Abstract
Complicated schemes of classical breeding and their drawbacks, environmental risks imposed by agrochemicals, decrease of arable land, and coincident escalating damages of pests and pathogens have accentuated the necessity for highly efficient measures to improve crop protection. During co-evolution of host-microbe interactions, antimicrobial peptides (AMPs) have exhibited a brilliant history in protecting host organisms against devastation by invading pathogens. Since the 1980s, a plethora of AMPs has been isolated from and characterized in different organisms. Nevertheless the AMPs expressed in plants render them more resistant to diverse pathogens, a more orchestrated approach based on knowledge of their mechanisms of action and cellular targets, structural toxic principle, and possible impact on immune system of corresponding transgenic plants will considerably improve crop protection strategies against harmful plant diseases. This review outlines the current knowledge on different modes of action of AMPs and then argues the waves of AMPs' ectopic expression on transgenic plants' immune system.
Key words: antimicrobial peptides, durable resistance, gene technology, immune system, metchnikowin, sustainable agriculture
Introduction
Cationic as well as anionic antimicrobial peptides (AMPs) are peptides serving as constitutive or inducible defense barriers against microbial infections in plants, insects, amphibians and mammals including human.1–3 They might additionally have the ability to boost the host immunity by functioning as immunomodulators.4,5 Plenty of AMPs exist to cope with practically all potential infection sources. In general, the amphipathic peptides consist of positively charged residues, predominantly arginine and lysine, or else histidine in acidic setting, and a substantial ratio of hydrophobic amino acids.6,7 The best-known antimicrobial peptide families are (1) linear α-helical peptides comprising cecropins8 and magainins9 exhibiting generally antibacterial activities, (2) multiple Cystein-bridge-containing defensins showing antibacterial and antifungal activities,10,11 (3) Pro-rich peptides with activity against bacteria and filamentous fungi,2,12 and (4) the Gly-rich peptides active mainly against Gram-negative and occasionally Gram-positive bacteria.13,14
Major environmental concerns have been leading to prohibition of a huge part of existing agrochemicals all around the world, and coincident growing demands for sustainable strategies in crop protection have inspired the idea of recruiting AMPs for improvement of plant health.15 This review covers the diverse modes of action, by which AMPs impede assaulting pathogens and overviews the impact of expression of AMPs in corresponding transgenic plants in terms of modulating the plant's different immune pathways.
AMPs; General Aspects
Controlled by only one single gene, AMPs can be produced rather quickly upon infection with narrow energy consumption;16 nonetheless, some are constitutively expressed. Despite the fact that AMPs in insects and mammals may modulate the innate and adaptive immune reactions,17 common persuasion on their most important function is elimination of infectious microorganisms.18,19 Several kinds of classification have been proposed for AMPs; however, consistent with their secondary structures AMPs are generally categorized into four clusters:20
linear α-helical peptides containing cationic amphipathic helices that perform inhibitory activities generally against bacteria. Many peptides of this group have been classified as “pore-forming” such as alamethicin, cecropin, PGLa, magainin, melittin and mastoparan.21,23
cyclic peptides with β-sheet structure that form predominantly β-sheets including coupled β-strands due to the presence of two or more disulfide bonds. Although being antifungal in some cases,11,24 they are often characterized as antibacterial peptides. The β-sheets are stably assembled by either disulfide bonds, as in cases of tachyplesins,25 defensins,26 protegrins,27 and gallerimycin,28 or circling of the peptide backbone, as in cases of polymyxin B,29 tyrocidines,30 arenicins.31
peptides with β-hairpin or looped configuration that include those containing a looped structure due to the presence of a single disulfide bond and/or circling of the peptide chain. Thanatin from Podisus maculiventris is an example of this kind of peptides.32 The best-characterized molecules amongst this group are lantibiotics produced by Gram-positive bacteria.
linear peptides with unusual bias in particular amino acids, e.g., drosocin, metchnikowin, apidaecin, abaecin, formaecin, lebocin, pyrrhocoricin and metalnikowin all rich in proline,12,33 indolicidin rich in tryptophan,34 histatin rich in histidine,35 tritrpticin rich in arginine or tryptophan,36 and diptericins and attacins rich in glycine.33 These peptides, predominantly found in Class Insecta, are active against bacteria and fungi.2,37 The most recognized small Pro-rich AMPs are apidaecins that structurally consist of two domains: the conserved domain in charge of general antibacterial activity, and the variable one responsible for the antibacterial spectrum. They are lethal to many Gram-negative bacteria.38 Of interest, several linear AMPs are amorphous in free solution and fold into their final configuration upon partitioning into biological membranes.39
AMPs; Modes of Action
Although mechanisms of action of antimicrobial peptides have been frequently reviewed33, 38, 40–42, there are yet open questions regarding their heterologous functions. Generally speaking, functions of these peptides vary from membrane permeabilization to actions on an array of intracellular target molecules including immuno-modulatory activities (Table 1).
Table 1.
Effects of native and transgenic AMPs in different organisms
| AMPs | Organism | Target molecule | Outcome |
| Native AMPs | Bacteria | Membrane phospholipids/LPS | Prevention of vital microbial homeostasis because of pore formation in membranes64 |
| DnaK; receptor/docking/transporter molecule; GroEL | Inhibition of natural ATPase activity; Inhibition of chaperon-assisted protein folding68–70 | ||
| Ribosome | Protein synthesis inhibition69 | ||
| Fungi | Membrane glycosyl ceramide | Membrane permeabilization779,80 | |
| Redox signaling cascade | Induction of ROS, membrane damage, organelle breakdown and cell death24,82 | ||
| MAP kinase signaling cascades | Regulation of fungal genes important for overcoming plant defense83 | ||
| Chitin in cell wall | Interference with chitin synthesis85,86 | ||
| Plants | Diverse molecules | Modification of host gene expression88,89 | |
| Induction of cell death in other plants90 | |||
| Association with epigenetic somaclonal variation events91 | |||
| Conferring zinc tolerance92 | |||
| Mammals | Nucleus | Cell cycle impairment in rat retinal neuroblasts81 | |
| L-type Ca2+ channel | Blockade of mammalian L-type Ca2+ channel84 | ||
| Diverse molecules | Playing as chemokines93,94 and/or induction of chemokine production95 | ||
| Inhibition of pro-inflammatory cytokine production induced by LPS96 | |||
| Wound healing promotion97 | |||
| Inhibitory activities toward tumor cells and HIV-1 reverse transcriptase98 | |||
| Modulation of adaptive immune responses4,5 | |||
| Transgenic AMPs in plants | Microbes | Diverse molecules | Interference with microbial fitness and virulence establishment2,99 |
| Plants | Diverse molecules | Primed status of transgenic plants due to more activated ISR and SAR and higher redox potential2,99,100 | |
| Alteration of processes of synthesis, folding, and stabilization of proteins which enter to the secretory pathway101 | |||
| Alteration of translational machinery101 | |||
| Alteration of components of vesicle-associated transport machinery101 | |||
| Improved protection against oxidative stress24,82,99–101 |
Pore-forming activity.
