Abstract
The rise in prevalence of obesity, diabetes, metabolic syndrome, and fatty liver disease has been linked to increased consumption of fructose-containing foods or beverages. Our aim was to compare the effects of moderate consumption of fructose-containing and non-caloric sweetened beverages on feeding behavior, metabolic and serum lipid profiles, and hepatic histology and serum liver enzymes, in rats. Behavioral tests determined preferred (12.5–15%) concentrations of solutions of agave, fructose, high fructose corn syrup (HFCS), a combination of HFCS and Hoodia (a putative appetite suppressant), or the non-caloric sweetener Stevia (n=5/gp). HFCS intake was highest, in preference and self-administration tests. Groups (n=10/gp) were then assigned to one of the sweetened beverages or water as the sole source of liquid at night (3 nights/wk, 10wks). Although within the normal range, serum cholesterol was higher in the fructose and HFCS groups, and serum triglycerides were higher in the Agave, HFCS, and HFCS/Hoodia groups (vs. water-controls, p<0.05). Liver histology was normal in all groups with no evidence of steatosis, inflammation, or fibrosis; however serum alanine aminotransferase was higher in the fructose and HFCS groups (vs. water-controls, p<0.05). Serum inflammatory marker levels were comparable among Stevia, agave, fructose, HFCS, and water-consuming groups, however levels of IL-6 were significantly lower in association with the ingestion of Hoodia. There were no differences in terminal body weights, or glucose tolerance assessed by 120-min IVGTTs performed at the end of the 10-week regimen. We conclude that even moderate consumption of fructose-containing liquids may lead to the onset of unfavorable changes in the plasma lipid profile and one marker of liver health, independent of significant effects of sweetener consumption on body weight.
Keywords: High fructose corn syrup, Agave, Stevia, Hoodia, Liver
1. Introduction
There is an alarming rise in the prevalence of obesity, type 2 diabetes mellitus, and Metabolic Syndrome in children and adults in the U.S. and around the world, related to increasing availability of energy-dense, high-calorie foods, and perhaps to increased consumption of sugar- and particularly fructose-sweetened beverages [1]. Bray and colleagues reported that in the United States, the consumption of high fructose corn syrup (HFCS) has increased by more than 1000% over the last 30 years which is parallel to the increase in obesity [2]. Average total calories from sweetened beverages has increased from 70 kcal to 189 kcal per day. Sugared beverage intake has partly replaced dairy beverage intake in children and teenagers – as sugared beverage consumption increased, milk consumption dropped by 38% between 1971 and 1996 [1,3,4]. However, lack of longitudinal data and variation in beverages tested make the role of sweet calories from obesity somewhat uncertain [5]. Non-alcoholic fatty liver disease (NAFLD), a hepatic manifestation of the Metabolic Syndrome and associated inflammation, is the most common liver disease in both obese adults and children [6,7]. The pathophysiology of NALFD is not fully understood, but recent studies likewise point to a possible role for consumption of fructose-containing beverages and foods in NALFD development.
The use of alternative caloric and non-caloric sweeteners, and also natural ‘appetite suppressants’ such as Hoodia, has become increasingly popular over the past several years. We tested whether moderate consumption of fructose-containing and non-caloric sweeteners led to metabolic changes that would predispose to increased risk for cardiovascular disease; abnormal glucose tolerance; or structural or functional hepatic changes. Two such sweeteners are agave, a product of the cactus plant, which has fructose as a main sugar, and Stevia, which does not contain useable carbohydrates for humans. Agave plants are common in the American southwest, Mexico, central and tropical South America, some parts of India, and the Mediterranean. Agave syrup has a low glycemic index because of its 90% fructose content and is used as a sugar alternative. We selected Agave because of its potential to serve as an abundant natural source of fructose. Stevioside is a natural sweet-tasting glycoside isolated from the herb Compositae (Stevia rebaudiana), growing in South America. Stevioside is 200–350 times sweeter than sucrose and has been used as a natural sweetener and antihyperglycemic agent in Brazil and Japan for decades [8–10]. Another plant extract of current popular interest is derived from Hoodia gordonii, a succulent plant growing in South Africa and Namibia, which has been reported to contain compounds that cause decreased appetite, weight loss, and improvement of glucose tolerance. In recent studies performed in rats [11], different compounds isolated from H. gordonii were tested for their appetite suppressant properties. One compound resulted in a decrease of food consumption and body weight over an eight day period. We decided to examine the effects of Hoodia extract in our rat model, because the mechanism(s) of the putative appetite-suppressant effects of Hoodia are unknown.
We evaluated iso-caloric solutions of agave, fructose, high fructose corn syrup (HFCS), and HFCS with Hoodia (HFCS/Hoodia), and Stevia, in a rat model of moderate intake. HFCS was included as it has been a recent focus of metabolic studies, and regimens where HFCS represents a significant source of daily caloric intake result in rapid development of acute and chronic pathological blood lipid profiles. The HFCS/Hoodia condition was designed to simulate a self-medicating weight loss regimen, i.e., the practice of taking a putative weight-loss pharmaceutical while continuing to consume high quantities of sugar or fat. Rats had ad libitum access to these solutions for three nights/week, with water being the sole source of liquid for the other nights, and during the daytime. We used this design to reflect the facts that sweetened beverages, and drinks in general, are most frequently taken with food during the conventional ‘awake’ hours (humans, during the day and rats, at night); and that for most individuals, sweetened beverages do not represent the sole source of beverage, with water, and other non-sweetened beverages (coffee, tea) being consumed on a regular basis as well. We felt that although some individuals do consume excessive amounts of fructose in foods or beverages, evaluation of a model of regular but more moderate intake would provide a realistic metabolic ‘snapshot’ of the effects of these beverages on body weight and metabolic health. Limited evaluation of these compounds has been done in a realistic model that simulates intake by the average person, or includes comprehensive metabolic evaluation. Thus, the aim of this study was to carry out behavioral and metabolic evaluation of rats that had chronic but more limited access to a variety of sweeteners in drinking water. Collectively, our findings suggest that early changes in lipid and liver metabolism may occur with moderate sweetener intake, independent of, and preceding, any changes of body weight.
