Abstract
Aims
To examine whether overweight and obesity indicators – body mass index, waist circumference, and waist circumference-to-height ratio – predict progression of periodontal disease in men.
Materials and Methods
Participants were 1038 medically healthy, non-Hispanic, white males in the VA Dental Longitudinal Study who were monitored with triennial oral and medical examinations between 1969 and 1996. Periodontal disease progression in an individual was defined as having 2 or more teeth advance to levels of alveolar bone loss ≥40%, probing pocket depth ≥5mm, or clinical attachment loss ≥5mm after baseline. Extended Cox regression analyses estimated hazards of experiencing periodontal disease progression events due to overweight/obesity status, controlling for age, smoking, education, diabetes, recent periodontal treatment, recent prophylaxis, and number of filled/decayed surfaces.
Results
Body mass index and waist circumference-to-height ratio were significantly associated with hazards of experiencing periodontal disease progression events regardless of periodontal disease indicator. Adjusted hazard ratios for periodontal disease progression were 41-72% higher in obese men (body mass index ≥ 30 kg/m2) relative to men with both normal weight and waist circumference-to-height ratio (≤50%).
Conclusion
Both overall obesity and central adiposity are associated with an increased hazards of periodontal disease progression events in men.
Keywords: Overweight, Obesity, Body Mass Index, Waist Circumference, waist circumference-to-height ratio, Periodontal Disease
INTRODUCTION
Periodontal disease is a chronic disease that affects approximately half of US adults aged 30 and older (Albandar, 2011), and is one of the primary causes of tooth loss (Murray et al. 1997, Phipps & Stevens 1995). Most recent cross-sectional studies and a meta-analysis of obesity and periodontal disease have found significant positive associations between obesity status and periodontal disease prevalence (Al-Zahrani et al. 2003, Wood et al. 2003, Saito et al. 2001 & 2005, Linden et al. 2007, Sarlati et al. 2008, Khader et al. 2009, Chaffee & Weston, 2010). In several studies, measures of abdominal obesity such as high waist circumference (WC) and waist-to-hip ratio appeared to be more strongly related than overall obesity (body mass index, or BMI) to higher periodontal disease prevalence (Kim et al., 2010, Al-Zahrani et al. 2003, Wood et al. 2003).
The growing prevalence of overweight and obesity among US adults and children has raised interest in using obesity-related indices to predict risks of many chronic health conditions, including periodontal disease. Fat distribution, more so than total body fat, has been postulated as a key predictor of disease risk. Although overweight and obesity in adults are commonly defined by BMI, this index is not capable of describing body fat distribution. Another marker used to predict obesity-related disease risk, WC, is a potentially useful predictor as it is a measure of abdominal fat content and is associated with visceral fat deposition. WC was reported to be an independent predictor of all-cause mortality, diabetes mellitus (DM), and Coronary Artery Disease (CAD) in both men and women in a preventive cardiology population (Bajaj et al. 2009). BMI was only associated with DM, but the association disappeared when WC was added to the model.
WC is limited in that it does not account for differences in body height, and the ratio of WC to height (waist-height ratio, or WHtR) has been proposed as a better screening tool for cardiovascular risk, mortality, and intra-abdominal fat compared to BMI, WC and waist-hip ratio (Schneider et al. 2007, Lin et al. 2002). Moreover, the degree of abdominal fat mass can vary significantly within a narrow range of BMI (WHO, 2/12/2009) so that WHtR can be used to identify subjects who are at higher risk of metabolic diseases even within the normal weight category of BMI.
It is not clear whether measures of fat distribution are better predictors of periodontal disease than BMI status. Furthermore, there is a lack of prospective studies evaluating obesity status and periodontal disease incidence. The primary aim of this study was to examine the association of multiple obesity-related characteristics of male participants in the Department of Veterans Affairs Dental Longitudinal Study (DLS) with periodontal disease progression, controlling for multiple risk factors. A second aim was to evaluate whether WC and WHtR are related to periodontal disease independently of BMI, which may suggest a specific role of visceral fat. Several clinical and radiographic measures of periodontal disease indicators were available for analysis in this cohort.
MATERIALS and METHODS
We examined 1038 white males in the DLS, a unique population of men who have continuously had their oral health, weight status, medical health and lifestyle monitored since the 1960s. The DLS is a closed-panel prospective study of aging and oral health that began in 1969 by recruiting 1231 male participants from the ongoing VA Normative Aging Study (Kapur et al. 1972). The Normative Aging Study (NAS) participants were all men between the ages of 21 and 84 years who were initially free of chronic disease and lived in the greater Boston metropolitan area. The participants were screened prior to NAS enrollment in the mid 1960s to exclude those who had pre-existing cardiovascular disease, cancer and diabetes. While the majority of the participants are veterans, they have not been patients of the VA healthcare system and have received dental and medical care from private health care providers during the study follow-up. Participants attend dental examinations approximately every 3 years for assessments of periodontal status and oral health. The protocols were approved by the Institutional Review Boards of the VA Boston Healthcare System and Boston University Medical Campus. All participants gave informed consent on approved forms prior to each examination.
