Abstract
Background
Diabetes and obesity, which confer an increased risk of sudden cardiac death, are associated with cardiomyocyte lipid accumulation and altered cardiac electrical properties, manifested by prolongation of the QRS duration and QT interval. It is difficult to distinguish the contribution of cardiomyocyte lipid accumulation versus the contribution of global metabolic defects to the increased incidence of sudden death and electrical abnormalities.
Methods and Results
In order to study the effects of metabolic abnormalities on arrhythmias without the complex systemic effects of diabetes and obesity, we studied cardiac-specific transgenic mice expressing PPARγ1 via the cardiac α-myosin heavy-chain promoter. The PPARγ-transgenic mice develop abnormal accumulation of intracellular lipids and die as young adults, prior to a significant reduction in systolic function. Using implantable ECG telemeters, we found that these mice have prolongation of the QRS and QT intervals, and spontaneous ventricular arrhythmias, including polymorphic ventricular tachycardia and ventricular fibrillation. Isolated cardiomyocytes demonstrated prolonged action potential duration caused by reduced expression and function of the potassium channels responsible for repolarization. Short-term exposure to pioglitazone, a PPARγ agonist, had no effect on mortality or rhythm in WT mice, but further exacerbated the arrhythmic phenotype and increased the mortality in the PPARγ TG mice.
Conclusions
Our findings support an important link between PPARγ activation, cardiomyocyte lipid accumulation, ion channel remodeling and increased cardiac mortality.
Keywords: arrhythmia, metabolism, ion channels
Introduction
Diseases that affect cardiac energy metabolism and increase cardiomyocyte lipid stores, such as diabetes and obesity, are frequently associated with altered mechanical and electrical function in the heart, a syndrome termed lipotoxic cardiomyopathy 1-3. After adjusting for other cardiovascular risk factors, both diabetes and obesity confer an increased risk of sudden cardiac death 4-8, and are associated with altered cardiomyocyte electrical properties, manifested by prolongation of the QRS and QT intervals 9-11. The molecular mechanisms responsible for alterations in the electrical properties of cardiomyocytes and the increased incidence of sudden cardiac death have not been well elucidated. An essential question is whether the diabetes-induced cardiomyocyte lipid accumulation or the diabetes-induced global metabolic defects cause the increased incidence of sudden death.
In contrast to diabetic patients, several models of cardiac lipid accumulation have not shown increased mortality. Transgenic (TG) mice with cardiac-restricted over-expression of the peroxisome proliferation-activated receptor α (PPARα) exhibit a cardiac metabolic phenotype that is similar to that of the diabetic heart: increased fatty acid utilization and decreased uptake and oxidation of glucose 12. These mice, which develop cardiomyocyte lipid accumulation, demonstrate reduced potassium (K+) channel repolarizing currents. In contrast to diabetic patients, however, TG-PPARα mice do not have significant prolongation of the cardiac action potential duration (APD) and do not have increased incidence of sudden death 13. Mice with cardiac-restricted over-expression of the fatty-acid transport protein 1 (FATP1), which develop cardiomyocyte lipid accumulation, demonstrate prolongation of the QTc interval due to a reduction in repolarizing voltage-gated K+ currents 14. These mice only have increased mortality when pregnant.
PPARγ, a transcription factor that causes lipid accumulation, insulin sensitivity and reduced inflammation in the vessel wall 15, 16, is typically expressed at relatively low levels in the heart. PPARγ suppresses cardiac growth and embryonic gene expression 17. PPARγ is expressed at higher levels in the human heart, especially in humans with metabolic syndrome, than in the murine heart 18, 19. PPARγ is activated by rosiglitazone and pioglitozone, drugs that are associated with heart failure and, in the case of rosiglitazone, greater cardiac mortality 20. Mice with cardiac-restricted overexpression of PPARγ have abnormal accumulation of intracellular lipids in cardiomyocytes, gradually develop a dilated cardiomyopathy and die suddenly in young adulthood, often prior to a reduction in systolic function; this premature demise is exacerbated by treatment with rosiglitazone 18. We found that these mice have spontaneous ventricular tachyarrhythmias causing sudden death, secondary to electrical remodeling. Although short-term exposure to pioglitazone, a more commonly used PPARγ agonist, had no effect on mortality and spontaneous ventricular arrhythmias in WT mice, pioglitazone further exacerbated the arrhythmic phenotype and increased the mortality in the TG-PPARγ mice. Our findings support an important link between cardiac PPARγ activation, cardiomyocyte lipid accumulation and the cardiac electrophysiological remodeling, and describe a model for the greater incidence of sudden death in patients with diabetes.
Methods
A detailed description of methods and reagents used is provided in the online Supplement.
Telemetry, ECG analysis and Monophasic Action Potential (MAP) Recordings
Telemetry devices (Data Sciences International, model EA-F20) were implanted in 10 week-old mice. Recordings were begun 1 week after implantation. Intervals were measured manually using Ponemah 3 software. For in vivo MAP recordings, under general anesthesia, a thoracotomy was made between the ribs of the left side of the thorax, and a 0.25 mm-tip electrode was pressed lightly against the anterior surface of the left ventricle (LV). The ground electrode was pressed against the inner surface of the rib cage. Signals were amplified and filtered as described21.
Echocardiography
Transthoracic echocardiography was performed on isoflurane-anesthetized mice using a high-resolution imaging system with a 30-MHz imaging transducer (Vevo 770; VisualSonics) 18.
Isolation of Cardiomyocytes and Cellular Electrophysiology
Cardiomyocytes were isolated using methods previously described 22. Membrane currents, of non-contracting rod-shaped cells with clear striations, were measured by the whole-cell patch-clamp method 23 using a MultiClamp 700B amplifier (Axon Instruments, Union City, CA). Solutions and voltage clamp methodologies are further described in the supplement.
Optical mapping
High-resolution optimal mapping experiments were performed on 16-week old TG-PPARγ and WT littermate control mice as previously described 24-26. Briefly, hearts were isolated and perfused by the Langendorff method with warm (37°C) oxygenated Tyrode's solution. After stabilization, the heart was stained with the voltage-sensitive dye Di-4-ANEPPs (8μL of 2-mmol/L stock solution dissolved in DMSO), and contraction was inhibited with blebbistatin (5μM). The heart was stimulated with a platinum electrode at 100 ms intervals.
Real-time PCR
Samples of ventricular tissue from 10-12 week old PPARγ and WT littermate mice were used for RT-PCR. Real-time PCR was performed using an Applied Biosystems StepOne Plus Real-Time PCR system and inventoried primers (Applied Biosystems). PCR reactions were performed, in duplicate, for 40 cycles with automated detection of crossing threshold.
Immunoblots
The preparation and immunoblotting of heart homogenates was performed as described 27. Chemiluminescence signal was obtained using a Kodak Image Station and signal intensities quantified using ImageJ software.
Immunohistochemistry
Heart tissue was fixed with 4% paraformaldehyde, embedded in paraffin wax, and then sectioned. Sections were incubated with anti-Cx43 (1:200) or non-immune rabbit polyclonal IgG at 4°C overnight. For DAB staining, sections were exposed sequentially to 0.3% H2O2, anti-rabbit swine antibody conjugated to biotin (1:500, DakoCytomation) for 1 hr, peroxidase-labeled ABC (VECTASTAIN ABC Kit, Vector Laboratories), and finally developed with DAB solution (ImmPACT DAB Peroxidase Substrate, Vector Laboratories). Sections were counterstained with hematoxylin. For immunofluorescent staining, after reaction with anti-rabbit donkey antibody conjugated to Alexa Fluor 488 (1:500, Invitrogen), sections were counterstained with DAPI.
Statistical Analysis
Results are presented as mean ±SEM. The nonparametric Mann–Whitney U-test was used for comparisons with n<10, and the unpaired t-test with equal variances was used for comparisons of larger groups. A 2-tailed value of P<0.05 was considered statistically significant, except for ECG intervals and PVC burden where the Bonferroni correction was used for multiple comparisons. Linear regression analysis was performed using GraphPad Software.
Results
Two lines of TG-PPARγ1 mice have been developed and reported18, showing similar phenotypes, albeit with different severities. The high-expressing line (MHC-PPARγ1H) demonstrated a reduced ejection fraction by 4 months of age. The MHC-PPARγ1H mice had significantly reduced lifespan, with 50% mortality at 3 months of age, prior to the clinical development of heart failure18. The MHC-PPARγ1H mouse line was used for all experiments.