2. Materials and methods
2.1. Animal model and experimental design
We studied male young adult (2 mo) Albino rats (Rattus rattus, Simonsen, CA) and all procedures were approved by the VA Puget Sound Health Care System IACUC. Rats were maintained on ad libitum rat chow (5001 Rodent Diet) except as indicated. In an initial study, behavioral tests were carried out to determine optimally-preferred concentrations of solutions of Stevia, agave, fructose, HFCS, and HFCS/Hoodia (n =5) and to test the reinforcing value of these solutions. Once these concentrations were determined, in a second cohort, rats were given the sweetened beverages (n =10/gp) as the sole source of liquid at night, an average of three nights/week. On these nights, beverage intake and 24-h chow intake were quantitated. The remaining nights, and during the daytime, only water was available. Rats were weighed weekly. Some liquid intake data were deemed unreliable because of faulty set-ups, therefore only intake data obtained reliably for the entire 10-week beverage/kcal measurements were analyzed although all animals in each group were given beverage access for the entire study (final ‘n’s ‘total kcal’ and beverage intake data: Stevia, 10; agave, 7; fructose, 8; HFCS, 8; HFCS-Hoodia, 9; water, 10). After ten weeks of this regimen, rats had chronic intravenous (IV) cannulas implanted according to our established methodology [12]. After a week of recovery, rats were taken through a conscious 120 min IVGTT procedure [13] for assessment of glucose tolerance. Other than an overnight fast prior to the IVGTT, rats were maintained on their sweetened beverage routine. The subsequent week, rats were fasted overnight, anesthetized with isoflurane, and euthanized with trunk blood and tissue collection. Liver was evaluated histologically for lipid inclusion and morphology. Retroperitoneal fat pads (n =5 from each group) were collected quantitatively as one estimate of body adiposity. Trunk blood was separated for multiple measurements in serum or plasma.
2.2. Behavioral tests
We conducted two behavioral tests to determine optimal concentrations of solutions to provide for the chronic intake study. First, we determined preferred concentrations of Stevia (NOW Foods: Nutrition for Optimal Wellness), agave (NOW Foods), fructose (NOW Foods) and HFCS (NaturesFlavors.com) in preference tests where trios of concentrations (2.5, 5, 7.5, 10, 12.5, 15, 20, 30, or 40%) were tested for relative intake of the most preferred of each trio. Overlapping concentration trials were carried out, to validate the final selections. We added Hoodia (“Slim 400”, 12.5–18 mg/kg) to HFCS at a concentration that we anticipated using for the chronic study. The concentrations of Hoodia were based upon the report of its weight loss effects in rats [11], and a normative estimate of the volume that rats would drink overnight, with the goal that the rats would ingest a minimal amount of Hoodia-containing solution that had weight loss effects. These preferred concentrations were used for the subsequent, chronic intake study, and were, in fact, calorically matched (12.5% agave, 0.57 kcal/ml; 12.5% fructose, 0.5 kcal/ml; 15% HFCS, 0.5 kcal/ml) based upon caloric information from the respective manufacturers. Stevia (12.5%) served as a sweet but non-caloric solution.
Motivating strength of the iso-caloric solutions was tested with progressive ratios self-administration, using our published methodology [14]. As established in our laboratory, the protocol included 3 phases: autoshaping; fixed ratio (FR) training; and progressive ratios (PR) training using the PR algorithm of Richardson and Roberts [15]. The PR algorithm requires 1, 2, 4, 6, 9, 12, 16, 20, 28, 36, 48, 63, 83, 110, 145, 191, 251, 331, 437, 575, 759, 999 (etc.) lever presses for succeeding reward deliveries within a session [15]. Rats were trained to self-administer their respective sweetener solution (0.5 ml reward) delivered into a liquid drop receptacle. The operant boxes, controlled by a Med Associates (Georgia, VT) system, had two levers, but only one lever (an active, retractable lever) activated the infusion pump. Presses on the other lever (an inactive, stationary lever) were also recorded. As we have observed previously, the number of presses on the inactive lever was very low (less than 10 presses/session). The solution was delivered into a liquid drop receptacle for oral consumption (Med Associates). Initial training was conducted during one-h sessions for 10 days under a continuous reinforcement schedule (FR1: each lever press was reinforced), with a maximum possible of 50 rewards delivered per session. Each session began with the insertion of the active lever and the illumination of a white houselight that remained on for the entire session. A 5-s tone (2900 Hz, 20 dB above background)+ light (7.5 W white light above the active lever) discrete compound cue accompanied each reward delivery, followed by a 40-s time out after each sweetener delivery. PR training was carried out for 3 h/day for ten days. Sessions ended after 30 min of no active lever press responding, at which point the house light was turned off and the active lever retracted.
2.3. IV glucose tolerance test
Conscious IVGTTs were carried out in rats with chronically implanted IV cannulas, that were fasted overnight prior to study, and the methodology was based upon Frangioudakis et al. [13]. Bilateral intravenous cannulas were implanted two weeks prior to study, as per our established methodology [12]. Rats were recovered and on a positive weight gain trajectory before IVGTTs were carried out. Baseline samples were drawn at t-10 min (1.5 ml for determination of insulin, glucose, and corticosterone) and t0 min (0.5 ml). Rats received an infusion of 1 g glucose/2 ml/kg over 15–20 s followed by 0.5 ml flush of saline. Blood samples (0.5 ml) were taken at 5, 15, 30, 60, 90, and 120 min. Owing to some plugging of catheters during the procedure (hence, inability to obtain blood samples), final ‘n’s for the baseline/IVGTT data presented are: Stevia, 10/10; agave, 9/8; fructose, 9/8; HFCS, 8/8; HFCS-Hoodia, 8/8; and water 8/7. Plasma insulin was determined using Linco rat insulin RIA kits (#RI-13 K and SRI-13 K, Linco) and plasma glucose was determined on a YSI Glucose Analyzer. Area under the curve (AUC) for the response from baseline was calculated at 5 min and 120 min. The HOMA index was calculated as fasting (glucose [mM] × insulin [mU/L])/22.5.