Oral examinations
Alveolar bone loss (ABL) was measured on dental radiographs by superimposing a transparent ruler on the tooth image with reference points at the cemento-enamel junction (CEJ) and root apex. In the DLS, a modified Schei ruler method is used (Schei 1959) in which ABL was recorded in increments of 20% of the distance from the CEJ to the tip of the root. Probing pocket depth (PPD) was measured by calibrated examiners in millimeters using a Williams probe at six sites per tooth (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, and distolingual. In the first 6 examination cycles, probing pocket depth was measured on a continuous scale but the maximum PPD for each tooth was recorded on an interval scale (0 = ≤2mm; 1 = >2-≤3mm; 2 = >3-<5mm; 3 = ≥5mm). At examination cycles 7 through 9, the measures were recorded as continuous millimeters. However, we categorized them for data analysis according to the earlier interval scale. Clinical attachment loss (CAL) measures were first recorded in 1981-1985 (examination cycle 5) using the same instrument and scales as PPD measurements.
Restorations and caries on each surface were noted by the examiner and recorded. Three levels of caries were distinguished: level 1 refers to caries of enamel only whereas levels 2 and 3 involve enamel and dentin. For data analysis, the total number of surfaces with restorations (including crowns) and/or any level of caries was computed for each participant.
Third molars were excluded from all tooth counts.
Anthropometry
At each NAS examination, height was measured in inches on a stadiometer, and weight (pounds) on a balance beam scale. The measurements were converted to meters and kilograms for computation of body mass index (kg/m2). Waist circumference was measured with the subject wearing little clothing and standing erect, relaxed, with arms at the sides and feet together. The examiner faced the subject and placed an inelastic tape measure around the narrowest part of the torso keeping the tape horizontal to the floor. The tape was kept snug but did not compress the skin. The circumference measure, in units of 0.1 cm, was taken at the end of a normal expiration. Categories of BMI were calculated based on the accepted cut-off values of ideal BMI (18.1-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese >30 kg/m2). Waist circumference was categorized as desirable WC (≤102 cm) or high WC (>102 cm). Waist-height ratio (WHtR) percentage was calculated by dividing WC (cm) by height (cm), and categorized as desirable (≤50%) or high WHtR (>50%) based on studies by Ashwell et al. (2005) and Schneider et al. (2007).
Other information
Smoking status, frequency of toothbrushing and frequency of dental visitswere ascertained by questionnaire at each dental examination. Participants reported the numbers of packs of cigarettes smoked each day, and the number of dental visits and reasons for dental treatment received in the year preceding the DLS examination. Current smoking status was defined as use of any cigarettes within the previous year. Two treatment variables, for any periodontal disease in past year (yes/no) and for any prophylaxis in past year (yes/no), were used in the statistical analyses. Educational status was collected only at the baseline examination and categorized as education beyond high school (yes/no). Information on medication use, coronary heart disease diagnosis, and diabetes diagnosis was obtained during medical examinations conducted by Normative Aging Study physicians. Diabetes diagnosis (yes/no) was determined as positive if the participant was being treated for diabetes by his personal physician or if he was taking insulin or an oral hypoglycemic drug. Although diabetics were excluded at the NAS baseline, 6 men had developed the disease by 1969 when the DLS began.
For this study, men were excluded if they had no teeth at baseline (n=73), no follow-up examinations after baseline (n=113), baseline body mass index below 18.2 kg/m2 (n=3), no information on body weight after baseline (n=2), or all teeth at baseline had the maximum scores of probing pocket depth (≥5mm) and alveolar bone loss (≥40%) and therefore had no teeth at risk of progression (n=2).
Statistical Analysis
For each periodontal disease measure, a periodontal disease progression event was defined as having 2 or more teeth advance to the specified thresholds (>40% ABL, or >=5mm PPD or CAL) subsequent to the baseline dental examination. Teeth that were lost before ever reaching that threshold were counted as progression events if they had a mild/moderate level of the index (ABL=20%, PPD or CAL >3mm but <5mm) at all prior study examinations. Inclusion of lost teeth in the definition of periodontal disease was done to avoid underestimation of periodontal disease progression, since periodontal disease is one of the major causes of tooth loss in adults (Murray et al. 1997, Phipps and Stevens 1995). Time to progression was defined as the number of years between the baseline dental examination and first examination where the cumulative number of teeth with periodontal disease above the threshold exceeded the baseline number of affected teeth by 2 or more. For analyses of CAL, baseline was redefined as the dental examination conducted in examination cycle 5.
Extended Cox regression analyses were used to estimate the hazards of experiencing periodontal disease progression events attributable to body weight and each of the 3 overweight indicators: BMI, WC and WHtR. Failure time, i.e., time to a periodontal disease event, and total follow-up time were measured in whole years. Ties in failure time were accounted for by the approximate likelihood method of Efron (1977). Covariates in all models included education (high school graduate or less, vs. college and higher), age, cigarette status (non-smoker, smoker), diabetes (yes/no), periodontal treatment in past year (yes/no), prophylaxis in past year (yes/no), and number of filled/decayed surfaces. All independent variables except education were treated as time-dependent covariates. The criterion for retaining these covariates was p value <0.05. The anthropometric measures used in the Cox models were those collected at the NAS examination closest in time to the dental examination preceding any onset of periodontal disease progression (1969-1972) to ensure that obesity/overweight status was achieved prior to any progression of periodontal disease. Because BMI and WC were strongly correlated (R=0.86), they were not included as independent predictors in the same models. Analyses were conducted with SAS 9.1.
