Abstract
Intrauterine growth restriction (IUGR) increases the risk of respiratory compromise throughout postnatal life. However, the molecular mechanism(s) underlying the respiratory compromise in offspring following IUGR is not known. We hypothesized that IUGR following maternal food restriction (MFR) would affect extracellular matrix deposition in the lung, explaining the long-term impairment in pulmonary function in the IUGR offspring. Using a well-established rat model of MFR during gestation to produce IUGR pups, we found that at postnatal day 21, and at 9 months of age the expression and abundance of elastin and alpha smooth muscle actin (αSMA), two key extracellular matrix proteins, were increased in IUGR lungs when compared to controls (p<0.05, n = 6), as determined by both Western and immunohistochemistry analyses. Compared to controls, the MFR group showed no significant change in pulmonary resistance at baseline, but did have significantly decreased pulmonary compliance at 9 months (p<0.05 vs control, n=5). In addition, MFR lungs exhibited increased responsiveness to methacholine challenge. Furthermore, exposing cultured fetal rat lung fibroblasts to serum deprivation increased the expression of elastin and elastin-related genes, which was blocked by serum albumin supplementation, suggesting protein deficiency as the predominant mechanism for increased pulmonary elastin deposition in IUGR lungs. We conclude that accompanying the changes in lung function, consistent with bronchial hyperresponsiveness, expression of the key alveolar extracellular matrix proteins elastin and αSMA increased in the IUGR lung, thus providing a potential explanation for the compromised lung function in IUGR offspring.
Keywords: intrauterine growth restriction, maternal food restriction, extracellular matrix, elastin, α smooth muscle actin, pulmonary function
INTRODUCTION
Epidemiologic studies of infants, children and adults indicate that prenatal compromises that restrict feto-placental growth, causing intrauterine growth restriction (IUGR), affect the postnatal development and function of many organs.1–3 There is only limited information on the effect of this phenomenon, termed “fetal programming”, on the lungs of the offspring. However, in general, IUGR increases the risk of respiratory compromise throughout postnatal life. Although the molecular mechanisms underlying this respiratory compromise are not well established, general cellular and molecular effects of IUGR on the developing lung have been described.4–15 These include an overall reduction in lung weight, DNA, and protein content,4–6 reduced surfactant content and activity,7–9 impaired maturation of the alveolar type II (ATII) cell,10 decreased alveolar formation,11–13,15 reduced alveolar surface area for gas exchange,11,14 an immature and thicker air-blood barrier, and a thicker alveolar wall.10,14
In this study, we hypothesized that IUGR would affect deposition of key lung extracellular matrix proteins such as elastin and alpha smooth muscle actin (αSMA), explaining the long-term impairment of pulmonary function in IUGR offspring. Elastin is an important structural protein that is a key component of the extracellular matrix (ECM) of the lung. Elastin is intimately involved in the process of alveolarization, as indicated by the finding that alveoli cannot form in the absence of tropoelastin expression and elastin deposition in the alveolar septa.16 In humans, elastin is laid down in the lung parenchyma, predominantly during late fetal and early postnatal life when the alveoli are forming.17 Elastin deposition occurs at the apex of the secondary septal crests during the process of alveolarization.18 The synthesis of elastin in the developing lung can be influenced by a number of factors, including hypoxia,19 corticosteroids,20,21 growth factors,22,23 retinoic acid,24 and nutrient restriction.5,25
To test the hypothesis that IUGR affects lung extracellular matrix deposition, we utilized a well established rat model of maternal food restriction (MFR) during gestation to produce IUGR. This model is associated with neonatal growth restriction and adult-onset obesity, diabetes, and hypertension.26,27
MATERIALS AND METHODS
Rat Model of Maternal Food Restriction
All studies were approved by the Animal Research Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, in accordance with the American Association for the Accreditation of Laboratory Animal Care and the National Institutes of Health guidelines. First-time-pregnant Sprague Dawley rat dams (Charles River Laboratories, Inc., Hollister, CA) were housed in a facility with constant temperature and humidity, in a controlled 12 h light-12 h dark cycle. At 10 days of gestation, the dams were provided either an ad libitum diet of standard laboratory chow (LabDiet 5001, Brentwood, MO, USA: protein 23%, fat 4.5%, metabolizable energy 3030 kcal/kg) or a 50% food restricted diet, as determined by the quantification of the normal intake in the ad libitum fed rats. Following delivery, the pups were allowed to breast feed ad libitum. In order to eliminate the effect of MFR on lactation, offspring from food restricted dams were cross-fostered to rat dams fed ad libitum during pregnancy. The control group, i.e., those fed ad libitum during pregnancy, offspring were also cross fostered to control for the study design. In all studies, the number of pups/dam (=8) was kept similar between the experimental groups. At postnatal day 21 (PND21), all the offspring were weaned to an ad libitum diet and housed individually. Animals were killed and their lungs collected for further processing at PND21 and 9 months (9M).
