Abstract
Atopic dermatitis is a chronic inflammatory skin disease. Multiple genetic and environmental factors are thought to be responsible for susceptibility to AD. In this study, we collected 2,478 DNA samples including 209 AD patients and 729 control subjects from Taiwanese population and 513 AD patients and 1027 control subject from Japanese population for sequencing and genotyping ORAI1. A total of 14 genetic variants including 3 novel single-nucleotide polymorphisms (SNPs) in the ORAI1 gene were identified. Our results indicated that a non-synonymous SNP (rs3741596, Ser218Gly) associated with the susceptibility of AD in the Japanese population but not in the Taiwanese population. However, there is another SNP of ORAI1 (rs3741595) associated with the risk of AD in the Taiwanese population but not in the Japanese population. Taken together, our results indicated that genetic polymorphisms of ORAI1 are very likely to be involved in the susceptibility of AD.
Introduction
Atopic dermatitis (AD) or childhood eczema is a chronic relapsing inflammatory skin disease [1] that usually associated with a family history of atopic disorders such as allergic rhinitis and bronchial asthma [2], [3], [4]. There has been a dramatic increase in the prevalence of AD in the last decade. Although the pathogenesis of AD remains elusive, multiple genetic and environmental factors are thought to contribute to the disease onset [2], [3], [4]. Genes associated with skin-barrier formation and adaptive immunity have been implicated in the development of AD. For example, filaggrin (FLG) is essential for the maintenance of the skin-barrier function. Genetic mutations in FLG are significantly associated with the risk of AD and elevated immunoglobulin E (IgE) levels [5]. In addition, single nucleotide polymorphisms (SNPs) in Toll-like receptors (TLRs), ST2, IL-3, IL-4, IL-5, IL12RB1, and IL-13 have been shown to be associated with the pathogenesis of AD [6], [7], [8], [9], [10], [11], [12]. The results from a genome-wide association study (GWAS) have indicated the complex involvement of multiple loci in the susceptibility of human AD [13].
Despite all the knowledge, the treatment of severe AD remains a challenge. Clinical trials have indicated that cyclosporine is an effective treatment option in children with AD. Short-term treatment with cyclosporine has shown to alleviate disease activity [14]. Cyclosporine, an immunosuppressant, functions as a phosphatase inhibitor that prevents the translocation of calcium-dependent transcription factor—nuclear factor of activated T cells (NFAT). The influx of calcium through store-operated calcium (SOC) channels is one of the major pathways to increase the intracellular calcium concentration in non-excitable cells such as the mast cells and T lymphocytes [15]. In mast cells, short-term SOC influx has been shown to result in the secretion of inflammatory molecules such as arachidonic acids and leukotriene C4 [16], [17]. In T lymphocytes, the nuclear translocation of NFAT could be driven by the SOC-mediated calcineurin signaling pathway in order to control immune responses [18]. The molecular components of the SOC channels were first identified in patients with severe combined immune deficiency (SCID) syndrome [19]. ORAI1 gen encoding Orai1 protein is one of the major proteins of SOC channels. A point mutation created in the ORAI1 gene resulted in the reduction of calcium influx through the SOC channels and dysfunction of the immune system. ORAI1-knockout mice exhibited defective mast cells and attenuated cytokine release [20].
In our study, we first conducted LD (linkage disequilibrium) mapping of the ORAI1 gene, performed a case-control association study and showed a haplotype analysis. We then tested the correlation between the ORAI1 genetic polymorphisms and the expression level of the ORAI1 transcript. Our results support a functional role of ORAI1 polymorphisms in the susceptibility of human AD in both Japanese and Taiwanese population.
Materials and Methods
Subjects
A total of 513 atopic dermatitis patients were recruited in Takao Hospital and the University of Tokyo. All subjects with atopic dermatitis were diagnosed according to the criteria of Hannifin and Rajka [21]. There are four major criteria, including pruritus, chronic or relapsing dermatitis, dermatitis affecting flexural surfaces in adults, and a personal or family history of cutaneous or respiratory atopy. There are 23 minor criteria, such as hypopigmented patch, infraorbital darkening, cheilitis, hyperlinerized palm, and elevated IgE, etc. To be included, the patients had to meet three major criteria plus at least one minor criterion or two major criteria plus at least three minor criteria. Japanese subjects with AD were recruited from several hospitals and diagnosed according to the criteria of Hanifin and Rajka by dermatologists. A total of 839 adult control individuals who had no history of bronchial asthma, allergic rhinitis and atopic dermatitis were recruited by detailed doctors' interviews. A total of 188 healthy individuals who had no history of atopic dermatitis based on a questionnaire were recruited in Fukui University (3).