RESULTS
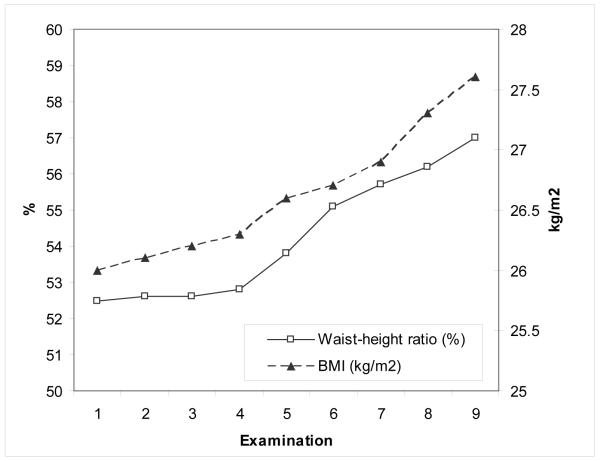
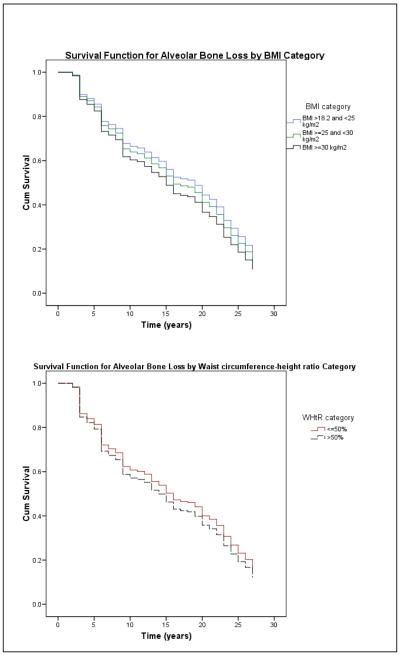
Baseline characteristics of the study cohort and changes in periodontal disease and overweight/obesity status are shown in Table 1. The mean BMI increased 1.6 kg/m2 and waist circumference-height ratio increased 4.5% during follow-up (Figure 1). Over the same time period, the number of teeth that remained free of periodontal disease declined, more so in men who were obese. Figure 2 shows the associations of alveolar bone loss with BMI and WHtR; survival curves for PPD and CAL were similar. In multivariate Cox analyses, weight was not statistically associated with the hazard of experiencing a periodontal disease progression event regardless of which periodontal disease indicator was used to define the event (Table 2). Each unit increase in BMI was associated with a 5% increase in the hazard of experiencing an ABL progression event. An increment of 1 cm waist circumference was associated with 1-2% increases in the hazards of experiencing PPD and CAL progression events, and each 1% increment in baseline WHtR was associated with 3% increase in the hazard of experiencing periodontal disease progression events as defined by all three periodontal disease indicators.
TABLE 1.
Demographics of study participants (n=1038).
| Variable | Mean (SD) or % |
|---|---|
|
Baseline descriptive data (1969-1972, unless otherwise
indicated) |
|
| Age (yrs) | 47.8±8.7 |
| Weight (kg) | 80.1±10.3 |
| Body Mass Index (kg/m2) | 26.1±2.8 |
| Ideal (18.2-24.9 kg/m2) | 36% |
| Overweight (25-29.9 kg/m2) | 56% |
| Obese (≥30 kg/m2) | 8% |
| Waist Circumference (cm) | 91.9±7.1 |
| Waist circumference >102 cm | 9% |
| Waist circumference -height ratio (%) | 52.5±4.1 |
| Waist-height ratio >50% | 74% |
| Number of teeth | 22.5±5.3 |
| Number teeth with alveolar bone loss ≥40% | 0.7±1.7 |
| Number teeth with probing pocket depth ≥5mm | 1.2±2.2 |
| Number teeth with clinical attachment loss ≥5mm (n)* | 1.6±3.0 (675) |
| Number decayed/filled surfaces | 33.4±15.5 |
| % Participants with education beyond High School | 68% |
| Smoking Status | |
| Never smoked | 55% |
| Quit prior to 1969-1972 baseline examination | 18% |
| Current smoker at 1969-1972 baseline examination | 27% |
| Periodontal treatment in past year | 8% |
| Had professional cleaning in past year | 84% |
| Had periodontal treatment in past year | 8% |
| Positive diabetes diagnosis | 0.5% |
|
| |
| Changes since baseline | |
| % Ideal weight (BMI 18.2-24.9 kg/m2) at last examination | 29% |
| % Overweight (BMI 25-29.9 kg/m2) at last examination | 54% |
| % Obese (BMI (≥30 kg/m2) at last examination | 17% |
| % With waist circumference >102 cm at last examination | 25% |
| % With waist circumference -height ratio >50% at last examination | 86% |
| Number teeth/decade advanced to level of ABL ≥40% | 2.5±2.8 |
| Number teeth/decade advanced to level of probing pocket depth ≥5mm |
1.5±2.7 |
| Number teeth/decade advanced to level of clinical attachment loss ≥5mm |
4.7±5.3 |
Measurements began in 1981.
Figure 1.
