Abstract
This review highlights a potential signaling pathway of CO2-dependent stimulation in root hair development. Elevated CO2 firstly increases the carbohydrates production, which triggers the auxin or ethylene responsive signal transduction pathways and subsequently stimulates the generation of intracellular nitric oxide (NO). The NO acts on target Ca2+ and ion channels and induces activation of MAPK. Meanwhile, reactive oxygen species (ROS) activates cytoplasmic Ca2+ channels at the plasma membrane in the apex of the root tip. This complex pathway involves transduction cascades of multiple signals that lead to the fine tuning of epidermal cell initiation and elongation. The results suggest that elevated CO2 plays an important role in cell differentiation processes at the root epidermis.
Key words: elevated CO2, root hairs, carbohydrate, auxin, ethylene, NO, ROS, Ca2+, genetic elements
Increasing concentration of atmospheric CO2 in the 21st century will impact many aspects of the human and natural world. Elevated CO2 has some beneficial physiological effects on plants but nutrient limitation has generally been found to suppress these beneficial effects.1 Therefore, under conditions of suboptimal supply of nutrients and elevated CO2, the plants need to develop adaptive mechanisms to enhance nutrient acquisition, among which the plasticity of root development is of crucial importance.
Root hairs make a significant contribution to increasing root surface area and facilitating physical anchorage to a substrate and providing a large interface for nutrient uptake.2 Root-hair cells are highly polarized cellular structures resulting from tip growth of specific epidermal cells, which are controlled by multiple cellular factors and genetic processes.3,4 Previous studies have shown that root hair development can influenced by various environmental factors, such as nutritional status,5 mycorrhizal infection and water stress,6 salinity7 and light intensity.8 Our current research has demonstrated a profound effect of elevated CO2 on development of root hairs in Arabidopsis, which works through the well-characterized auxin signal transduction pathway.9 Since root hairs are an efficient strategy to alleviate the limitation of nutrients, one promising area of future research will be to discover the pathway that control root hair differentiation in crops under elevated CO2. In this paper, we discussed a layer pathway in the interaction between CO2 and some classical signals on regulating gene regulatory network to control development of root hairs.
Process of Root Hair Development
Root-hair morphogenesis, which forms a model system for studying polarized plant cell growth, can be subdivided into three major stages: swelling formation (referred to hereafter as root-hair initiation), the transition to tip growth and tip growth.10 The patterning of such a process is highly regulated. Numerous experimental observations indicate that root hair initiation and tip growth are controlled by multiple factors, such as phytohormones, ABA, cellular and extracellular signals like expansins, cytoplasmic and cell wall pH, actin cytoskeleton and microtubules.11–14 A tip-focussed cytoplasmic calcium (Ca2+) gradient forms during root hair growth as it does in all other tip growing cells,15 These process requires tip-localized ROS produced by an NADPH oxidase through activation of hyperpolarisation-activated calcium channels.16 The maintenance of this tip-focused Ca2+ gradient during hair growth is dependent on microtubules.12 Besides, potassium channel is the major osmotically active ion in many plant cells and the translocation of potassium is vital for root hair growth.17
Genetic analyses have resulted in identification of a number of genes that control root hair development at various stages. Genes including CPC, TRY, ETC1, TTG, GL2, GL3/EGL3, WER, RHL1, RHL2, RHL3, ERH1, ERH3 and ERH2 4,14 are identified to be involved in the early phase of root epidermal cell specification. SCM, a leucinerich repeat receptor-like kinase (LRR-RLK), has recently been shown to be required for position dependent pattern of epidermal cells. In scm mutants, the formation of N and H cells is not correlated to their position.18 After the emergence of a bulge outside an epidermal cell (root hair initiation stage), genes RHD6, TRH1, RHD1, TIP1, AtEXP7 and AtEXP18 can affect the number of swellings on each hair cell, and hair outgrowth and elongation.4,13,14,17 Following initiation, numerous genes are activated for the correct direction and extent of root-hair-tip growth. Root hairs without functional RHD2, SHV1, SHV2, SHV3, TRH1, ROP2, KJK or AKT1 genes stop growing before this stage.14,17,19,20 These results suggest that all of these genes are important for successful establishment of hair cell elongation and tip growth. Mutations affecting the CEN1, CEN2, CEN3, SCN1, BST1 and TIP1 genes can also stop hair growth before this stage, but only in certain double-mutant combinations.19 Additionally, SCN1, COW1, TIP1, CEN2, CEN1, CEN3, BST1, RHD3 or RHD4 genes can induce more branched hairs in Arabidopsis.4,19 LRX1, PFN1 and Sec1 protein KEULE are required for normal root hair development.4,14
How does Elevated CO2 Regulate Root Hairs Development?
