Maternally human immunodeficiency virus (HIV)-exposed but seronegative Zambian infants had reduced length and psychomotor development associated with human cytomegalovirus infection. Slower growth was also associated with human cytomegalovirus in HIV-unexposed infants. We conclude that human cytomegalovirus impacts negatively on child health in HIV-endemic regions of sub-Saharan Africa.
Abstract
Background. Human immunodeficiency virus (HIV) and human cytomegalovirus (HCMV) coinfections have been shown to increase infant morbidity, mortality, and AIDS progression. In HIV-endemic regions, maternal HIV-exposed but HIV-uninfected infants, which is the majority of children affected by HIV, also show poor growth and increased morbidity. Although nutrition has been examined, the effects of HCMV infection have not been evaluated. We studied the effects of HCMV infection on the growth, development, and health of maternally HIV-exposed and unexposed infants in Zambia.
Methods. Infants were examined in a cohort recruited to a trial of micronutrient-fortified complementary foods. HIV-infected mothers and infants had received perinatal antiretroviral therapy to prevent mother-to-child HIV transmission. Growth, development, and morbidity were analyzed by linear regression analyses in relation to maternal HIV exposure and HCMV infection, as screened by sera DNA for viremia at 6 months of age and by antibody for infection at 18 months.
Results. All HCMV-seropositive infants had decreased length-for-age by 18 months compared with seronegative infants (standard deviation [z]-score difference: −0.44 [95% confidence interval {CI}, −.72 to −.17]; P = .002). In HIV-exposed infants, those who were HCMV positive compared with those who were negative, also had reduced head size (mean z-score difference: −0.72 [95% CI, −1.23 to −.22]; P = .01) and lower psychomotor development (Bayley test score difference: −4.1 [95% CI, −7.8 to −.5]; P = .03). HIV-exposed, HCMV-viremic infants were more commonly referred for hospital treatment than HCMV-negative infants. The effects of HCMV were unaffected by micronutrient fortification.
Conclusion. HCMV affects child growth, development, and morbidity of African infants, particularly in those maternally exposed to HIV. HCMV is therefore a risk factor for child health in this region.
Human cytomegalovirus (HCMV) commonly infects children, establishing a lifelong latent infection. It is mainly a pathogen in an immunocompromised setting, particularly for human immunodeficiency virus (HIV)/AIDS and transplantation patients, where HCMV can reactivate. HCMV is the main infectious cause of mental retardation and neurodevelopmental impairment in neonates; this includes hearing loss, chiefly from congenital infection in utero [1–4]. In transplantation patients, HCMV-associated pneumonitis has a high mortality rate that is controlled primarily with ganciclovir. Prior to antiretroviral therapy (ART) in adult HIV/AIDS patients, HCMV was a major opportunistic infection that correlated with retinitis, leading to blindness as well as disseminated infections and in turn mortality [5]. In childhood HIV, HCMV coinfection can be particularly severe; studies in the United States showed increased neurological disease, AIDS progression, and mortality [6, 7]. In HIV-endemic sub-Saharan Africa, pediatric ART is restricted and HCMV complications are only beginning to be addressed; even the extent of HCMV effects on the general child population has not been established. In Kenyan infants, HIV infection was associated with impaired control of HCMV replication [8]. In Zambia, autopsy studies of HIV-positive children who died from respiratory disease identified HCMV as a frequent infection, often together with tuberculosis [9, 10].
HCMV may also affect infants born to HIV-positive mothers, even if the infants remain HIV-negative. HIV-positive mothers can have reduced maternal passive immunity to protect their infants against HCMV disease. This includes protective cytokines or antibodies, immunoglobulin G (IgG) transcytosed across the placenta in utero to the fetus, or immunoglobulin A (IgA) secreted in breast milk to protect against gut infection in the newborn. Moreover, immunosuppression in the HIV-positive mother can lead to increased HCMV levels due to reactivation or secondary infection. Little is known about how these effects impact childhood HCMV in HIV-endemic African populations. The number of children who are maternally HIV exposed but not infected is increasing due to the epidemic spread of HIV and also ART introduction to decrease mother-to-child HIV transmission.
We previously reported that in Zambia, where HIV is endemic, children are exposed to a complex collection of HCMV strains, as exists elsewhere, but also show increased prevalence of higher loads transmitted from HIV-positive mothers [10]. HIV-exposed infants do not grow as well as unexposed infants, have increased morbidity, and are particularly vulnerable when they transition from breastfeeding [11, 12]. Here we report the effects of HCMV infection on growth, development, and health of HIV-exposed and unexposed infants in Zambia who were recruited to a double-blind, randomized controlled trial of micronutrient-fortified infant foods [13]. The results identify HCMV as a pathogen associated with poor development including evidence of increased morbidity, irrespective of diet.
METHODS
Study Design, Population, and Recruitment
A total of 811 infants were recruited for a trial of micronutrient-fortified complementary foods. The trial was conducted in Chilenje at the University Teaching Hospital (UTH), Lusaka, Zambia, from October 2005 to July 2009, registered as ISRCTN37460449 (www.controlled-trials.com/mrct), with 77% completion rates as described [13]. The infants were representative of the region, as mothers were recruited during their infant’s standard care checks for weighing or vaccinations, and coverage for these services is high in Lusaka.
Follow-up, Anthropometry, and Sera Collection
Anthropometry and standard deviation z scores were calculated using World Health Organization (WHO) growth reference data, as described [13]. Stunting was recorded as length/age z <−2. Venous blood was collected in plain vacutainers at age 6 months and 18 months; sera were separated within 2 hours, aliquoted, and stored at −80°C.
