose and subsequently for insulin.
Insulin was assayed by RIA (Linco Human Insulin RIA, Millipore Corporation, Billerica, MA, USA). The sensitivity of the insulin assay was 1 µIU/ml and the intra-assay coefficient of variation was 2.7%. Glucose was assayed using the YSI 2300 Stat Plus (YSI Inc., Yellow Springs, OH, USA).
Timeline
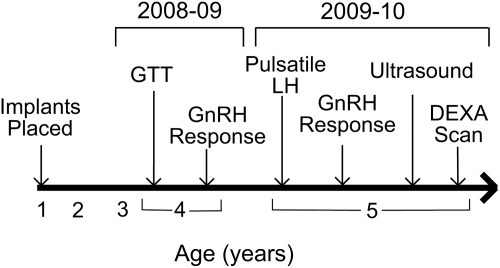
Figure 1 depicts the experimental timeline for this study. GTT was performed when the animals were 3.5 years old and LH responsiveness to GnRH was measured during the midfollicular phase (D8–10) of a menstrual cycle when the animals were 4 years of age (i.e. during the 2008–2009 breeding season). When the animals were 5 years old, pulsatile LH secretion was measured during the early follicular phase (D2–3) of a menstrual cycle, and LH response to GnRH was measured during the midfollicular phase (D8–10) of a separate menstrual cycle. Animals were tested 2–3 or 8–10 days after onset of menses, regardless of ovulatory status. Ovarian ultrasounds and DEXA scanning were also performed when the animals were 5 years of age (i.e. during the 2009–2010 breeding season).
Figure 1.
Schematic diagram of the experimental timeline indicating ages at which experiments were performed. Note that the timeline does not have a uniform scale.
Statistical analyses
LH pulses were identified using the Pulsar algorithm that was developed by Merriam and Wachter (1982), and used previously to detect LH pulses in monkeys (Cameron and Nosbisch, 1991; Ramaswamy et al., 2007). The following G-values were used: G(1): 50.00, G(2): 1.0, G(3): 0.40, G(4): 0.40 and G(5): 0.40. For all Pulsar analyses, values below the level of detectability for the assay were assigned the minimum detectable concentration of the assay.
The MINMOD Millenium computer program was used to determine glucose effectiveness, insulin sensitivity, acute insulin response and disposition index values (Boston et al., 2003). This program was designed to calculate these values based on the GTT protocol that was described by Bergman et al. (1979) and that was used in this study.
A Fisher's exact test was used when analyzing the presence or absence of follicles over 2.0 mm in diameter, and an independent t-test was used to analyze number of LH pulses occurring when the animals were 5 years old, age at first nighttime rise in LH, age at menarche and testosterone levels. Due to abnormally distributed data, the non-parametric Mann–Whitney U-test was used to assess LH response to GnRH. Ovarian data were analyzed by two-way analysis of variance (ANOVA) (ovary size × treatment) or one-way ANOVA (total follicles per female). Statistical analyses were performed using Prediction Application Software Statistics 17 (SPSS Inc., Chicago, IL, USA). Values are presented as means ± SEM. P < 0.05 was considered significant.
Results
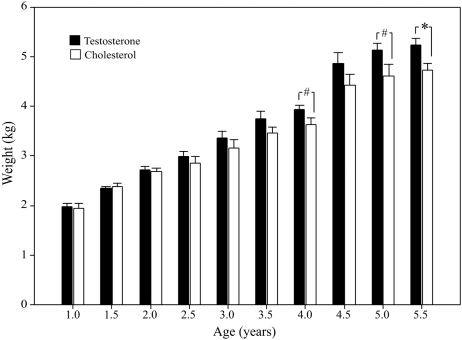
Plasma testosterone concentrations in the testosterone-treated animals were maintained in a narrow range at 3.7 ± 0.2-fold higher than in the control animals from the time of first implant at 1 year of age through 5.5 years of age (testosterone-treated: 1.73 ± 0.02 ng/ml; control: 0.50 ± 0.05 ng/ml, P = 0.001). Implants were replaced on a regular schedule, every 8.0 ± 0.4 weeks throughout the study. At 1, 2 and 3 years of age, the two groups of monkeys did not differ in body weight (Fig. 2). However, there was a trend toward heavier weights in testosterone-treated monkeys at 4 and 5 years of age (P = 0.077 and P = 0.075, respectively), and a significant difference between the groups at 5.5 years, with testosterone-treated animals weighing significantly more than controls (testosterone-treated: 5.2 ± 0.1 kg; control: 4.7 ± 0.2 kg; P = 0.03, Fig. 2).
Figure 2.
