Abstract
Traction forces increase after microtubule depolymerization; however, the signaling mechanisms underlying this, in particular the dependence upon myosin II, remain unclear. We investigated the mechanism of traction force increase after nocodazole-induced microtubule depolymerization by applying traction force microscopy to cells cultured on micropatterned polyacrylamide hydrogels to obtain samples of homogeneous shape and size. Control cells and cells treated with a focal adhesion kinase (FAK) inhibitor showed similar increases in traction forces, indicating that the response is independent of FAK. Surprisingly, pharmacological inhibition of myosin II did not prevent the increase of residual traction forces upon nocodazole treatment. This increase was abolished upon pharmacological inhibition of FAK. These results suggest two distinct pathways for the regulation of traction forces. First, microtubule depolymerization activates a myosin-II-dependent mechanism through a FAK-independent pathway. Second, microtubule depolymerization also enhances traction forces through a myosin-II-independent, FAK-regulated pathway. Traction forces are therefore regulated by a complex network of complementary signals and force-generating mechanisms.
Key words: Traction force, Microtubule, FAK, Nocodazole
Introduction
The interior of a cell contains intricately organized and well-regulated cytoskeletal networks that serve both as structural support, enabling the cell to maintain a specified shape and polarity, and as a crucial mechanism for transducing and regulating mechanical signals. Disruptions of these mechanical signals affect the most basic functions of the cell, including migration (Pelham and Wang, 1999; Doyle et al., 2009; Discher et al., 2005), proliferation (Chen et al., 1997; Wang et al., 2000) and differentiation (Engler et al., 2006).
Among the most profound functions of the cytoskeleton are maintaining a proper cell shape and facilitating mechanical interactions with the outside environment. The production of traction forces – forces that are transmitted from the actin cytoskeleton through focal adhesions to the substrate (Harris et al., 1980) – is necessary for the cell to perform these vital tasks. The actin cytoskeleton, in conjunction with the motor protein myosin II, is responsible for the production of traction forces (Pelham and Wang, 1999; Beningo et al., 2006). Probably due to the association of many signaling factors with cytoskeletal elements, proper regulation of traction forces is essential for many mechanosensing phenomena, including the responses of cell migration, growth and differentiation to substrate compliance (Engler et al., 2006; Lo et al., 2004; Ulrich et al., 2009) and to cell shape (McBeath et al., 2004). Conversely, the maintenance of traction forces is also sensitive to such factors as substrate rigidity (Lo et al., 2000), cell shape (Rape et al., 2011a), and adhesive ligand density (Reinhart-King et al., 2003). Interestingly, traction forces are also regulated by the presence of a microtubule network (Danowski, 1989), suggesting that cross-talk between cytoskeletal systems is crucial for proper cell behavior. However, despite the importance of microtubule-based control of traction forces, there remain gaps in the knowledge of pathways involved in mediating this control.
Recent studies have begun to shed light on the nature of the cross-talk between microtubules and the actin cytoskeleton. Although microtubules might function as a sponge for sequestering factors that stimulate contractility, such as activators of the small GTPase Rho (Krendel et al., 2002; Zhou et al., 2010), there is evidence for direct mechanical coupling between the actin and microtubule cytoskeletons (Wang et al., 2001). Adding to the complexity is the recently discovered role of microtubules in regulating focal adhesion size (Erzatty et al., 2005), which has been shown to positively correlate with traction forces (Balaban et al., 2001). Microtubule depolymerization might signal an increase in focal adhesion size that enhances traction forces.
We have initially sought to characterize the response of traction forces in adherent cells to microtubule depolymerization, using traction force microscopy in conjunction with pharmacological and genetic manipulations to systematically quantify the increase of traction forces in single, micropatterned cells. Our investigations unexpectedly unveiled two distinct mechanisms for the generation of traction forces. The first mechanism is independent of focal adhesion kinase (FAK) but dependent on myosin II; the second mechanism is independent of myosin II but regulated by FAK. Therefore, although both respond to the depolymerization of microtubules, they might perform complementary functions and respond to distinct signals.
Results
To dissect the mechanisms underlying a microtubule depolymerization-induced increase in traction forces, we first measured this qualitatively well-known phenomenon using NIH 3T3 cells plated on polyacrylamide hydrogels with defined adhesive regions. Because traction forces are regulated by the shape and size of cells (Rape et al., 2011a), this patterning approach allowed us to quantify the results in a more homogenous population. Traction force microscopy was used to obtain the average traction stress for cells both before and after the depolymerization of microtubules. As shown in Fig. 1, traction forces in untreated cells were concentrated at the corners of the cell. Upon treatment with 10 μM nocodazole for 30 minutes, the corners were strongly pulled inward relative to the sides of the square, resulting in the cell becoming more circular. Quantitative analysis confirmed that traction force increased around the periphery by approximately twofold relative to untreated counterparts (Fig. 1). The effect was reversible upon nocodazole wash-out (Fig. 1G).
Fig. 1.