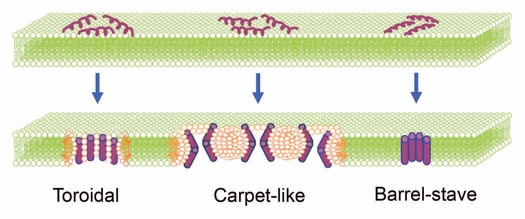
Ability to interact with membranes is a classical countenance of AMPs;43 nonetheless, membrane permeabilization is not an absolute feature. Earlier reports indicate an utter correlation between antibiotic effects of defensins and membrane permeabilization.44,45 Notable hydrophilic positively charged domains facilitate the peptides to interact with the negatively charged microbial surfaces and head groups of bilayer phospholipids leading to cell membrane penetration. Three pore-forming mechanisms are described to explain the effects of α-helical membrane peptides.1,40 A simplistic schematic illustration of different pore formation mechanisms is provided in Figure 1 and a selection of peptides showing different pore-forming mechanisms is given in Table 2. The so-called “carpet-like” mechanism refers to destruction of membrane assembly by collaborative action of peptides.40 Peptides self-associate onto the acidic phospholipid-rich regions of lipid bilayers and once their concentrations reach a certain threshold they permeate into the membrane by mounting the bilayer positive potential.38 The second pore-formation mechanism “barrel-stave”63 is symbolized in alamethicin;60 it inserts into the membrane hydrophobic area and creates a pore by forming trans-membrane helical bundles. In the third mechanism, the “toroidal” model, the peptide builds toroidal pores in lipid bilayers. Pore construction is, intriguingly, managed by the lipid polar head groups and the helix bundles that orient vertically to the membrane exterior. More precisely, the attached peptides aggregate and induce the lipid monolayers to bend continuously through the pore so that both the inserted peptides and the lipid head groups line the water core.1 Pores act as non-selective channels for ions, toxins and metabolites, thus preventing the microbe from maintaining the vital homeostasis.64 Conditional on experimental settings, α-helical membrane peptides can take on different pore-forming mechanisms. For instance, the pore-forming mechanism differs appropriate to the type of membranes and pH, which indicates the essence of experimental settings in the studies on AMPs' modes of action.65 On the other hand, making the Magainin2 tetravalent and octavalent largely increases the pore-forming capability of peptide leading to decreasing the peptide minimal inhibitory concentration to low nanomolar ranges.66
Figure 1.
Schematic illustration of three pore-forming mechanisms to explain the α-helical membrane peptides. In “toroidal” model, the peptide builds toroidal pores in lipid bilayers. Pore construction is managed by the lipid polar head groups and the helix bundles that orient vertically to the membrane exterior. In other words, the attached peptides aggregate and tempt the lipid monolayers to bend continuously through the pore so that both the inserted peptides and the lipid head groups line the water core. “Carpet-like” mechanism refers to destruction of membrane assembly by collaborative action of peptides. Peptides self-associate onto the acidic phospholipids-rich regions of lipid bilayers, and as soon as their concentrations reach to a certain threshold, they permeate into the membrane. This is assisted by escalating the positive potential of bilayer. Via “barrel-stave” mechanism, peptide inserts into the membrane hydrophobic substance, flips inward and creates a pore by forming transmembrane helical bundles. (Scheme is modified after refs. 1 and 46).
Table 2.
Classification of different α-helical AMPs according to their membrane permeabilization mechanisms
| Pore-forming mechanism | Peptide | Origin | Reference |
| Carpet-like (detergent-like) | Cecropins | Hyalophora cecropia | 47 |
| PGLa | Xenopus laevis | 48 | |
| Dermaseptins | Phyllomedusa spp. | 49 | |
| Ovispirin | Ovis aries | 50 | |
| Latarcins | Lachesana tarabaevi | 51 | |
| RL-37 | Macaca mulatta | 52 | |
| Toroidal (wormhole) | Mastoparan X | Vespa xanthoptera | 53 |
| Magainin 2 | Xenopus laevis | 54, 55 | |
| LL-37 | Homo sapiens | 52, 56 | |
| Melittin | Xenopus laevis | 57 | |
| Piscidin | Morone saxatilis | 58 | |
| Barrel-Stave | Pardaxin | Pardarchirus marmoratus | 59 |
| Alamethicin | Trichoderma viride | 60 | |
| Amphotricin B | Streptomyces nodosus | 61 | |
| Ceratotoxin | Ceratitis capitata | 62 |
Inhibition of DNA and protein functions.
It is postulated that the positive charge in the short Pro-rich AMPs boosts bacterial cell access67 and the existing prolines may perhaps inhibit helix formation and hence, toxicity to the host. Nevertheless, apidaecins belonging to short Pro-rich peptides are of distinction by lacking the pore-inducing action.38 Eradication of bacteria by apidaecins commences by an ambiguous interaction of the peptide with an outer membrane component like LPS and DnaK,68 and consequently, its entrance into the periplasmic space. Next, peptide traverses inner membrane specifically by an irrevocable band with either a receptor/docking/transporter molecule69 or the 60-kDa bacterial chaperone GroEL.68 Ultimately, the peptide is displaced into the cell where it runs into its certain target that is either ribosome leading to protein synthesis inhibition69 or DnaK leading to protein folding inhibition.68,70 Evidently, pyrrhocoricin, a relative of apidaecins, inhibits natural ATPase activity and chaperon-assisted protein folding of E. coli's DnaK, whereas it lacks any activity on human HSP70.68,70 In contrast, PR-39 kills bacteria by stopping their DNA and protein syntheses and gives rise to degradation of these components.71 Interestingly, PR-39 does pass through membranes without any apparent damage. The molecule can induce the synthesis of syndecans involved in wound healing72 and hamper the NADPH-dependent redox reactions.73
Prokaryotic DnaK recognizes extended peptide constituents as well as positively charged residues inside and outside of its substrate-binding furrow.74 This might also occur to similar sequence motifs in typical members of Pro-rich peptides family, i.e., pyrrhocoricin, drosocin, apidaecin and metchnikowin. It is evident that metchnikowin can be triggered by both major pathways of fruit fly immune system, imd and Toll,75,76 which makes it unique in terms of extreme immune capacity to almost all potential microbes, e.g., fungi, bacteria and even viruses. As well, obstruction of chaperone-assisted protein folding by Prorich cationic peptides70 prospects a gallant approach to combating microbial infections.
Disturbance of other intracellular targets.
It is documented that plant defensins, contrary to their mammalian and insect orthologs, neither induce ion permeable pores in artificial phospholipidic membranes nor change their electrical status, making evident that these defensins do not interact directly with plasma membrane phospholipids.77,78 Though it was reported that the antifungal defensin NaD1 from Nicotiana alata induces membrane permeabilization, its activity may not only be restricted to the hyphal membrane, but it also enters cells and affects intracellular targets.24
Defensins of different origins exhibit no clear similarities in their modes of mechanism; nevertheless, fungal membrane sphingolipid glycosyl ceramide is the most common key target of a number of defensin-called peptides. Glycosyl ceramide was identified as specific target for the antifungal plant defensin RsAFP2 and the insect defensin-like peptide heliomicin.79 Consistently, a Fusarium graminearum mutant deficient in glycosyl ceramide was resistant to both radish RsAFP2 and alfalfa MsDef1 defensins, corroborating the idea that glycosyl ceramide is also the target of MsDef1.80 On the contrary, pea defensin, Psd1, directs cell cycle impairment and causes Neurospora crassa conidia to undergo endoreduplication. Furthermore, Psd1 regulates interkinetic nuclear migration from Synthesis (S) to Mitosis (M) phase of cell cycle in rat retinal neuroblasts.81 Additionally, some defensins such as RsAFP282 and NaD124 modulate the intracellular signaling cascades, specifically, induction of reactive oxygen species (ROS) that upon accumulation may cause membrane damage, organelle breakdown and eventually cell death. Pertinent support for this modulatory activity was found in certain mutants of F. graminearum disrupted in some MAPKKK(s) genes that were hypersensitive to alfalfa MsDef1, barrel clover MtDef2, and radish RsAFP2. MAP kinase signaling cascades in F. graminearum regulate the fungus sensitivity to plant defensins.83 It is giving proof that plant defensins can act as stimuli to launch MAP kinase signaling cascades involved in regulating the fungal genes important for overcoming the plant defense. It would seem, upon binding of defensins to their receptors, activation of MAPKKK(s) occurs owing to physical interaction and/or phosphorylation by either the receptor itself or intermediary factors or an interlinking kinase.83 Moreover, MsDef1 has been characterized to block the mammalian L-type Ca2+ channel in a manner akin to structurally unrelated antifungal toxin KP4 from Ustilago maydis.84 It is, as well, documented that bamboo defensins-like AMPs, PpAMP1 and PpAMP2, bind to chitin in microbial cell walls,85 and Aspergillus giganteus defensin, AFP, inhibits the chitin biosynthesis in susceptible fungi.86 Consistently, chitin synthase mutants of Fusarium oxysporum and Aspergillus oryzae are less susceptible to AFP. Presumably, AFP causes cell wall stress by interfering with the chitin synthesis and disturbing the cell integrity in sensitive fungi.86
Several lines of evidence from different studies indicate that microbial membrane permeabilization or/and cell wall disruption are within modes of mechanism of defensins.87 However, the issue whether the lytic action (membrane disturbance) is a phenomenon actively processed by AMPs to kill the microbes remains an enigma.