2.4. Terminal blood chemistries
Fasting trunk blood was separated into serum or plasma (1 ml, for Luminex determinations) for subsequent assays. A lipids panel (Northwest Lipid Research Center) measured serum cholesterol, triglycerides, VLDL, HDL, and LDL. Liver status was evaluated by measurements of alanine-amino-transferase, serum bilirubin, and serum albumin. Kidney status was evaluated by measurement of serum creatinine, alkaline phosphatase, and blood urea nitrogen (BUN). Luminex xMAP technology (Luminex Corp., Austin, TX) was used to simultaneously measure plasma levels of leptin, and peptide YY (PYY), using a kit from Millipore (Billerica MA) to reflect neuroendocrine anorexic signaling. Determinations of serum levels of markers of inflammation (IL6, MCP1, TNFα, and IL-1b) were made with the Luminex multiplex instrument. The Luminex intra-assay CV was determined by replicate analysis (n=8) of two different samples, the results being 7.8% and 7.5%, respectively. Final ‘n’s for all chemistry determinations were: Stevia, 10; agave, 9; fructose, 10; HFCS, 10; HFCS-Hoodia, 9; and water, 9.
2.5. Liver histology
Sudan black staining of frozen liver tissue was employed to identify and quantitate hepatic steatosis. Hematoxylin and eosin stains and Masson’s trichrome stains were used to formally assess hepatic architecture, inflammation, and fibrosis [16].
2.6. Statistical analysis
The main focus of our study was a comparison of the effect of each sweetener vs. the water-control condition. One-way ANOVA tested for overall effect of beverage on any specific experimental parameter. 2-tailed Student’s t-test compared each sweetener with the water-control condition (except where noted, one-tailed comparisons tested the specific hypothesis that plasma lipid levels would be elevated with caloric sweeteners vs. water). Additional comparisons for specific parameters, between sweetener groups, are described below. Data are expressed as mean+/− the standard error of the mean.
3. Results
3.1. Behavioral tests
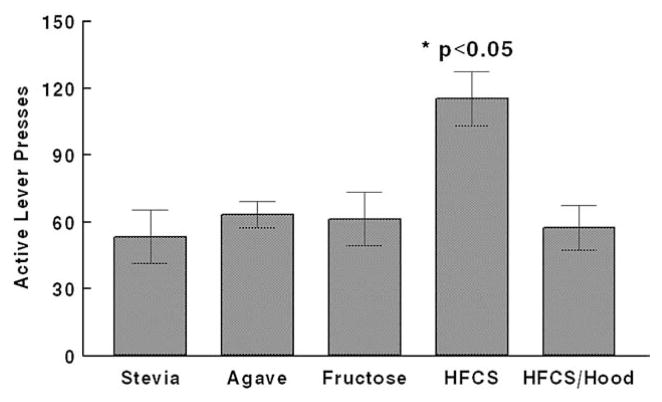
A peak of comparable preference at 12.5% and 15% concentrations was observed for rats drinking fructose, agave, Stevia, and HFCS (Fig. 1). HFCS intakes were significantly higher than those of the Stevia, agave, and fructose solutions at both concentrations (overall effect of beverage, F[4,20]=3.40 p =.028, and F[4,20]=20.64 p <.001, for 12.5% and 15%, respectively; p <0.05 for post-hoc comparisons). Adding Hoodia to the HFCS resulted in a significant decrease of intake at 12.5%, but intakes between HFCS and HFCS/Hoodia were comparable at 15%. Because the caloric density of the 15% HFCS solution (based upon manufacturer’s information) matched the caloric density of the agave and fructose 12.5% solutions (0.5, 0.57, and 0.5 kcal/ml respectively), we selected these as final concentrations. Intake of water (“0% solutions”) was minimal across all sweetener groups. To determine the motivating qualities of the solutions, rats were trained to self-administer the solutions in a progressive-ratios paradigm. At the chosen isocaloric concentrations, the HFCS was more reinforcing than the other sweeteners (overall effect of beverage, F[4,20]=5.725 p=.003; p<0.05 for individual comparisons) (Fig. 2).
Fig. 1.
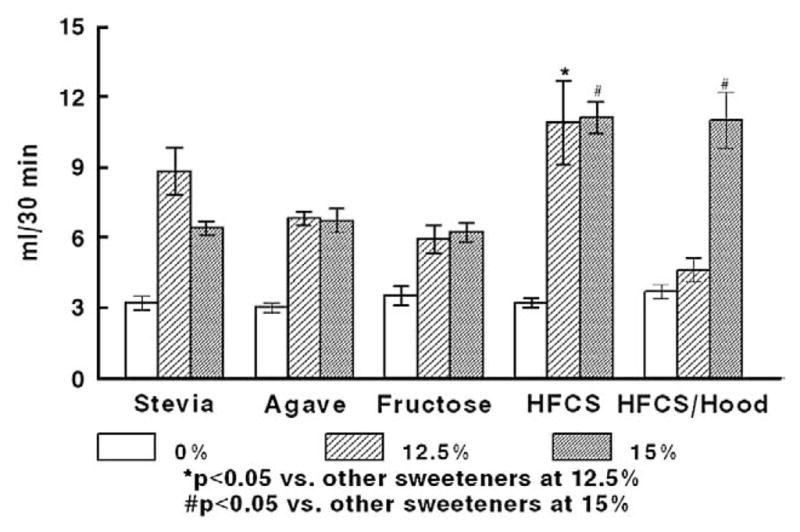
Preference test showing 30-min intakes of sweetened solutions in different concentrations, n=5/group. *Preferred intake of 12.5% HFCS was significantly greater (p<0.05) compared with all other sweetener groups. #Preferred intake of 15% HFCS with or without Hoodia was significantly greater (p<0.05) than that of Stevia, agave, or fructose.
Fig. 2.
Active lever presses (progressive ratios self-administration) of sweetened solutions of isocaloric concentration (Agave and fructose, 12.5%; HFCS and HFCS/Hoodia, 15%), and Stevia (12.5%) n=5/group. *The number of active lever presses in the HFCS group was significantly greater (p<0.05) vs. each other group.