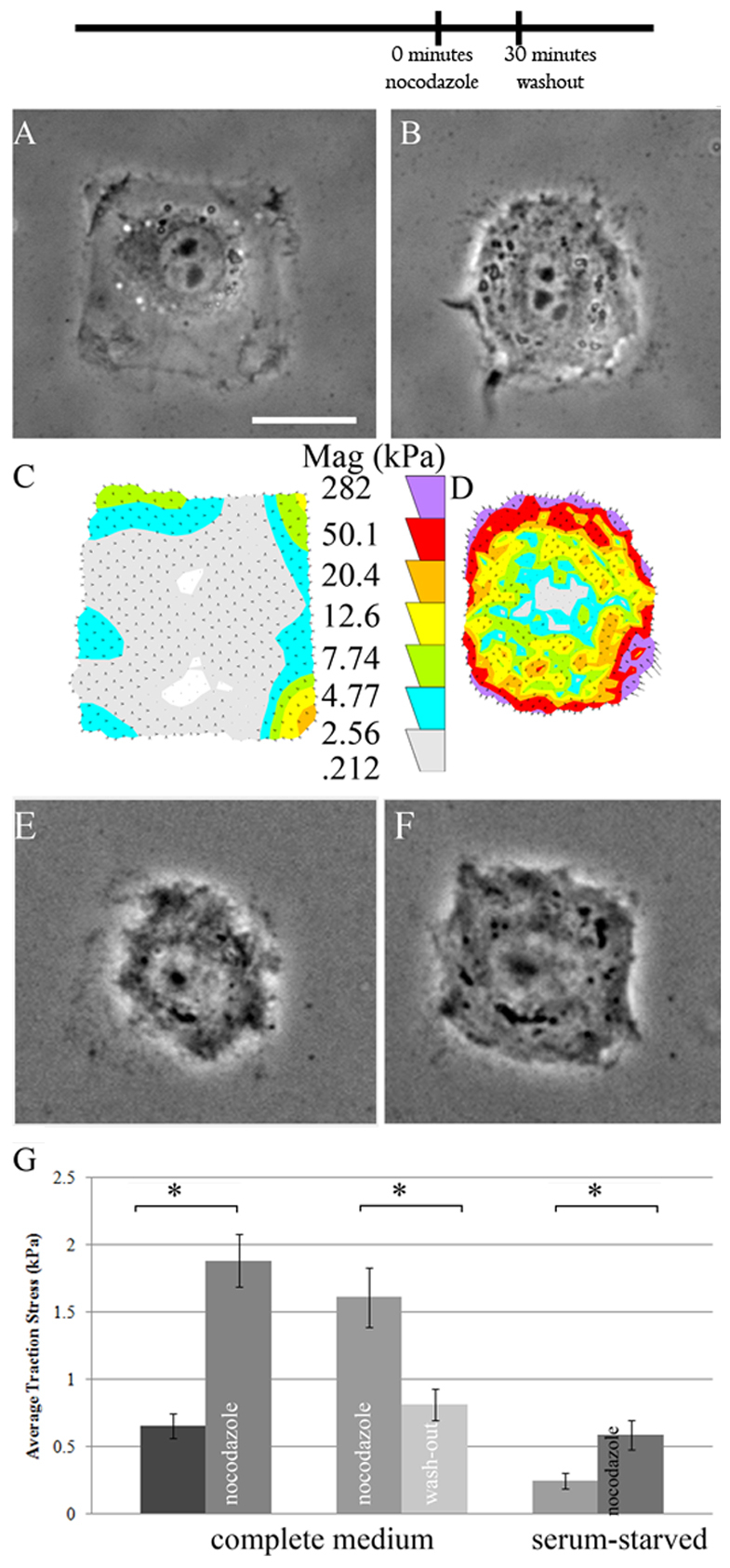
Nocodazole-induced traction stress increase in NIH 3T3 cells. Cells were cultured on micropatterned polyacrylamide gels in order to standardize cell shape, and phase contrast images taken to show cell morphology. (A,B) Untreated cells reliably occupied the micropatterned squares (A). Upon addition of 10 μM nocodazole, cells became more circular (B). (C,D) Magnitude of traction forces (Mag) in the cells shown in A and B. Circular shape of nocodazole-treated cells is probably due to the markedly increased traction stress at the periphery (D) as opposed to untreated cells (C). (E,F) Nocodazole-treated cells (E) re-spread upon wash-out of the drug (F), and concomitantly diminished their traction stress. (G) Quantification of the average stress exerted by individual cells confirmed the significant increase in traction stress upon nocodazole addition, as well as its reversibility by washout. Similarly, serum-starved cells also markedly increased the traction stress from the basal levels after nocodazole addition. *P<0.05. Error bars indicate s.e.m. Scale bar: 25 μm. n=14, 15, 17, 17 cells for control, nocodazole-treated, pre-nocodazole washout, and nocodazole washout, respectively. The timeline indicates the temporal sequence of drug manipulation.
Because the bulk of traction forces have been shown to be generated by myosin-II-mediated contractility (Beningo et al., 2006), we expected blebbistatin, a potent inhibitor of myosin II ATPase, to inhibit not only traction forces, but also any increase in traction force upon microtubule depolymerization. Blebbistatin treatment caused a sixfold decrease in average traction stress in patterned cells. In addition, cells pretreated for 1 hour with blebbistatin prior to nocodazole treatment showed a similar percentage increase in traction stress upon microtubule depolymerization to cells with uninhibited myosin II (Fig. 2C), implicating a myosin-II-independent force generation mechanism that is similarly regulated by microtubules. Treatment with taxol, a microtubule stabilizing agent, failed to induce a significant change in traction force in blebbistatin-treated cells (Fig. 2C), which argues against the involvement of microtubule dynamics.
Fig. 2.
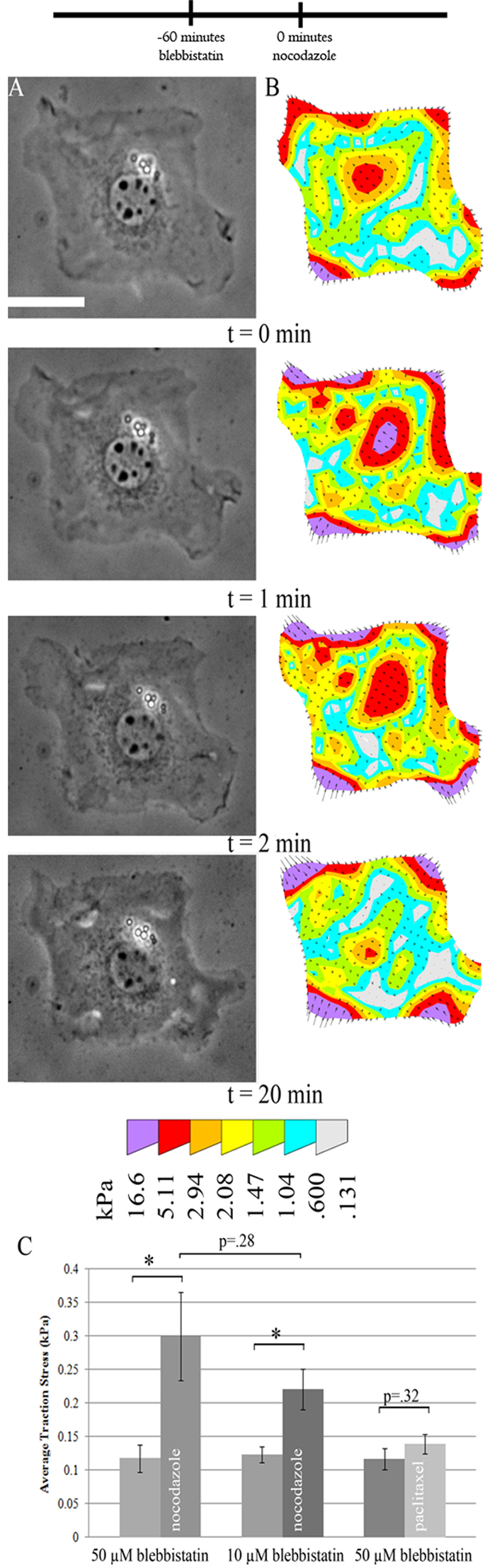
Nocodazole-induced traction stress increase in myosin-II-inhibited cells. Cells were pretreated with 50 μM blebbistatin and then nocodazole added at t=0. (A) Cell morphology as shown by phase contrast images was unaffected by nocodazole addition (time indicates minutes after nocodazole addition). (B) The traction stress increased rapidly, appearing to saturate within a few minutes. (C) Average traction stress increased by a factor of 2–3 after nocodazole addition. Similar effects were observed using a lower dosage of blebbistatin, although treatment with the microtubule-stabilizing drug taxol after blebbistatin caused no significant change in traction stress. *P<0.05. Error bars indicate s.e.m. Scale bar: 25 μm. n=10, 11, 13, 15, 11, 14 cells for 50 μM blebbistatin, 50 μM blebbistatin and nocodazole, 10 μM blebbistatin, 10 μM blebbistatin and nocodazole, 50 μM blebbistatin, and 50 μM blebbistatin and nocodazole, respectively. The timeline indicates the temporal sequence of drug manipulation.