Tissue Preparation Protocol
Rat lungs were inflated in situ with 4% paraformaldehyde (PFA) in phosphate buffer (PBS) at a standard inflation pressure of 20 cm of H2O. The trachea was ligated and the lungs were removed and placed in 4% PFA for approximately 4 hours at 4°C. The lungs were subsequently transferred to PBS containing 30% sucrose (w/v) until equilibrated (4°C). The lung tissue was frozen in Optimal Cutting Temperature compound (Sakura Finetek, Torrance, CA) and cut at 8-µm thickness.
Protein Abundance by Western Blotting
In brief, tissue samples (100 mgs or less) were collected in T-PER lysis buffer (Pierce Protein Research Products, Rockford, IL), containing protease inhibitors. Samples were homogenized, and the resulting lysates were clarified by centrifugation. Protein concentration was determined by BCA Protein Assay (Pierce Protein Research Products, Rockford, IL). Twenty µgs of protein lysate from each sample were resolved by SDS-PAGE gel electrophoresis, and electro-transferred to polyvinylidene difluoride membranes. A lysate-positive control was used for each gel. Non-specific antibody binding was blocked with blocking buffer for 1 hour at room temperature. Membranes were washed twice with Tris buffered saline (TBS) wash buffer. The membranes were incubated with the appropriate primary antibody in blocking buffer, washed in TBS buffer, and incubated with secondary antibody goat anti-rabbit IgG conjugated to horse-radish peroxidase for 1 h. The membranes were washed 3 times with TBS wash buffer, developed with Super Signal West Pico reagents (Pierce Protein Research Products, Rockford, IL), and exposed to X-ray film. The blots were subsequently stripped and reprobed with antiGAPDH antibody (1:10,000, Chemicon, Temecula, CA), and protein values were normalized to GAPDH. The densities of the specific protein bands were quantified using a scanning densitometer (Eagle Eye II still video system, Stratagene, La Jolla, CA). Results are expressed as the normalized mean +/− SE, and considered significant at p < 0.05. The specific primary antibodies used included elastin (1:200, cataglog # Sc-17581, Santa Cruz, CA), and αSMA (1:10,000, catalog # A2547, Sigma, St. Louis, MO).
Real Time RT-PCR
In brief, samples were collected, treated with RNA later preservative (Ambion/Applied Biosystems, Foster City, CA), and total RNA was isolated using the RNAqueous-4PCR kit (Ambion/Applied Biosystems, Foster City, CA). RNA was DNase-treated and quantitated by absorbance spectrometry using a nanodrop spectrophotometer (Nanodrop Instruments, Wilmington, DE). The purity of the RNA was assessed based on the visual appearance of the ethidium bromide-stained ribosomal bands following fractionation on a 1.2% (wt/vol) agarose-formaldehyde gel, and quantitated by absorbance at 260 nm.