Body weight across time. Data are expressed as mean ± SEM. #Indicates a trend toward a group difference (P = 0.05–0.1). *Indicates a significant difference between groups (P = 0.03).
There were no differences in age at first nighttime rise in LH (testosterone-treated: 33.0 ± 2.8 months; control: 33.3 ± 3.6 months) or age at menarche (testosterone-treated: 32.1 ± 1.4 months; control: 32.4 ± 2.5 months). The groups also did not differ in the number of menstrual cycles or in the number or percentage of ovulatory menstrual cycles that occurred when they were 3–5 years of age (Table I).
Table I.
Numbers of menstrual cycles per year in control and testosterone-treated macaques after 3 years of age.
| Animal | Third year |
Fourth year |
Fifth year |
||||
|---|---|---|---|---|---|---|---|
| Number menstrual periods | Number ovulatory cycles | Number menstrual periods | Number ovulatory cycles | Number menstrual periods | Number ovulatory cycles | ||
| Control | 1 | 2 | 0 | 3 | 0 | 4 | 0 |
| 2 | 6 | 0 | 7 | 5 | 9 | 4 | |
| 3 | 0 | 0 | 8 | 0 | 6 | 4 | |
| 4 | 2 | 0 | 8 | 1 | 7 | 3 | |
| 5 | 4 | 0 | 4 | 0 | 7 | 3 | |
| 6 | 6 | 1 | 5 | 0 | 9 | 7 | |
| Mean | 3.3 ± 1.0 | 0.2 ± 0.2 | 5.8 ± 0.9 | 1 ± 0.8 | 7.0 ± 0.8 | 3.5 ± 0.9 | |
| Testosterone-treated | 7 | 7 | 0 | 8 | 1 | 9 | 3 |
| 8 | 6 | 0 | 7 | 3 | 6 | 4 | |
| 9 | 5 | 0 | 5 | 0 | 4 | 0 | |
| 10 | 4 | 0 | 7 | 1 | 9 | 7 | |
| 11 | 3 | 0 | 7 | 3 | 10 | 5 | |
| 12 | 3 | 0 | 6 | 0 | 8 | 2 | |
| Mean | 4.7 ± 0.7 | 0 | 6.7 ± 0.4 | 1.3 ± 0.6 | 7.7 ± 0.9 | 3.5 ± 1.0 | |
Menstrual periods are presented as recorded from September to May, as rhesus monkeys may be acyclic or anovulatory in the summer months. Therefore, number of menstrual periods ≥9 is considered normal for adults. Data are presented as mean ± SEM. There were no significant differences between groups for any measure.
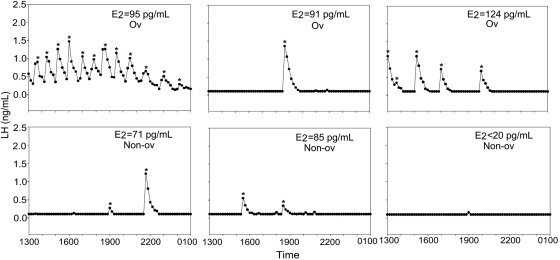
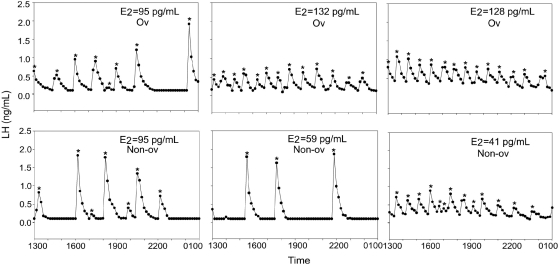
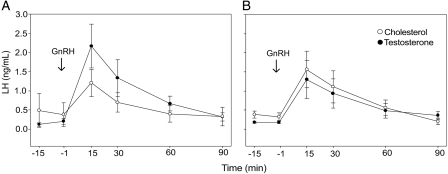
At 5 years of age, testosterone-treated animals had a significantly greater number of LH pulses than control animals on D2–3 of the menstrual cycle (testosterone-treated: 9.7 ± 1.8 pulses; control: 3.7 ± 1.8 pulses; P = 0.04; Figs. 3 and 4), though there was considerable individual variation in pulse frequency within each group. There was no correlation between LH pulse frequency and whether the monkey had ovulated in the prior menstrual cycle, and there were no group differences in estrogen levels when pulsatile LH secretion was assessed (testosterone-treated: 92 ± 15 pg/ml; control: 81 ± 14 pg/ml; P = 0.6; Figs 3 and 4). Testosterone-treated animals also had a significantly greater LH response to GnRH compared with control monkeys on D8–10 of a menstrual cycle when the monkeys were 4 years of age (testosterone-treated: 73.9 ± 20.3 ng/ml/90 min; control: 17.9 ± 10.8 ng/ml/90 min; P = 0.042; Fig. 5). However, there was no difference between the groups in LH response to GnRH when the monkeys were 5 years of age (testosterone-treated: 47.7 ± 22.4 ng/ml/90 min; control: 44.4 ± 24.0 ng/ml/90 min; P = 0.57).