Information of these participants is provided in Table S1. All individuals were unrelated Japanese and gave written informed consent to participate in the study according to the rules of the process committee at the Center for Genomic Medicine, The Institute of Physical and Chemical Research (RIKEN).
A total of 209 AD patients were recruited from a dermatological clinic at Kaohsiung Medical University hospital and National Taiwan University hospital in Taiwan from January 1st to December 15th in 2008. A diagnosis of AD patients was also based on criteria proposed by Hanifin and Rajka [21]. Information of these participants is provided in Table S2. We took a medical history and performed physical examinations and blood biochemistry exams to exclude other diseases causing pruritus, such as contact dermatitis, asteatotic dermatitis, and metabolic diseases. Suspected cases were biopsied to exclude other mimicking cutaneous diseases, including cutaneous T cell lymphoma. Patients were excluded if they had received any topical treatments for at least 2 weeks or systemic treatment for 2 months prior to the study. Patients were excluded if they had active skin diseases other than AD, including HIV infection and cancers of any origin. Patients visiting the same hospital in the Department of Preventive Medicine for medical diseases other than atopic diseases were referred to the Department of Dermatology were hospital-based controls. They were excluded if they had active skin diseases, past history of AD, allergic asthma, allergic rhinitis, allergic conjuntivitis, cancers of any origin, or HIV infection or were taking oral corticosteroids. A board-certified dermatologist took a medical history, examined the whole surface the body, and assigned a SCORAD severity index score for each subject. Venous peripheral blood was drawn and the serum was stored at −70°C until assayed. IgE levels from AD patients were measured in a College of American Pathologists (CAP) accredited laboratory in the same hospital. The study was approved by the Institutional Review Board of the Hospital. All clinical assessments and specimen collections were conducted according to Declaration of Helsinki principles. Each participant signed an informed written consent form before entering the study. Patients or controls who did not sign the inform consent were excluded.
Selection of human ORAI1 polymorphisms for genotyping
Genomic DNA was prepared from peripheral blood samples using standard protocols. To identify SNPs in the human ORAI1 gene, we sequenced all exons, including a minimum of 200 bases of the flanking intronic sequence, 2 kb of the 5′ flanking region, and a 2 kb continuous 3′ flanking region of the last exon except for regions with interspersed repeats from 24 subjects as described in Japanese population. Pairwise LD was calculated as D′/LOD and r2 and the Tag SNPs were selected among 10 SNPs with a frequency of greater than 10% by using the Haploview 4.2 program (http://www.broad.mit.edu/mpg/haploview/). Genotyping of SNPs was performed by the TaqMan allele-specific amplification (TaqMan-ASA) method (Applied Biosystems, Foster City, CA).
In Taiwanese population, we sequenced all exons of ORAI1 gene in 10 subjects, however, none of SNP was found. We further choose five ORAI1 tagging SNPs (rs12320939, rs6486795, rs3741595, rs3825175 and rs712853) with a minor allele frequency >10% in the Han Chinese population were selected from the HapMap database (http://www.hapmap.org). Genotyping was performed using the TaqMan Allelic Discrimination Assay (Applied Biosystems, Foster city, CA, USA). The polymerase chain reaction (PCR) was carried out using the ABI7900 Thermal Cycler. After PCR, fluorescence from reaction products was measured and analyzed using the System SDS software version 1.2.3.
Real-time quantitative RT-PCR
Total RNA from normal human tissues was purchased from Clontech (Mountain View, CA). Each RNA was reverse transcribed with Superscript III reverse transcriptase and oligo dT primers (Invitrogen, Carlsbad, CA). The expression of ORAI1 transcripts was determined by real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) using SYBR Premix Ex Taq (Takara, Shiga, Japan) with specific primers (5′- ACCTCGGCTCTGCTCTCC -3′ and 5′- GATCATGAGCGCAAACAGG -3′). In all experiments, the amounts of cDNA were standardized by quantification of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistical analysis
We tested agreement with Hardy-Weinberg Equilibrium using a χ2 goodness-of-fit test at each locus in both populations. In Japanese population, we then compared differences in genotype frequencies of the polymorphisms between case and control subjects by the Cochran-Armitage trend test, and calculated odds ratios (ORs) with 95 percent confidence intervals (95% CI). We applied Bonferroni correction; the multiplication of the P values by four, the number of tag SNPs. In the association study, P values of less than 0.05 were considered to be statistically significant. Haplotype frequencies for three loci were estimated, and haplotype association tests were performed using Haploview 4.2. Total IgE levels and expression levels of ORAI1 transcripts between genotypic groups were tested with the Jonckheere-Terpstra test.Chi-square test was then used to compare differences in allele frequencies and genotype distribution of the polymorphisms between AD and controls.
