Abstract
A series of novel 2-nitro-1H-imidazole- and 3-nitro-1H-1,2,4-triazole-based aromatic and aliphatic amines were screened for anti-trypanosomal activity and mammalian cytotoxicity by the Drugs for Neglected Diseases initiative (DNDi). Out of 42 compounds tested, eighteen 3-nitro-1,2,4-triazoles and one 2-nitroimidazole displayed significant growth inhibitory properties against T. cruzi amastigotes (IC50 ranging from 40 nM to 1.97 μM), without concomitant toxicity towards the host cells (L6 cells), having selectivity indices (SI) 44 to 1320. Most (16) of these active compounds were up to 33.8-fold more potent than the reference drug benznidazole, tested in parallel. Five novel 3-nitro-1,2,4-triazoles were active against bloodstream form (BSF) T. b. rhodesiense trypomastigotes (IC50 at nM levels and SI 220 to 993). An NADH-dependent nitroreductase (TbNTR) plays a role in the anti-parasitic activity, since BSF T. b. brucei trypomastigotes with elevated TbNTR levels were hypersensitive to tested compounds. Therefore, a novel class of affordable 3-nitro-1,2,4-triazole-based compounds with antitrypanosomal activity has been identified.
Keywords: nitrotriazoles, amines, T. cruzi, Chagas disease, antitrypanosomal agents
Introduction
The protozoan parasites Trypanosoma cruzi (T. cruzi)a, Trypanosoma brucei (T. brucei), and various Leismania species, also referred to as trypanosomatids, are the causative agents of Chagas disease, Human African Trypanosomiasis (HAT) and different forms of leishmaniasis, respectively. Over 10 million people are infected by T. cruzi and 50,000 to 80,000 by T.b. gambiense or T.b. rhodesiense, resulting in more than 40,000 deaths per year.1 Chagas disease is transmitted by blood sucking triatomine insects and occurs mainly in Latin America. Although over the past 20 years the number of incidences has declined, primarily due to vector control initiatives,2 the number of cases in non-endemic regions such as the United States is on the rise.3 Reasons for this rise include population migration, drug usage and medical practices. With no immediate prospect for vaccines, chemotherapy is the only way to fight the parasite in the patient.
Currently two nitroheterocycle prodrugs, Nifurtimox (4-(5-nitrofurfurylidenamino)-3-methylthio-morpholine-1,1-dioxide) (Nfx) and Benznidazole (N-benzyl-2-(2-nitro-1H-imidazol-1-yl)acetamide) (Bnz) (Chart 1), are used to treat Chagas disease.4 However, their use is problematic as both can cause side effects, have limited efficacy, while some strains are refractory to treatment.5 In addition, the large quantities of medication required render it expensive, and the recommended long course of treatment is often not completed, resulting in the development of resistance. Therefore, the need for new drugs to treat this disease is urgent.
Chart 1.
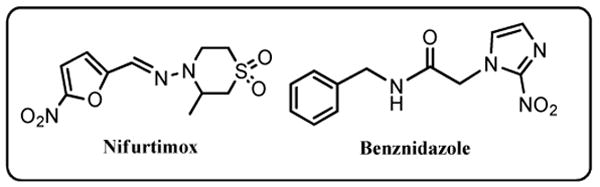
As with most nitroheterocyclic compounds, Nfx and Bnz both function as prodrugs and must undergo activation before mediating their cytotoxic effects. Initially it was proposed that the trypanocidal action of Nfx was due to its ability to induce oxidative stress within the parasite5-7 and several trypanosomal flavoproteins have been shown to mediate the 1-electron reduction of this prodrug's conserved nitro-group that subsequently promotes formation of superoxide anions via a futile cycle.7-9 However, although this reaction does occur in parasite cells, the available functional data suggests that it does not occur at levels that are toxic to the trypanosome.10 Recently, an alternative reduction pathway has been elucidated involving the activity of a type I nitroreductase (NTR).11 This enzyme can mediate a series of 2 electron reduction reactions of both Nfx and Bnz resulting in fragmentation of the heterocyclic ring and production of toxic metabolites.10,12
Recent reports about several new nitroheterocycles having trypanocidal activities with no or low toxicity,13-18 in conjunction with the fact that the activation of nitroheterocyclic prodrugs can be catalyzed by the type I NTR, which is normally absent in most eukaryotes, with trypanosomes being a major exception, have led to a renewed interest in the use of these compounds as antiparasitic agents.
In collaboration with the Drugs for Neglected Diseases initiative (DNDi), we have found that 9-[(3-nitro-1H-1,2,4-triazolyl)-propylamino]acridine hydrochloride (NTLA-1 or NLA-6, 1; Chart 2), a compound that was originally designed as a DNA-targeting anti-cancer agent,19,20 and which was screened against T. cruzi, T. b. rhodesiense and L. donovani, was significantly and selectively active against T. cruzi amastigotes in infected L6 myoblasts, without showing toxicity for the host cells.21 Thus, NTLA-1 demonstrated an IC50 of 140 nM for the parasite and a selectivity index (SI = IC50 for L6 cells/IC50 for T. cruzi) of 146.21 NTLA-1, given at just 2 mg/kg/day, for 50 days in mice infected with T. cruzi, in the acute phase of infection, resulted in a rapid and persistent drop in peripheral parasite levels and in a fraction of cures (20%).22 Importantly, there was an absolute correlation between treatment efficacy as determined parasitologically and the increase in the fraction of T. cruzi-specific CD8+ T cells with a T central memory phenotype in the peripheral blood of treated mice.22 However, NTLA-1, which inhibits topoisomerase I and II,20 demonstrated toxicity at 15 mg/kg given i.p. for 30 days. Therefore, a more thorough investigation was initiated for the development of less toxic and more efficacious nitrotriazole- and nitroimidazole-based compounds as trypanocidal agents. Here we describe the synthesis and in vitro evaluation of 3-nitro-1H-1,2,4-triazole-based and 2-nitroimidazole-based aromatic and aliphatic amines as antiparasitic agents.
Chart 2.
Chemistry
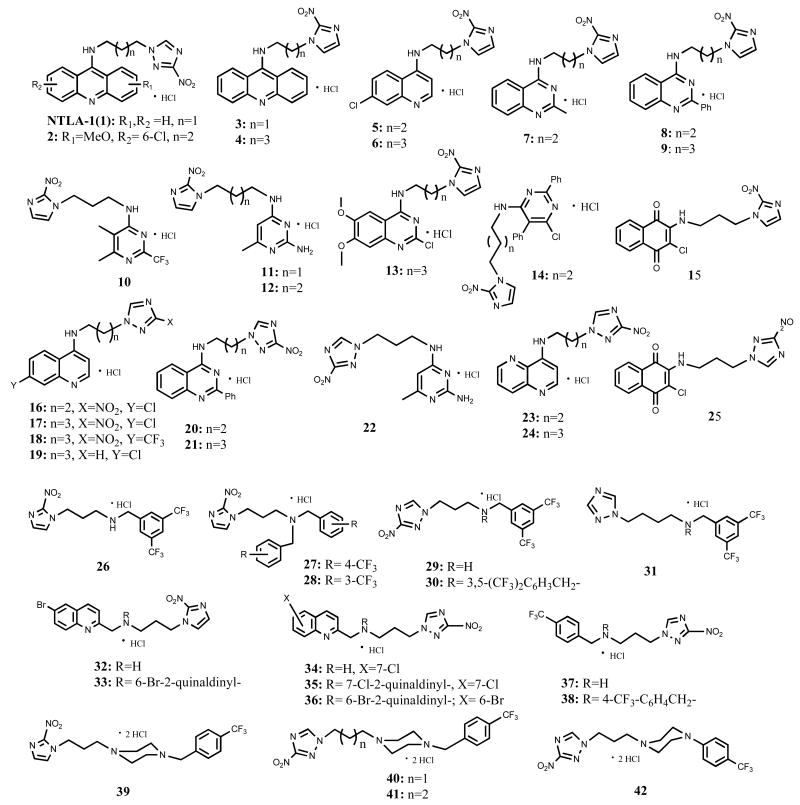
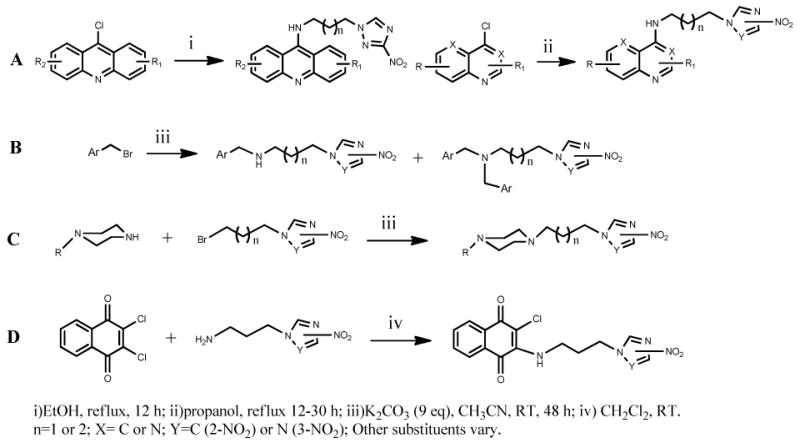
The structure of all compounds is depicted on Chart 2. Their synthesis is straightforward and based on well-established chemistry, outlined in Scheme 1. Aromatic amines 1-14 and 16-24 were synthesized by coupling the appropriate nitrotriazole- or nitroimidazole alkyl amine19 with the appropriate chloro- or fluoro-aromatic chromophore23 by nucleophilic aromatic substitution (Scheme 1A). The yields were in general moderate to good with the exception of 18. Aliphatic secondary and tertiary amines 26-38 were synthesized via the same reaction by nucleophilic attack of the appropriate nitrotriazole- or nitroimidazole alkyl amine to a chosen bromide in the presence of K2CO3 (Scheme 1B). In most cases the monoalkylated product was the dominant one, since 1 equivalent of the required halide was used. Piperazine derivatives 39-42 were synthesized similarly, by nucleophilic attack of the appropriate, commercially available mono-substituted piperazine to the appropriate nitrotriazole- or nitroimidazole alkyl bromide24 (Scheme 1C). Finally, enamines 15 and 25 were synthesized from 2,3-dichloro-1,4-naphthoquinone and 2-nitroimidazole-propylamine or 3-nitro-1,2,4-triazole-propylamine, respectively, by nucleophilic substitution (Scheme 1D).