There is accumulating evidence that elevated CO2 can accelerate plant growth and development by affecting cell division, elongation and differentiation within apical meristems.21,22 These cellular processes are regulated by a suite of classical signaling including auxin, ethylene, jasmonates (JAs), gibberellins (GAs), cytokinins (CKs), NO, abscisic acid (ABA), ROS, phospholipids and cytoplasmic Ca2+.16,23 Interestingly, elevated CO2 increases carbohydrate production,24 auxin level and response in plants,9,25 ethylene production,26 NO accumulation27 and our (unpublished data) and abscisic acid concentration.25 Thus, changes in levels and/or responses of these factors may play an important role in regulating the development of root hairs grown under elevated CO2.
To further discuss the pathway in which elevated CO2 affects root hair growth, we need find more convincing evidence to support the above hypothesis. In fact, many studies have shown that plants grown in elevated CO2 usually have an increased concentration of carbohydrates, such as soluble sugar and starch, in leaves because of carbohydrate assimilation in excess of consumption.24 The conclusion is in accordance with the results found in many other plants. It has been recognized that an increased accumulation of carbohydrates in plants would increase the production of auxin.28 Thus, elevated CO2 might thus increase concentrations of auxin in the plants via an increase in carbohydrate production. Alternatively, elevated CO2 could enhance ethylene production,26 while ethylene could stimulate IAA synthesis and transport in root tips. However, Rahman et al.29 reported that auxin plays a compensating role in the process of root hair development in Arabidopsis in the absence of ethylene. Both auxin and ethylene can interact on their biosynthesis and the response pathways, or sometimes independently regulate the same target genes.3 The correlation between auxin and ethylene signalling in root development is complex. Moreover, it has also reported that JAs promote root hair formation in Arabidopsis, through an interaction with ethylene.23 This implies that there exists interplay among phytohormones in mediating root hair development. These issues require further investigation. Recent studies have shown that elevated CO2 could increase auxin levels which then induced NO accumulation.27 In addition, NO was involved in the growth and development of root hairs, of which underlying mechanisms were under the control of auxin.22 Thus NO may act downstream of CO2, carbohydrates auxin, ethylene or probably JAs.
Recently, NO has been proved as a multipurpose signaling messenger that accomplishes its biological functions through its action on multiple targets. The available data illustrate that NO can directly influence the activity of target proteins through nitrosylation and has the capacity to act as a Ca2+-mobilizing intracellular messenger.30 Meanwhile, NO-dependent signals can be modulated through protein phosphorylation upstream of intracellular Ca2+ release. They implicate a target for protein kinase control in ABA signalling that feeds into NO-dependent Ca2+ release.31 As broadly known, a high concentration of cytoplasmic Ca2+ at the root tip is required for maintaining its growth rate. Furthermore, Samaj et al.,5 have assembled these components into a model in which ROS produced by NADPH oxidase activates Ca2+ channels at the plasma membrane in the apex of the root tip, leading to a tip-focused Ca2+ concentration gradient and subsequent signaling inherent to root hair growth. The Ca2+-permeable channel modulated by ROS has been demonstrated in Vicia faba guard cells and Arabidopsis root hairs.16 Additionally, root hair growth was associated with ROS production through the activation of the MAPK cascade.32 Interestingly, NO has also been shown to be involved in the activation of a MAPK cascade during adventitious root formation.33 These implies that NO play a fundamental role in outgrowth through MAPK cascade activation.