One µg of total RNA was reverse-transcribed into single-stranded cDNA using the TaqMan Gold RT-PCR Kit (Applied Biosystems, Life Technologies Corp., Carlsbad, CA) at 50°C for 30 min in a total volume of 20 µls. The PCR reaction mixture consisted of 1 µl of 10-fold diluted cDNA, PCR Gold DNA polymerase reagent mix, and optimized forward and reverse gene-specific primers (900 nMs each) with a gene-specific probe (250 nM, FAM dye label). All primer and probe sets were purchased as pre-designed or, if needed, custom-designed TaqMan Gene Expression Assays (Applied Biosystems, Life Technologies Corp., Carlsbad, CA). Real-Time PCR reactions were run in triplicate in 384 well plates using an ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems, Life Technologies Corp., Carlsbad, CA). Reactions proceeded by activation of DNA polymerase at 95°C for 10 min, followed by 38 PCR cycles of denaturing at 95°C for 15 sec, and annealing/extension at 60°C for 1 min. The normalization control was the 18S ribosomal RNA TaqMan Gene Expression Assay (Applied Biosystems, Life Technologies Corp., Carlsbad, CA). Data were analyzed to select a threshold level of fluorescence that was in the linear phase of the PCR product accumulation [the threshold cycle (CT) for that reaction]. The CT value for 18S ribosomal RNA was subtracted from the CT value of the gene to obtain a delta CT (ΔCT) value. The relative fold-change for each gene was calculated using the ΔΔCT method (21). Results were expressed as the mean +/− SE, and considered significant at p < 0.05. RT PCR probes used included- Rat lox, F5’-AAACGGAAAAACAACAAAGAAGGT-3’; R5’-TGCTGATTTAAACACTCAAAATCCA-3’ (product 83 bp); rat lox1-1, 5F’-AGGGAGTGAACATGGACCAAAC-3’; R5’-TTAAAACCAACAACAGACAAAATGG-3’ (102 bp); rat fibrillin 1, F5’-ACCTGGAACCTGCTACAACACTCT-3’; R5’-CCGTTATAGCTTCTGTAGCAAAAGC-3’ (126 bp); rat fibulin 5, F5’-TCCTCTATCTCTTGCTGCATTGC-3’; R5’-ACGTGTTCCCATAGCCTTCTCA-3’ (113 bp); rat tropoelastin, F5’-AGAAGCCTCGACATTAGATTTGGT-3’; R5’-GGAGCTATTCCCAGTGTGAGAAGT-3’ (139 bp).
Immunohistochemistry (IHC) for Elastin and αSMA
In situ protein expression was assessed by IHC performed with a UniTect ABC Kit (Calbiochem-EMD Biosciences, Gibbstown, NJ). Five-micrometer paraffin sections were deparaffinized in xylene, and rehydrated by a sequential ethanol wash. Endogenous avidin and biotin activities and nonspecific antibody binding to tissue were inhibited as per the manufacturer's instructions. Tissue was incubated with primary antibody overnight at 4°C in a humidified chamber. For elastin staining, rabbit anti-elastin antibody (1: 100, Santa Cruz Biotechnology, Santa Cruz, CA), and for α-SMA staining (1:100, Sigma- Aldrich, St. Louis, MO) antibody were used as primary antibodies. The next day, following PBS washes at room temperature, the tissues were incubated sequentially with appropriate biotinylated secondary antibody, ABC reagent, diaminobenzidine substrate (DAB; Sigma- Aldrich, St. Louis, MO), in the dark. Sections were rinsed with water and counterstained with hematoxylin. Finally, tissue slices were dehydrated in xylene and permanently mounted with VectaMount (Vector Laboratories, Inc., Burlingame, CA). Immunostained sections were examined under a microscope (Axioskop 40; Carl Zeiss Microimaging LLC, Thornwood, NY) at 20× magnification.