Figure 3.
Pulsatile LH secretion in the six cholesterol-treated animals on D2–3 of the menstrual cycle. E2 at the time of blood sampling is indicated for each animal. Each experiment is also labeled as occurring during either an ovulatory (Ov) or anovulatory (Non-ov) cycle. *Indicates LH pulse as detected by Pulsar analysis.
Figure 4.
Pulsatile LH secretion in the six testosterone-treated animals on D2–3 of the menstrual cycle. E2 at the time of blood sampling is indicated for each animal. Each experiment is also labeled as occurring during either an ovulatory (Ov) or anovulatory (Non-ov) cycle. *Indicates LH pulse as detected by Pulsar analysis.
Figure 5.
LH response to a bolus injection of GnRH at 4 years of age (A) and 5 years of age (B). GnRH (250 ng/kg, i.v.) was infused at time 0. Testosterone-treated animals showed a significantly greater response measured as area under the curve at 4 years of age (P = 0.042) but not at 5 years of age (P = 0.57). Data are expressed as mean ± SEM.
Ovarian ultrasounds were performed on D1–3 of a menstrual cycle when the animals were 5 years of age. There were no statistically significant group differences in ovarian area, circumference or diameter. There were also no differences in number of visible antral follicles on each ovary, the mean, minimum and maximum size of the antral follicles on each ovary, or the total number of antral follicles (Table II). There was a trend toward control animals being more likely to have a follicle over 2.0 mm in diameter compared with testosterone-treated animals (P = 0.09), and follicles of this size were only present in control animals.
Table II.
Ovarian parameters as measured by ultrasound on D2–3 of the menstrual cycle.
| Avg. ovary diameter (mm) | Avg. ovary area (cm2) | Avg. # follicles (both ovaries) | Max follicle size (mm) | # Animals with follicles >2 mm | |
|---|---|---|---|---|---|
| Testosterone-treated | 2.8 ± 0.2 | 0.046 ± 0.004 | 5.2 ± 0.5 | 1.4 ± 0.1 | 0 |
| Control | 3.7 ± 0.4 | 0.053 ± 0.008 | 6.7 ± 0.9 | 2.0 ± 0.4 | 3* |
Data are presented as mean ± SEM.
*Trend toward difference between groups (P = 0.09).
At 5 years of age, there were no differences between the groups in overall percent fat (testosterone-treated: 1.7 ± 0.3%; control: 1.5 ± 0.5%; P = 0.6) or percent lean mass (testosterone-treated: 95.2 ± 0.7%; control: 95.4 ± 0.5%; P = 0.4). The percent of fat which was stored in the trunk also showed no difference between the groups (testosterone-treated: 25.1 ± 12.3%; control: 22.5 ± 7.3%; P = 0.7). When the animals received GTT at 3.5 years of age, there were no differences in baseline or peak glucose, baseline or peak insulin, or insulin sensitivity, glucose effectiveness, acute insulin response or disposition index as calculated by the MINMOD Millennium program (all P > 0.1).
Discussion
Female monkeys treated with low doses of testosterone during pubertal development (resulting in a 3.7-fold increase in testosterone over controls) showed increased pulsatile LH secretion, as well as increased LH responsiveness to GnRH in early adulthood, both of which are key neuroendocrine features associated with various hyperandrogenic states in women (Rebar et al., 1976; Levin et al., 1991; Pastor et al., 1998; Blank et al., 2009). This suggests that a modest increase in circulating androgen levels during the peri-pubertal interval in female monkeys can play a causal role in the greater activation of the central neural drive to the reproductive axis during adulthood, and supports the hypothesis that HA may be pivotal in the development of reproductive dysfunction associated with obesity, PCOS and CAH.
