Abstract
Algae, like other plants, produce a variety of remarkable compounds collectively referred to as secondary metabolites. They are synthesized by these organisms at the end of the growth phase and/or due to metabolic alterations induced by environmental stress conditions. Carotenoids, phenolic compounds, phycobiliprotein pigments, polysaccharides and unsaturated fatty acids are same of the algal natural products, which were reported to have variable biological activities, including antioxidant activity, anticancer activity, antimicroabial activity against bacteria-virus-algae-fungi, organic fertilizer and bioremediation potentials.
Key words: algae, biological activities, active ingredients
Introduction
Phytoplankton comprises organisms such as diatome, dinoflagellates and macrophytes including green, red and brown algae. As photosynthetic organisms, these groups play a key role in productivity of the ocean and constitute the basis of marine food chain. On the other hand, the use of macroalgae as a potential source of high value chemicals and in therapeutic purpose has a long history. Recently, macroalgae have been used as a novel food with potential nutritional benefits in industry and medicine for various purposes. Furthermore, macroalgae have shown to provide a rich source of natural bioactive compounds with antiviral, antifungal, antibacterial, antioxidant, anti-inflammatory, hypercholesterolemia and hypolipidemic and antineoplasteic properties.1 Thus, there is a growing interest in the area of research on the positive effect of macroalgae on human health and other benefits. In Egypt, the macroalgae is self-grown on the craggy surface near the seashore of the Mediterranean and Red Sea. Macroalgae have not been used as healthy food, while in Japan and China the macroalgae are tradionally used in folk medicine and as healthy foods.2 There is a current worldwide interest in finding new and safe antioxidants from natural sources, such as plant material, to prevent oxidative deterioration of food and to minimize oxidative damage to living cells Experimental and epidemiological evidence suggests the participation of free radicals in tissue damage and pathological processes such as cardiovascular disease and cancer. The use of synthetic antioxidants has decreased due to their suspected activity as promoters of carcinogenesis as well as a general consumer rejection of synthetic food additives. The term “phytochemicals” refers to a wide variety of compounds produced by plants. They are found in fruits, vegetables, beans, grains and microorganisms including microalgae. Scientists have identified thousands of phytochemicals, although only a small fraction have been studied closely. Some of the more commonly known phytochemicals include beta carotene, ascorbic acid (vitamin C), folic acid and vitamin E. The potential uses of algal biomass for the benefit of mankind have been intensively reviewed in the last few years. Algae are considered to be the most efficient biological system for harvesting solar energy and for the production of organic compounds via the photosynthetic process. Many species of algae can be induced to produce particularly high concentrations of chosen compounds (proteins, carbohydrates, lipids and pigments) that of commercial value.3
Algal biomass and algae-derived compounds have a very wide range of potential applications,4,5 from animal feed and aquaculture to human nutrition and health products. Some algae are considered as rich sources of natural antioxidants, although macroalgae have received much attention as potential natural antioxidants.6
Microalgae may serve as a continuous and reliable source of natural products, including antioxidants, because they can be cultivated in bioreactors on a large scale. Furthermore, the qualities of the microalgal cells can be controlled, so that they contain no herbicides and pesticides, or any other toxic substances, by using clean nutrient media for growing the microalgae. The value of microalgae as a source of natural antioxidants is further enhanced by the relative ease of purification of target compounds.7
Microalgae represent an almost untapped resource of natural antioxidants, due to their enormous biodiversity, much more diverse than higher plants. However, not all groups of microalgae can be used as natural sources of antioxidants, due to their widely varied contents of target products, growth rate or yields, ease of cultivation, and/or other factors. Reports on the antioxidant activity of microalgae are limited. Cyanobacteria have been identified as one of the most promising group of organisms from which novel and biochemically active natural products are isolated. Cyanobacteria such as Spirulina, Anabaena, Nostoc and Oscillatoria produce a great variety of secondary metabolites.3 The only comparable group is actinomycetes, which has yielded a tremendous number of metabolites. The rate of discovery from traditional microbial drug producers like actinomycetes and hyphomycetes, which are in the focus of pharmaceutical research for decades, is decreasing and it is time to turn to cyanobacteria and exploit their potential. This is of paramount importance to fight increasingly resistant pathogens and newly emergent diseases.8 Because cyanobacteria are largely unexplored, they represent a rich opportunity for discovery; the expected rate of rediscovery is far lower than for other better-studied groups of organisms.
Cyanobacteria produce a wide variety of bioactive compounds, which include 40% lipopeptides, 5.6% amino acids, 4.2% fatty acids, 4.2% macrolides and 9% amides. Cyanobacterial lipopeptides include different compounds like cytotoxic (41%), antitumor (13%), antiviral (4%), antibiotics (12%) and the remaining 18% activities include antimalarial, antimycotics, multi-drug resistance reversers, antifeedant, herbicides and immunosuppressive agents;9 besides the immune effect, blue-green algae improves metabolism. Blue-green algae have a cholesterol-lowering effect in animals and humans. The level of the total cholesterol, LDL and VLDL cholesterol in rat serum was reduced when a high cholesterol diet was supplemented with blue-green algae. It was found that adopohepatosis caused by a high cholesterol diet was cured by a diet supplemented with algae. This was due to the activity of lipoprotein lipase, an enzyme for metabolism of triglyceride rich lipoproteins.10
Cyanobacteria are also known to produce antitumor, antiviral and antifungal compounds. Many of the pharmaceutically interesting compounds in cyanobacteria are peptides, including cyanobacterial toxins and important candidates for anti-cancer drugs. Peptide synthetases are common in cyanobacteria and responsible for the production of cyanobacterial hepatotoxins and other peptides. Polyketide synthetases are also involved in the biosynthesis of certain cyanobacterial bioactive compounds (e.g., microcystins). Photobioreactor technology has advanced to the point where it is relatively easy to scale up cultures to produce enough material for research purposes. Cyanobacteria also have good potential as a food. Dried Arthrospira (Spirulina) is sold in the market as a health food with annual sales estimated at $40 million. Cyanobacteria can also have application as fuel producers.
In this review article, we focus on many desirable chemicals are the products of secondary metabolism triggered under conditions not conducive to fast growth. For those chemicals to be produced by microalgae, one needs to develop new strains (faster growth, higher substrate tolerance, etc.,) by classical selection or genetic manipulation so microalgal biomass can be produced consistently. Highlight the role of dietary antioxidants and their potential benefits in health and disease directly or indirectly by the plant nutrition and animal feed to produce healthy organic food. Investigate the different biological activities of algae and the relations with its biochemical composition, pigments and different constituents which may vary with salt stressed culture conditions and describe the antioxidant characteristics of algae.
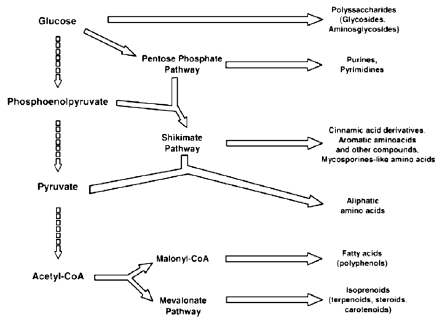
The current application of chemical compounds isolated from diverse classes of algae is enormous. Since 1975, three areas of research in aquatic natural products were emerged: toxins, byproducts and chemical ecology. Over 15,000 novel compounds were chemically determined. Focusing on bio-products, recent trends in drug research from natural sources suggested that algae are a promising group to furnish novel biochemically active substances.11 To survive in a competitive environment, freshwater and marine algae developed defense strategies that resulted in a significant level of structural-chemical diversity from different metabolic pathways.12,13 The exploration of these organisms for pharmaceutical purposes revealed important chemical prototypes for the discovery of new agents stimulated the use of sophisticated physical techniques and new syntheses of compounds with biomedical application. Moreover, algae were promising organisms for providing both novel biologically active substances and essential compounds for human nutrition.9,14 Therefore, an increasing supply for algal extracts, fractions or pure compounds for the economical sector was needed.15 In this regard, both secondary and primary metabolisms were studied as a prelude to future rational economic exploitation as show in Figure 1.
Figure 1.
A schematic representation of processes involved in the uptake, sequestration and translocation of Cd++.
Fresh seaweeds, both wild and cultivated, are commonly eaten as food in the Hawaiian Islands. Before western contact, Limu (seaweed) was a regular part of the diet, and was thought to have contributed vitamins and essential mineral nutrients not found in otherstaple food items. Different species of edible Hawaiian macroalgae were analyzed for protein, lipid, carbohydrate and ash content.16
Eleven species of macroalgae such as Corallina sp. and Porphyra sp. were analyzed for moisture, ash, fat, protein, crude fiber, calorific value and calcium content. At the extremes of the nutritional values, Corallina sp. had low calorific value (2.7 ± 0.3 MJkg−1), high ash content (77.8 ± 0.2% dw), low protein (6.9 ± 0.1% dw) and high calcium content (182 ppm); whereas the exploited Porphyra sp. had high calorific value (18.3 ± 1.8 MJkg−1), low ash content (9.3 ± 0.2% dw), high protein (44.0 ± 1.2% dw) and low calcium content (19.9 ppm).17
Biological Activity of Macroalgae
Algae have mainly been used in west countries as raw material to extract alginates (from brown algae) and agar and carragenates (from red algae). However, as shows in Table 1 it is concluded that algae also contain multitude of bioactive compounds that might have antioxidant, antibacterial, antiviral, anticarcinogenic, etc., properties. Some of them are outlined later due to their special interest as possible functional ingredients.18
Table 1.
