Abstract
Using freeze-fracture electron microscopy we have recently shown that non-photochemical quenching (NPQ), a mechanism of photoprotective energy dissipation in higher plant chloroplasts, involves a reorganization of the pigment-protein complexes within the stacked grana thylakoids.1 Photosystem II light harvesting complexes (LHCII) are reorganized in response to the amplitude of the light driven transmembrane proton gradient (ΔpH) leading to their dissociation from photosystem II reaction centers and their aggregation within the membrane.1 This reorganization of the PSII-LHCII macrostructure was found to be enhanced by the formation of zeaxanthin and was associated with changes in the mobility of the pigment-protein complexes therein.1 We suspected that the structural changes we observed were linked to the ΔpH-induced changes in thylakoid membrane thickness that were first observed by Murikami and Packer.2,3 Here using thin-section electron microscopy we show that the changes in thylakoid membrane thickness do not correlate with ΔpH per se but rather the amplitude of NPQ and is thus affected by the de-epoxidation of the LHCII bound xanthophyll violaxanthin to zeaxanthin. We thus suggest that the change in thylakoid membrane thickness occurring during NPQ reflects the conformational change within LHCII proteins brought about by their protonation and aggregation within the membrane.
Key words: nonphotochemical quenching, photoprotection, LHCII, photosystem II, thylakoid membrane
NPQ is Associated with Ultrastructural Changes in Grana Membrane Organization
The ultrastructural changes in the thylakoid membrane accompanying NPQ formation were studied by thin-section electron microscopy on intact spinach chloroplasts, prepared in a range of states. The aim was to see if the changes reported by Murakami and Packer2,3 related to xanthophyll cycle activity and NPQ or only to ΔpH formation. For this, spinach chloroplasts were harvested from either dark-adapted leaves (Vio) or leaves preilluminated to accumulate zeaxanthin (Zea) (Table 1). In Zea chloroplasts an NPQ amplitude of 2.1 ± 0.1 was obtained following 5 min illumination, while in Vio chloroplasts the NPQ amplitude was 0.6 ± 0.1. The level of 9-aminoacridine (9-AA) quenching confirmed that the amplitude of ΔpH was the same in each type of chloroplast (Fig. 1A). Samples of chloroplasts were either taken immediately after 5 min of illumination (Light) or following a further 5 min period of darkness (Dark) to allow relaxation of the ΔpH dependent component of NPQ (qE). Four samples of chloroplasts were thus obtained for thin-section analysis hereafter referred to as Dark Vio, Light Vio, Dark Zea and Light Zea. The ultrastructure of the thylakoid membrane within the intact chloroplasts of each of the four samples was quite different (Fig. 1B–E). In the Dark Vio and Dark Zea chloroplasts the lumen of the thylakoid membranes was swollen meaning large spaces were present between the pairs of stacked membranes (Fig. 1B and D, arrows), as observed previously in reference 2–4. In Light Vio and Light Zea chloroplasts fixed with the same procedure, the volume of the thylakoid lumen was much less, resulting in a decreased spacing between thylakoid membranes (Fig. 1C and E). While there are inevitably dehydration artifacts associated with the chemical fixation used which give rise to the non-uniform lumen thickness observed in each sample the qualitative differences between dark and light samples treated in the same way is clear. Indeed, we note that the study of Pfeiffer and Krupinska using high-pressure freezing and freeze substitution also showed that the thylakoid lumen is swollen in dark adapted chloroplasts compared to those that are light treated.4 To confirm this change was reproducible measurements were made on 10 grana within each of 10 separate micrographs from three different fixation experiments, the data are shown in Figure 2A. The apparent lumen thickness is reduced by ∼30% by illumination in both Light Zea and Light Vio chloroplasts compared to Dark Vio and Dark Zea chloroplasts that were allowed a further 5 min of darkness prior top fixation (Fig. 2A). When chloroplasts were illuminated for 5 min in the presence of the uncoupler nigericin (Light + nigericin) and then immediately fixed no change in apparent lumen thickness was observed confirming that the change required the presence of ΔpH (Fig. 2A). No significant differences however were observed between Light Vio and Light Zea chloroplasts indicating that the change in lumen thickness was not directly related to NPQ.