Scheme 1.
Results and Discussion
Anti-proliferative effects of nitrotriazole and nitroimidazole compounds
The in vitro growth inhibitory properties of all compounds against T. b. rhodesiense bloodstream form trypomastigotes, T. cruzi amastigotes (in infected L6 myoblasts), axenically cultured L. donovani amastigotes and rat skeletal myoblasts (L6 cells) were evaluated by using standard drug screens25. From resultant dose response curves, IC50 values in μM were determined (Table 1). The criteria for activity were set as follows: For T.b. rhodesiense, compounds that gave an IC50 < 0.5 μM, were designated as ‘active’, while those yielding an IC50 = 0.5-6.0 μM or an IC50 > 6.0 μM were designated ‘moderately active’ and ‘inactive’, respectively. For T. cruzi, IC50 < 4.0 μM, ‘active’; IC50 = 4.0-60 μM, ‘moderately active’; IC50 > 60 μM, ‘inactive’. For L. donovani, IC50 < 1 μM, ‘active’; IC50 = 1.0-6.0 μM, ‘moderately active’; IC50 > 6.0 μM, ‘inactive’. Based on these criteria, all but compounds 19 and 31 were active or moderately active against T. cruzi, about 66 % of all compounds were active or moderately active against T. brucei rhodesiense, and only 6 compounds (∼ 14%) were active or moderately active against L. donovani parasites. However, for a compound to be considered for further in vivo investigation, the growth inhibitory effect against the mammalian cell line L6 has to be evaluated from which a measure of a compound's cytotoxicity can be deduced. Thus, the selectivity index (SI), namely the ratio of IC50 against L6 cells to IC50 against each parasite, is also an important parameter. This SI must be ≥ 100 for T.b. rhodesiense, ≥ 50 for T. cruzi and ≥ 20 for L. donovani axenic amastigotes.
Table 1. In vitro screening data against three different trypanosomatids.
| All values as μM | Comp. Typee |
|||||||
|---|---|---|---|---|---|---|---|---|
| Compound | T.b.rhodesiensea | T. cruzib | L. donovani axen. c | Cytotox. L6d | ||||
| IC-50 | SI | IC-50 | SI | IC-50 | SI | IC-50 | ||
| 1 | 0.611 | 34 | 0.140 | 147 | 35.37 | 1 | 20.52 | Nitro-Trz |
| 2 | 0.134 | 74 | 0.151 | 66 | 10.15 | 1 | 9.935 | Nitro-Trz |
| 3 | 0.996 | 0 | 0.996 | 0 | 9.09 | 0 | 0.308 | Nitro-Im |
| 4 | 0.601 | 3 | 0.926 | 2 | 14.77 | 0 | 1.675 | Nitro-Im |
| 5 | 1.397 | 18 | 1.041 | 24 | 54.35 | 0 | 24.95 | Nitro-Im |
| 6 | 1.872 | 36 | 3.508 | 19 | 39.06 | 2 | 66.83 | Nitro-Im |
| 7 | 3.455 | 9 | 2.097 | 15 | 68.29 | 0 | 31.56 | Nitro-Im |
| 8 | 3.922 | 22 | 1.973 | 45 | 10.11 | 9 | 87.82 | Nitro-Im |
| 9 | 2.756 | 12 | 3.628 | 9 | 6.17 | 5 | 32.46 | Nitro-Im |
| 10 | 15.16 | >16 | 4.28 | >55 | 20.05 | >12 | >236.5 | Nitro-Im |
| 11 | 10.271 | >28 | 7.081 | >41 | >95.69 | ∼3 | >287.0 | Nitro-Im |
| 12 | 11.782 | >23 | 41.82 | >7 | 98.07 | >3 | >274.7 | Nitro-Im |
| 13 | 2.257 | 7 | 0.968 | 17 | 3.63 | 5 | 16.48 | Nitro-Im |
| 14 | 15.46 | 1 | 9.67 | 1 | 12.43 | 1 | 11.71 | Nitro-Im |
| 15 | 0.621 | 6 | 30.12 | 0 | 0.516 | 8 | 3.91 | Nitro-Im |
| 16 | 0.309 | 463 | 0.607 | 236 | 45.78 | 3 | 143.22 | Nitro-Trz |
| 17 | 0.193 | 708 | 0.14 | 976 | 64.49 | 2 | 136.6 | Nitro-Trz |
| 18 | 0.117 | 973 | 0.305 | 373 | 136.56 | 1 | 113.83 | Nitro-Trz |
| 19 | 21.82 | >14 | 92.14 | >3 | >298.5 | ∼1 | >298.5 | Trz |
| 20 | 1.417 | 68 | 1.48 | 66 | 9.48 | 10 | 96.99 | Nitro-Trz |
| 21 | 0.562 | 137 | 1.74 | 44 | 8.69 | 9 | 77 | Nitro-Trz |
| 22 | 2.191 | >131 | 33.7 | >8 | >95.39 | ∼3 | >286 | Nitro-Trz |
| 23 | 2.22 | 60 | 1.031 | 129 | 182.29 | 1 | 132.67 | Nitro-Trz |
| 24 | 0.435 | 220 | 0.46 | 208 | >275.5 | 0 | 95.77 | Nitro-Trz |
| 25 | 0.882 | 8 | 15.6 | 0 | 6.53 | 1 | 7.16 | Nitro-Trz |
| 26 | 13.8 | 10 | 24.48 | 6 | >69.36 | ∼2 | 139.65 | Nitro-Im |
| 27 | 23.04 | >7 | 3.77 | >46 | 6.49 | >27 | >172.25 | Nitro-Im |
| 28 | 21.57 | 4 | 8.02 | 10 | 4.27 | 19 | 79.62 | Nitro-Im |
| 29 | 7.84 | 18 | 0.169 | 816 | 11.07 | 12 | 137.9 | Nitro-Trz |
| 30 | 8.17 | 10 | 1.96 | 40 | 4.87 | 16 | 78.79 | Nitro-Trz |
| 31 | 32.30 | >7 | 123.40 | >2 | 172.25 | >1 | >223.6 | Trz |
| 32 | 8.56 | 16 | 6.05 | 23 | 80.28 | 2 | 137.03 | Nitro-Im |
| 33 | 14.23 | 7 | 4.67 | 20 | 18.67 | 5 | 93.6 | Nitro-Im |
| 34 | 1.05 | 136 | 0.311 | 460 | 63.81 | 2 | 143.13 | Nitro-Trz |
| 35 | 0.917 | 78 | 0.358 | 200 | 9.58 | 7 | 71.62 | Nitro-Trz |
| 36 | 0.463 | 99 | 0.32 | 144 | 9.33 | 5 | 46 | Nitro-Trz |
| 37 | 3.61 | 34 | 0.32 | 383 | 158.7 | 1 | 122.41 | Nitro-Trz |
| 38 | 0.271 | 339 | 0.145 | 634 | 0.348 | 264 | 91.88 | Nitro-Trz |
| 39 | 15.30 | >13 | 20.72 | >9 | >191.49 | ∼1 | >191.49 | Nitro-Im |
| 40 | 1.38 | >140 | 0.34 | >562 | 58.15 | >3 | >191.08 | Nitro-Trz |
| 41 | 1.2 | 99 | 0.412 | 287 | 44.45 | 3 | 118.37 | Nitro-Trz |
| 42 | 5.33 | 10 | 0.04 | 1320 | 25.92 | 2 | 52.79 | Nitro-Trz |
|
| ||||||||
| Melarsoprol | 0.01* | |||||||
| Benznidazole | 1.35* | |||||||
| Miltefosine | 0.44* | |||||||
|
| ||||||||
| active, | ||||||||
| moderate activity | ||||||||
| active, but cytotoxic, low specificity | ||||||||
| compounds have been previously synthesized.19, 23, 29-31 | ||||||||
STIB 900 trypomastigotes;
Tulahuen C4 amastigotes;
MHOM-ET-67/L82 amastigotes;
Cytotoxicity measurements;
Nitro-Trz: 3-nitro-1H-1,2,4-triazole; Nitro-Im: 2-nitro-1H-imidazole; Trz: not nitro triazole;
Median values from 43 measurements in parallel with each compound. SI = IC50 in L6 cells / IC50 in parasites.