Pulmonary Function Testing
For evaulating pulmonary function, we studied pulmonary mechanics [toal pulmonary resistance (Rrs) and compliance (Cdyn)] at baseline, and following methacholine challenge in control and MFR offspring at PND21 and 9M. Rats were anesthetized, tracheas were cannulated, and the animals, in the supine position, were ventilated at 8 ml/kg tidal volume in a whole-body plethysmograph for restrained animals ((Buxco Inc., Troy, NY) using a MiniVent (Harvard Apparatus, Holliston, MA) at a frequency of 150 breaths per minute for the duration of the procedure. Total pulmonary dynamic compliance (Cdyn, as mL/cm of H2O) and total pulmonary resistance (Rrs, as cm of H2O*s/mL) measurements were made at baseline and following methacholine (MCh) challenge at 2.0 mg/mL. The percent change (% decrease in Cdyn or % increase in Rrs) was calculated as the difference between the physiologic parameter (Cdyn or Rrs) measured at baseline and after MCh bronchial challenge, divided by the baseline value (for % decrease in Cdyn or % increase in Rrs).
Isolation of pulmonary fibroblasts
Neonatal rat lung fibroblasts were cultured according to our previously described method.28 Briefly, the lungs were trimmed to remove major airways, and rinsed with calcium- and magnesium-free Hanks' balanced salt solution (HBSS). Pooled lung tissue from 3 to 5 pups was minced into 1 to 2-mm3 pieces and was suspended in pre-warmed (37°C) digestion buffer containing 2.5 ml of heat-inactivated chicken serum (2.5 ml), Hepes (1.25 ml of 500 mM, pH 7.4), collagenase I (12.5 mg, Sigma), Collagenase 1A (12.5 mg, Sigma) in Waymouth's medium (final volume 25 ml). The tissue was triturated 100 times with a 10 ml pipette, 100 times with a 5 ml pipette, and 100 times with a 9” Pasteur pipette. The tissue was further dissociated in a 37°C water bath using a Teflon™ stirring bar to disrupt the tissue mechanically. Once the tissue was dispersed into a unicellular suspension, the cells were pelleted at 500 × g for 10 min at room temperature in a 50 ml polystyrene centrifuge tube. The supernatant was decanted, and the pellet was resuspended in Minimal Essential Medium (MEM) containing 20% fetal bovine serum (FBS) to yield a mixed cell suspension of ca. 3 × 108 cells, as determined by Coulter particle counter (Beckman-Coulter, Hayaleah, FL). The cell suspension was then added to culture flasks (75 cm2) for 30–60 min to allow for differential adherence of the lung fibroblasts. These cells are greater than 95% pure fibroblasts based upon their morphologic appearance when viewed at the light microscopic level, and by immunohistochemical staining for vimentin.
At 80–90% confluence, the cells were cultured in DMEM for 24h with (1, 5, or 10%) or without (0%) fetal bovine serum (FBS) supplementation. For some experiments cells were cultured without FBS, but with 5% fetal bovine albumin supplementation.
Statistical analysis
Analysis of Variance for multiple comparisons was used to analyze the experimental data and a p value of <0.05 was considered to indicate a statistically significant difference.
RESULTS
General
As reported previously, at postnatal day 1, MFR pups were significantly lighter (6.1 ± 0.3 g, p<0.05, n=6) compared to control pups (7.0 ± 0.3 g, n=6). However, at PND21 (49 ± 3 vs.. 41 vs. ± 3 g, p<0.05, MFR vs. control, n=6) and 9M (410 ± 18 vs.. 340 vs.. ± 20 g, p<0.05, MFR vs. control, n=6), the MFR pups weighed significantly more. The wet lung weights of the MFR offspring were higher at PND21 (0.48 ± 0.03 vs. 0.37 ± 0.02, MFR vs. control, p<0.05, n=6) and 9M (1.58 ± 0.07 vs. 1.39 ± 0.06 g, p<0.05, MFR vs. control, n=6). However, the lung weights, expressed as a percentage of body weight between the two groups, were not different at both time-points examined (p>0.05, n=6).