The doses of androgens used in this study were based on clinical findings that obese girls and women with PCOS often have levels of testosterone that are increased about 3–4 times above the levels seen in healthy girls and women (Eagleson et al., 2003; Silfen et al., 2003; Moran et al., 2004; McCartney et al., 2006). Although elevated, these increased levels of androgens are still relatively low compared with those typically present in men (Evans et al., 1971; Piro et al., 1973). We were able to successfully mimic this modest increase in testosterone in our female monkeys, with testosterone levels still remaining lower than those observed in male macaques (Goodman et al., 1974). Our findings of increased central neuroendocrine drive in testosterone-treated animals support previous findings, which showed that monkeys exposed to high doses of androgens during early fetal development had elevated LH levels in adulthood (Dumesic et al., 2002). Our study expands these findings by demonstrating that excess androgens do not need to be present during gestation in order for neuroendocrine changes to occur. Also, the doses of testosterone used in the current study were smaller than had been used previously, indicating that a modest increase in testosterone levels is sufficient to cause changes in neuroendocrine function.
There are several clinical conditions that produce both HA and increased pulsatile LH secretion, including PCOS, CAH and premature pubarche. Women with PCOS reportedly have a consistent, high rate of LH pulsatility (Rebar et al., 1976; Zumoff et al., 1983; Waldstreicher et al., 1988), while LH pulse frequency varies with the menstrual cycle in healthy women (Midgley and Jaffe 1971; Yen et al., 1972). The difference in pulsatile LH secretion between women with PCOS and healthy women is most apparent during the early follicular phase of the menstrual cycle, when PCOS subjects have about one LH pulse per hour, compared with healthy women who have about one LH pulse every 2h (McCartney et al., 2002). In one study comparing gonadotrophin release in patients with either PCOS or CAH, women with PCOS showed both elevated androgens and increased frequency of pulsatile LH secretion compared with healthy controls, while women with CAH had an intermediate phenotype, showing levels of androgens and LH pulse frequencies that were higher than controls but lower than PCOS patients (Levin et al., 1991). Other studies have also found elevated basal LH levels and an increased LH response to GnRH agonists in women with CAH (Barnes et al., 1994, Holmes-Walker et al., 1995), neuroendocrine changes that are similar to those seen in women with PCOS. Many CAH patients also experience premature pubarche, and in one study of women with premature pubarche, ∼45% went on to develop polycystic ovaries, oligomenorrhea and elevated LH levels postpubertally (Ibanez et al., 1993). These girls also had elevated androgen levels at the time of their premature pubarche diagnosis, suggesting that increased levels of androgens during puberty may play a role in the development of later neuroendocrine and ovarian dysregulation.
In the current study, we found that the testosterone-treated animals had significantly more pulses on D2–3 of the menstrual cycle when compared with control animals, despite the small sample size. There were no group differences in the percentage of menstrual cycles that were ovulatory (see Table I), and there was no correlation between LH pulse frequency and the incidence of ovulation in the previous menstrual cycle in individual monkeys. The control monkeys had approximately one LH pulse per 3h in the early follicular phase. This is similar to the pulse frequency reported in the early follicular phase in a closely related macaque species, Macaca fascicularis (Herod et al., 2010b). In contrast, the testosterone-treated monkeys showed almost one pulse per hour in the early follicular phase, a pulse frequency about 3-fold greater than in the control animals.
We also found that the testosterone-treated animals had a significantly higher LH response to exogenous GnRH than control animals when tested at 4 years of age, as would be expected from clinical findings that women with PCOS secrete more LH in response to GnRH than controls (Yen et al., 1975; Patel et al., 2004; Bachelot et al., 2007). This difference was not apparent when the animals were tested at 5 years of age. However, previous studies have shown that LH responsiveness to GnRH is normalized in women with PCOS after spontaneous ovulation occurs (Blankstein et al., 1987). Four out of the six testosterone-treated animals and two out of six controls had ovulatory cycles in the cycle before GnRH responsiveness was tested at 5 years of age (as indicated by elevated progesterone on D20 of the previous cycle), so it is possible that recent ovulation led to a normalization of the LH response in those four testosterone-treated animals.
Ovarian ultrasounds performed during the early follicular phase when these monkeys were 5 years of age showed no differences in numbers of small antral follicles or ovarian size between testosterone-treated and control monkeys. This may indicate that the amount of ovarian testosterone exposure in this study was not sufficient to induce changes in the ovaries. Studies in female-to-male transsexuals have shown that extremely high doses of testosterone can result in a PCOS phenotype replete with morphological changes in the ovaries (Pache et al., 1991). In addition, silastic implants that delivered substantial levels of testosterone to non-human primates also showed an increase in ovarian follicle formation (Vendola et al., 1998). The dose of testosterone in the current study was considerably lower than in these previous studies. By design in this study, we mimicked the circulating levels of testosterone seen in women with PCOS; however, the ovary is a major source of androgens in PCOS and it is possible that higher levels of localized testosterone or more prolonged exposure are needed to induce ovarian changes. Although no differences were found in follicle number, there was a trend toward control animals being more likely to display an antral follicle ≥2 mm, a size that is indicative of selection of the dominant follicle during the early follicular phase of the cycle in rhesus monkeys (Bishop et al., 2009). Unlike the control group, none of the testosterone-treated animals displayed antral follicles ≥2 mm. It is worth noting that pilot studies of adult breeding female rhesus monkeys indicated that the average diameter of ovaries, imaged during the early follicular phase of the menstrual cycle, to be 5.6 ± 0.6 mm (Bishop et al., unpublished data). This is much larger than ovaries of both control and testosterone-treated females in the current study (see Table II), suggesting the monkeys at five years of age are still developing reproductively. It is possible that ovarian changes resulting from increased testosterone exposure may not occur until later, when the ovaries reach a normal adult size.
