Abstract
Objectives To evaluate whether the use of adult heart rate (HR) criteria is appropriate for diagnosing Postural Tachycardia Syndrome (POTS) and orthostatic intolerance (OI) in children and adolescents and to establish normative data and diagnostic criteria for pediatric POTS and OI.
Study design 106 normal controls between the ages 8 and 19 years (14.5±3.3 years) underwent standardized autonomic testing, including 5 minutes of 70 degree head-up tilt. The orthostatic HR increment and absolute orthostatic HR were assessed and retrospectively compared with 654 pediatric patients of similar age (15.5±2.3 years), who were referred to our Clinical Autonomic Laboratory with symptoms of OI.
Results The HR increment was mildly higher in patients referred for POTS/OI but there was considerable overlap between patient and control group. 42% of normal controls had a HR increment of 30bpm or more. The 95th percentile for the orthostatic HR increment in normal controls was 42.9bpm. Absolute orthostatic HR showed a greater and more consistent difference between groups, although there was still considerable overlap.
Conclusions The diagnostic criteria for OI/POTS in adults are inadequate for children and adolescents. Based on our normative data, new criteria are proposed for the diagnosis of OI and POTS in children and adolescents.
Keywords: nervous system, autonomic, orthostatic intolerance, postural tachycardia, adolescent autonomic dysfunction
The diagnosis of Postural Tachycardia Syndrome (POTS) and orthostatic intolerance (OI) is based on a symptomatic, excessive orthostatic rise in heart rate (HR). Common symptoms of OI and POTS include lightheadedness, palpitations, presyncopal feelings, tremulousness, and leg weakness when assuming the upright position. These symptoms are felt to relate to a combination of reduced cerebral perfusion and increased sympathetic activation. Females predominate in these conditions over males in a ratio of about 5:1.[1]
For a diagnosis of OI, current criteria postulate the presence of an orthostatic HR increment of at least 30bpm within 5 minutes of active standing or passive head-up tilt, associated with symptoms of lightheadedness or faintness.[1–4] For a diagnosis of POTS, most authors postulate in a addition to the criteria for OI an absolute orthostatic HR of at least 120bpm.[1–4] Some authors use different criteria and definitions of OI and POTS but the above referenced criteria are the only published criteria that are based on normative data, which also show that the orthostatic HR increment is significantly influenced by age.[1]
The term “Adolescent Autonomic Dysfunction” has been created which describes the frequent association between chronic fatigue, headaches, abdominal pain, nausea, dizziness, and lightheadedness observed in adolescents.[5] Because of these recent observations and frequency of these symptoms among pediatric patients, we have seen increasing numbers of children and adolescents referred to our Autonomic Laboratories with a question of OI.
Adult diagnostic criteria for OI and POTS have been adopted in studies on OI in the pediatric population, although little is known about the normal range of orthostatic HR and HR increment in children and adolescents.[6–9] We and others have observed higher, asymptomatic orthostatic increases in HR in normal adolescents, an observation that was further underlined in a recently reported study among high-school students in the community of Rochester, MN, which reports the HR increment with active standing in normal high-school students to be as high as 48bpm.[10–12]
We therefore raise the question, whether it is appropriate to use adult criteria for a diagnosis of OI and POTS in pediatric and adolescent populations. The goals of this study were to assess orthostatic HR and HR increment with head-up tilt in a normal pediatric population in the controlled setting of an autonomic laboratory, to establish normative data and criteria suitable for the diagnosis of pediatric/adolescent OI and POTS, and to compare these normative data with a large cohort of young patients referred to our laboratories for OI.
METHODS
Normal control subjects under age 20 years (n=106) were recruited from communities within Southeastern Minnesota. All subjects were screened for conditions and medications that could affect autonomic testing and were required to have a normal neurological examination.
Our clinical database was searched for patients under age 20 years referred to our laboratories with referral diagnoses of orthostatic intolerance, lightheadedness, orthostatic tachycardia, and postural tachycardia.
Subjects were excluded from enrollment in the study if there was evidence of failure of organ systems or of systemic illness that could affect study results, autonomic function or the patient's ability to cooperate. Concomitant therapy with anticholinergic, adrenergic antagonists, vasoactive agents or other medications that could interfere with testing of autonomic function was also exclusive unless discontinued for at least five half-lives before the study.