Table 1.
Pigment composition and NPQ values for intact spinach chloroplasts
| Sample | Neo | Lut | XC | DEPs% | chl a/b | NPQ |
| Dark Vio | 45 ± 2.8 | 122 ± 3 | 50 ± 2.8 | 0 | 3.5 ± 0.1 | 0 |
| Light Vio | 44 ± 1.6 | 122 ± 2 | 49 ± 1.1 | 0 | 3.4 ± 0.09 | 0.6 ± 0.1* |
| Light Zea | 45 ± 1.1 | 121 ± 2 | 49 ± 1.3 | 35 ± 1.3* | 3.5 ± 0.1 | 2.1 ± 0.1* |
| Dark Zea | 46 ± 0.8 | 120 ± 3 | 49 ± 2.1 | 36 ± 3.7* | 3.5 ± 0.07 | 0.3 ± 0.03* |
| Light + nigericin | 44 ± 2.4 | 121 ± 3 | 50 ± 2.3 | 0 | 3.4 ± 0.1 | 0 |
Chloroplasts devoid of zeaxanthin and antheraxanthin (Vio) and chloroplasts enriched in zeaxanthin (Zea) were light treated for 5 min at 350 µmol photons m−2 s−1 to form NPQ and then either immediately frozen for pigment analysis (Light Vio and Light Zea chloroplasts) or given a further 5 min of darkness to allow NPQ to relax prior to freezing for pigment analysis. A separate sample of Vio chloroplasts were light treated at 350 µmol photons m−2 s−1 for 5 min in the presence of 2 µM nigericin (Light + nigericin). Data are expressed as mmoles carotenoids per mole chlorophyll a + b molecules and are means ± S.E.M. from four replicates. Neo, Lut, XC, DEPs and chl a/b, NPQ: correspond to neoxanthin, lutein, xanthophyll cycle carotenoids (violaxanthin, antheraxanthin, zeaxanthin), xanthophyll cycle de-epoxidation state [(Z + 0.5A)/(V + A + Z)]%, chlorophyll a/b ratio, the amount non-photochemical quenching in each sample as calculated from chlorophyll fluorescence traces as shown in Figure 1. Statistical confidence levels *indicates significantly different with respect to dark adapted sample p < 0.01, using an AN OVA analysis (Tukey contrast).
Figure 1.
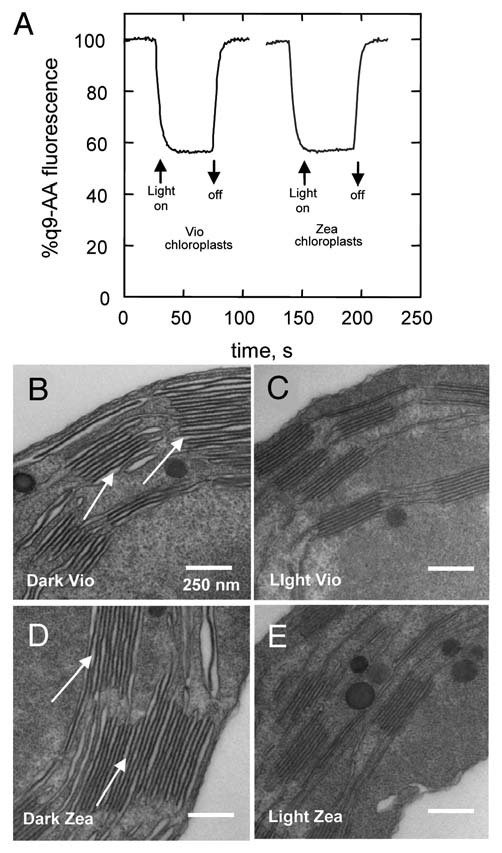
(A) Comparison of 9-aminoacridine quenching, reflecting amplitude of ΔpH, in Zea and Vio chloroplasts upon 350 µmolphotons m−2 s−1 light treatment. ΔpH was determined from the measurement of 9-aminoacridine fluorescence using a Jobin Yvon FluoroMax-3 spectrophotometer. Intact chloroplasts were illuminated in the presence of 1 µM 9-aminoacridine. Excitation was defined at 400 nm with a 2 nm spectral bandwidth, fluorescence emission was filtered using a Corning 4–96 filter and an OCLl Cyan T400-570 mirror and detected at 456 nm with a 5 nm slit width. The intensity of the 635 nm LED light used to induce ΔpH was 350 µmol photons m−2 s−1. The fluorescence kinetic integration time was 0.5 