Abstract
The plant cuticle, a cutin matrix embedded with and covered by wax, seals the aerial organ's surface to protect the plant against uncontrolled water loss. The cutin matrix is essential for the cuticle to function as a barrier to water loss. Recently, we identified from wild barley a drought supersensitive mutant, eibi1, which is caused by a defective cutin matrix as the result of the loss of function of HvABCG31, an ABCG full transporter. Here, we report that eibi1 epidermal cells contain lipid-like droplets, which are supposed to consist of cutin monomers that have not been transported out of the cells. The eibi1 cuticle is fragile due to a defective cutin matrix. The rice ortholog of the EIBI1 gene has a similar pattern of expression, young shoot but not flag leaf blade, as the barley gene. The model of the function of Eibi1 is discussed. The HvABCG31 full transporter functions in the export of cutin components and contributed to land plant colonization, hence also to terrestrial life evolution.
Key words: ABC transporter, cuticle, cuticular wax, drought resistance, inclusion
Inclusions in eibi1 Mutant Epidermal Cells
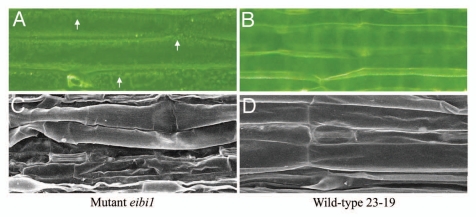
The drought-hypersensitive wild barley's (Hordeum spontaneum Koch) naturally occurring mutant eibi11 suffers from a particularly severe level of water loss and displays a defective cuticle with reduced cutin deposition (∼50% of the wild type) and a thin cuticle (∼25% of the wild type) and a similar amount of the major wax component, 1-hexacosanol.2 Protrusions of cytoplasm into the vacuole were a specific feature of elongation-zone epidermis cells in eibi1 leaves. These protrusions may be caused by the inclusions of cutin monomers failed to be secreted. When the cells become as large as mature cells in the non-elongation zone and the emerged blade, the inclusions may remain in cytoplasm as indicated by lipid-like droplets in the epidermis (Fig. 1A). The lipid-like droplets were not found in wild-type epidermis (Fig. 1B) since no protrusions or inclusions exist in wild-type epidermis cells. Similar protrusions have been noted in the stem epidermal cells of the Arabidopsis thaliana atabcg11 and atabcg12 mutants.3–7 These mutants are unable to export cutin and wax from the epidermis cells, leading to an accumulation of intracellular lipid, which is responsible for the formation of protrusions. In Arabidopsis pec1 mutant, the eibi1 ortholog, the inclusions in petals observed by TEM are also observed by light microscopy with nile red staining.8
Figure 1.
Light microscope (A and B) and scanning electron microscope (C and D) images of emerged leaf blades of eibi1 mutant (A and C) and wild type (B and D).
Fragile eibi1 Cuticle
A key role for EIBI1 is in cutin matrix formation. The EIBI1 protein was detected exclusively in the elongation zone where the cutin matrix was formed.2 The reduced cuticle thickness correlated well with the reduced amount of cutin in eibi1 mutant leaves. A fragile cuticle was observed in eibi1 leaves (Fig. 1C). The eibi1 cuticle was broken while the wild-type cuticle was kept intact during the process of sample preparation for SEM analysis (Fig. 1D). The thin and fragile cuticle might explain the excess water loss in eibi1 mutant leaves. Many Arabidopsis cutin mutants such as bdg, dcr, att1 and lacs2 have a disorganized cutin matrix and display increased cuticle permeability. A few cutin mutants in monocot species, such as the Sorghum bicolor bm2 (previously named bm22) mutants, show a reduced cuticle thickness and increased water loss.9,10 The rice wdl1 mutant shows increased water loss from wdl1 mutant leaves, which is associated with loose packing of the cuticle and an irregular thickness of the cell wall. These evidences demonstrate that a functional cuticle is required for water retention.
Expression of the Eibi1 Orthologous Gene from Rice
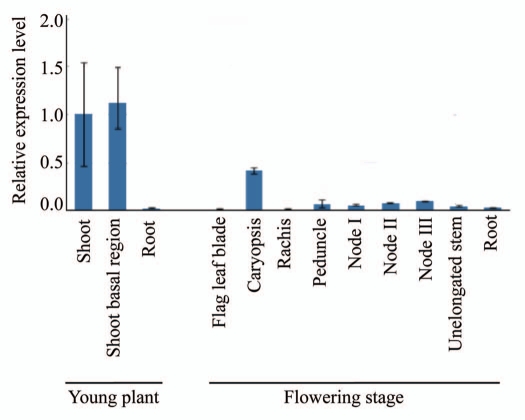
The Eibi1 gene was highly expressed in the elongation zone of the growing leaf (the site of cutin synthesis), and its gene product was also localized in developing, but not in mature tissue in barley.2 Similarly, the analysis of Eibi1 expression in rice showed the presence of abundant transcripts in young shoot, but only traces in flag leaf blade, rachis and root (Fig. 2). The gene was also expressed in young caryopsis, peduncle, nodes and unelongated stem. AtABCG32/Pec1, the closest Eibi1 homolog in Arabidopsis, is expressed in all organs of the shoot with a tendency toward higher expression levels in young, expanding tissues than in older ones.8 These results indicate that the Eibi1 expression pattern is highly conserved not only in monocot but also in dicot plants.
Figure 2.
Quantitative RT-PCR analysis of Eibi1 gene expression in different organs of rice plants.