Pulmonary function
We found that MFR altered lung function in the offspring (Figures 1a–1d). Measurement of pulmonary mechanics [total airway resistance (Rrs) and compliance (Cdyn)] at PND21 and 9M showed that, compared to control animals, in the MFR offspring, although there were no significant differences in Rrs at baseline, at either PND21 or 9M (Fig. 1a), Cdyn was significantly decreased in the MFR group at 9M (Fig. 1b) (p<0.05 vs. control, n=5). In response to a methacholine challenge, Rrs increased significantly (Fig. 1c) and Cdyn decreased significantly (Fig 1d) in the MFR group at both time points examined (p<0.05 vs. control, n=5). These results suggest that the lungs of the MFR offspring were both stiff and hyperresponsive.
Fig. 1.
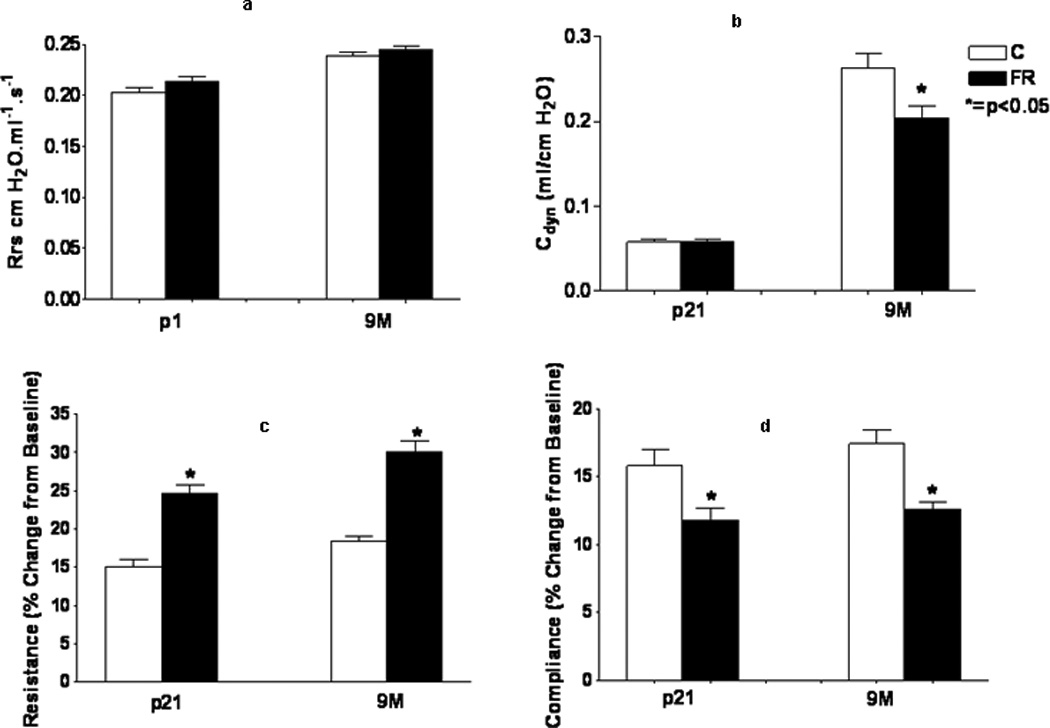
(a–d) Effects of MFR on pulmonary resistance and compliance at PND21 and 9M both at baseline (top panel) and in response to a methacholine challenge (bottom panel) are shown here. There were no significant differences in pulmonary resistance at baseline at either PND21 or 9M. However, pulmonary compliance was significantly decreased in the MFR group at 9M. On methacholine challenge, pulmonary resistance increased, and pulmonary compliance decreased significantly in the MFR group at both time points. (*p<0.05 vs. control; n=5)
Alveolar extracellular matrix deposition
The expression of elastin (Tropoelastin) and elastin-assembly-related genes (Lox 1 and 2, fibrillin 1 and fibulin 5) was clearly altered, with a significant increase in their expression at 9M (Fig. 2). Compared to controls, in MFR offspring there was a significant decrease in elastin expression at PND21, and a significant increase at 9M (Real-Time PCR, p<0.05 vs. control, n=4). The changes in elastin expression with an increase at 9M was corroborated by Western hybridization (Fig. 3) (p<0.05 vs. control, n=4). We also examined the expression of α-smooth muscle actin (αSMA), another key marker for chronic lung disease. Based on Western hybridization data, αSMA protein levels were 30% higher at PND21, and 110% higher at 9M (Fig. 4). To further evaluate the effects of MFR on the extracellular matrix, lung tissue sections were probed with fluorescent antibodies against elastin and αSMA (Fig. 5 A and B). Both the distribution and intensity of elastin and αSMA were increased at PND21 and 9M. It is interesting to note that although elastic fibers are relatively sparse in the distal portions of the alveolus (base/inter-septal region) in a normal adult lung, in the MFR lung elastic fibers predominated, indicating their abnormal localization.
