Abstract
Background
Not long after the Big Bang, iron began to play a central role in the Universe and soon became mired in the tangle of biochemistry that is the prima essentia of life. Since life’s addiction to iron transcends the oxygenation of the Earth’s atmosphere, living things must be protected from the potentially dangerous mix of iron and oxygen. The human being possesses grams of this potentially toxic transition metal, which is shuttling through his oxygen-rich humor. Since long before the birth of modern medicine, the blood—vibrant red from a massive abundance of hemoglobin iron—has been a focus for health experts.
Scope of Review
We describe the current understanding of iron metabolism, highlight the many important discoveries that accreted this knowledge, and describe the perils of dysfunctional iron handling.
General Significance
Isaac Newton famously penned, “If I have seen further than others, it is by standing upon the shoulders of giants”. We hope that this review will inspire future scientists to develop intellectual pursuits by understanding the research and ideas from many remarkable thinkers of the past.
Major Conclusions
The history of iron research is a long, rich story with early beginnings, and is far from being finished.
Introduction
This review is divided into four major sections. In the first section we discuss current views of iron metabolism followed by the history of iron and its homeostasis. A third section will present a brief history of the discovery and properties of human serum transferrin and the final major section will describe the pathophysiogy of iron metabolism.
1. Current Understanding of Iron Metabolism
1.1. Overview of Mammalian Iron Metabolism
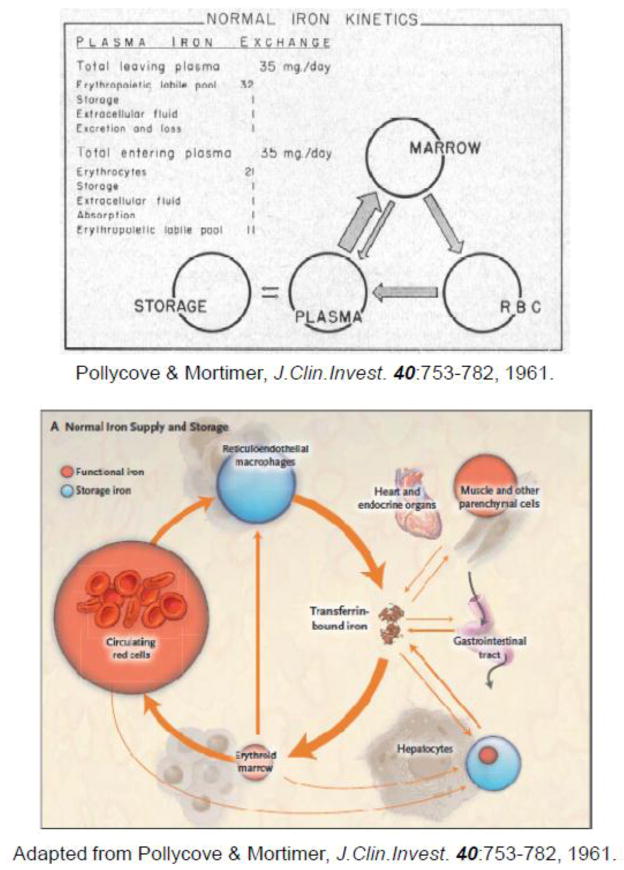
Figure 1 illustrates the iron cycle in humans and provides the approximate size of each iron pool. For historical interest, we have included an original version of the cycle, as determined by early ferrokinetic studies by Pollycove and Mortimer in 1961 [1] (Fig. 1A) in addition to a more recent adaptation of the former (Fig. 1B). Dietary iron enters the body primarily through duodenal enterocytes. As for the uptake of inorganic iron, these polarized cells express both DMT1 and the cytochrome b ferrireductase, Dcytb, on their apical membrane (the functions of DMT1 and Dcytb are defined later). Heme uptake, while constituting a significant iron source for many mammals, is poorly understood. It was proposed that enterocytes’ acquisition of heme requires the activity of the SLC46A1 (a.k.a., heme carrier protein 1) transporter [2]. However, Goldman and co-workers subsequently demonstrated [3] that a loss-of-function mutation in the SLC46A gene is the molecular basis for hereditary folate malabsorption. Additionally, the Km is approximately 100-fold lower for folate than heme, so the search for a high affinity intestinal heme transporter should continue. Although the path of iron through the enterocyte has not been completely elucidated, export of the metal from these cells occurs via the basolateral transporter, ferroportin, which transports ferrous iron. This export pathway is presumably driven by the oxidation of iron at the basolateral, exosolic leaflet by hephaestin. As will be discussed later, this seemingly membrane-bound multicopper oxidase exhibits considerable homology to the plasma protein ceruloplasmin (Cp).
Figure 1. Mammalian iron cycle.
(A) Scheme depicting quantitative aspects of normal iron kinetics based on measurements of an 59Fe disappearance curve, 59Fe incorporation into erythrocytes and its surface counting (reproduced from Pollycove and Mortimer [1], with permission). As discussed in section 1.2, it is unlikely that the “Erythropoietic labile pool” represents iron released from the interior of bone marrow erythroblasts. Rather, this pool probably represents iron derived from hemoglobin degraded in bone marrow macrophages as a result of normal ineffective erythropoiesis [408] and hemoglobin released during the enucleation of orthochromatic erythroblasts [409]. Ferrokinetic studies yield higher values for ineffective erythropoiesis (i.e., “erythropoietic labile pool”) than studies based on measurement of the incorporation of [15N]ALA into early labelled bilirubin; hence, it cannot be excluded that ferrokinetic studies (that include inaccurate radioactivity measurements over the body surface) produce inflated values. The Polycove-Mortimer scheme, published in 1961, is being reproduced or reprinted after minor modifications ever since: (B) shows one of the current versions [395].
The Fe3+ is then available to bind to free sites on plasma transferrin (Tf), which the body normally maintains at one-third saturation. In this context, and the historical standpoint, we feel compelled to emphasize the seminal discovery by Chaim Hershko and his co-workers [4] that patients with severe iron overload, such as those with-thalassemia major, whose Tf is fully saturated, contain a fraction of iron in their plasma that is not associated with Tf (non-transferrin bound iron; NTBI). They proposed that NTBI “… might be of relevance to the pathogenesis of tissue damage and the protective effect of iron chelating therapy in disease.” These are concepts that are now generally accepted. While dietary iron absorption is crucial to replace iron losses, maintaining the body in a homeostatic “zero balance”, it amounts to about one milligram per day—a small fraction of what is used daily (approximately 30 mg) to form iron-containing proteins. The remainder of the iron that enters the circulation is derived, for the most part, from reticuloendothelial macrophages turning over iron from senescent erythrocytes. At equilibrium, these cells release the approximately 30 mg of iron each day that is used to produce hemoglobin for about 200 billion erythrocytes. Thus, the pool of circulating Tf-iron, amounting to less than 3 mg, is the most dynamic iron compartment, turning over about ten times per day. Excess iron is stored in reticuloendotheial macrophages and hepatocytes in the form of ferritin. Ferritin is a ubiquitous, cytoslic iron storage protein, comprising 24 subunits of ferritin, heavy (H) and light (L) chains. The composition of ferritin (i.e., the ratio of L to H chains) varies between different tissues. H-ferritin contains ferroxidase activity responsible for efficient oxidation of Fe2+ for mineralization of iron within the protein, while L-ferritin is believed to promote efficient nucleation and mineralization of the iron. For a comprehensive review of the ferritins see [5], [6], and [7].
As Tf saturation is normally ~30%, any iron entering the circulation of healthy individuals will bind to Tf, making it the only iron source available to most cells. The exceptions to this are erythrophagocytes, enterocytes, and possibly cells of the brain where the cerebrospinal fluid Tf pool is generally fully saturated.
1.2. Cellular Iron Acquisition from Transferrin
Though binding to Tf overcomes the toxicity and (in)solubility constraints that elemental iron poses to the organism, the extraction of this iron into a bioavailable moiety is not a trivial undertaking for the cell. Not only must the cell release iron from its tightly bound association with Tf (vide infra), but, once unprotected by Tf, the metal must also be delivered to its site of use without generating harmful free radicals. For most of the bioavailable or active iron in a cell, this location of functional assimilation is the mitochondrion, the setting for both heme and iron-sulfur cluster (Fe/S) biogenesis.
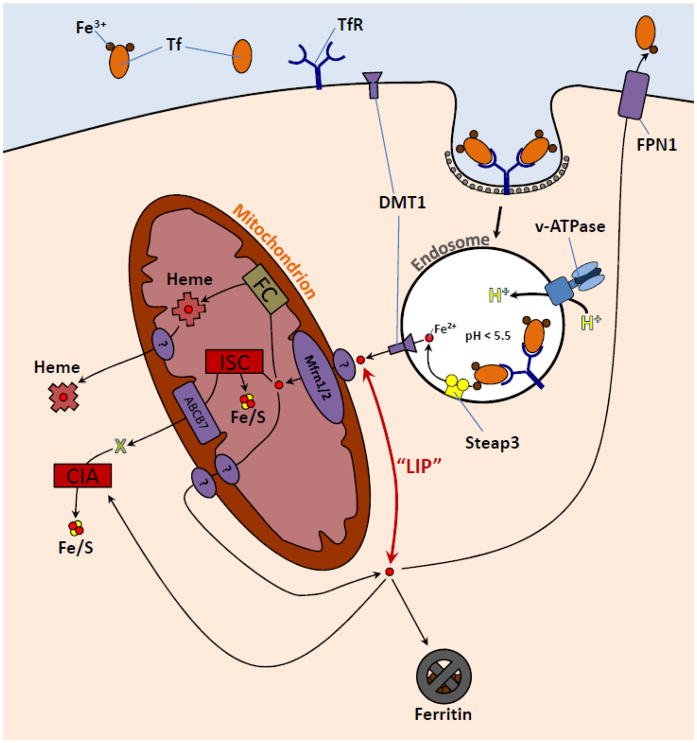
The passage of diferric Tf (Fe2Tf) into the cell is mediated by the Tf receptor 1 (TfR), which is presented to the plasma as a dimer on the cell surface, each monomer binding one holo-Tf molecule (Fig. 2). Receptor-mediated endocytosis is a complex process that involves coordination of a variety of proteins whose functions include targeting, signalling, docking, and movement (for review, see Mukherjee et al. [8], Maxfield and McGraw [9], and Grant and Donaldson [10]). In most cells a range of ligands are internalized through receptor-mediated endocytosis, though the handling of these materials ultimately varies depending on the nature of the ligand’s usage by the cell. The general pathway begins with the recruitment of clathrin through signalling by the cytoplasmic domains of the surface receptors to form a pit. After this pit is internalized to produce a clathrin-coated vesicle, the organelle loses its clathrin and combines with other new or already formed endosomes to form what is referred to as a sorting endosome. Following its presence in the sorting endosome, Tf has been observed in the endosomal recycling compartment (ERC), a collection of tubular structures associated with microtubules [9]. It is unclear, at this point, where iron is released from Tf, however it is generally believed that the iron-free Tf-TfR complexes are recycled from the ERC to the plasma membrane for reuse. It must also be noted that an additional TfR, TfR2, has been identified [11]. TfR2 seems to be a part of a complex system involved in monitoring organismal iron levels and implicated in the regulation of the expression of hepcidin, a master regulator of iron homeostasis in the body [12].
Figure 2. Cellular iron metabolism.
Most cells acquire iron by transferrin receptor (TfR)-mediated endocytosis of diferric transferrin (Tf) followed by the internalization of Tf-TfR complexes, eventuating in an acidified compartment whose pH promotes the release of iron from Tf. The iron is reduced by Steap3 and then released from Tf and then endosome through DMT1. The immediate fate of this released iron is unknown up to the point where it is transported across the inner mitochondrial membrane by Mfrn1 or Mfrn2. In mitochondria, iron is assembled into heme and iron sulfur clusters (Fe/S). Some of the heme is exported though an unknown mechanism for use by ER, peroxisomal, cytosolic, or nuclear hemoproteins. A not yet characterized intermediate (or possibly Fe/S cluster product) of the Fe/S biogenesis pathway (X) is exported through ABCB7 for the formation of extramitochondrial Fe/S cluster proteins. Iron outside of any subcellular compartment is comprised by the labile iron pool (“LIP”). Excess iron is stored in the ubiquitous iron storage protein, ferritin. Some cells may also export iron, also presumably from the LIP, by ferroportin (FPN).
Through endocytosis, the Fe2Tf eventually arrives at an endosomal compartment whose pH is decreased by the activity of the v-ATPase pump. This association of Tf with the TfR further facilitates the release of iron from Tf under this low pH (<5.6) [13] and, at this pH, the association of apoTf with the TfR remains very strong [14]. Also present is a member of the Steap family of ferrireductases [15,16]. Once reduced and released within the endosome, ferrous iron is available for export out of the endosomal compartment. The exit of iron from this organelle is mediated by the divalent metal transporter 1 (DMT1; a.k.a., Nramp2), utilizing the proton gradient as a driving force [17].
Following its egress from endosomes via DMT1, iron is transported to intracellular sites of use and/or storage in ferritin, but, in spite of approximately fifty years of research, this very important aspect of iron metabolism remains elusive. Different experimental strategies generated several different hypotheses and concepts. In the early sixties of the last century, Pollycove and co-workers [1,18] were the first to put forth a concept of an erythropoietic labile iron pool (LIP) in animals. They measured the two week plasma radioiron disappearance curve and the incorporation into red cells, using surface counting to follow the in vivo movement of the isotope. Unfortunately, these studies were unable to reveal whether or not radioiron returning back to plasma came from the interior of erythroblasts. Additionally, Noyes et al. [19] in subsequent, though similar, experiments found that approximately 90% of injected radioiron could be found in heme in biopsied marrow within an hour. Other researchers [20–23], mainly using cell fractionation and protein purification techniques, appeared to identify different Fe intermediates in hemoglobin (Hb)-producing tissues. Further characterization of these iron moieties was limited, and more recent investigations have demonstrated that when radioiron is supplied to erythroid tissues, virtually no intracellular radioactivity is present in a non-Tf, non-heme form [24,25]. Also in immature red cells, a transient interaction between endosomes and mitochondria has been demonstrated to result in an increase in chelatable iron within mitochondria (sites of iron utilization), suggesting that no appreciable cytosolic LIP is delivering iron to mitochondria for hemoglobin synthesis [26]. It is important to note that the pathway of iron from the cell surface to its intracellular targets in Hb-synthesizing cells may be distinct from that in non-erythroid cells [27]. Work by Jacobs in the late 1970’s in several cell lines demonstrated that a low molecular-weight, cytosolic intermediate may be present in non-erythroid cells [28,29]. However, Jacobs was unable to determine the nature of this intermediate and now, twenty-five years later, the chemical composition of the LIP still remains unresolved.
Another strategy used to assess iron levels in the LIP exploits iron-quenchable fluorescent probes [30,31]. However, all such probes are iron chelators and, therefore, can be expected to strip loosely bound iron from membrane-associated carriers; hence, this method would overestimate the levels of iron in the cytosolic LIP. Additionally, this approach neither determines the chemical nature of this entity nor the direction(s) of intracellular iron trafficking. Needless to say, the question of whither iron is delivered by DMT1 must be answered in order to fully understand the regulation of the intracellular Tf cycle.
Regardless of the nature of the LIP, it appears that the intracellular, bioavailable iron that is subject to chelation is that which is also sensed by the cellular iron regulatory proteins 1 and 2 (IRP1, IRP2). These are well-characterized mRNA-binding molecules that control the expression of molecules involved in iron uptake, utilization, and storage. Systemic regulation of iron absorption and cellular uptake is possible through both transcriptional and post-transcriptional mechanisms [32–34]. IRP1 and IRP2 are important components of post-transcriptional regulation. The IRPs bind to iron responsive elements (IREs), which are motifs present in the 3′- or 5′-untranslated regions (UTR) of mRNAs encoding proteins involved in the metabolism of iron. IRP1 is only active as an mRNA-binding protein when intracellular iron levels are low, as it otherwise contains a [4Fe-4S] cluster that prevents its binding to IREs. Similarly, IRP2 levels are severely decreased in iron-replete cells as it is degraded under this condition by the proteasome. For additional details on the IRE-IRP system we refer readers to section 2.2.4.1 below and Chapter X (R. Crichton) in this special edition.
1.3. Iron export from cells to transferrin
1.3.1. General comments
It is unknown whether all cells in the body can release iron. Because non-heme iron concentrations in erythrocytes are ~ 40,000-fold lower than those of heme iron [35,36], it is unlikely that mature red blood cells could release any iron into the circulation. Early studies [37–39] showed that radioactive iron taken up by reticulocytes in the presence of isoniazid, an inhibitor of heme synthesis, was released from the cells after they were reincubated with the chelator pyridoxal isonitotinoyl hydrazone or related substances. However, in the absence of chelators, negligible amounts of 59Fe were released from reticulocytes, containing high 59Fe radioactivity in their non-heme iron pool. Somewhat surprisingly, erythroid cells at earlier stages were shown to express ferroportin [40,41]. In this context it needs to be pointed out that Kailis and Morgan [42] examined radioiron release from bone marrow cells after incubation with 59Fe-Tf. Some of the 59Fe taken up by the cells could be released when the cells were washed and re-incubated with unlabeled diferric Tf. These results strongly suggested that the iron was released from the endosomes when they returned to the cell surface. Hence, radioiron had not passed through the endosomal membrane and its release would not require the activity of ferroportin. This could be, in part, the basis of the “erythropoietic labile iron pool”. It seems counterintuitive that developing erythroid cells would possess the capacity to purge themselves of iron, given their need to support such an astonishingly high rate of heme synthesis.
Importantly, there are specialized mammalian cells that are “professional” exporters of iron. Absorption of dietary iron for transfer to Tf in plasma requires iron efflux across the basolateral surface of the intestinal epithelia. A second major site of iron release is from macrophages where senescent or damaged red cells are degraded to export the metal from hemoglobin and provide it for binding to Tf. The third important cell type in this category is the hepatocyte, which stores iron in both healthy individuals and those with secondary iron overload; NTBI is likely to be a major contributor to hepatocyte iron loading under conditions of elevated Tf saturation. Iron is released from all cells, including the above mentioned, via ferroportin, which is under the control of circulating hepcidin levels (vide infra). Moreover, iron delivery from the circulation to the brain, placenta, and testis requires transport into the endothelial cells (representing various types of the blood-tissue barriers) as well as release of iron back to the peripheral circulation (reviewed in [43,44]).
Our previously vague knowledge of iron release from “donor cells” (primarily enterocytes and macrophages) was dramatically expanded about ten years ago, following a number of studies that provide new clues in this important area of iron metabolism. As discussed earlier, iron is exported from cells via ferroportin and the ferroxidase activity of hephaestin and ceruloplasmin facilitate the movement of iron across the membranes of enterocytes as well as macrophages and hepatocytes, respectively. Since the process of iron uptake by, and release from, duodenal enterocytes was sufficiently discussed above, in the following text we shall focus on the recycling of hemoglobin iron by macrophages.
1.3.2. The macrophage: A biological iron recycling plant
Macrophages are known as fundamental components of the innate immune system. However, in a much less heralded, but equally vital role macrophages are responsible for recycling hemoglobin iron acquired from the phagocytosis of senescent erythrocytes. This fact does not seem to have penetrated the territory controlled by “macrophagoligists” with immunological backgrounds. In contrast, the “iron people” are well aware that, at the end of its life, the erythrocyte is phagocytosed by macrophages of the reticuloendothelial system (i.e., splenic macrophages, Kupffer cells of the liver and bone marrow macrophages), and iron is liberated from the vice-like grip of the protoporphyrin ring by heme oxygenase-1 (HO-1). However, before going into the details of this process, we feel compelled to go back to history as mandated by the title of this review. Some readers of this treatise may be surprised, as we were, that an article entitled “Destruction of the red blood corpuscle in health and disease”, published in Physiological Reviews in 1923 [45], contains the following text:
THE EVIDENCE FOR A NORMAL DESTRUCTION OF BLOOD. So subtly is normal blood destruction conducted and the remains of the cells disposed of that were it not for indirect evidence one might suppose the life of most red corpuscles to endure with that of the body. But this evidence is convincing. The continuous activity of a broadly distributed hematopoietic tissue; the daily excretion through the bile of a pigment nearly if not precisely identical with one of the pigmented derivatives of hemoglobin; the appearance of this derivative in old hematomas and in the plasma after hemoglobin injection and liver exclusion; the apparently significant association of hemoglobin and bilirubin throughout the animal kingdom … there is no doubt that by some hook or crook of function or morphology the spleen often serves as a sort of midden for damaged erythrocytes.
This major landmark in the history of this subject of the time, written in a remarkably enlivened manner, was penned by Francis Peyton Rous who was, in 1966, awarded a Nobel Prize in Physiology or Medicine for his discovery, published in 1911, of the role of viruses in the transmission of certain types of cancer [46].
On another, somewhat related note, we wish to make a comment on heme oxygenase whose discovery is rightly attributed to Tenhunen et al. [47]. This enzyme, in an oxygen-dependent manner, attacks the α-methylene bridge of the heme macrocycle, generating biliverdin and releasing carbon monoxide (CO) and iron. However, this finding was preceded by Torgny Sjöstrand’s demonstration of endogenous formation of CO in man [48] and his discovery that this gas is generated as a result of hemoglobin catabolism [49] in the late forties and early fifties of the last century. However, in this context, we have to mention that CO was first detected in newborn children as early as 1901 [50].
Returning back to the current state of affairs, organelles containing engulfed senescent or pathological erythrocytes allegedly will merge with lysosomes, leading to the formation of erythrophagolysosomes. It is likely that here the globin moiety of hemoglobin is degraded, but the fate of heme, which has to undergo HO-1-mediated degradation, is obscure. Most of the comments on this phenomenon implicitly or explicitly indicate that heme is somehow moved towards the endoplasmic reticulum that allegedly harbors HO-1 [51,52]. However, no heme exporter from erythrophagolysosomes has ever been identified. There is an alternative possibility, i.e., that following erythrophagocytosis, HO-1, confined to vesicularized ER, is recruited by erythrophagolysosomes. This view is supported by recent observations demonstrating that both Nramp1 (vide infra) and DMT1 are involved in the export of hemoglobin-derived iron from macrophages [53–55]. One caveat of this model is that the catalytic domain of the ER-bound, insoluble HO-1 has been reported to be in the cytosol [56]. Further investigation is needed to not only add rigor to this latter assertion but also determine precisely where in the cell active HO-1 encounters its heme substrate.
The intra-macrophage fate of liberated iron destined for release by ferroportin is unknown. However, recent research yielded important new information about the regulation of ferroportin in hemoglobin iron recycling macrophages. Erythrophagocytosis was found to increases ferroportin expression in both macrophage-like cell lines [57] and bone-marrow derived macrophages [58]. Subsequently, Marro et al. [59] demonstated that heme, in the form of hemin or hemoglobin, activates ferroportin transcription in an iron-independent manner. This activation depends on the presence of a functional MARE (Maf recognition element) located 7 kb upstream of the ferroportin gene transcription start site. Inactivation of Bach1 (a transcription factor that belongs to the cap‘ri’collar type of basic region leucine zipper family) by siRNA strongly enhances ferroportin gene expression whereas its overexpression decreases ferroportin mRNA levels. Importantly, in the above experiments, ferroportin was shown to be transcriptionally co-regulated with HO-1 [59]. In our opinion, there is one caveat in this study since the transcription of ferroportin was activated not only by hemoglobin and heme, but also protoporphyrin, which exhibits totally different chemistry than heme and is not a product of HO-1 catalysis.
Moving to the whole organism, we would like to focus on a few important issues that are not adequately discussed in the current literature. After phagocytosis of erythrocytes by the reiculoendothelial macrophages, there is an initial processing period or lag phase, required for heme catabolism (approximately 1 h). Iron freed from hemoglobin is then either promptly returned to circulating Tf with a t1/2 of less than 35 min or is transferred to a more slowly exchanging pool (ferritin) within the macrophages, which releases its iron with a t1/2 of 7 days. These two distinct phases of release account for the entire exchange of iron between the reticuloendothelial macrophages and circulating plasma. Quantitatively similar data were obtained from studies on dogs [60] and humans [61]. Importantly, in iron deficient individuals the slow phase is totally absent and all the iron is released into circulation in the early phase with a t1/2 of 31 min [61] (reviewed by Hershko [62]).
One of our comments on the above process concerns the remarkable swiftness with which iron leaves macrophages during the early phase. This can probably be explained by a scenario in which hemoglobin-processing macrophages are “primed” to catabolize heme, i.e., their HO-1 is constantly turned on and does not require de novo induction of this enzyme. Additionally, the absence of hepcidin in iron deficient persons will likely lead to maximal levels of ferroportin expression, allowing a “waterfall” of iron to be poured into the circulation. In individuals with adequate iron levels, the expression of hepcidin could render ferroportin levels limiting for the amounts of iron generated in macrophages by HO-1. Hence, a fraction of iron, which fails to encounter ferroportin, will be diverted towards ferritin. However, the biphasic nature of the iron release process is more difficult to explain, if we assume that the quantity of iron released per time unit by both rapid and slow phases is equal to the quantity of iron delivered to hemoglobin per identical time unit. It is worth mentioning here that overexpression of ferroportin in cells in culture leads to a profound decrease of cellular ferritin levels [63]. Considering these data together, we must wonder what kinds of signals instruct ferritin to give up its iron, be it direct release from the protein or its degradation.
2. History of Iron and its Homeostasis
2.1. Preamble
The current cosmology tells us that the Universe was born around 13.7 billon years ago and entered its own “Iron Age” 200 million years after the Big Bang. Earth, which was formed together with the solar system some 9 billion years after the Big Bang, is estimated to be about 4.6 billion years old. Iron is the most abundant element in the planet Earth, forming much of Earth’s outer and inner core, and it is the fourth most abundant element in the Earth’s crust. This spinning iron at the center of the Earth generates a magnetosphere around the planet, protecting it from solar winds and radiation that would otherwise make the planet far less habitable. Life on earth started some 3.5 billion years ago when the atmosphere was virtually oxygen-free. Interestingly, this anaerobic atmosphere, together with a hyperthermic (near 100°C) and hyperbaric environment on Earth, was favorable for a cycle of chemical reactions that produce energy in a form that can be harnessed. Importantly, a key element in the development of life in these conditions was the transfer of electrons from mineral sources (notably ferrous iron, sulfide and hydrogen) to electron acceptors leading to the fixation of carbon oxides (CO and CO2) that both dissipated the redox gradient and simultaneously created organic compounds.
According to Günter Wächtershäuser [64,65], the principal proponent of the iron-sulfur world hypothesis, this early chemistry of life occurred on mineral surfaces, such as iron pyrites, near deep hydrothermal vents. This hypothesis postulates that an early form of metabolism predated genetics and that a primitive metabolic cycle could produce increasingly complex compounds. With all due respect to Wächtershäuser and his disciples, we cannot resist quoting RJP Williams’ prophetic remark, expressed in Nature, 1990, “Energy capture based on Fe/S compounds, now and perhaps before there was life, is as important as DNA in life’s history” [66]. Initially, soluble ferrous iron was plentiful and easily available for the first life forms, but this “ferrous paradise” began to slowly decrease approximately three billion years ago due to the insidious addition of oxygen into the atmosphere as a result of oxygen generation by photosensitizing cyanobacteria. Over the next ~1.5 billion years, this process led to 21% atmospheric oxygen content, dramatically affecting life on Earth. First, although oxygen is toxic to organisms that are not adapted to it, it can greatly increase the metabolic efficiency of oxygen-adapted organisms: anaerobic fermentation produces a net yield of two ATP molecules per molecule of glucose, while aerobic respiration produces a net yield of 36 ATP molecules. Second, the increased oceanic oxygen oxidized ferrous iron to virtually insoluble ferric iron. The gargantuan consequences of this event had an eternal impact on both the geology (“banded iron formations”) of our planet and the life on it: all aerobic organisms struggle to acquire, transfer and store this precious metal, iron.