Some examples of algae together with their functional ingredients and possible effect on human health
| Algae | Functional ingredients | Possible health effect |
| Sargassum vulgare | Alginic acid, xylofucans | Antiviral activity |
| Himanthalia elongate | PUFAs | Reduce risk of certain heart diseases |
| a-Tocoferol | Antioxidant activity | |
| Sterols | Reduce total and LDL cholesterol | |
| Soluble fiber | Reduce total and LDL cholesterol | |
| Undaria pinnatifida | PUFAs | Reduce risk of certain heart diseases |
| Sterols | Reduce total and LDL cholesterol | |
| Soluble fiber | Reduce total and LDL cholesterol | |
| Folates | Reduce risk of certain types of cancer | |
| Sulphated polysaccharides | Antiviral activity | |
| Fucoxanthin | Preventive effect on cerebrovascular diseases | |
| Phorphira spp. | PUFAs | Reduce risk of certain heart diseases |
| Sterols | Reduce total and LDL cholesterol | |
| Soluble fiber | Reduce total and LDL cholesterol | |
| Chondrus crispus | PUFAs (n-3) fatty acids | Reduce risk of certain heart diseases |
| Sterols | Reduce total and LDL cholesterol | |
| Soluble fiber | Reduce total and LDL cholesterol | |
| Sulphated polysaccharides (porphyrans) | Apoptotic activities | |
| Cystoseira spp. | Terpenes | Valuable curative properties |
| Sterols | Reduce total and LDL cholesterol |
Antioxidant activity.
Gordon and Magos19 suggested that sterols (gramisterol, sitosterol, campesterol and triterpene alcohol esters) were inhibited oxidation by acting as hydrogen donors. The absence of structural damage in the algae leads to consider that these organisms are able to generate the necessary compounds to protect themselves against oxidation. In this respect, algae can be considered as an important source of antioxidant compounds that could be suitable also for protecting our bodies against the reactive oxygen species formed e.g., by our metabolism or induced by external factors (as pollution, stress, UV radiation, etc.). In algae there are antioxidant substances of very different nature, among which vitamin E (or α-tocopherol) and carotenoids can be highlighted within the fat-soluble fraction, whereas the most powerful water-soluble antioxidants found in algae are polyphenols, phyco-biliproteins and vitamins (vitamin C).18 Also, Anggadiredja et al. studied the antioxidant activity of different extracts from fresh and dry specimens of Sargassum polycystum and Laminaria obtuse. The result showed that the extracts of fresh material had highest antioxidant activity than that in dry material. L. obtusa extracts exhibited higher antioxidant activity than those of S. polycystum. Their activity may be due to the present of higher carotenoids and triterpene contained in fresh algae material. The antioxidant activity of extracts of 17 species of seaweed collected from the Pacific Ocean21 was evaluated by determination of lipoxygenase activity and by DPPH scavenging method. Lipoxygenase activity was depressed in the presence of aqueous and ethanol extracts of four algae species; Sargassum species had the highest antioxidant activity of all the species examined. The ethanol extracts of one Sargassum species showed competitive inhibition with the substrate. The same species also showed DPPH radical scavenging activity. Comparison of these results showed no relationship between enzyme inhibition and radical scavenging activity. Jia-Zhi et al. found that polysaccharide of Sargassum fusiform consisting mainly of fucose, galactose and ester sulfate from. Further purification and fractionation on a DEAE cellulose anion exchange column yielded three major fractions (F1–F3). F1 and F2 were fucose-containing sulfate polysaccharides (fucose, xylose, mannose and galactose in the molar ratio 1:trace: 0.027:0.46 and 1:0.03:0.16:1.36, respectively). Antioxidant activities were determined using nitro blue tetrazolium (NBT) reduction. All 3 fractions showed activity, but it was strongest in F1.
β-sitosterol7 demonstrated quite strong antioxidant activity against DPPH radical.23 Novoa et al. studied the antioxidant activity exhibited by the aqueous extract of the red seaweed Bryothamnion triquetrum related to the specific compound that could be responsible for such biological activity. The result showed that the seaweed extract contains 8.08 mg of total polyphenols per gram of lyophilized extract and can prevent thiobarbituric acid reactive substances formation during spontaneous lipoperoxidation of rat brain homogenates with IC50 of 23.3 µg/ml. Fractionation of the extract was performed using chromatographic methods, it was possible to identify and quantify the trans-cinnamic, p-coumaric and ferulic acids, at 221.9, 4,187.3 and 442.3 µg/g of lyophilized extract, as the active compounds. The antioxidant activity of the lipid extracts of eight marine algae belonging to the Cystoseira genus were evaluated in a micellar model system by Ruberto et al. They found that the activity was ascribed to the presence of tetraprenyltoluquinols (9), which are tocopherol-like compounds characteristic of these algae. Jime'nez-Escrig et al.26 reported that the radical scavenging activity of a brown alga Fucus was decreased by 98% after drying at 50°C for 48 h. The same author found that extracts from Laminariales exhibited not only stable free radical scavenging activity, but also ferric ion reducing activity, albeit, the reducing activity was lower than of the red alga Porphyra umbilicalis. This latter evidence was related to the low levels of free phloroglucinol in kelps.
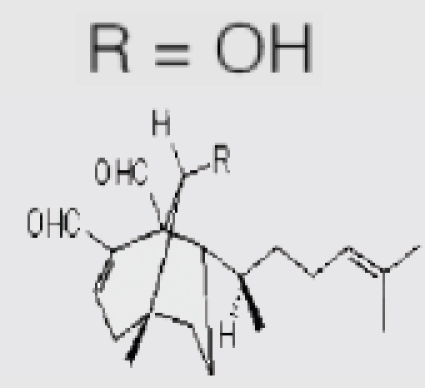
Nagai and Ukimoto27 reported that marine algae were considered to be a rich source of antioxidants. Some active antioxidant compounds from brown algae were identified as hylopheophytin in Eisenia bicyclis (arame),28 and fucoxantinein Hijikia fusiformis (hijiki).29 Several extracts from marine brown alga Saragassum micracanthum (Kuetzing) Endlicher were screened for their inhibitory activity on lipid peroxidation by Mori et al. They found that methanol extract (Sm-M), chloroform/methanol (3:1) extract and ethyl acetate fraction of Sm-M inhibited lipid peroxidation in rat liver homogenates with IC50 values of 0.70, 0.70 and 0.37 µg/ml, respectively. These inhibitions were stronger than vitamin C and E. The in vivo antioxidant activities of fucosterol8 isolated from the marine algae P. siliquosa (Silvetia siliquosa) were investigated by Lee-Saung et al. The results indicated that fucosterol exhibited a significant decrease in serum transaminase activities elevated by hepatic damage induced by CC14-intoxication in rats. Fucosterol inhibited the sGOT and sGPT activities in the blood serum by 25.57 and 63.16%, respectively. It increased also the anti-oxidation enzymes such as hepatic cytosolic superoxide dismutase (SOD), catalase and glutathione peroxide (GSH-px) activities by 33.89, 21.56 and 39.24%, respectively. These results suggest that fucosterol possess not only antioxidation, but also hepatoprotective activities in rats.
The in vitro antioxidation activity of four species of green seaweeds (Codium decorticatum, Enteromorpha intestinalis, Ulva fascita and Chaetomorpha anteninna) from Santa Catarina, Brazil was investigated through inhibition of peroxidation of linoleic acid. The most efficient species in terms of lipid peroxidation were E. intestinalis and Chaetomorpha anateninna, with inhibition of above 70%. The capacity of methanolic extracts to quench hydrogen peroxide was also estimated, and the mean values varied in the range 1.26–20.01%.31 Maehira et al. suggested that red alga of the Gracilaria sp. cultivated with deepsea water has the potential to ameliorate degenerative diseases of aging. Potential antioxidative activities of enzymatic extracts (carbohydrate degrading enzymes and five proteases) from seven species of brown seaweeds were evaluated using four different reactive oxygen species (ROS) scavenging assays by Heo et al. They found that the enzymatic extracts exhibited more prominent effects in hydrogen peroxide scavenging activity (approximately 90%) compared to the other scavenging activities which was even higher than that of the commercial antioxidants. Jang- Kyoung et al. isolated 16 new meroterpenods of the chromene class from the brown alga Sargassum siliquastrum. They found that this compound exhibited significant antioxidant activity in the DPPH assay (as shown in Table 2) also showed inhibitory activity toward butylcholine esterase.
Table 2.