Results
Association of ORAI1 SNPs with susceptibility to atopic dermatitis
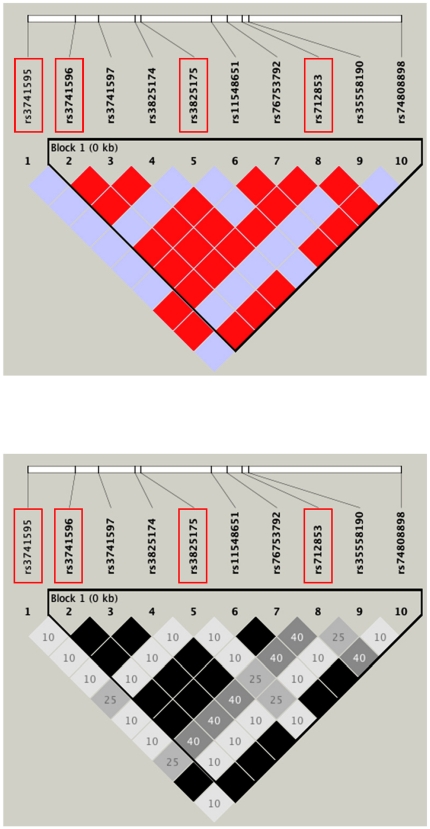
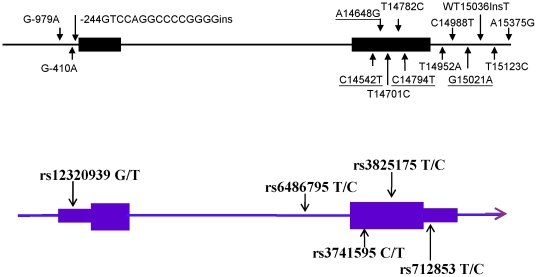
After extensive examination of ORAI1 by direct sequencing, we identified 14 polymorphisms (three SNPs in promoter region and five SNPs within the transcript) (Table 1). Eight polymorphisms were contained in the available public databases, NCBI dbSNP (http://www.ncbi.nlm.nih.gov/SNP/). We calculated pairwise linkage disequilibrium (LD) as r2 and selected four tag SNPs with a minor allele frequency (MAF) of >10% using the Haploview 4.2 program (http://www.broad.mit.edu/mpg/haploview/). The four tag SNPs captured 10 of the 10 alleles with a mean r2 of 0.98 (r2>0.76) (Figure 1 and Table S3). The locations of these four SNPs are shown in Figure 2 (upper). In Taiwanese population, five ORAI1 tagging SNPs with a minor allele frequency >10% in the Han Chinese population were selected from the HapMap database (http://www.hapmap.org). A graphical overview of the genotyped polymorphism is shown in Figure 2 (Lower).
Table 1. Locations and allele frequencies of polymorphisms in ORAI1.
| SNPa | Location | Amino acid | Allele frequency(%) | NCBIb | |
| Marker 1 | −979G/A | 5′ flanking region | 2 | ||
| Marker 2 | −410G/A | 5′ flanking region | 2 | rs116376569 | |
| Marker 3 | −244GTCCAGGCCCCGGGG×1/×2 | 5′ flanking region | 4 | ||
| Marker 4c | 14542C/T | exon2 | I182I | 33 | rs3741595 |
| Marker 5c | 14648A/G | exon2 | S218G | 17 | rs3741596 |
| Marker 6 | 14701T/C | exon2 | A235A | 17 | rs3741597 |
| Marker 7 | 14782T/C | exon2 | V262V | 17 | rs3825174 |
| Marker 8c | 14794C/T | exon2 | T266T | 33 | rs3825175 |
| Marker 9 | 14952T/A | 3′ UTR | 17 | rs11548651 | |
| Marker 10 | 14988C/T | 3′ UTR | 17 | rs76753792 | |
| Marker 11c | 1502A/G | 3′ UTR | 33 | rs712853 | |
| Marker 12 | 15036insT | 3′ UTR | 33 | rs35558190 | |
| Marker 13 | 15123T/C | 3′ UTR | 2 | ||
| Marker 14 | 15375A/G | 3′ flanking region | 17 | rs74808898 |
Numbering according to the genomic sequence of ORAI1 (AC140062.11).
Position 1 is the A of the initiation codon.
NCBI, Number from the dbSNP of NCBI (http://www.ncbi.nlm.nih.gov/SNP/).
SNPs were genotyped in both Taiwanese and Japanese population.
Figure 1. SNPs and pairwise LD map of the ORAI1 gene.
