Abstract
Zea mays L. exhibits a strong growth reduction in response to NaCl-induced stress that is attributable to a decline of cell division and elongation. Wall-loosening expansins are of major impact for cell wall extensibility and growth. This study provides an analysis of the impact of an 8-d 100 mM NaCl stress treatment on the mRNA abundance of the α- and β-expansin sub-families using real-time quantitative RT-PCR. Moreover, we provide a comparative study of plants that contrast in their degree of salt resistance in order to reveal contrasting features of physiological functions that may bear a causal relation to the differential response of plants to salt. In result, the transcript abundance of wall-loosening β-expansins was impaired in size-reduced leaves of the salt-sensitive hybrid but not in leaves of the salt-resistant hybrid that maintained growth. This indicates a role for the β-expansins in processes related to salt resistance.
Key words: salt stress, growth inhibition, α-expansin, β-expansin, wall-loosening, Zea mays L
Introduction
Soil salinity poses a major threat to agriculture, since most crop plants will not grow in high concentrations of salt.1 During the osmotic stress phase, the rate of shoot growth falls significantly immediately after the salt concentration around the roots increases to a threshold level of approximately 40 mM NaCl for most plants, largely attributable to the osmotic effect of the salt outside the roots.2 The osmotic effect not only reduces the expansion of growing leaves, but also causes new leaves to emerge more slowly.3,4 This growth reduction might be controlled by hormonal signals.2 However, the growth-related processes that are altered by this putative signal are not precisely known.4 Bernstein et al.5 have reported that salinity reduces the leaf growth rate by shortening the length of the leaf elongating zone and decreasing its growth intensity. This has led to the assumption that the leaf growth inhibition observed under salt stress might occur via an effect on this region.6 A modified capacity of cell walls to irreversibly expand has been suggested to be the major growth-limiting factor causing growth inhibition under salt stress.7
Cell-wall enlargement begins with wall stress relaxation, allowing the cells physically to enlarge. Following the acid growth theory, an auxin-mediated acidification of the leaf apoplast is the major requirement for an increase in wall extensibility.8 Expansins are a group of non-enzymatic cell-wall proteins that are thought to mediate acid-induced growth. Experimental evidence supports the idea that expansins intercalate within carbohydrate matrices in the cell wall, leading to a transient loosening of non-covalent interactions, and thus enhance the ability of these matrices to move relative to each other.9,10 Strong correlations between the presence of expansins, wall extensibility and cell elongation have been demonstrated.10,11 Interestingly, Pitann et al. have revealed a downregulation of growth-mediating β-expansin proteins in size-reduced shoots of NaCl-affected maize plants, whereas the β-expansin protein abundance was less affected in the leaves of a salt-resistant cultivar.
Elucidation of the way that salinity affects shoot growth is of great importance for a better understanding of processes that contribute to salt resistance. For this purpose, (1) shoot growth and (2) the abundance of growth mediating expansin were compared between the salt-sensitive maize hybrid Lector and the highly salt-resistant hybrid SR03 (developed by Schubert et al.) under saline conditions. Such a comparative study of plants belonging to the same species but contrasting in their degree of salt resistance was undertaken because it might reveal contrasting features of physiological functions that may bear a causal relation to the differential response of plants to salt.14
Salinity Differentially Affects Shoot Growth and β-expansin Abundance in Maize Cultivars that Differ in Salt Resistance
Our previously study showed that an 8-d 100 mM NaCl treatment of maize plants that differ in their degree of salt-resistance has revealed genotype-specific differences regarding the ability of the young shoots to maintain growth. The leaves of the salt-sensitive hybrid Lector exhibited a strong reduction in wall extensibility (Geilfus et al.15 Table 2 therein) and biomass accumulation,15,16 being characteristic for the first phase of salt stress. In contrast, the shoots of the salt-resistant hybrid SR03 were only marginally affected and maintained growth. The finding that, at saline conditions, the mRNA abundance of wall-loosening β-expansins (ZmEXPB2, ZmEXPB6 and ZmEXPB8, Geilfus et al.16) was differentially regulated within both maize hybrids, viz. downregulated in size-reduced shoots of the salt-sensitive Lector but upregulated in shoots of salt-resistant SR03 that maintained growth under NaCl stress, is indicative for an important role of these wall loosening agents in processes related to salt-resistance. The upregulation of the expansin transcripts in expanding shoots of the salt-resistant hybrid SR03 might contribute to a mechanism for improving wall extensibility under stress and thus might counteract growth reduction as occurs, for example, in the salt-sensitive hybrid. In favor of this assumption, these particular expansin transcripts (ZmEXPB2, ZmEXPB6 and ZmEXPB8) are downregulated in the salt-sensitive hybrid Lector.15,16
In general, β-expansins are more numerous and more abundantly expressed in maize tissue compared with α-expansins and are hypothesized to function in cell enlargement and other processes in which wall loosening is required.17 In order to estimate whether both expansin sub-families are affected equally by salinity, the transcript abundance of these wall-loosening agents was compared. This was done on the basis of purified poly (A)+ RNA by using the real-time qRT-PCR technique. In response to the 8-d 100 mM NaCl treatment, the transcript abundance of the α-expansin sub-family was increased in both genotypes. However, this upregulation was not noticeably different between the two genotypes (Fig. 1A). In contrast, the β-expansins have been shown to be opposingly regulated within the two genotypes, viz. downregulated in salt-sensitive Lector but upregulated in salt-resistant SR03, under NaCl stress (Fig. 1B). The finding that on the one hand the α-expansins were equally regulated in the two genotypes that contrast in their degree of salt resistance, but, on the other hand the β-expansin transcripts were opposingly regulated in both genotypes under stress suggests that the β-expansin sub-family is more important in respect of genotypic differences in terms of wall extensibility and shoot growth.