Besides direct elimination of microbes, AMPs have been shown to possess several immuno-modulatory functions such as modification of host gene expression,88,89 induction of cell death in other plants,90 association with epigenetic somaclonal variation events,91 conferring zinc tolerance,92 playing as chemokines,93,94 and/or induction of chemokine production,95 inhibition of proinflammatory cytokine production induced by LPS,96 wound healing promotion,97 inhibitory activities toward tumor cells and HIV-1 reverse transcriptase,98 and modulation of adaptive immune responses, e.g., by activation of human plasmacytoid dendritic cells in some auto-immune diseases.4,5
Modulation of different cascades of immune system in relevant transgenic plants.
There are some reports demonstrating that the host gene profiling alters after introgression of antimicrobial peptides.2,99,100,101 Expression of metchnikowin gene from Drosophila melanogaster in barley to codify a peptide with antimicrobial activity was recruited to improve plant defense against microbial attacks.2 Assessment of metchnikowin effects on powdery mildew fungus during its interaction with transgenic barley provided evidence that the antifungal peptide improves the resistance of plant as if it impedes the development of functional haustorium due to increased rate of hypersensitive response (HR) and development of cell wall apposition (CWA).2 Comprehensive study on possible latent influence of metchnikowin on the defense system of plant revealed that the SAR and ISR pathways as well as redox status of metchnikowin-expressing barley plants are potentiated during interaction with powdery mildew fungus.99 In Phenylpropanoid pathway, the PAL-1 gene expression profile demonstrated that in Bgh challenge the activity of phenylalanine ammonia-lyase is elevated in metchnikowin transgenic plants.99 Similar observation was reported by Distefano et al. (2008) for PAL gene,100 which suggests that highly activated ISR may be one of the causes of higher resistance in these transgenic plants. This suggestion is supported by elevated expression of PR-6 in metchnikowin plants compared with that in wild type individuals.99 The higher level of reactive oxygen species down to expression of antimicrobial peptides24,82,99,100 supports the notion that these peptides play some part of their roles by modulating the redox milieu, which might ultimately lead to cell death.
Examination of susceptibility factors, i.e., MLO and Bax inhibitor-1 in metchnikowin barley concluded that the susceptibility/resistance of those plants is independent of these factors.99 Comparative analysis of gene expression between cecropin A transgenic and wild-type rice plants grown under optimal conditions and during infection of rice with the rice blast fungus Magnaporthe oryzae revealed the overexpression of diverse genes involved in (1) protection against oxidative stress, (2) processes of synthesis, folding and stabilization of proteins that enter into the secretory pathway, (3) translational machinery, and (4) genes encoding components of the vesicle-associated transport machinery in cecropin A rice.101 Together, these reports imply the altered immune status of AMPs-expressing plants.
Using AMPs for Plant Disease Control
As demands for a better control of plant diseases increase, AMPs come into focus.15 To date, a multitude of gene constructions with coding sequences of AMPs have been expressed in planta leading to various extents of protection against fungal and bacterial pathogens (see ref. 99). Insect peptides seem especially suitable owing to their exceptionally broad antimicrobial potential for protecting their hosts against various biotic challenges. Growing knowledge on structure-function relationships and thus elucidation of essential peptide domains will press forward the use of synthetic AMPs in transgenic crop plants. Synthetic analogues of cecropins facilitated their ectopic expression for improvement of plant fitness in biotic stress circumstances.102 Interestingly, early attempts to express cecropin in tobacco for resistance induction against Pseudomonas syringae pv. tabaci were barely successful.103 Short persistence of cecropin B has been ascribed to proteinases in the cytosol mediating proteolysis through an initial endopeptidase cleavage.104 To put off such an interfering process, cecropin B-derived peptides were manipulated to be shorter in length. Alternatively, targeting the mature peptides using signal sequences from different origins into the intercellular spaces, in which proteinases are seldom present, could lead to relatively higher accomplishment.105 Accordingly, the antimicrobial activity of intercellular fluid of metchnikowin expressing barley plants2 and the green fluorescing background of intercellular spaces surrounding the faintly fluorescing barley epidermal cells that transiently express the GFP-fused metchnikowin peptide99 confirm the functionality of the fruit fly-origin signal sequence of metchnikowin peptide, in planta, to secrete the produced peptide into the intercellular space. As another strategy, molecular modeling for engineering the AMPs offers a dominant tool to engender peer synthetic and chimeric peptides with potentially superior properties.
Prospective for Future Endeavors
Dose-effect and synergistic activity.
Though it has been reported that the degree of antimicrobial activity is not dependent on the AMP production level in transgenic plants,106 most of the publications furnish clear evidence for peptides' dose-effect activity.82,100,107,108 Some investigators emphasize the possibility of synergism among different AMPs.109–111 In vivo data for different antibacterial peptides show that defensins and linear peptides work in-synergy,112,113 as exemplified by synergism between LL-37 and human β-defensin HBD-2.114 Consistently, concomitant expression of AMPs in plants usually leads to higher levels of induced resistance than their individual expression.115,116 This finding is reminiscing of the effect known from combining disease resistance genes (gene pyramiding) in traditional breeding, which often results in long-lasting, durable plant protection against pathogens.
Recruiting inducible promoters.
General propensity toward reducing fitness costs117 as well as downgrading the co-evolutionary collapse of resistance to microbes has weighted the generation of plants expressing AMPs on-demand, by exploiting synthetic or native inducible promoters activated upon pathogen attack.2,11,107,118,119 Employment of wounding and/or pathogen-inducible promoters ensures high expression level of the peptide upon mechanical wounding and/or microbial infections. This may assist to avoid the development of pathogenic microbes capable of circumventing induced disease resistance, by e.g., mutation and/or synthesis of proteolytic agents.
Approaching different strategies to alleviate existing drawbacks.