Fig. 2.
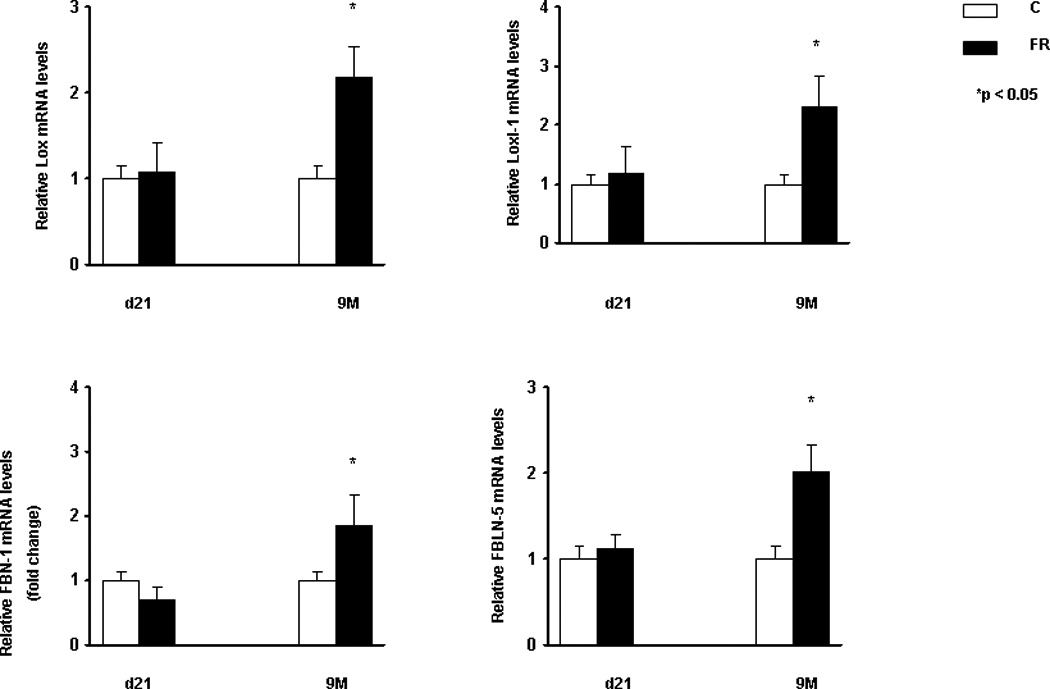
In response to MFR, the mRNA levels of elastin and elastin-related genes such as Lox-1, Loxl-2, Fibrillin-1, and Fibulin-5, were increased at 9M, as determined by Real-Time PCR. (*p<0.05 vs. control; n=4).
Fig. 3.