Figure 1.
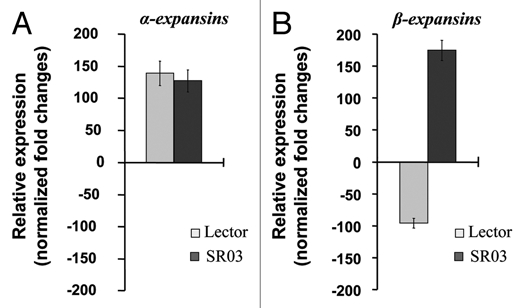
Effect of salt treatment on the relative α- and β-expansin transcript abundance as measured by SYBR-green-based real-time quantitative RT-PCR. Relative expression changes in response to an 8-d 100-mM NaCl treatment of (A) α-expansins and (B) β-expansin. Isoforms were detected with a family-specific primer pair. The transcript for ubiquitin-conjugating enzyme was used as the endogenous control in an Applied Biosystems 7300 real-time PCR system. Data show the effect of salt treatment on the relative transcript abundance with the control as the calibrator sample (+100 rel. expression = two-fold upregulation, −100 rel. expression = two-fold downregulation). Salt-sensitive Lector (light gray); salt-resistant SR03 (dark gray). Expression data correspond to means of three biological replicates, each being run in triplicate, ±SE.
Genotype-Specific Protein Abundance of β-expansins
It was recently demonstrated that genotype-specific effects of the salt treatment on β-expansin transcript abundance were also occurring on the level of the proteome: salinity did not affect the abundance of the vegetatively expressed β-expansins in the leaves of the salt-resistant maize hybrid SR03.16 However, β-expansin were downregulated in size-reduced leaves of the salt-sensitive cultivar Lector. The finding that growth-mediating β-expansins were downregulated in size-reduced leaves of the salt-sensitive maize but were not affected in leaves of the salt resistant SR03 maize that maintained growth is indicative of a role for the β-expansins in maintaining growth and thus of their contribution to salt resistance.
Concluding Remarks
A comparative study of plants that differ in their degree of salt resistance revealed contrasting physiological features with respect to the abundance of growth mediating β-expansins in expanding shoots. Wall-loosening β-expansins were impaired in size-reduced leaves of the salt-sensitive hybrid but not in leaves of the salt-resistant hybrid that maintained growth. This physiological difference is indicative for a role of these wall-loosening agents for salt-resistance and thus may be used for screening for salt-resistant plants.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Flowers TJ. Improving crop salt tolerance. J Exp Bot. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- 2.Munns R, James RA, Läuchli A. Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot. 2006;57:1025–1043. doi: 10.1093/jxb/erj100. [DOI] [PubMed] [Google Scholar]
- 3.Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 4.Munns R, Tester M. Mechanism of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein N, Silk WK, Läuchli A. Growth and development of sorghum leaves under conditions of NaCl stress: spatial and temporal aspects of leaf growth inhibition. Planta. 1993;191:433–439. [Google Scholar]
- 6.Lazof D, Bernstein N. The NaCl-induced inhibition of shoot growth: the case for disturbed nutrition with special consideration of calcium nutrition. Adv Bot Res. 1998;29:113–189. doi: 10.1016/S0065-2296(08)60311-0. [DOI] [Google Scholar]
- 7.Cramer GR, Bowman DC. Kinetics of maize leaf elongation. I: Increased yield threshold limits short-term, steady-state elongation rates after exposure to salinity. J Exp Bot. 1991;42:1417–1426. doi: 10.1093/jxb/42.11.1417. [DOI] [Google Scholar]
- 8.Hager A, Menzel H, Krauss A. Versuche und Hypothese zur Primärwirkung des Auxins beim Streckungswachstum. Planta. 1971;100:47–75. doi: 10.1007/BF00386886. (Ger). [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 11.Cho HT, Kende H. Expression of expansin genes is correlated with growth in deepwater rice. Plant Cell. 1997;9:1661–1671. doi: 10.1105/tpc.9.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitann B, Zörb C, Mühling KH. Comparative proteome analysis of maize (Zea mays L.) expansins under salinity. J Plant Nutr Soil Sci. 2009;172:75–78. doi: 10.1002/jpln.200800265. [DOI] [Google Scholar]
- 13.Schubert S, Neubert A, Schierholt A, Sümer A, Zörb C. Development of salt resistant maize hybrids: the combination of physiological strategies using conventional breeding methods. Plant Sci. 2009;177:196–202. doi: 10.1016/j.plantsci.2009.05.011. [DOI] [Google Scholar]
- 14.Epstein E, Norlyn JD, Rush DW, Kingsbury RW, Kelly DB. Saline culture of crops: a genetic approach. Science. 1980;210:399–404. doi: 10.1126/science.210.4468.399. [DOI] [PubMed] [Google Scholar]
- 15.Geilfus CM, Zörb C, Neuhaus C, Hansen T, Lüthen H, Mühling KH. Differential transcript expression of wall-loosening candidates in leaves of maize cultivars differing in salt resistance. J Plant Growth Regul. 2011 doi: 10.1007/s00344-011-9201-4. [DOI] [Google Scholar]
- 16.Geilfus CM, Zörb C, Mühling KH. Salt stress differentially affects growth-mediating β-expansin in resistant and sensitive maize (Zea mays L.) cultivars. Plant Physiol Biochem. 2010;48:993–998. doi: 10.1016/j.plaphy.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Meeley RB, Cosgrove DJ. Analysis and expression of the α-expansin and β-expansin gene families in mai. Plant Physiol. 2001;126:222–232. doi: 10.1104/pp.126.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]