We then characterized focal adhesions in control and myosin-II-inhibited cells before and after microtubule depolymerization. Despite the increase in traction stress upon nocodazole treatment, there was no significant change in focal adhesion size or intensity in control cells, on the basis of immunofluorescence staining of paxillin, phosphorylated tyrosine, or FAK (Fig. 3A,B). However, there were more subtle changes in other aspects of focal adhesions, such as the relative signal between focal adhesions along the periphery and in interior regions. Inhibition of myosin II is known to cause a striking reduction of focal adhesion size, resulting in small dot-like structures (Chrzanowska-Wodnicka and Burridge, 1996). Interestingly, unlike focal adhesions in control cells, these residual structures showed a significant increase in size and intensity upon microtubule depolymerization (Fig. 3C,D).
Fig. 3.
Nocodazole-induced increase in focal adhesion size in myosin-II-inhibited cells, but not in untreated cells. (A–D). Focal adhesions were stained with immunofluorescence against paxillin. The total area occupied by focal adhesions in nocodazole-treated cells (B) showed no significant difference from that in control cells (A). Cells treated with blebbistatin showed only dot-shaped focal complexes (C); large focal adhesions appeared upon nocodazole treatment (D). (E) Quantification of the average focal adhesion size was performed by manual segmentation. Similar results were obtained by staining for FAK and phosphotyrosine (pY). The increase in focal adhesions after nocodazole treatment in blebbistatin-treated cells was not observed in cells pretreated with a FAK-specific inhibitor PF 573228. Error bars indicate s.e.m. Scale bar: 25 μm. A total of at least 65 individual focal adhesions for each condition from at least four separate cells were analyzed for each condition. The timeline indicates the temporal sequence of drug manipulation.
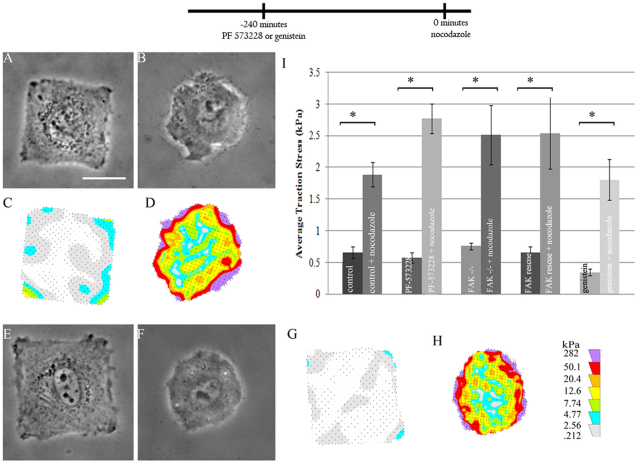
We hypothesized that tyrosine kinases (such as the Fyn-Yes-Src family) at focal adhesions might be involved in mediating the increase in traction forces upon microtubule depolymerization. Surprisingly, although application of a broad spectrum tyrosine kinase inhibitor (genistein) alone caused a slight decrease in traction stress, the addition of nocodazole after incubation with genistein caused the average traction stress to increase to a similar extent, regardless of the inhibition of tyrosine kinases (Fig. 4). Similarly, inhibition of FAK, which is required for microtubule-dependent control of focal adhesion size (Erzatty et al., 2005), with a specific inhibitor PF 573228 showed no effect on the increase in traction stress upon nocodazole treatment (Fig. 4) (Slack-Davis et al., 2007). Likewise, FAK knockout cells showed a large increase in traction stress upon nocodazole treatment, similar to that in FAK re-expressing cells (Fig. 4).
Fig. 4.
Lack of effect of FAK signaling on nocodazole-induced traction stress increase in cells with functional myosin II. (A,B,E,F) Phase contrast or (C,D,G,H) traction stress images show the effect of nocodazole on cells pretreated with 100 μM PF 573228 (A–D) or genistein (E–H). The pretreatment alone did not affect the square morphology (A,E), although genistein caused some decrease in the production of traction stress (C,G; compare with Fig. 1). Upon subsequent exposure to 10 μM nocodazole for 30 minutes, the cell became much more circular (B,F) due to an increased contraction of the gel at the corners (D,H). (I) Average traction stress measured in different samples, as labeled. *P<0.01. Error bars indicate s.e.m. Scale bar: 25 μm. n=28, 29, 11, 9, 29, 18, 21, 17, 14, 10 cells for control, nocodazole, PF 573228, PF 573228 and nocodazole, FAK−/−, FAK−/− with nocodazole, FAK−/− rescue, FAK−/− rescue with nocodazole, genistein, and genistein and nocodazole, respectively. The timeline indicates the temporal sequence of drug manipulation.