Four boxed polymorphisms were genotyped in the Japanese population. Pairwise D′/LOD (upper) and r2 (lower) for all combinations of SNP pairs are shown.
Figure 2. A graphical overview of ORAI1 gene polymorphisms identified in in the Japanese population (upper).
A graphical overview of tSNPs of ORAI1 gene selected for genotyping in the Taiwanese population.
We next carried out case-control association studies of the four and five SNPs in the 1540 Japanese subjects and 938 Taiwanese subjects. The control genotypes did not deviate from Hardy-Weinberg equilibrium. As shown in Table 2, a nonsynonymous ORAI1 SNP (rs3741596, Ser218Gly) showed significant associations with susceptibility to atopic dermatitis (P = 0.002, OR = 1.36, 95% CI 1.12–1.64) by the Cochran-Armitage trend test in Japanese population. Other variants did not show a significant association after Bonferroni correction. In Taiwanese population, rs6486795 and rs3741595 were significantly associated with susceptibility to atopic dermatitis under the dominant and allelic models.
Table 2. Genotype counts for ORAI1 and atopic dermatitis susceptibility in Japanese and Taiwanese population.
| Japanese (case-control association analysis) | Taiwanese (case-control association analysis) | |||||||||||||
| Genotypea | Allele*2 | Genogype | Allele*2 | |||||||||||
| db SNP ID | Allele 1/2 | Subjects | 1/1 | 1/2 | 2/2 | Frequency of allele 2 | P a | OR (95% c.i.) | 1/1 | 1/2 | 2/2 | Frequency of allele 2 | P | OR (95% c.i.) |
| rs12320939 | G/T | AD | 50 | 90 | 67 | 0.54 | 0.050 | 1.25 (1.00–1.55) | ||||||
| Control | 184 | 341 | 165 | 0.49 | ||||||||||
| rs6486795 | T/C | AD | 59 | 93 | 47 | 0.47 | 0.0004 | 1.50 (1.20–1.88) | ||||||
| Control | 277 | 320 | 98 | 0.37 | ||||||||||
| rs3741595 | C/T | AD | 323 | 160 | 25 | 0.21 | 0.275 | 0.90 (0.75–1.08) | 83 | 93 | 31 | 0.37 | 0.0001 | 1.59 (1.27–2.01) |
| Control | 622 | 343 | 58 | 0.22 | 370 | 275 | 53 | 0.27 | ||||||
| rs3741596 | A/G | AD | 315 | 177 | 19 | 0.21 | 0.002 | 1.36 (1.12–1.64) | ||||||
| Control | 717 | 283 | 27 | 0.16 | ||||||||||
| rs3825175 | C/T | AD | 197 | 243 | 71 | 0.38 | 0.245 | 0.91 (0.78–1.06) | 89 | 81 | 37 | 0.37 | 0.187 | 0.86 (0.69–1.08) |
| Control | 379 | 476 | 171 | 0.40 | 239 | 346 | 114 | 0.41 | ||||||
| rs712853 | G/A | AD | 165 | 266 | 80 | 0.42 | 0.027 | 1.19 (1.02–1.38) | 26 | 71 | 109 | 0.70 | 0.035 | 1.29 (1.02–1.64) |
| Control | 397 | 479 | 144 | 0.38 | 97 | 303 | 301 | 0.65 | ||||||
P values represent the Cochran-Armitage trend P for case-control comparisons.
Haplotypes of ORAI1 and their association with the disease occurrence of atopic dermatitis
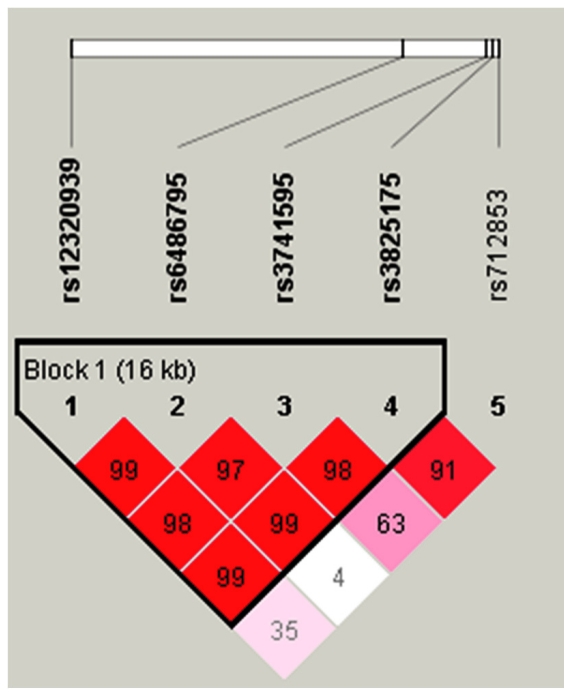
To further identify the effects of Haplotypes of ORAI1 to atopic dermatitis, we constructed the haplotypes of the three SNPs to estimate the frequency of each haplotype in controls in the Japanese population (Table 3). Three common haplotypes were identified in the Japanese population. Haplotype C-G-C-A (rs3741595, rs3741596, rs3825175, and rs712853) of ORAI1 was significantly associated with atopic dermatitis. A P value of 0.0017 was obtained by using the Haploview 4.2 program. In the Taiwanese population, the haplotype block structure of ORAI1 was shown in Figure 3. Among five haplotypes, subjects with T-C-T-C haplotype (rs12320939, rs6486795, rs3741595 and rs3825175) have a significant risk to AD (OR = 1.54, 95% CI = 1.18–2.01, P = 0.0014) (Table 4).