3.2. Food intake, beverage intake, and body weight
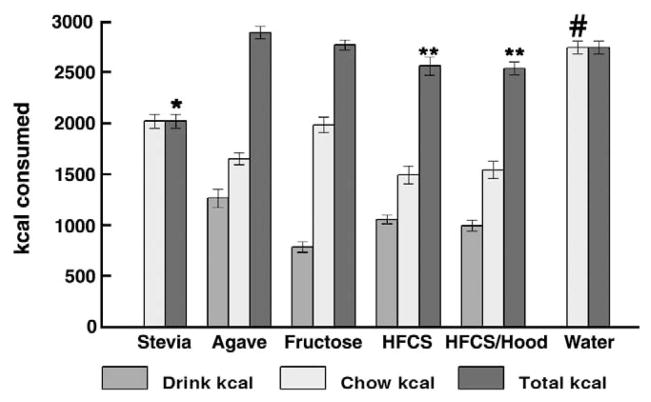
Chronic access to sweeteners did not result in any difference in final body weight or total weight gain of the rats (Table 1). Sweetened beverage intake volumes were all significantly elevated compared with intake of water (overall effect of beverage, F[5,48]=14.0 p<.01; p<0.05 for each post-hoc comparison); and among the sweeteners, volume of intake was greatest in the agave group (p≤0.05 vs. each other sweetener). Total calories consumed on the days that the rats had access to sweeteners were comparable for the agave, fructose, HFCS, HFCS/Hoodia and water groups (overall effect of beverage, F[5,46]=20.11, p<.01) (Fig. 3 and Table 1); total caloric intake of HFCS and HFCS/Hoodia was slightly but significantly lower vs. agave (p<0.05 for both comparisons). Total intakes were lower for the Stevia group (p<0.0001 vs. each other group), presumably reflective of consumption of a large volume of non-caloric sweet beverage along with chow. Retroperitoneal fat pad weight, as one adipose depot reflective of total body fat, did not differ among the six groups, at time of euthanasia.
Table 1.
Body weight, retroperitoneal fat weight, and caloric intake.
| Stevia | Agave | Fructose | HFCS | HFCS/Hoodia | Water | |
|---|---|---|---|---|---|---|
| Start BW | 288±3 | 287±3 | 287±3 | 287±4 | 288±4 | 287±4 |
| Final BW | 397±11 | 398±8 | 402±10 | 402±13 | 400±10 | 408±11 |
| Change BW | 108±10 | 111±8 | 115±9 | 115±12 | 115±12 | 121±10 |
| RP fat pad weight (mg) | 527±67 | 437±52 | 526±39 | 496±76 | 652±94 | 553±58 |
| Drink ml | 2099±142 | 2216±156a | 1568±104 | 2112±86 | 1990±106 | 1146±83 |
| Total cal | 2019±69a | 2891±61 | 2767±47 | 2561±88b | 2537±63 | 2742±63 |
| Food cal | 2019±69d | 1651±60 | 1983±75e | 1492±88 | 1542±84c | 2742±63 |
| Drink cal | 0 | 1263±89f | 784±52g | 1056±43 | 995±53 | 0 |
| Drink cal as %total | 0 | 44±1 | 29±2h | 42±2 | 40±3 | 0 |
p<0.001 vs. all other groups.
p<0.05 vs. Agave.
p<0.05 vs. water, fructose or Agave.
p<0.001 vs. Agave, HFCS, or HFCS/Hoodia.
p<0.01 vs. Agave, HFCS, or HFCS/Hoodia.
p<0.01 vs. fructose, HFCS, or HFCS/Hoodia.
p<0.05 vs. HFCS or HFCS/Hoodia.
p<0.01 vs. Agave, HFCS, or HFCS/Hoodia.
Fig. 3.
Caloric intake in association with sweetened beverages. *p<0.05 vs. any of the other groups for total kcal; # p<0.05 vs. any of the other groups for chow kcal; **p<0.05 vs. agave for total kcal.
3.3. Endocrine parameters and glucose tolerance
Baseline samples during the IVGTT (t-10 min and t0 min) allowed us to evaluate fasting levels of glucose and insulin. Fasting plasma glucose did not differ among the sweeteners groups, but fasting insulin levels were significantly decreased (p<0.05) in the HFCS group compared with the water group. The HOMA index (Table 2) did not differ among the groups. Based upon the IVGTTs, there were no major differences in glucose tolerance between the groups, confirmed by area under the curve (AUC) calculation for the first 5 min of the test (see Table 2) or the entire test period (0–120 min). There was no difference in the glucose excursion at t=5 min among the groups, although insulin release was significantly lower in the HFCS/Hoodia group compared with HFCS or water (overall effect of beverage, F[5,43]=2.58 p=0.04; p<0.05 for individual post-hoc comparisons). With regard to neuroendocrine signals of satiety or adiposity, there was no difference in PYY levels among all groups, reflecting satiety. There was no overall effect of beverage on plasma leptin levels.
Table 2.
IVGTT and terminal endocrines.
| Stevia | Agave | Fructose | HFCS | HFCS/Hoodia | Water | |
|---|---|---|---|---|---|---|
| IRI t-10 | 1.63±0.27 | 1.59±0.18 | 2.35±0.54 | 1.12±0.22a | 1.51±0.4 | 1.98±0.36 |
| IRI t0 | 1.83±0.2 | 1.75±0.25 | 2.65±0.64 | 1.08±0.17a | 1.58±0.42 | 1.96±0.35 |
| Glu t-10 | 99±3 | 100±4 | 92±3 | 88±3 | 99±1 | 91±3 |
| Glu t0 | 97±2 | 99±5 | 94±3 | 89±4 | 98±2 | 92±5 |
| IRI AUC 0–5 min | 2.44±0.34 | 2.9±0.44 | 2.2±0.44 | 3.19±0.55 | 1.31±0.16a,b | 2.31±0.27 |
| Glu AUC 0–5 min | 619±133 | 748±220 | 910±161 | 574±204 | 575±101 | 695±215 |
| HOMA | 13.5±1.6 | 13.3±2 | 10.6±2.3 | 8.5±1.3 | 11.4±3 | 14±2.7 |
| Leptin | 1212±177 | 927±97 | 1250±124 | 852±108 | 1007±134 | 1282±85 |
| Leptin/RP wt | 2.91±0.52 | 2.32±0.18 | 2.62±0.27 | 2.13±0.16 | 1.55±0.07c | 2.72±0.42 |
| PYY | 88±7 | 89±5 | 77±5 | 89±6 | 81±6 | 86±5 |
p<0.05 vs. control (water).
p<0.05 vs. HFCS.
p≤0.05 vs. all other groups.