Changes in BMI and waist circumference-height ratio during follow-up. Examination 1 was conducted in 1969-1973 and examination 9 in 1993-1998, with intervening examinations at 3-year intervals.
Figure 2.
Survival functions for progression of alveolar bone loss by BMI category and waist circumference-height ratio.
Table 2.
Continuous obesity-related predictors of periodontal disease progression. Adjusted hazard ratios and 95% CI.
| Periodontal Disease Indicator | |||
|---|---|---|---|
| Probing Depth ≥5mm* | Clinical Attachment Loss ≥5mm† | Alveolar Bone Loss ≥40%‡ | |
| Predictor | |||
| Weight (per kg) | 1.01 (0.99-1.02) | 1.01 (0.99-1.02) | 1.01 (0.99-1.02) |
| BMI (per kg/m2) | 1.03 (0.99-1.06) | 1.03 (0.99-1.06) | 1.05 (1.01-1.09) |
| Waist circumference (per cm) | 1.02 (1.00-1.03) | 1.01 (1.00-1.03) | 1.01 (0.99-1.03) |
| Waist-height ratio (per 1%) | 1.03 (1.01-1.05) | 1.03 (1.01-1.05) | 1.03 (1.01-1.06) |
All models adjusted for age, cigarette use (yes/no), education beyond high school (yes/no), number of decayed or filled surfaces, treatment for periodontal disease in past year (yes/no), prophylaxis in past year (yes/no), and diabetes diagnosis (yes/no). All independent variables except education were treated as time-dependent covariates.
Individual had 2 or more teeth that either progressed to ≥5mm or were lost after having >3 but <5mm probing depth at each prior examination
Individual had 2 or more teeth that either progressed to ≥5mm or were lost after having >3 but <5mm CAL at each prior examination
Individual had 2 or more teeth that either progressed to ≥40%or were lost after having >20% but <40% ABL at each prior examination
Hazards of experiencing PPD, CAL, and ABL progression events were 40%, 52% and 60% higher, respectively, among obese men relative to ideal weight men (Table 3). Neither of the commonly used categorical waist size indices was significantly related to hazards of periodontal disease progression events.
Table 3.
Categorical obesity-related predictors of periodontal disease progression. Adjusted hazard ratios and 95% CI.
| Periodontal Disease Event (≥2 teeth progressed to indicated level) | |||
|---|---|---|---|
| Predictor | Probing Depth ≥5mm* | CAL ≥5mm† | ABL ≥40%‡ |
| Baseline BMI category | |||
| Ideal (18.1–24.9 kg/m2) | 1.0 (reference) | 1.0 | 1.0 |
| Overweight (25–29.9 kg/m2) | 1.09 (0.92-1.30) | 1.13 (0.88-1.46) | 1.07 (0.83-1.38) |
| Obese (≥30kg/m2) | 1.40 (1.02-1.91) | 1.52 (1.05-2.21) | 1.60 (1.07-2.38) |
| Baseline waist circumference category | |||
| Normal (<102 cm) | 1.0 | 1.0 | 1.0 |
| High (≥102 cm) | 1.23 (0.95-1.61) | 0.99 (0.75-1.33) | 1.24 (0.88-1.75) |
| Baseline waist-height ratio category | |||
| Normal (<50%) | 1.0 | 1.0 | 1.0 |
| High (≥50%) | 1.15 (0.95-1.39) | 1.41 (1.01-1.97) | 1.26 (0.95-1.67) |
All models adjusted for age, cigarette use (yes/no), education beyond high school (yes/no), number of decayed or filled surfaces, treatment for periodontal disease in past year (yes/no), prophylaxis in past year (yes/no), and diabetes diagnosis (yes/no). All independent variables except education were treated as time-dependent covariates.
Individual had 2 or more teeth that either progressed to ≥5mm or were lost after having >3 but <5mm probing depth at each prior examination
Individual had 2 or more teeth that either progressed to ≥5mm or were lost after having >3 but <5mm CAL at each prior examination
Individual had 2 or more teeth that either progressed to ≥40%or were lost after having >20% but <40% ABL at each prior examination
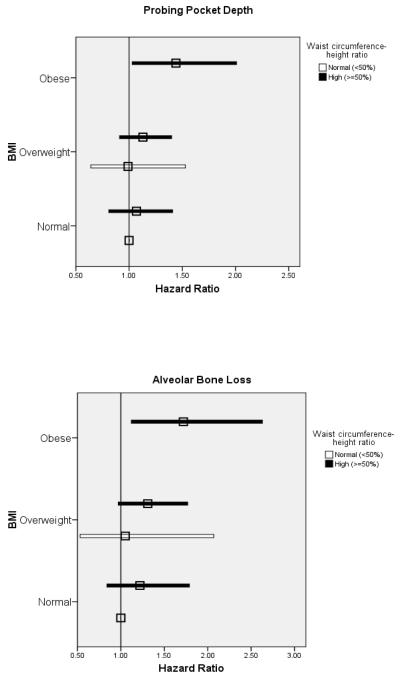
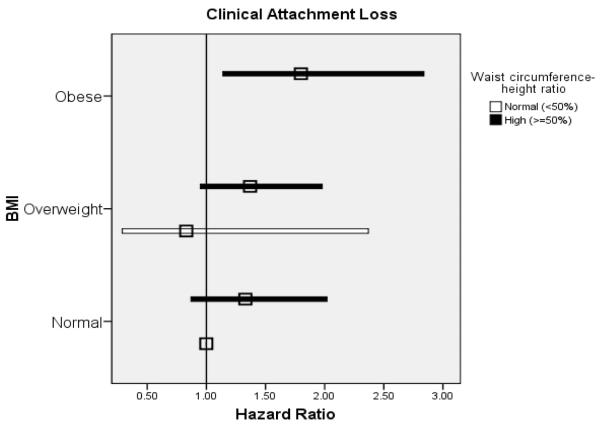
The concurrent use of BMI and WHtR to estimate hazard ratios revealed a trend for increased hazards of experiencing PPD, CAL and ABL progression events among men with a high waist-height ratio relative to low ratio in the absence of obesity (Figure 3).