Because the myosin-II-independent traction stress increase might involve a mechanism different to the myosin-II-dependent increase, the dependence on tyrosine phosphorylation might also differ. To investigate whether tyrosine phosphorylation was required for the increase of residual traction forces in myosin-II-inhibited cells upon microtubule depolymerization, cells were treated with a mixture of blebbistatin and genistein before the addition of nocodazole. In contrast to cells with normal myosin II activity, these cells showed no detectable increase in traction stress upon microtubule depolymerization (Fig. 5). Similarly, cells pretreated with a combination of blebbistatin and PF 573228 showed no increase in traction stress upon the addition of nocodazole; nor did blebbistatin-treated FAK−/− cells show an increase (Fig. 6). The growth of focal complexes upon nocodazole treatment was also inhibited in cells pretreated with a combination of blebbistatin and PF 573228 (Fig. 3E). Interestingly, FAK-inhibited cells showed both stronger traction stresses and larger focal adhesions upon blebbistatin treatment than control cells treated with blebbistatin (Fig. 2C, Fig. 3E and Fig. 6E). Re-expressing FAK in FAK−/− cells rescued the normal response. Because of the differential dependence of traction stress increase on tyrosine phosphorylation and FAK in control and myosin-II-inhibited cells, we suggest that microtubules regulate traction forces via two distinct pathways: the first is a tyrosine phosphorylation-independent pathway that involves the activation of myosin II; the second requires tyrosine phosphorylation and FAK, and is independent of myosin II.
Fig. 5.
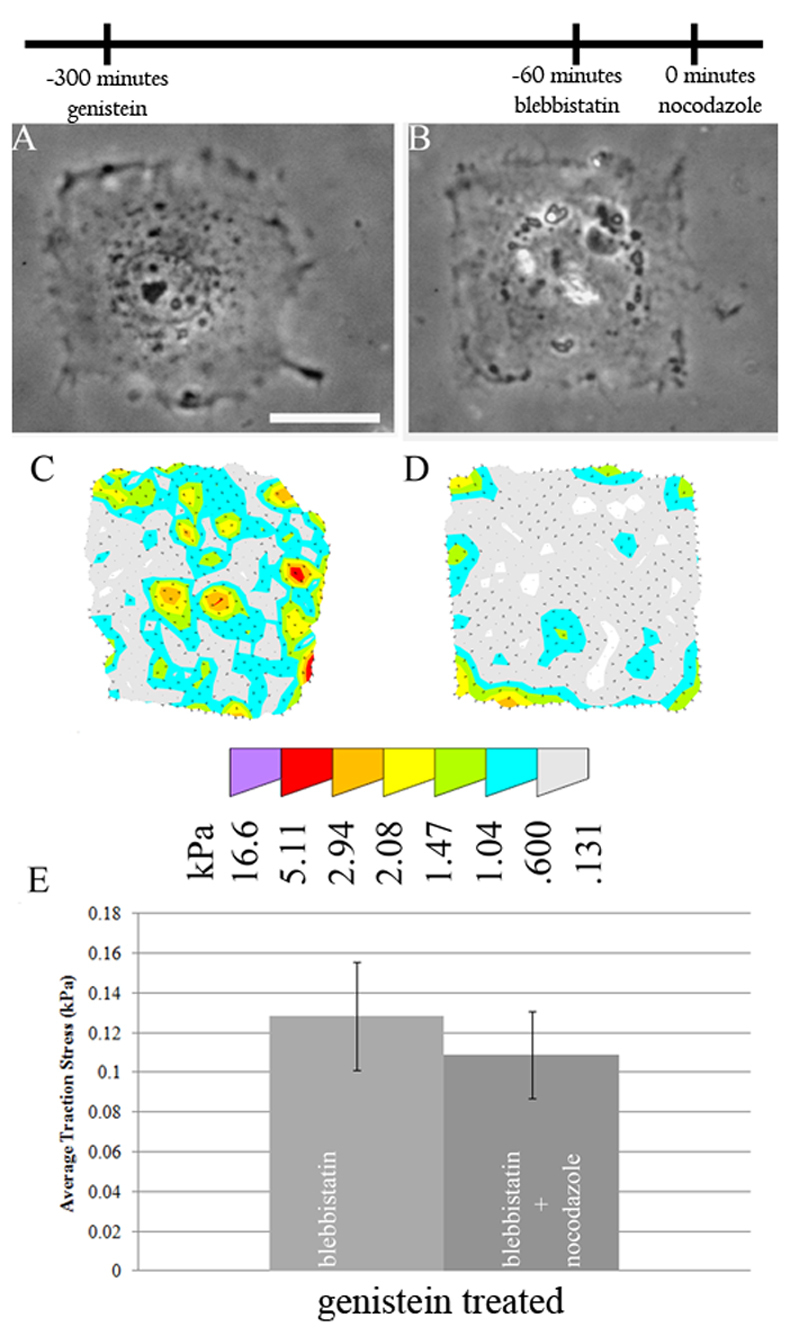
Requirement of tyrosine phosphorylation for nocodazole-induced traction stress increase in myosin-II-inhibited cells. (A–D) Cells were pretreated sequentially with genistein and blebbistatin (A,C), then with nocodazole (B,D). The addition of nocodazole caused neither apparent morphological effects, as shown by phase contrast images (A,B), nor significant changes in traction stress (C–E). Error bars indicate s.e.m. Scale bar: 25 μm. n=9, 10 cells for genistein and genistein and nocodazole, respectively. The timeline indicates the temporal sequence of drug manipulation.
Fig. 6.