HIV Antibody Testing
Serum samples at 18 months were tested for HIV antibodies using Determine HIV 1/2 (Inverness Medical). Negative results were recorded; positive results were further tested using Unigold HIV 1/2 (Trinity Biotech). If a negative, discordant result was shown, a third test, SD-Bioline HIV 1/2 (Standard Diagnostics), was used.
Human Cytomegalovirus Antibody and DNA Testing
The ETI-CYTOK-G PLUS enzyme-linked immunosorbent assay kit (DiaSorin) for detection of anti-HCMV IgG in human serum was used in accordance with the manufacturer’s instructions. Standard curves were plotted; control sera standards were used to interpolate sample IgG titers with positive antibody titers read above a cutoff of 0.4 IU/mL. Qualitative and quantitative polymerase chain reaction (PCR) was performed on DNA extracted from 200 μL of sera using the QIAamp DNA Blood Mini Kit (Qiagen). PCR was used to amplify HCMV glycoprotein gB (UL55), as described previously [10], with baseline and high-load cutoff sensitivities of 50 and 1000 copies/mL of sera, respectively.
Morbidity Assessments
The clinical officer (J. S.) examined children during scheduled or voluntary visits, with diagnoses and treatment given based on WHO guidelines for Integrated Management of Childhood Illness [13]. Prescriptions for antibiotics or antimalarials were provided at the study clinic. Hospital referrals were defined as those made to the local tertiary facility (UTH), with severe symptoms (inability to drink or breastfeed, severe vomiting, convulsions, respiratory distress, loss of consciousness, or severe lethargy), for surgery, or with an illness requiring consultation with a specialist.
Bayley Testing of Development
Child development tests were administered by 2 trained psychologists using Bayley scales of infant development (BSID II). The mental development index (MDI) and psychomotor development index (PDI) were used as standardized in the United States [14] and age-normalized to a population mean of 100 and SD of 15. Some items were adapted to local settings (doll appearance or house pictures) while keeping underlying constructs. The test was translated to local languages (Bemba and Nyanja). If a child was sick at the time of assessment, the mother was asked to bring the child back 1–2 weeks after recovery. For logistical reasons, testing was conducted on a subset, including all with HIV-positive mothers and alternate infants with HIV-negative mothers.
Statistical Analysis
Growth and reported illness were compared between infants with and without HCMV using χ2 tests. The impact of HCMV infection on length-for-age (LAZ), weight-for-age (WAZ), head circumference-for-age (HCZ), and arm circumference-for-age (ACZ) z scores at 18 months was analyzed as a continuous variable using linear regression and 95% confidence intervals (CIs) for regression coefficients. The impact on incidence of severe morbidity, defined as hospital referral or death, was estimated using rate ratios (RRs) and 95% CIs obtained using random-effects Poisson regression to account for repeated referrals among infants. Any planned surgeries were excluded (5 circumcisions, 4 hernias, and 4 other congenital abnormalities). It was decided a priori to perform analyses stratified by maternal HIV status; this excluded the 70 infants of HIV-unknown women. Maternal education, socioeconomic status (SES), and diet intervention arm were determined a priori as potential confounders. Adjustments were made for these a priori confounders as well as for breastfeeding duration (shorter or longer than 6 months), which was determined to be associated with HCMV infection in risk factor analyses. SES was measured by an asset index, as described previously [13]. Interobserver reliability of the Bayley test was obtained in 663 testing sessions during which the same child was scored by the 2 psychologists. The intraclass correlation coefficient (ICC) of the scores was calculated using 1-way analysis of variance. A good agreement between scorers was found for both MDI (ICC = 0.91 [95% CI, .89–.92]) and PDI (ICC = 0.91 [95% CI, .90–.92]). MDI and PDI scores were analyzed as continuous variables and checked for normality on a normal probability plot. The association of Bayley test scores and HCMV seropositive antibody at 18 months was examined using linear regression with subsequent adjustment for SES, mother’s education, diet intervention arm, and breastfeeding duration. A 2-sided P value ≤.05 was considered statistically significant. Stata software (version 11.1, StataCorp) was used in all analyses.
Multiple Imputation Analyses
Among the 811 children enrolled in the study, 561 (69%) were available for HCMV DNA at 6 months, 430 (53%) for HMCV DNA at 18 months, and 460 (57%) for HCMV antibody at 18 months. Any missing data were mainly due to limitations on the infant serum sample volume after the large number of previous metabolic assays, which were used to interpret the primary outcome analyses, as described [13]. Ninety samples were missing due to participant withdrawal from the study. In order to account for infants who were missing data, we used multiple imputations with chained equations methods using Stata software. Factors predictive of missing data (small child), risk factors for HCMV (mother’s education, SES, breastfeeding duration, and maternal HIV status), and the micronutrient diet intervention arm were used to impute 10 data sets. All continuous variables used in the imputation were normally distributed; SES and mother’s education were treated as ordered categorical variables. In addition to the main analyses of observed data, complete analyses were conducted with all imputed data.
Ethics
The study received approval from the ethics committees of the University of Zambia and the London School of Hygiene and Tropical Medicine. The funding source, the Bill and Melinda Gates Foundation, was not involved in the study or its interpretation.
RESULTS
Growth Effects
In order to analyze the effects of HCMV on growth, 2 measurements were made: HCMV viremia at recruitment (age 6 months; sera DNA) and HCMV infection at the end of the study (age 18 months; sera antibody). The antibody was used to measure infection occurring in the cohort by 18 months; the DNA assay detects HCMV viremia on recruitment at 6 months, either primary or earlier, uncontrolled infections.