3.4. Fasting plasma lipids and inflammatory marker levels
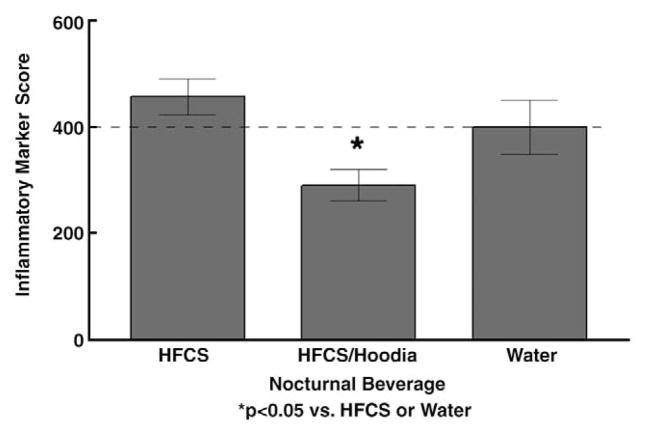
Chronic access to sweeteners in this moderate regimen did result in changes of fasting plasma lipids, although overall changes did not reach pathological levels (Table 3). However, for all of the calorie-containing sweeteners, levels of fasting lipids (triglyceride, total cholesterol, and VLDL) were elevated compared with the water-drinking controls (p<0.05 for comparisons summarized below). Serum cholesterol was significantly elevated in the fructose and HFCS groups. Serum triglycerides and VLDL were significantly elevated in the agave group, with significant elevations in the HFCS and HFCS/Hoodia groups, analyzed by one-tailed t-test. Mean levels of the inflammatory markers, MCP1, TNFα, and IL-1b, did not differ among Stevia-, agave-, fructose-, HFCS-, or water-consuming rats (Table 4). However, there was an overall effect of beverage on IL-6 levels (F [5,45]=2.61 p=.037) and consumption of Hoodia resulted in significantly decreased IL6 levels compared with water controls, Stevia, fructose, or HFCS groups (p≤0.05 for individual comparisons). A composite ‘score’ of the four inflammatory markers was calculated, with control levels set to 100% for each marker, then added together (maximum ‘score’ =400). As shown in Fig. 4, the cumulative score for the HFCS/Hoodia group was lower than that of the HFCS and water groups (overall effect of beverage, F[2,25]=4.65 p<0.01; p<0.05 for HFCS/Hoodia vs. HFCS and HFCS/Hoodia vs. water).
Table 3.
Terminal serum lipids, liver enzymes, and kidney enzymes.
| Stevia | Agave | Fructose | HFCS | HFCS/Hoodia | Water | |
|---|---|---|---|---|---|---|
| Cholesterol | 99±8 | 112±13 | 123±9a | 118±7a | 102±7 | 100±4 |
| TG (net) | 59±6 | 78±13a | 69±13 | 67±9b | 62±6b | 48±4 |
| VLDL | 12±1 | 16±2a | 14±3 | 13±2b | 12±1b | 9±0.9 |
| LDL | 5±1 | 6±2 | 12±4 | 8±3 | 3±1 | 6±1 |
| HDL | 84±6 | 93±10 | 99±6 | 98±4 | 87±6 | 85±3 |
| ALT | 81±4 | 75±4 | 101±7a | 90±5a | 86±7 | 76±3 |
| ALK | 129±7 | 114±4 | 133±8 | 118±6 | 116±9 | 107±7 |
p<0.05 vs. control (water).
p<0.05, one-tailed t-test.
Table 4.
Terminal inflammatory markers (plasma).
| Stevia | Agave | Fructose | HFCS | HFCS/Hoodia | Water | |
|---|---|---|---|---|---|---|
| TNF-alpha | 12.3±0.1 | 12.6±0.5 | 12.7±0.5 | 12.5±0.3 | 12.2±0.1 | 12.3±0.1 |
| IL-1beta | 15.6±3 | 12±2.5 | 16.2±3 | 10.8±1 | 8.5±1 | 15.3±4 |
| IL-6 | 319±84 | 158±45 | 281±62 | 432±95 | 74±22a | 387±140 |
| MCP-1 | 65±11 | 77±13 | 72±9 | 83±19 | 54±12 | 59±11 |
p<0.05 vs. Stevia, Fructose, HFCS, and water.
Fig. 4.
Composite inflammatory marker score. Mean plasma levels in the ‘water control group’ for each of the four inflammatory markers were set to 100 for a total of 400, and normalized values were then calculated for the HFCS and HFCS/Hoodia groups. *The composite score for the HFCS/Hoodia was significantly lower (p<0.05) than the HFCS or water-control groups.
3.5. Liver histology and function
Sudan black staining revealed no hepatic steatosis in any of the animals, with no between-group differences. There was preserved hepatic architecture in all animals with no significant inflammatory infiltrates, and no fibrosis. Serum albumin and bilirubin levels were similar between the sweetener groups and the control (water) group (data not shown). Alkaline phosphatase was elevated in the Stevia and fructose groups compared with the water group, but there was no overall effect of beverage (F[5,52]=1.56 p=0.18). Alanine aminotransferase was significantly elevated in the fructose and HFCS groups vs. water-drinking controls (overall effect of beverage, F[5,52]=3.55 p=.008; p<0.05 for individual comparisons). Finally, BUN and serum creatinine levels did not differ among the groups (data not shown), suggesting that renal function was normal.