2.2. From the Iron Age to the Golden Age of iron
2.2.1. Early premonitions
From the above period, an astronomically long time was needed for Homo sapiens to evolve—about 200,000 years ago. Relatively late in their sojourn on Earth, numerous civilizations entered into Iron Age (~1200–1000 BC). It is of great interest that early Iron Age civilizations in Assyria [67] and the Mediterranean area used iron for therapeutic purposes (readers can find an informative description of the early history of iron in medicine in: [68]). We can only fathom how wise men of the time conceived the idea of healing powers of iron since these men could not possibly be aware of the essentiality of iron for human bodies. Awareness of the biological importance of iron is much more recent, the metal having been discovered in the body by Lemmery and Geofgroy in 1713 [69]. A similar point can be made in regard to the declaration made by Nicholas Monarde, a 16th century physician of Seville, that iron was supposed to produce “great effectes and marvelous works” [70]. He used iron for a large number of unrelated ailments including gout, acne and alopecia [71].
2.2.2. Advent of science
The beginning of this era is inseparably wedded to Antoine Lavoisier (1743–1794), considered by many as the “father of modern chemistry”. He was a prominent chemist and leading scientist in the 18th century chemical revolution. He developed an experimentally based theory of the chemical reactivity of oxygen (he coined this term), iron’s intimate partner in innumerable biochemical processes. As will be discussed in more detail, the interaction of iron with oxygen can generate extremely toxic radical species, but organisms evolved a large series of shrewd mechanisms to prevent such toxicity, while using the reactivity of these two elements for their own benefits. Jean-Paul Marat, a leading creature in the Reign of Terror, despised Lavoisier since he publicly demeaned a ludicrous invention of Marat and prevented (together with Benjamin Franklin) his election to the French Academy. As a result, Antoine Lavoisier was executed by guillotine on May 8, 1794. Acknowledging Lavoisier’s scientific grandeur, Joseph-Louis Lagrange, a mathematician and astronomer, expressed his grief by the following adage: “Cela leur a pris seulement un instant pour lui couper la tête, mais la France pourrait ne pas en produire une autre pareille en un siècle.” (“It took them only an instant to cut off his head, but France may not produce another such head in a century.”)
As will be discussed, most iron in vertebrates is present in red blood cells (RBC). Hence, it is pertinent to note that the first observation of “globules” in blood was published in April 1674 in the Philosophical Transactions of the Royal Society of London where Antoni van Leeuwenhoek (1632–1723) recorded his description of human RBC: “I Have divers times endeavoured to see and to know, what parts the Blood consists of; and at length I have observed, taking some Blood out of my own hand, that it consists of small round globuls driven thorough a Crystalline humidity or water.” Hemoglobin, which accounts for over 90% of the dry weight of the erythrocyte, was discovered by FL Hünefeld in 1840 at Leipzig University. In 1851, Otto Funke (1828–1879) published a series of articles in which he described growing hemoglobin crystals by successively diluting red blood cells with a solvent such as pure water, alcohol or ether, followed by slow evaporation of the solvent from the resulting protein solution. Hemoglobin’s reversible oxygenation was described a few years later by Felix Hoppe-Seyler. Of note, Hoppe-Seyler founded (1877) and edited the first biochemical journal, Zeitschrift für Physiologische Chemie, which continues as Biological Chemistry.
Vincenzo Menghini (1704–1759) was the first to ascertain that iron in the blood is concentrated in the red corpuscles [72]. Although this discovery predated that of hemoglobin, we deem it more logical to first determine what makes blood red before understanding what comprises this red material. This discovery was communicated to the Academy of Sciences of the Bologna Institute on April 21, 1746. He and his co-workers, Ercole Lelli and Giandomenico Campedelli, examined samples of blood from mammals, birds, fish and humans. After they had separated the various components and observed them under the microscope, they “dried them”, burned them and looked for iron using a simple knife with a magnetic blade. Menghini’s words are revealing: “And thus I finally discovered that red globules themselves are the seat of iron, which I had been searching for high and low with wearisome and daily fatigue in other parts of animals”. In this context we have to mention the work of Louis René Lecanu (1800–1871), who studied the chemistry of the blood in the 1830’s. He determined that RBC contain “globuline” and red coloring matter, “hematosine”, that contained iron [73,74].
With regard to the clinical aspects of the history of iron, we must mention chlorosis (from the Greek, “χλωρις”, meaning “greenish-yellow”), also known as “green sickness” or “virgin’s disease” (morbus virgineus), that was a curse of adolescent females until approximately the twenties of the last century [75]. In addition to a pale complexion, symptoms included headaches, heart palpitations, loss of appetite, indigestion, exhaustion and “neurasthenia”. Although English physician Thomas Sydenham, in the second half of the seventeenth century, classified chlorosis as a “hysterical disease”, he advocated iron as a treatment. Neither Sydenham nor Blaud, who treated chlorosis with pills containing ferrous sulfate and potassium carbonate 150 years later [76], provided any rationale for this therapy. Pierre Blaud wrote “the treatment is ferruginous preparations, modifiers of the organism, which return to the blood the exciting principle which it has lost, that is to say the coloring substance”.
Although it is now generally accepted that the symptoms of chlorosis were caused by iron deficiency [71], the disappearance of this disease is somewhat mysterious. “Very little of the decrease in chlorosis can be attributed to direct medical intervention,” writes a medical historian Robert Hudson [77]. If the demise of chlorosis is not the self-conscious accomplishment of modern medicine, how then did it occur? Hudson claims that certain changes in the day-to-day life of Americans in the first few decades of the twentieth century had consequences for the future of chlorosis, specifically, a general improvement in nutrition, diminishing prejudice against the eating of meat, the abandonment of corsets or “tight lacing,” and a related increase in physical activity among women. The term “chlorosis” cannot be found in the current textbooks of hematology. So, why does Google return 906,000 hits using this term? Chlorosis is a plant disease in which normally green tissue is pale, yellow, or bleached. One of the factors causing it is iron deficiency.
Too much of a good thing may, however, be exceedingly dangerous. It was Henry John Horstman Fenton who, shortly before the end of the nineteen century, laid the grounds for the concept that under certain conditions iron can generate toxic species [78,79]. He demonstrated iron-salt-dependent decomposition of dihydrogen peroxide that leads to the generation of the highly reactive hydroxyl radical (OH·):
The attitude of biomedical scientists to the above “Fenton reaction” was initially lukewarm. However, the discovery of superoxide dismutase [80], which catalyzes the dismutation of superoxide into oxygen and dihydrogen peroxide, revolutionized the study of oxygen free-radicals in biochemistry (e.g., [81–84]).
The previous century witnessed several crucial advances in both basic and clinical aspects of iron homeostasis. The presence of iron in plasma was first established in the early twenties of the last century. Until then, the small quantities of iron demonstrated in serum was believed to originate from hemoglobin or to have been caused by slight hemolysis. In 1925, Fontès and Thivolle determined the serum iron concentration in horses and found that it is decreased in cases of iron deficiency [85]. In 1945, Holmberg and Laurell discovered the plasma iron-binding protein, Tf [86]. In 1944, Schade and Caroline [87] showed that raw egg white inhibited the growth of several bacteria and that iron could overcome this inhibition. The egg white protein was subsequently found to be identical with conalbumin [88]. Schade and Caroline [89] then demonstrated that human plasma also contained an iron-binding protein with antibacterial properties. This protein was later named siderophilin. By 1949, it became obvious that Tf and siderophilin were identical proteins [90], but the term “siderophilin” did not survive.
2.2.3. Classical Era
According to our nolens volens subjective classification, this period started when two cardinal protagonists of iron metabolism were identified and to some degree characterized: the above discussed Tf and the intracellular iron-storage protein, ferritin which was crystallized by the Czech Physiologist, Vilém Laufberger in 1937 [91]. The most important characteristic of this period was a major leap in methodology based on the use of radioactive iron that was exploited initially in studies of organismal iron homeostasis and, with a considerable delay, in investigations devoted to the understanding of the mechanisms of cellular iron acquisition and its regulation. We should be endlessly grateful for this marvelous implement, 59Fe, to John Livingood, Fred Fairbrother, and Glenn Seaborg who created it in 1937, using one of Lawrence’s advanced cyclotrons (reviewed in [92]). Of interest, Glenn Theodore Seaborg and Edwin Mattison MacMillan shared the Nobel Prize for Chemistry in 1951.
One of the milestone studies of the Classical era that revealed crucial principles of organismal iron homeostasis was not based on the use of radioactive iron. In 1938, McCance and Widdowson [93] demonstrated that iron is not excreted by the body. This study has been fully confirmed by subsequent assessments of iron balance using 59Fe or 55Fe, which have definitively demonstrated that losses of iron from the body are minuscule [94–96]. Collectively, these studies revealed that the regulation of iron absorption serves as the principal mechanism maintaining a normal quantity of iron in the organism. Studies conducted during this period could not reveal mechanisms involved in iron absorption, since the players involved in this process would emerge only sixty or more years later.
A number of intriguing ideas and concepts about the regulation of iron absorption came out from these early studies. One of them is a hypothesis published in 1943 by Hahn et al., known as the “mucosal block mechanism”, that was proposed to regulate iron absorption [97]. The authors of this study observed that “Ordinary doses of iron given 1 to 6 hours before radio-iron will cause some “mucosa block” - that is an intake of radio-iron less than anticipated” and concluded that the first dose of iron had “blocked” the absorption of iron from the second dose. They seemed to believe that this mechanism operated by virtue of the fact that mucosal cells became saturated with ferritin after the first dose of iron was provided. This study and its interpretation was repeatedly criticized by Ernest Beutler who pointed out that the study did not have appropriate controls and was performed only on three animals (dogs) (e.g., [71]). In addition to Beutler’s reservations, we feel the major problem with this study was that it was inappropriately designed. As they indicate in Table 3 of their paper, Hahn et al. measured “Radio-iron absorption”. However, what should ideally have been measured was the total iron (59Fe plus 56Fe) absorption. The previously administered dose of “cold” iron (56Fe) can be predicted to dilute the second dose consisting of “hot” iron (59Fe) inside enterocytes. Hence, less 59Fe will be transported into an organism even if the extent of iron absorption does not change. It is important to note that, despite the caveats associated with the study of Hahn et al., current literature still accepts this as a bona fide regulatory mechanism [98–100].
Some of the early studies of this era provide valuable information relevant to the regulation of iron absorption. Anemia, produced by bleeding or by hemolytic agents, was shown to greatly augment the absorption of iron [97,101]. Hypoxia [102], and iron depletion [103] also enhanced the absorption of standard doses of iron. On the other hand, transfusional polycythemia was shown to depress the absorption of iron, as did loading with parenteral iron [101–103]. For further information about early iron homeostasis studies readers are referred to an outstanding monograph published by Bothwell et al. in 1979 [104].
The “classical” period was also marked by efforts of numerous researchers to investigate iron homeostasis at the cellular level. Some milestone and seminal contributions deserve special attention. In 1949 Finch and coworkers [105] were the first investigators to demonstrate that reticulocytes (but not erythrocytes) take up iron from plasma and incorporate it into hemoglobin in vitro. Of historical interest, the second author on this study, Donnall Thomas, was awarded The Nobel Prize in Physiology or Medicine in 1990 for his discoveries concerning bone marrow transplantation. Ten years later, Bessis and Breton-Gorius [106] presented electron micrographs depicting erythroblastic islands in the bone marrow, in which a central reticulum cell (“nurse cell”) was surrounded by a ring of erythroblasts. In the region of contact between these cells the authors observed ferritin and proposed that it was transferred from the reticulum cell (macrophage) to the erythroblasts by a form of micropinocytosis termed “rhopheocytosis”. However, somewhat later, Jandl, Katz, and coworkers [107,108] showed that immature erythroid cells take up iron from Tf and suggested the existence of a membrane-bound TfR which may be involved in uptake; trypsinization of reticulocytes curtailed the subsequent uptake of 59Fe from 59Fe bound to plasma Tf [107]. The many studies and discussions that followed have led to a general consensus that Tf, not ferritin, is the source of iron for hemoglobin-synthesizing cells (reviewed in [27,109]). In 1958 Paoletti et al. [110] (Fr. title, “Absence de consommation de la sidèrophiline au cours de la synthèse de l’hémoglobine in vitro”) demonstrated that reticulocytes remove iron from Tf without catabolizing the protein. This concept was confirmed by Katz in his experiments showing that Tf disappears from the plasma compartment much more slowly than the rate at which iron is cleared [111].
Between 1969 and 1971, Evan Morgan and his co-workers [112,113] provided the first evidence for the internalization of Tf by cells. This discovery represents a “paradigm shift” not only for the field of iron metabolism but cell biology as a whole, because the Morgan-Appleton study provided the very first example of plasma protein internalization that is essential for the protein’s function. Morgan [114] then demonstrated that the inhibitors of intravesicular acidification decreased the release of iron from Tf within reticulocytes and Schulman and coworkers [14,115] showed that apoTf-receptor complexes remained stable at pH 5.0. In 1981 Morgan published an extensive review on Tf [116] that contained a scholarly discussion on the mechanisms of cellular iron acquisition from Tf. Some statements from his review are worth quoting: “Collectively, the above experiments show conclusively that transferrin is taken up by immature erythroid cells by endocytosis.” and “However, one difference stands out between transferrin endocytosis and receptor mediated endocytosis of other substances. Whereas transferrin is not degraded by the endocytotic process, the endocytosis of the other substances results in intracellular catabolism within secondary lysosomes as a result of fusion of the endocytotic vesicles with lysosomes.” Thus, virtually all aspects of the Tf cycle were reasonably well worked out before 1980.
However, around that time, the concept of Tf endocytosis and its importance for cellular iron uptake was still highly controversial. The concept of Tf internalization was not well received at various meetings, and the Morgan & Appleton Nature paper (1969) was seldom cited with at least some notable examples to the contrary as follows: [24,117–121]. Most of these studies contain experiments whose results have supported the idea of Tf internalization.
In this context we feel compelled to bring to the attention of readers an unfortunate development that came about at the beginning of the eighties of the last century. In 1983 two esteemed laboratories published a series of articles that rehashed the results of Morgan and Appleton, describing the now well-accepted Tf cycle [122–124]. As of April 2011, collectively, these papers have been cited almost 1,900-times, in contrast to 144 citations for the Morgan and Appleton paper. In fact, the work of the Morgan laboratory was not mentioned in the aforementioned often-cited studies.
Critically important to the field was the development of protocols to conduct cell binding studies utilizing radioisotopes 125I and 59Fe to label Tf. The double label allowed simultaneous determination of both binding and iron uptake [111] and was used in virtually all of the studies cited above. Equilibrium binding studies yield affinity constants and the number of TfR per cell. Kinetic studies allow estimates of the timing of the steps in the uptake and release process. In non-erythroid cells, the full cycle has been reported to take ~16 min, while iron removal occurs early in the cycle with estimates ranging from 2–6 min [123,125].
The general acceptance of the concept of Tf endocytosis was followed by an explosion of research that exploited Tf (primarily labeled by fluorescent dyes) to examine intracellular trafficking of endosomes. These studies revealed the movement of TfR-containing endosomes to various intracellular structures, such as Golgi complexes, endoplasmic reticulum and perinuclear structures (reviewed in [9,126,127]). Unfortunately, most of these studies do not consider the actual function of the Tf protein. As no function of the long known holo-Tf other than iron transport and delivery has ever been shown, it must be assumed that the movement of Tf-containing endosomes toward various intracellular structures is related to iron delivery to these organelles.
At the present time, virtually nothing is known about intracellular iron trafficking after iron is released from Tf and pumped out of endosomes by divalent metal transporter 1 (DMT1, originally known as “natural resistance-associated macrophage protein 2” [128]). The only solid evidence about some aspects of the intracellular iron movement comes from in vitro studies with erythroid cells (either primary cells or cell lines) showing that most of the Tf-borne iron ends up in hemoglobin and non erythroid cells that seem to sequester most of the acquired iron into ferritin. The latter finding raises concerns about the value of in vitro studies. It is counterintuitive that the purpose of the cellular iron-uptake system would be to simply transfer iron to storage. This brings us to the intracellular role of ferritin, which is generally regarded not only as an iron depository, but also a ready source of iron for the formation of heme and non-heme iron proteins. To the best of our knowledge, Mazur and Carleton [129] were the first to propose that “ferritin iron plays an active role as an intermediate between iron originating from the plasma and the heme synthesized by marrow and reticulocytes”. This concept received some support [130,131], but an overwhelming number of reports [22,23,132,133] failed to show that iron from ferritin could be used for hemoglobin synthesis. Additionally, murine erythroleukemia cells constitutively expressing ferritin exhibit significantly decreased iron incorporation into hemoglobin, suggesting that high levels of ferritin ‘steal’ iron destined for hemoglobin [134]. Importantly, the conditional deletion of ferritin heavy-chain in adult mice did not cause any decrease in hematocrit or hemoglobin levels [135]. The above observations are not compatible with the conclusion that ferritin is an intermediate in iron delivery to hemoglobin. It seems likely that the only way ferritin’s iron can be liberated is as a result of this protein’s degradation. However, it is unclear whether iron is released from ferritin prior to its degradation [136] or whether ferritin degradation is a downstream result of iron export out of the cell by ferroportin [137]. Iron can be readily released from isolated ferritin by small reductants (e.g., dithionite), assisted by Fe(II) chelators such as 2,2′-bipyridine, bathophenanthroline sulphonate or ferrozine [6]. However, as discussed above, there has only been evidence to the contrary that this happens in vivo.
This era was also marked by a notable development relevant to the TfR, which was identified and biochemically characterized in the late seventies and early eighties [14,138–140]. A common practice of that time was to use monoclonal antibodies to analyze (a) cell surface protein(s). Two studies identified a 95–100 kDa protein with a widespread distribution on various types of human tumor cells and normal proliferating cells [141,142]. This protein was not readily detectable on non-proliferating cells. In 1981, two groups of investigators, using two different monoclonal antibodies (OKT9: [143]; and B3/25: [144]), reported that these antibodies specifically bound to the above mentioned “proliferation associated antigen”. Their biochemical analyses revealed that this antigen was, in fact, the receptor for Tf. This finding represented a strong stimulus for research on TfR [145] and its role in cell proliferation. Again, virtually none of these studies considered the well-defined function of Tf; one paper [146] from this period reported that TfR forms a molecular complex with ras proteins and proposed that this interaction regulates cell proliferation. Shortly afterwards, Harford demonstrated that the apparent association of a ras gene product and the TfR is an artifact of the immunoprecipitation technique [147]. Collectively, the above studies were proposing that the Tf-receptor association is analogous to the interaction of growth factors with their receptors to promote cell proliferation. However, since the only clearly defined function of Tf is iron transport and delivery to cells, it may merely serve as an iron donor and not as a growth factor. This issue was resolved unequivocally by studies exploiting iron bound to acyl hydrazones such as salicylaldehyde isonicotinoyl hydrazone (Fe-SIH), which can efficiently supply iron to cells without using physiological TfR pathway [148]. These studies demonstrated that the inhibited cell growth, caused by blocking antibodies against TfR, could be rescued upon the addition of Fe-SIH. This chelate was also shown to deliver iron to cells in the presence of receptor-blocking antibodies [149].
In our opinion, the “classical” era ended abruptly in 1986 when the wise men and women of the American Society of Hematology terminated a scientific “Subcommittee on Nutritional Anemia” whose mandate included iron. We strongly feel that some aspects of this epoch should be revitalized, namely the use of radioactive iron to trace the movement of this metal inside cells. This strategy can never be substituted by the current approaches which are based on measurements of mRNA or protein levels or fluorescent dyes. There is some hope. A recent study [150] developed a novel in vivo method employing the radioisotope 55Fe to directly examine the incorporation of iron into complex I and other respiratory complexes. It is the first study that allowed visualization of radioactive iron association with proteins other than hemoglobin, Tf, or ferritin.
2.2.4. Golden Age
Similarly to the previous period, this era also started with the availability of new tools in the form of molecular biology technology. Once again, several developments during our “golden times” represent ramifications of seminal discoveries made in the past. A good example of this is the long saga of iron-mediated regulation of the expression of ferritin and TfR. In 1946, Granick wrote “When 10-mg. of ferrous iron were fed per day (to guinea pigs), a marked increase in the content of ferritin was noted all along the (gastrointestinal) tract” [151]. In 1955, Fineberg and Greenberg [152,153] reported that administration of iron to guinea pigs caused a rapid stimulation of ferritin protein synthesis in the liver as measured by the incorporation of [14C]leucine or [14C]glycine into isolated ferritin. Apparently, the first product was iron-poor apoferritin. In accordance with the above observations, ferritin synthesis in cells cultured in vitro was shown to be inhibited by iron chelators [154]. The results of Fineberg and Greenberg were confirmed by Drysdale and Munro [155] who, however, included an additional experimental variable to their experiments. If the animal was treated prior to iron injection with actinomycin D at levels adequate to suppress transcription, the response to iron administration was maintained. This crucial observation gave birth to the concept of the post-transcriptional regulation of ferritin expression by iron; a concept that was further elaborated in Munro’s laboratory. In 1976, Zahriger et al. [156] demonstrated that iron administration to actinomycin-treated animals caused a two-fold increase in the amount of ferritin mRNA in the polyribosomal fraction, while at the same time a cytoplasmic pool of inactive ferritin mRNA was diminished.
2.2.4.1. IRP/IRE consonance
Some ten years after it was established that ferritin expression was under the control of a post-transcriptional mechanism, Aziz and Munro [157] reported that the response of ferritin mRNA to iron was eliminated by deleting a part of the 5′ UTR containing a conserved 28-base sequence from ferritin mRNA. This observation suggested that iron-sensitive factor(s) in the cytoplasm may bind to this sequence and regulate the availability of ferritin mRNAs for translation. This effort was crowned by Leibold and Munro [158] who, in 1988, reported that this conserved sequence forms complexes with proteins in the cytoplasmic extracts of rat tissues and cells and that these complexes respond to iron treatment in parallel with the time of translational activation of H- and L-ferritin mRNAs. Today, the conserved mRNA sequences are known as iron-responsive elements (IREs) while the cytoplasmic proteins that interact with IREs have been named iron regulatory proteins (IRPs).
Early experiments examining TfR regulation revealed that iron excess inhibited its expression [159], whereas iron deprivation was associated with an increase in receptor levels [160]; the effect of chelators on TfR mRNA is even more pronounced [161]. Hence, responses of ferritin and TfR to changes in cellular iron levels, probably more precisely in a mysterious “labile iron pool”, are totally opposite.
Needless to say, a detailed molecular explanation of this regulation became feasible only when the TfR was cloned and sequenced [162,163]. It was initially proposed that the iron-mediated regulation of TfR occurred at the transcriptional level [164,165]. However, Owen and Kühn [161], in 1987, had provided compelling evidence that sequences within the 3′ UTRs are required for the iron-dependent feedback regulation of TfR expression, whereas the presence of the TfR promoter region is not necessary. Importantly, deletion of a 2.3 kb fragment within the 2.6 kb 3′ non-coding region of the TfR cDNA was shown to abolish the regulation by iron and to increase the constitutive level of receptor expression. This seminal work has revealed that the principal regulation of the expression of TfR was also post-transcriptional. It then took no time to document that the 3′ UTR of the TfR mRNA contains five IREs that show high similarity to the IRE present in the 5′ UTR of the ferritin mRNA [166]. Further research then confirmed predictions that IREs present at ferritin and TfR mRNAs bind an identical cytosolic protein, at that time termed the IRE-binding protein (IRE-BP) [167,168]. Presently, there are two IRPs known with moderately different structures conferring divergent molecular mechanisms of regulation, but similar overall function; their detailed discussion is beyond the scope of this review. Interested readers are referred to a recent, extensive review [33].
The physiological implications of this elegant regulatory system are truly remarkable: Excess iron results in an increase in the translation of ferritin mRNA and a decrease in the stability of TfR mRNA. These harmonized regulatory actions are mediated by IREs present within the 5′ UTR of the ferritin mRNA and the 3′ UTR of the TfR mRNA. The IREs from both transcripts interact with cytoplasmic proteins, IRP1 and IRP2. When cells contain high iron levels IRP1 assumes a conformation that does not allow its binding to IREs and IRP2 degradation becomes induced. The interaction between IRPs and the ferritin IRE results in attenuation of translation, and a similar interaction with TfR mRNA protects the transcript from rapid degradation mediated by a rapid turnover determinant within the 3′ UTR. Exactly the opposite scenario occurs when cells are iron-starved.
There are several other proteins that contain IREs in their messages and, therefore, seem to be regulated by the above described IRE/IRP mechanism [34]. An IRE in the 3′ UTR is also present in the mRNA for DMT1 (divalent metal transporter). An IRE in the 5′ UTR is present also in the mRNAs for ferroportin and erythroid-specific 5-aminolevulinic acid synthase (ALA-S2) [169,170]. Both DMT1 and ferroportin will be discussed later; here we shall consider only ALA-S2.
2.2.4.2. Why our blood is red …
The first step of heme synthesis occurs in the mitochondria and involves the condensation of succinyl CoA and glycine to form 5-aminolevulinic acid (ALA), catalyzed by ALA-S. There are two different genes for this first enzyme in the pathway. One of these is expressed ubiquitously (ALA-S1, or housekeeping ALA-S) and is encoded on chromosome 3 [171], while the expression of the other is specific to erythroid cells (ALA-S2, or erythroid ALA-S) and is encoded on the X chromosome [172]. These two genes are responsible for the occurrence of ubiquitous and erythroid-specific mRNAs for ALA-S and, consequently, two corresponding isoforms of the enzyme. In hepatocytes, heme feedback inhibits ALA-S1 biosynthesis. This is the principal factor that renders ALA-S1 the rate limiting and controlling step in heme synthesis in hepatocytes (and probably other non-erythroid cells). In contrast, neither the activity nor the production of ALA-S2 is inhibited by heme (reviewed in [27]).
There are several important implications of the 5′ UTR IRE on the ALA-S2 mRNA. First, since the translation of ALA-S2 mRNA depends on the availability of iron, in erythroid cells it is iron acquisition rather than ALA production which is the rate-limiting step in heme synthesis. Of historical interest, the hypothesis that a step in the pathway of iron from extracellular Tf to protoporphyrin IX limits the overall rate of heme synthesis in erythroid cells was proposed long before the discovery of the erythroid-specific ALA-S. This conclusion was based on several observations: 1) Reticulocytes with an artificially increased non-heme iron pool in mitochondria (following their pre-incubation with Fe2-Tf and a heme synthesis inhibitor) incorporate more [2-l4C]glycine into heme than untreated reticulocytes [173]. 2) Exogenously added ALA, which can be utilized for heme synthesis in reticulocytes, is unable to increase 59Fe incorporation (from 59Fe-Tf) into heme [174]. 3) Fe-SIH (vide supra), when added in relatively high concentrations, can stimulate heme synthesis in erythroid cells to above the levels seen in the presence of saturating concentrations of Fe2-Tf [148,175,176]. Collectively, these studies supported the concept of erythroid-specific regulation of iron metabolism and heme synthesis in erythroid cells [177–179]. Second, this regulatory mechanism guarantees a coupling of levels of protoporphyrin IX (which is toxic) with iron availability. At this moment we can only marvel about the evolution of the gene for erythroid-specific ALA-S and about the astonishing mechanisms that conveyed the IRE into its gene; questions of broad biological significance.
In summary, while the basic principles of the regulation of heme synthesis in the liver (and probably other non-erythroid cells) and erythroid cells are conceptually totally divergent, these differences are extremely well-tailored for the needs of the respective tissues. Compared to developing red blood cells, the liver produces heme with much lower rates, but in quantities that satisfy the requirements for the synthesis of hepatic hemoproteins. This is achieved by two major factors: the rate-limiting nature of ALA-S1 and the fact that the production of this enzyme is feedback inhibited by heme. In contrast, an extremely efficient mechanism involved in the targeting of Tf-derived iron towards ferrochelatase together with the lack of ALA-S2 inhibition by heme are key reasons that the synthesis of heme in erythroid cells is comparable to “breaking a dam”. This, at least in part, explains “… why our blood is red …”, a question John Donne (1572–1631) asked in his The Progresse of the Soule some four hundred years ago: “… Why grasse is greene, or why our blood is red, Are mysteries which none have reach’d unto.”