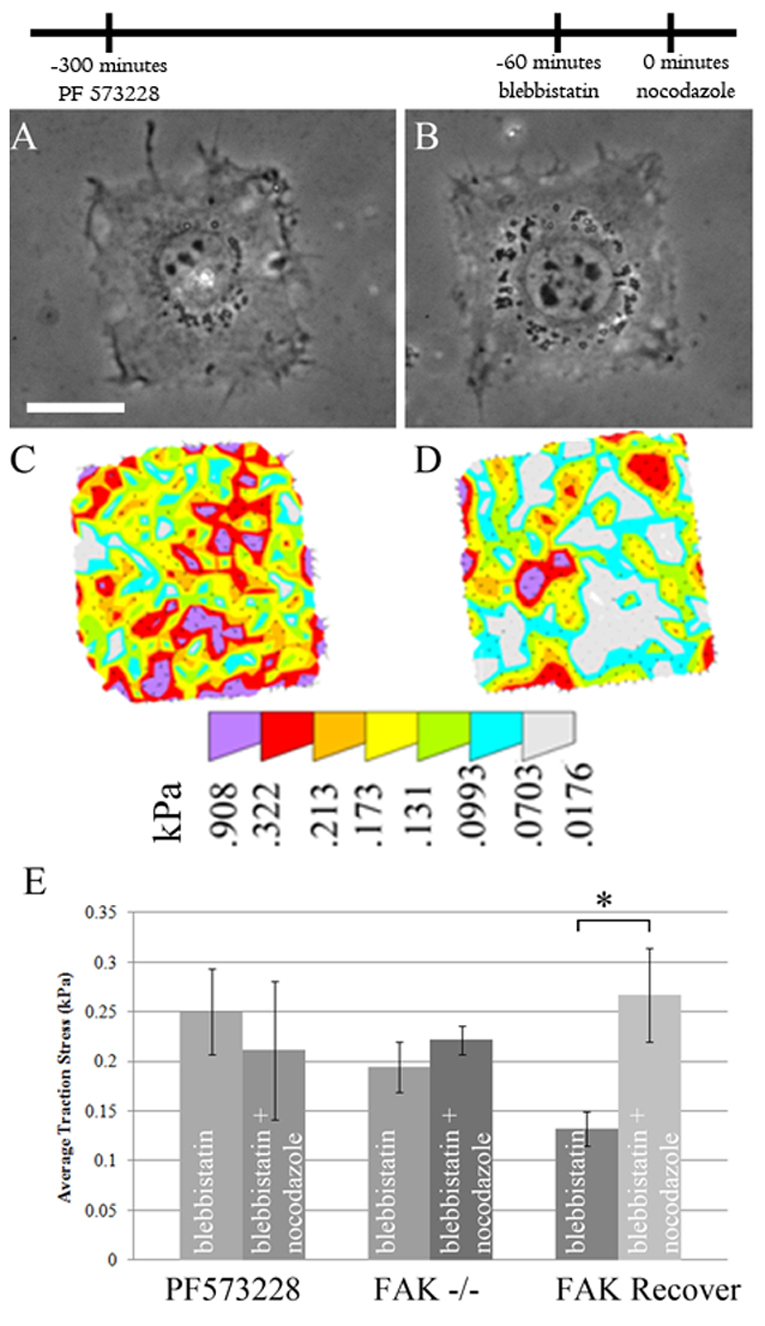
Requirement of FAK signaling for nocodazole-induced traction stress increase in myosin-II-inhibited cells. (A–D) Cells were pretreated sequentially with PF 573228 and blebbistatin to inhibit both FAK and myosin II (A,C), then with nocodazole (B,D). The addition of nocodazole caused neither morphological responses, as shown by phase contrast images (A,B), nor an increase in traction stress (C,D). (E) Quantification of average stress. Similar results were obtained using FAK knockout cells However, knockout cells re-expressing FAK showed a significant increase in traction stress after the addition of nocodazole. *P<0.05. Error bars indicate s.e.m. Scale bar: 25 μm. n=12, 13, 37, 39, 13 and 22 cells for PF 573228, PF 573228 and nocodazole, FAK−/−, FAK−/− and nocodazole, FAK rescue, and FAK rescue and nocodazole, respectively. The timeline indicates the temporal sequence of drug manipulation.
Because one of the first steps in the activation of FAK is auto-phosphorylation at Tyr397 (Owen et al., 1999), we investigated whether this activity was necessary for nocodazole-induced traction stress increase in myosin-II-inhibited cells. Using FAK−/− cells re-expressing a non-phosphorylatable FAK by replacing Tyr397 with phenylalanine, we found that phosphorylation at Tyr397 is necessary for a traction stress increase after microtubule depolymerization in myosin-II-inhibited cells (supplementary material Fig. S1).
Finally, we looked at whether traction stress and its increase upon microtubule depolymerization were dependent on actin filaments. We used a low dose of cytochalasin D to disrupt the actin cytoskeleton while maintaining the conformity of cell shape to the square micropattern. Cytochalasin D caused a significant inhibition of traction stresses, but did not completely abolish the forces at corners (supplementary material Fig. S2). Addition of nocodazole to these cells caused no apparent increase in residual traction stress, indicating that the increase in traction stress required an intact actin cytoskeleton, regardless of myosin II activity.
Discussion
Previous studies have indicated a strong dependence of traction forces on myosin II (Beningo et al., 2006; Gardel et al., 2008). Patterning cells allows more precise quantification and has indicated an incomplete inhibition of traction stress upon blebbistatin treatment. In the present study, we have performed quantitative analysis of the increase in these forces upon microtubule depolymerization. Our results suggest two distinct mechanisms of traction force increase upon microtubule depolymerization (Fig. 7). The first mechanism, which occurs in the presence of active myosin II, is independent of tyrosine phosphorylation. The second mechanism, which becomes dominant upon the inhibition of myosin II, is found only in the presence of functional FAK. Although both mechanisms are dependent upon an intact actin cytoskeleton, the differential dependence on tyrosine phosphorylation suggests that they are driven by different mechanisms.
Fig. 7.
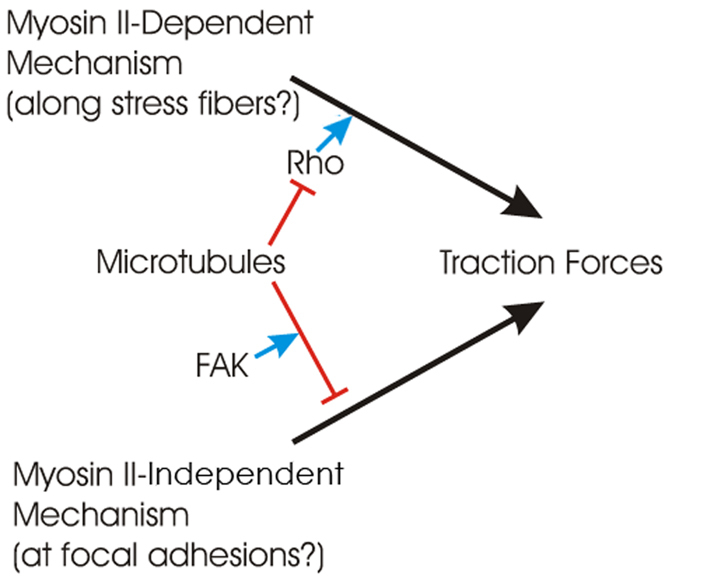
Two distinct pathways for microtubule depolymerization to induce an increase in traction forces. Traction forces are generated by both myosin-II-dependent and -independent mechanisms, and both are down regulated by microtubules. The former, which has been reported in the literature, is believed to be dependent on the activation of the small GTPase Rho but not tyrosine kinases. Because myosin II is not concentrated at focal adhesions, the regulation might take place outside of focal adhesions. The latter pathway is myosin-II-independent and requires FAK activity for regulation. The regulation might take place at focal adhesions where FAK is localized.
