Abstract
Although zinc is an essential micronutrient for all living organisms, zinc is harmful to cells at high levels. In the presence of excess zinc, plants exhibit several major symptoms, including root growth inhibition, abnormal root hair morphology and chlorosis. To dissect the molecular mechanisms underlying the effects of excess zinc on plant cells, we used an iTRAQ-based quantitative proteomics approach to analyze the microsomal protein profiles of Arabidopsis roots from wild-type (WT) plants and de-etiolated 3-1 (det3-1), a vacuolar H+-ATPase (V-ATPase) subunit C-defective mutant. A comparative analysis of the iTRAQ data from WT and det3-1 plants exposed to excess zinc suggests that the reduction in V-ATPase subunit levels and its activity are the cause of the symptoms of zinc toxicity, including the inhibition of cell expansion. Provided that reduced V-ATPase activity in the trans-Golgi network (TGN) alone can inhibit cell expansion, it is possible that the det3-1 mutant phenotype is caused mainly by a defect in TGN acidification, leading to reduced cell wall component trafficking and cell expansion in the presence of excess zinc. To evaluate the contribution of V-ATPase activity to vacuolar acidification under excess zinc, the vacuolar pH was measured. Our results indicate clear alkalinization of Deep cell vacuoles treated with 300 µM ZnSO4.
Key words: zinc toxicity, root growth inhibition, iTRAQ, V-ATPase, det3-1 mutant, trans-golgi network, vacuole, Arabidopsis
The plant V-ATPase, together with H+-pyrophosphatase (V-PPase), plays a central role in vacuole acidification, energizing the active transport of ions across the tonoplast and coordinately regulating turgor pressure and cell elongation.1,2 On the other hand, as a strategy to avoid the toxicity of several ions, plant cells take advantage of proton gradients generated across the tonoplast to sequester ions inside vacuoles.3 While V-PPase consists of a single polypeptide, V-ATPase is a multimeric complex composed of 28 subunits.4 Moreover, while V-PPase is thought to be exclusively localized in the tonoplast, V-ATPase is also found in the endosome.5 Finally, data from mutants of several V-ATPase subunits suggest that some of them may contribute differently to the acidification of vacuoles or endosomes.6,7
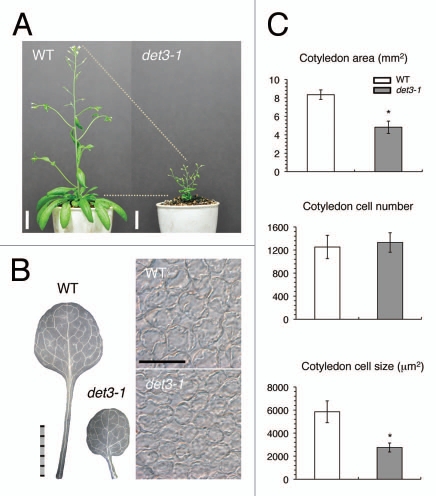
In normal cell expansion, the V-ATPase plays an important role in the TGN, which functions in the trafficking of proteins involved in the biosynthesis of cell wall components, such as cellulose.8 The partial dysfunction of the V-ATPase in det3-1, which is defective in V-ATPase subunit C, causes defects in cell elongation in the hypocotyl, petioles and inflorescence stems, resulting in severe plant dwarfism (Fig. 1).6 In Arabidopsis thaliana, V-ATPase subunit C is encoded by a single-copy gene that is essential for plant survival, and which is fundamentally important for V-ATPase function at both the tonoplast and endosome.4 Curiously, the extent of cell expansion inhibition in several organs was proportional to the reduction in V-ATPase subunit C mRNA and protein expression, and to the level of V-ATPase activity in the det3-1 mutant (Fig. 1). For example, the first leaf area (40.1 cotyledon area and cotyledon cell size were all reduced by ∼50% in the det3-1 mutant compared with the WT (Fig. 1C).
Figure 1.
Gross morphology and cellular phenotype of the det3-1 mutant. (A) WT and det3-1 mutant plants grown for 46 d after sowing (DAS). Bar = 4 cm. (B, left parts) Mature first leaves from WT and det3-1 mutant plants (25 DAS). Bar = 5 mm. (B, right parts) Images of paradermal palisade tissue cells from the mature first leaves of WT and det3-1 mutant plants (25 DAS). Bar = 100 µm. (C) Cotyledon area, cell number and cell size in WT and det3-1 mutant plants (25 DAS). The data shown are the means and standard deviation (n = 8). Asterisk, p < 0.0001 (two-tailed Student's t-test).