Table 3. Haplotype frequency of four SNPs in ORAI1 in Japanese population.
| n | Frequency | Odds ratio | ||||||||
| rs3741595 | rs3741596 | rs3825175 | rs712853 | AD | Control | AD | Control | P value | (95% c.i.) | |
| Haplotype 1 | C | A | T | G | 386 | 819 | 0.38 | 0.40 | ||
| Haplotype 2 | T | A | C | G | 212 | 459 | 0.21 | 0.22 | ||
| Haplotype 3 | C | A | C | A | 211 | 435 | 0.20 | 0.21 | ||
| Haplotype 4 | C | G | C | A | 216 | 337 | 0.21 | 0.16 | 0.0017 | 1.36 (1.12–1.64) |
| 1026 | 2050 | 1.00 | 1.00 |
Figure 3. ORAI1 gene LD and haplotype block structure in AD.
The number on the cell is the LOD score of D′.
Table 4. Haplotype frequency of four SNPs in ORAI1 in Taiwanese population.
| n | Frequency | Odds ratio | ||||||||
| rs12320939 | rs6486795 | rs3741595 | rs3825175 | AD | Control | AD | Control | P value | (95% c.i.) | |
| Haplotype 1 | T | C | T | C | 148 | 370 | 0.38 | 0.27 | 0.0014 | 1.54 (1.18–2.01) |
| Haplotype 2 | T | C | C | C | 36 | 130 | 0.09 | 0.10 | ||
| Haplotype 3 | T | T | C | C | 33 | 154 | 0.08 | 0.11 | ||
| Haplotype 4 | G | T | C | C | 30 | 142 | 0.08 | 0.10 | ||
| Haplotype 5 | G | T | C | T | 143 | 550 | 0.36 | 0.41 | ||
| 390 | 1346 | 0.99 | 0.99 |
If the frequencies less than 1% were excluded.
Expression of ORAI1 mRNA in skin, immune tissues
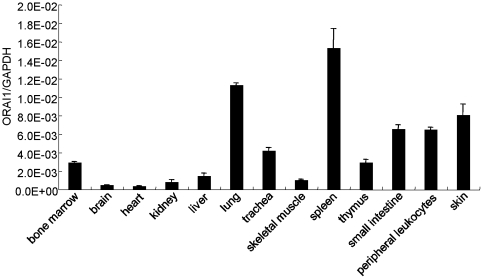
To investigate the ORAI1 mRNA expression in various human tissues, we conducted real-time quantitative RT-PCR. As shown in Figure 4, ORAI1 mRNA was expressed abundantly in all tissues examined, especially in the tissues of lung, spleen, and skin.
Figure 4. Expression of ORAI1 mRNA in different tissues.
Quantities of total RNA extracted from normal human tissues were determined by real-time quantitative reverse transcription polymerase chain reaction (RT-PCR). The results were normalized to GAPDH transcripts.
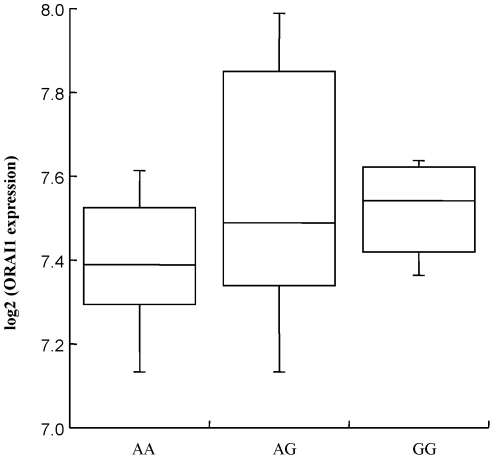
rs3741597 is associated with the expression level of the ORAI1 transcript
We next assessed whether the rs3741597 genotype correlated with the mRNA levels of ORAI1 transcript (NM_032790). The ORAI1 mRNA expression data for the EBV-transformed lymphoblastoid cell lines from 42 JPT HapMap subjects were analyzed by the Jonckheere-Terpstra trend test. As shown in Figure 5, the expression level of transcripts of NM_032790 was positively correlated with the rs3741597 genotype (P = 0.040). These results suggested that rs3741597 or other SNPs, 14648A/G (rs3741596), 14782T/C (rs3825174), 14952T/A (rs11548651), 14988C/T (rs76753792), and 15375A/G (rs74808898), in strong linkage disequilibrium with rs3741597 might influence susceptibility to atopic dermatitis through higher expression of an ORAI1 transcript.