s. Thin-section electron micrographs of (B) Dark Vio, (C) Light Vio, (D) Dark Zea and (E) Light Zea intact spinach chloroplasts. For explanation of arrows see text. Chloroplasts were fixed in 0.45 M Sorbitol, 5 mM MgCl2, 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.2, post-fixed in 0.45 M Sorbitol, 5 mM MgCl2, osmium tetroxide in 0.1 M sodium cacodylate buffer pH 7.2 and embedded in TAA B resin. Ultrathin-sections (∼70 nm thickness) were cut and stained with uranyl acetate and lead citrate. Thin-sections were examined with a Hitachi H7600 or FEI Tecnai 12 BioTWIN electron microscope at a range of magnifications.
Figure 2.
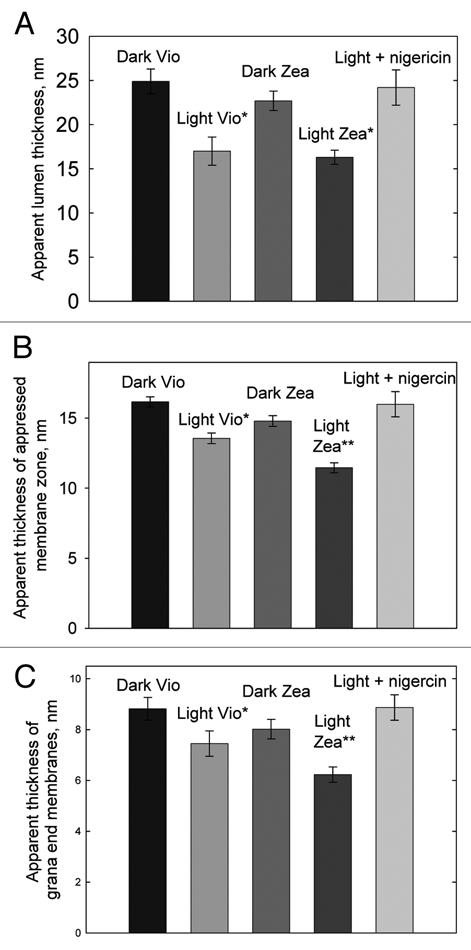
Analysis of thin-section electron micrographs of intact spinach chloroplasts. (A) Apparent lumen thickness, (B) apparent thickness of appressed membrane zone and (C) apparent thickness of grana end membranes in Dark Vio, Light Vio, Dark Zea, Light Zea and Light + nigericin intact spinach chloroplasts determined from measurements of 10 grana within each of 10 separate micrographs from three different fixation experiments ±SEM %, *significantly different from Dark Vio sample, **significantly different from Dark Vio, Dark Zea and Light Vio samples (Anova analysis, Tukey contrast, 99% confidence). Analysis of thin-section images of thylakoid grana membranes within intact chloroplasts was performed using ImagePro Plus software (Media Cybernetics, Bethesda, MA ) with about 0.72 nm accuracy, which is the spatial resolution (single pixel size in XY direction) of our electron micrographs.
In addition to measuring the apparent lumen thickness in the four chloroplast samples we also measured the apparent thickness of the appressed membrane zone (i.e., the apparent thickness of two stacked grana membranes and the stromal space in between). As with the apparent lumen thickness illumination resulted in a reduction in the apparent thickness of the appressed membrane zone in Light Vio and Light Zea chloroplast compared to Dark Vio and Dark Zea chloroplasts (Fig. 2B). In contrast to apparent lumen thickness however the thickness of the appressed membrane zone was significantly different in the Light Zea and Light Vio chloroplasts (Fig. 2B). In the Light Zea chloroplasts the thylakoid membrane thickness was reduced by 16% compared to Light Vio chloroplasts and by as much as 30% compared to the Dark Vio sample (Fig. 2B).