Reported biochemical compounds from different algal species as antioxidant activity
| Algal species | Cpd No. | Name of reported compound | Structure | References |
| Taonia atomaria (Brown algae) | 1 | taondiol | 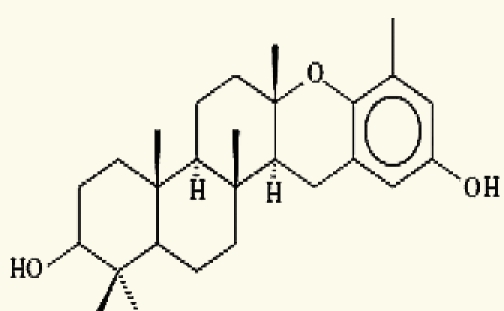 |
Nahas, et al. (2006)35 |
| 2 | isoepitaondiol | 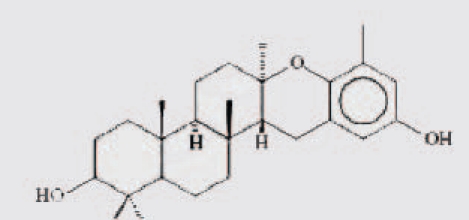 |
||
| 3 | stypodiol | 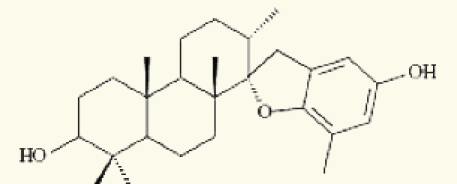 |
||
| 4 | stypoldione | 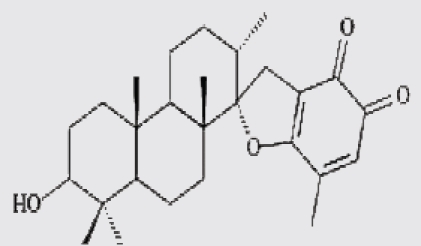 |
||
| 5 | sargaquinone | 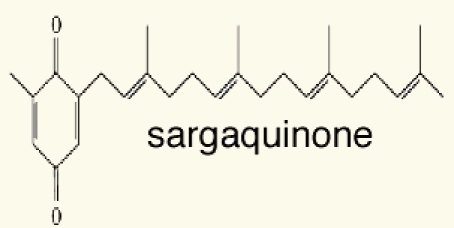 |
||
| 6 | sargaol | 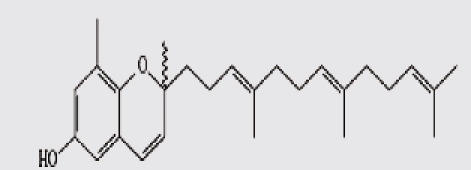 |
||
| Salvia plebeia | 7 | ß-Sitosterol | 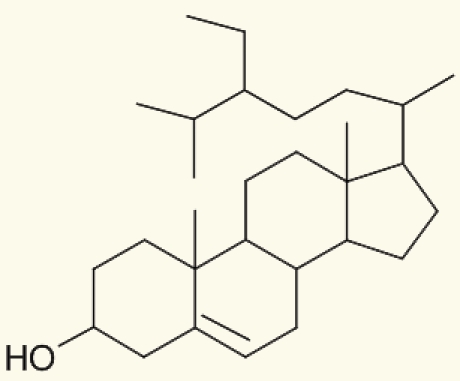 |
Weng and Wang. (2000)23 |
| P. siliquosa (Brown algae) | 8 | Fucosterol | 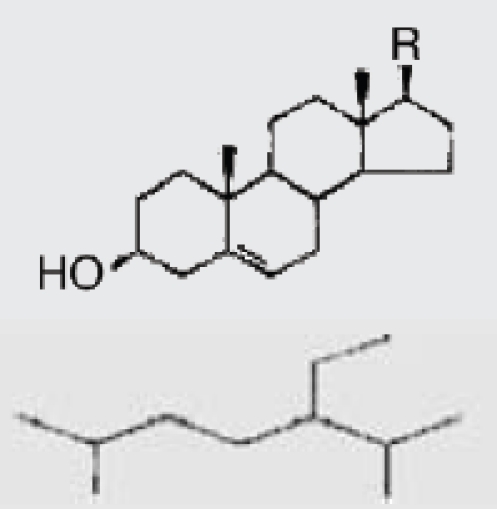 |
Lee-SangHyun, et al. (2003)2 |
| Cystoseira sp. | 9 | tetraprenyltoluquinols | 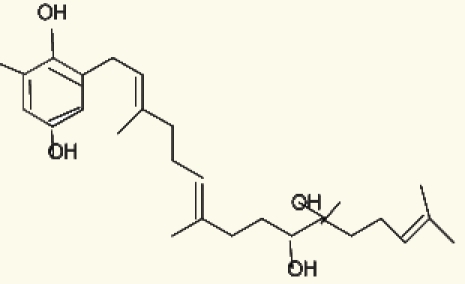 |
Ruberto, et al. (2001)25 |
Thirteen algae from the Aegean Sea were examined for radical scavenging activity (RSA) using the DPPH and chemiluminescence (CL) tests by Nahas et al. They found that the extracts of the brown alga Taonia atomaria exhibited the best RSA in comparison to the extracts of the other investigated species and approached the activity of powerful antioxidant standards. Separation of T. atomaria extract by chromatography methods resulted in the isolation of six metabolites: taondiol (1), isoepitaondiol (2), stypodiol (3), stypoldione (4), sargaquinone (5) and sargaol (6) which were found to possess marked RSA. Total phenolic content (TPC) and antioxidant activity (AOA) of 50% aqueous methanol extracts of the marine algae, Padina antillarum, Caulerpa racemosa and Kappaphycus alvarezzi were studied by Chew et al. They found that P. antillarum had the highest TPC, and exhibited the highest reducing power, and highest chelating ability than that in C. racemosa and K. alvarezzi. In vitro antioxidant activities of three selected Indian red seaweeds, Euchema kappaphycus, Gracilaria edulis and Acanthophora spicifera were evaluated by Ganesan et al. They found that ethyl acetate fraction of A. spicifera exhibited higher total antioxidant activity among all the fractions. Higher phenolic content (16.26 mg gallic acid equivalent/g extract) was noticed in petrolum ether fraction of G. edulis. Reducing power and hydroxyl radical scavenging activity of E. kappaphycus were higher compared to standard antioxidant (α-tocopherol). In vitro antioxidant activities of methanol extracts of all the three seaweeds exhibited dose dependency; and increased with increasing concentration of the extract.
Anticancer activity.
Screening results of potential antitumor promoting properties of 36 edible/common marine algae, collected in the sea near Maizuru, Kyoto, Japan, were described by Ohigashi.38 They reported that strong inhibitory activities were found in Wakame seaweed (Undaria pinnatifida) and in Laminaria and Saragassm spp. The extracts of marine algae are evaluated in a series of mechanism based anticancer screens, including protein kinase C (PKC), protein tyrosine kinase (PTK) and inosine monophosphate dehydrogenase (IMPDH). More that 501 extracts have been screened in these three assay areas, and have yielded 23 active to IMPDH, 9 to PTK. Poteriochromonas malhamensis, has yielded a novel chlorosulfolipid.14 Rashid et al. Fractionated an extract from the marine alga Laurencia majuscule which provided three brominated chamigrane sesquiterpenes.11,12 These compounds produced a novel pattern of antitumour activity against the national Cancer Institutes in vitro cell line panel. They were cytotoxic to certain cell lines in the colon cancer sub panel at concentrations 10- to 100-fold lower than the mean cytotoxic concentration observed in the other tumour sub panels. In vitro selective antitumour activity as a general screening parameter for biologically active substances from a wide range of marine algae from Kyushu, Island, Japan was carried out by Harada et al. Algae extracts prepared by phosphate buffered saline and then by methanol were tested for in vitro activity against murine lymphoid leukaemia L1210 cells and for cytotoxicity against mouse NIH-3T3 normal cell. The results revealed that some algae showed specifically strong antitumor activity with low cytotoxicity to normal cells. In particular, methanol extracts from green algae Cladophoropsis vaucheriaeformis was found to have the highest selective effect against leukemic cell lines. Deslandes et al. isolated an unusual sulfated fucan-like polysaccharide containing amino sugar constituent from the brown Fucales, Turbinaria ornate. They studied its antiproliferative effect on asynchronous cells of a human non-small-cell bronchopulmonarycarcinoma line (NSCL-N6) in vitro. The results showed the cell growth was inhibited in G1 phase. Harada et al. found that palmitic acid extract from marine red algae, Amphiroa zonata, showed selective cytotoxic activity to human leukaemic cells, but no cytotoxicty to normal human dermal fibroblast (HDF) cells in vitro. One molecular target of palmitic acid in tumor cells is DNA topoisomerase I; however, interestingly, it did not affect DNA topoisomerase II, they suggested that palmitic acid may be a lead compound of anticancer drugs. Sahara et al. reported that 3′-sulfonoquinovosyl- 1′-monoacylglycerol (A-5, 10) extracted from sea urchin intestine was effective in suppressing the growth of solid tumors. Although the major fatty acid component of A-5 was a saturated C16 acid, there were five other fatty acids, 14:0, 18:0, 14:1, 16:1 and 18:1, which constitute minor components of A-5. The same authors reported that the solid tumors showed hemorrhagic necrosis after treatment with A-5. (IC50 More than 10−5 M). Xu et al. screened 39 species of marine algae collected from the coast of China for their antitumor activities. They found that eight species Leathesia difformes, Polysiphonia urcedata, Scytosiphon lomentarius, Gloiopeliis furcata, Punctaria latifolia, Symphyocladia latiuscula, Rhodomela confervoides and Ulva pertusa have potent cytotoxic activities. More than 30 compounds were isolated and purified, and 14 bromophenols,11,12 one steroid13 and one carotene were identified. Amongst the 16 identified compounds, 7 showed vigorously selective activities against KB.-Be17402 and A549 cancer cells. Of which, six new compounds were identified as bromophenol.