Approximately 80% of women with PCOS are overweight or obese (Azziz et al., 2004), conditions that can aggravate PCOS symptoms (Legro, 2000; Chang, 2007). We did not see any differences between the groups in percent fat or percent lean mass in this study, which is not surprising because the animals were maintained on a diet that is low in fat (15% of calories from fat). It may therefore be not unexpected that differences between the groups in insulin sensitivity were not apparent with GTT when the animals were 3.5 years of age. Interestingly, the testosterone-treated animals are becoming significantly heavier than controls at 5.5 years of age. Since there were no differences in percent fat or percent lean mass, the increased body weight is not due to preferential deposition of either fat or muscle, but rather an overall increase in body mass. Whether persistence of this testosterone-associated weight gain may eventually lead to metabolic dysfunction such as insulin resistance and type 2 diabetes is unclear and continued monitoring of these animals is planned.
In summary, the increased pulsatile LH secretion and LH response to GnRH that we observed in testosterone-treated monkeys indicate that HA during puberty could play a causal role in altering the neural drive to the reproductive axis, such as is seen in girls with HA and women with PCOS. Further studies with these animals will help to determine downstream changes in ovarian function that may occur as a result of chronic testosterone exposure and/or increased neuroendocrine drive to the reproductive axis, and to assess whether elevated testosterone levels lead to metabolic changes that are common in PCOS, particularly as the animals gain weight.
Acknowledgements
The authors thank Dr Skyla Herod, Mandi Bulechowsky, Dr Theodore Molskness, Jon Reyes, Chris Cannon, Marcelo Bernuci and Paul Loprinzi for their superb technical assistance, and express appreciation to the ONPRC Division of Animal Resources, surgical and veterinary staff for the excellent care of the animals in this study. Consultations with Dave Hess, PhD, of the ONPRC Endocrine Services Core, were invaluable for validating a highly sensitive testosterone RIA that was critical for the performance of this study. Gratitude is also expressed for assistance with assays from the ONPRC Endocrine Services Core and the Assay Core of the Center for Reproductive Physiology at the University of Pittsburgh. The authors thank Elinor Sullivan, PhD for consultation on metabolic measurements made in this study.
Authors’ roles
W.K.M. was involved in all aspects of the study, including the design, collection of neuroendocrine and hormonal data, data analysis and writing of the paper. C.V.B. collected ovarian function data and assisted with design of ovarian assessments, data analysis and writing of the paper. A.B. was involved in the design of the study, collection of hormonal data and data analysis. C.R.P. performed pulse analyses with LH data. F.K.P. oversaw the development and validation of the hormone assays. R.J.C. and J.C.M. provided clinical expertise in hyperandrogenemia for the design and interpretation of experiments. R.L.S. oversaw the design and interpretation of the ovarian assessments, as well as monitored the progress of the entire study. J.L.C. oversaw the design and interpretation of the neuroendocrine and metabolic assessments, as well as monitored the progress of the entire study.
Funding
This study was funded by a grant from the OHSU Center for Women's Health Circle of Giving, pilot funds from RR0163, HD18185, the Eunice Kennedy Shriver NICHD/NIH through cooperative agreements, U54 HD 28934 and U54 HD 12303 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, as well as by an R21 grant from NCRR (RR030276). W.K.M. was supported by a fellowship from the OHSU ARCS Foundation and NIH grant T32 HD07133. A.B. was supported by fellowship DK007674-13.
Conflict of interest
None declared.