4. Discussion
A main finding of this study was that ingestion of both caloric and non-caloric sweeteners caused acute and chronic changes of feeding patterns. Furthermore, we observed modest but significant changes in some lipid and liver enzyme measurements, consistent with the more striking observations of altered plasma lipid profiles and hepatic pathology in human or animal models of more extensive fructose-based sweetener feeding. These changes were independent of increased body weight, adiposity, or glucose metabolism, as chronic moderate consumption of isocaloric solutions of agave, fructose, or HFCS with or without Hoodia did not result in altered body weight or overt changes in glucose tolerance and insulin secretion in our rat model. Our findings suggest that even moderate sweetener consumption, in lean individuals, can lead to the onset of altered lipid, and perhaps liver, metabolism. The observation of decreased insulin secretion and low concentrations of inflammatory markers in Hoodiafed animals is novel and needs further investigation.
In the first part of our study, preferred concentrations of either the non-caloric sweetener Stevia, or fructose-containing agave extract, fructose, HFCS, or HFCS combined with Hoodia, were determined, and their reinforcing capability tested in a behavioral paradigm of self-administration. Comparable preference was observed at concentrations of 12.5% and 15% for each sweetener solution, except the HFCS/Hoodia combination. Whereas 12.5% HFCS/Hoodia intake was comparable to water intake, 15%HFCS/Hoodia intake was comparable to that of 15% HFCS alone. Further, 15% HFCS was calorically matched to 12.5% agave or 12.5% fructose. Thus, these isocaloric solutions were tested for reinforcing capability and were used for the second study, as well. HFCS was quantitatively more preferred (absolute intake in the preference test) as well as more reinforcing, compared with any of the other sweetened solutions. Since this pattern was not exactly mirrored by the free-choice ad libitum intakes of the second part of the study, our finding suggests that there may be differential acute reinforcing efficacy of HFCS which is less meaningful in circumstances where an individual or animal does not have to work to attain the sweetened beverage.
In the second part of our study in a second group of rats, metabolic changes were studied after a 10-week exposure to the different sweeteners. We demonstrated that even moderate consumption of fructose-containing liquids led to some unfavorable changes in lipid parameters and liver enzymes. Although levels of alanine amino transferase and alkaline phosphatase were within the normal range, the results nonetheless suggest a trend towards the onset of liver pathology. Interestingly, for all groups consuming fructose-containing beverages, additional energy taken in from calorie-containing beverages was adequately compensated by reduction of normal chow intake. Some evidence from human studies has suggested that the increased caloric intake by beverages is not sufficiently compensated by reduced intake calories of solid food, although this assertion has been challenged [5]. Only in the Stevia (non-caloric sweetener) group, the total calorie intake was less than in all fructose groups or the water control group on sweetener days (Fig. 3). Nevertheless, at the end of the experiment there was no increased or decreased weight gain in any of the sweetener groups relative to the controls, nor any difference among the groups for retroperitoneal fat pad weights. This suggests that the Stevia-consuming rats may have been over-consuming on days when they did not have access to Stevia, and thus compensated for overall intake. Further, rats consuming moderate amounts of fructose do not develop excessive adiposity if they are fed with normal (i.e., low fat) chow. High dietary fructose leads to blunted responses of important satiety hormones insulin and leptin [17]; with our paradigm, we did not find an overall effect on fasting insulin or leptin, in groups drinking fructose-containing beverages.
Intermittent consumption of the different sweetened solutions over a period of ten weeks did not lead to any histologically observable hepatic steatosis, inflammation, or fibrosis. This suggests that moderate differences in the proportion of total ingested calories that are made up by different liquid carbohydrate drinks have little effect on hepatic morphology or development of overt liver damage. However, our finding that serum ALT levels were slightly elevated in the fructose and HFCS groups compared to the water-control group, suggests that there may be the initiation of some minor liver injury in these groups, which did not, however, translate to any persistent chronic liver damage as assessed by liver histology.
In our experiments, drinking water sweetened by Stevia led to an increased fluid intake but decreased total calorie intake compared to the control group. Stevioside is the major sweet active component in this non-caloric sweetener and is isolated from Stevia rebaudiana. An antihyperglycemic action of stevioside has been ascribed to effects on insulin secretion [18] as well as increased insulin sensitivity (glucose uptake) at muscle [9,19]. The aglycone steviol is also an active compound: it is an inhibitor of intestinal glucose absorption [20], and decreases glucose production [21,22] and monosaccharide transport in the rat liver [23]. Our studies with intermittent access to Stevia demonstrated decreased caloric intake which we ascribe to the displacement of chow consumption by the large volumes of Stevia ingested overnight. Across a 10-week period, however, there was no net effect on either body weight or insulin sensitivity.
Agave nectar is gaining popularity as a healthful natural sweetener alternative. The juice from the Agave plant is heated or enzymatically treated to hydrolyze the complex carbohydrates to sugars and concentrated to a syrup [24]. The taste and consistency of agave nectar are similar to corn syrup and the major part of its sugar content is fructose which explains its low glycemic index [25]. But by high-performance liquid chromatography (HPLC), glucose, sucrose, xylose, and maltose have also been identified in agave juice [26]. It is expected that agave syrup contains beneficial antioxidant nutrients, trace elements, or phytochemicals. All these other components might explain the effects of chronic agave drinking on ALT levels, which were lower than the levels in the pure fructose group in our experiments, and were normal.
Chronic intake of HFCS with added Hoodia did not differ from intake of HFCS without Hoodia. Hoodia is characterized as slightly bitter in taste [27]. As bitter receptors (T2Rs) are expressed in the same gut L-cells as sweet receptors, we postulated that Hoodia consumption might lead to higher secretion of PYY, but we did not observe this. The main active compound in H. gordonii is a substance called pregnane glycoside, known as P57 [28,29]. In a rat study of Hoodia compounds, Hoodia compound administration resulted in a reduction in food intake over the study period, with a concomitant overall decrease in body weight [11]. In our study, chronic Hoodia in combination with HFCS had no net effect on body weight, or retroperitoneal fat pad size. This may have been dose-related as we estimated our Hoodia intake to span the published dose range of efficacious treatment for the Hoodia compound, but we did not have an opportunity to determine blood levels of Hoodia compounds. We cannot rule out the possibility that Hoodia may decrease food intake on an acute basis, which we did not assess, although the HFCS/Hoodia solution was less reinforcing than HFCS alone (Fig. 2). The IVGTT results of a lower insulin level following intravenous glucose (Table 2), suggest increased insulin sensitivity. Thus, although our study does not corroborate an effect to lower body weight, moderate consumption of Hoodia may have protective or beneficial effects on glucose tolerance. Further, a calculation of the ratio of plasma leptin/retroperitoneal fat pad weight (Table 2) revealed a significantly lower ratio in the HFCS-Hoodia group vs. the HFCS group (and all other groups). One interpretation of this is that leptin sensitivity may be increased by Hoodia, a concept that lends itself to further experimentation. Finally, consumption of Hoodia resulted in a drop below control levels, of plasma inflammatory markers. Collectively these observations suggest that moderate Hoodia consumption does not have metabolically toxic effects, and is either neutral, or metabolically beneficial.