Based on the above, only 6 compounds (16-18, 24, 36, 38) were active and selective against T.b. rhodesiense, whereas 18 compounds (1, 2, 16-18, 20, 23, 24, 27, 29, 34-38, 40-42) were active and selective against T. cruzi (Table 1). Only one compound, 38, was active and selective against L. donovani. Therefore, the antichagasic activity of these compounds is of the greatest interest based on the number of active molecules.
Evaluation of Structure-Activity Relationships: Analysis of the nitroheterocyclic ring
As a large set of the compounds showed significant anti-T. cruzi activity, we were able to conduct a detailed structure-activity relationship. Analysis of the trypanocidal activity in relation to the nitroheterocyclic ring revealed that compounds (3-9, 13, 21, 27, 30) that were active against T. cruzi (IC50< 4 μM) but not sufficiently specific (SI < 50), were exclusively 2-nitroimidazole derivatives except for compounds 21 and 30. Similarly, moderately active compounds with low specificity against T. cruzi (11, 12, 14, 15, 22, 25, 26, 28, 32, 33, 39) were seen mainly in the 2-nitroimidazole series. In contrast, all 3-nitrotriazoles, with the exception of 22 and 25, demonstrated significant in vitro anti-T. cruzi activity coupled with excellent selectivity (Table 1). In all cases where an active/moderately active trypanocidal effect was observed, irrespective of SI values, the 3-nitrotriazole derivatives (1, 16, 17, 20, 21, 29, 38) always had a greater effect (1.3 - 45 fold) on parasite growth as compared to their 2-nitroimidazole counterparts (3, 5, 6, 8, 9, 26, 27), and no toxicity: compare 1 with 3, 16 with 5, 17 with 6 etc (Table 1). Similar results are seen even with the moderately active and not specific 3-nitrotriazole 25 (a naphthoquinone derivative) which is 2-fold more potent than its 2-nitroimidazole analog 15.
To determine whether the nitro-group was important in the anti-parasitic activity of the triazoles, two non-nitro compounds (19 and 31) were synthesized and their growth inhibitory properties against T. cruzi compared with that of their nitro-analogs 17 and 29 (note: 31 has an extra methylene group as compared to 29). In both cases, the removal of the nitro-group led to inactivity (IC50 > 60 μM) and the IC50 value was significantly increased (658- and 730-fold, respectively) compared to the nitro-containing analog (Table 1). The anti-HAT activity of 19 and 31 was also reduced compared to that of 17 and 29, but to a lesser degree. Therefore, the nitro group present on the triazole ring is essential in mediating the anti-parasitic activity of these compounds.
Analysis of aromatic amines
A closer look at the SARs for all antichagasic compounds is given in Table 2. In the subclass of 3-nitrotriazole bearing aromatic amines (1, 2, 16-18, 20, 21, 23, 24), activity decreases in the following order: acridines (1, 2) ≥ quinolines (16-18) > 1,5-naphthyridines (23, 24) > quinazolines (20, 21). The 2-nitroimidazole linked quinazoline derivative 8 demonstrates similar activity with the 3-nitrotriazole analogs 20 and 21.
Table 2. Biological and physical properties of analogs active against T. cruzi amastigotes.
| Compound | T. cruzi IC-50 (μM) |
SI | Bzn/Compa | clogP | pKa | Lipinski Rule of 5 | PSA (Å2) |
|---|---|---|---|---|---|---|---|
| 1 | 0.14 | 147 | 9.6 | 3.20 | 9.20 | S | 101.45 |
| 2 | 0.15 | 66 | 8.9 | 4.16 | 8.84 | S | 110.68 |
| 8 | 1.97 | 45 | 0.7 | 4.56 | 5.06 | S | 101.45 |
| 16 | 0.61 | 236 | 2.2 | 2.43 | 7.31 | S | 101.45 |
| 17 | 0.14 | 976 | 9.6 | 2.95 | 7.31 | S | 101.45 |
| 18 | 0.31 | 373 | 4.4 | 3.22 | 7.53 | S | 101.45 |
| 20 | 1.48 | 66 | 0.9 | 4.05 | 5.06 | S | 114.34 |
| 21 | 1.74 | 44 | 0.8 | 4.52 | 5.06 | S | 114.34 |
| 23 | 1.03 | 129 | 1.3 | 0.99 | 6.81 | S | 114.34 |
| 24 | 0.46 | 208 | 2.9 | 1.51 | 6.81 | S | 114.34 |
| 29 | 0.17 | 816 | 8.0 | 3.51 | 9.44 | S | 88.56 |
| 34 | 0.31 | 460 | 4.3 | 2.60 | 8.76 | S | 101.45 |
| 35 | 0.36 | 200 | 3.8 | 5.55 | 6.87 | V (2) | 105.55 |
| 36 | 0.32 | 144 | 4.2 | 5.88 | 6.87 | V (2) | 105.55 |
| 37 | 0.32 | 383 | 4.2 | 2.63 | 9.65 | S | 88.56 |
| 38 | 0.15 | 634 | 9.3 | 5.62 | 8.79 | V (2) | 79.77 |
| 40 | 0.34 | >562 | 4.0 | 2.86 | 8.33 | S | 83.01 |
| 41 | 0.41 | 287 | 3.3 | 3.38 | 8.52 | S | 83.01 |
| 42 | 0.04 | 1320 | 33.8 | 3.03 | 7.85 | S | 83.01 |
| Bnz | 1.35* | 1.0 | 1.32 | S | 92.74 |
Bzn/Comp: IC50 of Bnz / IC50 of Comp. PSA: Polar surface area.
Median values from 43 measurements in parallel with each compound. All physical properties were predicted by using the Marvin Calculator (www.chemaxon.com).
An extra methylene group in the linkage in compound 2 and the chloro- substituent in the acridine ring increased lipophilicity and toxicity, compared to its analog 1, but did not decrease activity. It is assumed that the acridine compounds 1 and 2 demonstrate increased toxicity and lack of sufficient selectivity due to DNA-intercalation19 and topoisomerase I/II inhibition.20 Thus, compound 1, which was tested in vivo for Chagas, could not be given at sufficient doses for an extended period of time, due to the observed toxicity.22
Comparing the quinoline analogs 16 and 17, it is observed that increased lipophilicity in 17, due to an extra methylene group in the linkage, slightly increased toxicity (Table 1); however, at the same time activity was also increased, resulting in an improved SI (by a factor of 4) compared to 16. Comparing the analogs 17 and 18, it is observed that the replacement of chlorine in 17 with a trifluoromethyl group increases lipophilicity and toxicity in 18, however the activity remains at low nM concentrations, slightly less than that in 17, but still better than the one in 16. All three quinoline compounds show excellent selectivity, significantly higher than the threshold of 50 we have set, and are candidates for in vivo studies.
Comparing the quinazoline systems 20 and 21 (Tables 1 and 2), it is observed that in this case an extra methylene group in the linkage of 21 did not improve the antichagasic activity but increased lipophilicity and thus toxicity, lowering thus the SI from 66 to 44. Similar results, but significantly more prominent, can be seen with the 2-nitroimidazole-based quinazoline systems 8 and 9, which are the corresponding analogs of 20 and 21, respectively; in this case 9 was totally inactive, whereas 8 is more comparable with 21 rather than 20 with regard to its antichagasic activity and selectivity (Table 2).
Finally, in the case of the two naphthyridine compounds 23 and 24, the beneficial effect of an extra methylene group in the linkage of 24 is reflected in its improved activity and selectivity, despite its increased toxicity (Tables 1 and 2).
It is worth mentioning that while alteration in the length of the linkage between the nitrotriazole/imidazole ring and aromatic chromophore in the aromatic amines can not always predict the direction of changes in the antichagasic activity, it is clear in all cases (2, 17, 18, 21, 24) that four methylene groups in the linkage favor anti-HAT activity (Table 1).
Analysis of aliphatic amines
The 3-nitrotriazole-based benzylamines 29, 37 and 38 are all active against T. cruzi and demonstrate very good SI values (Table 2). The dibenzylated derivative 38 is significantly more lipophilic and thus more toxic than the monobenzylated analog 37, violating twice the Lipinski rule of 5 (Table 2). However, its increased antichagasic activity balances out its toxicity, so it appears with a better SI value than 37 (Tables 1 and 2). Interestingly, 38 is the only compound active across all parasites tested (Table 1). Compound 29, although more lipophilic (due to 2 trifluoromethyl groups) than 37, appears less toxic, perhaps because the trifluoromethyl groups being in meta positions offer a better compound-stability compared to 37.
The 3-nitrotriazole-based quinaldinamines 34, 35 and 36 demonstrate similar antichagasic activity and their SI corresponds inversely to their clogP value and toxicity (Table 1 and 2). All three analogs have similar activity with the p-trifluoromethylbenzylamine 37, but the monoalkylated chloro-quinaldine analog 34 demonstrates a superior SI value, presumably due to its decreased toxicity compared to 37, despite the fact that both 34 and 37 have similar clogP values. As was expected, the dialkylated analogs 35 and 36 also violate the Lipinski rule of 5.