Among 666 patients identified, 12 were found to have orthostatic hypotension and those patients were also excluded from further analysis.
The study was carried out with prospective normative data collection and retrospective analysis of patient data. The study protocol was approved by the Institutional Review Board and was carried out in accordance with the Declaration of Helsinki. Patients, whose charts were reviewed and data accessed gave written permission to allow the use of their medical records for research purposes. Normal subjects undergoing testing as part of this study and their legal guardian gave specific written informed consent before entering the study. The study was carried out at controlled ambient room temperature (23°C) in a quiet room dedicated to autonomic testing. All study subjects were fasting at least 4 hours prior to testing. No caffeine, nicotine, or alcohol was permitted on the day of testing prior to study completion.
All subjects underwent standardized autonomic testing, including a head-up tilt study. Tilt table testing consisted of supine rest of at least 30 minutes, followed by 70° passive head-up tilt. We routinely tilt patients with concern about OI for 10 minutes, so that the majority of patients had a 10 minute tilt study. Considering above described definitions of OI in adults, the first 84 control subjects were tilted for 5 minutes. To provide additional data for prolonged head-up tilt, the last 22 control subjects enrolled were tilted for 10 minutes. Orthostatic symptoms were recorded. Blood pressure responses were reviewed to exclude patients with orthostatic hypotension and to document the frequency of presyncope.
Primary endpoints were: a) Normative values of orthostatic HR and HR increment in a large cohort of normal controls younger than 20 years of age, and b) Comparison of orthostatic HR and HR increment between normal controls and a large cohort of pediatric patients with symptoms of orthostatic intolerance. Secondary endpoints were: (1) normative values of supine HR and comparison with patients; (2) influence of age, sex, and body mass index (BMI) on HR measurements; and (3) frequency of tilt-induced symptoms and presyncope.
Beat-to-beat BP was continuously recorded using the photoplethysmographic volume clamp method (Finapres Model 2300, Ohmeda, Englewood, CO, or Finometer Model 1.22, Finapres Medical Systems, Arnhem, Netherlands). The analog BP signal was sampled at 250 Hz and the maximal and minimal points occurring between the QRS pulse derived as systolic and diastolic BP.
Instantaneous HR was calculated from the RR-interval using continuous 3-lead EKG recordings. Respiratory excursions were measured using a nasal thermistor or a chest expansion bellows.
Data Analysis
HR was determined at supine rest (30 seconds before tilt), and during tilt (at 1 minute, 5 minutes and – where available – at 10 minutes) in two different ways: 1) as the “spot HR” recorded by the technician during the study at the time points of interest using the HR calculation of the EKG monitor (2 second average), and 2) as “average HR” using data averaging of 30 second time windows at the time points of interest in order to minimize the influence of spontaneous signal oscillations. The HR change from baseline was calculated for each time point during tilt.
All data are expressed as mean ± SD. Linear regression analysis was performed to assess the influence of age and BMI on variables. Chi Squared Analysis was performed to assess for the influence of sex. Normative ranges were determined using percentile calculations. Mann-Whitney Wilcoxon testing was performed to assess for differences of variables between patients and controls. Significance was accepted at the 5% level.
RESULTS
106 normal control subjects were included with a mean age of 14.5±3.3 years, and an age range between 8 and 19 years. 52 subjects were female, 55 were male. 654 patients were included with a mean age of 15.5±2.3 years. 476 were female, 178 were male.