Figure 5. Differential expression of an ORAI1 transcript (NM_032790) of EBV-transformed lymphoblastoid cell lines from HapMap-JPT (Japanese in Tokyo) subjects for each rs3741597 genotype.
P value was calculated by the Jonckheere-Terpstra test.
Discussion
We screened the polymorphisms of ORAI1 and performed a case-control association study and a haplotype analysis. A total of 14 genetic variants was identified from Japanese population, 3 SNPs are novel. Our results showed a significant association between AD and a non-synonymous SNP (rs3741596, Ser218Gly) in the human ORAI1 gene in the Japanese population. rs3741596 was genotyped in the Taiwanese population, however, the MAFs of this SNP is less than 1% which is different from the allele frequency of Japanese population (data not shown). In addition, rs3741595 of ORAI1 is associated with the risk of AD in the Taiwanese population but not in the Japanese population. We also performed I2 index to assess heterogeneity on three SNPs which were genotyped in both Taiwan and Japan. The I2 indexes of rs3825175 and rs712853 showed no heterogeneity on these locus. The heterogeneity only occurred on rs3741595 (I2 Index = 93%) We attribute this to the different genetic backgrounds in the populations, due to variation in allele frequencies, population admixture, heterogeneity of the phenotype between populations.
The rs3741597 genotype correlated with the mRNA levels of the ORAI1 transcript. Another three SNPs (14952T/A, 14988C/T, and 15375A/G) located in the 3′ flanking region, which are in strong LD with rs3741597, may influence higher expression of an ORAI1 transcript. MicroRNAs (miRNA) are small non-coding RNAs that control gene expression by preferentially binding to the 3′-untranslated regions (3′-UTR) of the target genes [22]. Therefore, further functional analysis of the 3′-UTR of the ORAI1 gene should be conducted in order to clarify the mechanism underlying this susceptibility.
Several diseases have been linked to the ORAI1-mediated calcium influx. Feske et al. identified a mutation in the Orai1 from SCID patients. This missense mutation caused a reduction in the SOC influx and a decrease in immune response [19]. Studies from the Orai1-knockout mice have shown an important role of this gene in the activation of inflammatory reactions in mast cells [20], [23]. To study the role of ORAI1, we analyzed the tissue distribution of ORAI1. The highest expression of ORAI1 was found in the spleen, an organ involved in immune function. The tissue distribution of ORAI1 in our study revealed the potential importance of ORAI1 in the regulation of immune system—a finding consistent with that reported previously. In addition, the expression of ORAI1 was found to be higher in the proliferative vascular smooth muscle cells [24]. Furthermore, results obtained from ORAI1 knockdown cells indicated a reduction of cell proliferation in both endothelial and breast cancer cells [25], [26]. Studies using keratinocytes have shown that calcium is an important regulator of cell proliferation and differentiation [27]. Dysregulated apoptosis in keratinocytes contributes to the progress of AD [28]. Hence, genetic polymorphisms may result in the changes of gene expression level of ORAI1 that further contribute to the dysregulated growth in keratinocytes, leading to a defective skin-barrier formation. Cell-based physiological studies in the keratinocytes are required to identify the role of ORAI1 in AD.
STIM1 and ORAI1 are the two major components in the regulation of calcium entry through store-operated calcium channels. Co-expression of STIM1 and Orai1/CRACM1 results in the amplification of store-operated Ca2+ influx signals [1], [29], [30]. The SOC entry pathway is influenced by ORAI1 or STIM1 knockdown [31], [32]. Importantly, the overexpression of orai1 also causes the attenuation of store-operated Ca2+ influx [33]. Two possible mechanisms were proposed. The oligomers formed by the overexpressed ORAI1 may lose sensitivity to the signals released from calcium store [34]. Soboloff et al. suggested that the coupling stoichiometry between Orai1 and STIM1 is not unity, therefore, overexpression of Orai1 may influence the functional compositions of SOC [30]. Combined the findings from other groups [34], our results propose that the genetic polymorphisms in the 3′-UTR of the ORAI1 gene may change the expression level of Orai1, which, in turn, may cause dysfunction of calcium channels and immune responses. However, the coupling stoichiometry between the different expression levels of ORAI1 should also be further considered.