2.2.4.3. Tales of membrane transporters
The identification of membrane transporters for iron was anticipated for a long time and many researchers in the field were increasingly frustrated by a lack of evidence for iron transporters due to the unavailability of methods that could identify such entities. The discovery of the first ever membrane transporter for iron has a remarkable history that started at McGill University (Alma Mater for one of us [ADS] and an enduring supportive milieu for another [PP] for more than 30 years). Research conducted in the late 1980’s revealed that natural resistance to infection with intracellular parasites, such as Salmonella typhimurium and Leishmania donovani, was controlled, in mice, by a single locus on chromosome 1 called Ity and Lsh, respectively [180]. In 1981, Emil Skamene, Philippe Gros and their co-workers at McGill University added Mycobacterium bovis as another intracellular parasite to the above group of intracellular pathogens [181,182]. It took more than ten years for this same group of investigators to isolate the actual gene coding for the Ity/Lsh/Bcg locus and show that mouse strains sensitive to these parasites had a point mutation in this gene [183]. Since this gene was expressed only in professional phagocytic cells such as macrophages, it was named “natural macrophage-associated protein” (Nramp). In 1995, Gros and co-workers [128] identified and characterized the second mouse Nramp gene, named Nramp2, while the host resistance gene was denoted Nramp1. These investigators demonstrated that Nramp2 mRNA was expressed ubiquitously, but the function of Nramp2 protein remained unknown for several years.
Importantly, keeping with the historical aims of this tractate, it was discovered in 1996 that a point mutation in the Nramp2 gene was responsible for the severe hypochromic, microcytic anemia observed in mk/mk mice [184]. A parallel report in the same year revealed that Nramp2 (in the cited study named DCT1 [divalent-cation transporter]) was involved in ferrous iron uptake by duodenal enterocytes [17]. Research conducted at McGill by Gruenheid et al. revealed, in 1999 [185], that Nramp2 colocalized with the plasma iron carrier Tf in recycling endosomes, a finding which has led to the currently accepted conclusion that Nramp2 is also involved in the export of Tf-derived iron from endosomes in all cell types. The finding that Nramp2 was involved in iron transport suggested that Nramp1 could also be involved in the transport of divalent metals across the phagosomal membrane in macrophages. In fact, Gros and his colleagues provided experimental evidence for this, and have proposed [186] that Nramp1 promotes host resistance by efficiently depleting the phagosome of divalent metals, thus depriving intracellular pathogens of vital nutrients and inhibiting their growth. We cannot resist making a comment on an unfortunate nomenclature in this field. Shortly after the discovery of the Nramp2’s role in iron export from endosomes and iron import into duodenal enterocytes, the term Nramp2 was, somewhat fervently, changed to DMT1 (defined earlier). Should we, with the current evidence that Nramp1 is also involved in the membrane transport of metals, now rename this protein “DMT2”?
Until relatively recently, iron release from “donor cells” (primarily enterocytes and macrophages) to plasma Tf was poorly understood. This situation was rectified when, about ten years ago, three groups of investigators, using three different strategies, discovered the second known iron transporter to be described. This protein pumps iron out of cells. Abboud and Haile [187] employed a library of mRNA sequences enriched for IRP1 binding and, using IRP1 affinity chromatography, obtained IRE-containing mRNA sequences. This allowed them to identify a novel iron-regulated iron transporter, called MTP1 (metal transporter protein 1), that localized to the basolateral membrane of the duodenal enterocytes and a cytoplasmic compartment of macrophages. Importantly, overexpression of MTP1 in tissue culture cells was shown to cause intracellular iron depletion. McKie et al. [188] used a subtractive cloning strategy (through a duodenal cDNA library from hypotransferrinemic mice) designed to identify genes involved in the iron absorptive pathway. This approach enabled them to isolate a cDNA (termed Ireg1) encoding a new duodenal membrane protein. The IREG1 protein was shown to mediate iron efflux in the Xenopus laevis oocyte expression system. Finally, ferroportin (this term is now commonly used) was identified using a positional gene cloning strategy to study hypochromic anemia in zebrafish [189]. The birth of ferroportin, in particular with the discovery of hepcidin that regulates ferroportin levels, has had a vast impact on iron metabolism research (vide infra).
2.2.4.4. Iron reduction is essential for trafficking
Although the redox form of iron for export has never been determined, the term “ferroportin” suggests that this protein transports Fe2+. Roots of this issue go back to Laurell and Holmberg who should be credited for not only their discovery of Tf (vide supra), but also the identification of the copper-containing plasma protein ceruloplasmin (after the Latin caeruleus for blue, the color of the purified protein) [190]. They also were the first to describe ceruloplasmin’s oxidase activity [191]. The oxidase activity of this protein was confirmed by many studies that followed (e.g., [192,193]), but ceruloplasmin’s ferroxidase activity was demonstrated only in the mid sixties [194]. Somewhat later, several reports revealed that ceruloplasmin facilitates the mobilization of iron from the liver [195,196]. Recently, these early observations were agreeably complemented by studies of mice with the targeted disruption of the ceruloplasmin gene [197]. Ceruloplasmin−/− mice showed a striking impairment in the iron release from macrophages and hepatocytes associated with a significant increase in the iron content of the liver and spleen and a prominent elevation in plasma ferritin levels. In this context it is of interest to mention that a membrane-bound form of ceruloplasmin is expressed by astrocytes [198]. It is a glycosylphosphatidylinositol (GPI)-anchored form of ceruloplasmin generated by alternative RNA splicing [199]. Subsequently, Jeong and David [200] provided evidence that the capacity of astrocytes isolated from ceruloplasmin-deficient mice to purge themselves of iron is compromised. Additionally, ferroxidase activity seems to be required for the stability of ferroportin in cells expressing GPI-ceruloplasmin [200]. In this context it is of interest to mention that Kono et al. [201] recently demonstrated that under low hepcidin levels mutant GPI-ceruloplasmin with impaired ferroxidase activity could not stabilize ferroportin on the cell surface. In contrast, the wild-type GPI-ceruloplasmin inhibited ferroportin internalization by competing with hepcidin.
The precise mechanism by which ceruloplasmin promotes iron efflux from macrophages and other cells remains uncertain. A prevailing opinion is that the ferroxidase activity of ceruloplasmin is required for efficient insertion of iron into apoTf [202], as Tf binds the metal in the oxidized valence state (ferric). However, in our opinion, it should be considered that the ferroxidase activity of ceruloplasmin may be necessary for oxidation of ferrous ions following their transfer to the cell surface via ferroportin; the ferroxidation may foster shedding of iron from the cell membrane and “drive” the egress of ferrous ions from the intracellular milieu. Of interest in this context, a multi-copper oxidase, Fet3 with homology to ceruloplasmin has been identified in yeast [203]. In contrast to ceruloplasmin, which facilitates iron release from vertebrate cells, Fet3 is required for the oxidation of ferrous iron in the extracellular environment in preparation for iron uptake by the yeast membrane permease, Ftr1 [204]. Hence, although the yeast systems are very valuable in providing cues for research in mammals [205,206], they need to be interpreted with caution.
More recently, Vulpe et al. [207] discovered a ceruloplasmin homolog called hephaestin (named after the Greek god of fire and metals, Hephaestos), which is highly expressed in the intestine; in contrast to ceruloplasmin, it is membrane-bound. Mutation of the hephaestin gene is responsible for the defect in sex-linked anemia (sla) mice. The sla mice take up iron by enterocytes but the metal is not efficiently released, indicating that hephaestin plays a role in dietary iron assimilation.
Recent evidence indicates that ceruloplasmin and hephastin are not the only ferroxidases involved in iron homeostasis. Chen et al. [208] recently identified a gene encoding a new member of the multicopper oxidase family most closely related to hephaestin. The authors proposed calling this protein “zyklopen” after the Cyclopes, mythical one-eyed iron workers in Greek mythology who helped Hephaestus in the forge of the gods. Moreover, Duce et al. [209] recently reported that the Alzheimer’s Disease β-amyloid protein precursor (APP) possesses ferroxidase activity mediated by a conserved H-ferritin-like active site, which is inhibited specifically by Zn2+. This important finding is compatible with a proposal that this protein’s defects could lead to neurodegenerative disorders arising from iron accumulation under impaired iron export.
The above topic is conceptually related to another, equally important issue. All available evidence indicates that in vertebrates, prior to its transport across biological membranes, iron must be reduced to its more malleable and soluble ferrous (Fe2+) form. The only iron substrate for DMT1, which transports Tf-derived iron across the endosomal membrane and is involved in the acquisition of iron from food by duodenal enterocytes, is Fe2+. The mechanism of ferric iron reduction within endosomes was a long-awaited occurrence until Ohgami et al. [15] identified a gene, Steap3 (six transmebrane, epitheilal antigen of the prostate3). The protein coded by this gene is highly expressed in hematopoietic tissues, is present in endosomes and colocalizes with Tf, TfR, and DMT1. Significantly, Steap3 contains an oxido-reductase domain, based on the presence of a flavin-NAD(P)H binding structure and a putative heme binding site. The results of this study indicate that Steap3 is an endosomal ferrireductase required for efficient Tf-dependent iron uptake in erythroid cells. Mice with selective knock-out of Steap3 develop the expected anemia, but remain alive [15]. This seems to suggest that one or more other Steap proteins [16] may participate in iron reduction in the endosome.
The situation regarding iron reduction for duodenal DMT1 is less straightforward. The duodenal brush border contains a ferric reductase, Dcytb [210] that has been proposed to play a role in the formation of Fe2+ before its transport into the enterocyte. However, Andrews and coworkers inactivated the murine Dcytb gene and showed that Dcytb deficiency did not impair intestinal iron absorption when mice were fed normal food [211]. Hence, Dcytb does not seem to be an essential component of the intestinal iron absorption system in mice.
We can occasionally encounter views that iron itself is not metabolized in a classical sense, but the above examples that at least two enzymes, ceruloplasmin and Steap3, proteins involved in catalyzing iron’s redox states, are essential for iron homeostasis, indicate that iron is, in fact, metabolized. In this context, it may be of interest to bring up an article entitled “The relation of the spleen to blood destruction and regeneration and to hemolytic jaundice. XI. The influence of the spleen on iron metabolism” by J Harold Austin and Richard M Pearce (from the John Herr Musser Department of Research Medicine of the University of Pennsylvania, Philadelphia), which as early as 1914 [212], used the term “iron metabolism”. They probably derived this term from its German equivalent, “Eisenstoffwechsel”, that was used by L Asher and H Grossenbacher in their paper published in Beitrage zur Physiologie der Drüsen in 1909 (cited by JHA & RMP). Hence, in our opinion, there is no need to remove the term “iron metabolism” from the vocabulary of iron research.
2.2.4.5. Unexpected players
The unearthing of many of the above-discussed proteins was anticipated; their absence was felt as a gap in our knowledge about iron metabolism. What makes the current epoch of iron metabolism so exciting is that several recently discovered genes and their products, initially unforeseen to be linked to iron, turned out to be important players in the metabolism of this metal. We shall discuss two such proteins, frataxin and huntingtin. Friedreich ataxia is an autosomal recessive neurodegenerative disease and the most common of the heritable human ataxias. The defective gene in this disease is FRDA (encoded by a nuclear gene) which codes for the mitochondrial protein, frataxin. About 98% of mutant alleles have an expansion of the GAA trinucleotide repeat in intron 1 resulting in a marked reduction in frataxin levels [213]. Important clues to the role of frataxin in iron metabolism emerged from studies on the yeast FRDA ortholog, Yfh1. Mitochondria in Yfh1 mutants accumulate approximately an order of magnitude more iron that do wild-type mitochondria [214]. This process appears to be reversible as reintroduction of wild-type Yfh1 results in the rapid export of mitochondrial non-heme iron to the cytosolic compartment [215]. Although the precise function of frataxin is unknown, recent research bolsters the notion that frataxin is an essential component of complex machinery responsible for the synthesis of Fe-S clusters in mitochondria [216].
Huntington’s disease, a progressive, fatal, neurodegenerative disorder, is caused by a CAG triplet repeat expansion in the huntingtin gene, which encodes an expanded polyglutamine stretch in the huntingtin protein [217]. Following the finding that huntingtin immunoreactivity overlaps with that of TfR in membrane vesicles isolated from human and rat cortex synaptosomes [218], this protein was found to be involved in perinuclear TfR trafficking and mitochondrial cluster-tethering [219]. This study also revealed that iron deprivation causes an increase in huntingtin levels. More recently, huntingtin-deficient zebrafish were shown to develop hypochromic erythrocytes [220]. Co-injection of iron-dextran with huntingtin morpholino at the one-cell stage rescued the hypochromic phenotype of huntingtin-deficient embryos. Considering the above report by Hilditch-Maguire et al., the phenotype seen in huntingtin-deficient zebrafish can be explained by impaired Tf-receptor trafficking causing inadequate delivery of iron to developing erythroid cells.
2.2.4.6. Cellular smithy
An extremely important cellular organelle with respect to iron metabolism is the mitochondrion, which is involved in both heme biosynthesis and the assembly of iron-sulfur clusters. Heme synthesis and its regulation are reasonably well comprehended [27,221–223]. Nevertheless, some important recent developments should be highlighted. The group of Paw has demonstrated that mammalian homologs to yeast Mrs3/Mrs4 [205,206], dubbed “mitoferrins”, are responsible for iron import, probably in its Fe2+ form, through the mitochondrial inner membrane [224,225]. Mitoferrin 1 exerts its function primarily in erythroid cells, but mitoferrin 2, which is expressed ubiquitously, cannot support hemoglobinization [225]. Of considerable interest in this context is a recent discovery [226] that mitoferrin 1 physically interacts with ferrochelatase and Abcb10 (formerly known ABC-me; [227], a mitochondrial inner membrane ATP-binding cassette transporter with unknown function, which is highly induced during erythroid maturation. Of considerable clinical interest is the very recent finding that abnormal expression of mitoferrin 1 causes a variant type of erythropoietic protoporphyria [228], which is typically due to a partial deficiency of the last enzyme of the heme biosynthetic pathway, ferrochelatase. Some patients with an erythropoietic protoporphyria phenotype do not have such a defect. Hence, this report enhances our knowledge about the molecular pathogenesis of this disease.
Our understanding of iron-sulfur cluster assembly is far from complete. Nevertheless, it is an active area of research and the most recent developments in the field can be found in these recent reviews: [216,229,230]. At this time, more than twenty proteins have been implicated in the biogenesis of Fe/S proteins, however the molecular mechanisms involved in this pathway are only beginning to be understood. Furthermore, it appears that specific factors involved in the Fe/S maturation of functional Fe/S proteins have yet to be discovered. Of these putative components, to date only four have been identified: Ind1 (required for complex I maturation) [150] and three proteins involved in [4Fe-4S] protein maturation (ISCA1, ISCA2, and IBA57) [231–233] (Sheftel et al., manuscript in preparation). Additionally, novel Fe/S proteins are still being found, many of which perform essential cellular functions. The most notable recent additions to the collection of Fe/S proteins are those involved in DNA repair. The FANCJ and XPD genes encode Fe/S-containing helicases essential for DNA repair [234]. Respectively, these proteins have been shown to be mutated in cases of Fanconi anemia and xeroderma pigmentosum, diseases of DNA instability. Consistent with this notion, the Gottschling laboratory has recently demonstrated, through genetic analyses, an intimate connection between mitochondrial function and nuclear DNA integrity [235], further solidifying the link between Fe/S protein biogenesis and genomic DNA stability. It is important to mention here that Fe/S proteins (or Fe/S protein-modified enzymes) have also been shown to be associated with mitochondrial DNA [236,237] and, in at least one case [237], its stability. The discussion of mitochondrial iron metabolism must include an important discovery by Arosio and co-workers who have recently identified a mitochondrial ferritin isoform that can store iron within a shell of homopolymers, although the function and regulation of this protein is not yet understood [238–240]. Notwithstanding, it has recently been demonstrated that the overexpression of mitochondrial ferritin causes the redistribution of iron from cytosolic ferritin to mitochondrial ferritin [241] and leads to the inhibition of cancer cell growth [242]. Interestingly, mitochondrial ferritin shows very low expression in all tissues except for testis [238].
Additionally, although mitochondrial ferritin is not expressed in normal erythroblasts, it is expressed in ring sideroblasts of patients with sideroblastic anemia [243]. In our opinion, this protein deserves serious consideration and additional study.
2.2.4.7. Iron and nitric oxide: a precious bond
One of the seminal discoveries in the present “golden” epoch is the revelation that living systems generate nitric oxide (NO), a gaseous molecule that has remarkably important functions in virtually all organisms. In 1846, Italian chemist Ascanio Sobrero synthesized nitroglycerin [244] without knowing that this chemical releases NO; this was discovered only in 1977 by Ferid Murad [245]. Nitroglycerin, which had initially been shown to cause flushing, was later found to effectively relieve anginal chest pain with remarkable speed. Even the Swedish scientist Alfred Nobel, who invented dynamite, the major ingredient of which is nitroglycerin, was given a prescription for this drug by his physician. Nobel refused this type of treatment. The prelude to the currently accepted fact that NO, the notoriously known toxic environmental pollutant, may be a biologically relevant molecule, emerged in the eighties and early nineties of the previous century. Louis Ignarro became interested by the observation of Murad that NO and some other nitro-compounds, which might release NO (e.g., nitroglycerin), activate the cytosolic guanylyl cyclase that, in turn, catalyses the formation of cyclic GMP from guanylyl triphosphate (GTP). Murad has demonstrated that when nitric oxide binds to the iron in the heme group of guanylate cyclase, the enzyme is activated (reviewed in [246]) and the resulting cGMP affects numerous cellular processes, one of them being vasorelaxation. Actually, numerous biological effects of NO can be attributed to the fact that NO avidly binds iron within many proteins and enzymes. Some examples are other heme-containing proteins, such as cytochromes [247], NO synthase [248], the rate-limiting enzyme of DNA synthesis ribonucleotide reductase [249], Fe-S cluster proteins involved in energy metabolism such as mitochondrial aconitase, and those that are part of complexes I and II of the electron transport chain [250,251]. In terms of iron metabolism as such, one of the most important examples is the effect of NO on IRPs (reviewed in [252]). Hence, it is not at all surprising that Science [253]) proclaimed NO “Molecule of the Year” in 1992. The December 18, 1992 issue of Science published a highly informative review on our knowledge of NO in biology [254]. It needs to be added that Robert Furchgott discovered a substance in endothelial cells that relaxes blood vessels [255], calling it endothelium-derived relaxing factor (EDRF). By 1986, he determined that EDRF was, in fact, NO. In 1998, the Nobel Prize in Physiology or Medicine was awarded jointly to Robert Furchgott, Louis Ignarro and Ferid Murad “for their discoveries concerning nitric oxide (NO) as a signalling molecule in the cardiovascular system”. The decision of the Nobel Committee to choose scientists involved in the NO-related research to be crowned with the Prize must have been a tremendously difficult task. It should not be overlooked, however, that other investigators in the late 1980’s and early1990’s generated seminal reports of the various roles of NO in life [256–262], despite the Nobel committee’s decision that NO’s role in the cardiovascular system was the most significant.
2.2.4.8. Long-lasting conundrum resolved
Two milestone discoveries, which are especially relevant to clinical medicine and deserve to be brought to light, are related to the hereditary iron overload disorders and the remarkable developments related to the regulation of organismal iron homeostasis in both physiology and pathology. The first subject has its beginnings in the “pre-classical” era, going back to the mid 1800’s, when the French physician Armand Trousseau [263] described a patient with diabetes who, based on the autopsy examination, exhibited a “bronze-like” appearance. In 1889, the German pathologist Friedrich Daniel von Recklinghausen (also to be credited for the first description of neurofibromatosis type I) described a similar patient exhibiting a bronze-like color of organs and a grayish or brown skin tone, which he attributed to blood-borne pigments. In fact, hyperpigmentation of the skin is now known to be caused by increased melanogenesis with increased melanin in the epidermis and in dermal melanophages as well as iron deposits in the deeper dermis [264]. Von Recklinghausen coined the term “hämochromatose” [265]. In 1935, Joseph Sheldon published a monograph [266] describing 311 cases of hemochromatosis that existed in the literature up to that time. He concluded that hemochromatosis is an inherited disorder and that its pathology and symptoms stem from excessive iron deposition in various tissues.
In 1976, Marcel Simon and his colleagues [267] clearly established the autosomal recessive inheritance of hemochromatosis and its close linkage with the major histocompatibility class I A3 on the short arm of the chromosome 6. To pay tribute to Dr. Simon’s critical contributions, the Marcel Simon Prize was created, and is awarded for excellence in the research of genetic hemochromatosis at the biannual Meetings of the International BioIron Society (http://www.bioiron.org/about/awards.aspx).
Subsequent confirmation of this discovery by investigators from all over the world revealed a prevalence of homozygotes with hemochromatosis to be 1 in 250 to 1 in 300 of the Caucasian population (reviewed in [12]). At this time, a race to identify a gene responsible for hereditary hemochromatosis began. Anecdotal testimony for this can be provided by one of the authors (PP) who, together with Herb Schulman and Robert Woodworth, co-organized the “Eighth International Conference on Proteins of Iron Transport and Storage” held in Chateau Montebello, Québec, in May of 1987. Earlier that year, the meeting organizers received letters from three different scientists who proclaimed a discovery of the hemochromatosis gene. Hence a decision was made to set a special session to present these findings. However, due to curiously suspicious failings (e.g., “we lost the clones”, etc.) the session had to be cancelled.
The defective hemochromatosis gene (HFE; initially named HLA-H) was eventually discovered almost ten years later by Feder et al. [268], using a positional cloning technique in families with hereditary hemochromatosis. HFE is a non-classical major histocompatibility complex class I type molecule. Over 80% of hereditary hemochromatosis patients have the same mutation in the α3 domain of HFE that prevents its heterodimerization with β2-microglobulin [269]. The C282Y mutation has been shown to prevent cell surface expression and, presumably, function of the protein [269,270]. As will be discussed, the precise physiologic role of HFE is not yet fully understood, but mouse knockout studies have demonstrated that HFE is essential for normal control of iron absorption. Mice homozygous for HFE gene disruption have the same biochemical and histologic features of iron overload as patients with hereditary hemochromatosis [271].
2.2.4.9. “A master regulator”
The second landmark discovery was the identification of hepcidin by three groups investigating either novel anti-microbial peptides [272,273] or the recognition of the fact that it is highly expressed during iron overload [274]. Tomáš Ganz and his co-workers, who discovered that this peptide was associated with inflammation, named it “hepcidin” after observing that it was produced in the liver (“hep-”) and seemed to have bactericidal properties. Compelling links between hepcidin and iron metabolism emanated from a report showing that USF2 (Upstream Stimulatory Factor 2 gene) knockout mice, which fail to express either copy of the duplicated hepcidin genes immediately downstream of USF2, exhibit iron overload that the authors attributed to the absence of hepcidin [275]. In a second publication [276] the same group demonstrated that transgenic mice expressing hepcidin under the control of the liver-specific transthyretin promoter are born with pale skin and die within a few hours after birth. “These transgenic animals had decreased body iron levels and presented severe microcytic hypochromic anemia”. Subsequent research revealed that hepcidin limits iron entry into the plasma from macrophages, intestinal enterocytes and other cells. The molecular mechanism of this phenomenon involves the binding of hepcidin to the sole iron-export protein ferroportin (vide infra) and facilitating its degradation [63]. Hence, hepcidin regulates iron entry into the circulation by promoting ferroportin’s destruction. As will be discussed in more detail later, iron stores and inflammation positively regulate hepcidin levels, whereas hypoxia and increased erythropoiesis downregulate the hormone’s expression. The unusually active research effort by investigators scattered all over the world has revealed that hepcidin is a “master regulator” of organismal iron homeostasis [277–279].
If Nancy Andrews’ review [280] is not sufficient evidence, the definitive proof that the Golden Age of iron was upon us appeared in 1996, when the American Society of Hematology decided to reinstate iron-related topics under the umbrella “Ad Hoc Scientific Subcommittee on Iron and Heme”. The program of the first Subcommittee session, entitled “Recent Advances in Iron and Heme Metabolism”, was as follows: The Control of Cellular Iron Metabolism: New Developments (Tracey Rouault); Identification of the Hereditary Hemochromatosis Gene (Roger Wolff); Role of Heme in Nitric Oxide Production and Function (James Stone); NO Interactions with Hemoglobin: Biological Chemistry and Physiological Function (Jonathan Stamler). After humble beginnings, with only handful people in the audience, this Committee now flourishes, with two well-attended sessions at the ASH annual meetings. This act was followed by the “bioiron community” that, at their biannual conference in Bethesda in 2003, made a decision to establish the International BioIron Society (IBIS; http://www.bioiron.org) where the first President, Antonello Pietrangelo, and other officials of IBIS were elected. However, this Society’s move did not appear from out of thin air. The very first “International Conference on Iron Storage and Transport Proteins” was held at University College Hospital in London, July 12–14, 1973, organized by Pauline Harrison, Phil Aisen and Ernie Huehns.
Many proteins discussed here, and some additional ones that have not been mentioned, are listed in Table 1. The broadness of this theme, “History of iron homeostasis”, did not allow us to discuss some aspects of iron metabolism in an adequate depth. We feel fortunate that we can refer readers to an engaging and informative review composed by one of the most respected investigators in this field [280].
Table 1.
Selected Proteins Involved in Iron Metabolism
| Protein | Function | Result of deficiency | References |
|---|---|---|---|
| ABCB7 | Involved in Fe-S biosynthesis | X-linked sideroblastic anemia with ataxia | [391,410,411] |
| ABCB10 (ABC-me) | Mitochondrial transport function related to heme synthesis; interacts with mitoferrin 1 and enhances its stability in erythroid cells | Unknown | [226,227] |
| ALA-S2/eALA-S | 1st enzyme of heme synthesis; erythroid-specific | X-linked sideroblastic anemia (XLSA) | [221,412] |
| Bone morphogenic proteins 2/4/6 (BMP 2/4/6) | Positive regulator of hepcidin | Iron overload (for BMP6) | [413,414] |
| Ceruloplasmin (CP) | Ferroxidase; involved in promoting release of Fe from cells | Neurodegeneration; iron loading of brain and liver; microcytic anemia | [415,416] |
| DMT1 | Membrane transporter of Fe2+ | Hypochromic, microcytic anemia | [128,184,382,383,417–419] |
| Duodenal cytochrome b (Dcytb) | Ferric reductase | No noticeable defect | [210,211] |
| Ferritin (H & L) | Intracellular iron storage proteins | H ferritin: Embryonic lethality | [420,421] |
| Ferrochelatase | Enzyme responsible for the insertion of Fe2+ into protoporphyrin IX in the final step of heme synthesis | Erythropoietic protoporphyria | [27,221–223,422] |
| Ferroportin/MTP1/Ireg1 | Cellular iron exporter | Type IV hemochromatosis (Ferroportin disease) | [187–189,423] |
| Feline leukemia virus-C receptor (FLVCR) | Putative heme exporter (export of endogenously formed heme has never been demonstrated) | Red cell aplasia (in cats) | [424,425] |
| Frataxin | Fe-binding chaperone for Fe-S cluster synthesis | Freiderich’s ataxia | [426,427] |
| GDF15 | Contributes to the suppression of hepcidin during β-thalassemia | Unknown | [428,429] |
| Heme carrier protein (HCP) | Proposed to be an intestinal heme transporter; more likely involved in folate transport than iron metabolism | Unknown | [2,3] |
| HFE | Positively regulates hepcidin expression | Type 1 hemochromatosis (variable penetrance) | [268,271,430] |
| Heme oxygenase-1 (HO-1) | Catalyzes the release of iron from protoporphyrin IX; involved in the recycling of hemoglobin Fe | Anemia and inflammation | [431–435] |
| Hemojuvelin (HJV/HFE2/RGMc) | Co-receptor for BMP2 and 4; binds neogenin | Juvenile hemochromatosis | [413,436,437] |
| Hepcidin | Negative regulator of iron absorption; causes the internalization and degradation of ferroportin | Juvenile hemochromatosis | [63,273,438] |
| Hephaestin | Membrane-bound ferroxidase; Cp homologue | Hypochromic, microcytic anemia; sex-linked anemia in mice | [207] |
| Huntingtin | TfR trafficking | Huntingtin knockout zebrafish have microcytic anemia | [219,220] |
| IRP (1&2) | Sensors of intracellular iron; bind cognate IREs | IRP1: Elevated ferritin in brown fat and kidney IRP2: microcytic anemia, erythropoietic protoporphyria, neurodegeneration | [372,373,439,440] |
| Lactoferrin | Fe-binding glycoprotein; member of Tf family | No major abnormalities | [441–443] |
| Mitochondrial ferritin (MtFt) | Mitochondrial Fe storage (?);high expression in ring sideroblasts and testes | Unknown | [238,241,242,444] |
| Mitoferrin 1 (erythroid specific) Mitoferrin 2 (ubiquitous) | Involved in mitochondrial iron delivery | Hypochromic, microcytic anemia; erythroid maturation arrest | [224,225] |
| Mon1a | Involved in trafficking of ferroportin | Associated with macrophage iron loading | [445] |
| Neogenin | Required for the processing and release of HJV | Altered growth patterns of neuronal axons | [437,446,447] |
| Sec15l1 | Promotes cycling of transferrin in erythroid precursors | Hemoglobin-deficit mouse (hbd/hbd); hypochromic, microcytic anemia | [375,376,380,448] |
| Sideroflexin | Mitochondrial transport related to heme synthesis | Siderocytic anemia | [449] |
| Steap3 (six-transmembrane epithelial antigen of the prostate 3) | Endosomal ferrireductase | Hypochromic, microcytic anemia | [15,450] |
| TMPRSS6 | Serine protease regulating the processing of hepcidin | Microcytic anemia; generalized Fe overload | [451,452] |
| Transferrin (Tf) | Fe(III)-carrier in plasma | Severe anemia | [43,453] |
| Tf receptor 1 (TfR) | Membrane receptor for Fe2-Tf | Embryonic lethality | [126,127,370] |
| TfR2 | Required for the regulation of hepcidin | Type 3 hemochromatosis | [11,454,455] |
| ZIP14 | Putative mediator of non-transferrin bound iron uptake in hepatocytes | Unknown | [456] |
3. A brief history of human serum transferrin
3.1. Overview
Since its discovery over six decades ago (see Section 2.2.2), human serum Tf (Tf) has been extensively studied. The natural abundance of Tf in the blood (~35 μM), in addition to its unique spectral properties, has long fascinated scientists. In particular the ability of Tf to bind ferric iron (Fe+3) and other metals in two homologous lobes, tightly but reversibly, begs an explanation at a molecular level. As previously described [281], Tf and the two other founding family members, lactoferrin (lTf) and ovotransferrin (oTf), have served as model systems for development and verification of numerous experimental techniques. Because this volume contains chapters dealing with the other family members, this section of the current review will focus primarily on human serum Tf. Although it is not possible to list everyone who has contributed to the effort, a number of comprehensive reviews have appeared over the years and provide an excellent background for those who wish to follow the field from its inception until now [43,116,127,281–296]. In such a mature field, this is an interesting and enlightening exercise which highlights the controversies which have often led to progress as scientists focused on devising definitive experiments to prove or disprove a hypothesis.