Young adult PPARγ mice have normal echocardiograms and histology
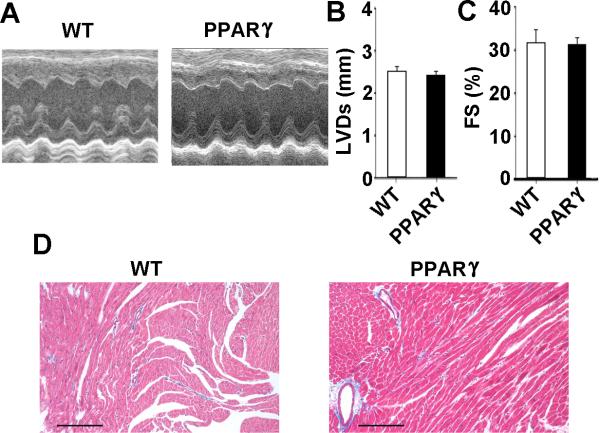
We performed echocardiography of 10-12 week old MHC-PPARγ and WT littermate mice. LV systolic dimension and fractional shortening were within normal limits28 for PPARγ mice at this age (Figure 1A-C). Histological examination did not demonstrate increased fibrosis (Figure 1D). These results suggest that the increased mortality is not related to systolic dysfunction or fibrosis. Gross structural analysis revealed that the thickness and fiber direction across the ventricular walls did not change significantly in the 10-12 week old PPARγ mice compared WT littermate controls (Figure 1D). The PPARγ mice have mild cardiac hypertrophy, since the heart-weight:body-weight ratio is modestly increased, 13% above WT (Supplemental Figure 1), consistent with prior reports from PPARγ–agonist treated mice17.
Figure 1.
Normal cardiac function in 10-12 week old PPARγ mice. A, Representative M-mode echocardiographic images from a wild-type (WT) littermate control and PPARγ mice. B and C, Graphs of left ventricular systolic diameter (LVDs) and fractional shortening. The values are presented as mean + SEM; (n= 6 for WT and 11 for PPARγ). D, Representative Masson's trichrome stain of ventricular tissue from WT littermate and PPARγ mice. Collagen appears as a light blue stain. Scale bars: 70 μm.
PPARγ mice have fatal ventricular arrhythmias
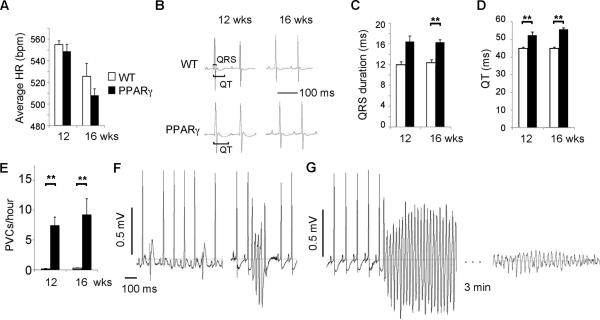
We hypothesized that an arrhythmia was the most likely cause of the sudden death, and implanted telemeters to monitor the heart rhythm of 10 week-old MHC-PPARγ and WT littermate mice. Echocardiograms, performed prior to telemeter-implantation, demonstrated normal LV size and function. The average daily heart rate of the TG-PPARγ mice was similar to WT mice at 12 and 16 weeks of age (Figure 2A). The QRS duration was significantly prolonged for 16 week-old TG-PPARγ mice compared to age-matched WT littermates (Figure 2B, 2C). The QT interval was significantly prolonged at 12 and 16 weeks in TG-PPARγ mice compared with WT (Figure 2B, 2D).
Figure 2.
Electrocardiograms of PPARγ mice. A, Average daily heart rate of 12 week and 16 week old mice, 3 animals in each group. Mean + SEM. B, Representative ECGs of PPARγ mice and WT littermates. QRS and QT intervals are indicated by brackets. C-D, Graphs of QRS duration and QT interval from WT and PPARγ mice. The values are presented as mean + SEM. ** p<0.025 by U-test. E, Graph of PVCs per hour, recorded in unrestrained mice. The values are presented as mean + SEM. n= 4 or 5 mice per group. ** p<0.025 by U-test. F, Representative telemetry recordings of PPARγ mice showing frequent PVCs (left) and non-sustained ventricular tachycardia (right). G, Fatal ventricular tachycardia followed by an agonal rhythm in a PPARγ mouse.
The TG-PPARγ mice had significantly increased ventricular arrhythmias compared to WT at 12 and 16 weeks of age. Premature ventricular complexes (PVCs) were frequent in the PPARγ mice, averaging 7.4 PVCs/hour in 12 week-old mice, compared to less than 0.2 PVCs/hour (approximately 5 PVCs/day) in age-matched WT mice (Figure 2E). Complex ectopy, such as paired PVCs or non-sustained VT, occurred frequently in TG-PPARγ mice (0.08/hour at 12 weeks, 1.2/hour at 16 weeks), and was never observed in WT littermates. Sustained VT was the cause of death in two of three TG-PPARγ mice undergoing long-term-monitoring; the third mouse died from bradycardia. Death due to bradycardia is common in TG mouse models of heart disease, whereas death from spontaneous VT is unusual29, 30.
Prolonged action potential duration (APD) and decreased K+ current density in PPARγ mice
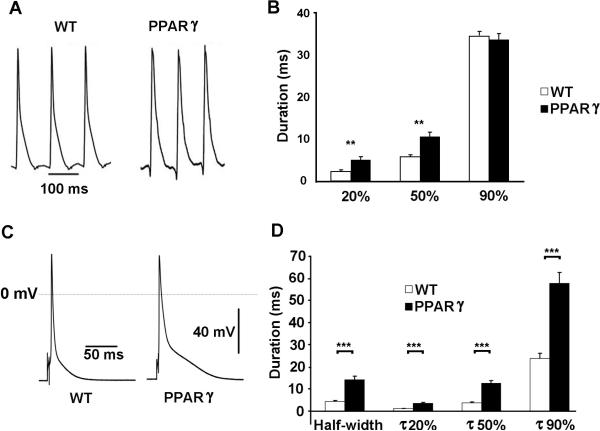
The prolongation of the QT interval may be due to abnormalities in cardiomyocyte depolarization and/or repolarization. Monophasic action potentials (MAP), which are extracellular waveforms, were used to quantify ventricular repolarization. We measured the MAP in WT and TG-PPARγ mice through a small thoracotomy, by placing an electrode on the anterolateral surface of the beating heart during normal sinus rhythm. The APD measurements in WT mice are similar to the in vitro findings previously reported for murine heart 21. We found that the APD20 (PPARγ: 5.2 ± 0.7 ms; WT 2.4 ± 0.3 ms; p<0.01) and APD50 (PPARγ: 10.7 ± 1.0 ms; WT 5.9 ± 0.4 ms; p<0.01) were significantly prolonged in 12-week-old TG-PPARγ mice compared to age-matched littermates (Figure 3A, 3B). The APD90 was not significantly different in TG-PPARγ and WT mice, p=0.44. In the TG-PPARγ mice, the longer phase 2 of the action potential likely causes increased activation of K+ currents in phase 3 of the action potential, enabling a compensatory faster phase-3 repolarization. The prolongation of early repolarization (APD20 and APD50), without significant prolongation of the APD90, can be arrhythmogenic31.
Figure 3.
Action potential waveforms in WT and PPARγ mice. A, Representative in vivo monophasic action potential recordings from WT and PPARγ mice, mean RR interval 117 ms and 113 ms respectively. B, Graph of MAP recordings at 20%, 50% and 90% repolarization. WT: n=6; PPARγ: n=5. Mean ± SEM; **=p<0.01. C, Representative cardiomyocyte action potential waveforms from WT and PPARγ mice. D, Graph of APD at half-width, 20%, 50% and 90% repolarization. WT: n=24; PPARγ: n=39. Mean ± SEM; *** p<0.001.
The action potential waveforms, measured using the perforated patch clamp technique at 35°C and stimulating at 1000ms intervals, were prolonged in the ventricular myocytes from 10-12 week-old TG-PPARγ mice compared to age-matched WT littermate controls (Figure 3C). Consistent with the in vivo MAP recordings, the APD20 and APD50 in the TG-PPARγ cardiomyocytes were significantly increased compared to control cardiomyocytes (p<0.001; Figure 3D). The APD90 in the TG-PPARγ cardiomyocytes was also significantly increased compared to control cardiomyocytes (p<0.001; Figure 3B). The difference in rate between the MAP recordings and patch-clamp recording may explain the difference in APD90 estimates, since rate can modify repolarization currents and calcium handling.