A Model of Eibi1 Function
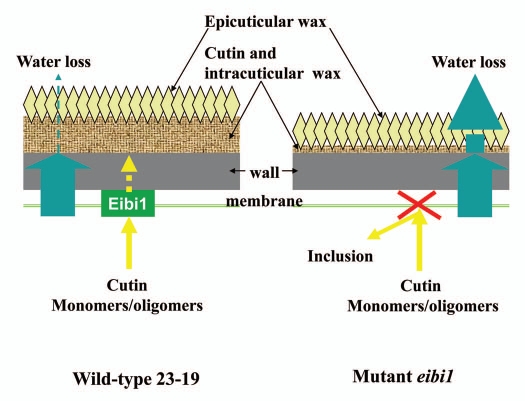
The deposition of cutin and wax are under independent control in monocots during leaf emergence. Eibi1 is hypothesized to function as a transporter involved in the secretion of cutin monomers or oligomers in elongating epidermal cells in a young growing leaf (Fig. 3). Cutin monomers or oligomers are transported out of the cells and across the cell wall to form a cutin matrix. A functional cuticle is synthesised by filling and covering the cutin matrix with waxes. In eibi1 mutant leaves, the cutin monomers or oligomers appear as inclusions in epidermis because they are not transported out of the cells when there is loss of function of eibi1, which leads to a thin and fragile cuticle. Therefore, eibi1 mutant leaves are unable to retain water as effectively as wild type can. Eibi1 encodes an ABCG full transporter. There are three half ABCG transporters that have been identified in Arabidopsis, AtABCG11, AtABCG12 and AtABCG13. ABCG11 is required for the export of cutin precursors as well as wax molecules.4–7 ABCG12 is required for the secretion of cuticular wax.3 ABCG13 is involved in cutin formation in flowers.11 However, ABCG32, the Eibi1 ortholog in Arabidopsis, has a function in the export of cuticular components distinct from the three half transporters.8 Homologs of HvABCG31 were found in green algae, moss and lycopods, indicating that this full transporter is highly conserved in land plants thereby contributing to land plant colonization and evolution.
Figure 3.
Putative model of Eibi1 function in barley.
Acknowledgments
This work was supported by the “One Hundred Talents” Project of the Chinese Academy of Sciences (O827751002), the National Natural Science Foundation of China (30970449, 31170369), Genomics for Agricultural Innovation Grant TRG1004 from the Ministry of Agriculture, Forestry and Fisheries of Japan, postdoctoral Grant P10511 from the Japan Society for the Promotion of Science, the Ancell Teicher Research Foundation for Genetics and Molecular Evolution.
References
- 1.Chen G, Sagi M, Weining S, Krugman T, Fahima T, Korol AB, et al. Wild barley eibi1 mutation identifies a gene essential for leaf water conservation. Planta. 2004;219:684–693. doi: 10.1007/s00425-004-1277-7. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Komatsuda T, Ma JF, Nawrath C, Pourkheirandish M, Tagiri A, et al. An ATP-binding cassette subfamily G full transporter is essential for the retention of leaf water in both wild barley and rice. Proc Natl Acad Sci USA. 2011;108:12354–12359. doi: 10.1073/pnas.1108444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, et al. Plant cuticular lipid export requires an ABC transporter. Science. 2004;306:702–704. doi: 10.1126/science.1102331. [DOI] [PubMed] [Google Scholar]
- 4.Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, et al. Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J. 2007;52:485–498. doi: 10.1111/j.1365-313X.2007.03252.x. [DOI] [PubMed] [Google Scholar]
- 5.Luo B, Xue XY, Hu WL, Wang LJ, Chen XY. An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion. Plant Cell Physiol. 2007;48:1790–1802. doi: 10.1093/pcp/pcm152. [DOI] [PubMed] [Google Scholar]
- 6.Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Hàfer R, et al. The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol. 2007;145:1345–1360. doi: 10.1104/pp.107.105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ukitsu H, Kuromori T, Toyooka K, Goto Y, Matsuoka K, Sakuradani E, et al. Cytological and biochemical analysis of COF1, an Arabidopsis mutant of an ABC transporter gene. Plant Cell Physiol. 2007;48:1524–1533. doi: 10.1093/pcp/pcm139. [DOI] [PubMed] [Google Scholar]
- 8.Bessire M, Borel S, Fabre G, CarraÁa L, Efremova N, Yephremov A, et al. A member of the PDR-family of ABC transporters is required for the formation of a functional cuticle in Arabidopsis. Plant Cell. 2011;23:1958–1970. doi: 10.1105/tpc.111.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenks MA, Joly RJ, Peter PJ, Rich PJ, Axtell D, Ashworth EN, et al. Chemically induced cuticle mutation affecting epidermal conductance to water vapor and disease susceptibility in Sorghum bicolor (L.) Moench. Plant Physiol. 1994;105:1239–1245. doi: 10.1104/pp.105.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters PJ, Jenks MA, Rich PJ, Axtell JD, Ejeta G. Mutagenesis, selection and allelic analysis of epicuticular wax mutants in Sorghum. Crop Sci. 2009;49:1250–1258. [Google Scholar]
- 11.Panikashvili D, Shi JX, Schreiber L, Aharoni A. The Arabidopsis ABCG13 transporter is required for flower cuticle secretion and patterning of the petal epidermis. New phytol. 2011;190:113–124. doi: 10.1111/j.1469-8137.2010.03608.x. [DOI] [PubMed] [Google Scholar]