Very briefly, as for all members of the Tf family, Tf consists of a single polypeptide chain (MW ~80 kDa) which folds into two globular, homologous halves referred to as the N- and C-lobes. The iron within each lobe of Tf resides in a deep cleft formed by two subdomains within each lobe. The Fe+3 is coordinated by four amino acid ligands (two tyrosines, a histidine and an aspartic acid) with two additional oxygen ligands provided by a synergistic anion identified as carbonate (Fig. 3). A conserved arginine residue, in addition to a threonine residue, and the dipole of an adjacent α-helix stabilize the binding of the synergistic anion.
Figure 3. Close-up of ligands and second shell residues in human serum transferrin.
N-lobe ligands are on the left and colored green (PDB 1A8E). Second shell residues are depicted in yellow. The conserved arginine is in purple. C-lobe ligands from porcine Tf are shown on the right with the same color scheme and with human numbering (1H76). Figure produced with PyMOL by Ashely N. Steere.
As in any family, Tf has had its share of disagreements over the years. Some, including the nomenclature, have been relatively minor, while others have had more substance. For example the Fletcher Huehn’s hypothesis which advocated functional differences between the two lobes was hotly debated for a number of years. Addressing issues related to whether Tf is a single polypeptide chain, whether iron is bound and released sequentially, whether it enters a cell or is delivered at the cell surface and whether iron delivery to erythroid cells differs from iron delivery to non-erythroid cell provided major impetuses driving research efforts for many years. Cooperativity between the two lobes, the role of the specific Tf receptor, the role of anions in binding and release and the precise mechanism of how iron is released and delivered within the cell (trafficking) continue to be discussed and studied.
Here we will endeavor to provide a brief overview of some important milestones in the history of Tf research, with reference to seminal papers. Given that the main function of Tf is iron transport and delivery to cells, the specific TfR must also be included in the history as an essential piece of the story (although it is treated in more depth in other chapters of this volume).
3.2. Physicochemical properties of transferrin
Following the identification and isolation of Tf, from Cohn fraction IV-3,-4 (the -globulin fraction of plasma) many studies focused on establishing its physical and chemical properties. Feeney and coworkers established that iron saturated Tf is considerably more resistant to attack by heat, chemicals, and proteolysis than apoTf [297] and is a single polypeptide [298]. This later finding was verified by analytical ultracentrifugation studies in the Tanford laboratory [299]. A seminal paper from the Aisen laboratory used equilibrium dialysis to establish that at pH 7.4, the C-lobe binds iron with an affinity constant that is about 6-times stronger than the N-lobe [300]. Additionally, the important concept that thermodynamic and kinetic properties both influence the binding and release of iron from each lobe of Tf was suggested. The work employed urea gels which had been fortuitously discovered a couple of years earlier [301]. Urea gels provide the simplest method to simultaneously view the four possible species of Tf: apoTf, monoferric Tf with iron in either the N-lobe, FeNTf or the C-lobe, FeCTf and diferric Tf designated Fe2Tf. These gels have been used to show that in normal serum the saturation level is only ~30% and to estimate the composition of Tf in the circulation [302–304]. An instructive primer on urea gel preparation is provided in [285]. More recently commercially available mini gels have simplified their use [305–307]. Notably, the lack of carbohydrate in recombinant Tf increases the sharpness of the bands in the mini gel format.
As mentioned above the unique spectral properties of Tf have been the basis of consternation and inquiry. Binding of iron increases absorbance in both the UV and visible portions of the spectrum. The increase has been attributed to energy transfer between Fe+3 and the two liganding tyrosine residues in each lobe which create a ligand to metal charge transfer band. The visible spectrum has a maximum centered around 466 nm resulting in the characteristic salmon pink hue of Tf [308,309]. In contrast to absorption, binding of iron to Tf quenches the intrinsic fluorescence attributed to excited state energy transfer from certain tryptophan residues in each lobe to the absorbance band [310]. Thus when iron is released the fluorescent signal increases and the absorbance decreases. In a testament to the power of recombinant expression in combination with mutagenesis (see below), the contribution of each of the well-distributed individual tryptophan residues (3 in the N-lobe and 5 in the C-lobe) has been determined by making single and double point mutants [311,312]. Recent work provides evidence that, in addition to Forster resonance energy transfer, static quenching contributes to the change in the fluorescent signal when iron is released from the N-lobe [311].
A challenging aspect of the unique spectral properties of Tf is the need to be able to determine accurate extinction coefficients for both the iron and apo species. Due to subtle differences in the geometry of the sites, the addition of iron does not follow Beer’s law. Particularly with respect to mutants (see below) that affect the iron binding site, it is essential to have a simple method to obtain an accurate estimate of the protein concentration without expending large amounts of sample. Such a method has been established [313]. Importantly the results compare favorably to values reported previously.
The marked effects of anion binding on the spectroscopic, kinetic, and thermodynamic, properties of Tf have been studied throughout its history (and are the subject of another chapter in this volume). Notably, experimental exclusion of a suitable synergistic anion leads to irreversible hydrolysis of ferric iron, precluding binding to Tf. In the seminal work of Bates and coworkers, a variety of other anions has been shown to substitute for the physiological carbonate anion [314,315]. Substitutes must have a carboxylate group available to bind to an amino acid residue in Tf (now identified as Arg124 in the N-lobe and Arg456 in the C-lobe of Tf) and an electron donor group, one or two carbon atoms away, which binds to the ferric iron.
Non-synergistic anions comprise a second distinct class which do not participate in binding of iron but which affect iron release by binding to specific sites and promoting conformational alterations [316,317]. The work of the Raymond laboratory experimentally established that no iron will be released in the absence of salt [318]. As recently described [319], techniques including EPR [285,316,320–322], NMR [323], and UV-difference spectra [324–328] have been used to identify possible binding sites and to document the effect of salt. As reviewed in [291], the equilibrium constants for binding of thirteen anions to apoTf shows a range of affinities.
3.3. Primary Sequence Determination
An important development in the history of Tf was the determination of the primary amino acid sequence. MacGillivray et al. in the Brew laboratory obtained the sequence using the brute force approach of producing overlapping peptides [329,330]. A year later the sequence was confirmed in the Bowman laboratory by sequencing the cDNA [331]. Availability of the sequence resolved ongoing controversy with regard to the mass and composition of Tf. The thirty-eight cysteine residues all engaged in disulfide bonds, and the clear homology between halves were both documented. The sequence of the TfR was reported by two different laboratories [163,332].
3.4. Transferrin structure
3.4.1. Structures with impact on the history of transferrin
In 1987, in a truly seminal achievement, Baker et al. [333] reported the structure of family member lactoferrin (lTf), providing the first look at the iron deep within each cleft and quelling all arguments with regard to the ligands holding the iron in place [289]. Significantly, the structure revealed the presence of the spectroscopically silent aspartate ligand on one side of the binding cleft. The great distance between the binding ligands in the primary structure (unprecedented in metalloproteins), explained why identification of these ligands was so challenging. A structure of rabbit Tf from Lindley and coworkers in 1988 showed that its disposition in 3-dimensional space was similar to lTf [334]. Higher resolution structures of rabbit (79% identical to human Tf) and pig Tf (71% identical to human Tf) have since become available [335].
A persistent problem in the field has been the failure to obtain crystals of human Fe2Tf, that diffract to provide a structure. The impressive solubility of Fe2Tf (80–100 mg/mL) may be a contributing factor. Tantalizingly, an unrefined, unpublished structure of monoferric Tf, FeCTf, from the doctoral thesis of Zuccola began circulating in 1993 [336]. In 1998, high resolution structures of the recombinant Tf N-lobe with [337] and without iron [338] became available from the Baker laboratory revealing the large conformational change (~60°) that results from iron binding or release. Additionally these structures confirmed the presence of the so called “dilysine trigger” originally identified in the structure of the N-lobe of oTf [339] (see chapter XX of this special issue for further details). This unique feature has been shown to be essential to the efficient release of iron from the N-lobe [340]. A reverse γ turn was also identified in this structure and is a feature found in each lobe of most family members. The residues that make up the turn appear in the disallowed region of the Ramachandran plot. Additionally, capture of the carbonate and the conserved arginine to which it is attached, in two different positions, provided a suggestion that protonation of the synergistic carbonate might be an early event in the pathway to iron release. Significantly the structure of apoTf published in 2006 revealed that, like the N-lobe, the C-lobe undergoes a similar large conformation change [341].
It is very satisfying when a structure provides insight into unexplained behavior. Three compelling examples from work on the N-lobe of Tf include an explanation of why, in contrast to two other mutants in which the liganding histidine was replaced by alanine or glutamine, the H249E mutant stabilizes the site [342], how the conserved arginine at position 124 helps control iron release [343], and why it is more difficult to remove iron when oxalate is substituted for carbonate [344]. Briefly, in the first example, the glutamate substitution disables the dilysine trigger because Glu249 binds to Lys296. In the second example, the ability of Arg124 to move into two different positions is directly connected to the iron release rate. In the third example the almost perfect geometry of the oxalate molecule in the binding site greatly restricts access to the iron, locking it in. Obviously a recombinant expression system (see below) allowing production of mutants is critical to each of these examples.
The role, or lack thereof, of glycosylation in Tf function has been elucidated by the ability to produce nonglycosylated species. The presence or absence of carbohydrate has no impact on the binding of iron to Tf or the binding of Tf to the TfR [345]. Removal of one or more of the four terminal sialic acids from the two biantenary glycans of Tf may target them for removal from the circulation by liver asialoreceptors [346].
3.4.2. Structures with impact on defining the interaction of transferrin with the transferrin receptor
The first crucial step toward understanding how Tf might interact with the receptor required a crystal structure of the soluble portion of the TfR [347]. A second relevant structure was that of the HFE protein bound to the TfR [348], which clearly showed that the structure of the TfR changes as a result of the binding of HFE (and predicted that the same might be true for Tf binding). Of great significance the 7.5 Å cryo-EM model based on the crystal structure of isolated TfR with human N-lobe and rabbit C-lobe soaked into the isolated TfR [349], provided the first and, to date, only view of the complex. Very recently a 3.22 Å crystal structure of hTF (with iron only in the N-lobe) bound to the soluble portion of TfR has become available [Eckenroth et al., Proc. Natl. Acad. Sci USA, in press]. The structure reveals how binding to the TfRaccelerates iron release for the C-lobe, slows release from the N-lobe, and stabilizes the binding of apo hTf at endosomal pH (see kinetics section below).
3.5. Recombinant expression systems for transferrin and transferrin receptor
In the past 20 years the field has been advanced by recombinant expression which allows mutation of individual amino acids providing a precise determination of the contribution of a given residue to the function. The impressive number of expression systems for oTf, lTf and Tf were reviewed in 2002 [295]. Notably the failure of bacterial systems to produce functional protein due to folding issues was discussed as well as successful expression of Tf in insect cells and yeast. To date, the most optimized, well documented and widely available system utilizes baby hamster kidney (BHK) cells transfected with a plasmid in which the presence of the native signal peptide for human Tf allows secretion of the recombinant Tf into the tissue culture medium [305,345,350–352]. Since 2002, a strain of yeast (S. cerevisiae) has been developed and optimized for commercial production of large amounts of recombinant, nonglycosylated Tf, termed deltaferrin [353,354]. More recently production of Tf in rice (Oryza sativa) has also been reported [355]. This nonglcosylated Tf is called Optiferrin and is also commercially available. Although neither of these recombinant Tf entities is completely homogenous by mass spectrometry analysis, they do support growth of various tissue culture cells, which is the target market. It is not immediately obvious whether it is would be possible or practical to use these constructs to produce mutants of Tf.
The soluble portion of the TfR has been produced in Chinese hamster ovary cells [347] and in an insect cell baculovirus system established by Dr. Peter Snow in the Björkman expression facility at the California Institute of Technology [356–358]. Additionally, the soluble portion of the TfR has been secreted and purified from BHK cells using similar protocols to those used for production of recombinant Tf [359]. Giannetti et al. [358], in an truly impressive study, produced and purified ~30 single point TfR mutants and determined the ability of each to bind to HFE, and Fe2- and apoTf by surface plasmon resonance imaging.
3.6. Kinetic studies with and without the transferrin receptor
It is simpler to measure iron release than to measure iron uptake. This is especially true for Tf mutants with weaker iron binding affinity since all binding protocols require the use of a chelator to keep the iron in solution. In essence, the Tf competes with the chelator to bind the iron in a two step process. A large number of kinetic studies have been carried out over several decades with many different cell types. In addition to the variability of the experimental system the conditions to measure iron release from Tf, including pH, salt, chelators, and temperature, have varied so greatly that comparisons are difficult. In addition it has become increasingly clear that the behavior of Tf in solution in the absence of the TfR differs considerably from the behavior of Tf when bound to the TfR. The Aisen lab has led the way in establishing techniques to provide data to validate this contention [360–366]. More recently recombinant Tf with well defined properties have allowed the most complete study of the interaction of Tf with the TfR carried out to date [367]. Kinetic constants for each step in the transition from Fe2Tf to apoTf with and without the TfR have been reported as described and discussed in another chapter in this special issue. The only caveat is that, since the precise endosomal milieu remains unknown, direct application of these in vitro findings to describe the mechanism of iron release within cells remains speculative.
4. Pathophysiology of Iron Metabolism
4.1. Diseases of Iron Deficiency
Nearly a billion people are afflicted with iron deficiency anemia. This condition arises when there is insufficient iron available for hemoglobin formation in erythroid cells. With suboptimal heme synthesis, the red blood cells in these patients possess less red color and are smaller than normal. While these hypochromic, microcytic erythrocytes are the most striking feature of iron deficiency anemia, severe iron deficiency can affect multiple organs, as iron is ubiquitously required for many other heme and non-heme proteins. It must be noted that microcytic anemia is not pathognomonic for iron deficiency anemia, as retention of iron in macrophages (e.g., anemia of chronic disease, functional iron deficiency), defective protoporphryin synthesis, or decreased globin synthesis can all result in hypochromic, microcytic anemia. Each of these conditions is associated with decreased production of hemoglobin that is responsible for this phenotype [368].
Dietary iron insufficiency is the most common cause of iron deficiency in infants and young children, while blood loss is responsible for iron deficiency in older children and adults. This blood loss is usually occult bleeding in the upper gastrointestinal tract (GI). Globally, the most common cause of GI bleeding is hookworm infection, although occult GI blood loss can also be caused by hiatal hernia, diverticulosis, peptic ulceriation, GI tumors, angiodysplasia of the colon, adenomas and polyps, esophageal varices and ingestion of salicylates, steroids, non-steroidal anti-inflammatory drugs, and heavy endurance exercise. In premenopausal women, menstrual bleeding is the most common cause of iron deficiency [369].
4.2. Hereditary disorders of iron trafficking: of mice, men, and fish
4.2.1. Introduction
Iron deficient erythropoiesis can be the result of intrinsic defects in premature red blood cells. Several of these states have been generated or identified in humans, rodents, and zebrafish. Although extremely rare and/or restricted to animal models, these conditions provide valuable information from which further understanding of iron metabolism may be extracted. Importantly, animal models may also predict the symptomology that future patients with similar defects may present. However, an open mind must be kept, as some animal models have exhibited significant deviation from the human condition as in the case of DMT1 deficiency described below.
4.2.2. Transferrin receptor defects
Transgenic animals without any functional TfR die in utero [370]. As expected, these animals have defective erythropoiesis but, somewhat surprisingly, TfR-null animals also demonstrate abnormalities in neurologic development. This intriguing finding needs further investigation to determine whether the neurological defect is caused by the lack of iron per se or by the lack of TfR that may have some unexpected developmental function unrelated to iron transport. Mice containing only one functional copy of the TfR gene had normal hemoglobin levels, but had microcytic, hypochromic erythrocytes [370]. This finding strongly suggests that normal hemoglobinization of erythroblasts on the one hand requires “full” transcriptional activation of the TfR gene and, on the other hand, is limited by receptor levels. Clearly, post-transcriptional regulatory mechanisms (vide supra) are unable to maintain TfR at adequate levels if only one allele contains a functional receptor gene.
It bears mentioning that, as of yet, no human pathology has been associated with mutations in TfR. However, one study [371] reported a patient with an acquired microcytic anemia and highly elevated levels of “free protoporphyrin” (likely to have been mostly Zn-protoporphyrin) in red cells. This study found an antibody in the serum of the patients that both blocked iron incorporation into heme in cultured cells and immunoprecipitated human TfR. The data of this case study suggest that an anti-TfR antibody was responsible for the patient’s anemia. Further, treatment with the immunosuppressant azathioprine and prednisone led to complete remission.
It has rigorously been shown that mice lacking IRP2 have microcytic anemia that develops despite normal or even elevated serum iron levels and Tf saturation [372,373]. In these animals, TfR protein [372,373] and mRNA [373] were shown to be diminished, likely to be the cause of the anemia as IRP binding to the IREs in TfR mRNA stabilizes the level of the message. Cooperman et al. reported increases in free- and zinc-protoporphyrin in these mice which would be expected since ALA-S2, the first enzyme in protoporphyrin synthesis, is also controlled by IRP binding (in a manner opposite to that of TfR) [372]. Of great interest, in this mouse line, the Rouault laboratory previously identified a progressive neurodegeneration [374]. This latter phenotype is consistent with the above mentioned observation that neurodevelopment is severely impaired in TfR knockout mouse embryos [370]. Interestingly, the IRP2 ablated mice from Hentze’s group do not show neurodegeneration. No unequivocal explanation for the discrepancies between the two IRP2 mouse line phenotypes has been provided.
4.2.3. Transferrin cycling defect: the hbd/hbd mouse
In 2005, two groups [375,376] identified the defect in a mouse line containing a spontaneous, autosomal recessive “haemoglobin deficit” mouse [377]. These mice are characterized by a seemingly erythron-restricted phenotype: hypochromic microcytic anemia, hyperferremia, increased red cell porphyrin levels, and reticulocytosis. While the number of TfR on the reticulocytes of these mice are normal, the uptake of Tf-bound iron is decreased in these cells [377–379]. The genetic basis for this disease, as simultaneously reported by Lim et al. [375] and White et al. [376] is a mutation in the SEC15l1 gene. Because it is a homologue of yeast Sec15, it is expected that Sec15l1 performs a function in the human exocyst pathway. Contrary to the hypothesis of Lim et al. that Sec15l1 mutation increases the recycling of TfR to the cell surface, it was later shown that the entire Tf cycle was decelerated in hbd reticulocytes [380]. As proposed by White et al., it is likely that Sec15l1 functions in vesicular trafficking, docking, fusing, and/or cargo delivery. The finding that an exocyst pathway member slows Tf cycling implies that a regulatory mechanism may balance endocytosis with exocytosis.
4.2.4. Intravesicular ferrireductase deficiency
Chromosome 1 of the spontaneous mutant nm1054 mice is missing approximately 400 kb. The pleiotropic effects resulting from ablation of the six genes contained in this region include a hypochromic microcytic anemia [381]. One of the genes in this region, designated six-transmembrane epithelial antigen of the prostate (Steap3) has been demonstrated to be an endosomal ferrireductase that reduces endosomal iron for export by DMT1 [15]. Interestingly, the nm1054 mice are only iron deficient in their erythroid tissues. Consistent with this observation, Ohgami et al. observed an enriched expression of Steap3 in hematopoietic tissues.
Ohgami et al. generated a Steap3 null mouse, the reticulocytes of which exhibited a severe diminishment of Tf-derived iron uptake. If Steap3 were the sole provider of electrons to endosomal, Tf-derived Fe3+, one would expect Steap3 gene inactivation to be incompatible with life, as was the TfR knockout mouse (see above). It was therefore speculated that other members of the Steap family may perform overlapping roles with Steap3, thus accounting for not only the viability of these mice but also the lack of an extra-erythroid iron phenotype in nm1054 mice [15,16].
4.2.5. DMT1 deficiency
As mentioned above, the function of DMT1 to transport iron across both the endosomal membrane and the intestinal brush border was originally discovered in mice (mk/mk) [184] and later in rats (Belgrade) [382], both of which harbored homologous mutations (G185R) in the protein sequence. Eight years after the identification of DMT1 as the mutation in these rodents, the first human DMT1 mutation was described in a Czech patient [383]. This mutation results in a E399D substitution which, while not interfering with the function or expression of the full length protein, causes a preferential skipping of exon 12, whose transcript shows compromised expression levels [384]. Strikingly, while rats and mice exhibit a marked hypochromic microcytic anemia and systemic iron deficiency, the patient, while possessing the same anemia, had increased plasma iron levels, modestly increased serum ferritin levels, and hepatic iron overload. Iron accumulation was apparent in both liver parenchymal cells and Kupffer cells. Since the discovery of this first patient, several others with different DMT1 mutations, but the same phenotype, have been identified [385,386]. It is interesting that decreased DMT1 levels have differing effects between rodents and humans. One possible explanation for this phenomenon, as proposed by Priwitzerova et al. [387], is the difference in dietary heme levels between rodents and man. (Further details on the characterization and possible treatment strategies for patients afflicted by DMT1 mutations are reviewed in [388].)
4.2.6. Mitochondrial iron transport defects
Foury and Rotanti were the first to identify the, as yet, only known mitochondrial iron import proteins: SLC transporters, Mrs3 and Mrs4 [205]. They demonstrated that deletion of these two duplicate yeast genes prevented the accumulation of iron in mitochondria normally associated with the deletion of the yeast frataxin homolog (Yfh1). In addition, Foury and Roganti showed that Mrs4 overexpression could accelerate heme biosynthesis in yeast. A few years later, Shaw et al. uncovered a zebrafish homolog (frascati) and, subsequently, a mammalian homolog (mitoferrin 1; MFRN1, SLC25A37) to Mrs3/4 that was required for efficient erythroid development in these two species [224]. These proteins were found to be highly expressed in the hematopoietic tissues of zebrafish and mice. The frascati zebrafish had a profound hypochromic anemia and mitoferrin 1-deficient embryonic eryroblasts exhibited severely impaired iron incorporation in to heme. Importantly, a second SLC25A family member, designated MFRN2 (SLC25A28), has been shown to assume the mitochondria iron uptake role in non-erythroid cells [224,225].
4.2.7. Deficient porphyrin synthesis
The production of protoporphyrin IX (PPIX), the setting into which an Fe2+ “gem” is embedded by mitochondrial ferrochelatase, is catalyzed by seven enzymes. In general, deficiency in any of the enzymes, except the first, ALA-S2, in this pathway results in a correspondent porphyria. Discussion of the porphrias is beyond the scope of this work but is reviewed in [223]. Mutation of the first enzyme in this pathway in erythroid cells, ALA-S2, causes X-linked sideroblastic anemia (XLSA; the sideroblastic anemias are reviewed in [389] and [390]). The hallmark of sideroblastic anemia is the ring sideroblast: an erythroblast containing iron-laden mitochondria that encircle the nucleus. The accumulation of iron in the mitochondria of these cells underscores how remarkable developing red blood cells are. Under normal circumstances, these cells extract iron from the pool of plasma diferric Tf, which is effectively approximately ~3 μM, and amass an effective concentration of 20 mM within the mature erythrocyte. When these cells are unable to produce ALA, and therefore PPIX, iron continues to flow unchecked into the mitochondria. Furthermore, since “uncommitted” heme provides a negative feedback to iron acquisition, iron uptake is even accelerated under these conditions (reviewed in [27]).
While XLSA is the most common of the inherited sideroblastic anemias, there have been other mutations identified that lead to the formation of ring sideroblasts. In the first of these, X-linked sideroblastic anemia with ataxia (XLSA/A) the hematological defect is overshadowed by a prominent neurodegeneration. Patients afflicted by XLSA/A harbor a mutation in the gene encoding the ATP-binding cassette transporter ABCB7 [391]. This protein has been shown to be involved in the production of extramitochondrial Fe/S proteins (reviewed in [230]). Although the molecular mechanism leading to mitochondrial iron accumulation has not yet been determined in these patients, there are two possible explanations for this phenomenon: 1) the delivery of reduced iron to ferrochelatase is compromised; 2) the inability to produce an Fe/S on IRP1 results in increased IRP1-mediated stabilization of the TfR mRNA. It must be noted here that Fe/S protein deficiency is often associated with the accumulation of iron in mitochondria [230,392]. In fact, mutation of the mitochondrial, monothiol glutaredoxin, GLRX5, which plays an essential role in the biogenesis of Fe/S proteins, results in another of the inherited sideroblastic anemias, most likely through a convergent mechanism as that caused by ABCB7 deficiency [393]. The most recently identified hereditary sideroblastic anemia was shown to be the result of a deficiency in an uncharacterized transporter of the SLC25A family. Guernsey et al. [394] showed that SLC25A38 is an erythroid-specific, mitochondrial transporter required for efficient heme synthesis. This study further demonstrated that the heme deficiency of yeast lacking the SLC25A38 could be rescued by glycine or ALA supplementation, suggesting that this transporter is involved in the delivery of glycine into mitochondria for heme synthesis. Thus, the pathogenesis of SLC25A38-deficiency sideroblastic anemia is predicted to be similar that that of XLSA.
4.3 Diseases of Iron Overload
As with virtually any nutrient, too much can be harmful to the human body. With respect to iron metabolism, it is characterized by a limited external exchange and by an efficient reutilization from internal sources. Because of the limited ability of the body to excrete excess Fe, iron overload can develop as a result of prolonged hyperabsorption of dietary iron, by administration of excess iron parenterally (e.g., via blood transfusions), or by a combination of both factors. Patients with iron overload accumulate excessive amounts of iron in various organs including the liver, pancreas, and heart and, consequently, they suffer from conditions that include cirrhosis, diabetes, and heart dysfunction [12,395]. Without treatment, iron overload leads to death. Secondary iron overload develops in transfusion-dependent patients, such as those with thalassemia, aplastic anemia, and myelodysplastic syndrome. Repeated transfusions lead to rapid Fe loading since each unit of blood contains 200 to 250 mg of iron (1 ml of red blood cells contains approximately 1 mg of iron) that cannot be excreted. Because this iron is derived from red cells, reticuloendothelial macrophages become iron-loaded before hepatocytes and other parenchymal cells. The only treatment for secondary iron overload is administration of iron chelating agents, such as desferrioxamine [395].