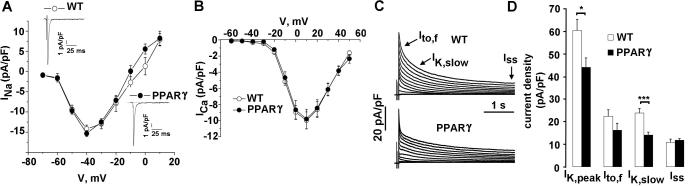
The repolarization phase of the cardiac action potential is dependent upon a balance between inward depolarizing Ca2+ and Na+ currents, and outward repolarizing K+ currents. Whole-cell currents were recorded at room temperature from 10-12 week-old TG-PPARγ mice and corresponding age-matched littermate controls. Voltage-dependent Na+ and Ca2+ current densities and current-voltage (I-V) relationship were similar in the TG-PPARγ and WT mice (Figure 4A, 4B). Boltzmann fits of the activation and inactivation revealed no changes in Na+ and Ca2+ channels’ V50 for activation and for inactivation. A late, persistent Na+ current was not observed in the TG-PPARγ mice (Figure 4A, insets). The voltage-dependent K+ currents, in contrast, were significantly altered in the PPARγ mice compared to the WT mice (Figure 4C). In mice at least four distinct voltage-dependent K+ currents have been identified 32, 33. Peak outward K+ current (IK,peak) was reduced in the PPARγ mice (44.2 ± 4.2 pA/pF) compared to WT mice (60.4 ± 5 pA/pF; p=0.02). The decay phases of the voltage-dependent K+ currents in adult mouse cardiomyocytes may be fit 13 by the sum of two exponentials, which denote the fast transient K+ current, Ito,f, a rapidly activating, very slowly inactivating current, IK,slow, and a non-inactivating current, Iss32, 33. Analysis of the decay phases demonstrated a statistically significant reduction in the current density of IK, slow (WT: 23.8 ± 2.1 pA/pF; PPARγ : 14.1 ± 1.3 pA/pF; p<0.001), and a non-statistically significant reduction in current densities of Ito,f (WT: 22.4 ± 2.8 pA/pF; PPARγ: 16.2 ± 3 pA/pF; p=0.15) (Figure 4C). There was no significant change in the current density of Iss (WT: 10.8 ± 0.8 pA/pF; PPARγ: 11.8 ± 0.8 pA/pF; p=0.34) (Figure 4C, 4D) or the current density of the inward rectifying current, IK1 (not shown), in the TG-PPARγ mice compared to WT mice. Cardiomyocytes had the same average size as shown by whole-cell membrane capacitance; the resting membrane potential and action potential amplitude (APA) were not significantly different in the PPARγ and WT littermate controls (see Supplement table 1.).
Figure 4.
Properties of Na+, Ca2+ and K+ currents. A, I-V relationship for Na+ current in WT and PPARγ mice. Insets: WT and PPARγ current traces. B, I-V relationship for Ca2+ current. C, Voltage-dependent K+ current tracings for WT and PPARγ mice. Cardiomyocytes were held at a holding potential of -80 mV. A brief (15 ms) voltage step to -40 mV was used to activate and inactivate Na+ channels. Following a brief return to -80 mV, voltage-dependent K+ currents were elicited with voltage steps between -60 mV and +50 mV in 10 mV-increment, for 4 sec with 10 sec interval. D, Graph of current density of IK, peak, Ito,f, IK,slow and Iss currents. The decay phases of the outward K+ currents evoked during 4.0 sec depolarizing voltage step to +40 mV were fit by a double exponential function. Mean + SEM. WT: n=43; PPARγ: n=31. * =p <0.05, *** =p <0.001
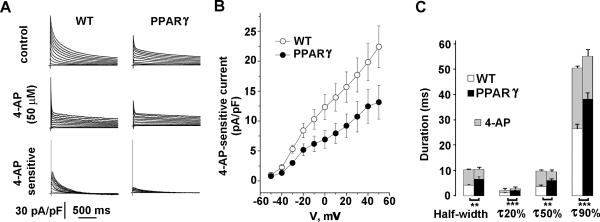
The reduction in the IK,slow current density in the TG-PPARγ mice was confirmed using a pharmacological approach. After exposure to 50 μmol/L 4-AP, WT cardiomyocytes showed a marked reduction in IK,slow (Figure 5A). The 4-AP-sensitive IK,slow current, however, was markedly reduced in cardiomyocytes from PPARγ mice (Figure 5A, 5B). Linear regression of the 4-AP-sensitive current demonstrated a significant difference in the slopes (pA/pF/mV) for the WT and PPARγ mice (WT: 0.211 ± 0.002; PPARγ: 0.126 ± 0.002; p=0.002; n=21 and 26, respectively). These data suggest that the prolongation of APD in PPARγ mice was primarily due to a reduction in IK,slow current. We also measured the effect of 4-AP inhibition on APD concurrently, by switching from voltage-clamp to current clamp mode. Intracellular calcium was buffered to 10 nM, at a temperature of 22°C to optimize cardiomyocyte condition. Under these conditions, the APD in PPARγ was still prolonged. Blocking 4-AP-sensitive current in WT cardiomyocytes prolonged the APD to a larger extent than in PPARγ cardiomyocytes. After exposure to 4-AP, the APD was not significantly different in WT and PPARγ mice (Figure 5C).
Figure 5.
Reduced IK,slow is responsible for APD prolongation in TG-PPARγ mice. A, Representative voltage-dependent K+ current tracings from WT and PPARγ mice (top), after exposure to 50 μM 4-AP (middle) and the 4-AP sensitive current (bottom). Patch clamp protocols are described in Methods and Figure 4. 4-AP sensitive current was obtained by off-line digital subtraction of records before and after 4-AP application. B, Graph of current density of 4-AP sensitive current for WT and TG-PPARγ mice. WT: n=26; PPARγ: n=21. Mean ± SEM. C, Graph of APD before and after (grey) 4-AP exposure. Prior to 4-AP exposure, differences at half-width, 20%, 50% and 90% repolarization between WT and PPARγ are statistically significant (** p<0.01, *** p<0.001). After 4-AP (grey bars), differences between WT and PPARγ are not statistically significant. WT: n=34; PPARγ: n=22. Mean + SEM.
Reduced expression of voltage-dependent K+ channels in PPARγ hearts
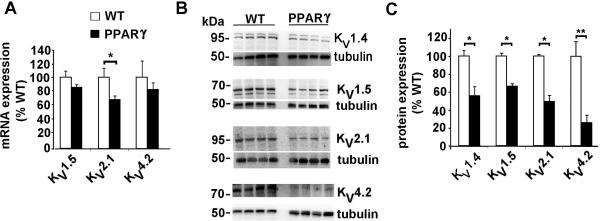
Gene expression was determined by real-time quantitative PCR of RNA extracted from the ventricular tissue of 10-12 week mice. The mRNA expression of KV2.1, which contributes to IK,slow34, 35 was significantly reduced in the TG-PPARγ mice, as compared to WT littermates. The mRNA expression of KV4.2, which encodes Ito,f36 was not significantly reduced. In contrast to larger animals, KV4.3 is not required for functional Ito,f in the mouse 37. Protein expression of the voltage-dependent K+ channels was measured using ventricular homogenates. In contrast to the modest or absent changes in mRNA expression, the protein expression of these channels was markedly reduced in the TG-PPARγ mice (Figure 6B, 6C). The protein levels of KV1.5 and KV2.1, that form IK,slow in mouse, was significantly reduced to 67% and 50%, respectively, of WT littermate controls. These reductions in protein expression are consistent with the findings of reduced IK,slow current density (Figure 5). The protein expression of KV4.2, which forms Ito,f, was also markedly reduced in the PPARγ mice, to 27% of the control level. Although the current density of Ito,f was reduced in the PPARγ mice, the reduction in protein expression of this subunit is far greater, suggesting up-regulation of activity of the remaining KV4.2. The Kv1.4 channel, which also contributes to Ito, is also reduced significantly.
Figure 6.
mRNA and protein expression of K+ channels. A, mRNA expression, measured by real-time qPCR of K+ channel pore-forming α subunits of IK,slow and Ito,f. n= 4 or 5 animals in each group. * p<0.05 by U-test. B, Immunoblots of K+ channels with tubulin loading controls. C, Bar graph of protein expression of indicated K+ channel subunits in PPARγ mice (black bars) relative to WT littermate mice (white bars). Mean image intensity in arbitrary units (measured using ImageJ) was normalized to tubulin signal and expressed as percent of WT control mice. Mean + SEM. * p<0.05; ** p<0.01 by U-test
Prolongation of APD does not cause triggered activity
To better understand the mechanisms of ectopy and arrhythmia, ventricular myocytes were paced through the patch pipette in perforated configuration by current pulses (amplitude 0.1 - 0.4 nA, 3 ms duration) at intervals from 1000 to 250 msec. Under these conditions, we did not observe any early after-depolarizations (EADs) or delayed after-depolarizations (DADs) in PPARγ or WT cardiomyocytes (n=32, from 3 mice in each group).