Undesirably, application of AMPs to engineer pathogen resistance in plants suffers from some limitations, namely species- and race-specificity of the peptides,120 slight enhanced resistance,106 induction of infertility,121 and leakage of conferred resistance after a while due to resiliency of disease-causing microbes.38 To alleviate these drawbacks as well as to increase the antimicrobial potency of existing peptides, several approaches have been proposed to follow. Engineering crop plants for disease resistance via chloroplast genome instead of nuclear genome is proposed to achieve high levels of expression and to prevent pollen-mediated escape of transgenes.122 In addition, synthesis/manipulation of peptides for base(s) substitution/deletion and AMPs' chimeric hybridization result in improved disease resistance in plants.120,123,124 Expression of antibody-AMP fusion proteins has been shown to control microbial pathogens, more efficiently and durably.125,126 Also, targeting the AMPs into endoplasmic reticulum instead of intercellular spaces dramatically drops the probability of infertility.121 Moreover, expression of a cocktail of AMPs with different modes of action demotes the possibility of resistance depletion attributable to microbial escape. Notably, insects synthesize concurrently a continuum of low-molecular-mass inhibitors against microbial proteases in company with AMPs.127 It is anticipated that these inhibitors annihilate the digestive action of proteolytic enzymes secreted from plant pathogenic fungi.128 Consequently, coincident transmission/expression of insect AMPs and inhibitors of microbial proteases will possibly avert the selection of pathogens, which can negate the foreign peptides in transgenic plants.
Biosafety remarks.
From the ecological point of view, the issue whether or not transfer of AMPs into plants imperils the mutualistic interactions between plants and beneficial microbes should be addressed. There are reports stating target specificity for AMPs among the kingdom of fungi. The phyla Glomeromycota and Basidiomycota that accommodate many symbiotic fungi might be less sensitive to AMPs than Ascomycota.2,129–131 However some AMPs affect Basidiomycota rather well, this might not be taken as a rule.108,115,119,124,132 Regarding specificity, the activity of metchnikowin on orchid mycorrhiza Piriformospora indica133 has been studied in detail.2 Growth and development of this fungus was not demolished in transgenic plants producing the metchnikowin, whilst ascomycete fungi were impaired.2 Despite the fact that the definite cause for specificity has not been elucidated yet, these observations do prospect a promising approach: utilizing AMPs to diminish the devastating consequences of diseases and pests without affecting the plant's essential mutualistic interactions with beneficial symbionts. Clearly, these symbionts are of vital importance for plants in terms of presenting a better fitness via supporting water and mineral uptake as well as strengthening disease resistance.134 However, it must be stressed that more research is needed to identify differential targets of AMPs in fungi in order to explain AMP's specificity on the molecular level.
AMPs potential for combating viruses and pests.
Since antiviral activities are within the panel of AMP's properties,135–137 it will be interesting to assess the potential of AMPs for controlling countless viral diseases in plants. However, since it is known that Toll pathway, one of the major pathways in immune response in Drosophila, is required for efficient inhibition of virus replication in infected flies,138 expectation to restrain viruses in plants should be considered, critically. Finally, in order to manage the pest damages on plants, one might think of expressing AMPs under the control of plant tissue specific promoters, for instance, those for in-phloem expression to construct some lethal peptides to devastating pests, though the biosafety aspects must be, yet again, well thought-out.
Overall, the provided data on endogenous genes expression in AMPs transgenic plants suggest that antimicrobial genes play their roles in disease resistance in part via modulation of different resistance mechanisms. They might also join forces of various plant immune pathways culminating in a primed status. The provided evidence on the involved pathway(s) in AMPs-induced resistance will improve our knowledge concerning the impacts of antimicrobial peptides, expressed in diverse plant species, on the immune system of transgenic plants. This helps going beyond the current notion that antimicrobial peptides-derived resistance refers solely to the direct noxious effects of these peptides on pathogenic microbes. Certainly, entire clarity of this important issue demands elaborate and comprehensive experiments, namely the use of transcriptomics to explore the impact of transgene expression in plants.
Acknowledgments
The research in my lab is financially supported by grants from Shahid Bahonar University of Kerman (Iran).
References
- 1.Brogden K. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 2.Rahnamaeian M, Langen G, Imani J, Khalifa W, Altincicek B, von Wettstein D, et al. Insect peptide metchnikowin confers on barley a selective capacity for resistance to fungal ascomycetes pathogens. J Exp Bot. 2009;60:4105–4114. doi: 10.1093/jxb/erp240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris F, Dennison SR, Phoenix DA. Anionic antimicrobial peptides from eukaryotic organisms. Curr Protein Pept Sci. 2009;10:585–606. doi: 10.2174/138920309789630589. [DOI] [PubMed] [Google Scholar]
- 4.Allaker R. Host defense peptides—a bridge between the innate and adaptive immune responses. Trans R Soc Trop Med Hyg. 2008;102:3–4. doi: 10.1016/j.trstmh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Eken C, Gasser O, Zenhaeusern G, Oehri I, Hess C, Schifferli JA. Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J Immunol. 2008;180:817–824. doi: 10.4049/jimmunol.180.2.817. [DOI] [PubMed] [Google Scholar]
- 6.Sitaram N, Nagaraj R. Host-defense antimicrobial peptides: importance of structure for activity. Curr Pharmaceut Design. 2002;8:727–742. doi: 10.2174/1381612023395358. [DOI] [PubMed] [Google Scholar]
- 7.Papagianni M. Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function and applications. Biotechnol Adv. 2003;21:465–499. doi: 10.1016/s0734-9750(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 8.Vilcinskas A, Matha V. Antimycotic activity of lysozyme and its contribution to antifungal humoral defense reactions in Galleria mellonella. Anim Biol. 1997;6:19–29. [Google Scholar]
- 9.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuhmann B, Seitz V, Vilcinskas A, Podsiadlowski L. Cloning and expression of Gallerimycin, an antifungal peptide expressed in immune response of greater wax moth larvae, Galleria mellonella. Arch Insect Biochem Physiol. 2003;53:125–133. doi: 10.1002/arch.10091. [DOI] [PubMed] [Google Scholar]
- 11.Langen G, Imani J, Altincicek B, Kieseritzky G, Kogel KH, Vilcinskas A. Transgenic expression of gallerimycin, a novel antifungal insect defensin from the greater wax moth Galleria mellonella, confers resistance to pathogenic fungi in tobacco. Biol Chem. 2006;387:549–557. doi: 10.1515/BC.2006.071. [DOI] [PubMed] [Google Scholar]