In the presence of functional myosin II, focal adhesions in stationary cells are well developed. Depolymerization of microtubules increases traction stress without causing an apparent increase in the size of these focal adhesions, suggesting that microtubules regulate activities downstream of focal adhesion assembly, for example by activating myosin II located away from focal adhesions through activation of the Rho family of GTPases (Liu et al., 1998; Zhou et al., 2010). Alternatively, microtubules, due to their rigidity, might absorb some of the forces from a contractile actin cytoskeleton and keep the forces from being transmitted to the substrate. Disassembly of microtubules then allows the mechanical load to be transferred to focal adhesions (Wang et al., 2001) independently of chemical signals. In either case, as most focal adhesions in stationary cells are fully assembled when myosin II is active, microtubule depolymerization has no significant effect on their size. The difference from previous studies, in which microtubule depolymerization was found to cause an increase in focal adhesion size (Liu et al., 1998), might be explained by the formation of more stable, full-sized focal adhesions in immobilized cells in the present study, whereas unconstrained migrating cells form smaller focal adhesions due to the need to actively reorganize adhesions.
Equally important is the response of residual traction forces and focal adhesions to nocodazole in cells treated with blebbistatin. Blebbistatin causes focal adhesions to shrink into dot-like focal complexes while traction forces decrease dramatically (Fig. 3A and Fig. 4A,B), probably as a consequence of the loss of ‘inside-out’ signaling, whereby intracellular myosin-II-dependent contractility enhances the assembly of focal adhesions (Burridge and Chrzanowska-Wodnicka, 1996; Gilmore and Burridge, 1996; Ginsberg et al., 2005). We found that microtubule depolymerization allows these residual focal complexes and traction forces to recover partially. FAK is involved not only as a physical component of focal adhesions but also as a regulatory component for this myosin-II-independent increase in traction forces, because both its kinase activity and regulation through Tyr397 are required for the response.
Interestingly, FAK-inhibited cells showed larger focal adhesions and higher traction forces upon blebbistatin treatment than control cells treated with blebbistatin. Because FAK has been suggested to mediate the interaction between microtubules and focal adhesions (Erzatty et al., 2005), it might be required for microtubule-dependent downregulation of myosin-II-independent traction forces (Fig. 7, lower mechanism). In blebbistatin-treated cells, depolymerization of microtubules would therefore have no further effect on either the size of focal adhesions or the magnitude of traction forces. In addition, FAK has also been shown to promote focal adhesion turnover and disassembly (Ren et al., 2000; Hamadi et al., 2005), which might explain why focal adhesions in FAK-inhibited cells become more resistant to blebbistatin and maintain stronger traction forces than focal complexes in control cells upon the inhibition of myosin II.
As for the myosin-II-dependent mechanism, removal of compression-bearing microtubules causes the transfer of compressive forces to focal adhesions in blebbistatin-treated cells. Increased tension in the focal adhesion might activate one or many of the proteins in the adhesome that are proposed to be mechanosensitive, including zyxin, p130Cas and FAK (Yoshigi et al., 2005; Sawada et al., 2006; Yano et al., 1996), inducing a signal transduction cascade that leads to the amplification of focal adhesion size and traction forces. In addition, Rho family GTPases have also been shown to activate FAK and control the size of focal adhesions as well as traction forces, and might be involved in a feedback mechanism (Machesky and Hall, 1996).
Regardless of the microtubule-sensitive trigger, several mechanisms have been proposed to explain actin-dependent, myosin-II-independent force generation. For example, actin polymerization and filament bundling at these focal complexes might be able to generate contractile mechanical forces (Gardel et al., 2008; Sun et al., 2010). Members of the myosin superfamily other than myosin II might also contribute to traction forces, as some of these motor molecules are known to localize at the protruding edge of cells (Buss et al., 1998; Wang et al., 1996). Under normal conditions, such myosin-II-independent forces might be responsible for probing mechanical properties of the substrate at the very early stage of focal adhesion formation. The ensuing positive feedback might then cause the growth of focal adhesions and the assembly of myosin II onto the associated actin bundles to generate stronger traction forces.
Traction forces are believed to play multiple important roles in adherent cells, including the organization of extracellular matrix (Lemmon et al., 2009; Baneyx et al., 2002), the detection of substrate mechanical properties (Discher et al., 2005; Rape et al., 2011b), and the propulsion of cell migration against strong adhesions (Munevar et al., 2001). The majority of these functions involve coordination over a long range, e.g. between the tip and tail of a polarized cell. The extensive network of microtubules serves the ideal role for such long-range coordination. Our present results suggest that microtubules function as a negative regulator in multiple phases of traction force generation, including the initial phase of focal complexes, as reflected in blebbistatin-treated cells, and the subsequent thrust phase near the lead edge. Conceivably, the low density of microtubules in newly extended lamellipodia and lamella allows newly formed focal adhesions to exert maximal traction forces in the anterior region (Beningo et al., 2001). Previous studies have suggested that microtubules deliver disassembly signals to mature focal adhesion to trigger their release (Kaverina et al., 1999; Erzatty et al., 2005). The combination of these mechanisms is sufficient to explain why microtubules are crucial for maintaining cell polarity (Bershadsky et al., 1991).
In conclusion, this study has found that there are two distinct mechanisms of traction force, both under the regulation of microtubules. The first mechanism involves the activation of myosin II and is independent of tyrosine phosphorylation, whereas the second mechanism generates traction forces independently of myosin II activity and is regulated by FAK. We conclude that traction forces are generated and controlled by a complex network of not only signaling factors but also force-generating mechanisms.