Previous studies have revealed the significant association between genetic polymorphisms of ORAI1 and inflammatory diseases such as ankylosing spondylitis and calcium nephrolithiasis [35], [36]. In this study, we identified 14 genetic variants including 3 novel SNPs in the ORAI1 gene. In a total of 2,478 subjects (938 Taiwanese and 1540 Japanese), our results indicated that different genetic polymorphisms of ORAI1 are associated with AD susceptibility in the Japanese, and Taiwanese populations. This is the first report to state the relationship between the genetic polymorphisms of ORAI1 and allergic diseases. Given the polygenic nature of allergic diseases such as AD, the susceptibility gene ORAI1 could provide a new clue in the pathogenesis of AD. The prevalence of atopic dermatitis in Taiwan is around 6.7%, therefore, this study should reach a power level of 0.97 (case 209; control 729). Although further replication studies in larger Taiwanese population is needed, it is likely to variants in the ORAI1 gene play a role in susceptibility to AD in both Japanese and Taiwanese populations. Further study on the relationship between the genotype of ORAI1 and the downstream functional relevance during dermal inflammation should be conducted in order to understand the etiology of AD.
Supporting Information
Basal characteristics of patients with Atopic Dermatitis (AD) and of normal controls in Japanese population.
(DOC)
Basal characteristics of patients with Atopic Dermatitis (AD) and of normal controls in Taiwanese population.
(DOC)
Pairwise linkage disequilibrium for all possible two-way comparisons among 10 polymorphisms in ORAI1 with 24 Japanese volunteers.
(DOC)
Acknowledgments
We thank Makiko T. Shimizu, Hiroshi Sekiguchi, Aya I. Jodo and Nami Kawaraichi for technical assistance. We are grateful to Prof. Anant Parekh (University of Oxford) for the functional study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors are grateful to the members of The Rotary Club of Osaka-Midosuji District 2660 Rotary International in Japan for supporting our study. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and from the Ministry of Health, Labor, and Welfare, Japan. This study was also partly supported by funding from NHRI-100A1-PDCO-03000001, Excellence for Cancer Research Center grant, Department of Health, Executive Yuan, Taiwan, ROC (NO. DOH100-TD-C-111-002) and from the National Science Council, Taiwan, ROC (NSC 98-2320-B-037-028-MY2; 100-2320-B-037-002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill PB, Hillier A, Olivry T. The ACVD task force on canine atopic dermatitis (VI): IgE-induced immediate and late-phase reactions, two inflammatory sequences at sites of intradermal allergen injections. Vet Immunol Immunopathol. 2001;81:199–204. doi: 10.1016/s0165-2427(01)00299-9. [DOI] [PubMed] [Google Scholar]
- 3.Olivry T, Hill PB. The ACVD task force on canine atopic dermatitis (XVIII): histopathology of skin lesions. Vet Immunol Immunopathol. 2001;81:305–309. doi: 10.1016/s0165-2427(01)00305-1. [DOI] [PubMed] [Google Scholar]
- 4.Vickery BP. Skin barrier function in atopic dermatitis. Curr Opin Pediatr. 2007;19:89–93. doi: 10.1097/MOP.0b013e328012315a. [DOI] [PubMed] [Google Scholar]
- 5.Enomoto H, Hirata K, Otsuka K, Kawai T, Takahashi T, et al. Filaggrin null mutations are associated with atopic dermatitis and elevated levels of IgE in the Japanese population: a family and case-control study. J Hum Genet. 2008;53:615–621. doi: 10.1007/s10038-008-0293-z. [DOI] [PubMed] [Google Scholar]
- 6.Akdis M. Healthy immune response to allergens: T regulatory cells and more. Curr Opin Immunol. 2006;18:738–744. doi: 10.1016/j.coi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Oh DY, Schumann RR, Hamann L, Neumann K, Worm M, et al. Association of the toll-like receptor 2 A-16934T promoter polymorphism with severe atopic dermatitis. Allergy. 2009;64:1608–1615. doi: 10.1111/j.1398-9995.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 8.Oiso N, Fukai K, Ishii M. Interleukin 4 receptor alpha chain polymorphism Gln551Arg is associated with adult atopic dermatitis in Japan. Br J Dermatol. 2000;142:1003–1006. doi: 10.1046/j.1365-2133.2000.03485.x. [DOI] [PubMed] [Google Scholar]