Our recent iTRAQ analysis revealed that the levels of V-ATPase subunits A, C and E1 were significantly decreased in the presence of excess zinc in WT plants.9 Consistent with this, the protein expression of six V-ATPase subunits, including A, C and E1, was also significantly decreased in the det3-1 mutant.9 Alternatively, given that the morphologies of the Golgi stacks and Golgi-derived vesicles in vha-A and vha-E1 were abnormal, decreased levels of VHA-A and VHA-E1 expression under conditions of excess zinc might be explained by growth inhibition due to deficient acidification of the TGN.10,11 In support of this hypothesis, Arabidopsis VHA-E1 and VHA-E3, but not VHA-E2, were found to functionally complement a vma4 zinc-sensitive mutant with a mutation in the single yeast subunit E isoform.12 If we assume that reduced V-ATPase activity in the TGN alone can inhibit cell expansion, it is likely that the severe phenotype observed in the det3-1 mutant in the presence of excess zinc is the result of impaired TGN acidification leading to cell wall defects.
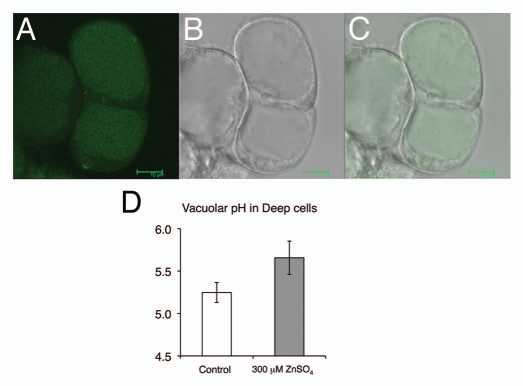
Is vacuolar V-ATPase activity also critical for zinc tolerance? To answer this question, we measured the vacuolar pH using suspension-cultured cells of Arabidopsis (strain Deep) and the ratiometric pH indicator 2′,7′-bis-(2-caroxyethyl)-5-(and-6)-carboxyfluorescein, diacetoxymethylester (BCECF-AM).13 Deep cells were treated with water as a control solution or MS medium containing 300 µM ZnSO4 for 16 h and then analyzed by confocal laser-scanning microscopy. Interestingly, while the vacuolar pH was 5.25 in the untreated Deep cells, it was 5.66 in the zinc-treated cells (Fig. 2). This suggests that the pH was increased due to the inhibition of V-ATPase activity by excess zinc in the vacuole. Actually, the root cell vacuoles were alkalinized in the vha-a2 vha-a3 double mutant of Arabidopsis, which lacks both tonoplast-localized isoforms of the membrane-integral V-ATPase subunit VHA-a.7 Furthermore, based on our iTRAQ data, the tonoplast Zn2+/H+ antiporter MTP3, which appears to transport excess zinc from the cytosol to the vacuole, was increased 4.7-fold, probably to alleviate zinc toxicity, although the level of transport activity under conditions of excess zinc has not been reported in reference 9. It would be interesting to analyze the response to excess zinc in other mutants with defects in other V-ATPase subunits, such as those reported recently by Krebs et al. (2010), in a way that addresses their roles in the TGN and vacuole.7
Figure 2.
ZnSO4 treatment causes alkalinization of the vacuolar pH in Deep cells. Confocal laser-scanning microscopic (CLSM, Leica TCS SP5) images of Deep cells stained with BCECF-AM. (A) Deep cell vacuoles loaded with BCECF-AM, (B) Nomarski images and (C) merged images. Bar = 10 µm. (D) Six-day-old cultured Deep cells were treated with water as a control or MS medium containing 300 µM ZnSO4 for 16 h, after which the vacuolar pH was measured. ZnSO4 treatment increased the vacuolar pH by 0.4 pH units. The standard deviation values provide an estimate of the variation in mean pH for 100 cells under each set of conditions.
In a recent report, we focused on a limited number of highly responsive proteins that are either directly related to zinc transport or which have a closely related function. However, the contribution of a substantial number of the zinc-responsive proteins identified from our iTRAQ data remains elusive. Given that under our experimental conditions seedlings were grown for 10 d in the presence of excess zinc, our data may include proteins that are not directly related to zinc tolerance and/or homeostasis. Moreover, we found that plants suffer an iron deficiency following long-term exposure to excess zinc.9 Therefore, it is important to explore zinc-responsive proteins following short-term treatment.