References
- Abbott DH, Dumesic DA, Eisner JR, Colman JR, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9:62–67. doi: 10.1016/s1043-2760(98)00019-8. doi:10.1016/S1043-2760(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. doi:10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Levine JE, Padmanabhan V, Dumesic DA, Jacoris S, Tarantal AF. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79:154–163. doi: 10.1095/biolreprod.108.067702. doi:10.1095/biolreprod.108.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter D, Cacciatore B, Alfthan H, Stenman U. Serum luteinizing hormone concentrations increase 100-fold in females from 7 years to adulthood, as measured by time-resolved immunofluorometric assay. J Clin Endocrinol Metab. 1989;68:53–57. doi: 10.1210/jcem-68-1-53. doi:10.1210/jcem-68-1-53. [DOI] [PubMed] [Google Scholar]
- Apter D, Butzow T, Laughlin GA, Yen SSC. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79:119–125. doi: 10.1210/jcem.79.1.8027216. doi:10.1210/jc.79.1.119. [DOI] [PubMed] [Google Scholar]
- Asuncion M, Calvo RM, san Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. doi:10.1210/jc.85.7.2434. [DOI] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. doi:10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- Bachelot A, Laborde K, Bresson JL, Plu-Bureau G, Raynaud A, Bertagna X, Mogenet A, Mansour M, Lucas-Jouy V, Gayno J-P, et al. Luteinizing hormone pulsatility in patients with major ovarian hyperandrogenism. J Endocrinol Invest. 2007;30:636–646. doi: 10.1007/BF03347443. [DOI] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler JF, Levitsky LL, Rosenthal IM. Ovarian hyperandrogenism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. doi:10.1210/jc.79.5.1328. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ider ZY, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- Bishop CV, Sparman ML, Stanley JE, Bahar A, Zelinski MB, Stouffer RL. Evaluation of antral follicle growth in the macaque ovary during the menstrual cycle and controlled ovarian stimulation by high-resolution ultrasonography. Am J Primatol. 2009;71:384–392. doi: 10.1002/ajp.20664. doi:10.1002/ajp.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank SK, Helm KD, McCartney CR, Marshall JC. Polycystic ovary syndrome in adolescence. Ann N Y Acad Sci. 2008;1135:76–84. doi: 10.1196/annals.1429.005. doi:10.1196/annals.1429.005. [DOI] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, Marshall JC. Modulation of GnRH pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls-Implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94:2360–2366. doi: 10.1210/jc.2008-2606. doi:10.1210/jc.2008-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankstein J, Rabinovici J, Goldenberg M, Shaley J, Mehta A, Serr DM, Mashiach S. Changing pituitary reactivity to follicle-stimulating hormone and luteinizing hormone-releasing hormone after induced ovulatory cycles and after anovulation in patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1987;65:1164–1167. doi: 10.1210/jcem-65-6-1164. doi:10.1210/jcem-65-6-1164. [DOI] [PubMed] [Google Scholar]
- Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: A computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. doi:10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 1991;128:1532–1540. doi: 10.1210/endo-128-3-1532. doi:10.1210/endo-128-3-1532. [DOI] [PubMed] [Google Scholar]
- Chang RJ. The reproductive phenotype in polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3:688–695. doi: 10.1038/ncpendmet0637. doi:10.1038/ncpendmet0637. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Yang WS, Li L, Chen X. The relationship between anti-Mullerian hormone, androgen and insulin resistance on the number of antral follicles in women with polycystic ovary syndrome. Hum Reprod. 2008;23:952–957. doi: 10.1093/humrep/den015. doi:10.1093/humrep/den015. [DOI] [PubMed] [Google Scholar]
- Clark MK, Dillon JS, Sowers M, Nichols S. Weight, fat mass, and central distribution of fat increase when women use depot-medroxyprogesterone acetate for contraception. Int J Obes (Lond) 2005;29:1252–1258. doi: 10.1038/sj.ijo.0803023. doi:10.1038/sj.ijo.0803023. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. doi:10.1210/jc.87.3.1111. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. doi:10.2337/diabetes.38.9.1165. [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Bellows AB, Hu K, Gingrich MB, Marshall JC. Obese patients with polycystic ovary syndrome: evidence that metformin does not restore sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by ovarian steroids. J Clin Endocrinol Metab. 2003;88:5158–5162. doi: 10.1210/jc.2003-030167. doi:10.1210/jc.2003-030167. [DOI] [PubMed] [Google Scholar]