The most common hereditary disease in people of European descent is hereditary hemochromatosis (HH). Table 2 lists the various types of HH. Approximately 1 in 250 people of Northern European origin are homozygous for the autosomal, recessive disorder known as type 1 HH, where the protein encoded by the HFE gene bears a C282Y substitution [12]. The consequences of this mutation are to prevent expression of the HFE protein, which closely resembles an HLA molecule, at the cell surface. Although the molecular function of HFE has not yet been definitively demonstrated, it has been suggested that the protein plays a direct role in sensing Tf saturation by interacting with TfR2 [396]. Congruent with this model, mutations in TfR2 underlie type 3 HH, which exhibits a similar pathophysiology as type 1. Disorders in which hepcidin levels are more directly affected comprise the juvenile hemochromatoses (type 2 HH) in which there is either a mutation in repulsive guidance molecule c, hemojuvelin, (type 2A) or hepcidin itself (type 2B). Type 4 HH (or ferroportin disease), is the only dominantly inherited HH, in which mutations in ferroportin affect either ferroportin’s function or its regulation by hepcidin (reviewed in [393]; c.f., Table 2). In most cases of HH, the levels of circulating hepcidin are low. However, one important exception from this pattern is a type 4 HH patient with high hepcidin concentrations in whom hepcidin binding to ferroportin was shown to be disrupted [397]. This finding supports the view that HH with a parenchymal iron-loading phenotype can be caused by either hepcidin deficiency or resistance to the effect of hepcidin. At this time, however, there is still no satisfactory model linking plasma iron levels (and the regulation thereof) with hepcidin secretion.
Table 2.
Hereditary Iron Overload
| Disease | Inheritance* | Mutated Gene | Population Frequency | Main Clinical Features | References |
|---|---|---|---|---|---|
| Hereditary hemochromatosis (HH) type 1 | AR | HFE | 1:250 | Liver disease | [12,268] |
| HH type 2 (Juvenile H) | AR AR |
a) Hemojuvelin b) Hepcidin |
Rare Rare |
Heart disease Heart disease |
[436] [438] |
| HH type 3 | AR | TfR2 | Rare | Liver disease | [457] |
| HH type 4 | AD | Ferroportin 1 | Rare | Liver disease (↑ Fe in macrophages) | [458,459] |
| Atransferrinemia | AR | Transferrin | Very rare | Liver disease | [460] |
| African Fe overload | AR | Unknown | Common | Liver disease | [461] |
AR, autosomal recessive; AD, autosomal dominant
4.4 Defects in iron release from cells to transferrin
While the above disorders result from inadequate or excessive organismal iron levels, conditions exist in which the overall systemic levels of iron are adequate, however the metal is not delivered to the sites of usage. One such condition is anemia of chronic disease (a.k.a., anemia of inflammation), in which iron is not exported from cells which normally cede their iron to apoTf: reticuloendothelial macrophages and duodenal enterocytes. This arises during inflammation caused by infection or malignancy through at least two mechanisms: 1) elevated circulating hepcidin (reviewed in [398]) and 2) increased NO production that leads to a rapid IRP2 degradation resulting in a consequent, dramatic upregulation of the synthesis of ferritin, which sequesters iron [399]. Another condition in which iron is not efficiently transferred to the plasma is functional iron deficiency, in which an elevated erythropoietic demand strips the plasma of iron faster than iron can be mobilized from its sites of storage. Hypochromic anemia of this type usually occurs only in patients who are administered erythropoietin.
5. Perspectives
What we know about iron homeostasis is likely only a small fraction of what we do not know. Table 3 lists some of the pieces of iron homeostasis that we know we do not know. The most obscure aspect of iron homeostasis is its intracellular trafficking and the enigma of how this metal encounters proteins. Hemoglobin is the only protein that has been extensively scrutinized in this regard, but even in this case our knowledge is far from complete. What we do know is that iron inserts into protoporphyrin IX at the matrix side of the inner mitochondrial membrane. However, we do not know how heme gets out of mitochondria or whether there is a chaperone, which escorts it to globin chains residing in the cytosol. The reaction between heme and apoglobin is very rapid [400,401]. This, together with a report documenting a co-translational heme binding to nascent globin chains [402] suggests a close proximity, or even interaction, of mitochondria and polyribosomes. If the “kiss-and-run” model of iron delivery from endosomes to mitochondria is undeniably proven, than one can envisage a huge molecular/organellar machine, which conveys Tf-borne iron from endosomes to the hemoglobin molecule. Our knowledge about the delivery of iron to ferritin was recently enhanced by a study [403] identifying a protein, poly (rC)-binding protein 1 (PCBP1), that increases the amount of iron loaded into ferritin when expressed in yeast, which do not express this protein. However, no evidence was provided that this protein behaves as a chaperone, but exhibits features indicating that it is a facilitator of iron entry into ferritin. Needless to say, a detailed understanding of intracellular iron/heme trafficking is essential for a full comprehension of human iron disorders.
Table 3.
What we know we don’t know about iron metabolism
I. Mechanisms
|
II. Regulation
|
We currently witness vigorous research efforts around the basolateral transporter, ferroportin, which transports ferrous iron. This export pathway is driven by the oxidation of iron at the basolateral, exosolic leaflet by hephaestin, a membrane-bound, multicopper oxidase. It can be expected that important information will soon emerge about the two new feroxidases, zyklopen and APP. Another area of intracellular iron metabolism is the assembly of Fe/S clusters. This important endeavor will predictably continue since the current state in this field still resembles a “jigsaw puzzle”, with many molecular mechanisms remaining poorly understood.
There are numerous unanswered questions related to genetically based iron deficiencies and overloads. For example, the incidence of IRIDA (iron-refractory, iron-deficiency anemia) is unknown and the molecular pathophysiology of African iron overload still awaits elucidation. The survival of patients with severe secondary iron overload depends on iron chelation therapy for survival. However, none of the available chelators is fully satisfactory; the search for more appropriate drugs will likely continue. Additionally, another clinically important field that will expectantly continue to flourish is brain iron homeostasis and its disturbance as a pathogenic factor in neurodegenerative diseases.
The role of iron in cell proliferation and cancer has received considerable attention over the last forty years [126]. Cancer cells appear to require a larger quantity of iron than normal proliferating cells [404], and there are attempts to develop iron chelating agents tailored to selectively eliminate cancer cells. We can expect an enhanced research activity in this area following a recent important report from the laboratories of Suzy and Frank Torti [405]. Their studies revealed that ferroportin protein levels are decreased in malignant breast cell lines when compared with normal mammary epithelial cells. This is caused by a decrease in ferroportin mRNA and an increase in hepcidin expression in breast cancer cells. Reduced ferroportin appears to be responsible for aggressive growth, because reintroduction of ferroportin into breast cancer cells reduced their growth following implantation into mice. The low concentrations of ferroportin protein expressed in breast cancer cells were found to be associated with higher concentrations of the LIP, suggesting that iron itself is the “culprit”. Perhaps even more importantly, ferroportin protein levels were also reduced in malignant tissue from breast cancer patients. High ferroportin levels in breast cancer patients is a very favorable indicator since such patients have a 90% 10-year survival rate.
Needless to say, “there are things we don’t know we don’t know.” This and the previous discussion indicate that the research on iron, which now spans many disciplines of medicine, will undoubtedly continue to be very active in the future. Hence, it would be premature to write the epitaph “… My fire’s extinct, my forge decayed … My coals are spent, my iron’s gone, My Nails are Drove, My Work is done.” (On William Strange, blacksmith. Epitaph, Nettlebed churchyard, England June 6, 1746).
6. Epilogue
We started this treatise with cosmological thoughts about the beginning of the Universe and our solar system, presenting the hypothesis that an early form of metabolism, driven by iron-mediated catalysis, predated genetics. Hence, it would seem appropriate to end this work by discussing a possible role of iron in the ultimate fate of the Universe. Astronomers presume that the Universe will gradually erode, provided it keeps on expanding and does not re-collapse under the pull of its own gravity. During the Stellar Era, that will end 100 trillion years in the future, most of the energy generated by the Universe is in the form of stars burning hydrogen and other elements in their cores. This will be followed by The Degenerate Era that extends to ten trillion trillion trillion years after the Big Bang. In this period, most of the mass that we can currently see in the Universe will have been locked up in degenerate stars, those that have blown up and collapsed into black holes and neutron stars. The neutron stars will also collapse eventually into black holes (1038 to 10100 years in the future), following which the black holes will slowly evaporate (due to Hawking radiation). Iron will play an important role here. It represents an intermediate stage in this process, since a lot of stars will burn up to iron before they collapse into either black holes or neutron stars. The Black Hole Era, which will end 10100 years in the future, will be followed by the Dark Era. The Universe as we know it will dissipate … [406,407].
Highlights.
Iron has been a requirement for nearly all life from the very first organisms.
We document the history of iron metabolism in health and disease.
Unique and specific systems for handling iron evolved to control its catalytic nature.
The plasma protein transferrin plays a key role in iron metabolism.
Disturbed iron homeostasis can result in catastrophic consequences for humans.
Acknowledgments
The authors thank Dr. Evan Morgan, Dr. Mark Koury, Dr. Gil Holder, Dr. Alex Rabinovitch, and Dr. Jiří Grygar for their valuable comments. We are also grateful to two anonymous reviewers for their valuable comments. This work was supported in part by the Canadian Institutes for Health Research (PP, ADS) and a USPHS Grant R01 DK21739 (ABM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollycove m, Mortimer r. the quantitative determination of iron kinetics and hemoglobin synthesis in human subjects. j Clin Invest. 1961;40:753–782. doi: 10.1172/JCI104310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shayeghi m, latunde-dada go, oakhill js, laftah ah, Takeuchi k, Halliday n, Khan y, Warley a, mccann fe, hider rc, frazer dm, anderson gj, vulpe cd, simpson rj, mckie at. identification of an intestinal heme transporter. cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Qiu a, Jansen m, Sakaris a, min sh, Chattopadhyay s, Tsai e, Sandoval c, Zhao r, akabas mh, goldman id. identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Hershko c, Graham g, bates gw, rachmilewitz ea. non-specific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. br J Haematol. 1978;40:255–263. doi: 10.1111/j.1365-2141.1978.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 5.harrison pm, hoy tg, macara ig, hoare rj. ferritin iron uptake and release. Structure-function relationships. biochem J. 1974;143:445–451. doi: 10.1042/bj1430445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.harrison pm, Arosio p. the ferritins: molecular properties, iron storage function and cellular regulation. biochimica et biophysica acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 7.Bou-abdallah f. the iron redox and hydrolysis chemistry of the ferritins. biochim Biophys Acta. 2010;1800:719–731. doi: 10.1016/j.bbagen.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee s, ghosh rn, maxfield fr. endocytosis. physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 9.maxfield fr, mcgraw te. endocytic recycling. nat Rev Mol Cell biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 10.grant bd, donaldson jg. pathways and mechanisms of endocytic recycling. nat Rev Mol Cell biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawabata h, Yang r, Hirama t, vuong pt, Kawano s, gombart af, koeffler hp. molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. j Biol Chem. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 12.Pietrangelo a. hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. gastroenterology. 2010;139:393–408. doi: 10.1053/j.gastro.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Dhungana s, taboy ch, Zak o, Larvie m, crumbliss al, Aisen p. redox properties of human transferrin bound to its receptor. biochemistry. 2004;43:205–209. doi: 10.1021/bi0353631. [DOI] [PubMed] [Google Scholar]
- 14.Ecarot-charrier b, grey vl, Wilczynska a, schulman hm. reticulocyte membrane transferrin receptors. can J Biochem. 1980;58:418–426. doi: 10.1139/o80-055. [DOI] [PubMed] [Google Scholar]
- 15.ohgami rs, campagna dr, greer el, Antiochos b, Mcdonald a, Chen j, sharp jj, Fujiwara y, barker je, fleming md. identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ohgami rs, campagna dr, Mcdonald a, fleming md. the steap proteins are metalloreductases. blood. 2006;108:1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunshin h, Mackenzie b, berger uv, Gunshin y, romero mf, boron wf, Nussberger s, gollan jl, hediger ma. cloning and characterization of a mammalian proton-coupled metal-ion transporter. nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 18.Pollycove m, Maqsood m. existence of an erythropoietic labile iron pool in animals. nature. 1962;194:152–154. doi: 10.1038/194152a0. [DOI] [PubMed] [Google Scholar]
- 19.noyes wd, Hosain f, finch ca. incorporation of radioiron into marrow heme. j Lab clin Med. 1964;64:574–580. [PubMed] [Google Scholar]
- 20.Falbe-hansen i, Lothe k. in vivo incorporation of 59 fe into nonhem iron and hemoglobin of red blood cells. acta physiol scand. 1962;54:97–104. doi: 10.1111/j.1748-1716.1962.tb02333.x. [DOI] [PubMed] [Google Scholar]
- 21.greenough wb, iii, Peters t, jr, thomas ed. an intracellular protein intermediate for hemoglobin formation. j Clin Invest. 1962;41:1116–1124. doi: 10.1172/JCI104563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.zail ss, charlton rw, torrance jd, bothwell th. studies on the formation of ferritin in red cell precursors. j Clin Invest. 1964;43:670–680. doi: 10.1172/JCI104952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.primosigh jv, thomas ed. studies on the partition of iron in bone marrow cells. j Clin Invest. 1968;47:1473–1482. doi: 10.1172/JCI105841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-medellin j, schulman hm. the kinetics of iron and transferrin incorporation into rabbit erythroid cells and the nature of stromal-bound iron. biochim Biophys Acta. 1972;264:272–274. doi: 10.1016/0304-4165(72)90291-7. [DOI] [PubMed] [Google Scholar]
- 25.zhang as, sheftel ad, Ponka p. intracellular kinetics of iron in reticulocytes: evidence for endosome involvement in iron targeting to mitochondria. blood. 2005;105:368–375. doi: 10.1182/blood-2004-06-2226. [DOI] [PubMed] [Google Scholar]
- 26.sheftel ad, zhang as, Brown c, shirihai os, Ponka p. direct interorganellar transfer of iron from endosome to mitochondrion. blood. 2007;110:125–132. doi: 10.1182/blood-2007-01-068148. [DOI] [PubMed] [Google Scholar]
- 27.Ponka p. tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. blood. 1997;89:1–25. [PubMed] [Google Scholar]
- 28.Jacobs a. an intracellular transit iron pool. ciba found Symp. 1976:91–106. doi: 10.1002/9780470720325.ch5. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs a. low molecular weight intracellular iron transport compounds. blood. 1977;50:433–439. [PubMed] [Google Scholar]
- 30.Breuer w, Shvartsman m, cabantchik zi. intracellular labile iron. int J Biochem Cell biol. 2008;40:350–354. doi: 10.1016/j.biocel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 31.esposito bp, Epsztejn s, Breuer w, cabantchik zi. a review of fluorescence methods for assessing labile iron in cells and biological fluids. anal Biochem. 2002;304:1–18. doi: 10.1006/abio.2002.5611. [DOI] [PubMed] [Google Scholar]
- 32.De domenico i, mcvey wd, Kaplan j. regulation of iron acquisition and storage: consequences for iron-linked disorders. nat Rev Mol Cell biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 33.muckenthaler mu, Galy b, hentze mw. systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (ire/irp) regulatory network. annu Rev Nutr. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- 34.hentze mw, muckenthaler mu, Galy b, Camaschella c. two to tango: regulation of mammalian iron metabolism. cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 35.koury mj, Ponka p. new insights into erythropoiesis: the roles of folate, vitamin b12, and iron. annu Rev Nutr. 2004;24:105–131. doi: 10.1146/annurev.nutr.24.012003.132306. [DOI] [PubMed] [Google Scholar]
- 36.Ponka p, sheftel ad. erythroid iron metabolism. In: anderson gj, mclaren gd., editors. iron physiology and pathophysiology in humans. humana press; 2011. in press. [Google Scholar]
- 37.Ponka p, Borova j, Neuwirt j, Fuchs o. mobilization of iron from reticulocytes. Identification of pyridoxal isonicotinoyl hydrazone as a new iron chelating agent. febs lett. 1979;97:317–321. doi: 10.1016/0014-5793(79)80111-8. [DOI] [PubMed] [Google Scholar]
- 38.Ponka p, Borova j, Neuwirt j, Fuchs o, Necas e. a study of intracellular iron metabolism using pyridoxal isonicotinoyl hydrazone and other synthetic chelating agents. biochim Biophys Acta. 1979;586:278–297. doi: 10.1016/0304-4165(79)90100-4. [DOI] [PubMed] [Google Scholar]
- 39.morgan eh. chelator-mediated iron efflux from reticulocytes. biochim Biophys Acta. 1983;733:39–50. doi: 10.1016/0005-2736(83)90089-5. [DOI] [PubMed] [Google Scholar]
- 40.Cianetti l, Segnalini p, Calzolari a, Morsilli o, Felicetti f, Ramoni c, Gabbianelli m, Testa u, sposi nm. expression of alternative transcripts of ferroportin-1 during human erythroid differentiation. haematologica. 2005;90:1595–1606. [PubMed] [Google Scholar]
- 41.zhang dl, hughes rm, Ollivierre-wilson h, ghosh mc, rouault ta. a ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. cell metab. 2009;9:461–473. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.kailis sg, morgan eh. transferrin and iron uptake by rabbit bone marrow cells in vitro. br J Haematol. 1974;28:37–52. doi: 10.1111/j.1365-2141.1974.tb06638.x. [DOI] [PubMed] [Google Scholar]
- 43.richardson dr, Ponka p. the molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. biochimica et biophysica acta. 1997;1331:1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- 44.Ponka p. hereditary causes of disturbed iron homeostasis in the central nervous system. ann N Y Acad Sci. 2004;1012:267–281. doi: 10.1196/annals.1306.022. [DOI] [PubMed] [Google Scholar]
- 45.Rous p. destruction of the red blood corpuscles in health and disease. physiol Rev. 1923;3:75–105. [Google Scholar]
- 46.Rous p. a sarcoma of the fowl transmissible by an agent separable from the tumor cells. j Exp Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tenhunen r, marver hs, Schmid r. the enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjöstrand t. endogenous formation of carbon monoxide in man. nature. 1949;164:580. doi: 10.1038/164580a0. [DOI] [PubMed] [Google Scholar]
- 49.Sjöstrand t. formation of carbon monoxide in connexion with haemoglobin catabolism. nature. 1951;168:1118–1119. doi: 10.1038/1681118a0. [DOI] [PubMed] [Google Scholar]
- 50.Nicloux m. sur la prèsence de l’oxyde de carbone dans le sang du nouveau-né. c R Hebd Acad Sci. 1901;132:1501–1504. [Google Scholar]
- 51.maines md. the heme oxygenase system: past, present, and future. antioxid Redox Signal. 2004;6:797–801. doi: 10.1089/ars.2004.6.797. [DOI] [PubMed] [Google Scholar]
- 52.maines md, gibbs pe. 30 some years of heme oxygenase: from a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. biochem Biophys Res Commun. 2005;338:568–577. doi: 10.1016/j.bbrc.2005.08.121. [DOI] [PubMed] [Google Scholar]
- 53.Soe-lin s, sheftel ad, Wasyluk b, Ponka p. nramp1 equips macrophages for efficient iron recycling. exp Hematol. 2008;36:929–937. doi: 10.1016/j.exphem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Soe-lin s, apte ss, Andriopoulos b, jr, andrews mc, Schranzhofer m, Kahawita t, Garcia-santos d, Ponka p. nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. proc Natl Acad Sci U S A. 2009;106:5960–5965. doi: 10.1073/pnas.0900808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soe-lin s, apte ss, mikhael mr, kayembe lk, Nie g, Ponka p. both nramp1 and dmt1 are necessary for efficient macrophage iron recycling. exp Hematol. 2010;38:609–617. doi: 10.1016/j.exphem.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida t, Sato m. posttranslational and direct integration of heme oxygenase into microsomes. biochem Biophys Res Commun. 1989;163:1086–1092. doi: 10.1016/0006-291x(89)92332-2. [DOI] [PubMed] [Google Scholar]
- 57.knutson md, vafa mr, haile dj, Wessling-resnick m. iron loading and erythrophagocytosis increase ferroportin 1 (fpn1) expression in j774 macrophages. blood. 2003;102:4191–4197. doi: 10.1182/blood-2003-04-1250. [DOI] [PubMed] [Google Scholar]
- 58.Delaby c, Pilard n, Hetet g, Driss f, Grandchamp b, Beaumont c, Canonne-hergaux f. a physiological model to study iron recycling in macrophages. exp Cell res. 2005;310:43–53. doi: 10.1016/j.yexcr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Marro s, Chiabrando d, Messana e, Stolte j, Turco e, Tolosano e, muckenthaler mu. heme controls ferroportin1 (fpn1) transcription involving bach1, nrf2 and a mare/are sequence motif at position −7007 of the fpn1 promoter. haematologica. 2010;95:1261–1268. doi: 10.3324/haematol.2009.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fillet g, cook jd, finch ca. storage iron kinetics. Vii. A biologic model for reticuloendothelial iron transport. j Clin Invest. 1974;53:1527–1533. doi: 10.1172/JCI107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fillet g, Beguin y, Baldelli l. model of reticuloendothelial iron metabolism in humans: abnormal behavior in idiopathic hemochromatosis and in inflammation. blood. 1989;74:844–851. [PubMed] [Google Scholar]
- 62.Hershko c. storage iron regulation. prog Hematol. 1977;10:105–148. [PubMed] [Google Scholar]
- 63.Nemeth e, tuttle ms, Powelson j, vaughn mb, Donovan a, ward dm, Ganz t, Kaplan j. hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 64.Wächtershäuser g. evolution of the first metabolic cycles. proc Natl Acad Sci U S A. 1990;87:200–204. doi: 10.1073/pnas.87.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wächtershäuser g. origin of life. Life as we don’t know it. science. 2000;289:1307–1308. doi: 10.1126/science.289.5483.1307. [DOI] [PubMed] [Google Scholar]
- 66.williams rj. biomineralization: iron and the origin of life. nature. 1990;343:213–214. doi: 10.1038/343213a0. [DOI] [PubMed] [Google Scholar]
- 67.Pleiner r, bjorkman jk. assyrian iron age - history of iron in assyrian civilization. proceedings of the american philosophical society. 1974;118:283–313. [Google Scholar]
- 68.fairbanks vf, fahey jl, Beutler e. clinical disorders of iron metabolism. grune & stratton; new york & london: 1971. [Google Scholar]
- 69.Vanotti a, Delachaux a. iron metabolism and its clinical significance. grune & stratton; new york: 1949. [Google Scholar]
- 70.Monarde n. joyful newes out of the newe founde worlde. new york: 1925. [Google Scholar]
- 71.Beutler e. history of iron in medicine. blood cells mol Dis. 2002;29:297–308. doi: 10.1006/bcmd.2002.0560. [DOI] [PubMed] [Google Scholar]
- 72.Busacchi v. [vincenzo menghini and the discovery of iron in the blood] bull Sci Med (bologna) 1958;130:202–205. [PubMed] [Google Scholar]
- 73.lecanu lr, l’hématosine de. o matière colorante du sang. ann Chim. 1830;45:5–27. [Google Scholar]
- 74.lecanu lr. nouvelle recherches sur le sang. ann Chim. 1831;48:308–327. [Google Scholar]
- 75.brumberg jj. chlorotic girls, 1870–1920: a historical perspective on female adolescence. child dev. 1982;53:1468–1477. [PubMed] [Google Scholar]
- 76.Blaud p. sur les maladies chlorotiques, et sur un mode de traitement specifique dans ces affections. rev Med Franc Etrang. 1832;45:341–367. [Google Scholar]
- 77.hudson rp. the biography of disease: lessons from chlorosis. bull Hist med. 1977;51:448–463. [PubMed] [Google Scholar]
- 78.fenton hjh. on a new reaction or tartaric acid. chemical news. 1876;33:190. [Google Scholar]
- 79.fenton hjh. the oxidation of tartaric acid in presence of iron. chem Soc Proc. 1893;9:113. [Google Scholar]
- 80.mccord jm, Fridovich i. superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) j Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 81.gutteridge jmc, Halliwell b. iron and oxygen: a dangerous mixture. In: Ponka p, schulman hm, woodworth rc., editors. iron transport and storage. crc press; boca raton: 1990. pp. 55–65. [Google Scholar]
- 82.Halliwell b. the wanderings of a free radical. free radic Biol Med. 2009;46:531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 83.Halliwell b. free radicals and antioxidants - quo vadis? trends pharmacol Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 84.murphy mp, Holmgren a, larsson ng, Halliwell b, chang cj, Kalyanaraman b, rhee sg, thornalley pj, Partridge l, Gems d, Nystrom t, Belousov v, schumacker pt, winterbourn cc. unraveling the biological roles of reactive oxygen species. cell metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fontés g, Thivolle l. sur la teneur du sérum en fer non hémoglobinique et sur sa diminution au cours de l’anémie expérimentale. compt Rend Soc Biol. 1925;96:687. [Google Scholar]
- 86.holmberg cg, Laurell c-b. studies on the capacity of serum to bind iron - a contribution to our knowledge of the regulation mechanism of serum iron. acta physiol Scand. 1945;10:307. [Google Scholar]
- 87.schade al, Caroline l. raw hen egg white and the role of iron in growth inhibition of shigella dysenteriae, staphylococcus aureus, escherichia coli and saccharomyces cerevisiae. science. 1944;100:14–15. doi: 10.1126/science.100.2584.14. [DOI] [PubMed] [Google Scholar]
- 88.Alderton g, ward wh, fevold hl. identification of the bacteria-inhibiting iron-binding protein of egg white as conalbumin. arch Biochem. 1946;11:9–13. [PubMed] [Google Scholar]
- 89.schade al, Caroline l. an iron-binding component in human blood plasma. science. 1946;104:340–341. doi: 10.1126/science.104.2702.340. [DOI] [PubMed] [Google Scholar]
- 90.schade al, reinhart rw, Levy h. carbon dioxide and oxygen in complex formation with iron and siderophilin, the iron-binding component of human plasma. arch Biochem. 1949;20:170–172. [PubMed] [Google Scholar]
- 91.Laufberger v. sur la cristallisation de la ferritine. bull Soc Chim Biol. 1937;19:1575–1582. [Google Scholar]
- 92.Schuh a, Fritsch a, Heim m, Shore a, Thoennessen m. discovery of the iron isotopes. atomic data and nuclear data tables. 2010;96:817–823. [Google Scholar]