The piperazine systems (40-42) showed significant antichagasic activity in vitro (Table 1). However, the 1-phenyl-piperazine 42 showed about 10-fold increased activity (IC50 at low nM concentrations) compared to the 1-benzyl-piperazines 40 and 41. Although the lipophilicity of 42 was between that of 40 and 41, its toxicity was higher than both of them. Despite an increased toxicity (Table 1), the SI of 42 was 1320, the highest of all tested compounds, making 42 a good candidate for in vivo studies. Comparing the substituted benzylpiperazine derivatives 40 and 41, it is observed that an extra methylene in 41, in the linkage between 3-nitrotriazole and the piperazine ring, decreased potency and increased lipophilicity and toxicity, resulting in a lower SI value compared to 40 (Table 2).
It can be observed that all compounds with antichagasic activity in Table 2 have a polar surface area < 140 and >60 Å2, which means good cell-membrane permeability and presumably absence of neurotoxicity since they can not cross the blood-brain barrier. In addition, all but compounds 8, 20 and 21 (all 2-phenylquinazolines), demonstrate a better antichagasic activity (1.3-33.8 fold) than the reference compound benznidazole, tested in parallel. It appears that increased antichagasic activity is observed with increased basicity in the amines of Table 2.
Evaluating the mechanism of action of nitrotriazoles
As was mentioned earlier, nitroheterocyclic prodrugs must undergo enzyme-mediated activation within the pathogen to have cytotoxic effects. These enzymes are most likely nitroreductases, although other reducing enzymes specific to the parasite, such as trypanothione reductase8,26 or NADH-fumarate reductase27, could be involved. Both Nfx and Bnz are activated by the NADH-dependent, oxygen insensitive, mitochondrially localized, bacterial-like, type I NTR, and down-regulation of this enzyme explains how resistance emerges.10-12 Therefore, we investigated the role of recombinant T. brucei NTR (TbNTR) in the activation of selected nitrotriazoles and the nitroimidazole 8 (Fig. 1; supplemental material), as well as the susceptibility of bloodstream form T. brucei brucei, engineered to overexpress tetracycline-inducible TbNTR, to these compounds (Table 3). The reduction potentials (E1/2) of the active compounds towards bloodstream form T. b. brucei were also measured by cyclic voltametry, to elucidate if there is any correlation between enzymatic activity and redox properties, and are shown in Table 3. Compounds from all sub-categories (aromatic and aliphatic amines, as well as piperazinic derivatives) have been chosen for these studies.
Table 3.
The effect of type I Nitroreductase (TbNTR) on the activity of selected compounds against bloodstream-form T. brucei brucei parasites.
| ID No | T.b. bruceia | TbNTRb | TbNTRb | Ratio | E1/2c |
|---|---|---|---|---|---|
| IC-50 (μM) | -tet | +tet | -tet/+tet | (V) | |
| 8 | 7.47 ± 0.71 | 7.58 ± 0.19 | 0.95 ± 0.11 | 8.00 | -1.03 |
| 17 | 0.17 ± 0.04 | 0.44 ± 0.06 | 0.10 ± 0.04 | 4.00 | -1.18 |
| 20 | 2.63 ± 0.25 | 4.48 ± 0.19 | 0.07 ± 0.02 | 64.00 | -1.04 |
| 23 | > 10 | nd | nd | nd | nd |
| 29 | 7.83 ± 0.32 | 11.08 ± 2.50 | 0.76 ± 0.16 | 14.00 | -1.07 |
| 38 | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.10 ± 0.02 | 2.00 | -1.06 |
| 40 | > 10 | nd | nd | nd | nd |
| 41 | > 10 | nd | nd | nd | -1.04 |
| 42 | 2.30 ± 0.10 | 2.63 ± 0.12 | 0.21 ± 0.01 | 13 | nd |
| Nifurtimoxd | 1.71 ± 0.06 | 0.13 ± 0.04 | 13 | -0.88 | |
Bloodstream form wild type T. brucei brucei parasites;
bloodstream form T. b. brucei, engineered to over-express type I nitroreductase in the presence of tetracycline (tet).
Reduction potential of each compound was measured in DMSO (except for 17, in CH3CN) by cyclic voltammetry relative to Ag/AgCl.
The E1/2 value is taken from ref. [32].
With regard to anti-HAT activity, it is observed that compounds 17 and 38 that were very active against T.b. rhodesiense (Table 1), were similarly active against bloodstream form T. b. brucei (Table 3), whereas compounds that were inactive (8, 29, 42) or moderately active (20, 23, 40, 41) against T.b. rhodesiense (Table 1), were in general more inactive against bloodstream form T. b. brucei (Table 3). With regard to the tetracycline (+tet)-inducible TbNTR overexpression system, it is observed that parasites induced to overexpress TbNTR are more susceptible to all nitrotriazoles/nitroimidazole tested, with compounds being moderately active against bloodstream form T. b. brucei showing a greater difference than the most active 17 and 38. As a general rule of thumb, if a -tet/+tet ratio is >5, then it is assumed that the major growth inhibitory activity of a compound is via NTR activation. For compounds with a -tet/+tet ratio <5, alternative systems may be involved or the NTR generated reduction products are extremely trypanocidal.
There was no correlation between trypanocidal activity and enzymatic activity (see supplemental material). Furthermore, no conclusive data were obtained by comparing the enzymatic activity with reduction potentials (E1/2), although there was a trend suggesting an increasing activity at more negative E1/2 values, values that possibly lie outside the normal range of mammalian redox systems. If this is true, then the mutagenic potential of these compounds may be low,28 something that has been confirmed in limited Ames studies with 16, 20 and 29 (data not shown).
In conclusion, nine nitrotriazole-based compounds (16-18, 24, 29, 34, 40-42) have been identified from Table 2 as potential candidates for in vivo studies in T. cruzi infected mice, and further development against Chagas. All of them have demonstrated significant antichagasic activity at low to intermediate nmolar concentrations, SI values of ≥200, and satisfy the Lipinski's rule of 5. In addition, compound 38 may also warrant additional attention as it displays significant anti-parasitic activity against T. cruzi, both T. brucei sub-species and L. major with high selectivity, although this compound does violate two of the Lipinski's rule of 5.
Experimental
All starting materials and solvents were purchased from Sigma-Aldrich (Milwaukee, WI), were of research-grade quality and used without further purification. Solvents used were anhydrous and the reactions were carried out under a nitrogen atmosphere and exclusion of moisture. Melting points were determined by using a Mel-Temp II Laboratory Devices apparatus (Holliston, MA) and are uncorrected. Elemental Analyses were obtained by Midwest Microlab, LLC (Indianapolis, IN). Proton NMR spectra were obtained on a Varian Inova-500 or a Bruker Avance-III-500 spectrometer at 500 MHz and are referenced to Me4Si or to the corresponding protonated solvent, if the solvent was not CDCl3. High-resolution electrospray ionization (HRESIMS) mass spectra were obtained on a Agilent 6210 LC-TOF mass spectrometer at 11000 resolution. Thin-layer chromatography was carried out on aluminum oxide N/UV254 or polygram silica gel G/UV254 coated plates (0.2 mm, Analtech, Newark, DE). Chromatography was carried out on preparative TLC alumina GF (1000 microns) or silicagel GF (1500 microns) plates (Analtech). All the amines were purified by preparative TLC chromatography on alumina plates (≥ 95% purity). The results from elemental analysis for C, H and N were within 0.4 of the theoretical value.
The synthesis of compounds 1, 3-7, 10 and 15 has been described before.19, 23, 29-31 Compounds 2 and 25 were synthesized in a similar manner with 119 and 15,31 respectively.
General Synthetic Procedure of aromatic amines
For compounds 8-14, 16-24: The appropriate chloro-aromatic starting material (commercially available in most cases) (1.24 mmol) was coupled with 2-nitro-1H--imidazolyl-alkylamine (1.24 mmol)19 or 3-nitro-1,2,4-triazolyl-alkylamine (1.24 mmol),19 by refluxing in absolute propanol (7-10 ml) for 12 -30 h. In the case of compounds 16, 17 and 19, the 4,7-dichloroquinoline was first converted to 7-chloro-4-fluoroquinoline23 before coupling. In the case of compound 19, 4-(1H-1,2,4-triazol-1-yl)butylamine was first synthesized as in ref. [19], to be then coupled with 7-chloro-4-fluoroquinoline. In the case of compound 18, 4-fluoro-7-trifluoromethyl quinoline could not be synthesized from the corresponding 4-chloro-7-trifluoromethylquinoline. In most cases the hydrochloride salt of the final product was precipitated upon cooling of the reaction mixture and separated by filtration. In some cases, the free amine of the desired product was isolated by preparative TLC on alumina, dissolved in ethyl acetate and converted to its HCl salt by treating with 1 M HCl in diethyl ether. In the case of compounds 23 and 24, the starting material 4-chloro-1,5-naphthyridine was synthesized in 4 steps as described previously.29
General Synthetic Procedure of mono- and dialkylated aliphatic amines 26-38
The appropriate bromide (1.035 mmol) was added dropwise (15 min) to a solution of 2-nitro-1H-imidazolyl-alkylamine (1.035 mmol) or 3-nitro-1H-1,2,4-triazolyl-alkylamine (1.035 mmol)19 in the presence of potassium carbonate (9.52 mmol) in dry acetonitrile (15 mL) and the reaction mixture was stirred under a nitrogen atmosphere, at room temperature for 48 h. In the case of 31, 4-(1H-1,2,4-triazol-1-yl)butylamine was used. The reaction mixture was then filtered, the solids were washed with acetonitrile, the organic filtrate was evaporated and the residue extracted from water-chloroform. The organic layer was separated and dried over anhydrous Na2SO4. The solvent was evaporated and the residue was separated by preparative TLC on alumina plates with ethyl acetate : petroleum ether mixture. Monoalkylated and dialkylated products were obtained in the same reaction at varying ratios for each case. The separated products were dissolved in ethyl acetate and converted to their HCl salts by treating with HCl gas in dry ether (1 M solution).