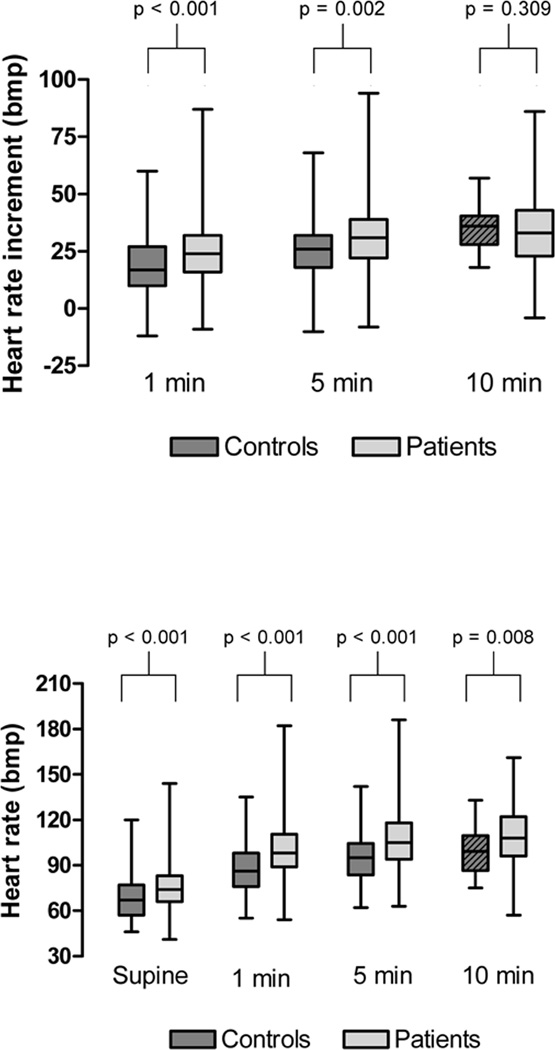
The HR increment from baseline at 5 minutes of tilt was significantly higher in patients referred for POTS/OI compared with the control group (32±14bpm versus 27±13bpm, p=0.002, Figure 1), though there was considerable overlap. The same was true for the 1 minute time-point, but the HR increment was not different between groups at the 10 minute time point. However, as most control subjects were tilted for 5 minutes only, the number of observations for that time point was small (n=22 versus n=106 for all other time points; Figure 1).
FIGURE 1.
Orthostatic HR increment (top panel) and absolute HR at supine rest and during head-up tilt (bottom panel) in normal controls (dark grey bars) and patients with symptoms of OI (light grey bars). Note the considerable overlap between patient and control group. The box for controls at the 10 minute time point is shaded to emphasize the smaller number of observations at that time point (n=22 versus n=106).
At 5 minutes of passive head-up tilt, 42% of normal controls had a HR increment of 30bpm or more using spot HR. Using averaged HR, the fraction of controls with a HR increment of at least 30bpm was still 33%. In the patient group, 54.5% had a HR increment of 30bpm or more at 5 minutes.
The 95th percentile for the 5 minute orthostatic HR increment among normal controls was 51bpm using spot HR and 43bpm using average HR (Table).
Table.
HR increment and orthostatic HR at 5 minutes of head-up tilt.
| Percentile (spot HR) |
Percentile (average HR) |
|||||
|---|---|---|---|---|---|---|
| 90th | 95th | 97.5th | 90th | 95th | 97.5th | |
| Orthostatic HR increment (bpm) | ||||||
| Age 8–19 years | 44.0 | 51.3 | 55.6 | 39.0 | 42.9 | 52.7 |
| Absolute orthostatic HR (bpm) | ||||||
| Age 8–13 years | 130.0 | 131.2 | 142.0 | 127.6 | 136.9 | 139.1 |
| Age 14–19 years | 107.0 | 119.5 | 123.5 | 106.7 | 116.0 | 120.6 |
Within the age group studied, there was no significant influence of age, sex, or BMI on the HR increment. There was an influence of age on orthostatic HR resulting in different values for different age groups.