Among all children there was no significant effect of HCMV viremia on growth from age 6 months to 18 months. However, when the population was stratified for maternal HIV exposure, there were significant associations, but in opposing directions (Table 1). Among HIV-exposed children with HCMV viremia at 6 months, there was reduced LAZ (P = .04), with evidence for interaction of HCMV viremia with maternal HIV on LAZ (interaction, P = .007). Similar but nonsignificant directions were seen for WAZ and HCZ. With multiple imputations, the effect on LAZ in HIV-exposed infants remained (regression coefficient of −0.40 to −0.28; interaction, P = .07). When HIV-positive children were removed from the analysis, the reduced LAZ effect remained for the maternally HIV-exposed uninfected infants.
Table 1.
Effect of Human Cytomegalovirus Viremia (Sera DNA) at 6 Months on Anthropometric z Scores at 18 Months
| Mean (SD) |
||||||||
| HCMV Negative | HCMV Positive | Unadjusted Regression Coefficient (95% CI) | P Value | Adjusted Regression Coefficienta (95% CI) | P Value | Adjusted Regression Coefficientb (95% CI) | P Value | |
| All children | n = 336 | n = 226 | ||||||
| Length | −1.05 (1.17) | −1.03 (1.07) | 0.02 (−.19 to .23) | .87 | 0.05 (−.15 to .25) | .61 | 0.06 (−.14 to .26) | .55 |
| Weight | −0.50 (1.23) | −0.50 (1.14) | 0.00 (−.23 to .22) | .99 | 0.03 (−.18 to .25) | .77 | 0.04 (−.18 to .25) | .72 |
| Head circumference | 0.57 (0.97) | 0.61 (1.06) | 0.05 (−.14 to .23) | .64 | 0.08 (−.11 to .26) | .40 | 0.10 (−.09 to .28) | .30 |
| Arm circumference | 0.20 (1.15) | 0.01 (1.06) | −0.20 (−.41 to .01) | .07 | −0.17 (−.37 to .04) | .11 | −0.16 (−.36 to .05) | .13 |
| Maternal HIV negative | n = 241 | n = 152 | ||||||
| Length | −1.06 (1.19) | −0.81 (1.00) | 0.25 (.00–.51) | .06 | 0.24 (.00 to .48) | .05 | 0.24 (.00 to .48) | .05 |
| Weight | −0.48 (1.25) | −0.28 (1.07) | 0.19 (–.08 to .47) | .17 | 0.19 (–.07 to .45) | .16 | 0.19 (−.07 to .46) | .15 |
| Head circumference | 0.62 (1.00) | 0.84 (1.01) | 0.22 (−.01 to .45) | .06 | 0.23 (.00 to .46) | .05 | 0.24 (.01 to .46) | .04 |
| Arm circumference | 0.27 (1.13) | 0.16 (0.95) | −0.11 (−.37 to .15) | .40 | −0.10 (−.35 to .15) | .43 | −0.10 (−.35 to .15) | .43 |
| Maternal HIV positive | n = 65 | n = 55 | ||||||
| Length | −1.09 (1.07) | −1.54 (1.14) | −0.45 (−.89 to –.02) | .04 | −0.40 (−.80 to .01) | .05 | −0.40 (−.80 to .00) | .05 |
| Weight | −0.58 (1.16) | −0.94 (1.30) | −0.36 (−.82 to .10) | .13 | −0.31 (−.75 to .13) | .16 | −0.32 (−.76 to .13) | .16 |
| Head circumference | 0.42 (0.91) | 0.25 (1.09) | −0.17 (−.56 to .22) | .40 | −0.13 (−.51 to .25) | .50 | −0.12 (−.50 to .26) | .54 |
| Arm circumference | 0.01 (1.28) | −0.36 (1.27) | −0.36 (−.80 to .07) | .10 | −0.33 (−.75 to .09) | .13 | −0.33 (−.75 to .09) | .12 |
P values for interaction HCMV viremia with maternal HIV: length-for-age = .007, weight-for-age = .05, head circumference-for-age = .11, and arm circumference-for-age = .35. Infants of women with unknown HIV status were excluded from the stratified analyses.
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; HIV, human immunodeficiency virus; SD, standard deviation.
Adjusted for maternal socioeconomic status and education.
Additionally adjusted for diet treatment arm and breastfeeding duration.
In contrast, among children of HIV-negative mothers, HCMV viremia at 6 months was associated with increased LAZ and HCZ. Effects of HCMV viremia in all infants were independent of nutrition, either micronutrient intervention or breastfeeding duration, as adjustments did not alter the associations. Adjustment for hemoglobin had no effect (data not shown).
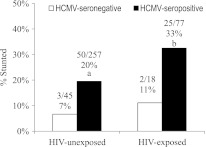
Next, the effects of HCMV infection on growth by 18 months (sera antibody) were analyzed. HCMV infection affected LAZ, with reduced measures in all children (Table 2). The effects remained after adjusting for SES, education, breastfeeding duration, and diet treatment arm (adjusted regression coefficient, −0.33 [95% CI, −.60 to −.06]; P = .01; adjusted regression coefficient was 0.04 after Bonferroni correction). The results were similar after multiple imputations for missing data (data not shown). There was 3-fold more stunting (LAZ <−2) associated with HCMV infection in maternally HIV-exposed infants and unexposed infants (Figure 1). The other anthropometric measures were also negatively associated with HCMV infection by 18 months; only results for HCZ were statistically significant, with the greatest effects among HIV-exposed children (regression coefficient, −0.71; interaction P = .02). This HCZ effect was similar after multiple imputations, although significance was borderline (regression coefficient, −0.57; P = .09). Because maternal HIV exposure has been shown to increase the prevalence of congenital HCMV infection [7, 15], and this may affect head size, an additional adjustment was made for viremia at 6 months, and this would include congenital infection. Adjustment strengthens the association (regression coefficient, −0.80 [95% CI, −1.40 to −.20]), suggesting that overall HCMV infection from 6 to 18 months is a main effect.