Piperazine derivatives (39-42) were synthesized from the commercially available appropriate monoalkylated piperazines (1.44 mmol) and the appropriate 2-nitro-1H-imidazolyl-alkylbromide or 3-nitro-1H-1,2,4-triazolyl-alkylbromide (1.485 mmol)24 in the presence of potassium carbonate (13.24 mmol) in dry acetonitrile (25 mL) as above.
6-Chloro-2-methoxy-N-[4-(3-nitro-1H-1,2,4-triazol-1-yl)butyl]acridin-9-amine hydrochloride (2)
Yellow powder (35%): mp 214-216 °C; 1H NMR (500 MHz, CD3OD) d: 8.58 (s, 1H), 8.44 (d, J=9.5 Hz, 1H), 7.78 (m, 3H), 7.68 (dd, J=8.0, 2.0 Hz, 1H), 7.50 (d, J=9.0 Hz, 1H), 4.40 (t, J=6.5 Hz, 2H), 4.18 (t, J=7.0, 2H), 4.01 (s, 3H), 2.11 (m, 2H), 1.99 (m, 2H). HRESIMS calcd for C20H20ClN6O3 m/z [M+H]+ 427.1286, found 427.1286.
N-[3-(2-Nitro-1H-imidazol-1-yl)propyl]-2-phenylquinazolin-4-amine hydrochloride (8)
Off white powder (44%): mp 174-176 °C (dec.); 1H NMR (500 MHz, CD3OD) d: 8.34 (d, J=8.5 Hz, 1H), 8.27 (d, J=7.5 Hz, 2H), 8.07 (t, J=7.5 Hz, 1H), 7.98 (d, J=8.0 Hz, 1H), 7.81-7.76 (m, 2H), 7.69 (t, J=8.0 Hz, 2H), 7.59 (s,1H), 7.13 (s, 1H), 4.68 (t, J=7.0 Hz, 2H), 4.04 (t, J=7.0 Hz, 2H), 2.45 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C20H19N6O2 m/z [M+H]+ 375.1570, found 375.1569.
N-[4-(2-Nitro-1H-imidazol-1-yl)butyl]-2-phenylquinazolin-4-amine hydrochloride (9)
Off white powder (51%). 1H NMR (500 MHz, CD3OD) d: 8.34-8.30 (m, 3H), 8.05 (t, J=7.5 Hz, 1H), 7.96 (d, J=8.5 Hz, 1H), 7.80-7.75 (m, 2H), 7.69 (t, J=8.0 Hz, 2H), 7.49 (s, 1H), 7.08 (s, 1H), 4.55 (t, J=7.0 Hz, 2H), 3.99 (t, J=7.0 Hz, 2H), 2.06 (quintet, J=7.0 Hz, 2H), 1.93 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C21H21N6O2 m/z [M+H]+ 389.1721, found 389.1729.
6-Methyl-4-N-[3-(2-nitro-1H-imidazol-1-yl)propyl]-pyrimidin-2,4-diamine hydrochloride (11)
Orange solid (34%): mp 178 °C (dec); 1H NMR (500 MHz, CD3OD) d: 7.53 (s, 1H), 7.17 (s, 1H), 5.89 (s, 1H), 4.55 (t, J=7.0 Hz, 2H), 3.52 (t, J=6.5, 2H), 2.25 (s, 3H), 2.19 (m, 2H). HRESIMS calcd for C11H16N7O2 m/z [M+H]+ 278.1366, found 278.1364.
6-Methyl-4-N-[4-(2-nitro-1H-imidazol-1-yl)butyl]-pyrimidin-2,4-diamine hydrochloride (12)
Off white powder (45%): mp 225-226 °C (dec); 1H NMR (500 MHz, CD3OD) d: 7.50 (s, 1H), 7.15 (s, 1H), 5.86 (s, 1H), 4.51 (t, J=7.5 Hz, 2H), 3.49 (t, J=6.5 Hz, 2H), 2.23 (s, 3H), 1.92 (quintet, J=7.5 Hz, 2H), 1.66 (quintet, J=7.5 Hz, 2H). HRESIMS calcd for C12H18N7O2 m/z [M+H]+ 292.1522, found 292.1530.
2-Chloro-6,7-dimethoxy-N-[4-(2-nitro-1H-imidazol-1-yl)butyl]quinazolin-4-amine (13)
Light yellowish powder (21%). 1H NMR (500 MHz, CDCl3) d: 7.23 (s, 1H), 7.17 (s, 1H), 7.14 (s, 1H), 6.91 (s, 1H), 5.93 (br t, 1H), 4.54 (t, J=7.5 Hz, 2H), 4.00 (s, 3H), 3.98 (s, 3H), 3.80-3.76 (m, 2H), 2.00 (quintet, J=7.5 Hz, 2H), 1.82 (quintet, J=7.5 Hz, 2H). HRESIMS calcd for C17H20ClN6O4 m/z [M+H]+ 407.1229, found 407.1236.
6-Chloro-N-[4-(2-nitro-1H-imidazol-1-yl)butyl]-2,5-diphenylpyrimidin-4-amine hydrochloride (14)
pale white powder (72%): mp 79-81 °C; 1H NMR (500 MHz, CD3OD) d: 8.21 (d, J=7.5 Hz, 2H), 7.68-7.56 (m, 6H), 7.46 (s, 1H), 7.39 (d, J=7.5 Hz, 2H), 7.08 (s, 1H), 4.49 (t, J=7.0 Hz, 2H), 3.66 (t, J=7.0, 2H), 1.91 (quintet, J=7.5 Hz, 2H), 1.71 (quintet, J=7.5 Hz, 2H). HRESIMS calcd for C23H22ClN6O2 m/z [M+H]+ 449.1487, found 449.1488.
7-Chloro-N-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]quinolin-4-amine hydrochloride (16)
White powder (84%): mp 240-242 °C; 1H NMR (500 MHz, D2O) d: 8.61 (s, 1H), 8.28 (d, J=7.0 Hz, 1H), 7.89 (d, J=9.0 Hz, 1H), 7.82 (s, 1H), 7.57 (d, J=9.0 Hz, 1H), 6.76 (d, J=7.0 Hz, 1H), 4.55 (t, J=6.5 Hz, 2H), 3.74 (t, J=6.5, 2H), 2.48 (m, 2H). HRESIMS calcd for C14H14ClN6O2 m/z [M+H]+ 333.0867, found 333.0866.
7-Chloro-N-[4-(3-nitro-1H-1,2,4-triazol-1-yl)butyl]quinolin-4-amine hydrochloride (17)
White powder (67%): mp 210-220 °C (dec); 1H NMR (500 MHz, D2O) d: 8.58 (s, 1H), 8.20 (d, J=7.0 Hz, 1H), 8.03 (d, J=9.0 Hz, 1H), 7.78 (s, 1H), 7.68 (d, J=9.0 Hz, 1H), 6.68 (d, J=7.0 Hz, 1H), 4.39 (t, J=6.5 Hz, 2H), 3.58 (t, J=7.0, 2H), 2.07 (m, 2H), 1.77 (m, 2H). HRESIMS calcd for C15H16ClN6O2 m/z [M+H]+ 347.1023, found 347.1019.
N-[4-(3-Nitro-1H-1,2,4-triazol-1-yl)butyl]-7-(trifluoromethyl)quinolin-4-amine hydrochloride (18)
White powder (14%). 1H NMR (500 MHz, CD3OD) d: 8.64 (s, 1H), 8.57 (d, J=8.5 Hz, 1H), 8.50 (d, J=7.0 Hz, 1H), 8.16 (s, 1H), 7.95 (d, J=8.5 Hz, 1H), 4.44 (t, J=6.5 Hz, 2H), 3.69 (t, J=7.0 Hz, 2H), 2.13 (m, 2H), 1.86 (m, 2H). HRESIMS calcd for C16H16F3N6O2 m/z [M+H]+ 381.1281, found 381.1286.