Figure 3.
Adjusted hazard ratios (squares) and 95% CI (total width of bars) for body mass index (BMI) and waist circumference-height ratio (WHtR) as predictors of probing pocket depth, alveolar bone loss, and clinical attachment loss progression. Reference group is Ideal BMI & Normal WHtR. No obese participants had WHtR <50%. All models adjusted for age, cigarette use (yes/no), education beyond high school (yes/no), number of decayed or filled surfaces, treatment for periodontal disease in past year (yes/no), prophylaxis in past year (yes/no), and diabetes diagnosis (yes/no).
DISCUSSION
Considerable attention has been given to the effects of obesity on chronic health conditions. The findings in this study support previous associations of height-adjusted overall obesity and central adiposity with the hazard of experiencing periodontal disease events. The two measures of central adiposity (WC and WHtR) were significant predictors of periodontal disease progression events when used as continuous measures but not when categorized according to currently accepted cut-off values. Given how the population has become heavier in the past several decades, these commonly-used values to dichotomize WC and WHtR may not have been appropriate for our historical data. In the first 10 years of the study, fewer than 10% of participants had a waist circumference >102 cm, and in the last 10 years, only 10-12% had a waist circumference-height ratio <=50%. These small sample sizes likely limited our power to detect statistically significant hazard ratios. In the concurrent analysis of BMI and WHtR status, the findings suggested an interactive effect on the hazards of experiencing periodontal disease events. That is, within each of the ideal and overweight BMI categories, hazard ratios tended to be greater in men with high WHtR than low WHtR.
The DLS participants offer an abundance of data from medical and dental evaluations and provide a long follow-up for monitoring disease progression. Multiple periodontal disease measures were available, including radiographic alveolar bone loss. Therefore, the major strengths of this study are the prospective design and long follow-up period, and control for multiple potential confounders. Risk assessment studies have identified several subject level characteristics including age, male gender, smoking and diabetes mellitus as being associated with periodontal disease severity and/or progression (Al-Shammari et al. 2005), and our study reaffirmed these characteristics as important predictors. A limitation of the study includes the all-male makeup of the cohort which leads to lack of ability to generalize these findings to a larger population. The DLS participants were participants in the Normative Aging Study who self-selected to join the DLS study, and were primarily white males. Baseline characteristics indicate much lower obesity prevalence (8%) in 1969-1971, a prevalence rate that is a bit lower than seen in the US population at that time (Flegal et al. 1998), and much lower than the current prevalence of adult obesity (~30%). Nevertheless, the increases in mean BMI and visceral adiposity mirror trends in the general male population over a similar time interval (Okosun et al. 2004). Additional limitations of this study include the change in methods for recording periodontal data during the study, potential residual confounding, the arbitrary definition of periodontal disease progression and the failure to account for multiple periodontal disease events in our models. Our threshold for defining periodontal disease progression was restricted by the interval scale on which data from the first several examination cycles were recorded and we could not examine other cut-off values. The dichotomization of smoking status may not have adequately controlled for the effect of different levels of cigarette dose on periodontal disease progression. We defined progression as occurring when an individual had 2 or more teeth advance to these threshold levels. This value was selected in order to balance the potential increase in misclassification, which is more likely to occur when the advancement of just one tooth is used as the definition, with the reduced number of events if the cut-off were set at 3 or more teeth. Moreover, there was a large range of baseline number of teeth in the DLS cohort. Although there were no differences in mean numbers of teeth among ideal, overweight and obese men at baseline, differences became more apparent at later follow-up examinations. The chance of having 2 or more teeth with periodontal disease progression is greater the more teeth one has, and the overall hazard ratios we report cannot be generalized to other populations. Finally, by only including the first occurrence of periodontal disease progression, we are underestimating the true rate of progression.