Table 2.
Association of Infant Human Cytomegalovirus Infection (Antibody) on Anthropometric z Scores at 18 Months
| Mean (SD) | ||||||||
| HCMV Negative | HCMV Positive | Unadjusted Mean Difference (95% CI) | P Value | Adjusted Mean Differencea (95% CI) | P Value | Adjusted Mean Differenceb (95% CI) | P Value | |
| All children | n = 76 | n = 384 | ||||||
| Length | −0.72 (1.07) | −1.17 (1.11) | −0.44 (–.72 to −.17) | .002 | −0.31 (–.58 to −.05) | .02 | −0.33 (−.60 to −.06) | .01 |
| Weight | −0.34 (1.10) | −0.64 (1.15) | −0.30 (–.58 to −.01) | .04 | −0.15 (−.42 to .13) | .29 | −0.17 (−.45 to .11) | .22 |
| Head circumference | 0.79 (1.00) | 0.49 (0.99) | −0.31 (–.55 to −.06) | .01 | −0.21 (−.45 to .04) | .10 | −0.25 (−.49 to .00) | .05 |
| Arm circumference | 0.05 (1.14) | 0.03 (1.06) | −0.01 (−.28 to .25) | .92 | 0.11 (−.15 to .38) | .40 | 0.06 (−.21 to .33) | .65 |
| Maternal HIV negative | n = 49 | n = 263 | ||||||
| Length | −0.63 (1.06) | −1.13 (1.13) | −0.49 (–.83 to −.15) | .01 | −0.38 (–.71 to –.06) | .02 | −0.38 (–.71 to –.06) | .02 |
| Weight | −0.26 (1.05) | −0.59 (1.15) | −0.33 (−.68 to .03) | .07 | −0.19 (−.53 to .16) | .29 | −.19 (−.53 to .16) | .29 |
| Head circumference | 0.74 (0.95) | 0.59 (1.00) | −0.15 (−.46 to .15) | .33 | −0.05 (−.35 to .26) | .77 | −0.04 (−.34 to .27) | .82 |
| Arm circumference | 0.18 (1.07) | 0.13 (1.00) | −0.06 (−.39 to .28) | .74 | 0.08 (−.25 to .40) | .65 | 0.08 (−.25 to .42) | .62 |
| Maternal HIV positive | n = 19 | n = 86 | ||||||
| Length | −0.85 (1.11) | −1.35 (1.10) | −0.49 (−1.05 to .06) | .08 | −0.29 (−.82 to .23) | .27 | −0.29 (−.83 to .25) | .30 |
| Weight | −0.56 (1.20) | −0.79 (1.22) | −0.23 (−.81 to .34) | .43 | −0.02 (−.57 to .53) | .93 | −0.08 (−.65 to .49) | .78 |
| Head circumference | 0.95 (1.19) | 0.25 (0.98) | −0.71 (−1.21 to −.21) | .01 | −0.60 (−1.08 to −.12) | .02 | −0.72 (−1.23 to −.22) | .01 |
| Arm circumference | −0.23 (1.38) | −0.21 (1.23) | 0.02 (−.52 to .56) | .93 | 0.17 (−.35 to .69) | .52 | 0.07 (−.48 to .62) | .80 |
P values for interaction HCMV antibody with maternal HIV: length-for-age = .76, weight-for-age = .76, head circumference-for-age = .02, and arm circumference-for-age = .97. Infants of women with unknown HIV status were excluded from the stratified analyses.
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; HIV, human immunodeficiency virus; SD, standard deviation.
Adjusted for maternal socioeconomic status and education.
Additionally adjusted for diet treatment arm and breastfeeding duration.
Figure 1.
Human cytomegalovirus (HCMV) seroprevalence correlates with stunting at 18 months. Stunting (length/age z <−2) prevalence at 18 months is tripled in HCMV-seropositive infants. Significance is by Pearson χ2: a Human immunodeficiency virus (HIV)–unexposed, P = .037; b HIV-exposed, P = .071.
Morbidity Effects
Table 3 shows effects of HCMV viremia and infection on infant hospitalization or death. Although no associations were statistically significant, the directions were consistent with HCMV viremia at recruitment (age 6 months), with fewer hospitalizations among HIV-unexposed infants and increased hospitalizations for HIV-exposed or HIV-infected infants. The increased hospitalizations were concentrated in infants coinfected with HIV and HCMV. Similar results are shown using a higher threshold for copy number of HCMV DNA in sera of 1000 copies/mL and after further sensitivity analyses (P = .09).
Table 3.