Materials and Methods
Preparation of patterned polyacrylamide hydrogels
Polyacrylamide hydrogels were prepared as described previously (Pelham and Wang., 1997; Wang and Pelham, 1998). Initially, 50 bloom gelatin at a concentration of 0.1% was activated by incubation with 3.6 mg/ml sodium m-periodate (Sigma, St Louis, MO) at room temperature for 30 minutes, as previously described (Rape et al., 2011a). Polydimethylsiloxane (PDMS) stamps were fabricated by standard soft lithography procedures. Briefly, a positive photoresist, SPR-220.3 (Microchem, Newton, MA), was spun on a glass coverslip. It was then exposed to UV light through a patterned photomask and developed for use as a mold. PDMS pre-polymers (Dow Corning, Midland, MI) were then mixed with catalyst and poured over the mold and cured at 60°C for 1 hour. The PDMS stamp was then cut away from the molding and incubated with activated gelatin solution for 30 minutes. Because of the hydrophobic nature of the PDMS stamp, a thin layer of molecular gelatin adsorbed to the stamp. Excess solution was blown away under a nitrogen stream and the stamp was brought into manual contact with a small square glass coverslip for 5 minutes, causing the transfer of molecular gelatin to the stamp.
Polyacrylamide was prepared at a final concentration of 5% acrylamide (BioRad, Hercules, CA), 0.1% bisacrylamide (BioRad) and a 1:1000 dilution of 0.2 μm fluorescent latex beads (Molecular Probes, Carlsbad, CA). A 30 μL aliquot of this solution, along with initiators ammonium persulfate and N,N,N′,N′ tetramethylethylenediamine (both from BioRad), was pipetted onto a large Bind-Silane (GE Healthcare, Waukesha, WI) activated coverslip. The gelatin-stamped coverslip was then placed, patterned side down, onto the acrylamide solution. After polymerization was complete, the top coverslip was gently removed. The final gel had an estimated Young's modulus of 5.8 kPa (Frey et al., 2007).
Cell culture and pharmacological inhibition
Patterned polyacrylamide hydrogels were mounted into chamber dishes and incubated in cell culture media for 30 minutes at 37°C. NIH 3T3 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma), supplemented with 10% donor calf serum (JHR Biosciences, Lenexa, KS), 2 mM l-glutamine, 50 μg/ml streptomycin and 50 U/ml penicillin (Gibco-BRL, Gaithersburg, MD).
FAK knockout cells were cultured in DMEM containing 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA) supplemented with 1% nonessential amino acids (GIBCO/BRL, Grand Island, NY). Tetracycline (Calbiochem, San Diego, CA) was added to the media at a concentration of 1 μg/ml to prevent expression of the FAK protein in FAK−/− experiments. To induce FAK expression, the same FAK−/− cells were transferred to media lacking tetracycline for at least 36 hours before experiments. Cells were plated on the hydrogels and allowed to spread overnight. Pharmacological interventions were performed as follows: Myosin II was inhibited using 50 μM blebbistatin (Calbiochem) for 1 hour. FAKwas inhibited using 10 μM PF 573228 (Tocris Biosciences, Ellisville, MO) for 4 hours. Tyrosine phosphorylation was inhibited using 100 μM genistein (Calbiochem) for 4 hours. The actin cytoskeleton was disrupted using 1 μM cytochalasin D (Sigma) for 1 hour. Microtubule depolymerization was induced using 10 μM nocodazole (Sigma) for 30 minutes. Microtubule stabilization was achieved using 500 nM taxol (Sigma) for 15 hours.
Traction force microscopy, immunofluorescence and image analysis
For traction force microscopy, phase contrast images of single mononucleated cells adhered to an island were collected using an Axiovert S100TV (Carl Zeiss, Thornwood, NY) microscope equipped with a 40× plan-Neofluar dry objective. Only cells that were nearly fully spread to the desired micropatterned domain were chosen for analysis. A fluorescence image of the beads near the ventral surface of the cell was acquired. A second fluorescent image of the beads in relaxed positions was acquired after removing the cell with a microneedle. Substrate displacement fields and the corresponding traction stress maps were computed as previously described using custom software and the LIBTRC package (Dembo and Wang, 1999). In the calculation of traction stresses, a boundary condition was imposed that confines traction forces to within the lateral border of the cell. This is a reasonable assumption because there is no physical basis for forces being exerted outside of the cell boundary. Substrate strains outside of cell domain are assumed to be a result of forces exerted inside the cell domain being transferred to the outside region via elastic coupling.
Immunofluorescence images were acquired after the cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in phosphate-buffered saline. Anti-tyrosine-phosphorylation antibodies were obtained from Upstate Biotechnology (Waltham, MA); anti-FAK and anti-paxillin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Images were collected using a Nikon Eclipse Ti microscope (Nikon, Melville, NY) equipped with a 100× Fluor oil immersion objective. Average focal adhesion size was quantified by manual segmentation of individual focal adhesions followed by integration of the area. Focal adhesions from at least four different cells for each condition were analyzed.
Supplementary Material
Acknowledgements
We thank Micah Dembo, Boston University, for providing the LIBTRC program package for computing traction forces, and Steve Hanks, Vanderbilt University, for providing tet-off FAK knockout cells.