The majority of cross-sectional studies have suggested a dose-response relationship between BMI category and extent of periodontal disease (Nishida et al. 2005, Dalla Vecchia et al. 2005, Khader et al. 2009). Only one cross-sectional study has noted an inverse trend between BMI and attachment loss (Kongstad et al. 2009). However, in each study, the extent or odds of periodontal disease were statistically significantly elevated among obese persons but not overweight persons. In our prospective analyses, we also found higher rates of periodontal disease progression in the obese men, but only slight increases in those who were overweight. Previous studies also reported significantly increased odds of periodontal disease associated with measures of central adiposity (Kim et al., 2010, Al-Zahrani et al. 2003, Reeves et al. 2006). Al-Zahrani et al. and Reeves et al. both found significant relationships between WC and periodontal disease, although Al-Zahrani et al. noted the magnitude of the association declined with increasing age. Reeves et al. confirmed that each 1 cm increase in WC was associated with 5% increase in odds of periodontitis. Kim et al. (2010) reported >35% increased odds of periodontal disease in persons with high WC (>102 cm for men, >88 cm for women) relative to normal WC. To our knowledge, no previous study of obesity and periodontal disease has used waist-height ratio (WHtR) as an obesity indicator. In the present study, the continuous WHtR was consistently associated with our three periodontal disease indices. Hsieh (2003) examined BMI, WC and WHtR in men and women and the correlation with metabolic/cardiovascular risk factors. Nearly all overweight men and women had a WHtR greater than 0.5 and 45.5% of men and 28.3% of women in the ideal weight BMI category had a WHtR greater than 0.5. It was concluded, of the 3 indices, WHtR was the best index for signaling metabolic risk in the ideal-weight subjects as well as the overweight subjects (Hsieh et al. 2003). Tseng also compared anthropometric indices of WC, Waist-Hip Ratios, WHtR, and BMI for indicators of risk in Type 2 diabetic Taiwanese patients and concluded that WHtR had a greater independent association with coronary artery disease and the highest magnitude of association than the other indices in both sexes (Tseng 2008). Heymsfield et al (2011) suggest further modification of the simple WHtR by squaring waist circumference or using the square root of height to improve the modeling of the relationships of health outcomes and body circumference-to-height measures.
Only three relatively simple measurements are required to compute BMI and WHtR. In the DLS cohort, the magnitudes of the hazard ratios were remarkably consistent for the BMI categories of overweight and obese, regardless of the periodontal disease indicator. At a minimum, data on height and weight should be collected in future studies of the association between adiposity and periodontal disease. Ideally, WC measurements should be made as well. There is a wide range of body fat distribution in lean, overweight and obese adults with upper body deposition being associated with visceral fat and abnormal metabolic profiles. It remains unclear which obesity indicator is the most sensitive in other populations, such as women and minorities who have different body fat distributions. Our data suggest that the nature of the association with periodontal disease progression might be refined by taking WHtR into account, but studies with larger sample sizes and more diverse populations are needed to confirm this. Therefore, it is imperative to continue to measure and examine multiple obesity indices.
Because all three of the periodontal disease measures in our study showed consistent associations with the BMI and the BMI-WHtR categories, this suggests the selection of an outcome measure in future studies can be based on available resources and the nature of the study question. Although alveolar bone loss is a hallmark of periodontal disease and measures cumulative bone tissue erosion, it is not always practical to obtain full-mouth radiographs measurements. CAL is considered a more accurate clinical indicator than PPD for measuring chronic disease, but CAL can also result from non-inflammatory causes. Therefore, measurements of both CAL and PPD are recommended, especially for studies of older adults (Page & Eke, 2007). Our results suggest that WC and WHtR, by way of the functions of visceral adipose tissue in relation to inflammation, may play a role in predicting future inflammatory disease development. Underlying biological mechanisms associating obesity with periodontal disease and other chronic inflammatory diseases likely involve adipocytokines and related hormones. Cytokines have broad molecular interactions that impact many aspects of immunity and inflammation and have wide ranging, as well as, overlapping functions (Preshaw et al. 2011). Recent studies have demonstrated that visceral adipose tissue is an active endocrine organ secreting bioactive substances. Many of these cytokines are secreted in proportion to the amount of adipose tissue present (Ritchie 2003). The balance of pro- and anti-inflammatory cytokines may determine the extent of periodontal tissue damage. Pro-inflammatory cytokines derived from adipose tissues may act on the periodontal tissues directly and/or systemically by initiating a host immune response. Cytokines likely work together in a network to modulate and regulate key cellular functions (Kinane et al. 2011). Some of the strongest evidence for cytokines functioning in networks exists for IL-1β, TNF- α, IL-6, and receptor activator of NF-κb ligan (RANKL) (Kinane et al. 2011). These adipocytokines are also postulated to play a role in leaving the subjects at greater risk for periodontal disease development and progression. It is also very likely that there is individual variation in cytokine expression and response that will impact the susceptibility, severity, and outcome of the disease process. The lack of adipocytokine data on our participants is another limitation of this study.
In conclusion, adult white males who were obese were more likely to exhibit moderate-to-severe periodontal disease progression events compared to ideal weight men. Disease progression events were also increased with increasing values of WHtR. These obesity measures may be potentially useful predictors of future periodontal disease progression by themselves or in combination. Future longitudinal studies are needed to confirm our findings and extend them to more diverse populations. A longitudinal assessment of changes in weight and adiposity with periodontal disease development over time is also needed to elucidate potential biologic mechanisms and cause-effect relationships that may exist.
Clinical Relevance.
Scientific Rationale for Study
Overweight, obesity, and markers of central adiposity have been associated with prevalent periodontal disease, but data from long-term prospective studies are lacking.
Principle Findings
Obesity (body mass index ≥ 30 kg/m2) and increased waist circumference-to-height ratio significantly predict greater hazards of periodontal disease development in men as measured by probing pocket depth, clinical attachment loss, and alveolar bone.