- Evans JI, MacLean AW, Ismail AAA, Love D. Concentrations of plasma testosterone in normal men during sleep. Nature. 1971;229:261–262. doi: 10.1038/229261a0. doi:10.1038/229261a0. [DOI] [PubMed] [Google Scholar]
- Foecking EM, Szabo M, Schwartz MB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72:1475–1483. doi: 10.1095/biolreprod.105.039800. doi:10.1095/biolreprod.105.039800. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Hotchkiss J, Karsch J, Knobil E. Diurnal variations in serum testosterone concentrations in the adult male rhesus monkey. Biol Reprod. 1974;11:624–630. doi: 10.1095/biolreprod11.5.624. doi:10.1095/biolreprod11.5.624. [DOI] [PubMed] [Google Scholar]
- Herod SM, Dettmer AM, Novak MA, Meyer JS, Cameron JL. Sensitivity to stress-induced reproductive dysfunction is associated with a selective but not a generalized increase in activity of the adrenal axis. Am J Physiol Endocrinol Metab. 2010a;300:E28–E36. doi: 10.1152/ajpendo.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 Antagonist Prevents Stress-Induced Suppression of the Central Neural Drive to the Reproductive Axis in Female Macaques. Am J Physiol Endocrinol Metab. 2010b;300:E19–E27. doi: 10.1152/ajpendo.00224.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger KM. Exercise therapy in polycystic ovary syndrome. Semin Reprod Med. 2008;26:93–100. doi: 10.1055/s-2007-992929. doi:10.1055/s-2007-992929. [DOI] [PubMed] [Google Scholar]
- Hunnell NA, Rockcastle NJ, McCormick KN, Sinko LK, Sullivan EL, Cameron JL. Physical activity of adult female rhesus monkeys (Macaca mulatta) across the menstrual cycle. Am J Physiol Endocrinol Metab. 2007;292:E1520–E1525. doi: 10.1152/ajpendo.00497.2006. doi:10.1152/ajpendo.00497.2006. [DOI] [PubMed] [Google Scholar]
- Holmes-Walker DJ, Conway GS, Honour JW, Rumsby G, Jacobs HS. Menstrual disturbance and hypersecretion of progesterone in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 1995;43:291–296. doi: 10.1111/j.1365-2265.1995.tb02034.x. doi:10.1111/j.1365-2265.1995.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Ibanez L, Potau N, Virdis R, Zampolli M, Terzi C, Gussinye M, Carrascosa A, Vicens-Calvet E. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76:1599–1603. doi: 10.1210/jcem.76.6.8501168. doi:10.1210/jc.76.6.1599. [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. doi:10.1210/jc.83.9.3078. [DOI] [PubMed] [Google Scholar]
- Legro RS. The genetics of obesity. Lessons for polycystic ovary syndrome. Ann N Y Acad Sci. 2000;900:193–202. doi: 10.1111/j.1749-6632.2000.tb06230.x. doi:10.1111/j.1749-6632.2000.tb06230.x. [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. doi:10.1210/jc.84.1.165. [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. doi:10.1016/S0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- Levin JH, Carmina E, Lobo RA. Is the inappropriate gonadotropin secretion of patients with polycystic ovary syndrome similar to that of patients with adult-onset congenital adrenal hyperplasia? Fertil Steril. 1991;56:635–640. doi: 10.1016/s0015-0282(16)54592-0. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Eagleson CA. Neuroendocrine aspects of polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:295–324. doi: 10.1016/s0889-8529(05)70071-2. doi:10.1016/S0889-8529(05)70071-2. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20:317–326. doi: 10.1055/s-2002-36706. doi:10.1055/s-2002-36706. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab. 2006;91:1714–1722. doi: 10.1210/jc.2005-1852. doi:10.1210/jc.2005-1852. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: Evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94:56–66. doi: 10.1210/jc.2008-1252. doi:10.1210/jc.2008-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- Midgley AR, Jr, Jaffe RB. Regulation of human gonadotropins. X. Episodic fluctuation of LH during the menstrual cycle. J Clin Endocrinol Metab. 1971;33:962–969. doi: 10.1210/jcem-33-6-962. doi:10.1210/jcem-33-6-962. [DOI] [PubMed] [Google Scholar]
- Moran C, Reyna R, Boots LS, Azziz R. Adrenocortical hyperresponsiveness to corticotropin in polycystic ovary syndrome patients with adrenal androgen excess. Fertil Steril. 2004;81:126–131. doi: 10.1016/j.fertnstert.2003.07.008. doi:10.1016/j.fertnstert.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril. 2002;77:1095–105. doi: 10.1016/s0015-0282(02)03111-4. doi:10.1016/S0015-0282(02)03111-4. [DOI] [PubMed] [Google Scholar]