Yuan and Walsh44 evaluated the effect of red alga, dulse (Palmaria palmata) and three kelp (Laminaria setchellii, Macrocystis integrifolia, Nereocystis leutkeana) extracts on human cervical adenocarcinoma cell line (HeLa cells) proliferation using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. They found that, The antiproliferative efficacy of these algal extracts were positively correlated with the total polyphenol contents, suggested a causal link related to extract content of kelp phlorotannins and dulse polyphenols including mycosporine-like amino acids (reviewed in ref. 15 and 16 and Table 3) and phenolic acids. The red alga Porphyra sp. contained sulphated polysaccharides (porphyrans) that have been showed to present potential antitumor activity through apoptotic mechanism.45 Kwon et al.46 found that the ethanolic extracts of Corallina pilulifera (EECP) showed cytotoxic activity against human cervical adenocarcinoma cell line, HeLa (IC50 = 250 µg/ml). Treatment of HeLa cells with various concentrations of EECP resulted in growth inhibition and induction of apoptosis in a dose-dependent manner. In western blot analysis, they showed that the apoptosis was associated with the release of cytochrome C from mitochondria into the cytosol, activation of caspase-3 and caspase-8, and proteolytic cleavage of PARP (poly (ADP-ribose) polymerase). Therefore; they suggested that EECP might be a potential candidate in the field of anticancer drug discovery.
Table 3.
Reported biochemical compounds from different algal species as anticancer activity
| Species | Cpd No. | Name of reported compound | Structure | References |
| Sea urchin intestine | 10 | 3-sulfonoquinovosyl-1-monoacylglycerol | 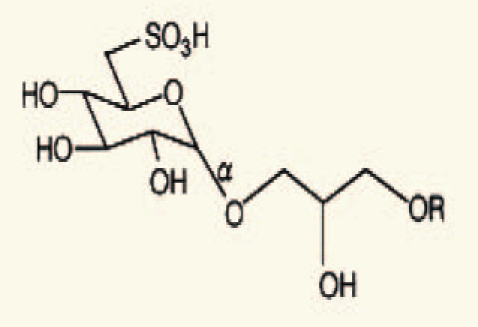 |
Sahara, et al. (2002)42 |
| Scytosiphon sp. | 11 | 3,4-dibromo-5-(ethoxymethyl) 1,2-benzenediol | 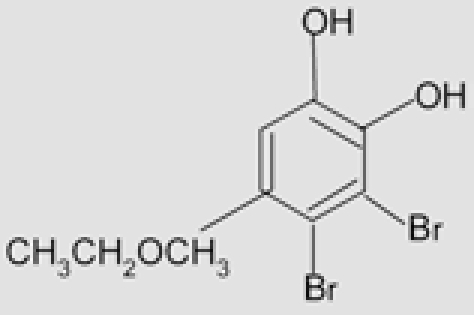 |
Xu, et al. (2004)43 |
| 12 | 2,3-dibromo-4,5dihydroxy-benzaldehyde | 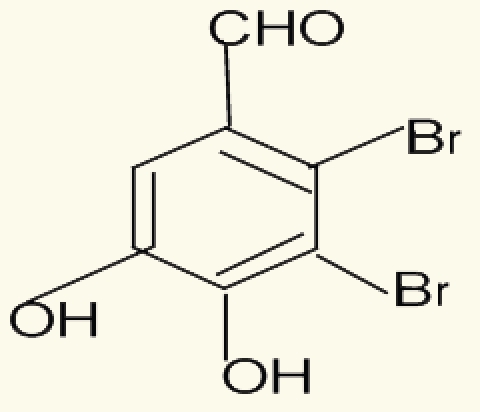 |
||
| 13 | Fucosterol | 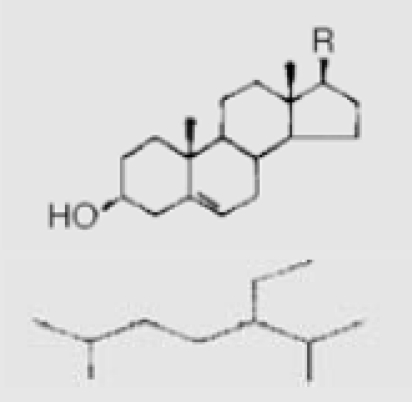 |
||
| Poteriochromomas sp. | 14 | Malhamensilipin A (IC50 = 35 µM) | 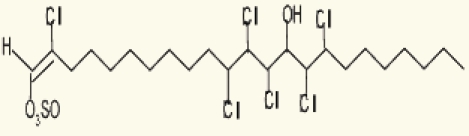 |
Gerwick, et al. (1994)90 |
| Palmaria palmata | 15 | Palythinol | 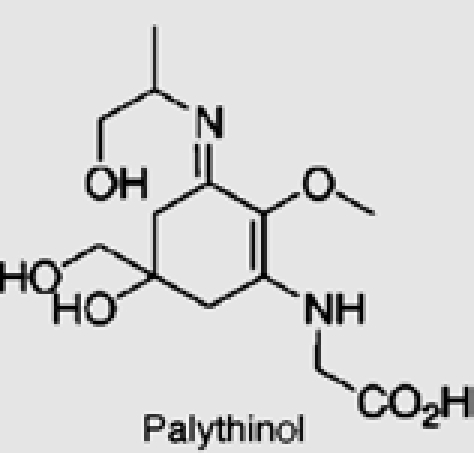 |
|
| 16 | Palythene | 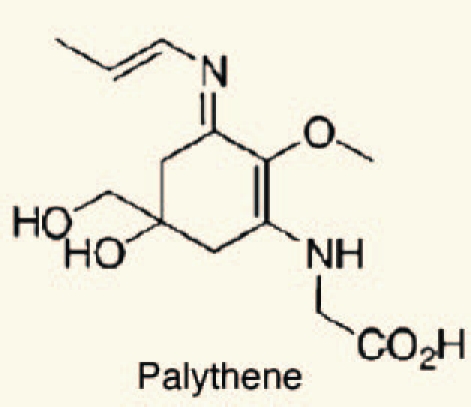 |
The polar and non-polar extracts of the brown alga Sargassum wightii (Greville) J. Agardh, collected during different seasons were evaluated for anti-inflammatory activity using carrageenaninduced paw edema in rats by Dar et al. They found that, all the extracts of winter collection exhibited significant anti-inflammatory property (80%) as compared to those of summer collection (34–59%). This difference could probably be related to seasonal variation due to nutrient availability thereby affecting synthesis of chemical constituents required for growth of the alga. Dichloromethane, ethanol and boiling water extracts of the brown seaweeds Sargassum fulvellum and Sargassum thunbergii were examined for antipyretic, analgesic and anti-inflammatory activities in mice by Kang et al. They reported that the dichloromethane extract (0.4 mg/ear) of Sargassum fulvellum inhibited an inflammatory symptom of mouse ear edema by 79.1%. These findings are consistent with various claims that these seaweeds can be used as remedies for inflammation-related symptoms.
Antimicrobial activities.
Antialgal activity. The discovery and application of natural and natural-based compounds has been tried to control harmful algae in aquatic systems as an alternative to synthetic algicides. The reported studies involve lysine and its analogs,49 ferulic acid, transcinnamic acid, anthraquinone,1,3-dichloronaphthoquinone,50 bacillamide,51 fischerellin B,52 12-epi-hapalindole F,53 oxygenated fatty acids and potassium ricinoleate,54 harmane and rutacridone epoxide.55 Decomposing barley straw has also been used to control blue-green algae.54,56 Kim et al.57 and58 found that extracts of the genus Polygonatum inhibited the growth of several freshwater algae (such as C. vulgaris, Scenedesmus sp and M. aeruginosa) as well as duckweed. The results demonstrated that L-2-azetidinecarboxylic acid (AZC) selectively inhibited algal growth at low concentrations. The allelopathic effects of three macroalgae, namely Ulva pertusa, Corallina pilulifera and Sargassum thunbergii, on the growth of the microalga Skeletonema costatum (Grev.) were evaluated by Wang et al.59 They demonstrated that the growth of S. costatum was strongly inhibited when fresh tissues, dry powder and aqueous extracts were used; (EC50 ranged from 0.45 to 3.50 g FW/L). Allelopathic effects of the green macroalgae Ulva lactuca on the growth of three species of red tide microalgae, Heterosigma akashiwo, Alexandrium tamarense and Skeletonema costatum were studied by Nan et al. They showed that U. lactuca exhibits negative allelopathic effects on harmful bloom-forming microalgae.
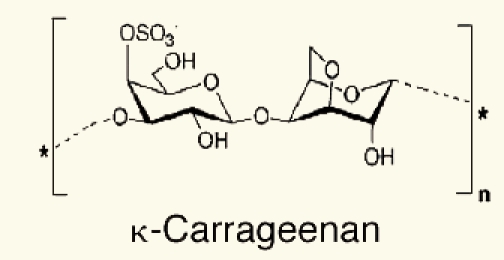
Antivirus activity. Gonzalez et al. found that carrageenan (reviewed in ref. 17 and Table 4), an algal polysaccharide, had no effect on virus attachment or penetration into host cells, but the synthesis of viral proteins inside the cells was inhibited. Similarly other sulfated algal polysaccharides selectively inhibited reverse transcriptase (RT) enzyme of human immunodeficiency virus (HIV) and its replication in vitro. Ivanova et al. Tested the extracts of air dried marine algae (Ulva lactuca), collected from beside the Bulgarian Black Sea, for their antiviral activity. They studied their effect on the reproduction of influenza virus (H1N1) in cultures of chorioallantoic membranes from 12- to 14-day-old chick embryos. The results showed an inhibitory effect on the reproduction of the virus, with reduction of the viral infectious titer. A number of biological and synthetic sulfated polyanions, such as heparin, inhibit the replication of various mammaline viruses.63 They suggested that these negatively charged molecules. Including the sulfated algal polysaccharides, exert their inhibitory effect by interacting with the positive charges on the virus or on the cell surface and thereby prevent the penetration of the virus into the host cells. Carlucci et al. reported that a sulfated galactan isolated from extracts of red algae was a selective inhibitor of herpes simplex virus (HSV-1 and HSV-2). The mode of action of sulfated galactan could be ascribed to an inhibitory action on virus adsorption as shown in Figure 2.