Footnotes
Funding
This work was supported by grant GM-32476 from the National Institutes of Health to Y.-L.W and an A.R.C.S. Award to A.R. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.090563/-/DC1
References
- Balaban N. Q., Schwarz U. S., Riveline D., Goichberg P., Tzur G., Sabany I., Mahalu D., Safran S., Bershadsky A., Addadi L., et al. (2001). Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466-472 [DOI] [PubMed] [Google Scholar]
- Baneyx G., Baugh L., Vogel V. (2002). Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc. Natl. Acad. Sci. USA 99, 5139-5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo K. A., Dembo M., Kaverina I., Small J. V., Wang Y.-L. (2001). Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 153, 881-888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo K. A., Hamao K., Dembo M., Wang Y.-L., Hosoya H. (2006). Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch. Biochem. Biophys. 456, 224-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A. D., Vaisberg E. A., Vasiliev J. M. (1991). Pseudopodial activity at the active edge of migrating fibroblasts is decreased after drug-induced microtubule depolymerization. Cell Motil. Cytoskeleton 19, 152-158 [DOI] [PubMed] [Google Scholar]
- Burridge K., Chrzanowska-Wodnicka M. (1996). Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463-518 [DOI] [PubMed] [Google Scholar]
- Buss F., Kendrick-Jones J., Lionne C., Knight A. E., Cote G. P., Luzio J. P. (1998). The localization of myosin VI at the Golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J. Cell Biol. 143, 1535-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S., Mrksich M., Huang S., Whitesides G. M., Ingber D. E. (1997). Geometric control of cell life and death. Science 276, 1425-1428 [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M., Burridge K. (1996). Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133, 1403-1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danowski B. A. (1989). Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J. Cell Sci. 93, 255-266 [DOI] [PubMed] [Google Scholar]
- Dembo M., Wang Y.-L. (1999). Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 76, 2307-2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher D. E., Janmey P., Wang Y.-L. (2005). Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139-1143 [DOI] [PubMed] [Google Scholar]
- Doyle A. D., Wang F. W., Matsumota K., Yamada K. M. (2009). One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184, 481-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677-689 [DOI] [PubMed] [Google Scholar]
- Erzatty E. J., Partridge M. A., Gunderson G. G. (2005). Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7, 581-590 [DOI] [PubMed] [Google Scholar]
- Frey M. T., Engler A. J., Discher D. E., Lee J., Wang Y.-L. (2007). Microscopic methods for measuring the elasticity of gel substrates for cell culture: microspheres, microindenters, and atomic force microscopy. Methods Cell Biol. 83, 47-66 [DOI] [PubMed] [Google Scholar]
- Gardel M. L., Sabass B., Ji L., Danuser G., Schwarz U. S., Waterman C. M. (2008). Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 183, 999-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A. P., Burridge K. (1996). Molecular mechanisms for focal adhesion assembly through regulation of protein-protein interactions. Structure 4, 647-651 [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Partridge A., Shattil S. J. (2005). Integrin Regulation. Curr. Opin. Cell Biol. 17, 509-516 [DOI] [PubMed] [Google Scholar]
- Hamadi A., Bouali M., Dontenwill M., Stoeckel H., Takeda K., Ronde P. (2005). Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J. Cell Sci. 118, 4415-4425 [DOI] [PubMed] [Google Scholar]
- Harris A. K., Wild P., Stopak D. (1980). Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208, 177-179 [DOI] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Small J. V. (1999). Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 146, 1033-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel M., Zenke F. T., Bokoch G. M. (2002). Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4, 294-301 [DOI] [PubMed] [Google Scholar]
- Lemmon C. A., Chen C. S., Romer L. H. (2009). Cell traction forces direct fibronectin matrix assembly. Biophys. J. 96, 729-738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. P., Chrzanowska-Wodnicka M., Burridge K. (1998). Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell Adhes. Commun. 5, 249-255 [DOI] [PubMed] [Google Scholar]
- Lo C. M., Wang H. B., Dembo M., Wang Y.-L. (2000). Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C. M., Buxton D. B., Chua G. C., Dembo M., Adelstein R. S., Wang Y.-L. (2004). Nonmuscle myosin IIB is involved in the guidance of fibroblast migration. Mol. Biol. Cell 15, 982-989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Hall A. (1996). Rho: a connection between membrane receptor signaling and the cytoskeleton. Trends Cell Biol. 6, 304-310 [DOI] [PubMed] [Google Scholar]
- McBeath R., Prone D. M., Nelson C. M., Bhadriraju K., Chen C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483-495 [DOI] [PubMed] [Google Scholar]
- Munevar S., Wang Y.-L., Dembo M. (2001). Distinct roles of frontal and rear cell-substrate adhesions in fibroblast migration. Mol. Biol. Cell 12, 3947-3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J. D., Ruest P. J., Fry D. W., Hanks S. K. (1999). Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 19, 4806-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham R. J., Wang Y.-L. (1997). Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 94, 13661-13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham R. J., Wang Y.-L. (1999). High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol. Biol. Cell 10, 935-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape A. D., Guo W. H., Wang Y-. L. (2011a). The regulation of traction force in relation to cell shape and focal adhesions. Biomaterials 32, 2043-2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape A. D., Guo W. H., Wang Y.-L. (2011b). Response of cells to adhesion mediated signals: a universal mechanism. In Mechanobiology of Cell-Cell and Cell-Matrix Interactions.. (ed. Wagoner Johnson A., Harley B.), pp. 1-10 USA: Springer; [Google Scholar]
- Reinhart-King C. A., Dembo M., Hammer D. A. (2003). Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir 19, 1573-1579 [Google Scholar]
- Ren X. D., Kiosses W. B., Sieg D. J., Otey C. A., Schlaepfer D. D., Schwartz M. A. (2000). Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 113, 3673-3678 [DOI] [PubMed] [Google Scholar]
- Sawada Y., Tamada M., Dubin-Thaler B. J., Cherniavskaya O., Sakai R., Tanaka S., Sheetz M. P. (2006). Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack-Davis J. K., Martin K. H., Tilghman R. W., Iwanicki M., Ung E. J., Autry C., Luzzia M. J., Cooper B., Kath J. C., Roberts W. G., et al. (2007). Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 282, 14845-14852 [DOI] [PubMed] [Google Scholar]
- Sun S. X., Walcott S., Wolgemuth C. W. (2010). Cytoskeletal cross-linking and bundling in motor-independent contraction. Curr. Biol. 20, R649-R654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich T. A., de Juan Parda E. M., Kumar S. (2009). The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167-4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. S., Wolenski J. S., Cheney R. E., Mooseker M. S., Jay D. G. (1996). Function of myosin-V in filopodial extension of neuronal growth cones. Science 273, 660-663 [DOI] [PubMed] [Google Scholar]
- Wang H. B., Dembo M., Wang Y. L. (2000). Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Cell Physiol. 279, C1345-C1350 [DOI] [PubMed] [Google Scholar]
- Wang N., Naruse K., Stamenovic D., Fredberg J. J., Mijailovich S. M., Tolic-Norrelykke I. M., Polte T., Mannix R., Ingber D. E. (2001). Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA 98, 7765-7770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-L., Pelham R. J. (1998). Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 298, 489-496 [DOI] [PubMed] [Google Scholar]
- Yano Y., Geibel J., Sumpio B. E. (1996). Tyrosine phosphorylation of pp125FAK and paxillin in aortic endothelial cells induced by mechanical strain. Am. J. Cell Physiol. 271, C635-C649 [DOI] [PubMed] [Google Scholar]
- Yoshigi M., Hoffman L. M., Jensen C. C., Yost H. J., Beckerle M. C. (2005). Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 171, 209-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Kim H. Y., Wang J. H. C., Davidson L. A. (2010). Macroscopic stiffening of the embryonic tissues via micortubules, RhoGEF, and the assembly of contractile bundles of actomyosin. Development 137, 2785-2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.