Connexin43 is reduced but conduction velocity is normal
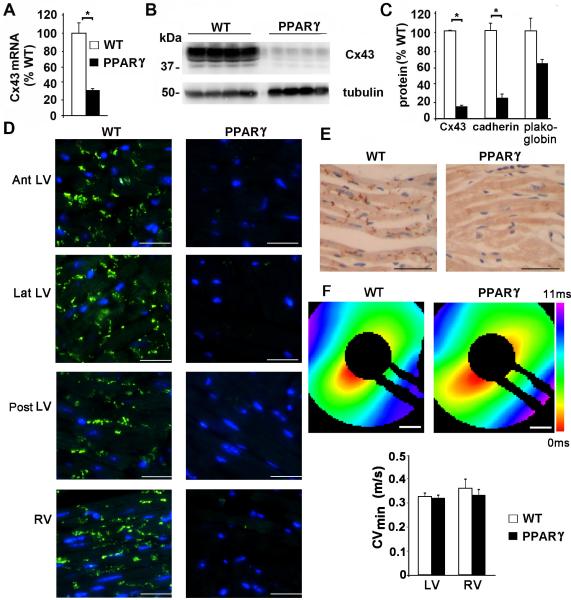
Connexin43 (Cx43, the major ventricular gap junction protein) mRNA and protein expression was significantly downregulated in the PPARγ mice, to 31% (Figure 7A) and 14% (Figure 7B, 7C) respectively of WT littermates. Other intercalated disk proteins, such as cadherin and plakoglobin were also reduced (Figure 7C). Cx43 was decreased throughout the heart, as determined by immunohistochemistry of sections isolated from the anterior (Ant), lateral (Lat), and posterior (Post) walls of the LV, and the right ventricle (RV) (Figure 7D). The reduction in Cx43 occurred prior to the development of systolic dysfunction. To assess the effect of Cx43 reduction on ventricular impulse propagation, we optically mapped cardiac activation patterns in the PPARγ and WT littermate mice. Surprisingly, the minimum conduction velocity (CVmin) was not significantly reduced in the PPARγ mice compared to the control littermate mice (p=0.55 for LV) (Figure 7F). Thus, it is not likely that the reduction in expression of Cx43 accounts for the increased propensity to develop ventricular arrhythmias and sudden death.
Figure 7.
Reduced Cx43 mRNA and protein expression in PPARγ mice. A, mRNA expression of Cx43, normalized to WT. n= 4 mice in each group. * p<0.05 by U-test. B, Immunoblot of Cx43 (with tubulin as a loading control) from mouse ventricular tissue, n=4 mice in each group. * p<0.05 by U-test. C, Graph of Cx43 expression in PPARγ mice, normalized to WT littermate control. D, Immunofluorescence of Cx43 protein (bright green) from indicated ventricular tissue. Scale bar: 75 μm. E, DAB staining for Cx43 (dark brown) from WT littermate and PPARγ mice. Scale bar: 45 μm. F, Representative activation maps showing conduction across the ventricular epicardium of control and TG-PPARγ mice. Scale bar: 1 mm. Lower, bar graph of LV and RV CVmin for WT and PPARγ mice.
PPARγ agonist, pioglitazone, increases ventricular arrhythmias in TG-PPARγ mice
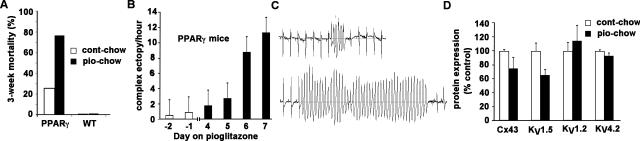
TG-PPARγ and WT littermates, 10-12 weeks of age, were fed standard mouse chow or pioglitazone-impregnated standard mouse chow for 3 weeks. Pioglitazone increased the mortality in the PPARγ mice (pioglitazone-chow: 75% vs. control-chow: 25%, n=8 in each group) during the 3-week period, but had no effect on mortality in the WT littermate animals (Figure 8A). To determine the mechanism(s) responsible for the pioglitazone-induced increased mortality in the TG-PPARγ mice, ECG telemeters were implanted into four 10-week-old TG-PPARγ mice and four WT littermate mice. Baseline telemetric measurements were initiated 4 days post-implantation. Mice were then fed pioglitazone for 7 days. For TG-PPARγ mice, within 4 days of starting pioglitazone, there was a significant increase in PVCs per hour; by day 6 of pioglitazone-chow the number of PVCs per hour was 15 times the baseline rate (p=0.0285 by U-test). Complex ventricular ectopy also increased approximately 15-fold over the same time period (Figure 8B, 8C). Linear regression modeling of the time course shows a slope that is significantly different from zero (F-test = 0.017). Complex ventricular arrhythmias were never observed in the WT littermate mice, in the absence or presence of pioglitazone ingestion, and even after 3 weeks of pioglitazone chow the WT mice did not have an increase in PVCs/hour. During the relatively short (and intermittent) monitoring period, sudden cardiac death was captured in one TG-PPARγ animal caused by spontaneous polymorphic ventricular tachycardia degenerating to ventricular fibrillation. We examined the ion channel protein expression from the hearts of TG-PPARγ mice fed either control-or pioglitazone-chow for 1 week. KV1.5, which encodes IKslow, was decreased by 35% (Figure 8C) in the pioglitazone-fed TG-PPARγ mice compared to control-chow-fed TG-PPARγ mice (p=0.11 by U-test). These results suggest that PPARγ-agonist induced activation of PPARγ, when over-expressed in the heart, has a deleterious effect on mortality and arrhythmogenesis.
Figure 8.
Pioglitazone increases mortality and ventricular arrhythmias in TG-PPARγ, but not WT mice. A, Bar graph of 3-week mortality of TG-PPARγ and WT mice fed either control-chow or pioglitazone-chow. n=8 in each group. B, Bar graph of average complex ventricular ectopy per hour in the PPARγ mice fed control (white bars) and pioglitazone-chow (black bars). Error bars are SEM. n=4 mice. C, Representative telemetry recordings of TG-PPARγ mice fed pioglitazone showing non-sustained ventricular tachycardia. D, Bar graph of protein expression of Cx43 and K+ channel subunits in TG-PPARγ mice fed pioglitazone (black bars) relative to TG-PPARγ mice fed control chow (white bars). Mean image intensity was normalized to tubulin signal and expressed as percent of control-chow fed TG-PPARγ. Mean + SEM, n=4 animals in each group.
Discussion
Despite the increasing prevalence of obesity and diabetes, little is known about the contribution of metabolic abnormalities to the pathophysiology of arrhythmias and sudden cardiac death. Increased cardiomyocyte lipid stores are observed in obese and diabetic patients and this may contribute to arrhythmias. In diabetic patients, plasma free fatty acid concentration correlates with the frequency of ventricular premature complexes, and patients with ischemic cardiomyopathy and obesity have more VT than non-obese patients with ischemic cardiomyopathy 38, 39. Diabetes and obesity are also associated with an increased risk of cardiomyopathy, independent of the presence of hypertension or coronary artery disease, which may represent a direct toxic effect of increased intracellular lipids 2, 40. We used a gain-of-function approach to show that cardiomyocyte-specific metabolic derangements associated with the cardiac-specific overexpression of PPARγ leads to ventricular arrhythmias and sudden cardiac death. The over-expression of PPARγ, which can regulate transcription of numerous targets, and/or the subsequent cardiomyocyte lipid accumulation led to reductions in the expression and current density of key repolarizing currents, prolongation of the APD in vitro and in vivo, and the reduced expression of intercalated disc proteins. The electrical remodeling of the heart ultimately caused a markedly increased incidence of malignant ventricular arrhythmias and sudden cardiac death, which occurred prior to the onset of systolic dysfunction, but after the development of cardiomyocyte lipid accumulation. Treating the TG-PPARγ mice, but not WT littermate controls, with a PPARγ agonist increased the incidence of arrhythmias and mortality.
Although PPARγ expression is normally relatively low in the heart, its expression is increased in several forms of heart disease including cardiomyopathy 41, cardiac hypertrophy 42, 43, and in the diabetic heart 44. Despite its low expression in the heart, tissue-specific loss of PPARγ leads to cardiac hypertrophy with preserved systolic function 17. PPARγ suppresses cardiac growth and embryonic gene expression and inhibits nuclear-factor κB activity in vivo 17 and may also suppress inflammation 45. The cardiac metabolic abnormalities found in the TG-PPARγ mice, specifically the lipid accumulation within cardiomyocytes 18, mimic many of the abnormalities found in the hearts of diabetic and obese/metabolic syndrome patients 19. The tissue-restricted TG over-expression of PPARγ enables the preferential shunting of plasma triglycerides and fatty acids to the heart. Pioglitazone-treatment in TG-PPARγ mice would further enhance cardiac PPARγ activity. The systemic effects of pioglitazone in WT mice, in contrast, by channeling a greater proportion of plasma triglycerides and fatty acids to adipose tissues, may actually reduce lipid uptake by the heart relative to the periphery 46. The differential activation of cardiac vs. peripheral PPARγ, and the shunting and accumulation of lipid in either the heart or the periphery likely account for the TG phenotype and effects of pioglitazone. In WT animals, pioglitazone had no effect on mortality or incidence of ventricular tachyarrhythmias because pioglitazone activation of adipose tissue PPARγ predominates. In the TG-PPARγ mice, although pioglitazone likely activated both cardiac and adipose PPARγ, the level of expression of the cardiac PPARγ likely favored additional cardiomyocyte lipid accumulation and increased electrical remodeling, as reflected by an increased incidence of ventricular tachyarrhythmias, reduced expression of K+ channels and increased mortality. In humans, the underlying ratio of adipose to cardiac PPARγ expression and the differential effects of different PPARγ agonists – e.g. pioglitazone versus rosiglitazone – likely modulates the beneficial versus toxic effects of these drugs on the heart.