Table 4.
Reported biochemical compounds from different algal species as antiviral activity
Figure 2.
Influenza virus reproduction.
Nowotny et al. found that the crude aqueous extract of a strain of Microcystis aeruginosa showed antiviral activity against influenza A virus. Virus specific protein synthesis decreased if the extract was present over the whole time of replication. Protease (proteinase) inhibitory activity was estimated for the crude aqueous extracts. Zhu et al.66 suggested that the antiviral mode of action of sulphated polysaccharide (SP2) isolated from the brown alga Sargassum patens on herpes simplex virus type 2 (HSV-2) could be ascribed to the inhibition of virus adsorption, which is different from that of the current drug of choice acyclovir. The antiviral effect of the CH2Cl2/MeOH-soluble fraction from the alga Dictyota menstrualis on HIV-1 replication was evaluated in vitro by Pereira et al.67 The results indicated that the antiretroviral activity was attributed to two diterpenes compounds18,19 and these compounds were affected an early step of the virus replicative cycle.
Antibacterial activity. Thirty five seaweed species from Sri Lanka were screened for antibacterial activity (Staphylococcus aureus and Escherichia coli) and antifungal activity (Cladosporium cladosporioides and Candida albicans), by Bandara et al. They found that 26 species exhibited antibacterial and/or antifungal activity. Extract with pronounced activity were obtained from Chondrococcus hornemanni and the active component was found in a mixture containing dihalogenated monotrepenes. Acrylic acid appeared to be responsible for the antimicrobial activity of Gracilaria corticata and Ulva lactuca. Extracts from 65 different sea water and fresh water algaewere screened for in vitro antimicrobial activity by Perez et al.69 The result indicated that nine algae showed activity against Staphylococcus aureus, nine against Staphylococcus pyogenes, four against Pseudomonas aeruginosa, one against Proteus vulgaris, three against Escherichia coli, three against Aspergillus fumigatus and two against Candida albicans. The most active extracts were those of the Rhodophytae. None of the extracts from the fresh water algae showed activity against microorganisms at concentration up to 50 mg/ml. The antimicrobial activities of extracts of three fresh water green algae (Spirogyra sp., Chara sp. and Cladophora sp.) and 11 marine algae green: Enteromorpha linza, Ulva lactuca, Cladophora coelothrix and Codium tomentosum; brown: Colpomenia sinuosa and Padina pavonica; red: Gelidium sp., Laurencia obtusa, Polysiphonia sp., Hypnea musciformis and Galaxuara rugosa were screened against Escherichia coli and Staphylococcus aureus. They found that the fresh water algal extracts had the highest antimicrobial activity against both bacteria. While the marine algal were active against S. aureus only.70 n-Hexane, ethyl acetate and methanol extract of Sargassum desfontainesii, Halopteris scoparia, Stypopodium zonale, Codium intertextum and Ulva rigida (collected from the littoral of Tenerife), were screened for antibacterial and antifungal activities by Febles et al. the results indicated that the methanol extracts showed the most potent antibacterial activity, particularly against Bacillus cereus and B. subtilis. The extracts of brown and green seaweed did not exhibit antifungal properties. The methanol extract of Codium intertextum showed activity against Saccharomyces cerevisiae and three species of Candida.
Algae as plant growth stimulators.
Crouch and Staden72 revealed that, seaweed concentrate (SWC) prepared from Ecklonia maxima (Osbeck) Papenfuss, significantly improved the growth of tomato seedlings when applied as a soil drench their application as a foliar spray had no effect on young plants. In a second experiment, SWC-treated plants exhibited early fruit ripening and total fruit fresh weight increase by 17%. The number of harvested fruit was also increased by about 10%. The Ascphyllum nodosum extracts (at 0, 1, 5 and 10%) were reported to improve germination, stimulate root growth, flower production, fruit set, crop quality and increase yield as well as enhancing stress and disease resistance. Greenhouse results indicated a slight increase in cabbage and tomato plant dry weight in the 5% treatment, followed by decreases at the higher rates. Zaccaro et al.73 documented that, the algal biofertilizers were likely to assume greater significance as complement and/or supplement to chemical fertilizers in improving the nutrient supplies to cereal crops because of high nutrient turn-over in the cereal production system, exorbitant cost of fertilizers and greater consciousness on environmental protection.
Cytotoxicity activity.
Penostatins compounds A-E (reviewed in ref. 29–33 and Table 5) was isolated from a strain of Penicillium sp., originally separated from the marine alga Enteromorpha intestinalis, and all of these compounds exhibited significant cytotoxicity against cultured P388 cells (lymphocytic leukemia).74
Table 5.
Reported biochemical compounds from different algal species as cytotoxic effect
| Species | Cpd No. | Name of reported compound | Structure | References |
| Red alga Jania rubens | 20 | 16_-hydroxy-5_-cholestane-3,6-dione | 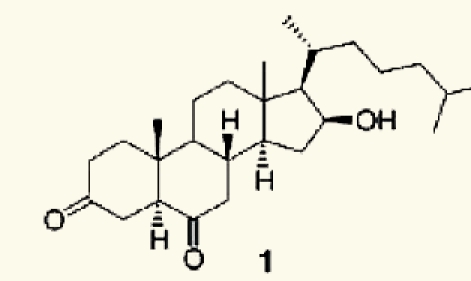 |
Denanc'e, et al. (2006)80 |
| Red alga Acantophora spicifera | 21 | 5_-cholestane-3,6-dione | 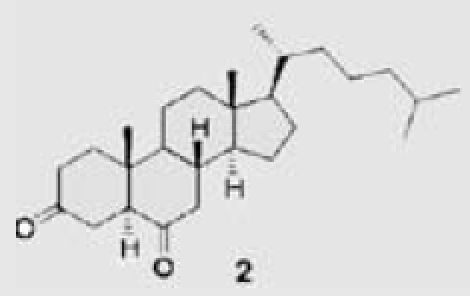 |
|
| Red alga Acantophora spicifera | 22 | 11-hydroxy-5_-cholestane-3,6-dione | 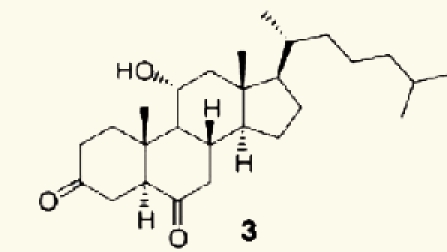 |
|
| Red alga Hypnea musciformis | 23 | 20-hydroxy-5_-cholest-22-ene-3,6-dione | 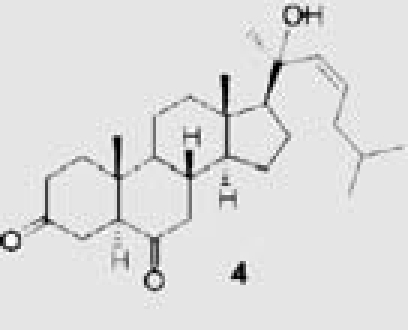 |