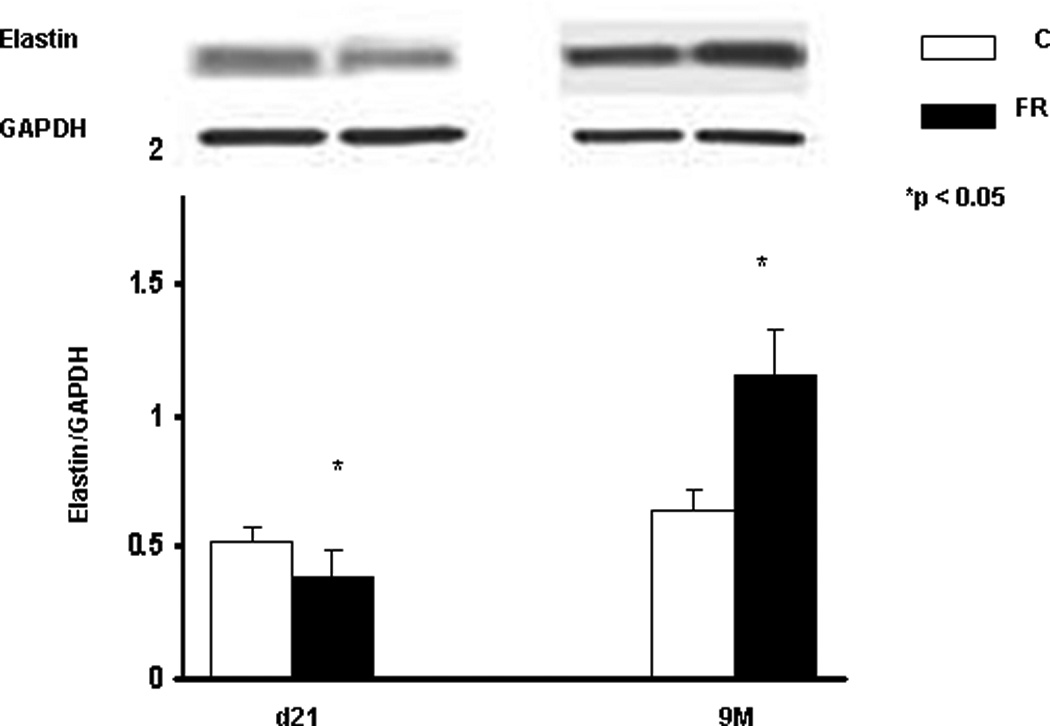
In response to MFR, the protein levels of pulmonary elastin was significantly increased at 9M, shown by Western blotting. (*p<0.05 vs. control; n=4).
Fig. 4.
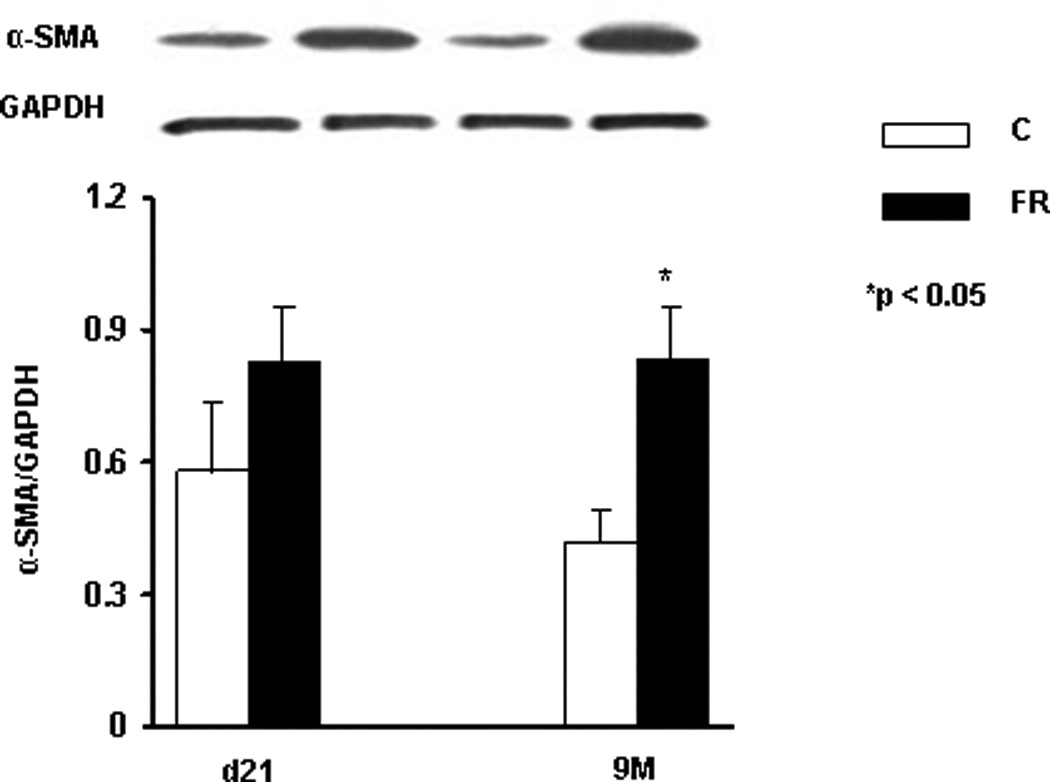