7-Chloro-N-[4-(1H-1,2,4-triazol-1-yl)butyl]quinolin-4-amine (19)
White powder (43%): mp 125-127 °C; 1H NMR (500 MHz, CDCl3) d: 8.54 (d, J=5.30 Hz, 1H), 8.10 (s,1H), 8.01 (s, 1H), 7.96 (d, J=2.1 Hz,1H), 7.73 (d, J=9.0, 1H), 7.37 (dd, J=8.9, 2.1 Hz, 1H), 6.39 (d, J=5.4 Hz, 1H), 5.32 (br s, 1H), 4.30 (t, J=6.8 Hz, 2H), 3.37 (m, 2H), 2.10 (m, 2H), 1.79 (m, 2H). HRESIMS calcd for C15H17ClN5O2 m/z [M+H]+ 302.1167, found 302.1169.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-2-phenyl-quinazolin-4-amine hydrochloride (20)
Yellow powder (71%): mp 246-248 °C (dec); 1H NMR (500 MHz, CD3SOCD3) d: 8.91 (s, 1H), 8.49 (br d, J=7.0 Hz,1H), 8.33 (d, J=7.5 2H), 8.08 (br s, 1H), 8.03 (br s, 1H), 7.75 (br s, 2H), 7.65 (br t, J=7.0 Hz, 2H), 4.52 (t, J=6.5 Hz, 2H), 3.90 (br m, 2H), 2.38 (t, J=6.5 Hz, 2H). HRESIMS calcd for C19H18N7O2 m/z [M+H]+ 376.1522, found 376.1523.
N-[4-(3-Nitro-1H-1,2,4-triazol-1-yl)butyl]-2-phenyl-quinazolin-4-amine hydrochloride (21)
Yellow powder (69%): mp >250 °C; 1H NMR (500 MHz, CD3SOCD3) d: 8.90 (s, 1H), 8.57 (d, J=8.0 Hz,1H), 8.39 (d, J=7.5 2H), 8.16 (d, J=8.0 Hz, 1H), 8.04 (t, J=7.5 Hz, 1H), 7.76 (t, J=7.0, 2H), 7.68 (t, J=7.5 Hz, 2H), 4.41 (t, J=7.0 Hz, 2H), 3.86 (br q, J=6.0 Hz, 2H), 2.012 (quintet, J=7.5 Hz, 2H), 1.78 (quintet, J= 7,0 Hz, 2H). HRESIMS calcd for C20H20N7O2 m/z [M+H]+ 390.1679, found 390.1681. Calculated analysis for C20H20ClN7O2: C, 56.41; H, 4.73; N, 23.02; Cl, 8.33. Found: C, 56.06; H, 5.01; N, 22.84; Cl, 9.06.
6-Methyl-4-N-[3-(2-nitro-1H-1,2,4-triazol-1-yl)propyl]-pyrimidin-2,4-diamine hydrochloride (22)
Off white powder (58%): mp 204-206 °C; 1H NMR (500 MHz, CD3OD) d: 8.60 (s, 1H), 5.85 (s, 1H), 4.42 (t, J=6.5 Hz, 2H), 3.53 (t, J=6.5, 2H), 2.28 (m, 2H), 2.24(s, 3H). HRESIMS calcd for C10H15N8O2 m/z [M+H]+ 279.1318, found 279.1319.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-1,5-naphthyridin-4-amine hydrochloride (23)
Yellowish powder (50%): mp 215-217 °C (dec); 1H NMR (500 MHz, CD3OD) d: 8.94 (d, J=3.5 Hz,1H), 8.65 (s, 1H), 8.47 (d, J=7.0 Hz, 1H), 8.27 (d, J=8.5 Hz,1H), 7.95 (dd, J=8.5, 4.5 Hz, 1H), 7.09 (d, J=7.0 Hz, 1H), 4.54 (t, J=6.5 Hz, 2H), 3.80 (t, J=7.0, 2H), 2.48 (m, 2H). HRESIMS calcd for C13H14N7O2 m/z [M+H]+ 300.1209, found 300.1206.
N-[4-(3-Nitro-1H-1,2,4-triazol-1-yl)butyl]-1,5-naphthyridin-4-amine hydrochloride (24)
Off white powder (61%). 1H NMR (500 MHz, CD3OD) d: 8.97 (d, J=4.0 Hz,1H), 8.64 (s, 1H), 8.43 (d, J=7.0 Hz, 1H), 8.26 (d, J=8.5 Hz,1H), 7.95 (dd, J=8.5, 4.5 Hz, 1H), 7.06 (d, J=7.5 Hz, 1H), 4.44 (t, J=7.0 Hz, 2H), 3.72 (t, J=7.0, 2H), 2.12 (m, 2H), 1.85(m, 2H). HRESIMS calcd for C14H16N7O2 m/z [M+H]+ 314.1360, found 314.1362.
2-Chloro-3-{[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]amino}-1,4-dihydronaphthalene-1,4-dione (25)
Dark red powder (74%): mp 137-138 °C; 1H NMR (500 MHz, CD3COCD3) d: 8.69 (s, 1H), 8.06 (d, J=8.0 Hz,1H), 8.02 (d, J=8.5 Hz, 1H), 7.84 (t, J=8.0 Hz,1H), 7.75 (t, J=8.0 Hz, 1H), 6.96 (br s, 1H), 4.61 (t, J=7.0 Hz, 2H), 4.04 (t, J=7.0 Hz, 2H), 2.44 (m, 2H). HRESIMS calcd for C15H13ClN5O4 m/z [M+H]+ 362.0651, 364.0627, found 362.0654, 364.0632.
{[3,5-bis(Trifluoromethyl)phenyl]methyl}[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]amine hydrochloride (29)
White powder (60-64%): mp 140-142 °C; 1H NMR (500 MHz, CD3OD) d: 8.68 (s, 1H), 8.20 (s, 2H), 8.12 (s, 1H), 4.52 (m, 2H), 4.44 (s, 2H), 3.27 (t, J=8.0 Hz, 2H), 2.40 (m, 2H). HRESIMS calcd for C14H14F6N5O2 m/z [M+H]+ 398.1052, found 398.1054.
bis({[3,5-bis(Trifluoromethyl)phenyl]methyl})[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl] amine hydrochloride (30)
White powder (8.5%): mp 138-140 °C (dec.); 1H NMR (500 MHz, CD3OD) d: 8.61 (s, 1H), 8.06 (s, 4H), 8.00 (s, 2H), 4.60-4.48 (br m, 6H), 3.29 (br m, 2H), 2.54 (br m, 2H). HRESIMS calcd for C23H18F12N5O2 m/z [M+H]+ 624.1263 found 624.1279.
{[3,5-bis(Trifluoromethyl)phenyl]methyl}[4-(1H-1,2,4-triazol-1-yl)butyl]amine hydrochloride (31)
White powder (37%): mp 120-123 °C; 1H NMR (500 MHz, CD3OD) d: 9.00 (s, 1H), 8.33 (s, 1H), 8.19 (s, 2H), 8.12 (s, 1H), 4.41 (br s, 4H), 3.17 (br t, J=5.8 Hz, 2H), 2.03 (m, 2H), 1.76 (m, 2H). HRESIMS calcd for C15H17F6N4 m/z [M+H]+ 367.1352, found 367.1338.
[(6-Bromoquinolin-2-yl)methyl][3-(2-nitro-1H-imidazol-1-yl)propyl]amine hydrochloride (32)
White powder (22%): mp 158-160 °C (dec.); 1H NMR (400 MHz, CD3OD) d: 8.33 (d, J=8.4 Hz, 1H), 8.19 (s, 1H), 7.99 (d, J=9.6 Hz, 1H), 7.89 (d, J=8.8 Hz, 1H), 7.53 (s, 1H), 7.52 (d, J=9.6 Hz, 1H), 7.17 (s, 1H), 4.61 (t, J=7.2 Hz, 2H), 4.59 (s, 2H), 3.34 (br t, 2H), 2.41 (m, 2H). HRESIMS calcd for C16H17BrN5O2 m/z [M+H]+ 390.0566, 392.0545 found 390.0569. 392.0551.
bis[(6-Bromoquinolin-2-yl)methyl][3-(2-nitro-1H-imidazol-1-yl)propyl]amine hydrochloride (33)
Pinkish powder (18%). 1H NMR (400 MHz, CD3OD) d: 8.36 (d, J=8.4, 2H), 8.2 (s, 2H), 7.91 (s, 4H), 7.57 (d, J=8.4 Hz, 2H), 7.47 (s, 1H), 7.09 (s, 1H), 4.95 (s, 4H), 4.63 (t, J=7.6 Hz, 2H), 3.66 (t, J=8.0 Hz, 2H), 2.60 (m, 2H). HRESIMS calcd for C26H23Br2N6O2 m/z [M+H]+ 609.0249, 611.0229, 613.0208, found 609.0251, 611.0233, 613.0210.