|
| Microcystis sp. | 24 | Microcystins | 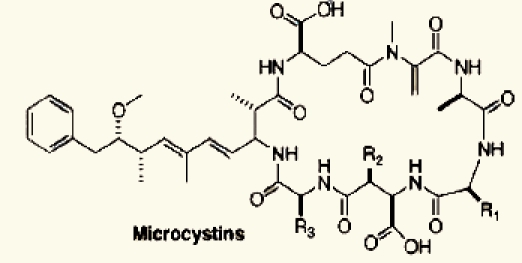 |
Codd, et al. (2005)76 |
| Nodularia sp. | 25 | Nodularins | 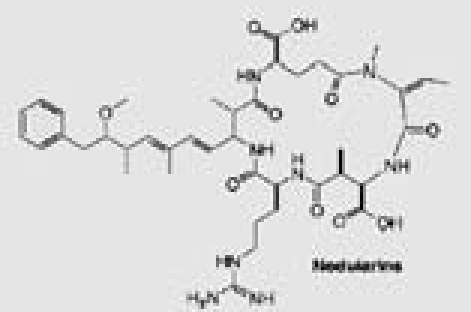 |
|
| Oscillatoria sp. | 26 | Anatoxin-a | 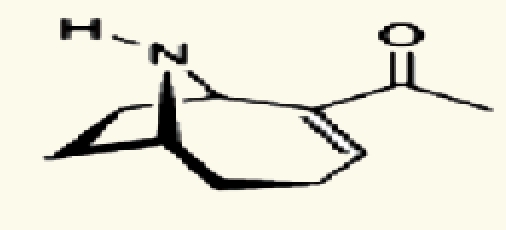 |
|
| Oscillatoria sp. | 27 | Anatoxin-a (S) | 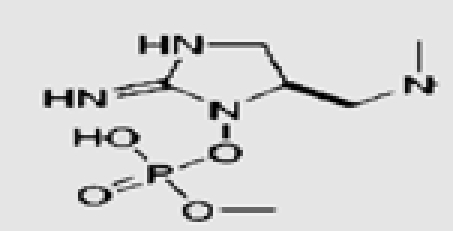 |
|
| Anabaena sp. | 28 | Cylindrospermopsin | 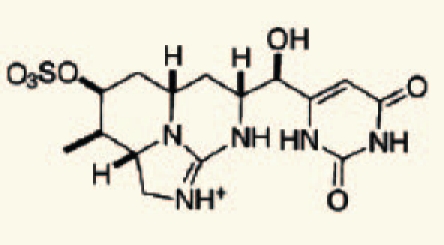 |
|
| From a Penicillium sp separated from an Enteromorpha alga | 29 | penostatins A–E | 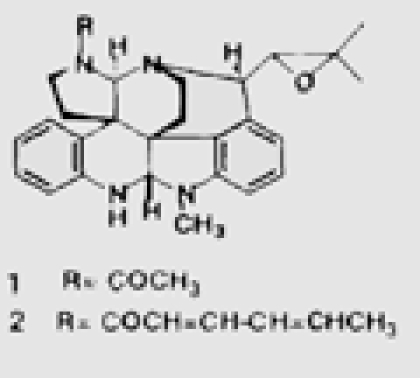 |
Iwamoto, et al. (1999)74 |
| 30 | 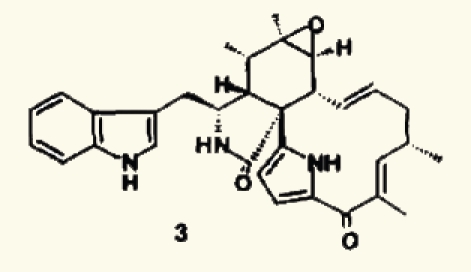 |
|||
| 31 | 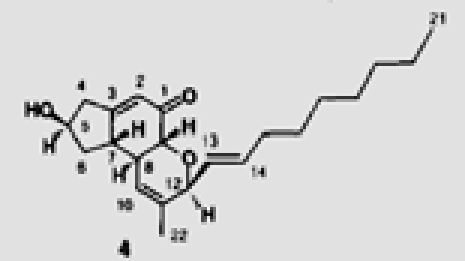 |
|||
| 32 | 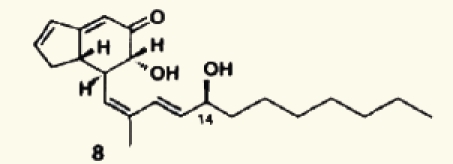 |
|||
| 33 | 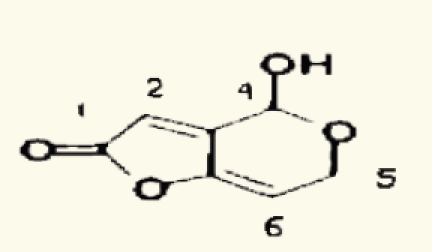 |
Marine and fresh water algal toxins are a varied group of compounds that can occur on the coast and off shore, in lakes and water reservoirs, especially in eutrophicated areas.75–77 Both marine and freshwater toxins can bioaccumulate in the food chain to very high concentration in seafood, mollusks, fish and other aquatic organisms.78 For these reasons, these compounds pose a health hazard for humans, domestic animals and wildlife with toxicological effects including neurotoxicity, hepatotoxicity, cytotoxicity and dermatotoxicity.79 Themicrocystins, cylindrospermopsin,28 homo- and anatoxin-a,26 anatoxin-a(s),27 and saxitoxins24 are the most common freshwater algae toxins and are associated with Microcystis, Anabaena, Oscillatoria and Nostoc species.76 Denance et al.80 mentioned that, a large of number of oxysterols occur as marine natural products with unusual functionalization.20–23 Among them the 16-hydroxy-5α-cholestane-3,6-dione, was isolated from the red alga Jania rubens and it exhibits a cytotoxic activity towards KB cells with an ID50 of 0.5 µg/ml.
Heavy metals and bioremoval.
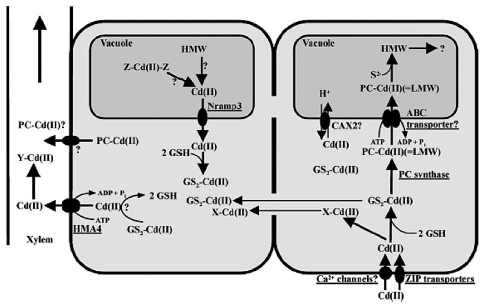
Awadalla and Pesic81 found that dead algal biomass has great biosorption capacity, probably due to the uncovering of maskedbinding sites. The two principle mechanism involved in biosorption appear to be: (1) ion exchange wherein ions such as Na, Mg and Ca become displaced by heavy metal ions and (2) complexation between metal ions and various functional groups such as carboxyl, amino, thiol, hydroxyl, phosphate and hydroxyl-carboxyl, that can interact in a coordinated way with heavy metal ions. Photosynthetic organisms have developed intra and extra cellular mechanisms for metal detoxification82 and some of them, such as formation of phytochelatins (PCs) may be used as biomarkers.83
Several mechanisms were described for heavy metal tolerance and detoxification in eukaryotes. The most important are: (a) exclusion performed by heavy. dependent ATPases; (b) chelation of heavy metals through the formation of complexes involving cysteine-rich proteins, called metallothioneins, and/or cysteinerich peptides, named phytochelatins; and (c) activation of antioxidant defenses to buffer the oxidative stress induced by heavy metals, mainly copper and iron.84 The molecular mechanisms of toxic metal (Cd) accumulation in plants and algae discussed by Clemens.85 Who reported that, as most other transition metal ions; Cd ions will preferentially bind to N and S donors. These can be functional groups in macromolecules and LMW ligands that are constitutively present or synthesized in response to Cd++ exposure. Additionally, Cd-ligand complexes can be transported into the vacuole or other organelles as show in Figure 3. Two red macroalgae species, Gracilaria cornea and Hondrophycus poiteaui, were evaluated for their intra and extra cellular Cd2+ accumulation capacity by Gracia-Rios et al.86 They reported that intracellular accumulation of Cd2+ by G. cornea was relatively low, only comprising 20% of total metal (intracellular and extra cellular). In contrast, C. poiteaui accumulated intracellularly close to 100% of total Cd2+.
Figure 3.
Main pathways of some secondary and primary metabolites biosynthesis.
A growing worldwide market for algae products.
The brown alga Undaria pinnatifida and the red alga Chondrus crispus could be used as a food supplement to help meeting the recommended daily intake of some minerals, macro elements (Na, K, Ca, Mg, ranging from 8.1 to 17.9 mg/100 g), and trace elements (Fe, Zn, Mn, Cu ranging from 5.1 to 15.2 mg/100 g) according to Ruperez.87
Algal products have been used in the food, cosmetic and pharmaceutical industries. An expanding market for these products is a fact and is facing a new challenge of growing algae on a large scale without harming any further the marine environment. Micro- and macroalgae are essential to the development of aquaculture since they provide the main micronutrients to many aquatic organisms, including vitamins, nitrogen-containing compounds, sterols, specific fatty acids, etc. Total aquaculture production in 2000 was reported to be 45.71 million metric tons (mmt) by weight, valued at $56.47 billion, with production up by 6.3% by weight and 4.8% by value since 1999.88
Use of macroalgae as fuel and its thermochemical behavior were studied by Ross et al. they reported that marine macroalgae is one such source of aquatic biomass and potentially represents a significant source of renewable energy. The average photosynthetic efficiency of aquatic biomass is 6–8%. Ash chemistryrestricts the use of macroalgae for direct combustion and gasification. Paralysis produces a range of pentosans and a significant proportion of nitrogen containing compounds. High char yields are produced.
