Abstract
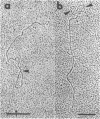
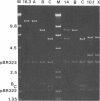
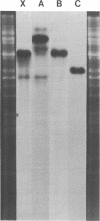
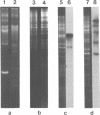
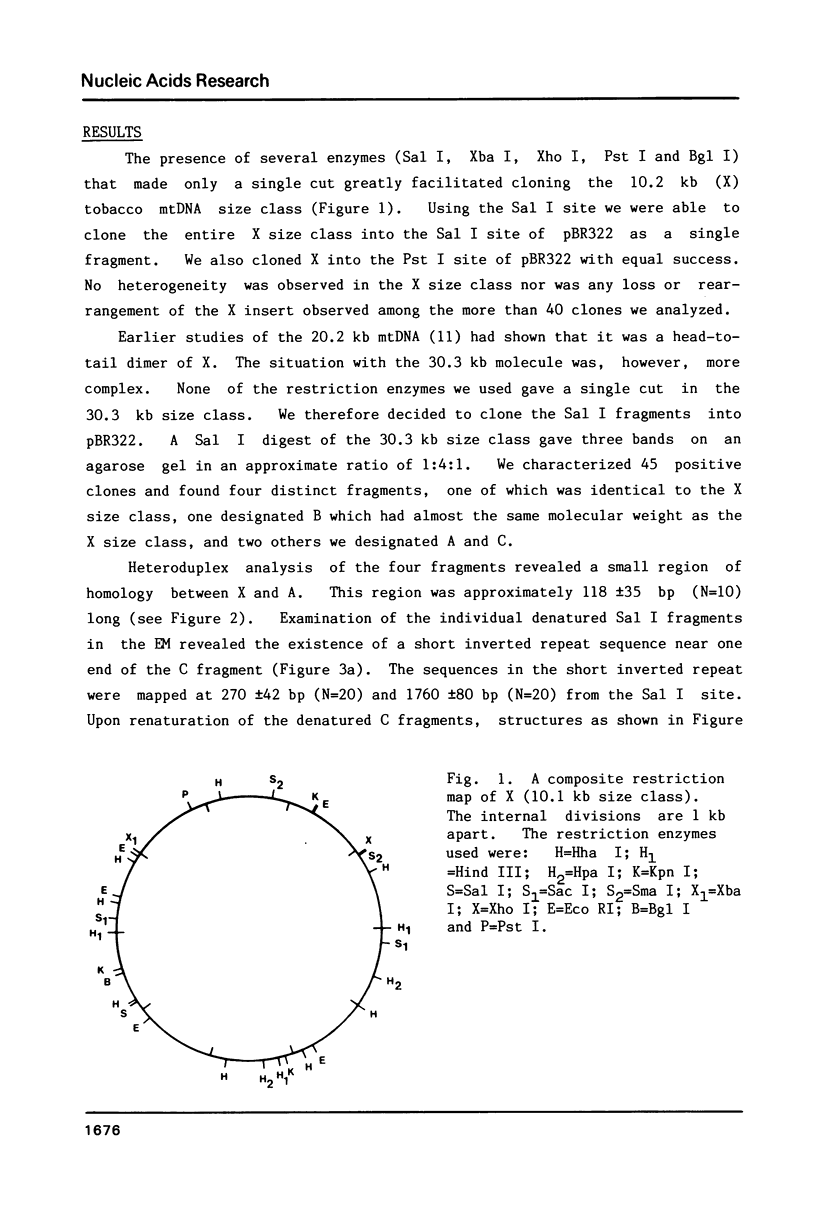
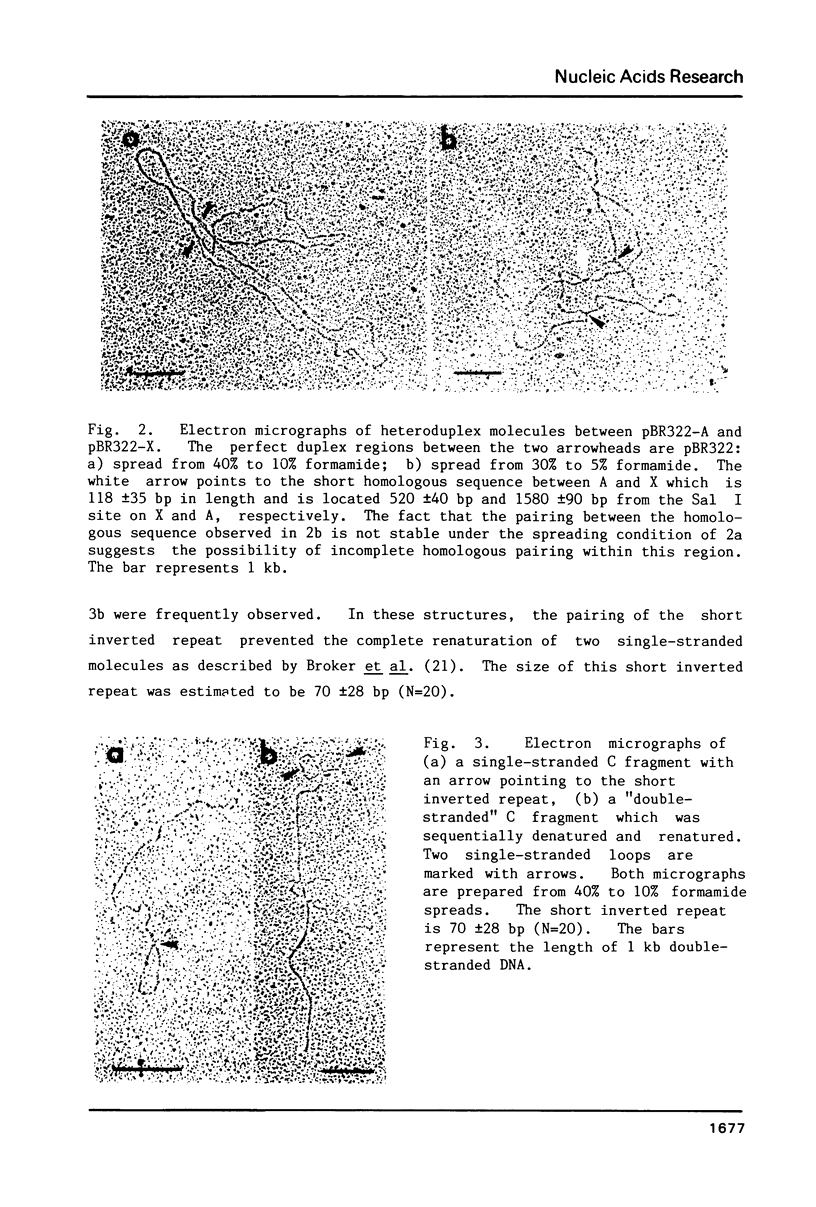
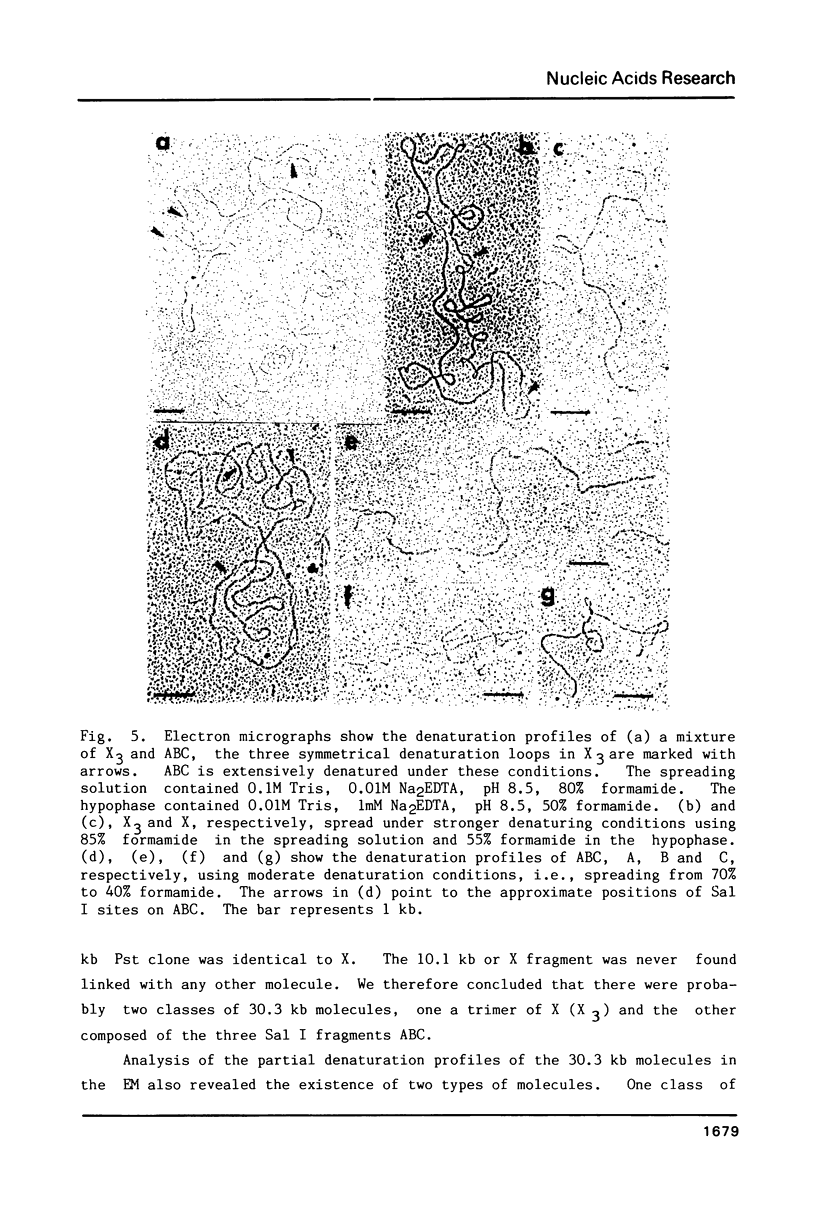
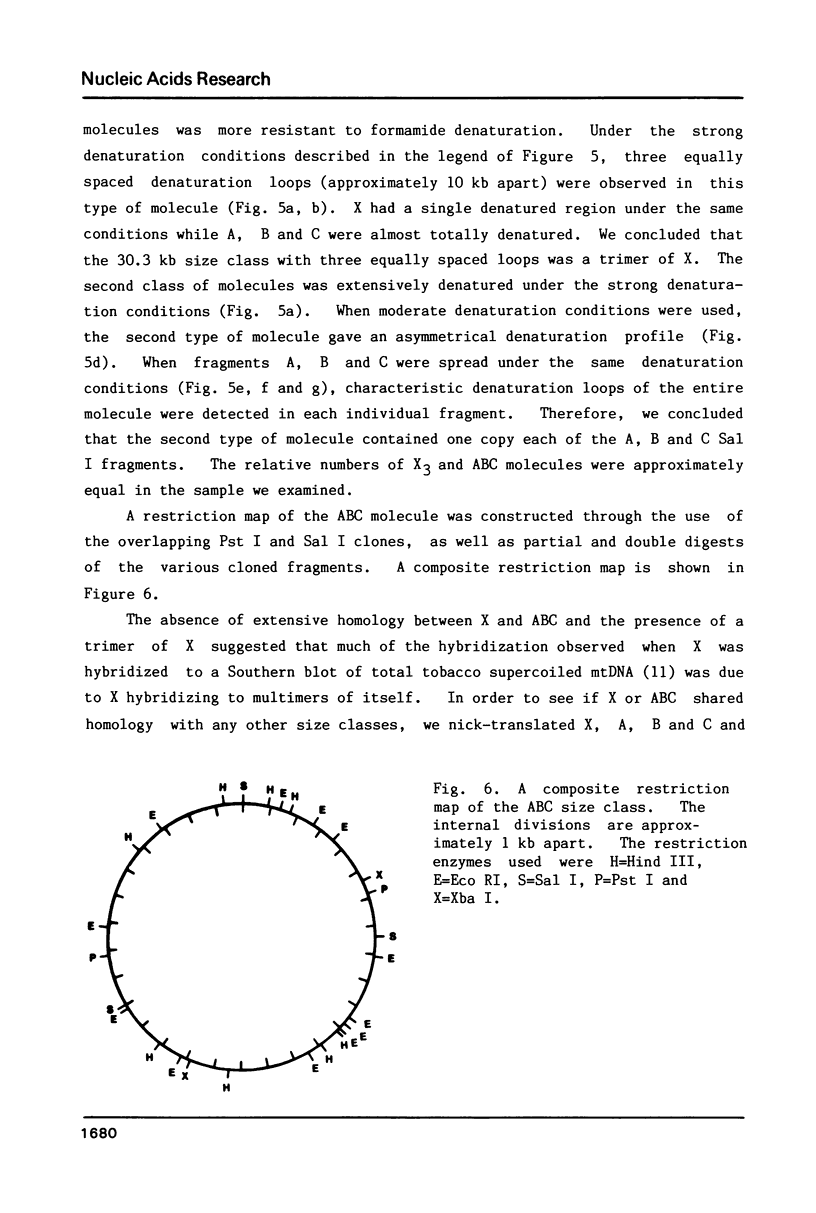
Supercoiled mtDNAs were isolated from tobacco suspension culture cells and three of the smallest size classes (10.1, 20.2 and 30.3 kb) were characterized through denaturation, heteroduplex and restriction mapping. The 20.2 molecule was found to be a head-to-tail dimer of the 10.1 or X size class, while the 30.3 kb size class was found to contain two kinds of molecules, a head-to-tail trimer of X (X3) and a second molecule, ABC. X and ABC had a 118 +/- 35 bp region of homology, and both size classes shared a degree of homology with at least one