[(7-Chloroquinolin-2-yl)methyl][3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]amine hydrochloride (34)
Beige powder (29%): mp 135 °C (dec.); 1H NMR (500 MHz, CD3OD) d: 8.68 (s, 1H), 8.42 (d, J=8.5 Hz, 1H), 8.12 (s, 1H), 7.99 (d, J=9.0 Hz, 1H), 7.64 (d, J=8.5 Hz, 1H), 7.54 (d, J=8.5 Hz, 1H), 4.64 (s, 2H), 4.55 (t, J=6.5 Hz, 2H), 3.35 (t, J=8.0 Hz, 2H), 2.50 (m, 2H). HRESIMS calcd for C15H16ClN6O2 m/z [M+H]+ 347.1018, 349.0994, found 347.1003. 349.0985.
bis[(7-Chloroquinolin-2-yl)methyl][3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]amine hydrochloride (35)
Off white powder (17%): mp 104-106 °C (dec.); 1H NMR (500 MHz, CD3OD) d: 8.61 (s, 1H), 8.55 (d, J=8.5 Hz, 2H), 8.14 (s, 2H), 8.03 (d, J=8.5 Hz, 2H), 7.69 (d, J=8.0 Hz, 4H), 4.91 (s, 4H), 4.52 (t, J=6.5 Hz, 2H), 3.56 (br t, 2H), 2.04 (m, 2H). HRESIMS calcd for C25H22Cl2N7O2 m/z [M+H]+ 522.1212, found 522.1216.
bis[(6-Bromoquinolin-2-yl)methyl][3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]amine hydrochloride (36)
Off white powder (16%): mp 128-130 °C (dec); 1H NMR (500 MHz, CD3OD) d: 8.60 (s, 1H), 8.41 (d, J=8.5 Hz, 2H), 8.22 (s, 2H), 7.92 (br s, 4H), 7.63 (d, J=8.5 Hz, 2H), 4.95 (s, 4H), 4.52 (t, J=6.5 Hz, 2H), 3.64 (t, J=8.0 Hz, 2H), 2.62 (m, 2H). HRESIMS calcd for C25H22Br2N7O2 m/z [M+H]+ 611.0235, 612.0181, 613.0215 found 611.0254, 612.0233, 613.0230.
[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]({[4-(trifluoromethyl)phenyl]methyl})amine hydrochloride (37)
White powder (12%): mp 127-128 °C; 1H NMR (500 MHz, CD3OD) d: 8.65 (s, 1H), 7.79 (d, J=8.5 Hz, 2H), 7.71 (d, J=8.0 Hz, 2H), 4.50 (t, J=6.5 Hz, 2H), 4.33 (s, 2H), 3.22 (t, J=8.0 Hz, 2H), 2.38 (m, 2H). HRESIMS calcd for C13H15F3N5O2 m/z [M+H]+ 330.1172 found 330.1179.
[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]bis({[4-(trifluoromethyl)phenyl]methyl})amine hydrochloride (38)
White powder (26%): mp 184-186 °C; 1H NMR (500 MHz, CD3OD) d: 8.59 (s, 1H), 7.78 (d, J=8.0 Hz, 4H), 7.70 (d, J=7.5 Hz, 4H), 4.53 (br s, 4H), 4.44 (t, J=6.0 Hz, 2H), 3.25 (br s, 2H), 2.52 (br m, 2H). HRESIMS calcd for C21H20F6N5O2 m/z [M+H]+ 488.1516 found 488.1513. Calculated analysis for C21H20F6ClN5O2: C, 48.13; H, 3.85; N, 13.37; Cl, 6.77. Found: C, 48.21; H, 3.93; N, 13.29; Cl, 6.88.
1-[3-(2-Nitro-1H-imidazol-1-yl)propyl]-4-{[4-(trifluoromethyl)phenyl]methyl}piperazine dihydrochloride (39)
White powder (67%): mp 185-187 °C; 1H NMR (500 MHz, D2O) d: 7.85 (d, J= 7.5 Hz, 2H), 7.70 (d, J=7.5 Hz, 2H), 7.49 (s, 1H), 7.22 (s, 1H), 4.58 (br t, J=7.0 Hz, 2H), 4.53 (s, 2H), 3.63 (br s, 8H), 3.34 (br s, 2H), 2.37 (br s, 2H). HRESIMS calcd for C18H23F3N5O2 m/z [M+H]+ 398.1798, found 398.1803. Calculated analysis for C18H24F3Cl2N5O2: C, 45.95; H, 5.15; N,14.89; Cl, 15.08. Found: C, 45.85; H, 5.05; N, 14.59; Cl, 15.12.
1-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-4-{[4-(trifluoromethyl)phenyl]methyl}piperazine dihydrochloride (40)
White powder (74%): mp 233-235 °C (dec); 1H NMR (500 MHz, D2O) d: 8.65 (s, 1H), 7.84 (d, J=8.0 Hz, 2H), 7.67 (d, J=8.0 Hz, 2H), 4.51 (t, J=6.0 Hz, 2H), 4.39 (s, 2H), 3.48 (br s, 8H), 3.27 (t, J=8.0 Hz, 2H), 2.41 (m, 2H). HRESIMS calcd for C17H22F3N6O2 m/z [M+H]+ 399.1751, found 399.1761. Calculated analysis for C17H23F3Cl2N6O2: C, 43.30; H, 4.92; N,17.83; Cl, 15.05. Found: C, 43.26; H, 4.91; N, 17.71; Cl, 15.42.
1-[4-(3-Nitro-1H-1,2,4-triazol-1-yl)butyl]-4-{[4-(trifluoromethyl)phenyl]methyl}piperazine dihydrochloride (41)
White powder (16%): mp 223-225 °C (dec); 1H NMR (500 MHz, CD3OD) d: 8.67 (s, 1H), 7.80 (s, 4H), 4.44 (t, J=7.0 Hz, 2H), 4.41 (s, 2H), 3.80-3.40 (br m, 10H), 2.06 (quintet, J=7.5 Hz, 2H), 1.86 (m, 2H). HRESIMS calcd for C18H24F3N6O2 m/z [M+H]+ 413.1907, found 413.1909.
1-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-4-[4-(trifluoromethyl)phenyl]piperazine dihydrochloride (42)
White powder: mp 225 °C (dec.); 1H NMR (500 MHz, CD3OD) d: 8.68 (s, 1H), 7.56 (d, J=8.5 Hz, 2H), 7.14 (d, J=8.5 Hz, 2H), 4.52 (t, J=6.5 Hz, 2H), 4.04 (d, J=13 Hz, 2H), 3.72 (d, J=11.5 Hz, 2H), 3.35 (t, J=8.0 Hz, 2H), 3.27-3.20 (m, 4H), 2.48 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C16H20F3N6O2 m/z [M+H]+ 385.1595, found 385.1606; calcd for C16H19F3N6NaO2 m/z [M+Na]+ 407.1414, found 407.1419. Calculated analysis for C16H21F3Cl2N6O2: C, 42.01; H, 4.63; N, 18.38; Cl, 15.51. Found: C, 42.29; H, 4.68; N, 18.79; Cl, 15.39.
In vitro biological evaluation
In vitro activity against T. cruzi, Trypanosoma b. rhodesiense, Leishmania donovani axenic amastigotes and cytotoxicity assessment using L6 cells (rat skeletal myoblasts) was determined using a 96-well plate format as previously described.25 Data were analyzed with the graphic program Softmax Pro (Molecular Devices, Sunnyvale, CA, USA), which calculated IC50 values by linear regression from the sigmoidal dose inhibition curves.
In vitro T. brucei brucei antiproliferating assays and susceptibility studies
T. brucei brucei bloodstream form parasites were seeded at 1 × 103 ml-1 in 200 μL of growth medium containing different concentrations of a nitrotriazole or nifurtimox. Where appropriate, induction of the TbNTR was carried out by adding tetracycline (1 μg/mL). After incubation for 3 days at 37 °C, 20 μL of Alamar blue was added to each well and the plates incubated for a further 16 h. The cell density of each culture was determined as described before11 and the IC50 established.
Enzymatic activity studies
Recombinant TbNTR was prepared and assayed as previously described.16 The activity of purified his-tagged TbNTR was assessed spectrophotometrically at 340 nm using various nitrotriazole substrates (100 μM) and NADH (100 μM) and expressed as nmol NADH oxidized min-1 mg-1 of enzyme.
Cyclic Voltametry
Reduction potentials (E1/2) were measured by cyclic voltametry and evaluated relative to the Ag/AgCl reference electrode. Supporting electrolyte was 0.1M of tetrabutyl ammonium hexafluorophosphate (TBAPF6), 98% purity from Sigma Aldrich. The working electrode was carbon mesh and the counter electrode Pt wire. The typical scan rate was 100 mV/sec.
Supplementary Material
Acknowledgments
The authors thank Dr. Howard Rosenzweig for reviewing the manuscript and his intellectual input, as well as Dr. Yuyang Wu for obtaining the NMR spectra of the compounds. This work was supported by an NIH Challenge Grant: 1R01AI082542 – 01, Subaward No: RU374-063/4693578.
Footnotes
Abbreviations: T. cruzi, Trypanosoma cruzi; T. brucei, Trypanosoma brucei; HAT, human African trypanosomiasis; Nfx, nifurtimox (4-(5-nitrofurfurylindenamino)-3-methylthio-morpholine-1,1-dioxide); Bnz, benznidazole (N-benzyl-2-(2-nitro-1H-imidazol-1-yl)acetamide); NTR, type I nitroreductase; TbNTR, T. brucei NTR; DNDi, Drugs for Neglected Diseases initiative; SI, selectivity index; SARs, structure-activity relationships; E1/2, reduction potential; tet, tetracycline.
References
- 1.Stuart K, Brun R, Croft S, Fairlamb A, Gürtler RE, McKerrow J, Reed S, Tarleton R. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 2008;118:1301–1310. doi: 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Report of the Scientific Working Group on Chagas Disease, WHO/TDR, 2005.