- 12.Levashina EA, Ohresser S, Bulet P, Reichhart JM, Hetru C, Hoffmann JA. Metchnikowin, a novel immuneinducible prolin-rich peptide from Drosophila with antimicrobial and antifungal properties. Eur J Biochem. 1995;233:694–700. doi: 10.1111/j.1432-1033.1995.694_2.x. [DOI] [PubMed] [Google Scholar]
- 13.Ando K, Natori S. Inhibitory effect of sarcotoxin IIA, an antibacterial protein of Sarcophaga peregrina, on growth of Escherichia coli. J Biochemistry. 1988;103:735–739. doi: 10.1093/oxfordjournals.jbchem.a122337. [DOI] [PubMed] [Google Scholar]
- 14.Asling B, Dushay MS, Hultmark D. Identification of early genes in the Drosophila immune response by PCR-based differential display: the Attacin A gene and the evolution of attacin-like proteins. Insect Biochemistry Mol Biol. 1995;25:511–518. doi: 10.1016/0965-1748(94)00091-c. [DOI] [PubMed] [Google Scholar]
- 15.Marcos JF, Muñoz A, Pérez-Payá E, Misra S, López-García B. Identification and rational design of novel antimicrobial peptides for plant protection. Ann Rev Phytopathol. 2008;46:273–301. doi: 10.1146/annurev.phyto.121307.094843. [DOI] [PubMed] [Google Scholar]
- 16.Hancock R. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infectious Dis. 2001;1:156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 17.Müller U, Vogel P, Alber G, Schaub GA. The innate immune system of mammals and insects. Contrib Microbiol. 2008;15:21–44. doi: 10.1159/000135684. [DOI] [PubMed] [Google Scholar]
- 18.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins and eosinophil-derived neurotoxin in host defense. Ann Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 19.Bowdish DM, Davidson DJ, Hancock RE. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci. 2005;6:35–51. doi: 10.2174/1389203053027494. [DOI] [PubMed] [Google Scholar]
- 20.van't Hof W, Veerman EC, Helmerhorst EJ, Amerongen AV. Antimicrobial peptides: properties and applicability. Biol Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 21.Boman H. Peptide antibiotics and their role in innate immunity. Ann Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 22.Mangoni ML, Rinaldi AC, Di Giulio A, Mignogna G, Bozzi A, Barra D, et al. Structure-function relationships of temporins, small antimicrobial peptides from amphibian skin. Eur J Biochem. 2000;267:1447–1454. doi: 10.1046/j.1432-1327.2000.01143.x. [DOI] [PubMed] [Google Scholar]
- 23.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 24.van der Weerden NL, Lay FT, Anderson MA. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J Biol Chem. 2008;283:14445–14452. doi: 10.1074/jbc.M709867200. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochimica Biophysica Acta. 1998;1462:1–10. doi: 10.1016/s0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 26.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochimica Biophysica Acta. 1999;1462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 27.Ostberg N, Kaznessis Y. Protegrin structure-activity relationships: using homology models of synthetic sequences to determine structural characteristics important for activity. Peptides. 2005;26:197–206. doi: 10.1016/j.peptides.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Seitz V, Clermont A, Wedde M, Hummel M, Vilcinskas A, Schlatterer K, et al. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev Comp Immunol. 2003;27:207–215. doi: 10.1016/s0145-305x(02)00097-6. [DOI] [PubMed] [Google Scholar]
- 29.Tsubery H, Ofek I, Cohen S, Fridkin M. Structure-function studies of polymyxin B nonapeptide: implications to sensitization of Gram-negative bacteria. J Med Chem. 2000;43:3085–3092. doi: 10.1021/jm0000057. [DOI] [PubMed] [Google Scholar]
- 30.Bu X, Wu X, Xie G, Guo Z. Synthesis of tyrocidine A and its analogues by spontaneous cyclization in aqueous solution. Organic Lett. 2000;4:2893–2895. doi: 10.1021/ol0263191. [DOI] [PubMed] [Google Scholar]
- 31.Ovchinnikova TV, Aleshina GM, Balandin SV, Krasnosdembskaya AD, Markelov ML, Frolova EI, et al. Purification and primary structure of two isoforms of arenicin, a novel antimicrobial peptide from marine polychaeta Arenicola marina. FEBS Lett. 2004;577:209–214. doi: 10.1016/j.febslet.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Imamura T, Yasuda M, Kusano H, Nakashita H, Ohno Y, Kamakura T, et al. Acquired resistance to the rice blast in transgenic rice accumulating the antimicrobial peptide thanatin. Transgenic Res. 2010;19:415–424. doi: 10.1007/s11248-009-9320-x. [DOI] [PubMed] [Google Scholar]
- 33.Otvos L. The short proline-rich antibacterial peptide family. Cell Mol Life Sci. 2002;59:1138–1150. doi: 10.1007/s00018-002-8493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falla TJ, Karunaratne DN, Hancock RE. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 35.Brewer D, Lajoie G. Structure-based design of potent histatin analogues. Biochemistry. 2002;41:5526–5536. doi: 10.1021/bi015926d. [DOI] [PubMed] [Google Scholar]
- 36.Yang ST, Shin SY, Lee CW, Kim YC, Hahm KS, Kim JI. Selective cytotoxicity following Arg-to-Lys substitution in tritrpticin adopting a unique amphipathic turn structure. FEBS Lett. 2003;540:229–233. doi: 10.1016/s0014-5793(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 37.Imler JL, Bulet P. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem immunol allergy. 2005;86:1–21. doi: 10.1159/000086648. [DOI] [PubMed] [Google Scholar]
- 38.Li WF, Ma GX, Zhou XX. Apidaecin-type peptides: Biodiversity, structure-function relationships and mode of action. Peptides. 2006;27:2350–2359. doi: 10.1016/j.peptides.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Wu M, Hancock RE. Improved derivatives of bactenecin, a cyclic dodecameric antimicrobial cationic peptide. Antimicrobial Agents Chemotherapy. 1999;43:1274–1276. doi: 10.1128/aac.43.5.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shai Y. Mode of action of membrane active antimicrobial peptide. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 41.Yount NY, Yeaman MR. Immunocontinuum: Perspectives in antimicrobial peptide mechanisms of action and resistance. Prot Peptide Lett. 2005;12:49–67. doi: 10.2174/0929866053405959. [DOI] [PubMed] [Google Scholar]
- 42.Aerts AM, François IE, Cammue BPA, Thevissen K. The mode of antifungal action of plant, insect and human defensins. Cell Mol Life Sci. 2008;65:2069–2079. doi: 10.1007/s00018-008-8035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hancock RE, Rozek A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol Lett. 2002;206:143–149. doi: 10.1111/j.1574-6968.2002.tb11000.x. [DOI] [PubMed] [Google Scholar]
- 44.Ludtke S, He K, Huang H. Membrane thinning caused by magainin 2. Biochemistry. 1995;34:16764–16769. doi: 10.1021/bi00051a026. [DOI] [PubMed] [Google Scholar]
- 45.van der Weerden NL, Hancock RE, Anderson MA. Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall-dependent process. J Biol Chem. 2010;285:37513–37520. doi: 10.1074/jbc.M110.134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tok O. Antimicrobial peptides: New candidates in the fight against bacterial infections. Curr Trends Pept Sci. 2005;80:717–735. doi: 10.1002/bip.20286. [DOI] [PubMed] [Google Scholar]