The molecular mechanisms responsible for the reduction in K+ channel expression in the TG-PPARγ mice are not known. The PPARγ over-expression and lipid loading may exert their effects via transcriptional, translational and/or post-translational processes. Cardiac-specific over-expression of PPARα, a key regulator of diabetes-induced lipid metabolic dysregulation, also induced ion channel remodeling, predominantly a reduction of Ito,f and a compensatory increase in Iss13. No change in IK,slow current was found in young TG-PPARα mice. In MHC-FATP TG mice, however, the Ipeak and IK,slow currents were selectively attenuated, without change in Ito,f and Iss14.. In our study of the cardiac-specific overexpression of MHC-PPARγ mice, Ipeak and IK,slow were significantly reduced, with a trend towards reduction in Ito,f current. The remodeling of K+ channels in the TG-PPARγ mice is similar to the remodeling found in the MHC-FATP mice. In the TG-PPARα and TG-FATP mice, the electrophysiological phenotypes were less severe than the TG-PPARγ mice, and sudden cardiac death (in non-pregnant animals) was not reported 13, 14. Different transcriptional and/or post-transcriptional pathways may be modified in these mice, which cause changes in distinct K+ currents. In all three animal models, fatty acid accumulation and utilization are increased. Glucose uptake and metabolism are decreased in the MHC-FATP and MHC-PPARα mice, whereas in the MHC-PPARγ mice, glucose uptake is increased.
Cardiac-specific loss of Cx43 is associated with significant conduction slowing and a higher propensity for the development of ventricular arrhythmias and sudden death 24, 26. Large reductions of Cx43 expression levels are required to significantly affect epicardial conduction velocities. A 50% reduction of Cx43 protein does not produce significant conduction slowing, but a 70% to 95% reduction of Cx43 protein results in reduced conduction velocity, increased dispersion of conduction, and enhanced arrhythmogenicity 47-49. The ~12 week-old TG-PPARγ mice demonstrate ~70% reduction in Cx43 mRNA and ~85% reduction in Cx43 protein amounts. The lack of significant slowing of the conduction velocity in the TG-PPARγ mice suggests that other proteins or post-translational modifications must be compensating for the marked reduction in Cx43 protein levels. Furthermore, the finding of normal conduction velocity implies that the reduction in Cx43 protein in the TG-PPARγ mice does not contribute significantly to the increased incidence of ventricular arrhythmias and mortality. The wide QRS may be due to damage to the His-Purkinje system rather than reduced Cx43 levels. The reduced levels of other intercalated disk proteins is probably not a direct effect of PPARγ since in some cancer models, PPARγ overexpression increases cadherins50. The reduction in intercalated disk proteins may contribute to the reduction in Cx43 protein.
In summary, we have found that overexpression of PPARγ in the heart is sufficient to induce action potential remodeling and to cause an acquired long-QT syndrome phenotype. The reduced repolarization reserve increases the incidence of spontaneous ventricular arrhythmias and sudden death. Pioglitazone-treatment increases the incidence of complex ventricular arrhythmias and sudden death in the TG-PPARγ mouse, but not WT littermates. Although an important limitation of this work is that the ion channels responsible for cardiac action potential repolarization in mice are different than in humans, the TG-PPARγ mouse recapitulates an arrhythmic phenotype observed in patients with diabetes and metabolic syndrome.
Supplementary Material
Mice with cardiac overexpression of PPARγ have impaired repolarization and spontaneous fatal ventricular arrhythmias
SUPPLEMENTAL MATERIAL
Supplemental Online Methods
Animal Care and Breeding
The PPARγ transgenic mouse has cardiac-specific overexpression of PPARγ driven by the myosin heavy chain (MHC) promoter as previously described 1. Animal protocols were approved by the Columbia University Institutional Animal Care and Use Committee and were carried out in accordance with the NIH guidelines for the care and use of laboratory animals. Pioglitazone-chow (30 mg/kg) was purchased from Research Diets2.
Telemetry and ECG analysis
Telemetry devices (Data Sciences International, model EA-F20) were implanted in 10 week-old mice using inhaled isoflurane anesthesia. The two subcutaneous leads were positioned to approximate limb lead II of a human ECG. The mice recovered for one week after surgery before initiating 24-hour recordings. ECG intervals were measured manually, blinded to genotype, using Ponemah 3 software from recordings with minimal artifact at heart rates of 520-550 bpm to avoid the issues of correcting QT for rate. Intervals were averaged from 4 consecutive beats. PVC and arrhythmias counts were tallied manually by review of daily telemetry recordings. Daily heart rates were averaged for 3 animals in each group.
Echocardiography
Transthoracic echocardiography was performed using a high-resolution imaging system with a 30-MHz imaging transducer (Vevo 770; VisualSonics, Toronto, ON, Canada). The mice were anesthetized with isoflurane throughout the procedure. Care was taken to minimize sedation by monitoring the heart rate and respiratory rate of the mice. Images were obtained using short-axis views at the level of papillary muscles, and each parameter was measured using M-mode view. Images were recorded in a digital format and were then analyzed off-line. Percent fractional shortening (%FS) was calculated as follows: %FS = (LVDd-LVDs)/LVDd X 100), where LVDd is left ventricular diastolic dimension and LVDs is left ventricular systolic dimension.
Isolation of Cardiomyocytes and Cellular Electrophysiology
Cardiomyocytes were isolated using methods previously described 3. Briefly, the mouse heart was removed and the aorta was cannulated. After perfusing Ca2+-free buffer for two min, a mixture of collagenase and protease was then perfused through the coronary arteries for 5-7 min (Blendzyme 4 or Liberase TH, 0.3 mg/mL, Roche) at a [Ca2+] =12.5 μM. The LV tissue was teased apart with fine forceps and briefly pipetted to release individual cells. After enzymatic dispersion, [Ca2+] in the buffer containing 3.5 mg/ml BSA was elevated in 4 steps up to 0.8 mM. Cells were transferred into experimental temperature controlled chamber (Delta T Culture Dish, Bioptechs Inc). Only non-contracting rod shaped cells with clear striations were used in this study. Experiments were performed on freshly isolated cardiomyocytes from left ventricle of PPARγ mice (10 animals) and their littermate controls (12 animals).
Membrane currents were measured by the whole-cell patch-clamp method 4 using a MultiClamp 700B amplifier (Axon Instruments, Union City, CA). For action potential duration (APD) measurements (Fig. 3), perforated, whole-cell patch-clamp was utilized, using amphotericin B (300 μg/ml; Sigma A9528) at 35°C. Micropipettes were pulled from borosilicate glass capillaries (BF150-110-7.5, Sutter Instruments, Novato CA) on a programmable horizontal puller (S-97; Sutter Instruments, Novato CA). The pipettes had inner tip diameters of about 1 to 1.5 μm. When filled with internal solutions, they had resistances of 1.5 to 2.5 MΩ. Data were filtered at 4 KHz with a four-pole low-pass Bessel filter and sampled at 10 KHz. All experiments were performed using pCLAMP 10.2 software (Axon Instruments, Union City CA). Boltzman's fits were performed as previously described 5.
To record action potentials and K+ currents, the pipette solution contained 130 mM K+ gluconate, 10 mM NaCI, 10 mM EGTA , 1 mM MgCl2, 2 mM Mg-ATP, 2.0 mM CaCl2 and 10 mM HEPES, adjusted to pH 7.2 with KOH. Cells were superfused at room temperature with HEPES-buffered Tyrode's containing 137 mM NaCl, 5.4 mM KCI, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM glucose, and 10 mM HEPES (pH was adjusted to 7.4 with NaOH). Voltage clamp correction for a liquid junction potential of -13.6 mV was made by configuring the recording files in CLAMPEX of pCLAMP 10.2. Series resistances were usually less than 2 MΩ after 60% compensation. All voltages in current clamp recording were also corrected for the junction potential.
The decay phases of the outward K+ currents evoked during 4.0 s depolarizing voltage steps were fitted by a double exponential function of the form:
where t is time, and are the decay time constants, A1 and A2 are the amplitudes of the inactivating current components (Ito,f and IK,slow), and B is the amplitude of the non-inactivating current component, Iss
To study L-type Ca2+ current, we used a bath solution containing 140 mM TEA-Cl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES (pH was adjusted to 7.4 with CsOH). In these experiments, pipettes were filled with solution contained 135 mM CsCI, 10 mM EGTA, 1 mM Mg Cl2, 2 mM Mg-ATP, 2.0 mM CaCl2 and 10 mM HEPES, adjusted to pH 7.2 with CsOH. Series resistance was usually less than 2 MΩ after 60% compensation. Leak currents and capacitance transients were subtracted by a P/4 protocol. Voltage clamp correction for a liquid junction potential of -3.6 mV was made by configuring the recording files in CLAMPEX of pCLAMP 10.2. To evaluate the steady state activation of Ca2+ currents, the cell membrane potential was held at -70 mV and stepped for 450 ms to -60 to +50 mV in 10 mV increments. The interval between pulses was 10 s. To study the steady state inactivation of Ca2+ currents, the cell membrane potential was held at -70 mV and stepped to -70 to +30 mV for 650 ms in 10 mV increments and then stepped to the test potential +10 mV for 650 ms. The interval between pulses was 10 sec. Na+ currents were measured as described previously 6.