Effect of Human Cytomegalovirus Viremia at 6 Months (Sera DNA) on Hospitalization
| No./Person-Years (Rate per 100 py) | ||||||||
| HCMV Negative | HCMV Positive | Unadjusted RR (95% CI) | P Value | Adjusted RR (95% CI)a | P Value | Adjusted RR (95% CI)b | P Value | |
| Standard threshold (50 copies/mL) | ||||||||
| All children | 61/288.5 (21.1) | 43/204.6 (21.0) | 1.00 (.65–1.53) | >.99 | 1.04 (.68–1.59) | .86 | 1.04 (.68–1.59) | .85 |
| Maternal HIV | ||||||||
| Negative | 44/202.4 (21.7) | 21/134.2 (15.7) | 0.72 (.41–1.25) | .24 | 0.75 (.43–1.30) | .31 | 0.74 (.43–1.29) | .29 |
| Positive | 15/59.9 (25.0) | 19/52.0 (36.5) | 1.49 (.70–3.16) | .30 | 1.62 (.77–3.43) | .21 | 1.53 (.72–3.27) | .27 |
| Child HIV | ||||||||
| Negative | 11/50.3 (21.9) | 7/43.1 (16.2) | 0.76 (.29–1.96) | .57 | 0.90 (.34–2.36) | .83 | 0.96 (.36–2.57) | .94 |
| Positive | 1/4.5 (22.4) | 5/6.3 (79.9) | 3.56 (.41–.80) | .25 | 3.11 (.35–27.40) | .31 | 5.08 (.55–47.30) | .15 |
| High threshold (>1000 copies/mL) | ||||||||
| All children | 61/288.5 (21.1) | 6/31.0 (19.4) | 0.93 (.38–2.31) | .88 | 0.81 (.32–2.03) | .65 | 0.86 (.34–2.18) | .75 |
| Maternal HIV | ||||||||
| Negative | 44/202.4 (21.7) | 2/18.5 (10.8) | 0.49 (.11–2.16) | .35 | 0.47 (.10–2.12) | .33 | 0.48 (.11–2.12) | .33 |
| Positive | 15/59.9 (25.0) | 4/11.5 (34.8) | 1.48 (.43–5.13) | .54 | 1.06 (.30–3.80) | .93 | 1.28 (.36–4.54) | .70 |
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; HIV, human immunodeficiency virus; py, person-years; RR, rate ratio.
Adjusted for mother's socioeconomic status and education.
Additionally adjusted for breastfeeding duration (<6 months/≥6 months) and diet treatment arm. Maternal HIV-exposure interaction with HCMV in a fully adjusted model has a P value = .10.
Development Effects
Mental and psychomotor development was assessed using Bayley tests. HIV-exposed infants with HCMV viremia at 6 months showed a borderline significance for decreased psychomotor skills (PDI) (Table 4). By 18 months, HIV-exposed infants with HCMV infection detected by antibody had significantly lower PDI after adjustment for SES, education, breastfeeding duration, and diet intervention (regression coefficient, −4.1 [95% CI ,−7.8 to −.5]; P = .03) (Table 5). HCMV did not affect PDI in HIV-unexposed infants or mental development in either subgroup.
Table 4.
Effect of Human Cytomegalovirus Viremia at 6 Months (Sera DNA) on Development at 18 Months
| Mean (SD) | ||||||
| HCMV Negative | HCMV Positive | Unadjusted Regression Coefficienta (95% CI) | P Value | Adjusted Regression Coefficientb (95% CI) | P Value | |
| All children | n = 155 | n = 121 | ||||
| MDI | 89.2 (7.8) | 89.2 (7.9) | 0.0 (−1.9 to 1.8) | .97 | −0.1 (−1.8 to 1.9) | .93 |
| PDI | 91.0 (5.3) | 90.4 (7.0) | −0.6 (−2.0 to .9) | .46 | −0.7 (−2.1 to .8) | .35 |
| Maternal HIV negative | n = 78 | n = 57 | ||||
| MDI | 89.3 (7.9) | 90.6 (6.9) | 1.3 (−1.3 to 3.9) | .32 | 1.0 (−1.6 to 3.6) | .45 |
| PDI | 91.1 (5.2) | 91.7 (5.9) | 0.6 (−1.3 to 2.5) | .53 | 0.2 (−1.7 to 2.2) | .82 |
| Maternal HIV positive | n = 55 | n = 49 | ||||
| MDI | 88.0 (6.9) | 87.4 (8.9) | −0.6 (−3.7 to 2.4) | .68 | −0.6 (−3.8 to 2.6) | .73 |
| PDI | 90.9 (5.7) | 88.6 (8.2) | −2.3 (−5.0 to .4) | .09 | −2.5 (−5.4 to .3) | .08 |
P values for interaction between human cytomegalovirus viremia and maternal human immunodeficiency virus on mental development index = 0.03, on psychomotor development index = 0.07.
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; HIV, human immunodeficiency virus; MDI, mental development index; PDI, psychomotor development index; SD, standard deviation.
Mean difference.
Adjusted for socioeconomic status, mother’s education, diet intervention arm, and breastfeeding duration.
Table 5.