- 47.Marassi FM, Opella SJ, Juvvadi P, Merrifield RB. Orientation of cecropin A helices in phospholipid bilayers determined by solid-state NMR spectroscopy. Biophysical J. 1999;77:3152–3155. doi: 10.1016/S0006-3495(99)77145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bechinger B. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochimica et Biophysica Acta. 1999;1462:157–183. doi: 10.1016/s0005-2736(99)00205-9. [DOI] [PubMed] [Google Scholar]
- 49.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochemical J. 1999:341. [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi S, Huster D, Waring A, Lehrer RI, Kearney W, Tack BF, et al. Orientation and dynamics of an antimicrobial peptide in the lipid bilayer by solid-state NMR spectroscopy. Biophysical J. 2001;81:2203–2214. doi: 10.1016/S0006-3495(01)75868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozlov SA, Vassilevski AA, Feofanov AV, Surovoy AY, Karpunin DV, Grishin EV. Latarcins, antimicrobial and cytolytic peptides from the venom of the spider Lachesana tarabaevi (Zodariidae) that exemplify biomolecular diversity. J Biol Chem. 2006;281:20983–20992. doi: 10.1074/jbc.M602168200. [DOI] [PubMed] [Google Scholar]
- 52.Morgera F, Vaccari L, Antcheva N, Scaini D, Pacor S, Tossi A. Primate cathelicidin orthologues display different structures and membrane interactions. Biochemical J. 2009;417:727–735. doi: 10.1042/BJ20081726. [DOI] [PubMed] [Google Scholar]
- 53.Matsuzaki K, Yoneyama S, Murase O, Miyajima K. Transbilayer transport of ions and lipids coupled with mastoparan X translocation. Biochemistry. 1996;35:8450–8456. doi: 10.1021/bi960342a. [DOI] [PubMed] [Google Scholar]
- 54.Matsuzaki K, Murase O, Fujii N, Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flipflop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996;35:11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 55.Matsuzaki K, Sugishita K, Ishibe N, Ueha M, Nakata S, Miyajima K, et al. Relationship of membrane curvature to the formation of pores by magainin 2. Biochemistry. 1998;37:11856–11863. doi: 10.1021/bi980539y. [DOI] [PubMed] [Google Scholar]
- 56.Henzler Wildman KA, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 57.Allende D, Simon SA, McIntosh TJ. Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores. Biophysical J. 2005;88:1828–1837. doi: 10.1529/biophysj.104.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campagna S, Saint N, Molle G, Aumelas A. Structure and mechanism of action of the antimicrobial peptide piscidin. Biochemistry. 2007;46:1771–1778. doi: 10.1021/bi0620297. [DOI] [PubMed] [Google Scholar]
- 59.Ehrenstein G, Lecar H. Electrically gated ionic channels in lipid bilayers. Quarterly Rev Biophysics. 1977;10:1–34. doi: 10.1017/s0033583500000123. [DOI] [PubMed] [Google Scholar]
- 60.North CL, Barranger-Mathys M, Cafiso DS. Membrane orientation of the N-terminal segment of alamethicin determined by solid-state 15N NMR. Biophysical J. 1995;69:2392–2397. doi: 10.1016/S0006-3495(95)80108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paquet MJ, Fournier I, Barwicz J, Tancrède P, Auger M. The effects of amphotericin B on pure and ergosterolor cholesterol-containing dipalmitoylphosphatidylcholine bilayers as viewed by 2H NMR. Chem Phys Lipids. 2002;119:1–11. doi: 10.1016/s0009-3084(02)00071-3. [DOI] [PubMed] [Google Scholar]
- 62.Bessin Y, Saint N, Marri L, Marchini D, Molle G. Antibacterial activity and pore-forming properties of ceratotoxins: a mechanism of action based on the barrel stave model. Biochimica Biophysica Acta. 2004;1667:148–156. doi: 10.1016/j.bbamem.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophysical J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boland MP, Separovic F. Membrane interactions of antimicrobial peptides from Australian tree frogs. Biochimica Biophysica Acta. 2006;1758:1178–1183. doi: 10.1016/j.bbamem.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Nomura K, Corzo G, Nakajima T, Iwashita T. Orientation and pore-forming mechanism of a scorpion pore-forming peptide bound to magnetically oriented lipid bilayers. Biophysical J. 2004;87:2497–2507. doi: 10.1529/biophysj.104.043513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnusch CJ, Branderhorst H, de Kruijff B, Liskamp RMJ, Breukink E, Pieters RJ. Enhanced membrane pore formation by multimeric/oligomeric antimicrobial peptides. Biochemistry. 2007;46:13437–13442. doi: 10.1021/bi7015553. [DOI] [PubMed] [Google Scholar]
- 67.Bencivengo AM, Cudic M, Hoffmann R, Otvos L., Jr The efficacy of the antibacterial peptide, pyrrhocoricin, is finely regulated by its amino acid residues and active domains. Lett Pept Sci. 2002;8:201–209. [Google Scholar]
- 68.Otvos L, Jr, O I, Rogers ME, Consolvo PJ, Condie BA, Lovas S, et al. Interaction between heat shock proteins and antimicrobial peptides. Biochemistry. 2000;39:14150–14159. doi: 10.1021/bi0012843. [DOI] [PubMed] [Google Scholar]
- 69.Castle M, Nazarian A, Yi SS, Tempst P. Lethal effects of apidaecin on Escherichia coli involve sequential molecular interactions with diverse targets. J Biol Chem. 1999;274:32555–32564. doi: 10.1074/jbc.274.46.32555. [DOI] [PubMed] [Google Scholar]
- 70.Kragol G, Lovas S, Varadi G, Condie BA, Hoffmann R, Otvos L., Jr The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry. 2001;40:3016–3026. doi: 10.1021/bi002656a. [DOI] [PubMed] [Google Scholar]
- 71.Boman HGAB, Boman A. Mechanisms of action on Escherichia coli of cecropin-P1 and PR-39, 2 antibacterial peptides from pig intestine. Infect Immun. 1993;61:2978–2984. doi: 10.1128/iai.61.7.2978-2984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, Bernfield M. Syndecans, cell surface heparin sulfate proteoglycans, are induced by a prolin-rich antimicrobial peptide from wounds. Proc Natl Acad Sci USA. 1994;91:11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi J, Ross CR, Minton JE, Ross CR, Blecha F. PR-39, a proline-rich antibacterial peptide that inhibits phagocyte NADPH oxidase activity by binding to src homology 3 domains of p47 (phox) Proc Natl Acad Sci USA. 1996;93:6014–6018. doi: 10.1073/pnas.93.12.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rüdiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levashina EA, Ohresser S, Lemaitre B, Imler JL. Two distinct pathways can control expression of the gene encoding the Drosophila antimicrobial peptide metchnikowin. J Mol Biol. 1998;278:515–527. doi: 10.1006/jmbi.1998.1705. [DOI] [PubMed] [Google Scholar]
- 76.Levashina EA, Landley E, Green C, Gubb D, Ashburner M, Hoffmann JA, Reichhart JM. Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science. 1999;285:1917–1919. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- 77.Thevissen K, Ghazi A, De Samblanx GW, Brownlee C, Osborn RW, Broekaert WF. Fungal membrane responses induced by plant defensins and thionins. J Biol Chem. 1996;271:15018–15025. doi: 10.1074/jbc.271.25.15018. [DOI] [PubMed] [Google Scholar]
- 78.Caaveiro JM, Molina A, Gonzalez-Manas JM, Rodriguez-Palenzuela P, Garcia-Olmedo F, Goni FM. Differential effects of five types of antipathogenic plant peptides on model membranes. FEBS Lett. 1997;410:338–342. doi: 10.1016/s0014-5793(97)00613-3. [DOI] [PubMed] [Google Scholar]