Action potential parameters, K+, Na+ and Ca2+ currents were measured and analyzed using pCLAMP 10.2, Excel and Origin 7.5 (OriginLab, Northampton, MA) software. V1/2 and k were calculated from Boltzmann function fitting for each cell. Statistical significance of observed differences were evaluated using TTEST (p<0.05). Regression analysis was done using GraphPad Software.
Real-time PCR
Samples of ventricular tissue from PPARγ mice and WT littermates, at 10-12 weeks of age, were used for harvesting RNA for RT-PCR. Cardiac tissue was homogenized with a Mini-BeadBeater (Glen Mills, Inc). RNA was then purified using a Qiagen RNeasy kit (item 74104). cDNA was synthesized using the Applied Biosystems high capacity RNA to cDNA kit (#4387406) and diluted to 10 ng/μL for use as a template (20 ng template was used for each 20 μL reaction). Real-time PCR was performed using an Applied Biosystems StepOne Plus Real-Time PCR system with StepOne Software v2.0 and inventoried primers from Applied Biosystems. PCR was performed for 40 cycles with automated detection of crossing threshold. PCR reactions were performed with duplicate wells with actin as a control reaction and no-template lanes for negative controls.
Immunoblots
Cardiac lysates were made by homogenizing ventricular tissue in buffer containing 1% (v/v) Triton X-100, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 10 mM EDTA, 10 mM EGTA, phosphatase inhibitor cocktail, and protease inhibitors (complete mini-tablet, calpain I and II inhibitors, Roche). Lysate were used for PAGE and then transferred to nitrocellulose membranes for immunoblots. The following antibodies were used: anti-Cx43 (Invitrogen), anti-KV1.5 and anti-KV2.1 (Alomone), anti-KV4.2 and anti-KV1.4 (Thermo-Scientific Pierce), and anti-tubulin (Santa Cruz Biotechnology). Chemiluminescence signal was obtained using a Kodak Image Station 400R Pro digital camera with Kodak Molecular Imaging Software v4.5.1. Signal intensity was quantified using ImageJ software (NIH). Blots for tubulin were performed to normalize loading of lanes, using the same membrane.
Immunohistochemistry
Heart tissue was fixed with 4% paraformaldehyde, embedded in paraffin wax, and then sectioned. Sections were deparaffinized and underwent antigen retrieval treatment (autoclaved with pH 9.0 Tris-buffer at 121°C for 15 min). For DAB staining, sections were treated with 0.3% H2O2 to block endogenous peroxidase. Sections were incubated with rabbit polyclonal antibodies against Cx43 (1:200 dilution, Invitrogen Corp.) or non-immune rabbit polyclonal IgG at the same concentration, at 4°C overnight. For DAB staining, after exposure to anti-rabbit swine antibody conjugated to biotin (1:500, DakoCytomation Denmark A/S, Glostrup, Denmark) for 1 hr in room temperature, the sections were treated with peroxidase-labeled ABC (VECTASATIN ABC Kit, Vector Laboratories, Inc., Burlingame, CA) and developed with DAB solution (ImmPACT DAB Peroxidase Substrate, Vector Laboratories, Inc.). After the reaction, the sections were counterstained with hematoxylin and observed by a light microscope. For immunofluorescent staining, after reaction with anti-rabbit donkey antibody conjugated to Alexa Fluor 488 (1:500, Invitrogen Corp.), the sections were counterstained with DAPI and observed by a fluorescent microscope. Tissues from two mice in each group (PPARγ overexpression and WT littermates) were used. Slides were photographed with a digital camera. Signal intensity was quantified using ImageJ software (NIH).
Supplement References:
1. Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791-2801.
2. Raphael KL, Strait KA, Stricklett PK, Baird BC, Piontek K, Germino GG, Kohan DE. Effect of pioglitazone on survival and renal function in a mouse model of polycystic kidney disease. Am J Nephrol. 2009;30:468-473.
3. O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271-296.
4. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85-100.
5. Yang L, Katchman A, Morrow JP, Doshi D, Marx SO. Cardiac L-type calcium channel (Cav1.2) associates with gamma subunits. FASEB J. 2011;25:928-936.
6. Knollmann BC, Knollmann-Ritschel BE, Weissman NJ, Jones LR, Morad M. Remodelling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J Physiol. 2000;525:483-498.
Supplemental Figure 1. Heart weight, body weight and heart weight:body weight ratio for WT and PPARγ mice. Mean + SEM. n=8, * indicates p<0.05 by U-test.
Supplemental Table 1. Additional patch-clamp data. WT: n=43; PPARγ: n=31. None of the comparisons were statistically significant by t-test
CLINICAL PERSPECTIVE.
Diabetes and obesity confer an increased risk of sudden cardiac death and are associated with cardiomyocyte lipid accumulation and altered cardiac electrical properties (demonstrated by prolongation of the QRS and QT intervals). In order to study the effects of metabolic abnormalities on arrhythmias without the complex systemic effects of diabetes and obesity, we studied a mouse model with cardiac-specific overexpression of PPARγ, a transcription factor that is a key regulator of glucose and lipid metabolism. These PPARγ-transgenic mice develop abnormal accumulation of intracellular lipids and die as young adults, prior to a significant reduction in systolic function. We found that these mice have prolongation of the QT interval, and spontaneous ventricular arrhythmias, including polymorphic ventricular tachycardia and ventricular fibrillation. Isolated cardiomyocytes demonstrated prolonged action potential duration caused by reduced potassium currents, which are responsible for repolarization. Short-term exposure to pioglitazone, a PPARγ agonist, had no effect on mortality or rhythm in WT mice, but further exacerbated the arrhythmic phenotype and increased the mortality in the PPARγ mice. Our findings support an important link between PPARγ activation, cardiomyocyte lipid accumulation, ion channel remodeling and increased cardiac mortality. This mouse model may help identify the molecular mechanisms leading to sudden death in diabetic and/or obese patients
Acknowledgments
Funding
JPM is supported by the Louis V. Gerstner, Jr. Foundation, Lewis Katz Cardiovascular Research Prize, and NIH K08HL105801. SOM is supported by NIH grants HL068093 and P01 HL081172, the Arlene and Arnold Goldstein Family Foundation, and the Lewis Katz Cardiovascular Research Prize. IJG is supported by NIH grants, HL45095 and HL73029. JD is supported by NIH grant, P01 HL081172. GEM is supported by NIH grant HL076751. CV is supported by NIH grant 1T32HL098129.