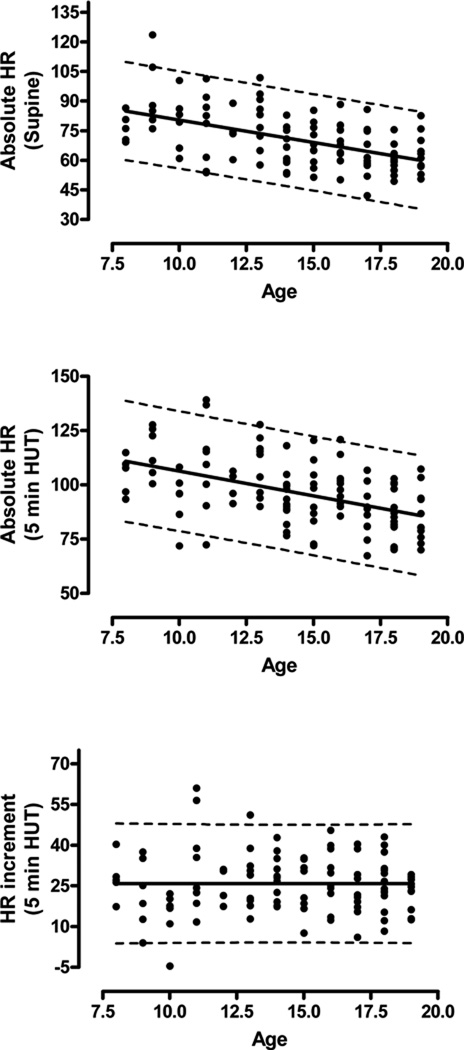
There was no influence of age, sex, or BMI on the orthostatic HR increment (Figure 2).
FIGURE 2.
Linear regression analysis with prediction intervals for absolute supine (top panel) and orthostatic HR (middle panel), as well as orthostatic HR increment (bottom panel) by age. Averaged HR is shown, although the findings for spot HR were similar apart from wider prediction intervals. Note the significant influence of age on supine and orthostatic HR but not on the orthostatic HR increment.
The absolute orthostatic HR showed a greater and more consistent difference between groups, although there was still considerable overlap with control subjects. This was true for all time points. The 5 minute orthostatic HR was 107±18bpm in patients vs. 95±16bpm in controls (p<0.001; Figure 1).
Although there was no influence of sex or BMI, the absolute orthostatic HR decreased significantly with age (Figure 2). Therefore, for the purposes of calculating normative values, the control group was divided into children (8–13 years) and adolescents (14 to 19 years). For children, the 95th percentile for the orthostatic HR at the 5 minute time point was 131 using spot HR and 137 using average HR. For adolescents, the 95th percentile was 120bpm (spot HR) and 116bpm (average HR; Table)
Supine HR was also higher in patients when compared with controls (75±14 versus 68±14, p<0.001, Figure 1). As for the orthostatic HR, there was a significant influence of age (lower HR with increasing age; Figure 2) but no influence of sex or BMI.
Six control subjects (5.7%) developed signs of presyncope requiring early termination of the tilt test. An additional 2 subjects reported orthostatic symptoms during tilt that were not associated with presyncope. None of the controls had a prior history of syncope or of orthostatic symptoms.
478 patients (74.2%) reported orthostatic symptoms during tilt. Rarely, these symptoms were related to presyncope (n=40, 6.2%).
DISCUSSION
The concept of Adolescent Autonomic Dysfunction and pediatric/adolescent OI and POTS is gaining increasing popularity and these syndromes are increasingly being recognized as common disorders of youth.[5, 6, 13] Our pediatric referral clinic sees overwhelming demand for evaluation and management of these disorders which have been reported to affect millions of patients, can be quite disabling and often have a significant impact on patients’ quality of life.[5, 6, 13] As these conditions are often chronic and refractory to treatment, the social and economic impact is considerable.[5, 6, 13]
This study was designed to fill an important gap in our knowledge of what is normal and abnormal when evaluating the HR and HR response with head-up tilt in children and adolescents in the autonomic laboratory. Even though it has been suggested that the criteria used to diagnose orthostatic intolerance in adults may not be appropriate to use in a pediatric population, children and adolescents have not only been diagnosed with and treated for POTS and OI but have also been enrolled in research studies on pediatric POTS based on those criteria, which we could be inappropriate for this age-group.[5–10, 12, 13]
Our study demonstrates that an orthostatic HR increment of 30bpm – the main diagnostic criterion for OI in adults – is still well within normal range for children and adolescents. If this is used as cut-off for diagnosing OI in a pediatric age group it would result in very low specificity. This observation is underlined by a recently published study on high-school students in the community of Rochester, Minnesota, in which orthostatic HR changes as high as 48bpm were observed.[10]
The presented data suggest that the HR increment in the age group studied is not significantly influenced by age, sex, or BMI. The study also shows the advantage of using data averages, as the range of normal could be narrowed considerably by eliminating the influence of substantial spontaneous HR fluctuations. Using this approach, an orthostatic HR increment of greater than 40 to 45bpm was shown to be excessive in this age group.