- 79.Thevissen K, Warnecke DC, Francois IE, Leipelt M, Heinz E, Ott C, Zahringer U, et al. Defensins from insects and plants interact with fungal glucosylceramides. J Biol Chem. 2004;279:3900–3905. doi: 10.1074/jbc.M311165200. [DOI] [PubMed] [Google Scholar]
- 80.Ramamoorthy V, Cahoon EB, Li J, Thokala M, Minto RE, Shah DM. Glucosylceramide synthase is essential for alfalfa defensin-mediated growth inhibition but not for pathogenicity of Fusarium graminearum. Mol Microbiol. 2007;66:771–786. doi: 10.1111/j.1365-2958.2007.05955.x. [DOI] [PubMed] [Google Scholar]
- 81.Lobo DS, Pereiraa IB, Fragel-Madeira L, Medeiros LN, Cabral LM, Faria J, Bellio M. Antifungal Pisum sativum defensins 1 interacts with Neurospora crassa cyclin F related to the cell cycle. Biochemistry. 2007;46:987–996. doi: 10.1021/bi061441j. [DOI] [PubMed] [Google Scholar]
- 82.Aerts AM, François I, Meert EMK, Li Q, Cammue BPA, Thevissen K. The antifungal activity of RsAFP2, a plant defensin from Raphanus sativus, involves the induction of reactive oxygen species in Candida albicans. J Mol Microbiol Biotechnol. 2007;13:243–247. doi: 10.1159/000104753. [DOI] [PubMed] [Google Scholar]
- 83.Ramamoorthy V, Zhao X, Snyder AK, Xu JR, Shah DM. Two mitogen-activated protein kinase signaling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum. Cell Microbiol. 2007;9:1491–1506. doi: 10.1111/j.1462-5822.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 84.Spelbrink RG, Dilmac N, Allen A, Smith TJ, Shah DM, Hockerman GH. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol. 2004;135:2055–2067. doi: 10.1104/pp.104.040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujimura M, Ideguchi M, Minami Y, Watanabe K, Tadera K. Amino acid sequence and antimicrobial activity of chitin-binding peptides, Pp-AMP 1 and Pp-AMP 2, from Japanese Bamboo shoots (Phyllostachys pubescens) Biosci Biotechnol Biochem. 2005;69:642–645. doi: 10.1271/bbb.69.642. [DOI] [PubMed] [Google Scholar]
- 86.Hagen S, Marx F, Ram AF, Meyer V. The antifungal protein AFP fro Aspergillus giganteus inhibits chitin synthesis in sensitive fungi. Appl Environmental Microbiol. 2007;73:2128–2134. doi: 10.1128/AEM.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thevissen K, Terra FR, Broekaert WF. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol. 1999;65:5451–5458. doi: 10.1128/aem.65.12.5451-5458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bowdish DM, Davidson DJ, Speert DP, Hancock RE. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol. 2004;172:3758–3765. doi: 10.4049/jimmunol.172.6.3758. [DOI] [PubMed] [Google Scholar]
- 89.Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, Rehaume L, Hancock RE. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 90.de Zélicourt A, Letousey P, Thoiron S, Campion C, Simoneau P, Elmorjani K, Marion D, et al. Ha-DEF1, a sunflower defensin, induces cell death in Orobanche parasitic plants. Planta. 2007;226:591–600. doi: 10.1007/s00425-007-0507-1. [DOI] [PubMed] [Google Scholar]
- 91.Tregear JW, Morcillo F, Richaud F, Berger A, Singh R, Cheah SC, et al. Characterization of a defensin gene expressed in oil palm inflorescences: induction during tissue culture and possible association with epigenetic somaclonal variation events. J Exp Bot. 2002;53:1387–1396. [PubMed] [Google Scholar]
- 92.Mirouze M, Sels J, Richard O, Czernic P, Loubet S, Jacquier A, et al. A putative novel role for plant defensins: a defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. Plant J. 2006;47:329–342. doi: 10.1111/j.1365-313X.2006.02788.x. [DOI] [PubMed] [Google Scholar]
- 93.Egesten A, Eliasson M, Johansson HM, Olin AI, Morgelin M, Mueller A, et al. The CXC chemokine MIG/CXCL9 is important in innate immunity against Streptococcus pyogenes. J Infectious Dis. 2007;195:684–693. doi: 10.1086/510857. [DOI] [PubMed] [Google Scholar]
- 94.Linge HM, Collin M, Nordenfelt P, Mörgelin M, Malmsten M, Egesten A. The human CXC-chemokine Granulocyte Chemotactic Protein 2 (GCP-2)/CXCL6 possesses membrane disrupting properties and is antibacterial. Antimicrob Agents Chemother. 2008;52:2599–2607. doi: 10.1128/AAC.00028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whiting D, Hsieh G, Yun JJ, Banerji A, Yao W, Fishbein MC, et al. Chemokine monokine induced by IFN-gamma/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J Immunol. 2004;172:7417–7424. doi: 10.4049/jimmunol.172.12.7417. [DOI] [PubMed] [Google Scholar]
- 96.Zhang JZ, Ward KW. Besifloxacin, a novel fluoroquinolone antimicrobial agent, exhibits potent inhibition of pro-inflammatory cytokines in human THP-1 monocytes. J Antimicrobial Chemotherapy. 2008;61:111–116. doi: 10.1093/jac/dkm398. [DOI] [PubMed] [Google Scholar]
- 97.Otte J, Werner I, Brand S, Chromik AM, Schmitz F, Kleine M, Schmidt WE. Human beta defensin 2 promotes intestinal wound healing in vitro. J Cell Biochem. 2008;104:2286–2297. doi: 10.1002/jcb.21787. [DOI] [PubMed] [Google Scholar]
- 98.Wong JH, Ng TB. Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides. 2005;26:1120–1126. doi: 10.1016/j.peptides.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Rahnamaeian M, Vilcinskas A. Defense gene expression is potentiated in transgenic barley expressing antifungal peptide metchnikowin throughout powdery mildew challenge. J Plant Res. 2011 doi: 10.1007/s10265-011-0420-3. [DOI] [PubMed] [Google Scholar]
- 100.Distefano G, La Malfa S, Vitale A, Lorito M, Deng Z, Gentile A. Defence-related gene expression in transgenic lemon plants producing an antimicrobial Trichoderma harzianum endochitinase during fungal infection. Transgenic Res. 2008;17:873–879. doi: 10.1007/s11248-008-9172-9. [DOI] [PubMed] [Google Scholar]
- 101.Campo S, Manrique S, García-Martínez J, San Segundo B. Production of cecropin A in transgenic rice plants has an impact on host gene expression. Plant Biotechnol J. 2008;6:585–608. doi: 10.1111/j.1467-7652.2008.00339.x. [DOI] [PubMed] [Google Scholar]
- 102.Jaynes JM, Xanthopoulos KG, Destefano-Beltran L, Dodds JH. Increasing bacterial resistance in plants utilizing genes from insects. BioEssays. 1987;6:263–270. [Google Scholar]
- 103.Hightower R, Baden C, Penzes E, Dunsmuir P. The expression of cecropin peptide in transgenic tobacco does not confer resistance to Pseudomonas syringae pv. tabaci. Plant Cell Rep. 1994;13:295–299. doi: 10.1007/BF00233324. [DOI] [PubMed] [Google Scholar]
- 104.Mills D, Hammerschlag FA, Nordeen R, Owens L. Evidence for the breakdown of cecropin B by proteinases in the intercellular fluid of peach leaves. Plant Sci. 1994;104:17–22. [Google Scholar]
- 105.Sharma A, Sharma R, Imamura M, Yamakawa M, Machii H. Transgenic expression of cecropin B, an antibacterial peptide from Bombyx mori, confers enhanced resistance to bacterial leaf blight in rice. FEBS Lett. 2000;484:7–11. doi: 10.1016/s0014-5793(00)02106-2. [DOI] [PubMed] [Google Scholar]
- 106.Balconi C, Lanzanova C, Conti E, Triulzi T, Forlani F, Cattaneo M, et al. Fusarium head blight evaluation in wheat transgenic plants expressing the maize b-32 antifungal gene. Eur J Plant Pathol. 2007;117:129–140. [Google Scholar]
- 107.Yevtushenko DP, Romero R, Forward BS, Hancock RE, Kay WW, Misra S. Pathogen-induced expression of a cecropin A-melittin antimicrobial peptide gene confers antifungal resistance in transgenic tobacco. J Exp Bot. 2005;56:1685–1695. doi: 10.1093/jxb/eri165. [DOI] [PubMed] [Google Scholar]