- 3.(a) Urbina J. Chemotherapy of Chagas disease. Curr Pharm Des. 2002;8:287–295. doi: 10.2174/1381612023396177. [DOI] [PubMed] [Google Scholar]; (b) Moncayo A, Silveira AC. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and healthy policy. Memorias do Instituto Oswaldo Cruz. 2009;104 1:17–30. doi: 10.1590/s0074-02762009000900005. [DOI] [PubMed] [Google Scholar]
- 4.(a) Murta SM, Gazzinelli RT, Brener Z, Romanha AJ. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol Biochem Parasitol. 1998;93:203–214. doi: 10.1016/s0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]; (b) Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Memorias do Instituto Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 5.Docampo R, Moreno SNJ. Free radical metabolism of antiparasitic agents. Fed Proc. 1986;45:2471–2476. [PubMed] [Google Scholar]
- 6.Docampo R. Sensitivity of parasite to free radical damage by antiparasitic drugs. Chem Biol Interact. 1990;73:1–27. doi: 10.1016/0009-2797(90)90106-w. [DOI] [PubMed] [Google Scholar]
- 7.Viode C, Bettache N, Cenas N, Krauth-Siegel RL, Chauviere G, Bakalara N, Perie J. Enzymatic reduction studies of nitroheterocycles. Biochem Pharmacol. 1999;57(5):549–557. doi: 10.1016/s0006-2952(98)00324-4. [DOI] [PubMed] [Google Scholar]
- 8.Blumenstiel K, Schoneck R, Yardley V, Croft SL, Krauth-Siegel RL. Nitrofuran drugs as common subversive substrates of Trypanosoma cruzi lipoamide dehydrogenase and trypanothione reductase. Biochem Pharmacol. 1999;58(11):1791–1799. doi: 10.1016/s0006-2952(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 9.Turrens JF. Oxidative stress and antioxidant defenses: a target for the treatment of diseases caused by parasitic protozoa. Mol Aspects Med. 2004;25:211–220. doi: 10.1016/j.mam.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Hall BS, Bot C, Wilkinson SR. Nifurtimox activation by trypanosomal type I nitroreductases generates cytotoxic nitrile metabolites. J Biol Chem. 2011;286(15):13088–13095. doi: 10.1074/jbc.M111.230847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. PNAS. 2008;105(13):5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson SR, Bot C, Kelly JM, Hall BS. Trypanocidal activity of nitroaromatic prodrugs: current treatments and future perspectives. Curr Top Med Chem. 2011;11:2072–2084. doi: 10.2174/156802611796575894. [DOI] [PubMed] [Google Scholar]
- 13.Baliani A, Gerpe A, Aran VJ, Torres de Ortiz S, Serna E, Vera de Bilbao N, Sanabria L, Yaluff G, Nakayama H, Rojas de Arias A, Maya JD, Morello JA, Cerecetto H, Gonzalez M. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. J Med Chem. 2005;48:5570–5579. doi: 10.1021/jm050177+. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez J, Aran VJ, Boiani L, Olea-Azar C, Lavaggi ML, Gonzalez M, Cerecetto H, Maya JD, Carrasco-Pozo C, Cosoy HS. New potent 5-nitroindazole derivatives as inhibitors of Trypanosoma cruzi growth: Synthesis, biological evaluation, and mechanism of action studies. Bioorg Med Chem. 2009;17:8186–8196. doi: 10.1016/j.bmc.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Boiani L, Gerpe A, Aran VJ, Torres de Ortiz S, Serna E, Vera de Bilbao N, Sanabria L, Yaluff G, Nakayama H, Rojas de Arias A, Maya JD, Morello JA, Cerecetto H, Gonzalez M. In vitro and in vivo antitrypanosomatid activity of 5-nitroindazoles. Eur J Med Chem. 2009;44:1034–1040. doi: 10.1016/j.ejmech.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Hall BS, Wu X, Hu L, Wilkinson SR. Exploiting the Drug-Activating Properties of a Novel Trypanosomal Nitroreductase. Antimicrob Agents Chemother. 2010;54:1193–1199. doi: 10.1128/AAC.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bot C, Hall BS, Bashir N, Taylor MC, Helsby NA, Wilkinson SR. Trypanocidal activity of aziridinyl nitrobenzamide prodrugs. Antimicrob Agents Chemother. 2010;54(10):4246–4252. doi: 10.1128/AAC.00800-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu L, Wu X, Han J, Chen L, Vass SO, Browne P, Hall BS, Bot C, Gobalakrishnapillai V, Searle PF, Knox RJ, Wilkinson SR. Synthesis and structure-activity relationships of nitrobenzyl phosphoramide mustards as nitroreductase-activated prodrugs. Bioorg Med Chem Lett. 2011;21(13):3986–3991. doi: 10.1016/j.bmcl.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulou MV, Bloomer WD. Nitroheterocyclic-linked acridines as DNA-targeting bioreductive agents. Drugs of the Future. 1993;18:231–238. [Google Scholar]
- 20.Rosenzweig HS, Papadopoulou MV, Bloomer WD. Interaction of strong DNA-intercalating bioreductive compounds with topoisomerases I and II. Oncol Res. 2005;15:219–231. doi: 10.3727/096504005776382288. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulou MV, Bourdin B, Bloomer WD, Brun R, Kaiser M, Torreele E. Novel nitroaromatic heterocycles as potential anti-trypanosomal drugs. Keystone Symposium on Molecular and Cellular Biology: “Drug Discovery for Protozoan Parasites”; Breckenridge, CO. March 22-26, 2009; Proceedings. [Google Scholar]
- 22.(a) Bustamante JM, Evans A, Papadopoulou MV, Tarleton R. Use of CD8+ T central memory characteristics as immunologic evidence for treatment efficacy in mice infected with Trypanosoma cruzi. 12th Woods Hole Immunoparasitology Meeting; Woods Hole, Massachusetts. April 27-29, 2008. [Google Scholar]; (b) Canavaci AMC, Bustamante JM, Padilla AM, Brandan CMP, Simpson LJ, Xu D, Boehlke CL, Tarleton RL. In vitro and in vivo high-throughput assays for the testing of anti-trypanosoma cruzi compounds. Plos Neglected Tropical Diseases. 2010;4(7):e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papadopoulou MV, Ji M, Rao MK, Bloomer WD. 4-[3-(2-Nitro-1-imidazolyl)-propylamino]-7-chloroquinoline hydrochloride (NLCQ-1), a novel bioreductive compound as a hypoxia-selective cytotoxin. Oncol Res. 2000;12:185–192. doi: 10.3727/096504001108747675. [DOI] [PubMed] [Google Scholar]
- 24.Cowan DSM, Panicucci R, McClelland RA, Rauth AM. Targeting radiosensitizers to DNA by attachment of an intercalating group: Nitroimidazole linked phenanthridines. Radiat Res. 1991;127:81–89. [PubMed] [Google Scholar]
- 25.Orhan I, Sener B, Kaiser M, Brun R, Tasdemir D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar Drugs. 2010;8:47–58. doi: 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonse S, Santelli-Rouvier C, Barbe J, Krauth-Siegel RL. Inhibition of Trypanosoma cruzi trypanothione reductase by acridines: Kinetic studies and structure-activity relationships. J Med Chem. 1999;42:5448–5454. doi: 10.1021/jm990386s. [DOI] [PubMed] [Google Scholar]
- 27.Turrens JF, Watts BP, Jr, Zhong L, Docampo R. Inhibition of Trypanosoma cruzi and T. b. brucei NADH fumarate reductase by benznidazole and antihelminthic imidazole derivatives. Mol Biochem Parasitol. 1996;82:125–129. doi: 10.1016/0166-6851(96)02722-3. [DOI] [PubMed] [Google Scholar]
- 28.Barry CE, Boshoff HIM, Dowd CS. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr Pharmaceut Design. 2004;10:3239–3262. doi: 10.2174/1381612043383214. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulou MV, Bloomer WD. Nitroimidazole-based bioreductive compounds bearing a quinazoline or a naphthyridine chromophore. Anti-Cancer Drugs. 2009;20(6):493–502. doi: 10.1097/CAD.0b013e32832cad9b. [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulou MV, Ji M, Bloomer WD. Novel Fluorinated Hypoxia-targeted Compounds as Non-invasive Probes for Measuring Tumor-hypoxia by 19F-Magnetic Resonance Spectroscopy (19F-MRS) Anticancer Res. 2006;26(5):3253–3258. [PubMed] [Google Scholar]
- 31.Papadopoulou MV, Bloomer WD. NLNQ-1, a 2-[3-(2-nitro-1-imidazolyl)-propylamino]-3-chloro-1,4-naphthoquinone as a hypoxia-selective cytotoxin and radiosensitizer. In Vivo. 2008;22:285–288. [PubMed] [Google Scholar]
- 32.Olea-Azar C, Rigol C, Mendizabal F, Morello A, Maya JD, Moncada C, Cabrera E, Di Maio R, Gonzalez M, Cerecetto H. ESR Spin Trapping Studies of Free Radicals Generated from Nitrofuran Derivative Analogues of Nifurtimox by Electrochemical and Trypanosoma cruzi Reduction. Free Radical Res. 2003;37(9):993–1001. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.