Thylakoid membranes are stacked by electrostatic screening of negatively charged amino acid side-chains by Mg2+ cations allowing the stromal side of one PSII containing membrane to interact with the one above.5 Thus, the decrease in membrane thickness either implies a decrease in the stromal space between the two membranes or a decrease in the actual vertical dimension of each individual membrane. While, the exact molecular composition of the grana end membranes and the grana core membranes varies slightly we felt that measurements of the membrane thickness of the single end membranes could give an indication of whether the membrane thickness is actually reduced by NPQ. The data confirmed that indeed the single grana end membranes also become thinner in response to ΔpH and moreover differences were observed between the Light Zea and Light Vio chloroplasts (Fig. 2C). Thus, de-epoxidation of violaxanthin to zeaxanthin is associated with an enhancement of the structural change occurring within the membrane that accompanies NPQ.
Relationship between the Ultrastructural Changes of the Grana Membranes and the Reorganization of PSII-LHCII
In the present study we have shown that the change in membrane thickness is correlated with the extent of NPQ rather than ΔpH per se, since the thickness of Light Zea membranes was significantly less than in Light Vio membranes despite identical levels of ΔpH in the two types of chloroplasts (Fig. 3A). The extent of the change in membrane thickness can also be linearly correlated with the extent of clustering of PSII and LHCII in freeze fracture replicas from the four types of chloroplast reported in our previous study (Fig. 3B).1 Thus, as we speculated the data suggest that the two phenomena may have a common origin. According to Daum et al. the thylakoid grana membrane is approximately 40 Å thick as determined by cryo-EM data,6 whereas we measure a thickness of single end membranes at ∼60–80 Å. It is likely that this difference in apparent thickness of the membrane is due to the fact that the thin-section rarely runs exactly perpendicular to the membrane plane and thus likely produces a systematic error in our measurements of membrane thickness. However, since all our samples were fixed in an identical way and multiple measurements on the relative differences are still informative about the nature of the change and the effects of NPQ and ΔpH upon it. Thus, the notion of a change in membrane properties upon protonation is established. Previously it has been demonstrated using LD spectroscopy that upon qE formation in vivo and upon LHCII aggregation in vitro that the xanthophylls adopt a more parallel orientation with respect to the plane of the membrane.7 We suggest that this “condensed” state of LHCII formed upon its aggregation may be the origin of the reduction in membrane thickness associated with qE. The fact that zeaxanthin enhanced the reduction in membrane thickness despite identical levels of ΔpH in the two types of chloroplasts is consistent with its ability to promote proton binding to LHCII (thus shifting the lumen pH versus qE titration curve to more alkaline values).8 In contrast, the differences observed in lumen thickness upon ΔpH formation, were similar in chloroplasts with and without zeaxanthin indicating they are unrelated to NPQ. This conclusion is consistent with the work of Murakami and Packer that showed that the change in spacing was related to purely osmotic effects, while the change in membrane thickness was only brought about by protonation.2,3 In contrast to our data there is a report that light treatment caused swelling of the thylakoid lumen in the ac46 ATP synthase mutant of Chlamydomonas reinhardtii, which suffers from photoinhibition and loss of PSII reaction centers in even moderate light. However, we note that in the wild-type cells identically treated did not undergo lumen swelling.9 Indeed, lumen swelling has previously been found to be associated with photoinhibition rather than NPQ.10 Our own freeze-fracture electron microscopy together with fluorescence recovery after photobleaching studies has shown that photoinhibition and NPQ have different effects on both PSII clustering and chlorophyll-protein mobility in intact chloroplasts.11
Figure 3.