We observed high chronic intakes of the fructose-containing solutions, agave and HFCS, which were statistically greater than intake of the fructose-only solution. The intakes therefore cannot be explained solely by the fructose content itself, but maybe by the mixture with glucose or other components in the agave syrup [26,30]. The effects of fructose consumption on whole-body energy metabolism were recently reviewed by Havel [31]. The de novo synthesis of fatty acids is stimulated by high intakes of fructose and glucose as simple sugars, especially in obese insulin resistant individuals. Roglans et al. showed that 10% glucose or fructose added daily to the drinking water did not lead to an increased weight gain in rats over a two week experimental period [32]. But in that study, fructose consumption led to increased hepatic triglyceride content and reduction of hepatic fatty-acid β-oxidation due to decreased PPARα activity. In another study, rats were provided sweetened beverages containing 13% high fructose corn syrup HFCS-55 (55% fructose), sucrose, or glucose, as a sole source of drinking liquid. Intake of the fructose-containing beverages led to reduced (compensatory) chow intake, but – in contrast to our study – to greater final body weights than the rats drinking water or glucose-containing beverages [33]. It has been shown that high dietary fructose consumption (fructose 60%/wt in chow) leads to a stress response in the liver, insulin resistance, and hyperlipidemia [34] as well as increased sympathetic neural activity, blood pressure, and elevated uric acid levels [35,36]. In contrast to those studies, we did not observe onset of steatosis or fibrosis of the liver, as mentioned above. In humans it could be demonstrated that combined sugars with a high fructose/glucose ratio resulted in higher triglyceride levels compared to lower ratios or glucose alone [37], and we likewise observed elevated fasting triglyceride levels in rats drinking agave or HFCS.
Our study has a few potential caveats. The first is that we studied the chronic effects of sweeteners at only a single concentration. This design was chosen because the final concentrations were both isocaloric and preferred by the rats. Second, we gave sweetened beverages only three nights per week. The rationale was that we aimed to mimic a more realistic scenario of moderate sweetened beverage intake than providing the rats with more than 50% sugar as total calorie intake. We recognize that there might be some metabolic compensation and especially reduction of hepatic lipid content during the nights between sweetened beverage intakes. Third, we did not include a glucose or sucrose group as we felt that the effects of fructose vs. glucose and sucrose have already been studied extensively. Finally, we measured lipid levels only in the fasting state. However, it is well established that in humans, the postmeal increase of triglyceride levels after high fructose consumption is even higher compared to controls [38]; we would anticipate a comparable finding if we measured post-prandial or post-absorptive levels of triglycerides in our model.
In conclusion we demonstrated that moderate consumption of sweeteners in drinking water leads to marked changes of drinking behavior and that fructose consumption leads to modest but significant changes in markers of liver and lipid metabolism. Although we did not observe excessive weight gain in all tested sweetener groups, the lipid and liver metabolism changes indicate that even moderate fructose consumption might contribute to the onset or development of the Metabolic Syndrome, which might be exacerbated by consumption of a Westernized high-fat diet, and needs to be investigated in future studies.
Acknowledgments
This work was carried out at the VA Puget Sound Health Care System, Seattle Division, and was supported by the Merit Review (DFL), Career Scientist (DFL) and Research Enhancement Award Program (REAP) (GI and DFL) Programs of the Dept of Veterans Affairs; NIH RO1 DK40963 (DFL); and by the CUMG Seed Funds of Seattle Children’s Hospital Research Institute and Seattle Children’s Hospital (CR). George Ioannou is supported by the Jan Albrecht Award from the American Liver Foundation and American Association for the Study of Liver Diseases. Christian Roth is supported by Seattle Children’s Hospital. The authors gratefully acknowledge the outstanding support and technical expertise of Connie Holmes West, Guochi Zhang, and Melissa Ralston. The generous and able assistance of Dr. Nicole Sanders, and student aides Marissa Haberlach, Kimberly Brennan, and Nick Shalygin, is acknowledged with grateful appreciation. The drug Hoodia was generously provided by Trade Avail Company, USA. We thank Dr. Tami Wolden-Hanson for the advice on fat pad dissection, and Dr. Alfred J. Sipols for assistance with the statistical analysis. The helpful discussion of Alan Bernstein, Dr. Janis Wignall, and Dr. Cindy Pekow is gratefully acknowledged.