- 93.mccance ra, widdowson em. the absorption and excretion of iron following oral and intravenous administration. j Physiol. 1938;94:148–154. doi: 10.1113/jphysiol.1938.sp003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dubach r, moore cv, Callender s. studies in iron transportation and metabolism. Ix. The excretion of iron as measured by the isotope technique. j Lab clin Med. 1955;45:599–615. [PubMed] [Google Scholar]
- 95.finch ca. body iron exchange in man. j Clin Invest. 1959;38:392–396. doi: 10.1172/JCI103813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.bonnet jd, orvis al, hagedorn ab, owen ca., jr rate of loss of radioiron from mouse and man. am J Physiol. 1960;198:784–786. doi: 10.1152/ajplegacy.1960.198.4.784. [DOI] [PubMed] [Google Scholar]
- 97.hahn pf, bale wf, ross jf, balfour wm, whipple gh. radioactive iron absorption by gastro-intestinal tract: influence of anemia, anoxia, and antecedent feeding distribution in growing dogs. j Exp Med. 1943;78:169–188. doi: 10.1084/jem.78.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.roy cn, andrews nc. anemia of inflammation: the hepcidin link. curr Opin Hematol. 2005;12:107–111. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 99.simpson rj, mckie at. regulation of intestinal iron absorption: the mucosa takes control? cell metab. 2009;10:84–87. doi: 10.1016/j.cmet.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 100.knutson md. iron-sensing proteins that regulate hepcidin and enteric iron absorption. annu Rev Nutr. 2010;30:149–171. doi: 10.1146/annurev.nutr.012809.104801. [DOI] [PubMed] [Google Scholar]
- 101.bothwell th, Pirzio-biroli g, finch ca. iron absorption. I. Factors influencing absorption. j Lab clin Med. 1958;51:24–36. [PubMed] [Google Scholar]
- 102.Krantz s, Goldwasser e, jacobson lo. studies on erythropoiesis. Xiv. The relationship of humoral stimulation to iron absorption. blood. 1959;14:654–661. [PubMed] [Google Scholar]
- 103.Pirzio-biroli g, finch ca. iron absorption. Iii. The influence of iron stores on iron absorption in the normal subject. j Lab clin Med. 1960;55:216–220. [PubMed] [Google Scholar]
- 104.bothwell th, charlton rw, cook jd, finch ca. iron metabolism in man. blackwell scientific publications; oxford, england: 1979. [Google Scholar]
- 105.walsh rj, thomas ed, chow sk, fluharty rg, finch ca. iron metabolism. Heme synthesis in vitro by immature erythrocytes. science. 1949;110:396–398. doi: 10.1126/science.110.2859.396. [DOI] [PubMed] [Google Scholar]
- 106.bessis mc, Breton-gorius j. ferrtin and ferruginous micelles in normal erythroblasts and hypochromic hypersideremic anemias. blood. 1959;14:423–432. [PubMed] [Google Scholar]
- 107.jandl jh, inman jk, simmons rl, allen dw. transfer of iron from serum iron-binding protein to human reticulocytes. j Clin Invest. 1959;38:161–185. doi: 10.1172/JCI103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.jandl jh, katz jh. the plasma-to-cell cycle of transferrin. j Clin Invest. 1963;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ponka p, richardson dr. can ferritin provide iron for hemoglobin synthesis? blood. 1997;89:2611–2613. [PubMed] [Google Scholar]
- 110.Paoletti c, Durand m, Gosse c, Boiron m. [absence of consummation of siderophilin during hemoglobin synthesis in vitro] rev Fr Etud Clin Biol. 1958;3:259–261. [PubMed] [Google Scholar]
- 111.katz jh. iron and protein kinetics studied by means of doubly labeled human crystalline transferrin. j Clin Invest. 1961;40:2143–2152. doi: 10.1172/JCI104440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.morgan eh, appleton tc. autoradiographic localization of 125-i-labelled transferrin in rabbit reticulocytes. nature. 1969;223:1371–1372. doi: 10.1038/2231371a0. [DOI] [PubMed] [Google Scholar]
- 113.Appleton tc, Morgan e, Baker e. a morphological study of transferrin uptake by reticulocytes. In: Trávníèek t, Neuwirt j., editors. the regulation of erythropoiesis and haemoglobin synthesis. universita karlova; prague: 1971. p. 310. [Google Scholar]
- 114.morgan eh. inhibition of reticulocyte iron uptake by nh4cl and ch3nh2. biochim Biophys Acta. 1981;642:119–134. doi: 10.1016/0005-2736(81)90143-7. [DOI] [PubMed] [Google Scholar]
- 115.Ecarot-charrier b, Grey v, Wilczynska a, schulman hm. the isolation of transferrin receptors from reticulocyte membranes. In: brown eb, Aisen p, Fielding j, crichton rr., editors. proteins of iron metabolism. grune & stratton; new york: 1977. p. 291. [Google Scholar]
- 116.morgan eh. transferrin: biochemistry, physiology and clinical significance. In: Baum h, Gergely j., editors. molecular aspects of medicine. pergamon press; oxford: 1981. pp. 1–123. [Google Scholar]
- 117.Borova j, Ponka p, Neuwirt j. study of intracellular iron distribution in rabbit reticulocytes with normal and inhibited heme synthesis. biochim Biophys Acta. 1973;320:143–156. doi: 10.1016/0304-4165(73)90174-8. [DOI] [PubMed] [Google Scholar]
- 118.sly da, Grohlich d, Bezkorovainy a. transferrin in the reticulocyte cytosol. biochim Biophys Acta. 1975;385:36–40. doi: 10.1016/0304-4165(75)90071-9. [DOI] [PubMed] [Google Scholar]
- 119.Isobe k, Isobe y, Sakurami t. cytochemical demonstration of transferrin in the mitochondria of immature human erythroid cells. acta haematol. 1981;65:2–9. doi: 10.1159/000207141. [DOI] [PubMed] [Google Scholar]
- 120.Karin m, Mintz b. receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. j Biol Chem. 1981;256:3245–3252. [PubMed] [Google Scholar]
- 121.Harding c, Heuser j, Stahl p. receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. j Cell biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.klausner rd, Ashwell g, van rj, harford jb, bridges kr. binding of apotransferrin to k562 cells: explanation of the transferrin cycle. proc Natl Acad Sci U S A. 1983;80:2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dautry-varsat a, Ciechanover a, lodish hf. ph and the recycling of transferrin during receptor-mediated endocytosis. proc Natl Acad Sci U S A. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ciechanover a, schwartz al, Dautry-varsat a, lodish hf. kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. j Biol Chem. 1983;258:9681–9689. [PubMed] [Google Scholar]
- 125.klausner rd, Van renswoude j, Ashwell g, Kempf c, schechter an, Dean a, bridges kr. receptor-mediated endocytosis of transferrin in k562 cells. j Biol Chem. 1983;258:4715–4724. [PubMed] [Google Scholar]
- 126.Kühn l, schulman hm, Ponka p. iron-transferrin requirements and transferrin receptor expression in proliferating cells. In: Ponka p, schulman hm, woodworth rc., editors. iron transport and storage. crc press; boca raton: 1990. pp. 149–191. [Google Scholar]
- 127.Ponka p, lok cn. the transferrin receptor: role in health and disease. int J Biochem Cell biol. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 128.Gruenheid s, Cellier m, Vidal s, Gros p. identification and characterization of a second mouse nramp gene. genomics. 1995;25:514–525. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- 129.Mazur a, Carleton a. relation of ferritin iron to heme synthesis in marrow and reticulocytes. j Biol Chem. 1963;238:1817–1824. [PubMed] [Google Scholar]
- 130.nunez mt, Glass j, robinson sh. mobilization of iron from the plasma membrane of the murine reticulocyte. The role of ferritin. biochim Biophys Acta. 1978;509:170–180. doi: 10.1016/0005-2736(78)90017-2. [DOI] [PubMed] [Google Scholar]
- 131.Vaisman b, Fibach e, konijn am. utilization of intracellular ferritin iron for hemoglobin synthesis in developing human erythroid precursors. blood. 1997;90:831–838. [PubMed] [Google Scholar]
- 132.grasso ja, hillis tj, mooney-frank ja. ferritin is not a required intermediate for iron utilization in heme synthesis. biochim Biophys Acta. 1984;797:247–255. doi: 10.1016/0304-4165(84)90128-4. [DOI] [PubMed] [Google Scholar]
- 133.richardson dr, Ponka p, Vyoral d. distribution of iron in reticulocytes after inhibition of heme synthesis with succinylacetone: examination of the intermediates involved in iron metabolism. blood. 1996;87:3477–3488. [PubMed] [Google Scholar]
- 134.Picard v, Renaudie f, Porcher c, hentze mw, Grandchamp b, Beaumont c. overexpression of the ferritin h subunit in cultured erythroid cells changes the intracellular iron distribution. blood. 1996;87:2057–2064. [PubMed] [Google Scholar]
- 135.Darshan d, Vanoaica l, Richman l, Beermann f, kühn lc. conditional deletion of ferritin h in mice induces loss of iron storage and liver damage. hepatology. 2009;50:852–860. doi: 10.1002/hep.23058. [DOI] [PubMed] [Google Scholar]
- 136.De domenico i, vaughn mb, Li l, Bagley d, Musci g, ward dm, Kaplan j. ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. embo j. 2006;25:5396–5404. doi: 10.1038/sj.emboj.7601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang y, Mikhael m, Xu d, Li y, Soe-lin s, Ning b, Li w, Nie g, Zhao y, Ponka p. lysosomal proteolysis is the primary degradation pathway for cytosolic ferritin and cytosolic ferritin degradation is necessary for iron exit. antioxid Redox Signal. 2010;13:999–1009. doi: 10.1089/ars.2010.3129. [DOI] [PubMed] [Google Scholar]
- 138.hu hy, Aisen p. molecular characteristics of the transferrin-receptor complex of the rabbit reticulocyte. j Supramol Struct. 1978;8:349–360. doi: 10.1002/jss.400080312. [DOI] [PubMed] [Google Scholar]
- 139.witt dp, woodworth rc. identification of the transferrin receptor of the rabbit reticulocyte. biochemistry. 1978;17:3913–3917. doi: 10.1021/bi00612a004. [DOI] [PubMed] [Google Scholar]
- 140.enns ca, sussman hh. physical characterization of the transferrin receptor in human placentae. j Biol Chem. 1981;256:9820–9823. [PubMed] [Google Scholar]
- 141.bramwell me, Harris h. an abnormal membrane glycoprotein associated with malignancy in a wide range of different tumours. proc R Soc Lond b biol Sci. 1978;201:87–106. doi: 10.1098/rspb.1978.0034. [DOI] [PubMed] [Google Scholar]
- 142.omary mb, trowbridge is, Minowada j. human cell-surface glycoprotein with unusual properties. nature. 1980;286:888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- 143.Sutherland r, Delia d, Schneider c, Newman r, Kemshead j, Greaves m. ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. proc Natl Acad Sci U S A. 1981;78:4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.trowbridge is, omary mb. human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. proc Natl Acad Sci U S A. 1981;78:3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.may ws, jr, Cuatrecasas p. transferrin receptor: its biological significance. j Membr Biol. 1985;88:205–215. doi: 10.1007/BF01871086. [DOI] [PubMed] [Google Scholar]
- 146.Finkel t, cooper gm. detection of a molecular complex between ras proteins and transferrin receptor. cell. 1984;36:1115–1121. doi: 10.1016/0092-8674(84)90062-x. [DOI] [PubMed] [Google Scholar]
- 147.Harford j. an artefact explains the apparent association of the transferrin receptor with a ras gene product. nature. 1984;311:673–675. doi: 10.1038/311673a0. [DOI] [PubMed] [Google Scholar]
- 148.Ponka p, schulman hm. acquisition of iron from transferrin regulates reticulocyte heme synthesis. j Biol Chem. 1985;260:14717–14721. [PubMed] [Google Scholar]
- 149.Laskey j, Webb i, schulman hm, Ponka p. evidence that transferrin supports cell proliferation by supplying iron for dna synthesis. exp Cell res. 1988;176:87–95. doi: 10.1016/0014-4827(88)90123-1. [DOI] [PubMed] [Google Scholar]
- 150.sheftel ad, Stehling o, pierik aj, netz dj, Kerscher s, elsasser hp, Wittig i, Balk j, Brandt u, Lill r. human ind1, an iron-sulfur cluster assembly factor for respiratory complex i. mol Cell biol. 2009;29:6059–6073. doi: 10.1128/MCB.00817-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Granick s. protein apoferritin and ferritin in iron feeding and absorption. science. 1946;103:107. [PubMed] [Google Scholar]
- 152.fineberg ra, greenberg dm. ferritin biosynthesis. Ii. Acceleration of synthesis by the administration of iron. j Biol Chem. 1955;214:97–106. [PubMed] [Google Scholar]
- 153.fineberg ra, greenberg dm. ferritin biosynthesis. Iii. Apoferritin, the initial product. j Biol Chem. 1955;214:107–113. [PubMed] [Google Scholar]
- 154.Bailey-wood r, white gp, Jacobs a. the use of chang cells cultured in vitro for the investigation of cellular iron metabolism. br J Exp Pathol. 1975;56:358–362. [PMC free article] [PubMed] [Google Scholar]
- 155.drysdale jw, munro hn. failure of actinomycin d to prevent induction of liver apoferritin after iron administration. biochim Biophys Acta. 1965;103:185–188. doi: 10.1016/0005-2787(65)90554-x. [DOI] [PubMed] [Google Scholar]
- 156.Zahringer j, baliga bs, munro hn. novel mechanism for translational control in regulation of ferritin synthesis by iron. proc Natl Acad Sci U S A. 1976;73:857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aziz n, munro hn. iron regulates ferritin mrna translation through a segment of its 5′ untranslated region. proc Natl Acad Sci U S A. 1987;84:8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.leibold ea, munro hn. cytoplasmic protein binds in vitro to a highly conserved sequence in the 5′ untranslated region of ferritin heavy- and light-subunit mrnas. proc Natl Acad Sci U S A. 1988;85:2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.ward jh, Jordan i, kushner jp, Kaplan j. heme regulation of hela cell transferrin receptor number. j Biol Chem. 1984;259:13235–13240. [PubMed] [Google Scholar]
- 160.ward jh, kushner jp, Kaplan j. regulation of hela cell transferrin receptors. j Biol Chem. 1982;257:10317–10323. [PubMed] [Google Scholar]
- 161.Owen d, kühn lc. noncoding 3′ sequences of the transferrin receptor gene are required for mrna regulation by iron. embo j. 1987;6:1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.kühn lc, Mcclelland a, ruddle fh. gene transfer, expression, and molecular cloning of the human transferrin receptor gene. cell. 1984;37:95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- 163.Schneider c, owen mj, Banville d, williams jg. primary structure of human transferrin receptor deduced from the mrna sequence. nature. 1984;311:675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- 164.Rao k, harford jb, Rouault t, Mcclelland a, ruddle fh, klausner rd. transcriptional regulation by iron of the gene for the transferrin receptor. mol Cell biol. 1986;6:236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.casey jl, di jb, rao kk, rouault ta, klausner rd, harford jb. deletional analysis of the promoter region of the human transferrin receptor gene. nucleic acids res. 1988;16:629–646. doi: 10.1093/nar/16.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.casey jl, hentze mw, koeller dm, caughman sw, rouault ta, klausner rd, harford jb. iron-responsive elements: regulatory rna sequences that control mrna levels and translation. science. 1988;240:924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- 167.koeller dm, casey jl, hentze mw, gerhardt em, chan ln, klausner rd, harford jb. a cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mrna. proc Natl Acad Sci U S A. 1989;86:3574–3578. doi: 10.1073/pnas.86.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.kühn lc, hentze mw. coordination of cellular iron metabolism by post-transcriptional gene regulation. j Inorg Biochem. 1992;47:183–195. doi: 10.1016/0162-0134(92)84064-t. [DOI] [PubMed] [Google Scholar]
- 169.cox tc, bawden mj, Martin a, may bk. human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mrna. embo j. 1991;10:1891–1902. doi: 10.1002/j.1460-2075.1991.tb07715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dandekar t, Stripecke r, gray nk, Goossen b, Constable a, johansson he, hentze mw. identification of a novel iron-responsive element in murine and human erythroid delta-aminolevulinic acid synthase mrna. embo j. 1991;10:1903–1909. doi: 10.1002/j.1460-2075.1991.tb07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.cotter pd, drabkin ha, Varkony t, smith di, bishop df. assignment of the human housekeeping delta-aminolevulinate synthase gene (alas1) to chromosome band 3p21.1 by pcr analysis of somatic cell hybrids. cytogenet Cell genet. 1995;69:207–208. doi: 10.1159/000133964. [DOI] [PubMed] [Google Scholar]
- 172.cotter pd, willard hf, gorski jl, bishop df. assignment of human erythroid delta-aminolevulinate synthase (alas2) to a distal subregion of band xp11.21 by pcr analysis of somatic cell hybrids containing x; autosome translocations. genomics. 1992;13:211–212. doi: 10.1016/0888-7543(92)90223-f. [DOI] [PubMed] [Google Scholar]
- 173.Ponka p, Neuwirt j. the use of reticulocytes with high non-haem iron pool for studies of regulation of haem synthesis. br J Haematol. 1970;19:593–604. doi: 10.1111/j.1365-2141.1970.tb01643.x. [DOI] [PubMed] [Google Scholar]
- 174.Ponka p, Neuwirt j, Borova j. the use of exogenous-aminolaevulinic acid for the studies of the regulation of haem synthesis in rabbit reticulocytes. biochim Biophys Acta. 1973;304:123–131. doi: 10.1016/0304-4165(73)90121-9. [DOI] [PubMed] [Google Scholar]
- 175.laskey jd, Ponka p, schulman hm. control of heme synthesis during friend cell differentiation: role of iron and transferrin. j Cell physiol. 1986;129:185–192. doi: 10.1002/jcp.1041290209. [DOI] [PubMed] [Google Scholar]
- 176.schmidt ja, Marshall j, hayman mj, Ponka p, Beug h. control of erythroid differentiation: possible role of the transferrin cycle. cell. 1986;46:41–51. doi: 10.1016/0092-8674(86)90858-5. [DOI] [PubMed] [Google Scholar]
- 177.Ponka p, schulman hm. regulation of heme biosynthesis: distinct regulatory features in erythroid cells. stem cells. 1993;11(suppl 1):24–35. doi: 10.1002/stem.5530110607. [DOI] [PubMed] [Google Scholar]
- 178.sadlon tj, Dell’oso t, surinya kh, may bk. regulation of erythroid 5-aminolevulinate synthase expression during erythropoiesis. int J Biochem Cell biol. 1999;31:1153–1167. doi: 10.1016/s1357-2725(99)00073-4. [DOI] [PubMed] [Google Scholar]
- 179.Schranzhofer m, Schifrer m, cabrera ja, Kopp s, Chiba p, Beug h, mullner ew. remodeling the regulation of iron metabolism during erythroid differentiation to ensure efficient heme biosynthesis. blood. 2006;107:4159–4167. doi: 10.1182/blood-2005-05-1809. [DOI] [PubMed] [Google Scholar]
- 180.plant je, blackwell jm, o’brien ad, bradley dj, glynn aa. are the lsh and ity disease resistance genes at one locus on mouse chromosome 1? nature. 1982;297:510–511. doi: 10.1038/297510a0. [DOI] [PubMed] [Google Scholar]
- 181.Gros p, Skamene e, Forget a. genetic control of natural resistance to mycobacterium bovis (bcg) in mice. j Immunol. 1981;127:2417–2421. [PubMed] [Google Scholar]
- 182.Skamene e, Gros p, Forget a, kongshavn pa, st cc, taylor ba. genetic regulation of resistance to intracellular pathogens. nature. 1982;297:506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- 183.vidal sm, Malo d, Vogan k, Skamene e, Gros p. natural resistance to infection with intracellular parasites: isolation of a candidate for bcg. cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 184.fleming md, trenor cc, iii, su ma, Foernzler d, beier dr, dietrich wf, andrews nc. microcytic anaemia mice have a mutation in nramp2, a candidate iron transporter gene. nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 185.Gruenheid s, Canonne-hergaux f, Gauthier s, hackam dj, Grinstein s, Gros p. the iron transport protein nramp2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. j Exp Med. 1999;189:831–841. doi: 10.1084/jem.189.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.forbes jr, Gros p. divalent-metal transport by nramp proteins at the interface of host-pathogen interactions. trends microbiol. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- 187.Abboud s, haile dj. a novel mammalian iron-regulated protein involved in intracellular iron metabolism. j Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 188.mckie at, Marciani p, Rolfs a, Brennan k, Wehr k, Barrow d, Miret s, Bomford a, peters tj, Farzaneh f, hediger ma, hentze mw, simpson rj. a novel duodenal iron-regulated transporter, ireg1, implicated in the basolateral transfer of iron to the circulation. mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 189.Donovan a, Brownlie a, Zhou y, Shepard j, pratt sj, Moynihan j, paw bh, Drejer a, Barut b, Zapata a, law tc, Brugnara c, lux se, pinkus gs, pinkus jl, kingsley pd, Palis j, fleming md, andrews nc, zon li. positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 190.holmberg cg, laurell cb. investigations in serum copper; nature of serum copper and its relation to the iron-binding protein in human serum. acta chem Scand. 1947;1:944–950. doi: 10.3891/acta.chem.scand.01-0944. [DOI] [PubMed] [Google Scholar]
- 191.holmberg cg, laurell cb. oxidase reactions in human plasma caused by coeruloplasmin. scand J Clin Lab invest. 1951;3:103–107. doi: 10.3109/00365515109060581. [DOI] [PubMed] [Google Scholar]
- 192.porter cc, titus dc, sanders be, smith ev. oxidation of serotonin in the presence of ceruloplasmin. science. 1957;126:1014–1015. doi: 10.1126/science.126.3281.1014. [DOI] [PubMed] [Google Scholar]
- 193.houchin ob. a rapid colorimetric method for the quantitative determination of copper oxidase activity (ceruloplasmin) clin Chem. 1958;4:519–523. [PubMed] [Google Scholar]
- 194.Osaki s, johnson da, Frieden e. the possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. j Biol Chem. 1966;241:2746–2751. [PubMed] [Google Scholar]
- 195.Osaki s, johnson da. mobilization of liver iron by ferroxidase (ceruloplasmin) j Biol Chem. 1969;244:5757–5758. [PubMed] [Google Scholar]
- 196.Osaki s, johnson da, Frieden e. the mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase i. j Biol Chem. 1971;246:3018–3023. [PubMed] [Google Scholar]
- 197.harris zl, durley ap, man tk, gitlin jd. targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. proc Natl Acad Sci U S A. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.patel bn, David s. a novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. j Biol Chem. 1997;272:20185–20190. doi: 10.1074/jbc.272.32.20185. [DOI] [PubMed] [Google Scholar]
- 199.patel bn, dunn rj, David s. alternative rna splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. j Biol Chem. 2000;275:4305–4310. doi: 10.1074/jbc.275.6.4305. [DOI] [PubMed] [Google Scholar]
- 200.jeong sy, David s. glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. j Biol Chem. 2003;278:27144–27148. doi: 10.1074/jbc.M301988200. [DOI] [PubMed] [Google Scholar]
- 201.Kono s, Yoshida k, Tomosugi n, Terada t, Hamaya y, Kanaoka s, Miyajima h. biological effects of mutant ceruloplasmin on hepcidin-mediated internalization of ferroportin. biochim Biophys acta. 2010;1802:968–975. doi: 10.1016/j.bbadis.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 202.hellman ne, gitlin jd. ceruloplasmin metabolism and function. annu Rev Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- 203.Askwith c, Eide d, van ha, bernard ps, Li l, Davis-kaplan s, sipe dm, Kaplan j. the fet3 gene of s. Cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. cell. 1994;76:403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 204.Stearman r, yuan ds, Yamaguchi-iwai y, klausner rd, Dancis a. a permease-oxidase complex involved in high-affinity iron uptake in yeast. science. 1996;271:1552–1557. doi: 10.1126/science.271.5255.1552. [DOI] [PubMed] [Google Scholar]
- 205.Foury f, Roganti t. deletion of the mitochondrial carrier genes mrs3 and mrs4 suppresses mitochondrial iron accumulation in a yeast frataxin-deficient strain. j Biol Chem. 2002;277:24475–24483. doi: 10.1074/jbc.M111789200. [DOI] [PubMed] [Google Scholar]
- 206.Mühlenhoff u, stadler ja, Richhardt n, Seubert a, Eickhorst t, schweyen rj, Lill r, Wiesenberger g. a specific role of the yeast mitochondrial carriers mrs3/4p in mitochondrial iron acquisition under iron-limiting conditions. j Biol Chem. 2003;278:40612–40620. doi: 10.1074/jbc.M307847200. [DOI] [PubMed] [Google Scholar]
- 207.vulpe cd, kuo ym, murphy tl, Cowley l, Askwith c, Libina n, Gitschier j, anderson gj. hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. nat Genet. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- 208.Chen h, attieh zk, syed ba, kuo ym, Stevens v, fuqua bk, andersen hs, naylor ce, evans rw, Gambling l, Danzeisen r, Bacouri-haidar m, Usta j, vulpe cd, mcardle hj. identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells. j Nutr. 2010;140:1728–1735. doi: 10.3945/jn.109.117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.duce ja, Tsatsanis a, cater ma, james sa, Robb e, Wikhe k, leong sl, Perez k, Johanssen t, greenough ma, cho hh, Galatis d, moir rd, masters cl, Mclean c, tanzi re, Cappai r, barnham kj, ciccotosto gd, rogers jt, bush ai. iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in alzheimer’s disease. cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.mckie at, Barrow d, latunde-dada go, Rolfs a, Sager g, Mudaly e, Mudaly m, Richardson c, Barlow d, Bomford a, peters tj, raja kb, Shirali s, hediger ma, Farzaneh f, simpson rj. an iron-regulated ferric reductase associated with the absorption of dietary iron. science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- 211.Gunshin h, starr cn, Direnzo c, fleming md, Jin j, greer el, sellers vm, galica sm, andrews nc. cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. blood. 2005;106:2879–2883. doi: 10.1182/blood-2005-02-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.austin jh, pearce rm. the relation of the spleen to blood destruction and regeneration and to hemolytic jaundice: xi. The influence of the spleen on iron metabolism. j Exp Med. 1914;20:122–129. doi: 10.1084/jem.20.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pandolfo m, Pastore a. the pathogenesis of friedreich ataxia and the structure and function of frataxin. j Neurol. 2009;256(suppl 1):9–17. doi: 10.1007/s00415-009-1003-2. [DOI] [PubMed] [Google Scholar]
- 214.Babcock m, de sd, Oaks r, Davis-kaplan s, Jiralerspong s, Montermini l, Pandolfo m, Kaplan j. regulation of mitochondrial iron accumulation by yfh1p, a putative homolog of frataxin. science. 1997;276:1709–1712. doi: 10.1126/science.276.5319.1709. [DOI] [PubMed] [Google Scholar]
- 215.radisky dc, babcock mc, Kaplan j. the yeast frataxin homologue mediates mitochondrial iron efflux. Evidence for a mitochondrial iron cycle. j Biol Chem. 1999;274:4497–4499. doi: 10.1074/jbc.274.8.4497. [DOI] [PubMed] [Google Scholar]
- 216.sheftel ad, Lill r. the power plant of the cell is also a smithy: the emerging role of mitochondria in cellular iron homeostasis. ann Med. 2009;41:82–99. doi: 10.1080/07853890802322229. [DOI] [PubMed] [Google Scholar]
- 217.ross ca, tabrizi sj. huntington’s disease: from molecular pathogenesis to clinical treatment. lancet neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 218.Difiglia m, Sapp e, Chase k, Schwarz c, Meloni a, Young c, Martin e, vonsattel jp, Carraway r, reeves sa. huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. neuron. 1995;14:1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 219.Hilditch-maguire p, Trettel f, passani la, Auerbach a, Persichetti f, macdonald me. huntingtin: an iron-regulated protein essential for normal nuclear and perinuclear organelles. hum Mol Genet. 2000;9:2789–2797. doi: 10.1093/hmg/9.19.2789. [DOI] [PubMed] [Google Scholar]
- 220.lumsden al, henshall tl, Dayan s, lardelli mt, richards ri. huntingtin-deficient zebrafish exhibit defects in iron utilization and development. hum Mol Genet. 2007;16:1905–1920. doi: 10.1093/hmg/ddm138. [DOI] [PubMed] [Google Scholar]
- 221.Ponka p. cell biology of heme. am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 222.dailey ha. terminal steps of haem biosynthesis. biochem Soc Trans. 2002;30:590–595. doi: 10.1042/bst0300590. [DOI] [PubMed] [Google Scholar]