Effect of Human Cytomegalovirus Infection (Antibody) on Development at 18 Months
| Mean (SD) | ||||||
| HCMV Negative | HCMV Positive | Unadjusted Regression Coefficienta (95% CI) | P Value | Adjusted Regression Coefficientb (95% CI) | P Value | |
| All children | n = 48 | n = 239 | ||||
| MDI | 89.2 (7.3) | 88.8 (7.8) | −0.4 (−2.8 to 2.0) | .73 | −0.1 (−2.7 to 2.5) | .97 |
| PDI | 92.2 (4.3) | 90.4 (6.2) | −1.8 (−3.6 to .0) | .05 | −2.0 (−4.0 to .0) | .05 |
| Maternal HIV negative | n = 22 | n = 121 | ||||
| MDI | 90.9 (6.6) | 88.9 (8.2) | −2.0 (−5.7 to 1.7) | .28 | −0.5 (−4.2 to 3.3) | .79 |
| PDI | 92.5 (4.4) | 90.8 (6.3) | −1.7 (−4.5 to 1.1) | .22 | −1.0 (−3.8 to 1.8) | .48 |
| Maternal HIV positive | n = 19 | n = 86 | ||||
| MDI | 87.5 (6.7) | 87.8 (7.1) | 0.3 (–3.2 to 3.8) | .86 | 0.8 (-3.4 to 5.0) | .71 |
| PDI | 92.1 (4.1) | 89.2 (6.5) | −2.8 (−5.9 to .2) | .07 | −4.1 (−7.8 to −.5) | .03 |
P values for interaction between human cytomegalovirus exposure and maternal human immunodeficiency virus on mental development index = 0.12, on psychomotor development index = 0.13.
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; HIV, human immunodeficiency virus; MDI, mental development index; PDI, psychomotor development index; SD, standard deviation.
Mean difference.
Adjusted for socioeconomic status, mother’s education, diet intervention arm, and breastfeeding duration.
DISCUSSION
The results show that HCMV is a widespread pathogen that affects healthy growth and development of infants in Zambia. In the maternally HIV-exposed infants, HCMV infection was also associated with increased prevalence of stunting, reduced head size, and decreased psychomotor development. Previous analyses of this cohort showed that reduced growth in the HIV-exposed infants was unaffected by micronutrient fortification but was affected by socioeconomic factors [13, 16]. The effects of HCMV on growth were also influenced by socioeconomic factors; however, the effects still existed after adjustments were made for socioeconomic factors, education level, breastfeeding duration, and diet. In Zambia we showed that HCMV infections occurred earlier, that is, during infancy, and were more prevalent (83% IgG seropositive in this cohort) than in many other geographic regions. Therefore, effects of HCMV on growth could affect the majority of the infant population. Moreover, growth effects at such an early age have been linked with lifetime risks of adult chronic disease and thus HCMV infection is a general public health concern [17]. In the United States, children studied from age 6 years, showed reduced growth associated with increased infectious burden, including HCMV (30%–50% seropositive at outset). This resulted in health disparities that persisted to middle age [18, 19].
HIV-unexposed infants with HCMV viremia at 6 months had higher length and head circumference z scores, whereas among maternally HIV-exposed infants, HCMV viremia was associated with lower z scores. This suggests that HIV-uninfected mothers, but not infected, provide some protective maternal immunity, permitting infants to tolerate HCMV infection. In the HIV-unexposed infants, HCMV may provide beneficial immune stimulation at this age, possibly contributing to the high HCMV seroprevalence in this population. There are similar examples with other sequential infections; for example, prior influenza infection affects outcome for subsequent respiratory syncytial virus infection [20]. HCMV encodes a number of immunomodulatory proteins that favor virus persistence, but these proteins may also affect responses to other infections and host survival [21–23]. However, even if beneficial in the short term, long-term HCMV immune stimulation during infancy can be detrimental by driving early immune senescence [24]. In West Africa, Malawi, and Gambia, HCMV-infected infants had expanded HCMV-specific T lymphocytes with markers of aged anergic cells, which have been previously identified in elderly European populations where HCMV is acquired later [25, 26]. HCMV immune senescence may compromise the ability to respond to new infections during infancy in Africa, as suggested for the elderly in Europe [24–26]. Therefore, increased overall burden of all infections may affect healthy growth. This is supported by the 18-month HCMV antibody data, which shows that when overall HCMV infection is considered at times when maternal immunity wanes, there is a negative effect on growth.
HCMV may also directly affect growth and development, depending on the route of infection. HCMV frequently reactivates in HCMV IgG-positive mothers during lactation, and the virus is detectable in milk whey. Furthermore, HCMV is a pathogen of the gastrointestinal tract of HIV/AIDS patients and so may alter nutrient absorption, and can invade the central nervous system, possibly affecting development [3, 4, 27, 28]. HCMV infection acquired in utero can cause neurological disease, while later postnatal infections, primarily via breast milk, are generally asymptomatic [5]. In utero infection may account for HCMV effects on reduced head size and psychomotor development in HIV-exposed infants because adjustments for breastfeeding increased the effect. However, in separate analyses, we showed that the major route for infection in this cohort is via breastfeeding and that its duration over age 6 months is a risk factor, particularly for HIV-exposed infants. Studies in Europe of preterm births show symptomatic HCMV infection acquired via breast milk is associated with a growth disadvantage [29, 30]. High HCMV load and prolonged excretion in breast milk are risk factors for acquisition [27]; both factors were apparent for this HIV-exposed Zambian cohort.
Maternal HIV exposure increased some adverse effects of HCMV. Our previous analyses indicated that maternal HIV exposure led to a greater prevalence of high-load viremic infant HCMV infections [10]. High-load HCMV viremia can correlate with symptomatic infection [4, 31, 32]. Studies in Kenya of HIV-positive mother/child pairs showed that detection of maternal HCMV plasma DNA was associated with infant mortality [33]. In our Zambian cohort, there was some evidence of increased hospitalization with HCMV viremia in the HIV-infected children, but this was not significant with maternal HIV exposure. Therefore, by affecting other diseases, the effects of HCMV on growth and development could be partially indirect; the effects appear to be a combination of direct and indirect causes. A limitation of our study was that the maternal CD4 count or HCMV status was not available and we could not determine the effects of HIV on maternal immunity or HCMV. Study strengths include analyses of HCMV effects in infants using both 6-month DNA for viremia and 18-month antibody for infection, with comparisons between maternally HIV-exposed and HIV-unexposed infants, controls for major confounding factors, and a large population-based study, the first in this region.