- 108.Almasia NI, Bazzini AA, Hopp HE, Vazquez-Rovere C. Overexpression of snakin-1 gene enhances resistance to Rhizoctonia solani and Erwinia carotovora in transgenic potato plants. Mol Plant Pathol. 2008;9:329–338. doi: 10.1111/j.1364-3703.2008.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zasloff M. Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol. 1992;4:3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]
- 110.Hoffmann J. Innate immunity of insects. Curr Opin Immunol. 1995;7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 111.McCafferty DG, Cudic P, Yu MK, Behenna DC, Kruger R. Synergy and duality in peptide antibiotic mechanisms. Curr Opin Chemical Biol. 1999;3:672–680. doi: 10.1016/s1367-5931(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 112.Levy O, Ooi CE, Weiss J, Lehrer RI, Elsbach P. Individual and synergistic effects of rabbit granulocyte proteins on Escherichia coli. J Clin Invest. 1994;94:672–682. doi: 10.1172/JCI117384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ong PY, Ohtake T, Brandt C, Strickland I, Bugoniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. New England J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 114.Boman H. Antibacterial peptides: basic facts and emerging concepts. J Internal Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 115.Jach G, Görnhardt B, Mundy J, Logemann J, Pinsdorf P, Leah R, et al. Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J. 1995;8:97–109. doi: 10.1046/j.1365-313x.1995.08010097.x. [DOI] [PubMed] [Google Scholar]
- 116.Xing H, Lawrence CB, Chambers O, Davies HM, Everett NP, Li QQ. Increased pathogen resistance and yield in transgenic plants expressing combinations of the modified antimicrobial peptides based on indolicidin and magainin. Planta. 2006;223:1024–1032. doi: 10.1007/s00425-005-0143-6. [DOI] [PubMed] [Google Scholar]
- 117.van Hulten M, Pelser M, van Loon LC, Pieterse CM, Ton J. Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:5602–5607. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moreno AB, Peñas G, Rufat M, Bravo JM, Estopa M, Messeguer J, et al. Pathogen-induced production of the antifungal AFP protein from Aspergillus giganteus confers resistance to the blast fungus Magnaporthe grisea in transgenic rice. Mol Plant-Microbe Interact. 2005;18:960–972. doi: 10.1094/MPMI-18-0960. [DOI] [PubMed] [Google Scholar]
- 119.Patkar RN, Chattoo BB. Transgenic indica rice expressing ns-LTP-Like protein shows enhanced resistance to both fungal and bacterial pathogens. Mol Breeding. 2006;17:159–171. [Google Scholar]
- 120.Osusky M, Zhou G, Osuska L, Hancock RE, Kay WW, Misra S. Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nature Biotechnol. 2000;18:1162–1166. doi: 10.1038/81145. [DOI] [PubMed] [Google Scholar]
- 121.Coca M, Peñas G, Gómez J, Campo S, Bortolotti C, Messeguer J, et al. Enhanced resistance to the rice blast fungus Magnaporthe grisea conferred by expression of a cecropin A gene in transgenic rice. Planta. 2006;223:392–406. doi: 10.1007/s00425-005-0069-z. [DOI] [PubMed] [Google Scholar]
- 122.DeGray G, Rajasekaran K, Smith F, Sanford J, Daniell H. Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol. 2001;127:852–862. [PMC free article] [PubMed] [Google Scholar]
- 123.Osusky M, Osuska L, Hancock RE, Kay WW, Misra S. Transgenic potatoes expressing a novel cationic peptide are resistant to late blight and pink rot. Transgenic Res. 2004;13:181–190. doi: 10.1023/b:trag.0000026076.72779.60. [DOI] [PubMed] [Google Scholar]
- 124.Osusky M, Osuska L, Kay W, Misra S. Genetic modification of potato against microbial diseases: in vitro and in planta activity of a dermaseptin B1 derivative, MsrA2. Theor Appl Genet. 2005;111:711–722. doi: 10.1007/s00122-005-2056-y. [DOI] [PubMed] [Google Scholar]
- 125.Peschen D, Li HP, Fischer R, Kreuzaler F, Liao YC. Fusion proteins comprising a Fusarium-specific antibody linked to antifungal peptides protect plants against fungal pathogens. Nature Biotechnol. 2004;22:732–738. doi: 10.1038/nbt970. [DOI] [PubMed] [Google Scholar]
- 126.Li HP, Zhang JB, Shi RP, Huang T, Fischer R, Liao YC. Engineering Fusarium head blight resistance in wheat by expression of a fusion protein containing a Fusarium-specific antibody and an antifungal peptide. Mol Plant-Microbe Interact. 2008;21:1242–1248. doi: 10.1094/MPMI-21-9-1242. [DOI] [PubMed] [Google Scholar]
- 127.Vilcinskas A, Götz P. Parasitic fungi and their interaction with the insect immune system. Adv Parasitol. 1999;43:267–313. [Google Scholar]
- 128.Vilcinskas A, Wedde M. Insect inhibitors of metalloproteinases. IUBMB Life. 2002;54:339–343. doi: 10.1080/15216540216040. [DOI] [PubMed] [Google Scholar]
- 129.Turrini A, Sbrana C, Pitto L, Castiglione MR, Giorgetti L, Briganti R, et al. The antifungal Dm-AMP1 protein from Dahlia merckii expressed in Solanum melongena is released in root exudates and differentially affects pathogenic fungi and mycorrhizal symbiosis. New Phytol. 2004;163:393–403. doi: 10.1111/j.1469-8137.2004.01107.x. [DOI] [PubMed] [Google Scholar]
- 130.Newhouse AE, Schrodt F, Liang H, Maynard CA, Powell WA. Transgenic American elm shows reduced Dutch elm disease symptoms and normal mycorrhizal colonization. Plant Cell Rep. 2007;26:977–987. doi: 10.1007/s00299-007-0313-z. [DOI] [PubMed] [Google Scholar]
- 131.Girlanda M, Bianciotto V, Cappellazzo GA, Casieri L, Bergero R, Martino E, et al. Interactions between engineered tomato plants expressing antifungal enzymes and nontarget fungi in the rhizosphere and phyllosphere. FEMS Microbiol Lett. 2008;288:9–18. doi: 10.1111/j.1574-6968.2008.01306.x. [DOI] [PubMed] [Google Scholar]
- 132.Oard SV, Enright FM. Expression of the antimicrobial peptides in plants to control phytopathogenic bacteria and fungi. Plant Cell Rep. 2006;25:561–572. doi: 10.1007/s00299-005-0102-5. [DOI] [PubMed] [Google Scholar]
- 133.Kumar V, Sahai V, Bisaria VS. High-density spore production of Piriformospora indica, a plant growthpromoting endophyte, by optimization of nutritional and cultural parameters. Bioresour Technol. 2011;102:3169–3175. doi: 10.1016/j.biortech.2010.10.116. [DOI] [PubMed] [Google Scholar]
- 134.Bonfante P, Genre A. Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends Plant Sci. 2008;13:492–498. doi: 10.1016/j.tplants.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 135.Marcos JF, Beachy RN, Houghten RA, Blondelle SE, Pérez-Payá E. Inhibition of a plant virus infection by analogs of melittin. Proc Natl Acad Sci USA. 1995;92:12466–12469. doi: 10.1073/pnas.92.26.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rudolph C, Schreier PH, Uhrig JF. Peptide-mediated broad-spectrum plant resistance to tospoviruses. Proc Natl Acad Sci USA. 2003;100:4429–4434. doi: 10.1073/pnas.0730832100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lopez-Ochoa L, Ramirez-Prado J, Hanley-Bowdoin L. Peptide aptamers that bind to a geminivirus replication protein interfere with viral replication in plant cells. J Virol. 2006;80:5841–5853. doi: 10.1128/JVI.02698-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci USA. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]