other size class. Restriction maps of both the X and ABC molecules are presented and the possible origin and role of the many plant mtDNA size classes are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Ojala D. Mitochondrial ribssome in HeLa cells. Nat New Biol. 1971 Feb 3;229(5):133–136. doi: 10.1038/newbio229133a0. [DOI] [PubMed] [Google Scholar]
- Bonen L., Gray M. W. Organization and expression of the mitochondrial genome of plants I. The genes for wheat mitochondrial ribosomal and transfer RNA: evidence for an unusual arrangement. Nucleic Acids Res. 1980 Jan 25;8(2):319–335. doi: 10.1093/nar/8.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Soll L., Chow L. T. Underwound loops in self-renatured DNA can be diagnostic of inverted duplications and translocated sequences. J Mol Biol. 1977 Jul 15;113(4):579–589. doi: 10.1016/0022-2836(77)90223-6. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Duesing J. H., Keene D. Supercoiled mitochondrial DNAs from plant tissue culture cells. Nucleic Acids Res. 1981 Sep 25;9(18):4583–4593. doi: 10.1093/nar/9.18.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M. Sequence homology among different size classes of plant mtDNAs. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4453–4457. doi: 10.1073/pnas.78.7.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- Greenfield L., Simpson L., Kaplan D. Conversion of closed circular DNA molecules to single-nicked molecules by digestion with DNAase I in the presence of ethidium bromide. Biochim Biophys Acta. 1975 Oct 15;407(3):365–375. doi: 10.1016/0005-2787(75)90104-5. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Pring D. R., Thornbury D. W. Molecular weights of maize mitochondrial and cytoplasmic ribosomal RNAs under denaturing conditions. Biochim Biophys Acta. 1975 Mar 10;383(2):140–146. doi: 10.1016/0005-2787(75)90255-5. [DOI] [PubMed] [Google Scholar]
- Sederoff R. R., Levings C. S., Timothy D. H., Hu W. W. Evolution of DNA sequence organization in mitochondrial genomes of Zea. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5953–5957. doi: 10.1073/pnas.78.10.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. F., Bonen L., Gray M. W. Primary sequence of wheat mitochondrial 5S ribosomal ribonucleic acid: functional and evolutionary implications. Biochemistry. 1981 Jul 7;20(14):4022–4029. doi: 10.1021/bi00517a011. [DOI] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Ward B. L., Anderson R. S., Bendich A. J. The mitochondrial genome is large and variable in a family of plants (cucurbitaceae). Cell. 1981 Sep;25(3):793–803. doi: 10.1016/0092-8674(81)90187-2. [DOI] [PubMed] [Google Scholar]