What may limit the effects of HCMV on infant growth and development in this region? Diagnostics for symptomatic congenital infections and their treatment with ganciclovir or its derivatives may be appropriate [34, 35]. Recent evidence shows that high-avidity HCMV IgG can cross the placenta and contribute to viral replication control [36]. This is an indication that hyperimmune globulin could also limit placental infection [35], and so improving maternal immunity could limit infection. In France, ART of HIV-positive women decreased HCMV congenital infection [37]. For HIV-positive mothers, limiting breastfeeding duration over 6 months could reduce infant HCMV infection; however, further study is required to assess the effects. In Zambia, HIV-positive women with advanced HIV/AIDS improved infant survival by decreasing breastfeeding, while those with less advanced HIV/AIDS improved survival of HIV-positive infants by increasing breastfeeding duration to longer than 4 months [38, 39]. Because HCMV is frequently secreted in urine, improved hygiene could limit horizontal transmission. Preliminary analyses showed lower HCMV infant infection with the presence of private indoor toilets compared with outdoor toilets. Ultimately, HCMV vaccination could control new infections or reduce viremia in those already infected. Early trials of a subunit vaccine show promise [40, 41].
In conclusion, HCMV infection in Zambia resulted in poor growth and development of maternally HIV-exposed infants and poor growth of HIV-unexposed children. Other African countries also have high HCMV childhood infection and endemic HIV. Therefore, efforts to improve child health will need to consider HCMV, particularly for effects on growth and development.
Notes
Acknowledgments.
We thank all the mothers and children who participated in the study and are grateful for the support of Lusaka District Health staff who permitted the project. Thanks are extended to the entire CIGNIS study team: Principal Investigator: Suzanne Filteau, London School of Hygiene and Tropical Medicine (LSHTM); Zambian Lead Investigator: Lackson Kasonka, University Teaching Hospital (UTH), Lusaka; Senior Investigators: Rosalind Gibson, University of Otago, New Zealand; Ursula A. Gompels, LSHTM; Shabbar Jaffar, LSHTM; Emmanuel Kafwembe, Tropical Diseases Research Centre, Ndola; Mwaka Monze, UTH; Moses Sinkala, Catholic Relief Services, Zambia; Andrew Tomkins, Institute of Child Health, University College, London; Rodah Zulu, National Institute of Science and Industrial Research, Zambia; Clinic Coordinator: Molly Chisenga; Clinical Officer: Joshua Siame; Data Manager: Hildah Banda Mabuda; Statisticians: Kathy Baisley, Helen Dale, Natasha Larke, Daniela Manno, Andrea Rehman; Research Fellows: Matthew Bates, Anne Mullen, Kunda Musonda, Marta Sanz-Ramos; Clinic Staff: Hellen Kangwa Bwalya, Margaret Chileshe, Priscilla Kangwa Kowa, Mabvuto Kumwenda, Munalula Likando, Sydney Mambwe, Mutinta Muzyamba, Anne Mwale, Lungowe Nyaywa; Laboratory Staff: Humphrey Bima, Julia Chibumbya, Laura Gosset, Louise Hackett, Abigail Jackson, Mirriam Kapambwe, Sydney Mwanza, Ida Ndumba, Eric Njunju; Data Entry: Concillia Kabanga, Natalia Shampwaya; Drivers and Cleaners: John Chobo, Winford Kapumba, Charity Musonda, Philip Soko.
Financial support.
This work was supported by the Bill and Melinda Gates Foundation, grant ID 37253.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr. 2008;153:84–8. doi: 10.1016/j.jpeds.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths PD, Walter S. Cytomegalovirus. Curr Opin Infect Dis. 2005;18:241–5. doi: 10.1097/01.qco.0000168385.39390.1b. [DOI] [PubMed] [Google Scholar]
- 3.Koyano S, Inoue N, Nagamori T, et al. Dried umbilical cords in the retrospective diagnosis of congenital cytomegalovirus infection as a cause of developmental delays. Clin Infect Dis. 2009;48:e93–5. doi: 10.1086/598506. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal LS, Fowler KB, Boppana SB, et al. Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. Pediatr Infect Dis J. 2009;28:515–20. doi: 10.1097/INF.0b013e318198c724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boppana SB, Fowler KB. Persistence in the population: epidemiology and transmission. In: Arvin A, Campadelli-Fiume G, Mocarski ES Jr, et al., editors. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge: Cambridge University Press; 2007. [PubMed] [Google Scholar]
- 6.Kovacs A, Schluchter M, Easley K, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle M, Atkins JT, Rivera-Matos IR. Congenital cytomegalovirus infection in infants infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1996;15:1102–6. doi: 10.1097/00006454-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Slyker JA, Lohman-Payne BL, John-Stewart GC, et al. Acute cytomegalovirus infection in Kenyan HIV-infected infants. AIDS. 2009;23:2173–81. doi: 10.1097/QAD.0b013e32833016e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet. 2002;360:985–90. doi: 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- 10.Bates M, Monze M, Bima H, Kapambwe M, Kasolo FC, Gompels UA. High human cytomegalovirus loads and diverse linked variable genotypes in both HIV-1 infected and exposed, but uninfected, children in Africa. Virology. 2008;382:28–36. doi: 10.1016/j.virol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14:276–87. doi: 10.1111/j.1365-3156.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 12.Koyanagi A, Humphrey JH, Ntozini R, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011;30:45–51. doi: 10.1097/INF.0b013e3181ecbf7e. [DOI] [PubMed] [Google Scholar]