As expected, absolute HR was found to be influenced by age and we could demonstrate both supine and orthostatic HR to decrease with age. An excessive orthostatic HR was shown to be greater than 130 to 140bpm in children up to 13 years of age, and greater than 120bpm in adolescents 14 years and older. The latter number is close to what has been reported as excessive orthostatic HR in young adults.[1] Younger children on the other hand clearly can have a higher orthostatic HR without being orthostatically intolerant and 120bpm is still a normal orthostatic HR at that age.
Our data raise many questions. Of note was the considerable overlap of the orthostatic rise in HR between control and patient group. As diagnostic criteria for pediatric OI and POTS have not been established, we purposely avoided circular logic by including only patients with a final diagnosis of OI/POTS which would have reflected diagnostic impressions based on adult HR criteria. Similarly, only including patients who fall outside of the now established normative range would have had the predictable outcome of a large difference between patients and controls. Rather, we included all patients in whom OI was clinically suspected and found that although a significantly higher orthostatic HR rise was seen, that difference was less than 5 bpm on average. This overlap is intriguing and raises the question whether HR increment alone can be relied upon when diagnosing OI in a pediatric setting. Considering that the absolute orthostatic HR showed a greater and more consistent difference between patients and controls, one could argue that the absolute orthostatic HR may be more important for the development of orthostatic symptoms and should be emphasized more in diagnosis of pediatric OI. One could ask the question whether OI at this age may not depend as much on HR as it does in adults or even whether the use of HR criteria in general may not be appropriate.
For the time being, HR criteria should be maintained in the diagnostic criteria for OI and POTS, as relying on symptoms alone cannot be satisfactory. The clinician should, however, keep in mind that not only can cardiovascular variables fluctuate with the time of the day, from day to day, and depending on factors such as hydration status and medication intake, but also that a given HR increment may be associated with symptoms in one but not in the other individual.[14–16] As with other disorders, diagnostic criteria can guide but cannot replace a clinician’s judgment. Diagnostic criteria are irreplaceable however, when scientific studies are being pursued.
Considering our normative data, and realizing that overly strict diagnostic guidelines are not desirable in these common but still poorly understood conditions, we suggest the following diagnostic criteria for pediatric OI/POTS:
Pediatric OI
1) Symptoms of OI such as lightheadedness and palpitations that frequently (>50% of the time) develop when assuming the upright position, and 2) Orthostatic HR increment ≥40bpm within 5 minutes of head-up tilt
Pediatric POTS
1) Symptoms and HR increment that fulfill criteria for pediatric OI, and 2) Absolute orthostatic HR ≥130bpm (for ages 13 years and younger), or ≥120bpm (for ages 14 years and older) within 5 minutes of head-up tilt.
A group of patients will remain with symptoms suggestive of orthostatic intolerance but without evidence of orthostatic tachycardia and orthostatic hypotension. The etiology of symptoms in these patients is unclear at this point and further studies are needed to evaluate this likely heterogeneous patient group. Possible mechanisms include low baseline blood pressure resulting in orthostatic symptoms despite relatively stable cardiovascular measures, hyperventilation resulting in reduced cerebral blood flow, bodily hypervigilance, anxiety disorders, migrainous phenomena, and vestibular disorders. Until this group of patients is better understood, we suggest using the term “orthostatic symptoms without tachycardia” which describes the phenomenon without implying knowledge of underlying pathophysiology.
These guidelines specifically refer to a diagnosis of pediatric OI and POTS and are not meant to replace the complex clinical concept of adolescent autonomic dysfunction. Tilt table testing alone cannot capture the full spectrum of symptoms that patients with this syndrome frequently experience, which range from orthostatic symptoms to chronic dizziness, fatigue, exercise intolerance, gastrointestinal dysmotility, and headaches. OI seems to be frequently associated with this syndrome, but is a dominant feature only in part of the spectrum of adolescent autonomic dysfunction. Further research is needed to help develop better diagnostic criteria for this complex syndrome, to delineate specific categories, and to ultimately find more effective treatment strategies for the large number of affected adolescents.