Practical Implications
Easily obtainable anthropometric measures used to estimate chronic disease risk and adiposity may be used to predict long-term periodontal disease development in males.
Acknowledgments
Conflict of Interest/Source of Funding: The Dental Longitudinal Study and Normative Aging Study are components of the Massachusetts Veterans Epidemiology Research and Information Center which is supported by the US Department of Veterans Affairs Cooperative Studies Program. Dr. Garcia was a recipient of a Veterans Affairs Career Development Award in Health Services Research from the VA Health Services Research and Development Service. Supported by National Institute of Dental and Craniofacial Research R01 DE019833 and K24 DE00419.
Footnotes
None of the authors report any personal or financial conflict of interest. The views expressed in this paper are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs.
Contributor Information
Andrea Gorman, School of Medicine Boston University 72 East Concord Street Boston, MA 02118 USA Phone Number: 401-444-5603 agorman@Lifespan.org.
Elizabeth Krall Kaye, Henry M. Goldman School of Dental Medicine Boston University Dept of Health Policy 715 Albany St., 560, Room 338 Boston, MA 02118 USA Phone Number: 617-638-6386 kralle@bu.edu.
Caroline Apovian, School of Medicine Boston University 72 East Concord Street Boston, MA 02118 USA Phone Number: 617-638-8556 capovian@bu.edu.
Teresa T. Fung, School of Nutrition Simmons College 300 The Fenway Boston, MA 02115 USA Phone Number: 617-521-2711 teresa.fung@simmons.edu
Martha Nunn, School of Dentistry Creighton University 2500 California Plaza Omaha, NE 68178 USA nunn@creighton.edu.
Raul I. Garcia, VA Boston Healthcare System 150 S. Huntington Avenue Boston, MA 02130 Henry M. Goldman School of Dental Medicine Boston University 560 Harrison Ave. Boston, MA 02118 USA Phone Number: 617-638-6385 rig@bu.edu
REFERENCES
- Albandar JM. Underestimation of periodontitis in NHANES surveys. Journal of Periodontology. 2011;82:337–341. doi: 10.1902/jop.2011.100638. [DOI] [PubMed] [Google Scholar]
- Al-Shammari KF, Al-Khabbaz AK, Al-Ansari JM, Neiva R, Wang HL. Risk indicators for tooth loss due to periodontal disease. Journal of Periodontology. 2005;76:1910–1918. doi: 10.1902/jop.2005.76.11.1910. [DOI] [PubMed] [Google Scholar]
- Al-Zahrani MS, Bissada NF, Borawski EA. Obesity and periodontal disease in young, middle-aged, and older adults. Journal of Periodontology. 2003;74:610–615. doi: 10.1902/jop.2003.74.5.610. [DOI] [PubMed] [Google Scholar]
- Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. International Journal of Food Sciences and Nutrition. 2005;56:303–307. doi: 10.1080/09637480500195066. [DOI] [PubMed] [Google Scholar]
- Bajaj HS, Brennan DM, Hoogwerf BJ, Doshi KB, Kashyap SR. Clinical utility of waist circumference in predicting all-cause mortality in a preventive cardiology clinic population: A PreCIS database study. Obesity. 2009;17:1615–1620. doi: 10.1038/oby.2009.44. [DOI] [PubMed] [Google Scholar]
- Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and metaanalysis. Journal of Periodontology. 2010;81:1708–1724. doi: 10.1902/jop.2010.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Vecchia CF, Susin C, Rösing CK, Oppermann RV, Albandar JM. Overweight and obesity as risk indicator for periodontitis in adults. Journal of Periodontology. 2005;76:1721–28. doi: 10.1902/jop.2005.76.10.1721. [DOI] [PubMed] [Google Scholar]
- Efron B. The efficiency of Cox’s likelihood function for censored data. Journal of the American Statistical Association. 1977;72:557–565. [Google Scholar]
- Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. International Journal of Obesity and Related Metabolic Disorders. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Heo M, Pietrobelli A. Are adult body circumferences associated with height? Relevance to normative ranges and circumferential indexes. American Journal of Clinical Nutrition. 2011;93:302–307. doi: 10.3945/ajcn.110.005132. [DOI] [PubMed] [Google Scholar]
- Hsieh SD, Yoshinaga H, Muto T. Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. International Journal of Obesity. 2003;27:610–616. doi: 10.1038/sj.ijo.0802259. [DOI] [PubMed] [Google Scholar]
- Kapur KK, Glass RL, Loftus ER, Alman JE, Feller RP. The Veterans Administration longitudinal study of oral health and disease. International Journal of Aging and Human Development. 1972;3:125–137. [Google Scholar]
- Khader YS, Bawadi HA, Haroun TF, Alomari M, Tayyem RF. The association between periodontal disease and obesity among adults in Jordan. Journal of Clinical Periodontology. 2009;36:18–24. doi: 10.1111/j.1600-051X.2008.01345.x. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Jin BH, Bae KH. Periodontitis and Obesity: A Study of the Fourth Korean National Health and Nutrition Examination Survey. Journal of Periodontology. 2010 doi: 10.1902/jop.2010.100274. (doi:10.1902/jop.2010.100274) [DOI] [PubMed] [Google Scholar]