- 13.CIGNIS Study Team Micronutrient fortification to improve growth and health of maternally HIV-unexposed and exposed Zambian infants: a randomised controlled trial. PLoS One. 2010;5:e11165. doi: 10.1371/journal.pone.0011165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lennon EM, Gardner JM, Kamel B. Encyclopedia of infant and early childhood development. 2008. Bayley scales of infant and early childhood development; pp. 145–56. [Google Scholar]
- 15.Duryea EL, Sanchez PJ, Sheffield JS, et al. Maternal human immunodeficiency virus infection and congenital transmission of cytomegalovirus. Pediatr Infect Dis J. 2010;29:915–8. doi: 10.1097/INF.0b013e3181e0ce05. [DOI] [PubMed] [Google Scholar]
- 16.Filteau S, Baisley K, Chisenga M, Kasonka L, Gibson RS, CIGNIS Study Team Provision of micronutrient-fortified food from 6 months of age does not permit HIV-exposed, uninfected Zambian children to catch up in growth to HIV-unexposed children: a randomised controlled trial. J Acquir Immune Defic Syndr. 2011;56:166–75. doi: 10.1097/QAI.0b013e318201f6c9. [DOI] [PubMed] [Google Scholar]
- 17.Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol. 2009;36:445–58. doi: 10.1080/03014460902980295. [DOI] [PubMed] [Google Scholar]
- 18.Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137:58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med. 2009;68:699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walzl G, Tafuro S, Moss P, Openshaw PJ, Hussell T. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J Exp Med. 2000;192:1317–26. doi: 10.1084/jem.192.9.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mocarski ES., Jr Immune escape and exploitation strategies of cytomegaloviruses: impact on and imitation of the major histocompatibility system. Cell Microbiol. 2004;6:707–17. doi: 10.1111/j.1462-5822.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson GW, Tomasec P, Stanton RJ, et al. Modulation of natural killer cells by human cytomegalovirus. J Clin Virol. 2008;41:206–12. doi: 10.1016/j.jcv.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boeckh M, Geballe AP. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest. 2011;121:1673–80. doi: 10.1172/JCI45449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19:47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Smith A, Gorak-Stolinska P, Floyd S, et al. Differences between naive and memory T cell phenotype in Malawian and UK adolescents: a role for cytomegalovirus? BMC Infect Dis. 2008;8:139. doi: 10.1186/1471-2334-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miles DJ, van der Sande M, Jeffries D, et al. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J Virol. 2007;81:5766–76. doi: 10.1128/JVI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jim WT, Shu CH, Chiu NC, et al. High cytomegalovirus load and prolonged virus excretion in breast milk increase risk for viral acquisition by very low birth weight infants. Pediatr Infect Dis J. 2009;28:891–4. doi: 10.1097/INF.0b013e3181a55c52. [DOI] [PubMed] [Google Scholar]
- 28.Meier J, Lienicke U, Tschirch E, Kruger DH, Wauer RR, Prosch S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol. 2005;43:1318–24. doi: 10.1128/JCM.43.3.1318-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamprecht K, Maschmann J, Jahn G, Poets CF, Goelz R. Cytomegalovirus transmission to preterm infants during lactation. J Clin Virol. 2008;41:198–205. doi: 10.1016/j.jcv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357:513–8. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 31.Kidd IM, Fox JC, Pillay D, Charman H, Griffiths PD, Emery VC. Provision of prognostic information in immunocompromised patients by routine application of the polymerase chain reaction for cytomegalovirus. Transplantation. 1993;56:867–71. doi: 10.1097/00007890-199310000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Lanari M, Lazzarotto T, Venturi V, et al. Neonatal cytomegalovirus blood load and risk of sequelae in symptomatic and asymptomatic congenitally infected newborns. Pediatrics. 2006;117:e76–83. doi: 10.1542/peds.2005-0629. [DOI] [PubMed] [Google Scholar]
- 33.Slyker JA, Lohman-Payne BL, Rowland-Jones SL, et al. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS. 2009;23:117–24. doi: 10.1097/QAD.0b013e32831c8abd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nassetta L, Kimberlin D, Whitley R. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J Antimicrob Chemother. 2009;63:862–7. doi: 10.1093/jac/dkp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adler SP, Nigro G, Pereira L. Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Semin Perinatol. 2007;31:10–8. doi: 10.1053/j.semperi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Nozawa N, Fang-Hoover J, Tabata T, Maidji E, Pereira L. Cytomegalovirus-specific, high-avidity IgG with neutralizing activity in maternal circulation enriched in the fetal bloodstream. J Clin Virol. 2009;46(Suppl 4):S58–63. doi: 10.1016/j.jcv.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guibert G, Warszawski J, Le Chenadec J, et al. Decreased risk of congenital cytomegalovirus infection in children born to HIV-1-infected mothers in the era of highly active antiretroviral therapy. Clin Infect Dis. 2009;48:1516–25. doi: 10.1086/598934. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn L, Aldrovandi GM, Sinkala M, et al. Differential effects of early weaning for HIV-free survival of children born to HIV-infected mothers by severity of maternal disease. PLoS One. 2009;4:e6059. doi: 10.1371/journal.pone.0006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn L, Aldrovandi GM, Sinkala M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–41. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191–9. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J Infect Dis. 2011;203:1534–40. doi: 10.1093/infdis/jir138. [DOI] [PMC free article] [PubMed] [Google Scholar]