Acknowledgments
Supported by National Institutes of Health (NS 32352; NS 065736), Mayo CTSA (MO1 RR00585), and Mayo Funds.
ABBREVIATIONS
- HR
heart rate
- POTS
Postural Tachycardia Syndrome
- OI
orthostatic intolerance
- BP
blood pressure
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Low PA, Sandroni P, Joyner MJ, Shen WK. Postural Tachycardia Syndrome. In: Low PA, Benarroch EE, editors. Clinical Autonomic Disorders. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 515–533. [Google Scholar]
- 2.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, et al. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 3.Low PA, Opfer-Gehrking TL, Textor SC, Schondorf R, Suarez GA, Fealey RD, et al. Comparison of the postural tachycardia syndrome (POTS) with orthostatic hypotension due to autonomic failure. Journal of the autonomic nervous system. 1994;50:181–188. doi: 10.1016/0165-1838(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 4.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 5.Antiel RM, Risma JM, Grothe RM, Brands CK, Fischer PR. Orthostatic intolerance and gastrointestinal motility in adolescents with nausea and abdominal pain. Journal of pediatric gastroenterology and nutrition. 2008;46:285–288. doi: 10.1097/MPG.0b013e318145a70c. [DOI] [PubMed] [Google Scholar]
- 6.Ojha A, Chelimsky TC, Chelimsky G. Comorbidities in Pediatric Patients with Postural Orthostatic Tachycardia Syndrome. The Journal of pediatrics. doi: 10.1016/j.jpeds.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1-7) production in postural tachycardia syndrome. Hypertension. 2009;53:767–774. doi: 10.1161/HYPERTENSIONAHA.108.127357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart JM, Weldon A. Reflex vascular defects in the orthostatic tachycardia syndrome of adolescents. J Appl Physiol. 2001;90:2025–2032. doi: 10.1152/jappl.2001.90.6.2025. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt BE, Fischer PR, Brands CK, Porter CB, Weaver AL, Yim PJ, et al. Exercise performance in adolescents with autonomic dysfunction. The Journal of pediatrics. 158:28–32. doi: 10.1016/j.jpeds.2010.07.020. e1. [DOI] [PubMed] [Google Scholar]
- 10.Skinner JE, Driscoll SW, Porter CB, Brands CK, Pianosi PT, Kuntz NL, et al. Orthostatic heart rate and blood pressure in adolescents: reference ranges. Journal of child neurology. 25:1210–1215. doi: 10.1177/0883073809359539. [DOI] [PubMed] [Google Scholar]
- 11.Dambrink JH, Imholz BP, Karemaker JM, Wieling W. Circulatory adaptation to orthostatic stress in healthy 10-14-year-old children investigated in a general practice. Clin Sci (Lond) 1991;81:51–58. doi: 10.1042/cs0810051. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS) The Journal of pediatrics. 2004;145:725–730. doi: 10.1016/j.jpeds.2004.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson JN, Mack KJ, Kuntz NL, Brands CK, Porter CJ, Fischer PR. Postural orthostatic tachycardia syndrome: a clinical review. Pediatric neurology. 42:77–85. doi: 10.1016/j.pediatrneurol.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Davis JE, Fortney SM. Effect of fluid ingestion on orthostatic responses following acute exercise. International journal of sports medicine. 1997;18:174–178. doi: 10.1055/s-2007-972615. [DOI] [PubMed] [Google Scholar]
- 15.Lewis NC, Atkinson G, Lucas SJ, Grant EJ, Jones H, Tzeng YC, et al. Diurnal variation in time to presyncope and associated circulatory changes during a controlled orthostatic challenge. American journal of physiology. 299:R55–R61. doi: 10.1152/ajpregu.00030.2010. [DOI] [PubMed] [Google Scholar]
- 16.Low PA, Sletten DM. Laboratory Evaluation of Autonomic Failure. In: Low PA, Benarroch EE, editors. Clinical Autonomic Disorders. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 130–163. [Google Scholar]