- 223.ajioka rs, phillips jd, kushner jp. biosynthesis of heme in mammals. biochim Biophys Acta. 2006;1763:723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 224.shaw gc, cope jj, Li l, Corson k, Hersey c, ackermann ge, Gwynn b, lambert aj, wingert ra, Traver d, trede ns, barut ba, Zhou y, Minet e, Donovan a, Brownlie a, Balzan r, weiss mj, peters ll, Kaplan j, zon li, paw bh. mitoferrin is essential for erythroid iron assimilation. nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 225.paradkar pn, zumbrennen kb, paw bh, ward dm, Kaplan j. regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. mol Cell biol. 2009;29:1007–1016. doi: 10.1128/MCB.01685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen w, dailey ha, paw bh. ferrochelatase forms an oligomeric complex with mitoferrin-1 and abcb10 for erythroid heme biosynthesis. blood. 2010;116:628–630. doi: 10.1182/blood-2009-12-259614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.shirihai os, Gregory t, Yu c, orkin sh, weiss mj. abc-me: a novel mitochondrial transporter induced by gata-1 during erythroid differentiation. embo j. 2000;19:2492–2502. doi: 10.1093/emboj/19.11.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang y, langer nb, shaw gc, Yang g, Li l, Kaplan j, paw bh, bloomer jr. abnormal mitoferrin-1 expression in patients with erythropoietic protoporphyria. exp Hematol. 2011 doi: 10.1016/j.exphem.2011.05.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.richardson dr, lane dj, becker em, huang ml, Whitnall m, rahmanto ys, sheftel ad, Ponka p. mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. proc Natl Acad Sci U S A. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sheftel a, Stehling o, Lill r. iron-sulfur proteins in health and disease. trends endocrinol Metab. 2010;21:302–314. doi: 10.1016/j.tem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 231.jensen lt, culotta vc. role of saccharomyces cerevisiae isa1 and isa2 in iron homeostasis. mol Cell biol. 2000;20:3918–3927. doi: 10.1128/mcb.20.11.3918-3927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kaut a, Lange h, Diekert k, Kispal g, Lill r. isa1p is a component of the mitochondrial machinery for maturation of cellular iron-sulfur proteins and requires conserved cysteine residues for function. j Biol Chem. 2000;275:15955–15961. doi: 10.1074/jbc.M909502199. [DOI] [PubMed] [Google Scholar]
- 233.Gelling c, dawes iw, Richhardt n, Lill r, Mühlenhoff u. mitochondrial iba57p is required for fe/s cluster formation on aconitase and activation of radical sam enzymes. mol cell biol. 2008;28:1851–1861. doi: 10.1128/MCB.01963-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rudolf j, Makrantoni v, ingledew wj, stark mj, white mf. the dna repair helicases xpd and fancj have essential iron-sulfur domains. mol Cell. 2006;23:801–808. doi: 10.1016/j.molcel.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 235.veatch jr, mcmurray ma, nelson zw, gottschling de. mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. cell. 2009;137:1247–1258. doi: 10.1016/j.cell.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.bogenhagen df, Wang y, shen el, Kobayashi r. protein components of mitochondrial dna nucleoids in higher eukaryotes. mol Cell proteomics. 2003;2:1205–1216. doi: 10.1074/mcp.M300035-MCP200. [DOI] [PubMed] [Google Scholar]
- 237.chen xj, Wang x, kaufman ba, butow ra. aconitase couples metabolic regulation to mitochondrial dna maintenance. science. 2005;307:714–717. doi: 10.1126/science.1106391. [DOI] [PubMed] [Google Scholar]
- 238.Levi s, Corsi b, Bosisio m, Invernizzi r, Volz a, Sanford d, Arosio p, Drysdale j. a human mitochondrial ferritin encoded by an intronless gene. j Biol Chem. 2001;276:24437–24440. doi: 10.1074/jbc.C100141200. [DOI] [PubMed] [Google Scholar]
- 239.Levi s, Arosio p. mitochondrial ferritin. int J Biochem Cell biol. 2004;36:1887–1889. doi: 10.1016/j.biocel.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 240.Santambrogio p, Biasiotto g, Sanvito f, Olivieri s, Arosio p, Levi s. mitochondrial ferritin expression in adult mouse tissues. j Histochem Cytochem. 2007;55:1129–1137. doi: 10.1369/jhc.7A7273.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nie g, sheftel ad, kim sf, Ponka p. overexpression of mitochondrial ferritin causes cytosolic iron depletion and changes cellular iron homeostasis. blood. 2005;105:2161–2167. doi: 10.1182/blood-2004-07-2722. [DOI] [PubMed] [Google Scholar]
- 242.Nie g, Chen g, sheftel ad, Pantopoulos k, Ponka p. in vivo tumor growth is inhibited by cytosolic iron deprivation caused by the expression of mitochondrial ferritin. blood. 2006;108:2428–2434. doi: 10.1182/blood-2006-04-018341. [DOI] [PubMed] [Google Scholar]
- 243.Cazzola m, Invernizzi r, Bergamaschi g, Levi s, Corsi b, Travaglino e, Rolandi v, Biasiotto g, Drysdale j, Arosio p. mitochondrial ferritin expression in erythroid cells from patients with sideroblastic anemia. blood. 2003;101:1996–2000. doi: 10.1182/blood-2002-07-2006. [DOI] [PubMed] [Google Scholar]
- 244.tfelt-hansen pc, Tfelt-hansen j. nitroglycerin headache and nitroglycerin-induced primary headaches from 1846 and onwards: a historical overview and an update. headache. 2009;49:445–456. doi: 10.1111/j.1526-4610.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 245.Katsuki s, Arnold w, Mittal c, Murad f. stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. j Cyclic Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 246.ignarro lj. regulation of cytosolic guanylyl cyclase by porphyrins and metalloporphyrins. adv Pharmacol. 1994;26:35–65. doi: 10.1016/s1054-3589(08)60050-2. [DOI] [PubMed] [Google Scholar]
- 247.khatsenko og, gross ss, rifkind ab, vane jr. nitric oxide is a mediator of the decrease in cytochrome p450-dependent metabolism caused by immunostimulants. proc Natl Acad Sci U S A. 1993;90:11147–11151. doi: 10.1073/pnas.90.23.11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.griscavage jm, rogers ne, sherman mp, ignarro lj. inducible nitric oxide synthase from a rat alveolar macrophage cell line is inhibited by nitric oxide. j Immunol. 1993;151:6329–6337. [PubMed] [Google Scholar]
- 249.Lepoivre m, Fieschi f, Coves j, Thelander l, Fontecave m. inactivation of ribonucleotide reductase by nitric oxide. biochem Biophys Res Commun. 1991;179:442–448. doi: 10.1016/0006-291x(91)91390-x. [DOI] [PubMed] [Google Scholar]
- 250.drapier jc, hibbs jb., jr murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. j Clin Invest. 1986;78:790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.drapier jc, hibbs jb., jr differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in l-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. j Immunol. 1988;140:2829–2838. [PubMed] [Google Scholar]
- 252.Pantopoulos k. iron metabolism and the ire/irp regulatory system: an update. ann N Y Acad Sci. 2004;1012:1–13. doi: 10.1196/annals.1306.001. [DOI] [PubMed] [Google Scholar]
- 253.Culotta e, koshland de., jr no news is good news. science. 1992;258:1862–1865. doi: 10.1126/science.1361684. [DOI] [PubMed] [Google Scholar]
- 254.stamler js, singel dj, Loscalzo j. biochemistry of nitric oxide and its redox-activated forms. science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 255.furchgott f, cherry pd, zawadzki jv, Jothianandan d. endothelial cells as mediators of vasodilation of arteries. j Cardiovasc Pharmacol. 1984;6(suppl 2):s336–s343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- 256.Bromberg y, Pick e. cyclic gmp metabolism in macrophages. I. Regulation of cyclic gmp levels by calcium and stimulation of cyclic gmp synthesis by no-generating agents. cell immunol. 1980;52:73–83. doi: 10.1016/0008-8749(80)90401-3. [DOI] [PubMed] [Google Scholar]
- 257.hibbs jb, jr, taintor rr, Vavrin z. macrophage cytotoxicity: role for l-arginine deiminase and imino nitrogen oxidation to nitrite. science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 258.palmer rm, ferrige ag, Moncada s. nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 259.Moncada s, radomski mw, palmer rm. endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. biochem Pharmacol. 1988;37:2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 260.Moncada s, palmer rm, higgs ea. the discovery of nitric oxide as the endogenous nitrovasodilator. hypertension. 1988;12:365–372. doi: 10.1161/01.hyp.12.4.365. [DOI] [PubMed] [Google Scholar]
- 261.Sakuma i, stuehr dj, gross ss, Nathan c, Levi r. identification of arginine as a precursor of endothelium-derived relaxing factor. proc Natl Acad Sci U S A. 1988;85:8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.stamler js, simon di, osborne ja, mullins me, Jaraki o, Michel t, singel dj, Loscalzo j. s-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. proc Natl Acad Sci U S A. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Trousseau a. glycosurie; dieabète sucré. clinique medicale de l’hotel de paris. 1865;2:663–698. [Google Scholar]
- 264.fistarol sk, itin ph. disorders of pigmentation. j Dtsch Dermatol Ges. 2010;8:187–201. doi: 10.1111/j.1610-0387.2009.07137.x. [DOI] [PubMed] [Google Scholar]
- 265.von recklinghausen fd. über hämochromatose. ärtze in heidelberg. 1889;62:324–325. [Google Scholar]
- 266.Sheldon j. haemochromatosis. oxford university press; oxford: 1935. [Google Scholar]
- 267.Simon m, Pawlotsky y, Bourel m, Fauchet r, Genetet b. [letter: idiopathic hemochromatosis associated with hl-a 3 tissular antigen] nouv Presse med. 1975;4:1432. [PubMed] [Google Scholar]
- 268.feder jn, Gnirke a, Thomas w, Tsuchihashi z, ruddy da, Basava a, Dormishian f, Domingo r, jr, ellis mc, Fullan a, hinton lm, jones nl, kimmel be, kronmal gs, Lauer p, lee vk, loeb db, mapa fa, Mcclelland e, meyer nc, mintier ga, Moeller n, Moore t, Morikang e, prass ce, Quintana l, starnes sm, schatzman rc, brunke kj, drayna dt, risch nj, bacon br, wolff rk. a novel mhc class i-like gene is mutated in patients with hereditary haemochromatosis. nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 269.feder jn, Tsuchihashi z, Irrinki a, lee vk, mapa fa, Morikang e, prass ce, starnes sm, wolff rk, Parkkila s, sly ws, schatzman rc. the hemochromatosis founder mutation in hla-h disrupts beta2-microglobulin interaction and cell surface expression. j Biol Chem. 1997;272:14025–14028. doi: 10.1074/jbc.272.22.14025. [DOI] [PubMed] [Google Scholar]
- 270.Waheed a, Parkkila s, zhou xy, Tomatsu s, Tsuchihashi z, feder jn, schatzman rc, britton rs, bacon br, sly ws. hereditary hemochromatosis: effects of c282y and h63d mutations on association with beta2-microglobulin, intracellular processing, and cell surface expression of the hfe protein in cos-7 cells. proc Natl Acad Sci U S A. 1997;94:12384–12389. doi: 10.1073/pnas.94.23.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.zhou xy, Tomatsu s, fleming re, Parkkila s, Waheed a, Jiang j, Fei y, brunt em, ruddy da, prass ce, schatzman rc, O’neill r, britton rs, bacon br, sly ws. hfe gene knockout produces mouse model of hereditary hemochromatosis. proc Natl Acad Sci U S A. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Krause a, Neitz s, magert hj, Schulz a, forssmann wg, Schulz-knappe p, Adermann k. leap-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. febs lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 273.park ch, valore ev, waring aj, Ganz t. hepcidin, a urinary antimicrobial peptide synthesized in the liver. j Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 274.Pigeon c, Ilyin g, Courselaud b, Leroyer p, Turlin b, Brissot p, Loreal o. a new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. j Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 275.Nicolas g, Bennoun m, Devaux i, Beaumont c, Grandchamp b, Kahn a, Vaulont s. lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (usf2) knockout mice. proc Natl Acad Sci U S A. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nicolas g, Bennoun m, Porteu a, Mativet s, Beaumont c, Grandchamp b, Sirito m, Sawadogo m, Kahn a, Vaulont s. severe iron deficiency anemia in transgenic mice expressing liver hepcidin. proc Natl Acad Sci U S A. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Nemeth e, Ganz t. regulation of iron metabolism by hepcidin. annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 278.bleackley mr, wong ay, hudson dm, wu ch, macgillivray rt. blood iron homeostasis: newly discovered proteins and iron imbalance. transfus Med Rev. 2009;23:103–123. doi: 10.1016/j.tmrv.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 279.Nemeth e, Ganz t. the role of hepcidin in iron metabolism. acta haematol. 2009;122:78–86. doi: 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.andrews nc. forging a field: the golden age of iron biology. blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.mason ab, everse sj. iron transport by transferrin. In: Fuchs h., editor. iron metabolism and disease. research signpost; kerala, india: 2008. pp. 83–123. [Google Scholar]
- 282.morgan eh, Jacobs a, Worwood m. transferrin and transferrin iron, iron in biochemistry and medicine. academic press; london: 1974. pp. 29–71. [Google Scholar]
- 283.Aisen p, brown eb. structure and function of transferrin. prog Hematol. 1975;9:25–56. [PubMed] [Google Scholar]
- 284.Aisen p, Listowsky i. iron transport and storage proteins. annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- 285.chasteen nd. transferrin: a perspective. adv Inorg Biochem. 1983;5:201–233. [PubMed] [Google Scholar]
- 286.brock jh, Harrison p. transferrins, metalloproteins part ii metal proteins with non-redox roles. macmillan press ltd; london: 1985. pp. 183–262. [Google Scholar]
- 287.huebers ha, finch ca. the physiology of transferrin and transferrin receptors. physiol Rev. 1987;67:520–582. doi: 10.1152/physrev.1987.67.2.520. [DOI] [PubMed] [Google Scholar]
- 288.harris dc, Aisen p, loehr tm. physical biochemistry of the transferrins, iron carriers and iron proteins. vch publishers, inc; new york: 1989. pp. 239–351. [Google Scholar]
- 289.Aisen p, loehr tm. iron carriers and iron proteins. vch publishers, inc; new york: 1989. physical biochemistry of the transferrins: update, 1984–1988; pp. 353–371. [Google Scholar]
- 290.baker en, lindley pf. new perspectives on the structure and function of transferrins. j Inorg Biochem. 1992;47:147–160. doi: 10.1016/0162-0134(92)84061-q. [DOI] [PubMed] [Google Scholar]
- 291.Sun h, Li h, sadler pj. transferrin as a metal ion mediator. chem Rev. 1999;99:2817–2842. doi: 10.1021/cr980430w. [DOI] [PubMed] [Google Scholar]
- 292.lindley pf. transferrins. In: Bertini i, Sigel a, Sigel h., editors. handbook of metalloproteins. marcel dekker; new york: 2001. pp. 793–811. [Google Scholar]
- 293.Aisen p, Enns c, Wessling-resnick m. chemistry and biology of eukaryotic iron metabolism. int J Biochem Cell biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 294.baker en, baker hm, kidd rd. lactoferrin and transferrin: functional variations on a common structural framework. biochem Cell biol. 2002;80:27–34. doi: 10.1139/o01-153. [DOI] [PubMed] [Google Scholar]
- 295.macgillivray rta, mason ab, templeton dm. transferrins, cell and molecular biology of iron transport. marcel dekker, inc; new york: 2002. pp. 41–69. [Google Scholar]
- 296.he qy, mason ab, templeton dm. molecular and cellular iron transport. marcel dekker, inc; new york: 2002. molecular aspects of release of iron from transferrins; pp. 95–123. [Google Scholar]
- 297.azari pr, feeney re. resistance of metal complexes of conalbumin and transferrin to proteolysis and to thermal denaturation. j Biol Chem. 1958;232:293–302. [PubMed] [Google Scholar]
- 298.greene fc, feeney re. physical evidence for transferrins as single polypeptide chains. biochemistry. 1968;7:1366–1371. doi: 10.1021/bi00844a018. [DOI] [PubMed] [Google Scholar]
- 299.mann kg, fish ww, cox ac, Tanford c. single-chain nature of human serum transferrin. biochemistry. 1970;9:1348–1354. doi: 10.1021/bi00808a008. [DOI] [PubMed] [Google Scholar]
- 300.Aisen p, Leibman a, Zweier j. stoichiometric and site characteristics of the binding of iron to human transferrin. j Biol Chem. 1978;253:1930–1937. [PubMed] [Google Scholar]
- 301.makey dg, seal us. the detection of four molecular forms of human transferrin during the iron binding process. biochim Biophys Acta. 1976;453:250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- 302.Leibman a, Aisen p. distribution of iron between the binding sites of transferrin in serum: methods and results in normal human subjects. blood. 1979;53:1058–1065. [PubMed] [Google Scholar]
- 303.Williams j, Moreton k. the distribution of iron between the metal-binding sites of transferrin in human serum. biochem J. 1980;185:483–488. doi: 10.1042/bj1850483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.chasteen nd, Williams j. the influence of ph on the equilibrium distribution of iron between the metal-binding sites of transferrin. biochem J. 1981;193:717–727. doi: 10.1042/bj1930717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.mason ab, halbrooks pj, larouche jr, briggs sk, moffett ml, ramsey je, connolly sa, smith vc, macgillivray rta. expression, purification, and characterization of authentic monoferric and apo-human serum transferrins. protein expr Purif. 2004;36:318–326. doi: 10.1016/j.pep.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 306.byrne sl, mason ab. human serum transferrin: a tale of two lobes. Urea gel and steady state fluorescence analysis of recombinant transferrins as a function of ph, time, and the soluble portion of the transferrin receptor. j Biol Inorg Chem. 2009;14:771–781. doi: 10.1007/s00775-009-0491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.steere an, byrne sl, chasteen nd, smith vc, macgillivray rt, mason ab. evidence that his349 acts as a ph-inducible switch to accelerate receptor-mediated iron release from the c-lobe of human transferrin. j Biol Inorg Chem. 2010;15:1341–1352. doi: 10.1007/s00775-010-0694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.gaber bp, Miskowski v, spiro tg. resonance raman scattering from iron(iii) and copper(ii) transferrin and an iron(iii) model compound. A spectroscopic interpretation of the transferrin binding site. j Am Chem Soc. 1974;96:6868–6873. doi: 10.1021/ja00829a010. [DOI] [PubMed] [Google Scholar]
- 309.patch mg, carrano cj. the origin of the visible absorption in metal transferrins. inorg Chim Acta. 1981;56:l71–l73. [Google Scholar]
- 310.lehrer ss. fluorescence and absorption studies of the binding of copper and iron to transferrin. j Biol Chem. 1969;244:3613–3617. [PubMed] [Google Scholar]
- 311.james ng, ross ja, mason ab, jameson dm. excited-state lifetime studies of the three tryptophan residues in the n-lobe of human serum transferrin. protein sci. 2009;19:99–110. doi: 10.1002/pro.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.james ng, byrne sl, steere an, smith vc, macgillivray rt, mason ab. inequivalent contribution of the five tryptophan residues in the c-lobe of human serum transferrin to the fluorescence increase when iron is released. biochemistry. 2009;48:2858–2867. doi: 10.1021/bi8022834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.james ng, mason ab. protocol to determine accurate absorption coefficients for iron containing transferrins. anal Biochem. 2008;378:202–207. doi: 10.1016/j.ab.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.schlabach mr, bates gw. the synergistic binding of anions and fe(iii) by transferrin. j Biol Chem. 1975;250:2182–2188. [PubMed] [Google Scholar]
- 315.bates gw, schalabach mr. the nonspecific binding of fe(iii) to transferrin in the absence of anions. j Biol Chem. 1975;250:2177–2181. [PubMed] [Google Scholar]
- 316.folajtar da, chasteen nd. measurement of nonsynergistic anion binding to transferrin by epr difference spectroscopy. j Am Chem Soc. 1982;104:5775–5780. [Google Scholar]
- 317.baker en. structure and reactivity of transferrins. adv Inorg Chem. 1994;41:389–463. [Google Scholar]
- 318.kretchmar sa, raymond kn. effects of ionic strength on iron removal from the monoferric transferrins. inorg Chem. 1988;27:1436–1441. [Google Scholar]
- 319.byrne sl, steere an, chasteen nd, mason ab. identification of a kinetically significant anion binding (kisab) site in the n-lobe of human serum transferrin. biochemistry. 2010;49:4200–4207. doi: 10.1021/bi1003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.price em, gibson jf. electron paramagnetic resonance evidence for a distinction between the two iron binding sites in transferrin and in conalbumin. j Biol Chem. 1972;247:8031–8035. [PubMed] [Google Scholar]
- 321.thompson cp, mccarty bm, chasteen nd. the effects of salts and amino group modification on the iron binding domains of transferrin. biochim Biophys Acta. 1986;870:530–537. doi: 10.1016/0167-4838(86)90262-1. [DOI] [PubMed] [Google Scholar]
- 322.grady jk, mason ab, woodworth rc, chasteen nd. the effect of salt and site-directed mutations on the iron(iii)-binding site of human serum transferrin as probed by epr spectroscopy. biochem J. 1995;309:403–410. doi: 10.1042/bj3090403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kubal g, mason ab, patel su, sadler pj, woodworth rc. oxalate- and ga 3+-induced structural changes in human serum transferrin and its recombinant n-lobe. 1 h nmr detection of preferential c-lobe ga 3+ binding. biochemistry. 1993;32:3387–3395. doi: 10.1021/bi00064a024. [DOI] [PubMed] [Google Scholar]
- 324.pecoraro vl, harris wr, carrano cj, raymond kn. siderophilin metal coordination. Difference ultraviolet spectroscopy of di-, tri-, and tetravalent metal ions with ethylenebis[(o-hydroxyphenyl)glycine] biochemistry. 1981;20:7033–7039. doi: 10.1021/bi00527a040. [DOI] [PubMed] [Google Scholar]
- 325.harris wr. thermodynamics of anion binding to human serum transferrin. biochemistry. 1985;24:7412–7418. doi: 10.1021/bi00346a057. [DOI] [PubMed] [Google Scholar]
- 326.harris wr, bali pk. effects of anions on the removal of iron from transferrin by phophonic acids and pyrophosphate. inorg Chem. 1988;27:2687–2691. [Google Scholar]
- 327.harris wr, cafferty am, Abdollahi s, Trankler k. binding of monovalent anions to human serum transferrin. biochim Biophys Acta. 1998;1383:197–210. doi: 10.1016/s0167-4838(97)00152-0. [DOI] [PubMed] [Google Scholar]
- 328.harris wr, Nesset-tollefson d, stenback jz, Mohamed-hani n. site selectivity in the binding of inorganic anions to serum transferrin. j Inorg Biochem. 1990;38:175–183. doi: 10.1016/0162-0134(90)84011-d. [DOI] [PubMed] [Google Scholar]
- 329.macgillivray rta, Mendez e, sinha sk, sutton mr, Lineback-zins j, Brew k. the complete amino acid sequence of human serum transferrin. proc Natl Acad Sci U S A. 1982;79:2504–2508. doi: 10.1073/pnas.79.8.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.macgillivray rta, Mendez e, shewale jg, sinha sk, Lineback-zins j, Brew k. the primary structure of human serum transferrin: the structures of seven cyanogen bromide fragments and the assembly of the complete structure. j Biol Chem. 1983;258:3543–3553. [PubMed] [Google Scholar]
- 331.Yang f, lum jb, mcgill jr, moore cm, naylor sl, van bragt ph, baldwin wd, bowman bh. human transferrin: cdna characterization and chromosomal localization. proc Natl Acad Sci U S A. 1984;81:2752–2756. doi: 10.1073/pnas.81.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Mcclelland a, kuhn lc, ruddle fh. the human transferrin receptor gene: genomic organization, and the complete primary structure of the receptor deduced from a cdna sequence. cell. 1984;39:267–274. doi: 10.1016/0092-8674(84)90004-7. [DOI] [PubMed] [Google Scholar]
- 333.anderson bf, baker hm, dodson ej, norris ge, rumball sv, waters jm, baker en. structure of human lactoferrin at 3.2 å resolution. proc Natl Acad Sci U S A. 1987;84:1769–1773. doi: 10.1073/pnas.84.7.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Bailey s, evans rw, garratt rc, Gorinsky b, hasnain ss, Horsburgh c, Jhoti h, lindley pf, Mydin a, Sarra r, watson jl. molecular structure of serum transferrin at 3.3-A resolution. biochemistry. 1988;27:5804–5812. doi: 10.1021/bi00415a061. [DOI] [PubMed] [Google Scholar]
- 335.hall dr, hadden jm, leonard ga, Bailey s, Neu m, Winn m, lindley pf. the crystal and molecular structures of diferric porcine and rabbit serum transferrins at resolutions of 2.15 and 2.60 a, respectively. acta crystallogr D Biol Crystallogr. 2002;58:70–80. doi: 10.1107/s0907444901017309. [DOI] [PubMed] [Google Scholar]
- 336.zuccola hJ. the crystal structure of monoferric human serum transferrin. georgia institute of technology; atlanta, ga: 1993. [Google Scholar]
- 337.macgillivray rta, moore sa, Chen j, anderson bf, Baker h, luo yg, Bewley m, smith ca, murphy me, Wang y, mason ab, woodworth rc, brayer gd, baker en. two high-resolution crystal structures of the recombinant n-lobe of human transferrin reveal a structural change implicated in iron release. biochemistry. 1998;37:7919–7928. doi: 10.1021/bi980355j. [DOI] [PubMed] [Google Scholar]
- 338.jeffrey pd, bewley mc, macgillivray rta, mason ab, woodworth rc, baker en. ligand-induced conformational change in transferrins: crystal structure of the open form of the n-terminal half-molecule of human transferrin. biochemistry. 1998;37:13978–13986. doi: 10.1021/bi9812064. [DOI] [PubMed] [Google Scholar]
- 339.dewan jc, Mikami b, Hirose m, sacchettini jc. structural evidence for a ph-sensitive dilysine trigger in the hen ovotransferrin n-lobe: implications for transferrin iron release. biochemistry. 1993;32:11963–11968. doi: 10.1021/bi00096a004. [DOI] [PubMed] [Google Scholar]
- 340.He q-y, mason ab, tam bm, macgillivray rta, woodworth rc. dual role of lys206-lys296 interaction in human transferrin n-lobe: iron-release trigger and anion-binding site. biochemistry. 1999;38:9704–9711. doi: 10.1021/bi990134t. [DOI] [PubMed] [Google Scholar]
- 341.Wally j, halbrooks pj, Vonrhein c, rould ma, everse sj, mason ab, buchanan sk. the crystal structure of iron-free human serum transferrin provides insight into inter-lobe communication and receptor binding. j Biol Chem. 2006;281:24934. doi: 10.1074/jbc.M604592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.macgillivray rta, bewley mc, smith ca, he qy, mason ab, woodworth rc, baker en. mutation of the iron ligand his 249 to glu in the n-lobe of human transferrin abolishes the dilysine “trigger” but does not significantly affect iron release. biochemistry. 2000;39:1211–1216. doi: 10.1021/bi991522y. [DOI] [PubMed] [Google Scholar]
- 343.adams te, mason ab, he qy, halbrooks pj, briggs sk, smith vc, macgillivray rt, everse sj. the position of arginine 124 controls the rate of iron release from the n-lobe of human serum transferrin. A structural study. j Biol Chem. 2003;278:6027–6033. doi: 10.1074/jbc.M210349200. [DOI] [PubMed] [Google Scholar]
- 344.halbrooks pj, mason ab, adams te, briggs sk, everse sj. the oxalate effect on release of iron from human serum transferrin explained. j Mol Biol. 2004;339:217–226. doi: 10.1016/j.jmb.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 345.mason ab, miller mk, funk wd, banfield dk, savage kj, oliver rwa, green bn, macgillivray rta, woodworth rc. expression of glycosylated and nonglycosylated human transferrin in mammalian cells. Characterization of the recombinant proteins with comparison to three commercially available transferrins. biochemistry. 1993;32:5472–5479. doi: 10.1021/bi00071a025. [DOI] [PubMed] [Google Scholar]
- 346.Regoeczi e, Bolyos m, chindemi pa. rat aglycotransferrin and human monoglycotransferrin: production and metabolic properties. arch Biochem Biophys. 1989;268:637–642. doi: 10.1016/0003-9861(89)90331-7. [DOI] [PubMed] [Google Scholar]
- 347.lawrence cm, Ray s, Babyonyshev m, Galluser r, borhani dw, harrison sc. crystal structure of the ectodomain of human transferrin receptor. science. 1999;286:779–782. doi: 10.1126/science.286.5440.779. [DOI] [PubMed] [Google Scholar]