Footnotes
Subjects: Arrhythmias, Energy metabolism, Animal models of human disease, Ion channels
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 2.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 3.Harmancey R, Wilson CR, Taegtmeyer H. Adaptation and maladaptation of the heart in obesity. Hypertension. 2008;52:181–187. doi: 10.1161/HYPERTENSIONAHA.108.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filippi A, Sessa E, Jr., Mazzaglia G, Pecchioli S, Jr., Capocchi R, Jr., Caprari F, Scivales A, Cricelli C. Out of hospital sudden cardiac death in Italy: a population-based case-control study. J Cardiovasc Med (Hagerstown) 2008;9:595–600. doi: 10.2459/JCM.0b013e3282f2c9d0. [DOI] [PubMed] [Google Scholar]
- 5.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 6.Balkau B, Jouven X, Ducimetiere P, Eschwege E. Diabetes as a risk factor for sudden death. Lancet. 1999;354:1968–1969. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 7.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 8.Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, Chakravarti A, Folsom AR, Siscovick DS, Rosamond WD. Diabetes and the risk of sudden cardiac death, the Atherosclerosis Risk in Communities study. Acta Diabetol. 2009;47:161–168. doi: 10.1007/s00592-009-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Noord C, Sturkenboom MC, Straus SM, Hofman A, Kors JA, Witteman JC, Stricker BH. Serum glucose and insulin are associated with QTc and RR intervals in nondiabetic elderly. Eur J Endocrinol. 2010;162:241–248. doi: 10.1530/EJE-09-0878. [DOI] [PubMed] [Google Scholar]
- 10.Nagaya T, Yoshida H, Takahashi H, Kawai M. Heart rate-corrected QT interval in resting ECG predicts the risk for development of type-2 diabetes mellitus. Eur J Epidemiol. 25:195–202. doi: 10.1007/s10654-009-9423-y. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Bai Y, Sun K, Xue H, Wang Y, Song X, Fan X, Song H, Han Y, Hui R. Patients with metabolic syndrome have prolonged corrected QT interval (QTc). Clin Cardiol. 2009;32:E93–99. doi: 10.1002/clc.20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marionneau C, Aimond F, Brunet S, Niwa N, Finck B, Kelly DP, Nerbonne JM. PPARalpha-mediated remodeling of repolarizing voltage-gated K+ (Kv) channels in a mouse model of metabolic cardiomyopathy. J Mol Cell Cardiol. 2008;44:1002–1015. doi: 10.1016/j.yjmcc.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 15.Marx N, Froehlich J, Siam L, Ittner J, Wierse G, Schmidt A, Scharnagl H, Hombach V, Koenig W. Antidiabetic PPAR gamma-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:283–288. doi: 10.1161/01.atv.0000054195.35121.5e. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Chawla A. Role of PPARgamma in macrophage biology and atherosclerosis. Trends Endocrinol Metab. 2004;15:500–505. doi: 10.1016/j.tem.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 18.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marfella R, Di Filippo C, Portoghese M, Barbieri M, Ferraraccio F, Siniscalchi M, Cacciapuoti F, Rossi F, D'Amico M, Paolisso G. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J Lipid Res. 2009;50:2314–2323. doi: 10.1194/jlr.P900032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 21.Knollmann BC, Katchman AN, Franz MR. Monophasic action potential recordings from intact mouse heart: validation, regional heterogeneity, and relation to refractoriness. J Cardiovasc Electrophysiol. 2001;12:1286–1294. doi: 10.1046/j.1540-8167.2001.01286.x. [DOI] [PubMed] [Google Scholar]
- 22.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 23.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 24.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroud DM, Gaussin V, Burch JB, Yu C, Mishina Y, Schneider MD, Fishman GI, Morley GE. Abnormal conduction and morphology in the atrioventricular node of mice with atrioventricular canal targeted deletion of Alk3/Bmpr1a receptor. Circulation. 2007;116:2535–2543. doi: 10.1161/CIRCULATIONAHA.107.696583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Doshi D, Morrow J, Katchman A, Chen X, Marx SO. Protein kinase C isoforms differentially phosphorylate Ca(v)1.2 alpha(1c). Biochemistry. 2009;48:6674–6683. doi: 10.1021/bi900322a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stypmann J, Engelen MA, Troatz C, Rothenburger M, Eckardt L, Tiemann K. Echocardiographic assessment of global left ventricular function in mice. Lab Anim. 2009;43:127–137. doi: 10.1258/la.2007.06001e. [DOI] [PubMed] [Google Scholar]
- 29.Monnig G, Wiekowski J, Kirchhof P, Stypmann J, Plenz G, Fabritz L, Bruns HJ, Eckardt L, Assmann G, Haverkamp W, Breithardt G, Seedorf U. Phytanic acid accumulation is associated with conduction delay and sudden cardiac death in sterol carrier protein-2/sterol carrier protein-x deficient mice. J Cardiovasc Electrophysiol. 2004;15:1310–1316. doi: 10.1046/j.1540-8167.2004.03679.x. [DOI] [PubMed] [Google Scholar]
- 30.Salama G, London B. Mouse models of long QT syndrome. J Physiol. 2007;578:43–53. doi: 10.1113/jphysiol.2006.118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Guo W, Nerbonne JM. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol. 1999;113:661–678. doi: 10.1085/jgp.113.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol. 2004;559:103–120. doi: 10.1113/jphysiol.2004.063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.London B, Guo W, Pan X, Lee JS, Shusterman V, Rocco CJ, Logothetis DA, Nerbonne JM, Hill JA. Targeted replacement of KV1.5 in the mouse leads to loss of the 4-aminopyridine-sensitive component of I(K,slow) and resistance to drug-induced qt prolongation. Circ Res. 2001;88:940–946. doi: 10.1161/hh0901.090929. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Barry DM, Li H, Brunet S, Guo W, Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 alpha subunit. Circ Res. 1999;85:623–633. doi: 10.1161/01.res.85.7.623. [DOI] [PubMed] [Google Scholar]
- 36.Guo W, Xu H, London B, Nerbonne JM. Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes. J Physiol. 1999;521:587–599. doi: 10.1111/j.1469-7793.1999.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niwa N, Wang W, Sha Q, Marionneau C, Nerbonne JM. Kv4.3 is not required for the generation of functional Ito,f channels in adult mouse ventricles. J Mol Cell Cardiol. 2008;44:95–104. doi: 10.1016/j.yjmcc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paolisso G, Gualdiero P, Manzella D, Rizzo MR, Tagliamonte MR, Gambardella A, Verza M, Gentile S, Varricchio M, D'Onofrio F. Association of fasting plasma free fatty acid concentration and frequency of ventricular premature complexes in nonischemic noninsulin- dependent diabetic patients. Am J Cardiol. 1997;80:932–937. doi: 10.1016/s0002-9149(97)00548-1. [DOI] [PubMed] [Google Scholar]
- 39.Pietrasik G, Goldenberg I, McNitt S, Moss AJ, Zareba W. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol. 2007;18:181–184. doi: 10.1111/j.1540-8167.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 40.Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 41.Harris GS, Lust RM, DeAntonio JH, Katwa LC. PPAR-gamma expression in animals subjected to volume overload and chronic Urotensin II administration. Peptides. 2008;29:795–800. doi: 10.1016/j.peptides.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Garre D, Herraiz M, Gonzalez-Rubio ML, Bernal R, Aragoncillo P, Carbonell A, Rufilanchas JJ, Fernandez-Cruz A. Activation of peroxisome proliferator-activated receptor-alpha and -gamma in auricular tissue from heart failure patients. Eur J Heart Fail. 2006;8:154–161. doi: 10.1016/j.ejheart.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T, Krek W. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Lee TI, Kao YH, Chen YC, Pan NH, Chen YJ. Oxidative stress and inflammation modulate peroxisome proliferator-activated receptors with regional discrepancy in diabetic heart. Eur J Clin Invest. 2010;40:692–699. doi: 10.1111/j.1365-2362.2010.02318.x. [DOI] [PubMed] [Google Scholar]
- 45.Pascual G, Sullivan AL, Ogawa S, Gamliel A, Perissi V, Rosenfeld MG, Glass CK. Anti-inflammatory and antidiabetic roles of PPARgamma. Novartis Found Symp. 2007;286:183–196. doi: 10.1002/9780470985571.ch16. [DOI] [PubMed] [Google Scholar]
- 46.Vikramadithyan RK, Hirata K, Yagyu H, Hu Y, Augustus A, Homma S, Goldberg IJ. Peroxisome proliferator-activated receptor agonists modulate heart function in transgenic mice with lipotoxic cardiomyopathy. J Pharmacol Exp Ther. 2005;313:586–593. doi: 10.1124/jpet.104.080259. [DOI] [PubMed] [Google Scholar]
- 47.Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol. 1999;10:1361–1375. doi: 10.1111/j.1540-8167.1999.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 48.Danik SB, Rosner G, Lader J, Gutstein DE, Fishman GI, Morley GE. Electrical remodeling contributes to complex tachyarrhythmias in connexin43-deficient mouse hearts. FASEB J. 2008;22:1204–1212. doi: 10.1096/fj.07-8974com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 50.Lee HJ, Su Y, Yin PH, Lee HC, Chi CW. PPAR(gamma)/PGC-1(alpha) pathway in E-cadherin expression and motility of HepG2 cells. Anticancer Res. 2009;29:5057–5063. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mice with cardiac overexpression of PPARγ have impaired repolarization and spontaneous fatal ventricular arrhythmias
SUPPLEMENTAL MATERIAL
Supplemental Online Methods
Animal Care and Breeding
The PPARγ transgenic mouse has cardiac-specific overexpression of PPARγ driven by the myosin heavy chain (MHC) promoter as previously described 1. Animal protocols were approved by the Columbia University Institutional Animal Care and Use Committee and were carried out in accordance with the NIH guidelines for the care and use of laboratory animals. Pioglitazone-chow (30 mg/kg) was purchased from Research Diets2.
Telemetry and ECG analysis
Telemetry devices (Data Sciences International, model EA-F20) were implanted in 10 week-old mice using inhaled isoflurane anesthesia. The two subcutaneous leads were positioned to approximate limb lead II of a human ECG. The mice recovered for one week after surgery before initiating 24-hour recordings. ECG intervals were measured manually, blinded to genotype, using Ponemah 3 software from recordings with minimal artifact at heart rates of 520-550 bpm to avoid the issues of correcting QT for rate. Intervals were averaged from 4 consecutive beats. PVC and arrhythmias counts were tallied manually by review of daily telemetry recordings. Daily heart rates were averaged for 3 animals in each group.
Echocardiography
Transthoracic echocardiography was performed using a high-resolution imaging system with a 30-MHz imaging transducer (Vevo 770; VisualSonics, Toronto, ON, Canada). The mice were anesthetized with isoflurane throughout the procedure. Care was taken to minimize sedation by monitoring the heart rate and respiratory rate of the mice. Images were obtained using short-axis views at the level of papillary muscles, and each parameter was measured using M-mode view. Images were recorded in a digital format and were then analyzed off-line. Percent fractional shortening (%FS) was calculated as follows: %FS = (LVDd-LVDs)/LVDd X 100), where LVDd is left ventricular diastolic dimension and LVDs is left ventricular systolic dimension.