References
- 1.El-Baroty GS, Moussa MY, Shallan MA, Ali MA, Sabh AZ, Shalaby EA. Contribution to the Aroma, Biological Activities, Minerals, Protein, Pigments and Lipid Contents of the Red Alga: Asparagopsis taxiformis (Delile) Trevisan. J App Sci Res. 2007;3:1825–1834. [Google Scholar]
- 2.Lee-Saung H, Lee-Yeon S, Jung-Sang H, Kang-Sam S, Shin-Kuk H. Antioxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch Pharm Res. 2003;26:719–722. doi: 10.1007/BF02976680. [DOI] [PubMed] [Google Scholar]
- 3.Shalaby EA, Shanab SMM, Singh V. Salt stress enhancement of antioxidant and antiviral efficiency of Spirulina platensis. J Med Plants Res. 2010;4:2622–2632. [Google Scholar]
- 4.Shalaby EAA. MSc. Thesis. Department of Biochemistry, Faculty of Agriculture, Cairo University; 2004. Chemical and biological studies on Spirulina species. [Google Scholar]
- 5.Shalaby EAA. Ph.D. Thesis. Department of Biochemistry, Faculty of Agriculture, Cairo University; 2008. Biochemical and Biotechnological studies on some marine algae. [Google Scholar]
- 6.Abou Elalla FM, Shalaby EA. Antioxidant Activity of Extract and Semi-Purified Fractions of Marine Red Macroalga, Gracilaria Verrucosa. Aust J Bas App Sci. 2009;3:3179–3185. [Google Scholar]
- 7.Shanab SM, Shalaby EA, El-Fayoumy E. Enteromorpha compressa exhibits potent antioxidant activity. J Biomed Biotech. 2011:72640. doi: 10.1155/2011/726405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hehmann A, Kaya K, Watanabe MM. Selective control of Microcystis sp using an amino acid a laboratory assay. J Appl Phycol. 2002;14:85–89. [Google Scholar]
- 9.Burja AM, Banaigs B, Abou-Mansour E, Burguess JG, Wright PC. Marine cyanobacteria-a profilic source of natural products. Tetrahedron. 2001;57:9347–9377. [Google Scholar]
- 10.Beker EW. Microalgae. Cambridge, NY: Cambridge University Press; 1994. [Google Scholar]
- 11.Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2005;22:15–61. doi: 10.1039/b415080p. [DOI] [PubMed] [Google Scholar]
- 12.Puglisi MP, Tan LT, Jensen PR, Fenical W. Capisterones A and B from the tropical green alga Penicillus capitatus: unexpected anti-fungal defenses targeting the marine pathogen Lindra thallasiae. Tetrahedron. 2004;60:7035–7039. [Google Scholar]
- 13.Barros MP, Pinto E, Sigaud-Kutner TCS, Cardozo KHM, Colepicolo P. Rhythmicity and oxidative/nitrosative stress in algae. Biol Rhythm Res. 2005;36:67–82. [Google Scholar]
- 14.Mayer AMS, Hamann MT. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis and antiviral activities; affecting the cardiovascular, immune and nervous system and other miscellaneous mechanisms of action. Mar Biotechnol. 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dos Santos MD, Guaratini T, Lopes JLC, Colepicolo P, Lopes NP. Plant cell and microalgae culture, In: Modern Biotechnology in Medicinal Chemistry and Industry. Kerala, India: Research Signpost; 2005. [Google Scholar]
- 16.McDermide K, Stuercke B. Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol. 2003;15:513–524. [Google Scholar]
- 17.Marsham S, Scott GW, Tobin ML. Comparison of nutritive chemistry of a range of temprate seaweeds. Food Chem. 2007;100:1331–1336. [Google Scholar]
- 18.Plaza M, Cifuentes A, Ibañez E. In the search of new functional food ingredients from algae. Food Sci Tech. 2008;19:31–39. [Google Scholar]
- 19.Gordon MH, Magos P. The effect of sterols on the oxidation of edible oils. Food Chem. 1983;10:141. [Google Scholar]
- 20.Anggadiredja J, Andyani R, Hayati M. Antioxidant activity of Sargassum polycystum (Phaeeophyta) and Laurencia obtus (Rhodophyta) from Seribu Island. J Appl Phycol. 1997;9:477–479. [Google Scholar]
- 21.Matsukawa R, Dubinasky Z, Kishimoto E, Masaki K, Masuda Y, Takeuchi T, et al. A comparison of screening methods for antioxidant activity in seaweeds. J Appl Phycol. 1997;9:29–35. [Google Scholar]
- 22.Jia-Zhi S, Wu-Jian M, Zheng-Hai L, Jiang, Yin B, Zhu-Hong Y. Isolation and composition analysis of fucose containing sulfated polysaccharides from the brown seaweed Sargassum fusiformis Setch J. Zhejiang Agriculture University. 1998:670–673. [Google Scholar]
- 23.Weng XC, Wang W. Antioxidant activity of compounds isolated from Salvia plebeian. Food chem. 2000;71:489–493. [Google Scholar]
- 24.Novoa V, Motidome M, Mancini-Filho J, Linares AF, Tanae MM, Torres LM, et al. Antioxidant activity related to phenolic acids in the aqueous extract of the marine seaweed Bryothamnion triquetrum (S.G. Gmelim) Howe. Revista-Brasilerra-deCiencias-Farmaceuticas. 2001:373–382. [Google Scholar]
- 25.Ruberto G, Baratta MT, Biondi DM, Amico V. Antioxidant activity of extracts of the marine algal genus Cystoseria in a micellar model system. J Appl Phycol. 2001;13:403–407. [Google Scholar]
- 26.Jimenez-Escrig A, Jimenez-Jimenez I, Pulido R, Saura-Calixto F. Antioxidant activity of fresh and processed edible seaweeds. J The Science of Food and Agriculture. 2001:530–534. [Google Scholar]
- 27.Nagai T, Ukimoto T. Preparation and functional properties of beverages made from Sea algae. Food Chem. 2003;81:327–332. [Google Scholar]
- 28.Cahyana AH, Shuto Y, Kinoshita Y. Pyropheophytin as an antioxidative substance from the marine alga, Arame (Eicenia bicyclis) Biosci Biotechnol Agrochem. 1992;56:1533–1535. [Google Scholar]
- 29.Yan XJ, Chuda Y, Suzuki M, Nagata T. Fucoxanthin as the major antioxidant in Hijikia fusiformis. Biosci Biotechnol Agrochem. 1999;63:605–607. doi: 10.1271/bbb.63.605. [DOI] [PubMed] [Google Scholar]
- 30.Mori J, Matsunaga T, Takahashi S, Hasegawa C, Saito H. Inhibition activity on lipid peroxidation of extracts from marine brown alga. Phytother Res. 2003;17:549–551. doi: 10.1002/ptr.1194. [DOI] [PubMed] [Google Scholar]
- 31.Raymundo MS, Horta P, Fett R. Antioxidant in vitro activity of extracts of some green seaweed (Chlorophyta) from Southern Brazilian coast. Revista-Brasileira-de-Ciencias-Farmaceuticas. 2004;40:495–503. [Google Scholar]
- 32.Maehira F, Motomura K, Nashiro T, Mekaru N, Sudo Y. Effects of red algae cultivated with deep-sea water on the oxidation-reduction status of liver, lung, brain and bone metabolism in SAMP1 and SAMR1. International Congress Series. 2004;1260:413–416. [Google Scholar]
- 33.Heo S, Park E, Lee K, Jeon Y. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour Technol. 2005;96:1613–1623. doi: 10.1016/j.biortech.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Jang-Kyoung H, Lee-Bong H, Choi-Byoung W, Lee-Hyi S, Shin-Jong H. Chromenes from the brown alga Sargassum siliquastrum. J Natural products. 2005;68:719–723. doi: 10.1021/np058003i. [DOI] [PubMed] [Google Scholar]
- 35.Nahas R, Abatis D, Anagnostopoulou MA, Kefalas P, Vagias C, Roussis V. Radical-scavenging activity of Aegean Sea marine algae. Food Chem. 2007;102:577–581. [Google Scholar]
- 36.Chew YL, Lim YY, Omar M, Khoo KS. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT. 2008;41:1067–1072. [Google Scholar]
- 37.Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol. 2008;99:2717–2723. doi: 10.1016/j.biortech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Ohigashi H, Sakai Y, Yamaguchi K, Umezaki I, Koshimizu K. Possible anti tumor promoting properties of marine algae and in vivo activity of Wakama seaweed extract. Biosci Biotechnol Biochem. 1992;56:994–995. doi: 10.1271/bbb.56.994. [DOI] [PubMed] [Google Scholar]
- 39.Rashid MA, Gustafson KR, Cardellina JHH. Brominated chamigrane sesquiterpene produce a noval profile of differentional cytotoxicity in the NCI in vitro screen. Nat Prod Lett. 1995;6:255–259. [Google Scholar]
- 40.Harada H, Yamashita U, Kurihara H, Fukushi E, Kawabata J, Kamei Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in marine red algae. Anticanc Res. 2002;22:2587–2590. [PubMed] [Google Scholar]
- 41.Deslandes E, Pondaven P, Auperin T, Rossakis C, Guézennec J, Stiger V, et al. Preliminary study of the in vitro antiproliferative effect of a hydroethanolic extract from the subtropical seaweed Turbinaria ornata (Turner J. Agardh) on a human non-small-cell bronchopulmonary carcinoma (NSCLC-N6) J Appl Phycol. 2000;12:257–262. [Google Scholar]
- 42.Sahara H, Hanashima S, Yamazaki T, Takahashi S, Ohtani S, Ishikawa M, et al. Anti-tumor effect of chemically synthesized sulfolipids based on Sea Urchin's natural sulfonoquinovosylmonoacylglycerols. Jpn J Cancer Res. 2002;93:85–92. doi: 10.1111/j.1349-7006.2002.tb01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu N, Fan X, Yan X, Tseng CK. Screening marine algae from China for their antitumor activities. J Appl Phycol. 2004;16:451–456. [Google Scholar]
- 44.Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxicol. 2006;44:1144–1150. doi: 10.1016/j.fct.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Kwon MJ, Nam TJ. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006;79:1956–1962. doi: 10.1016/j.lfs.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 46.Kwon H, Bae S, Kim K, Han C, Cho S, Nam S, et al. Induction of apoptosis in HeLa cells by ethanolic extract of Corallina pilulifera. Food Chem. 2007;1:196–201. [Google Scholar]
- 47.Dar A, Baig HS, Saifullah SM, Ahmad VU, Yasmeen S, Nizamuddin M. Effect of seasonal variation on the anti-inflammatory activity of Sargassum wightii growing on the N. Arabian Sea coast of Pakistan J. Exp Mar Biol Ecol. 2007;351:1–9. [Google Scholar]