Footnotes
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Lustig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 3.Isganaitis E, Lustig RH. Fast food, central nervous system insulin resistance, and obesity. Arterioscler Thromb Vasc Biol. 2005;25:2451–62. doi: 10.1161/01.ATV.0000186208.06964.91. [DOI] [PubMed] [Google Scholar]
- 4.Lustig RH. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the First Law of Thermodynamics. Nat Clin Pract Endocrinol Metab. 2006;2:447–58. doi: 10.1038/ncpendmet0220. [DOI] [PubMed] [Google Scholar]
- 5.Drewnowski A, Bellisle F. Liquid calories, sugar, and body weight. Am J Clin Nutr. 2007;85:651–61. doi: 10.1093/ajcn/85.3.651. [DOI] [PubMed] [Google Scholar]
- 6.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–57. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 7.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 8.Soejarto DD, Kinghorn AD, Farnsworth NR. Potential sweetening agents of plant origin. III. Organoleptic evaluation of Stevia leaf herbarium samples for sweetness. J Nat Prod. 1982;45:590–9. doi: 10.1021/np50023a013. [DOI] [PubMed] [Google Scholar]
- 9.Lailerd N, Saengsirisuwan V, Sloniger JA, et al. Effects of stevioside on glucose transport activity in insulin-sensitive and insulin-resistant rat skeletal muscle. Metabolism. 2004;53:101–7. doi: 10.1016/j.metabol.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Chen TH, Chen SC, Chan P, et al. Mechanism of the hypoglycemic effect of stevioside, a glycoside of Stevia rebaudiana. Planta Med. 2005;71:108–13. doi: 10.1055/s-2005-837775. [DOI] [PubMed] [Google Scholar]
- 11.van Heerden FR, Marthinus Horak R, Maharaj VJ, et al. An appetite suppressant from Hoodia species. Phytochemistry. 2007;68:2545–53. doi: 10.1016/j.phytochem.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Evans SB, Wilkinson CW, Bentson K, et al. PVN activation is suppressed by repeated hypoglycemia but not antecedent corticosterone in the rat. Am J Physiol. 2001;281:R1426–36. doi: 10.1152/ajpregu.2001.281.5.R1426. [DOI] [PubMed] [Google Scholar]
- 13.Frangioudakis G, Gyte AC, Loxham SJG, et al. The intravenous glucose tolerance test in cannulated Wistar rats: a robust method for the in vivo assessment of glucose-stimulated insulin secretion. J Pharmacol Toxicol Methods. 2008;57:106–13. doi: 10.1016/j.vascn.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Figlewicz DP, Bennett JL, Aliakbari S, et al. Insulin acts at different CNS sites to decrease acute sucrose feeding and sucrose self-administration in rats. Am J Physiol. 2008;295:R388–94. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 16.Lee LG. Manual of histologic staining methods of the Armed Forces Institute of Pathology. 3. McGraw Hill; 1968. [Google Scholar]
- 17.Teff KL, Elliott SS, Tschop M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 18.Jeppesen PB, Gregersen S, Poulsen CR, et al. Stevioside acts directly on pancreatic beta cells to secrete insulin: actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K+-channel activity. Metabolism. 2000;49:208–14. doi: 10.1016/s0026-0495(00)91325-8. [DOI] [PubMed] [Google Scholar]
- 19.Chang JC, Wu MC, Liu IM, et al. Increase of insulin sensitivity by stevioside in fructose-rich chow-fed rats. Horm Metab Res. 2005;37:610–6. doi: 10.1055/s-2005-870528. [DOI] [PubMed] [Google Scholar]
- 20.Toskulkao C, Sutheerawattananon M, Piyachaturawat P. Inhibitory effect of steviol, a metabolite of stevioside, on glucose absorption in everted hamster intestine in vitro. Toxicol Lett. 1995;80:153–9. doi: 10.1016/0378-4274(95)03391-w. [DOI] [PubMed] [Google Scholar]
- 21.Ishii EL, Bracht A. Glucose release by the liver under conditions of reduced activity of glucose 6-phosphatase. Braz J Med Biol Res. 1987;20:837–43. [PubMed] [Google Scholar]
- 22.Ferreira EB, de Assis Rocha Neves F, daCosta MA, et al. Comparative effects of Stevia rebaudiana leaves and stevioside on glycaemia and hepatic gluconeogenesis. Planta Med. 2006;72:691–6. doi: 10.1055/s-2006-931586. [DOI] [PubMed] [Google Scholar]
- 23.Ishii EL, Schwab AJ, Bracht A. Inhibition of monosaccharide transport in the intact rat liver by stevioside. Biochem Pharmacol. 1987;36:417–33. doi: 10.1016/0006-2952(87)90107-9. [DOI] [PubMed] [Google Scholar]
- 24.Mancilla-Margalli NA, Lopez MG. Generation of maillard compounds from inulin during the thermal processing of Agave tequilana Weber var. azul J Agric Food Chem. 2002;50:806–12. doi: 10.1021/jf0110295. [DOI] [PubMed] [Google Scholar]
- 25.Foster-Powell K, Holt SHA, Brand-Miller JC. International table of glycemic index and glycemic load values. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 26.Michel-Cuello C, Juárez-Flores BI, Aguirre-Rivera JR, et al. Quantitative characterization of nonstructural carbohydrates of mezcal Agave (Agave salmiana Otto ex Salm-Dick) J Agric Food Chem. 2008;56:5753–7. doi: 10.1021/jf800158p. [DOI] [PubMed] [Google Scholar]
- 27.van Heerden FR. Hoodia gordonii: a natural appetite suppressant. J Ethnopharmacol. 2008;119:434–7. doi: 10.1016/j.jep.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 28.MacLean DB, Luo LG. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004;1020:1–11. doi: 10.1016/j.brainres.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 29.Rumalla CS, Avula B, Shukla YJ, et al. Chemical fingerprint of Hoodia species, dietary supplements, and related genera by using HPTLC. J Sep Sci. 2008;31:3959–64. doi: 10.1002/jssc.200800441. [DOI] [PubMed] [Google Scholar]
- 30.Phillips KM, Carlsen MH, Blomhoff R. Total antioxidant content of alternatives to refined sugar. J Am Diet Assoc. 2009;109:64–71. doi: 10.1016/j.jada.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–57. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 32.Roglans N, Vila L, Farre M, et al. Impairment of hepatic Stat-3 activation and reduction of PPARα activity in fructose-fed rats. Hepatology. 2007;45:778–88. doi: 10.1002/hep.21499. [DOI] [PubMed] [Google Scholar]
- 33.Tsanzi E, Light HR, Tou JC. The effect of feeding different sugar-sweetened beverages to growing female Sprague–Dawley rats on bone mass and strength. Bone. 2008;42:960–8. doi: 10.1016/j.bone.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Kelley GL, Allan G, Azhar S. High dietary fructose induces a hepatic stress response resulting in cholesterol and lipid dysregulation. Endocrinology. 2004;145:548–55. doi: 10.1210/en.2003-1167. [DOI] [PubMed] [Google Scholar]
- 35.Valensi P. Hypertension, single sugars and fatty acids. J Hum Hypertens. 2005;19 (Suppl 3):S5–9. doi: 10.1038/sj.jhh.1001954. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 37.Parks EJ, Skokan LE, Timlin MT, et al. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138:1039–46. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1089–92. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]