- 348.bennett mj, lebron ja, bjorkman pj. crystal structure of the hereditary haemochromatosis protein hfe complexed with transferrin receptor. nature. 2000;403:46–53. doi: 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- 349.Cheng y, Zak o, Aisen p, harrison sc, Walz t. structure of the human transferrin receptor-transferrin complex. cell. 2004;116:565–576. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 350.funk wd, macgillivray rta, mason ab, brown sa, woodworth rc. expression of the amino-terminal half-molecule of human serum transferrin in cultured cells and characterization of the recombinant protein. biochemistry. 1990;29:1654–1660. doi: 10.1021/bi00458a043. [DOI] [PubMed] [Google Scholar]
- 351.mason ab, funk wd, macgillivray rta, woodworth rc. efficient production and isolation of recombinant amino-terminal half-molecule of human serum transferrin from baby hamster kidney cells. protein expres Purif. 1991;2:214–220. doi: 10.1016/1046-5928(91)90074-s. [DOI] [PubMed] [Google Scholar]
- 352.mason ab, he qy, halbrooks pj, everse sj, gumerov dr, kaltashov ia, smith vc, Hewitt j, macgillivray rta. differential effect of a his tag at the n- and c-termini: functional studies with recombinant human serum transferrin. biochemistry. 2002;41:9448–9454. doi: 10.1021/bi025927l. [DOI] [PubMed] [Google Scholar]
- 353.sargent pj, Farnaud s, Cammack r, zoller hm, evans rw. characterisation of recombinant unglycosylated human serum transferrin purified from saccharomyces cerevisiae. biometals. 2006;19:513–519. doi: 10.1007/s10534-005-5532-6. [DOI] [PubMed] [Google Scholar]
- 354.Keenan j, Pearson d, O’driscoll l, Gammell p, Clynes m. evaluation of recombinant human transferrin (deltaferrin(tm)) as an iron chelator in serum-free media for mammalian cell culture. cytotechnology. 2006;51:29–37. doi: 10.1007/s10616-006-9011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Zhang d, Nandi s, Bryan p, Pettit s, Nguyen d, santos ma, Huang n. expression, purification, and characterization of recombinant human transferrin from rice (oryza sativa l.) protein expr Purif. 2010;74:69–79. doi: 10.1016/j.pep.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.lebron ja, bennett mj, vaughn de, chirino aj, snow pm, mintier ga, feder jn, bjorkman pj. crystal structure of the hemochromatosis protein hfe and characterization of its interaction with transferrin receptor. cell. 1998;93:111–123. doi: 10.1016/s0092-8674(00)81151-4. [DOI] [PubMed] [Google Scholar]
- 357.west ap, jr, giannetti am, herr ab, bennett mj, nangiana js, pierce jr, weiner lp, snow pm, bjorkman pj. mutational analysis of the transferrin receptor reveals overlapping hfe and transferrin binding sites. j Mol Biol. 2001;313:385–397. doi: 10.1006/jmbi.2001.5048. [DOI] [PubMed] [Google Scholar]
- 358.giannetti am, snow pm, Zak o, bjorkman pj. mechanism for multiple ligand recognition by the human transferrin receptor. plos Biol. 2003;1:341–350. doi: 10.1371/journal.pbio.0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.byrne sl, Leverence r, klein js, giannetti am, smith vc, macgillivray rta, kaltashov ia, mason ab. effect of glycosylation on the function of a soluble, recombinant form of the transferrin receptor. biochemistry. 2006;45:6663–6673. doi: 10.1021/bi0600695. [DOI] [PubMed] [Google Scholar]
- 360.bali pk, Zak o, Aisen p. a new role for the transferrin receptor in the release of iron from transferrin. biochemistry. 1991;30:324–328. doi: 10.1021/bi00216a003. [DOI] [PubMed] [Google Scholar]
- 361.bali pk, Aisen p. receptor-modulated iron release from transferrin: differential effects on n- and c-terminal sites. biochemistry. 1991;30:9947–9952. doi: 10.1021/bi00105a019. [DOI] [PubMed] [Google Scholar]
- 362.bali pk, Aisen p. receptor-induced switch in site-site cooperativity during iron release by transferrin. biochemistry. 1992;31:3963–3967. doi: 10.1021/bi00131a011. [DOI] [PubMed] [Google Scholar]
- 363.Aisen p. entry of iron into cells: a new role for the transferrin receptor in modulating iron release from transferrin. ann Neurol. 1992;32(suppl):s62–s68. doi: 10.1002/ana.410320711. [DOI] [PubMed] [Google Scholar]
- 364.egan tj, Zak o, Aisen p. the anion requirement for iron release from transferrin is preserved in the receptor-transferrin complex. biochemistry. 1993;32:8162–8167. doi: 10.1021/bi00083a016. [DOI] [PubMed] [Google Scholar]
- 365.Zak o, Trinder d, Aisen p. primary receptor-recognition site of human transferrin is in the c-terminal lobe. j Biol Chem. 1994;269:7110–7114. [PubMed] [Google Scholar]
- 366.Aisen p. the transferrin receptor and the release of iron from transferrin. adv Exp Med Biol. 1994;356:31–40. doi: 10.1007/978-1-4615-2554-7_4. [DOI] [PubMed] [Google Scholar]
- 367.byrne sl, chasteen nd, steere an, mason ab. the unique kinetics of iron release from transferrin: the role of receptor, lobe-lobe interactions, and salt at endosomal ph. j Mol Biol. 2010;396:130–140. doi: 10.1016/j.jmb.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Iolascon a, de fl, Beaumont c. molecular basis of inherited microcytic anemia due to defects in iron acquisition or heme synthesis. haematologica. 2009;94:395–408. doi: 10.3324/haematol.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.massey ac. microcytic anemia. Differential diagnosis and management of iron deficiency anemia. med Clin North am. 1992;76:549–566. doi: 10.1016/s0025-7125(16)30339-x. [DOI] [PubMed] [Google Scholar]
- 370.levy je, Jin o, Fujiwara y, Kuo f, andrews nc. transferrin receptor is necessary for development of erythrocytes and the nervous system. nat Genet. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- 371.larrick jw, hyman es. acquired iron-deficiency anemia caused by an antibody against the transferrin receptor. n Engl J Med. 1984;311:214–218. doi: 10.1056/NEJM198407263110402. [DOI] [PubMed] [Google Scholar]
- 372.cooperman ss, meyron-holtz eg, Olivierre-wilson h, ghosh mc, mcconnell jp, rouault ta. microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. blood. 2005;106:1084–1091. doi: 10.1182/blood-2004-12-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Galy b, Ferring d, Minana b, Bell o, janser hg, Muckenthaler m, Schumann k, hentze mw. altered body iron distribution and microcytosis in mice deficient in iron regulatory protein 2 (irp2) blood. 2005;106:2580–2589. doi: 10.1182/blood-2005-04-1365. [DOI] [PubMed] [Google Scholar]
- 374.Lavaute t, Smith s, Cooperman s, Iwai k, Land w, Meyron-holtz e, drake sk, Miller g, Abu-asab m, Tsokos m, Switzer r, iii, Grinberg a, Love p, Tresser n, rouault ta. targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. nat Genet. 2001;27:209–214. doi: 10.1038/84859. [DOI] [PubMed] [Google Scholar]
- 375.lim je, Jin o, Bennett c, Morgan k, Wang f, trenor cc, fleming md, andrews nc. a mutation in sec15l1 causes anemia in hemoglobin deficit (hbd) mice. nat Genet. 2005;37:1270–1273. doi: 10.1038/ng1659. [DOI] [PubMed] [Google Scholar]
- 376.white ra, boydston la, brookshier tr, mcnulty sg, nsumu nn, brewer bp, Blackmore k. iron metabolism mutant hbd mice have a deletion in sec15l1, which has homology to a yeast gene for vesicle docking. genomics. 2005 doi: 10.1016/j.ygeno.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 377.Scheufler h. [an additional house mouse mutant with anemia (hemoglobin deficiency)] z Versuchstierkd. 1969;11:348–353. [PubMed] [Google Scholar]
- 378.bannerman rm, garrick lm, Rusnak-smalley p, hoke je, edwards ja. hemoglobin deficit: an inherited hypochromic anemia in the mouse. proc Soc Exp Biol Med. 1986;182:52–57. doi: 10.3181/00379727-182-42307. [DOI] [PubMed] [Google Scholar]
- 379.garrick lm, edwards ja, hoke je, bannerman rm. diminished acquisition of iron by reticulocytes from mice with hemoglobin deficit. exp Hematol. 1987;15:671–675. [PubMed] [Google Scholar]
- 380.zhang as, sheftel ad, Ponka p. the anemia of “haemoglobin-deficit” (hbd/hbd) mice is caused by a defect in transferrin cycling. exp Hematol. 2006;34:593–598. doi: 10.1016/j.exphem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 381.ohgami rs, campagna dr, Antiochos b, wood eb, sharp jj, barker je, fleming md. nm1054: a spontaneous, recessive, hypochromic, microcytic anemia mutation in the mouse. blood. 2005;106:3625–3631. doi: 10.1182/blood-2005-01-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.fleming md, romano ma, su ma, garrick lm, garrick md, andrews nc. nramp2 is mutated in the anemic belgrade (b) rat: evidence of a role for nramp2 in endosomal iron transport. proc Natl Acad Sci U S A. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.mims mp, Guan y, Pospisilova d, Priwitzerova m, Indrak k, Ponka p, Divoky v, prchal jt. identification of a human mutation of dmt1 in a patient with microcytic anemia and iron overload. blood. 2005;105:1337–1342. doi: 10.1182/blood-2004-07-2966. [DOI] [PubMed] [Google Scholar]
- 384.Priwitzerova m, Nie g, sheftel ad, Pospisilova d, Divoky v, Ponka p. functional consequences of the human dmt1 (slc11a2) mutation on protein expression and iron uptake. blood. 2005;106:3985–3987. doi: 10.1182/blood-2005-04-1550. [DOI] [PubMed] [Google Scholar]
- 385.Beaumont c, Delaunay j, Hetet g, Grandchamp b, De montalembert m, Tchernia g. two new human dmt1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. blood. 2006 doi: 10.1182/blood-2005-10-4269. [DOI] [PubMed] [Google Scholar]
- 386.Iolascon a, D’apolito m, Servedio v, Cimmino f, Piga a, Camaschella c. microcytic anemia and hepatic iron overload in a child with compound heterozygous mutations in dmt1 (scl11a2) blood. 2006;107:349–354. doi: 10.1182/blood-2005-06-2477. [DOI] [PubMed] [Google Scholar]
- 387.Priwitzerova m, Pospisilova d, prchal jt, Indrak k, Hlobilkova a, Mihal v, Ponka p, Divoky v. severe hypochromic microcytic anemia caused by a congenital defect of the iron transport pathway in erythroid cells. blood. 2004;103:3991–3992. doi: 10.1182/blood-2004-01-0225. [DOI] [PubMed] [Google Scholar]
- 388.Iolascon a, de fl. mutations in the gene encoding dmt1: clinical presentation and treatment. semin Hematol. 2009;46:358–370. doi: 10.1053/j.seminhematol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 389.burnell dj, apsimon jw, gilgan mw. variations in the levels of asterone and asterogenol, two steroids from the saponins of the starfish, asterias vulgaris (verrill) steroids. 1984;44:67–76. doi: 10.1016/s0039-128x(84)80017-3. [DOI] [PubMed] [Google Scholar]
- 390.sheftel ad, richardson dr, prchal jt, Ponka p. mitochondrial iron metabolism and sideroblastic anemia. acta haematologica. 2009;122:120–133. doi: 10.1159/000243796. [DOI] [PubMed] [Google Scholar]
- 391.Allikmets r, raskind wh, Hutchinson a, schueck nd, Dean m, koeller dm. mutation of a putative mitochondrial iron transporter gene (abc7) in x-linked sideroblastic anemia and ataxia (xlsa/a) hum Mol Genet. 1999;8:743–749. doi: 10.1093/hmg/8.5.743. [DOI] [PubMed] [Google Scholar]
- 392.sheftel ad, Stehling o, pierik aj, elsasser hp, Muhlenhoff u, Webert h, Hobler a, Hannemann f, Bernhardt r, Lill r. humans possess two mitochondrial ferredoxins, fdx1 and fdx2, with distinct roles in steroidogenesis, heme, and fe/s cluster biosynthesis. proc Natl Acad Sci U S A. 2010;107:11775–11780. doi: 10.1073/pnas.1004250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Camaschella c, Campanella a, De falco l, Boschetto l, Merlini r, Silvestri l, Levi s, Iolascon a. the human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. blood. 2007;110:1353–1358. doi: 10.1182/blood-2007-02-072520. [DOI] [PubMed] [Google Scholar]
- 394.guernsey dl, Jiang h, campagna dr, evans sc, Ferguson m, kellogg md, Lachance m, Matsuoka m, Nightingale m, Rideout a, Saint-amant l, schmidt pj, Orr a, bottomley ss, fleming md, Ludman m, Dyack s, fernandez cv, samuels me. mutations in mitochondrial carrier family gene slc25a38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. nat genet. 2009;41:651–653. doi: 10.1038/ng.359. [DOI] [PubMed] [Google Scholar]
- 395.brittenham gm. iron-chelating therapy for transfusional iron overload. n Engl J Med. 2011;364:146–156. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Gao j, Chen j, Kramer m, Tsukamoto h, zhang as, enns ca. interaction of the hereditary hemochromatosis protein hfe with transferrin receptor 2 is required for transferrin-induced hepcidin expression. cell metab. 2009;9:217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.sham rl, phatak pd, Nemeth e, Ganz t. hereditary hemochromatosis due to resistance to hepcidin: high hepcidin concentrations in a family with c326s ferroportin mutation. blood. 2009;114:493–494. doi: 10.1182/blood-2009-04-216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.lee pl, Beutler e. regulation of hepcidin and iron-overload disease. annu Rev Pathol. 2009;4:489–515. doi: 10.1146/annurev.pathol.4.110807.092205. [DOI] [PubMed] [Google Scholar]
- 399.Kim s, Ponka p. nitrogen monoxide-mediated control of ferritin synthesis: implications for macrophage iron homeostasis. proc Natl Acad Sci U S A. 2002;99:12214–12219. doi: 10.1073/pnas.192316099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.gibson qh, Antonini e. kinetic studies on the reaction between native globin and haem derivatives. biochem J. 1960;77:328–341. doi: 10.1042/bj0770328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.rose my, olson js. the kinetic mechanism of heme binding to human apohemoglobin. j Biol Chem. 1983;258:4298–4303. [PubMed] [Google Scholar]
- 402.komar aa, Kommer a, krasheninnikov ia, spirin as. cotranslational heme binding to nascent globin chains. febs lett. 1993;326:261–263. doi: 10.1016/0014-5793(93)81803-8. [DOI] [PubMed] [Google Scholar]
- 403.Shi h, bencze kz, stemmler tl, philpott cc. a cytosolic iron chaperone that delivers iron to ferritin. science. 2008;320:1207–1210. doi: 10.1126/science.1157643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.richardson dr, kalinowski ds, Lau s, jansson pj, lovejoy db. cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. biochim Biophys Acta. 2009;1790:702–717. doi: 10.1016/j.bbagen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 405.pinnix zk, miller ld, Wang w, D’agostino r, jr, Kute t, willingham mc, Hatcher h, Tesfay l, Sui g, Di x, torti sv, torti fm. ferroportin and iron regulation in breast cancer progression and prognosis. sci Transl Med. 2010;2:43ra56. doi: 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.dyson fj. time without end: physics and biology in an open universe. rev Mod Physics. 1979;51:447–460. [Google Scholar]
- 407.adams fc, Laughlin g. a dying universe: the long-term fate and evolution of astrophysical objects. rev Mod Physics. 1997;69:337–372. [Google Scholar]
- 408.Samson d, Halliday d, nicholson dc, Chanarin i. quantitation of ineffective erythropoiesis from the incorporation of [15n] delta-aminolaevulinic acid and [15n] glycine into early labelled bilirubin. I. Normal subjects. br J Haematol. 1976;34:33–44. doi: 10.1111/j.1365-2141.1976.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 409.koury st, koury mj, bondurant mc. cytoskeletal distribution and function during the maturation and enucleation of mammalian erythroblasts. j Cell biol. 1989;109:3005–3013. doi: 10.1083/jcb.109.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Bekri s, Kispal g, Lange h, Fitzsimons e, Tolmie j, Lill r, bishop df. human abc7 transporter: gene structure and mutation causing x-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation. blood. 2000;96:3256–3264. [PubMed] [Google Scholar]
- 411.burke ma, Ardehali h. mitochondrial atp-binding cassette proteins. transl res. 2007;150:73–80. doi: 10.1016/j.trsl.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 412.mclintock la, fitzsimons ej. erythroblast iron metabolism in sideroblastic and sideropenic states. hematology. 2002;7:189–195. doi: 10.1080/1024533021000013906. [DOI] [PubMed] [Google Scholar]
- 413.babitt jl, huang fw, wrighting dm, Xia y, Sidis y, samad ta, campagna ja, chung rt, schneyer al, woolf cj, andrews nc, lin hy. bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. nat genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 414.babitt jl, huang fw, Xia y, Sidis y, andrews nc, lin hy. modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. j clin invest. 2007;117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Yoshida k, Furihata k, Takeda s, Nakamura a, Yamamoto k, Morita h, Hiyamuta s, Ikeda s, Shimizu n, Yanagisawa n. a mutation in the ceruloplasmin gene is associated with systemic hemosiderosis in humans. nat Genet. 1995;9:267–272. doi: 10.1038/ng0395-267. [DOI] [PubMed] [Google Scholar]
- 416.patel bn, dunn rj, jeong sy, Zhu q, julien jp, David s. ceruloplasmin regulates iron levels in the cns and prevents free radical injury. j neurosci. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.garrick lm, dolan kg, romano ma, garrick md. non-transferrin-bound iron uptake in belgrade and normal rat erythroid cells. j Cell Physiol. 1999;178:349–358. doi: 10.1002/(SICI)1097-4652(199903)178:3<349::AID-JCP9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 418.Canonne-hergaux f, zhang as, Ponka p, Gros p. characterization of the iron transporter dmt1 (nramp2/dct1) in red blood cells of normal and anemic mk/mk mice. blood. 2001;98:3823–3830. doi: 10.1182/blood.v98.13.3823. [DOI] [PubMed] [Google Scholar]
- 419.zhang as, Canonne-hergaux f, Gruenheid s, Gros p, Ponka p. use of nramp2-transfected chinese hamster ovary cells and reticulocytes from mk/mk mice to study iron transport mechanisms. exp Hematol. 2008 Aug 30;:1227–1235. doi: 10.1016/j.exphem.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Ferreira c, Bucchini d, martin me, Levi s, Arosio p, Grandchamp b, Beaumont c. early embryonic lethality of h ferritin gene deletion in mice. j Biol Chem. 2000;275:3021–3024. doi: 10.1074/jbc.275.5.3021. [DOI] [PubMed] [Google Scholar]
- 421.Arosio p, Ingrassia r, Cavadini p. ferritins: a family of molecules for iron storage, antioxidation and more. biochim Biophysacta. 2009;1790:589–599. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 422.magness st, Maeda n, brenner da. an exon 10 deletion in the mouse ferrochelatase gene has a dominant-negative effect and causes mild protoporphyria. blood. 2002;100:1470–1477. doi: 10.1182/blood-2001-12-0283. [DOI] [PubMed] [Google Scholar]
- 423.De domenico i, ward dm, Nemeth e, vaughn mb, Musci g, Ganz t, Kaplan j. the molecular basis of ferroportin-linked hemochromatosis. proc natl acad sci u s a. 2005;102:8955–8960. doi: 10.1073/pnas.0503804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.quigley jg, Yang z, worthington mt, phillips jd, sabo km, sabath de, berg cl, Sassa s, wood bl, abkowitz jl. identification of a human heme exporter that is essential for erythropoiesis. cell. 2004;118:757–766. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 425.keel sb, doty rt, Yang z, quigley jg, Chen j, Knoblaugh s, kingsley pd, De domenico i, vaughn mb, Kaplan j, Palis j, abkowitz jl. a heme export protein is required for red blood cell differentiation and iron homeostasis. science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 426.bencze kz, kondapalli kc, cook jd, Mcmahon s, Millan-pacheco c, Pastor n, stemmler tl. the structure and function of frataxin. crit Rev Biochem Mol Biol. 2006;41:269–291. doi: 10.1080/10409230600846058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.rouault ta, tong wh. iron-sulfur cluster biogenesis and human disease. trends genet. 2008;24:398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Tanno t, bhanu nv, oneal pa, goh sh, Staker p, lee yt, moroney jw, reed ch, luban nl, wang rh, eling te, Childs r, Ganz t, leitman sf, Fucharoen s, miller jl. high levels of gdf15 in thalassemia suppress expression of the iron regulatory protein hepcidin. nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 429.Lakhal s, talbot np, Crosby a, Stoepker c, townsend ar, robbins pa, pugh cw, ratcliffe pj, mole dr. regulation of growth differentiation factor 15 expression by intracellular iron. blood. 2009;113:1555–1563. doi: 10.1182/blood-2008-07-170431. [DOI] [PubMed] [Google Scholar]
- 430.chua ac, herbison ce, drake sf, graham rm, olynyk jk, Trinder d. the role of hfe in transferrin-bound iron uptake by hepatocytes. hepatology. 2008;47:1737–1744. doi: 10.1002/hep.22180. [DOI] [PubMed] [Google Scholar]
- 431.abraham ng, Kappas a. pharmacological and clinical aspects of heme oxygenase. pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 432.ferris cd, jaffrey sr, Sawa a, Takahashi m, brady sd, barrow rk, tysoe sa, Wolosker h, baranano de, Dore s, poss kd, snyder sh. haem oxygenase-1 prevents cell death by regulating cellular iron. nat Cell biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 433.Yachie a, Niida y, Wada t, Igarashi n, Kaneda h, Toma t, Ohta k, Kasahara y, Koizumi s. oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. j Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.maines md. the heme oxygenase system: a regulator of second messenger gases. annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 435.maines md. heme oxygenase: clinical applications and functions. crc press; boca raton: 1992. [Google Scholar]
- 436.Papanikolaou g, samuels me, ludwig eh, macdonald ml, franchini pl, dube mp, Andres l, Macfarlane j, Sakellaropoulos n, Politou m, Nemeth e, Thompson j, risler jk, Zaborowska c, Babakaiff r, radomski cc, pape td, Davidas o, Christakis j, Brissot p, Lockitch g, Ganz t, hayden mr, goldberg yp. mutations in hfe2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 437.zhang as, Yang f, Meyer k, Hernandez c, Chapman-arvedson t, bjorkman pj, enns ca. neogenin-mediated hemojuvelin shedding occurs after hemojuvelin traffics to the plasma membrane. j Biol Chem. 2008;283:17494–17502. doi: 10.1074/jbc.M710527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Roetto a, Papanikolaou g, Politou m, Alberti f, Girelli d, Christakis j, Loukopoulos d, Camaschella c. mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. nat Genet. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 439.meyron-holtz eg, ghosh mc, Iwai k, Lavaute t, Brazzolotto x, berger uv, Land w, Ollivierre-wilson h, Grinberg a, Love p, rouault ta. genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. embo j. 2004;23:386–395. doi: 10.1038/sj.emboj.7600041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.muckenthaler mu, Galy b, hentze mw. systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (ire/irp) regulatory network. annu Rev Nutr. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- 441.ward pp, Mendoza-meneses m, cunningham ga, conneely om. iron status in mice carrying a targeted disruption of lactoferrin. mol Cell biol. 2003;23:178–185. doi: 10.1128/MCB.23.1.178-185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Legrand d, Pierce a, Elass e, Carpentier m, Mariller c, Mazurier j. lactoferrin structure and functions. adv Exp Med Biol. 2008;606:163–194. doi: 10.1007/978-0-387-74087-4_6. [DOI] [PubMed] [Google Scholar]
- 443.baker en, baker hm. a structural framework for understanding the multifunctional character of lactoferrin. biochimie. 2009;91:3–10. doi: 10.1016/j.biochi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 444.Napier i, Ponka p, richardson dr. iron trafficking in the mitochondrion: novel pathways revealed by disease. blood. 2005;105:1867–1874. doi: 10.1182/blood-2004-10-3856. [DOI] [PubMed] [Google Scholar]
- 445.Wang f, paradkar pn, custodio ao, Mcvey ward d, fleming md, Campagna d, roberts ka, Boyartchuk v, dietrich wf, Kaplan j, andrews nc. genetic variation in mon1a affects protein trafficking and modifies macrophage iron loading in mice. nat Genet. 2007;39:1025–1032. doi: 10.1038/ng2059. [DOI] [PubMed] [Google Scholar]
- 446.zhang as, west ap, jr, wyman ae, bjorkman pj, enns ca. interaction of hemojuvelin with neogenin results in iron accumulation in human embryonic kidney 293 cells. j Biol Chem. 2005;280:33885–33894. doi: 10.1074/jbc.M506207200. [DOI] [PubMed] [Google Scholar]
- 447.Conrad s, Genth h, Hofmann f, Just i, Skutella t. neogenin-rgma signaling at the growth cone is bone morphogenetic protein-independent and involves rhoa, rock, and pkc. j Biol Chem. 2007;282:16423–16433. doi: 10.1074/jbc.M610901200. [DOI] [PubMed] [Google Scholar]
- 448.garrick md, garrick lm. loss of rapid transferrin receptor recycling due to a mutation in sec15l1 in hbd mice. biochim Biophys acta. 2007;1773:105–108. doi: 10.1016/j.bbamcr.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 449.Zheng h, Ji c, Zou x, Wu m, Jin z, Yin g, Li j, Feng c, Cheng h, Gu s, Xie y, Mao y. molecular cloning and characterization of a novel human putative transmembrane protein homologous to mouse sideroflexin associated with sideroblastic anemia. dna seq. 2003;14:369–373. doi: 10.1080/10425170310001605491. [DOI] [PubMed] [Google Scholar]
- 450.Lambe t, simpson rj, Dawson s, Bouriez-jones t, crockford tl, Lepherd m, latunde-dada go, Robinson h, raja kb, campagna dr, Villarreal g, jr, ellory jc, goodnow cc, fleming md, mckie at, cornall rj. identification of a steap3 endosomal targeting motif essential for normal iron metabolism. blood. 2009;113:1805–1808. doi: 10.1182/blood-2007-11-120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Du x, She e, Gelbart t, Truksa j, Lee p, Xia y, Khovananth k, Mudd s, Mann n, moresco em, Beutler e, Beutler b. the serine protease tmprss6 is required to sense iron deficiency. science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.finberg ke, heeney mm, campagna dr, Aydinok y, pearson ha, hartman kr, mayo mm, samuel sm, strouse jj, Markianos k, andrews nc, fleming md. mutations in tmprss6 cause iron-refractory iron deficiency anemia (irida) nat Genet. 2008;40:569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kaplan j, Craven c, Alexander j, Kushner j, Lamb j, Bernstein s. regulation of the distribution of tissue iron. Lessons learned from the hypotransferrinemic mouse. ann N Y Acad Sci. 1988;526:124–135. doi: 10.1111/j.1749-6632.1988.tb55498.x. [DOI] [PubMed] [Google Scholar]
- 454.wallace df, Summerville l, lusby pe, subramaniam vn. first phenotypic description of transferrin receptor 2 knockout mouse, and the role of hepcidin. gut. 2005;54:980–986. doi: 10.1136/gut.2004.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.wallace df, Summerville l, subramaniam vn. targeted disruption of the hepatic transferrin receptor 2 gene in mice leads to iron overload. gastroenterology. 2007;132:301–310. doi: 10.1053/j.gastro.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 456.liuzzi jp, Aydemir f, Nam h, knutson md, cousins rj. zip14 (slc39a14) mediates non-transferrin-bound iron uptake into cells. proc Natl Acad Sci U S A. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Camaschella c, Roetto a, Cali a, De gobbi m, Garozzo g, Carella m, Majorano n, Totaro a, Gasparini p. the gene tfr2 is mutated in a new type of haemochromatosis mapping to 7q22. nat Genet. 2000;25:14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 458.njajou ot, Vaessen n, Joosse m, Berghuis b, van dongen jw, breuning mh, snijders pj, rutten wp, sandkuijl la, oostra ba, van duijn cm, Heutink p. a mutation in slc11a3 is associated with autosomal dominant hemochromatosis. nat Genet. 2001;28:213–214. doi: 10.1038/90038. [DOI] [PubMed] [Google Scholar]
- 459.Montosi g, Donovan a, Totaro a, Garuti c, Pignatti e, Cassanelli s, trenor cc, Gasparini p, andrews nc, Pietrangelo a. autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (slc11a3) gene. j Clin Invest. 2001;108:619–623. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Ponka p. rare causes of hereditary iron overload. semin Hematol. 2002;39:249–262. doi: 10.1053/shem.2002.35638. [DOI] [PubMed] [Google Scholar]
- 461.gordeuk vr. african iron overload. semin Hematol. 2002;39:263–269. doi: 10.1053/shem.2002.35636. [DOI] [PubMed] [Google Scholar]