Based on previous studies and our recent observations, a model could be proposed of how CO2 regulates the root hair formation (Fig. 1). This model is based on that proposed by Samaj et al.,5 Lombardo et al.,22 and Niu et al.9 Elevated CO2 firstly increases the carbohydrates production, which triggers the auxin or ethylene responsive signal transduction pathways and subsequently results in the generation of intracellular NO. NO modulate target Ca2+ and ion channels and MAPK signaling cascade that are proposed as control points of root hair development. Withal, ROS activates Ca2+ channels at the plasma membrane in the apex of the root tip. Then, these endogenous signals modulate the downstream genetic elements that control actin cytoskeleton vesicular and microtubules, which together regulate root hair development. Overall, future studies, including those focusing on molecular and physical mechanisms governing interactions among the cytoskeleton, plasma membrane and cell wall, must consider CO2 as a new and critical player to understand cell differentiation processes in the root epidermis.
Figure 1.
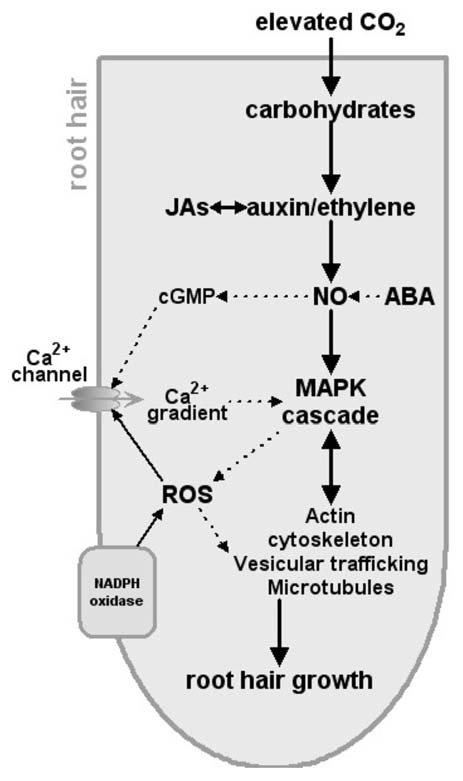
Conceptual model showing the potential elevated-CO2-target points in the signaling events those lead to the growth of root hairs. Solid arrows indicate links established in the induction of root hair development and broken arrows represent already established links in other systems but yet to be demonstrated in the growth of root hairs. NO, nitric oxide; cGMP, cyclic GMP; JAs, jasmonates; ROS, reactive oxygen species.
Acknowledgments
We thank Dr. Caixian Tang from Department of Agricultural Sciences, La Trobe University for critical reading and revision of the manuscript. This work was financially supported by the Project of Transformation Fund for Agricultural Scientific and Technological Achievements of China (2010GB23600669), the Natural Science Foundation of China (NSFC, No. 30871590) and Doctoral Fund of Ministry of Education of China (No. 200803350117).
References
- 1.Stitt M, Krapp A. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ. 1999;22:583–621. [Google Scholar]
- 2.Peterson RL, Farquhar ML. Root hairs: specialized tubular cells extending root surfaces. Bot Rev. 1996;62:1–40. [Google Scholar]
- 3.Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grierson C, Schiefelbein J. Root Hairs Plant Cell Monogr. Vol. 12. Berlin Heidelberg: Springer-Verlag; 2009. Genetics of root hair formation; pp. 1–15. [Google Scholar]
- 5.Samaj J, Baluška F, Menzel D. New signalling molecules regulating root hair tip growth. Trends Plant Sci. 2004;9:217–220. doi: 10.1016/j.tplants.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Bibikova T, Gilroy S. Root hair development. J Plant Growth Regul. 2003;21:383–415. [Google Scholar]
- 7.Wang YN, Zhang WS, Li KX, Sun FF, Han CY, Wang YK, Li X. Salt-induced plasticity of root hair development is caused by ion disequilibrium in Arabidopsis thaliana. J Plant Res. 2008;121:87–96. doi: 10.1007/s10265-007-0123-y. [DOI] [PubMed] [Google Scholar]
- 8.Okada K, Shimura Y. Modulation of root growth by physical stimuli. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 665–684. [Google Scholar]
- 9.Niu YF, Jin CW, Jin GL, Zhou QY, Lin XY, Tang CX, Zhang YS. Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2. Plant Cell Environ. 2011;34:1304–1317. doi: 10.1111/j.1365-3040.2011.02330.x. [DOI] [PubMed] [Google Scholar]
- 10.Dolan L, Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, et al. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development. 1994;120:2465–2474. [Google Scholar]
- 11.Miller DD, de Ruijter NCA, Bisseling T, Emons AMC. The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 1999;17:141–154. [Google Scholar]
- 12.Bibikova TN, Blancaflor EB, Gilroy S. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 1999;17:657–665. doi: 10.1046/j.1365-313x.1999.00415.x. [DOI] [PubMed] [Google Scholar]