Isolation of Cardiomyocytes and Cellular Electrophysiology
Cardiomyocytes were isolated using methods previously described 3. Briefly, the mouse heart was removed and the aorta was cannulated. After perfusing Ca2+-free buffer for two min, a mixture of collagenase and protease was then perfused through the coronary arteries for 5-7 min (Blendzyme 4 or Liberase TH, 0.3 mg/mL, Roche) at a [Ca2+] =12.5 μM. The LV tissue was teased apart with fine forceps and briefly pipetted to release individual cells. After enzymatic dispersion, [Ca2+] in the buffer containing 3.5 mg/ml BSA was elevated in 4 steps up to 0.8 mM. Cells were transferred into experimental temperature controlled chamber (Delta T Culture Dish, Bioptechs Inc). Only non-contracting rod shaped cells with clear striations were used in this study. Experiments were performed on freshly isolated cardiomyocytes from left ventricle of PPARγ mice (10 animals) and their littermate controls (12 animals).
Membrane currents were measured by the whole-cell patch-clamp method 4 using a MultiClamp 700B amplifier (Axon Instruments, Union City, CA). For action potential duration (APD) measurements (Fig. 3), perforated, whole-cell patch-clamp was utilized, using amphotericin B (300 μg/ml; Sigma A9528) at 35°C. Micropipettes were pulled from borosilicate glass capillaries (BF150-110-7.5, Sutter Instruments, Novato CA) on a programmable horizontal puller (S-97; Sutter Instruments, Novato CA). The pipettes had inner tip diameters of about 1 to 1.5 μm. When filled with internal solutions, they had resistances of 1.5 to 2.5 MΩ. Data were filtered at 4 KHz with a four-pole low-pass Bessel filter and sampled at 10 KHz. All experiments were performed using pCLAMP 10.2 software (Axon Instruments, Union City CA). Boltzman's fits were performed as previously described 5.
To record action potentials and K+ currents, the pipette solution contained 130 mM K+ gluconate, 10 mM NaCI, 10 mM EGTA , 1 mM MgCl2, 2 mM Mg-ATP, 2.0 mM CaCl2 and 10 mM HEPES, adjusted to pH 7.2 with KOH. Cells were superfused at room temperature with HEPES-buffered Tyrode's containing 137 mM NaCl, 5.4 mM KCI, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM glucose, and 10 mM HEPES (pH was adjusted to 7.4 with NaOH). Voltage clamp correction for a liquid junction potential of -13.6 mV was made by configuring the recording files in CLAMPEX of pCLAMP 10.2. Series resistances were usually less than 2 MΩ after 60% compensation. All voltages in current clamp recording were also corrected for the junction potential.
The decay phases of the outward K+ currents evoked during 4.0 s depolarizing voltage steps were fitted by a double exponential function of the form:
where t is time, and are the decay time constants, A1 and A2 are the amplitudes of the inactivating current components (Ito,f and IK,slow), and B is the amplitude of the non-inactivating current component, Iss
To study L-type Ca2+ current, we used a bath solution containing 140 mM TEA-Cl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES (pH was adjusted to 7.4 with CsOH). In these experiments, pipettes were filled with solution contained 135 mM CsCI, 10 mM EGTA, 1 mM Mg Cl2, 2 mM Mg-ATP, 2.0 mM CaCl2 and 10 mM HEPES, adjusted to pH 7.2 with CsOH. Series resistance was usually less than 2 MΩ after 60% compensation. Leak currents and capacitance transients were subtracted by a P/4 protocol. Voltage clamp correction for a liquid junction potential of -3.6 mV was made by configuring the recording files in CLAMPEX of pCLAMP 10.2. To evaluate the steady state activation of Ca2+ currents, the cell membrane potential was held at -70 mV and stepped for 450 ms to -60 to +50 mV in 10 mV increments. The interval between pulses was 10 s. To study the steady state inactivation of Ca2+ currents, the cell membrane potential was held at -70 mV and stepped to -70 to +30 mV for 650 ms in 10 mV increments and then stepped to the test potential +10 mV for 650 ms. The interval between pulses was 10 sec. Na+ currents were measured as described previously 6.
Action potential parameters, K+, Na+ and Ca2+ currents were measured and analyzed using pCLAMP 10.2, Excel and Origin 7.5 (OriginLab, Northampton, MA) software. V1/2 and k were calculated from Boltzmann function fitting for each cell. Statistical significance of observed differences were evaluated using TTEST (p<0.05). Regression analysis was done using GraphPad Software.
Real-time PCR
Samples of ventricular tissue from PPARγ mice and WT littermates, at 10-12 weeks of age, were used for harvesting RNA for RT-PCR. Cardiac tissue was homogenized with a Mini-BeadBeater (Glen Mills, Inc). RNA was then purified using a Qiagen RNeasy kit (item 74104). cDNA was synthesized using the Applied Biosystems high capacity RNA to cDNA kit (#4387406) and diluted to 10 ng/μL for use as a template (20 ng template was used for each 20 μL reaction). Real-time PCR was performed using an Applied Biosystems StepOne Plus Real-Time PCR system with StepOne Software v2.0 and inventoried primers from Applied Biosystems. PCR was performed for 40 cycles with automated detection of crossing threshold. PCR reactions were performed with duplicate wells with actin as a control reaction and no-template lanes for negative controls.
Immunoblots
Cardiac lysates were made by homogenizing ventricular tissue in buffer containing 1% (v/v) Triton X-100, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 10 mM EDTA, 10 mM EGTA, phosphatase inhibitor cocktail, and protease inhibitors (complete mini-tablet, calpain I and II inhibitors, Roche). Lysate were used for PAGE and then transferred to nitrocellulose membranes for immunoblots. The following antibodies were used: anti-Cx43 (Invitrogen), anti-KV1.5 and anti-KV2.1 (Alomone), anti-KV4.2 and anti-KV1.4 (Thermo-Scientific Pierce), and anti-tubulin (Santa Cruz Biotechnology). Chemiluminescence signal was obtained using a Kodak Image Station 400R Pro digital camera with Kodak Molecular Imaging Software v4.5.1. Signal intensity was quantified using ImageJ software (NIH). Blots for tubulin were performed to normalize loading of lanes, using the same membrane.
Immunohistochemistry
Heart tissue was fixed with 4% paraformaldehyde, embedded in paraffin wax, and then sectioned. Sections were deparaffinized and underwent antigen retrieval treatment (autoclaved with pH 9.0 Tris-buffer at 121°C for 15 min). For DAB staining, sections were treated with 0.3% H2O2 to block endogenous peroxidase. Sections were incubated with rabbit polyclonal antibodies against Cx43 (1:200 dilution, Invitrogen Corp.) or non-immune rabbit polyclonal IgG at the same concentration, at 4°C overnight. For DAB staining, after exposure to anti-rabbit swine antibody conjugated to biotin (1:500, DakoCytomation Denmark A/S, Glostrup, Denmark) for 1 hr in room temperature, the sections were treated with peroxidase-labeled ABC (VECTASATIN ABC Kit, Vector Laboratories, Inc., Burlingame, CA) and developed with DAB solution (ImmPACT DAB Peroxidase Substrate, Vector Laboratories, Inc.). After the reaction, the sections were counterstained with hematoxylin and observed by a light microscope. For immunofluorescent staining, after reaction with anti-rabbit donkey antibody conjugated to Alexa Fluor 488 (1:500, Invitrogen Corp.), the sections were counterstained with DAPI and observed by a fluorescent microscope. Tissues from two mice in each group (PPARγ overexpression and WT littermates) were used. Slides were photographed with a digital camera. Signal intensity was quantified using ImageJ software (NIH).
Supplement References:
1. Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791-2801.
2. Raphael KL, Strait KA, Stricklett PK, Baird BC, Piontek K, Germino GG, Kohan DE. Effect of pioglitazone on survival and renal function in a mouse model of polycystic kidney disease. Am J Nephrol. 2009;30:468-473.
3. O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271-296.
4. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85-100.
5. Yang L, Katchman A, Morrow JP, Doshi D, Marx SO. Cardiac L-type calcium channel (Cav1.2) associates with gamma subunits. FASEB J. 2011;25:928-936.
6. Knollmann BC, Knollmann-Ritschel BE, Weissman NJ, Jones LR, Morad M. Remodelling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J Physiol. 2000;525:483-498.
Supplemental Figure 1. Heart weight, body weight and heart weight:body weight ratio for WT and PPARγ mice. Mean + SEM. n=8, * indicates p<0.05 by U-test.
Supplemental Table 1. Additional patch-clamp data. WT: n=43; PPARγ: n=31. None of the comparisons were statistically significant by t-test