- Kinane DF, Preshaw PM, Loos BG. Host-response: understanding the cellular and molecular mechanisms of host-microbial interactions – consensus of the seventh European workshop on periodontology. Journal of Clinical Periodontology. 2011;38(suppl 11):44–48. doi: 10.1111/j.1600-051X.2010.01682.x. [DOI] [PubMed] [Google Scholar]
- Kongstad J, Hvidtfeldt UA, Grønbaek M, Stoltze K, Holmstrup P. The relationship between body mass index and periodontitis in the Copenhagen City Heart Study. Journal of Periodontology. 2009;80:1246–1253. doi: 10.1902/jop.2009.080559. [DOI] [PubMed] [Google Scholar]
- Lin WY, Lee LT, Chen CY, Lo H, Hsia HH, Liu IL, Lin RS, Shau WY, Huang KC. Optimal cut-off values for obesity: using simple anthropometric indices to predict cardiovascular risk factors in Taiwan. International Journal of Obesity. 2002;26:1232–1238. doi: 10.1038/sj.ijo.0802040. [DOI] [PubMed] [Google Scholar]
- Linden G, Patterson C, Evans A, Kee F. Obesity and periodontitis in 60-70 year old men. Journal of Clinical Periodontology. 2007;34:461–66. doi: 10.1111/j.1600-051X.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- Murray H, Clarke M, Locker D, Kay EJ. Reasons for tooth extractions in dental practices in Ontario, Canada according to tooth type. International Dental Journal. 1997;47:3–8. doi: 10.1111/j.1875-595x.1997.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Nishida N, Tanaka M, Hayashi N, Takeshita T, Nakayama K, Morimoto K, Shizukuishi S. Determination of smoking and obesity as periodontitis risks using the classification and regression tree method. Journal of Periodontology. 2005;76:923–28. doi: 10.1902/jop.2005.76.6.923. [DOI] [PubMed] [Google Scholar]
- Okosun IS, Chandra KM, Boev A, Boltri JM, Choi ST, Parish DC, Dever GE. Abdominal adiposity in U.S. adults: prevalence and trends, 1960-2000. Preventive Medicine. 2004;39:197–206. doi: 10.1016/j.ypmed.2004.01.023. [DOI] [PubMed] [Google Scholar]; Page R,C, Eke PI. Case definitions for use in population-based surveillance of periodontitis. Journal of Periodontology. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- Phipps KR, Stevens VJ. Relative contribution of caries and periodontal disease in adult tooth loss for an HMO dental population. Journal of Public Health Dentistry. 1995;55:250–252. doi: 10.1111/j.1752-7325.1995.tb02377.x. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? Journal of Clinical Periodontology. 2011;38(suppl 11):60–84. doi: 10.1111/j.1600-051X.2010.01671.x. [DOI] [PubMed] [Google Scholar]
- Reeves AF, Rees JM, Schiff M, Hujoel P. Total body weight and waist circumference associated with chronic periodontitis among adolescents in the United States. Archives of Pediatric and Adolescent Medicine. 2006;160:894–899. doi: 10.1001/archpedi.160.9.894. [DOI] [PubMed] [Google Scholar]
- Ritchie CS, Kinane DF. Nutrition, inflammation and periodontal disease. Nutrition. 2003;19:475–476. doi: 10.1016/s0899-9007(02)01043-2. [DOI] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between upper body obesity and periodontitis. Journal of Dental Research. 2001;80:1631–36. doi: 10.1177/00220345010800070701. [DOI] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, Yamashita Y. Relationship between obesity, glucose tolerance and periodontal disease in Japanese women: the Hisayama study. Journal of Periodontal Research. 2005;40:346–53. doi: 10.1111/j.1600-0765.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- Sarlati F, Akhondi N, Ettehad T, Neyestani T, Kamali Z. Relationship between obesity and periodontal status in a sample of young Iranian adults. International Dental Journal. 2008;58:36–40. [PubMed] [Google Scholar]
- Schei O. Alveolar bone loss as related to oral hygiene and age. Journal of Periodontology. 1959;30:7–16. [Google Scholar]
- Schneider HJ, Glaesmer H, Klotsche J, Böhler S, Lehnert H, Zeiher AM, März W, Pittrow D, Stalla GK, Wittchen HU. Accuracy of anthropometric indicators of obesity to predict cardiovascular risk. Journal of Clinical Endocrinology and Metabolism. 2007;92:589–594. doi: 10.1210/jc.2006-0254. [DOI] [PubMed] [Google Scholar]
- Tseng CH. Waist-to-height ratio and coronary artery disease in Taiwanese type 2 diabetic patients. Obesity. 2008;16:2754–2759. doi: 10.1038/oby.2008.430. [DOI] [PubMed] [Google Scholar]
- World Health Organization [accessed on 2 December 2009];Technical report series 894. Obesity: preventing and managing the global epidemic. [WWW document] URL http://whqlibdoc.who.int/trs/WHO_TRS_984.pdf. [PubMed]
- Wood N, Johnson RB, Streckfus CF. Comparison of body composition and periodontal disease using nutritional assessment technique: Third National Health and Nutrition Examination Survey (NHANES III) Journal of Clinical Periodontology. 2003;30:321–327. doi: 10.1034/j.1600-051x.2003.00353.x. [DOI] [PubMed] [Google Scholar]