- 13.Cho HT, Cosgrove DJ. Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell. 2002;14:3237–3253. doi: 10.1105/tpc.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grierson C, Schiefelbein J. The Arabidopsis Book. Root hairs. First published on April 4, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monshausen GB, Messerli MA, Gilroy S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in root cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008;147:1690–1698. doi: 10.1104/pp.108.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 17.Rigas S, Debrosses G, Haralampidis K, Vicente-Agullo F, Feldmann KA, Grabov A, et al. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell. 2001;13:139–151. doi: 10.1105/tpc.13.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak SH, Shen R, Schiefelbein J. Positional signaling mediated by a receptor-like kinase in Arabidopsis. Science. 2005;307:1111–1113. doi: 10.1126/science.1105373. [DOI] [PubMed] [Google Scholar]
- 19.Parker JS, Cavell AC, Dolan L, Roberts K, Grierson CS. Genetic interactions during root hair morphogenesis in Arabidopsis. Plant Cell. 2000;12:1961–1974. doi: 10.1105/tpc.12.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desbrosses G, Josefsson C, Rigas S, Hatzopoulos P, Dolan L. AKT1 and TRH1 are required during root hair elongation in Arabidopsis. J Exp Bot. 2003;54:781–788. doi: 10.1093/jxb/erg066. [DOI] [PubMed] [Google Scholar]
- 21.Taylor G, Tricker PJ, Zhang FZ, Alston VJ, Miglietta F, Kuzminsky E. Spatial and temporal effects of free-air CO2 enrichment (POPFACE) on leaf growth, cell expansion and cell production in a closed canopy of poplar. Plant Physiol. 2003;131:177–185. doi: 10.1104/pp.011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lombardo MC, Graziano M, Polacco JC, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav. 2006;1:28–33. doi: 10.4161/psb.1.1.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu CH, Gan LJ, Shen ZG, Xia K. Interactions between jasmonates and ethylene in the regulation of root hair development in Arabidopsis. J Exp Bot. 2006;57:1299–1308. doi: 10.1093/jxb/erj103. [DOI] [PubMed] [Google Scholar]
- 24.Long SP, Drake BG. Photosynthetic CO2 assimilation and rising atmospheric CO2 concentrations. In: Baker NR, Thomas H, editors. Crop photosynthesis: spatial and temporal determinants. Amsterdam, the Netherlands: Elsevier; 1992. pp. 69–95. [Google Scholar]
- 25.Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006;172:92–103. doi: 10.1111/j.1469-8137.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- 26.Dhawan KR, Bassi PK, Spencer MS. Effects of carbon dioxide on ethylene production and action in intact sunflower plants. Plant Physiol. 1981;68:831–834. doi: 10.1104/pp.68.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ. Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiol. 2009;150:272–280. doi: 10.1104/pp.109.136721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pritchard SHG, Rogers HOH, Prior SA, Peterson CTM. Elevated CO2 and plant structure: a review. Global Change Biology. 1999;5:807–837. [Google Scholar]
- 29.Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol. 2002;130:1908–1917. doi: 10.1104/pp.010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Courtois C, Besson A, Dahan J, Bourque S, Dobrowolska G, Pugin A, Wendehenne D. Nitric oxide signalling in plants: interplays with Ca2+and protein kinases. J Exp Bot. 2008;59:155–163. doi: 10.1093/jxb/erm197. [DOI] [PubMed] [Google Scholar]
- 31.Sokolovski S, Hills A, Gay R, Garcia-Mata C, Lamattina L, Blatt MR. Protein phosphorylation is a prerequisite for intracellular Ca2+ release and ion channel control by nitric oxide and abscisic acid in guard cells. Plant J. 2005;43:520–529. doi: 10.1111/j.1365-313X.2005.02471.x. [DOI] [PubMed] [Google Scholar]
- 32.Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, et al. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature. 2004;427:858–861. doi: 10.1038/nature02353. [DOI] [PubMed] [Google Scholar]
- 33.Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol. 2004;135:279–286. doi: 10.1104/pp.103.038554. [DOI] [PMC free article] [PubMed] [Google Scholar]