Very recently, Lan et al. (2011) performed an iTRAQ analysis to investigate the mechanisms of iron homeostasis under iron deficiency.14 They successfully identified 4,454 proteins and quantified 2,882. If this kind of pioneering technique is successfully applied to plant membrane proteomics, it may help dissect the intriguing response to excess zinc. The iTRAQ method is now being applied to plants to understand changes in protein profiles during normal growth/development or under a particular stress condition. Our next challenge is to identify a large enough number of proteins, including membrane proteins, to allow a more detailed comparison with the transcriptomic data available. This will boost our understanding of fundamentally important biological events and provide clues as to the integral components of the molecular network responsible for the phenotype of this interesting mutant.
Acknowledgments
The authors thank Dr. Noriko Inada (Nara Institute of Science and Technology) for providing technical advice and Yuka Nishimori (Nara Institute of Science and Technology) for providing technical assistance with the confocal laserscanning microscopy. This work was supported by Grant-in-Aid for Scientific Research from Nara Institute of Science and Technology supported by The Ministry of Education, Culture, Sports, Science and Technology, JAPAN; Grant-in-Aid for Organelle Differentiation as the Strategy for Environmental Adaptation in Plants for Scientific Research of Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology, JAPAN (1685101 to Y.F.). Grant-in-Aid for Young Scientists (B) (to A.F.).
References
- 1.Maeshima M. Vacuolar H(+)-pyrophosphatase. Biochim Biophys Acta. 2000;1465:37–51. doi: 10.1016/S0005-2736(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 2.Sze H, Schumacher K, Müller ML, Padmanaban S, Taiz L. A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H(+)-ATPase. Trends Plant Sci. 2002;7:157–161. doi: 10.1016/S1360-1385(02)02240-9. [DOI] [PubMed] [Google Scholar]
- 3.Dietz KJ, Tavakoli N, Kluge C, Mimura T, Sharma SS, Harris GC, et al. Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level. J Exp Bot. 2001;52:1969–1980. doi: 10.1093/jexbot/52.363.1969. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher K, Krebs M. The V-ATPase: small cargo, large effects. Curr Opin Plant Biol. 2010;13:724–730. doi: 10.1016/j.pbi.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schumacher K, Vafeados D, McCarthy M, Sze H, Wilkins T, Chory J. The Arabidopsis det3 mutant reveals a central role for the vacuolar H(+)-ATPase in plant growth and development. Genes Dev. 1999;13:3259–3270. doi: 10.1101/gad.13.24.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krebs M, Beyhl D, Görlich E, Al-Rasheid KAS, Marten I, Stierhof YD, et al. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci USA. 2010;107:3251–3256. doi: 10.1073/pnas.0913035107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brüx A, Liu TY, Krebs M, Stierhof YD, Lohmann JU, Miersch O, et al. Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth Inhibition in Arabidopsis. Plant Cell. 2008;20:1088–1100. doi: 10.1105/tpc.108.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukao Y, Ferjani A, Tomioka R, Nagasaki N, Kurata R, Nishimori Y, et al. iTRAQ analysis reveals mechanisms of growth defects due to excess zinc in Arabidopsis. Plant Physiol. 2011;155:1893–1907. doi: 10.1104/pp.110.169730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dettmer J, Schubert D, Calvo-Weimar O, Stierhof YD, Schmidt R, Schumacher K. Essential role of the V-ATPase in male gametophyte development. Plant J. 2005;41:117–124. doi: 10.1111/j.1365-313X.2004.02282.x. [DOI] [PubMed] [Google Scholar]
- 11.Strompen G, Dettmer J, Stierhof YD, Schumacher K, Jürgens G, Mayer U. Arabidopsis vacuolar H-ATPase subunit E isoform 1 is required for Golgi organization and vacuole function in embryogenesis. Plant J. 2005;41:125–132. doi: 10.1111/j.1365-313X.2004.02283.x. [DOI] [PubMed] [Google Scholar]
- 12.Dettmer J, Liu TY, Schumacher K. Functional analysis of Arabidopsis V-ATPase subunit VHA-E isoforms. Eur J Cell Biol. 2010;89:152–156. doi: 10.1016/j.ejcb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Matsuoka K, Higuchi T, Maeshima M, Nakamura K. A vacuolar-type H+-ATPase in a nonvacuolar organelle is required for the sorting of soluble vacuolar protein precursors in Tobacco cells. Plant Cell. 1997;9:533–546. doi: 10.1105/tpc.9.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan P, Li W, Wen TN, Shiau JY, Wu YC, Lin W, et al. iTRAQ protein profile analysis if Arabidopsis roots reveals new aspects critical for iron homeostasis. Plant Physiol. 2011;155:821–834. doi: 10.1104/pp.110.169508. [DOI] [PMC free article] [PubMed] [Google Scholar]