- 9.Rafatpanah H, Bennett E, Pravica V, McCoy MJ, David TJ, et al. Association between novel GM-CSF gene polymorphisms and the frequency and severity of atopic dermatitis. J Allergy Clin Immunol. 2003;112:593–598. doi: 10.1016/s0091-6749(03)01797-4. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu M, Matsuda A, Yanagisawa K, Hirota T, Akahoshi M, et al. Functional SNPs in the distal promoter of the ST2 gene are associated with atopic dermatitis. Hum Mol Genet. 2005;14:2919–2927. doi: 10.1093/hmg/ddi323. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi N, Akahoshi M, Matsuda A, Ebe K, Inomata N, et al. Association of the IL12RB1 promoter polymorphisms with increased risk of atopic dermatitis and other allergic phenotypes. Hum Mol Genet. 2005;14:3149–3159. doi: 10.1093/hmg/ddi347. [DOI] [PubMed] [Google Scholar]
- 12.Tsunemi Y, Saeki H, Nakamura K, Sekiya T, Hirai K, et al. Interleukin-13 gene polymorphism G4257A is associated with atopic dermatitis in Japanese patients. J Dermatol Sci. 2002;30:100–107. doi: 10.1016/s0923-1811(02)00065-8. [DOI] [PubMed] [Google Scholar]
- 13.Wood SH, Ke X, Nuttall T, McEwan N, Ollier WE, et al. Genome-wide association analysis of canine atopic dermatitis and identification of disease related SNPs. Immunogenetics. 2009;61:765–772. doi: 10.1007/s00251-009-0402-y. [DOI] [PubMed] [Google Scholar]
- 14.Berth-Jones J, Finlay AY, Zaki I, Tan B, Goodyear H, et al. Cyclosporine in severe childhood atopic dermatitis: a multicenter study. J Am Acad Dermatol. 1996;34:1016–1021. doi: 10.1016/s0190-9622(96)90281-9. [DOI] [PubMed] [Google Scholar]
- 15.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 16.Chang WC, Nelson C, Parekh AB. Ca2+ influx through CRAC channels activates cytosolic phospholipase A2, leukotriene C4 secretion, and expression of c-fos through ERK-dependent and -independent pathways in mast cells. FASEB J. 2006;20:2381–2383. doi: 10.1096/fj.06-6016fje. [DOI] [PubMed] [Google Scholar]
- 17.Chang WC, Parekh AB. Close functional coupling between Ca2+ release-activated Ca2+ channels, arachidonic acid release, and leukotriene C4 secretion. J Biol Chem. 2004;279:29994–29999. doi: 10.1074/jbc.M403969200. [DOI] [PubMed] [Google Scholar]
- 18.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 20.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermatol Venereol (Stockh) 1980;92:44–47. [Google Scholar]
- 22.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 23.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, et al. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Korkiamaki T, Yla-Outinen H, Koivunen J, Karvonen SL, Peltonen J. Altered calcium-mediated cell signaling in keratinocytes cultured from patients with neurofibromatosis type 1. Am J Pathol. 2002;160:1981–1990. doi: 10.1016/S0002-9440(10)61148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trautmann A, Akdis M, Blaser K, Akdis CA. Role of dysregulated apoptosis in atopic dermatitis. Apoptosis. 2000;5:425–429. doi: 10.1023/a:1009620329213. [DOI] [PubMed] [Google Scholar]
- 29.Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, et al. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, et al. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 31.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeHaven WI, Smyth JT, Boyles RR, Putney JW., Jr Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Lu J, Xu P, Xie X, Chen L, et al. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 35.Chou YH, Juo SH, Chiu YC, Liu ME, Chen WC, et al. A Polymorphism of ORAI1 Gene is Associated with the Risk and Recurrence of Calcium Nephrolithiasis. Journal of Urology. 2011;185(5):1742–6. doi: 10.1016/j.juro.2010.12.094. [DOI] [PubMed] [Google Scholar]
- 36.Wei CC, Yen JH, Juo SH, Chen WC, Wang YS, et al. Association of ORAI1 haplotypes with the Risk of HLA-B27 positive Ankylosing Spondylitis. PLos ONE. 2011;6(6):e20426. doi: 10.1371/journal.pone.0020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Basal characteristics of patients with Atopic Dermatitis (AD) and of normal controls in Japanese population.
(DOC)
Basal characteristics of patients with Atopic Dermatitis (AD) and of normal controls in Taiwanese population.
(DOC)
Pairwise linkage disequilibrium for all possible two-way comparisons among 10 polymorphisms in ORAI1 with 24 Japanese volunteers.
(DOC)