- Pache TD, Chadha S, Gooren LJG, Hop WCJ, Jaarsma KW, Dommerholt HBR, Fauser BCJM. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopath. 1991;19:445–452. doi: 10.1111/j.1365-2559.1991.tb00235.x. doi:10.1111/j.1365-2559.1991.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Antenucci D, Casimirri F, Venturoli S, Paradisi R, Fabbri R, Balestra V, Melchionda N, Barbara L. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab. 1989;68:173–179. doi: 10.1210/jcem-68-1-173. doi:10.1210/jcem-68-1-173. [DOI] [PubMed] [Google Scholar]
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83:582–590. doi: 10.1210/jcem.83.2.4604. doi:10.1210/jc.83.2.582. [DOI] [PubMed] [Google Scholar]
- Patel K, Coffler MS, Dahan MH, Malcom PJ, Deutsch R, Chang RJ. Relationship of GnRH-stimulated LH release to episodic LH secretion and baseline endocrine-metabolic measures in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60:67–74. doi: 10.1111/j.1365-2265.2004.01945.x. doi:10.1111/j.1365-2265.2004.01945.x. [DOI] [PubMed] [Google Scholar]
- Piro C, Fraioli F, Sciarra F, Conti C. Circadian rhythm of plasma testosterone, cortisol and gonadotropins in normal male subjects. J Steroid Biochem. 1973;4:321–329. doi: 10.1016/0022-4731(73)90056-3. doi:10.1016/0022-4731(73)90056-3. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148:3364–3370. doi: 10.1210/en.2007-0207. doi:10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- Rebar R, Judd HL, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976;57:1320–1329. doi: 10.1172/JCI108400. doi:10.1172/JCI108400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savabieasfahani M, Lee JS, Herkimer C, Sharma TP, Foster DL, Padmanabhan V. Fetal programming: testosterone exposure of the female sheep during midgestation disrupts the dynamics of its adult gonadotropin secretion during the periovulatory period. Biol Reprod. 2005;72:221–229. doi: 10.1095/biolreprod.104.031070. doi:10.1095/biolreprod.104.031070. [DOI] [PubMed] [Google Scholar]
- Shah B, Parnell L, Milla S, Kessler M, David R. Endometrial thickness, uterine, and ovarian ultrasonographic features in adolescents with polycystic ovarian syndrome. J Pediatr Adolesc Gynecol. 2010;23:146–152. doi: 10.1016/j.jpag.2009.07.002. doi:10.1016/j.jpag.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod. 2002;66:924–933. doi: 10.1095/biolreprod66.4.924. doi:10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- Silfen ME, Denburg MR, Manibo AM, Lobo RA, Jaffe R, Ferin M, Levine LS, Oberfield SE. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88:4682–4688. doi: 10.1210/jc.2003-030617. doi:10.1210/jc.2003-030617. [DOI] [PubMed] [Google Scholar]
- Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes. 1993;42:250–256. doi: 10.2337/diab.42.2.250. doi:10.2337/diabetes.42.2.250. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Bridson WE, Nass TE, Noonan JJ, Dierschke DJ. Developmental changes in the luteinizing hormone secretory pattern in peripubertal female rhesus monkeys: comparisons between gonadally intact and ovariectomized animals. Endocrinology. 1984;115:2233–2240. doi: 10.1210/endo-115-6-2233. doi:10.1210/endo-115-6-2233. [DOI] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. doi:10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF., Jr Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66:165–172. doi: 10.1210/jcem-66-1-165. doi:10.1210/jcem-66-1-165. [DOI] [PubMed] [Google Scholar]
- Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt DB, Nosbisch C, Cameron JL. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: abrupt transition to exercise-induced amenorrhea. Endocrinology. 2001;142:2381–2389. doi: 10.1210/endo.142.6.8113. doi:10.1210/en.142.6.2381. [DOI] [PubMed] [Google Scholar]
- Yen SS, Tsai CC, Naftolin F, Vandenberg G, Ajabor L. Pulsatile patterns of gonadotropin release in subjects with and without ovarian function. J Clin Endocrinol Metab. 1972;34:671–675. doi: 10.1210/jcem-34-4-671. doi:10.1210/jcem-34-4-671. [DOI] [PubMed] [Google Scholar]
- Yen SS, Lasley BL, Wang CF, Leblanc H, Siler TM. The operating characteristics of the hypothalamic-pituitary system during the menstrual cycle and observations of biological action of somatostatin. Recent Prog Horm Res. 1975;31:321–363. doi: 10.1016/b978-0-12-571131-9.50013-x. [DOI] [PubMed] [Google Scholar]
- Zumoff B, Freeman R, Coupey S, Saenger P, Markowitz M, Kream J. A chronobiologic abnormality in luteinizing hormone secretion in teenage girls with the polycystic-ovary syndrome. N Engl J Med. 1983;309:1206–1209. doi: 10.1056/NEJM198311173092002. doi:10.1056/NEJM198311173092002. [DOI] [PubMed] [Google Scholar]