- 48.Kang JY, Khan MNA, Park NH, Cho JY, Lee MC, Fujii H, et al. Antipyretic, analgesic and anti-inflammatory activities of the seaweed Sargassum fulvellumand Sargassum thunbergii in mice. J Ethnopharmacol. 2008;116:187–190. doi: 10.1016/j.jep.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 49.Hehmann A, Kaya K, Watanabe MM. Selective control of Microcystis sp using an amino acid a laboratory assay. J Appl Phycol. 2002;14:85–89. [Google Scholar]
- 50.Schrader KK, de Regt MQ, Tidwell PD, Tucker CS, Duke SO. Selective growth inhibitions of the mustyodor producing cyanobacterium Oscillatoria cf. chalybea by natural compounds. Bull Environ. Contam Toxicol. 1998;60:651–658. doi: 10.1007/s001289900676. [DOI] [PubMed] [Google Scholar]
- 51.Jeong SY, Ishida K, Ito Y, Okada S, Murakami M. Bacillamide, a novel algicide from the marine bacterium, Bacillus sp. SY-1 against the harmful dinoflagellate, Cochlodinium polykrikoides. Tetrahedron Lett. 2003;44:8005–8007. [Google Scholar]
- 52.Papke U, Gross EM, Francke W. Isolation, identification and determination of the absolute configuration of Fischerellin B. A new algicide from the freshwater cyanobacterium Fischerella muscicola (Thuret) Tetrahedron Lett. 1997;38:379–382. [Google Scholar]
- 53.Etchegaray A, Rabello E, Dieckmann R, Moon DH, Fiore MF, Dohren H, et al. Algicide production by the filamentous cyanobacterium Fischerella sp. CENA 19. J Appl Phycol. 2004;16:237–243. [Google Scholar]
- 54.Duke SO, Dayan FE, Rimando AM, Schrader KK, Aliotta G, Oliva A, et al. Chemicals from nature for weed management. Weed Sci. 2002;50:138–151. [Google Scholar]
- 55.Meepagala KM, Schrader KK, Wedge DE, Duke SO. Algicidal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs. Phytochemistry. 2005;66:2689–2695. doi: 10.1016/j.phytochem.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 56.Schrader KK, de Regt MQ, Tidwell PD, Tucker CS, Duke SO. Selective growth inhibitions of the mustyodor producing cyanobacterium Oscillatoria cf. chalybea by natural compounds. Bull Environ Contam Toxicol. 1998;60:651–658. doi: 10.1007/s001289900676. [DOI] [PubMed] [Google Scholar]
- 57.Kim JS, Hong KS, Lee BH, Kim JC, Cho KW. Herbicidal activities of Polygonatum spp. and their extracts against duckweed, freshwater algae and several paddy weeds. Kor J Weed Sci. 2005;25:103–111. [Google Scholar]
- 58.Kim JS, Kim J, Lee S, Lee B, Cho KY. Biological activity of L-2-azetidinecarboxylic acid, isolated from Polygonatum odoratum var. pluriflorum, against several algae. Aquat Bot. 2006;85:1–6. [Google Scholar]
- 59.Wang H, Liu Y, Gao X, Carter CL, Liu Z. The recombinant b subunit of C-phycocyanin inhibits cell proliferation and induces apoptosis. Cancer Lett. 2007;247:150–158. doi: 10.1016/j.canlet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Nan C, Zhang H, Lin S, Zhao G, Liu X. Allelopathic effects of Ulva lactuca on selected species of harmful bloom-forming microalgae in laboratory cultures. Aquat Bot. 2008;89:9–15. [Google Scholar]
- 61.Gonzalez M, Alarcon B, Carrasco L. Polysaccharides as antiviral agents: antiviral of carragenan. Antimicrob. Agents Chemother. 1987;31:1388–1393. doi: 10.1128/aac.31.9.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivanova V, Rouseva R, Kolarova M, Serkedjieva J, Rachev R, Manolova N. Isolation of a polysaccharide with antiviral effect from Ulva lactuca. Prep Biochem. 1991;24:83–97. doi: 10.1080/10826069408010084. [DOI] [PubMed] [Google Scholar]
- 63.Witvrouw M, De Clercq E. Sulfated polysaccharide extracted from Sea algae as potential antiviral drugs. Gen Pharmac. 1997;29:497–511. doi: 10.1016/s0306-3623(96)00563-0. [DOI] [PubMed] [Google Scholar]
- 64.Carlucci MJ, Pujol CA, Ciancia M, Noseda MD, Matulewiez MC, Damonte EB, et al. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: correlation between structure and biological activity. Int J Biol Macromol. 1997;20:97–105. doi: 10.1016/s0141-8130(96)01145-2. [DOI] [PubMed] [Google Scholar]
- 65.Nowotny N, Bascunana CR, Ballagi-Pordany A, Gavier-Widen D, Uhlen M, Betak S. Phylogenetic analysis of rabbit haemorrhagic disease and European brown hare syndrome viruses by comparison of sequences from capsid protein gene. Arch Virol. 1997;142:657–673. doi: 10.1007/s007050050109. [DOI] [PubMed] [Google Scholar]
- 68.Bandara BMR, Gunatilaka AAL, Kumer NS, Wimalasiri WR. Antimicrobial activity of some marine algae of Sri Lanka J. The National Science Council of Sri Lanka. 1988;16:209–221. [Google Scholar]
- 69.Perez GRM, Avila AJG, Perez GS, Martinez CA, Martinez CG. Antimicrobial activity of some American algae. J Ethnopharmacol. 1990;29:111–116. doi: 10.1016/0378-8741(90)90104-2. [DOI] [PubMed] [Google Scholar]
- 70.Mesmar MN, Abussaud M. The antibiotic activity of some aquatic plants and algal extracts from Jordan. Qater University Science J. 1991;11:155–160. [Google Scholar]
- 71.Febles CI, Arias A, Hardisson A, Sierra-Lopez A, Gil-Rodriguez MC. Antimicrobial activity of extracts from some Canary species of Phaeophyta and Chlorophyta. Phytother Res. 1995;9:385–387. [Google Scholar]
- 72.Crouch IJ, Staden V. Effect of seaweed concentrates on the establishment and yield of greenhouse tomato plants. J Appl Phycol. 1992;4:291–296. [Google Scholar]
- 73.Zaccro MC, Salazer C, Caire Z, Cans S, Stella AM. Lead toxicity in cyanobacteria porphyrin metaboilsm. Environ. Toxicol Water Qual. 2001;16:61–67. [PubMed] [Google Scholar]
- 74.Iwamoto C, Minoura K, Oka T, Ohta T, Hagishita S, Numata A. Absolute stereostructures of novel cytotoxic metabolites, penostatins A-E, from a Penicillium species separated from an Enteromorpha alga. Tetrahedron. 1999;55:14353–14368. [Google Scholar]
- 75.Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, et al. Literature review of Florida red tide: implications for human health effects. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Codd GA, Morrison LF, Metcalf JS. Cyanobacterial toxins: risk management for health protection. Toxicol Appl Pharmacol. 2005;203:264–272. doi: 10.1016/j.taap.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 77.Bittencourt-Oliveira MC, Kujbida P, Cardozo KHM, Carvalho VM, Moura AN, Colepicolo P, et al. A novel rhythm of microcystin biosynthesis is described in the cyanobacterium Microcystis panniformis. Biochem Biophys Res Commun. 2005;326:687–694. doi: 10.1016/j.bbrc.2004.11.091. [DOI] [PubMed] [Google Scholar]
- 78.Cazenave J, Wunderlin DA, Bistoni MDL, Ame MV, Krause E, Pflugmacher S, et al. Uptake, tissue distribution and accumulation of microcystin-RR in Corydoras paleatus, Jenynsiamultidentata and Odontesthesbonariensis a field and laboratory study. Aquat Toxicol. 2005;75:178–190. doi: 10.1016/j.aquatox.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 79.Kujbida P, Hatanaka E, Campa A, Colepicolo P, Pinto E. Effects of microcystins on human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 2006;341:273–277. doi: 10.1016/j.bbrc.2005.12.177. [DOI] [PubMed] [Google Scholar]
- 80.Denance M, Guyot M, Samadi M. Short synthesis of 16-hydroxy-5-cholestane-3,-dione a novel cytotoxic marine oxysterol. Steroids. 2006;71:599–602. doi: 10.1016/j.steroids.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 81.Awadalla FT, Pesic B. Biosorption of cobalt with the AMTTm metal removing agent. Hdrometallurgy. 1992;28:65–80. [Google Scholar]
- 82.Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp. Bot. 2002;53:1–11. [PubMed] [Google Scholar]
- 83.Ferrat L, Pergent-Martini C, Roméo M. Assessment of the use of biomarkers in aquatic plants for the evaluation of environmental quality: application to seagrasses. Aquat Toxicol. 2003;65:187–204. doi: 10.1016/s0166-445x(03)00133-4. [DOI] [PubMed] [Google Scholar]
- 84.Moenne A. Eucaryotic metallothioneins: protein structure, gene regulation and copper homeostasis. Cahiers Biologie Marine. 2001;42:125–135. [Google Scholar]
- 85.Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimic. 2006;88:1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 86.García-Ríos V, Freile-Pelegrín Y, Robledo D, Mendoza-Cózatl D, Moreno-Sánchez R, Gold-Bouchot G. Cell wall composition affects Cd2+ accumulation and intracellular thiol peptides in marine red algae. Aquatic Toxicology. 2007;81:166–170. doi: 10.1016/j.aquatox.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Ruperez P. Mineral content of edible marine seaweeds. Food Chemistry. 2002;79:23–26. [Google Scholar]
- 88.Cardozo KHM, Guaratini T, Barros MP, Falcao VR, Tonon AP, Lopes NP, et al. Metabolities from algae with economical impact. Comparative Biochemistry and Physiology. 2006;146:60–78. doi: 10.1016/j.cbpc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 89.Ross AB, Jones JM, Kubacki ML, Bridgeman L. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresource Technology. 2008;99:6494–6504. doi: 10.1016/j.biortech.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 90.Gerwick WH, Roberts MA, Proteau PJ, Chen J. Screening cultured marine microalgae for anticancer-type activity. J Appl Phycol. 1994;6:143–149. [Google Scholar]
- 91.Pereire HS, Leao-Ferreire LR, Moussatche N, Teixeira VL, Cavacanti DN, Costa LJ, Diaz R, Frugulhetti IC PP. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1) Antiviral Res. 2004;64:69–76. doi: 10.1016/j.antiviral.2004.06.006. [DOI] [PubMed] [Google Scholar]