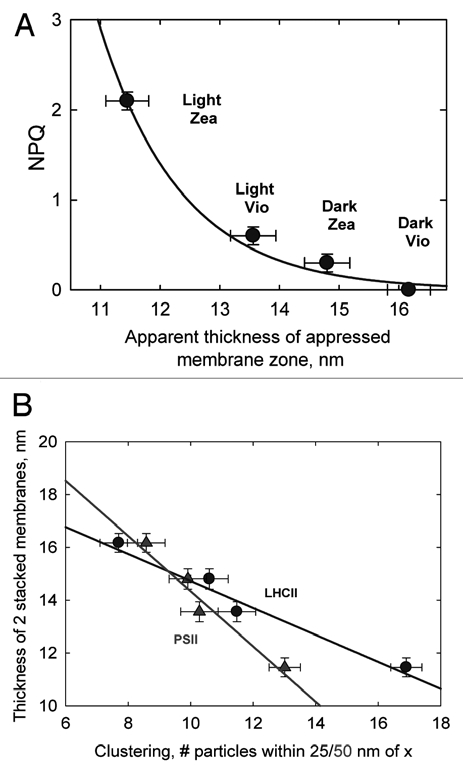
(A) Relationship between apparent thickness of appressed membrane zone and NPQ amplitude in Dark Vio, Light Vio, Dark Zea and Light Zea intact spinach chloroplasts. (B) Relationships between PSII (triangles) and LHCII (circles) particle clustering in freeze-fracture electron micrographs reported in Johnson et al.1 and the thickness of thickness of appressed membrane zone.
References
- 1.Johnson MP, Goral TK, Duffy CD, Brain AP, Mullineaux CW, Ruban AV. Photoprotective energy dissipation involves the reorganization of photosystem II light harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell. 2011;23:1468–1479. doi: 10.1105/tpc.110.081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami S, Packer L. Light-induced changes in the conformation and configuration of the thylakoid membrane of Ulva and Porphyra chloroplasts in vivo. Plant Physiol. 1970;45:289–299. doi: 10.1104/pp.45.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murakami S, Packer L. Protonation and Chloroplast Membrane Structure. J Cell Biol. 1970;47:332–351. doi: 10.1083/jcb.47.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeiffer S, Krupinska K. New Insights in Thylakoid Membrane Organization. Plant Cell Physiol. 2005;46:1441–1451. doi: 10.1093/pcp/pci156. [DOI] [PubMed] [Google Scholar]
- 5.Dekker JP, Boekema EJ. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim Biophys Acta. 2005;1706:12–39. doi: 10.1016/j.bbabio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Daum B, NiCastro D, Austin J, II, McIntosh JR, Kühlbrandt W. Arrangement of Photosystem II and ATP Synthase in Chloroplast Membranes of Spinach and Pea. Plant Cell. 2010;22:1299–1312. doi: 10.1105/tpc.109.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruban AV, Calkoen F, Kwa SLS, van Grondelle R, Horton P, Dekker JP. Characterisation of LHCII in the aggregated state by linear and circular dichroism spectroscopy. Biochim Biophys Acta. 1997;1321:61–70. [Google Scholar]
- 8.Ruban AV, Johnson MP, Duffy CDP. Photoprotective molecular switch in photosystem II. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbabio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Majeran W, Olive J, Drapier D, Vallon O, Wollman FA. The light sensitivity of ATP synthase mutants of Chlamydomonas reinhardtii. Plant Physiol. 2001;126:421–433. doi: 10.1104/pp.126.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topf J, Gong H, Timberg R, Mets L, Ohad I. Thylakoid membrane energization and swelling in photo-inhibited Chlamydomonas cells is prevented in mutants unable to perform cyclic electron flow. Photosynth Res. 1992;32:59–69. doi: 10.1007/BF00028798. [DOI] [PubMed] [Google Scholar]
- 11.Goral TK, Johnson MP, Brain APR, Kirchhoff H, Ruban AV, Mullineaux CW. Visualising the mobility and distribution of chlorophyll-proteins in higher plant thylakoid membranes: effects of photo-inhibition and protein phosphorylation. Plant J. 2010;64:948–959. doi: 10.1111/j.0960-7412.2010.04207.x. [DOI] [PubMed] [Google Scholar]