In response to MFR, the protein expression level of pulmonary α-SMA was significantly increased at PND21 and 9M, shown by Western blotting. (*p<0.05 vs. control; n=3).
Fig. 5.
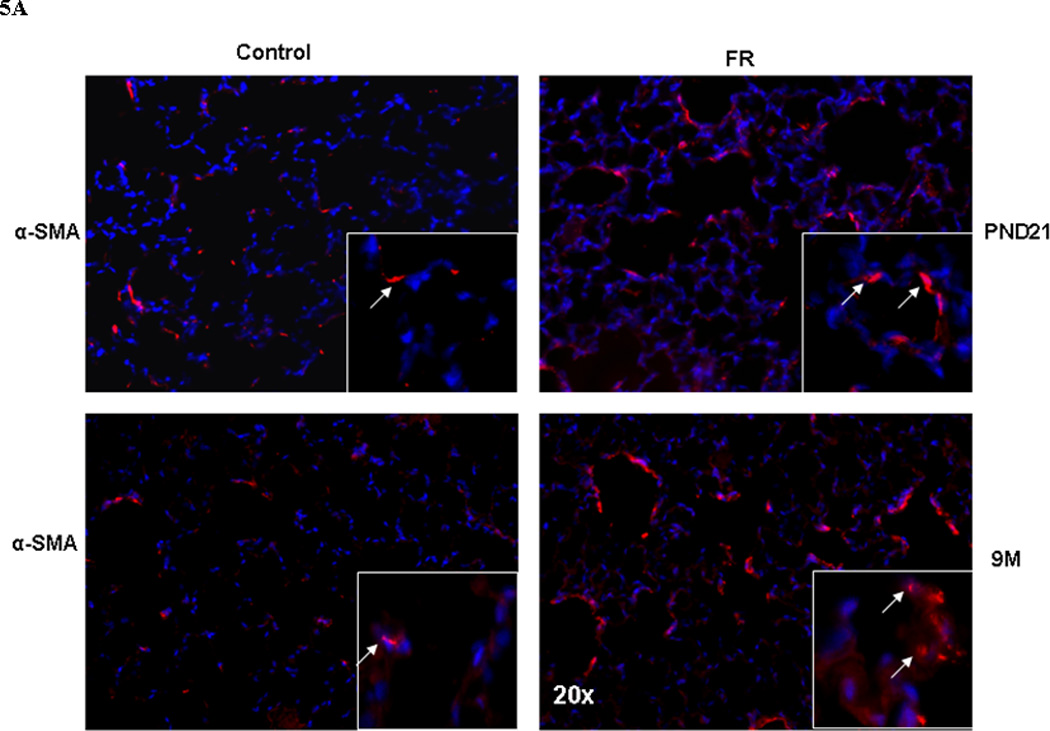
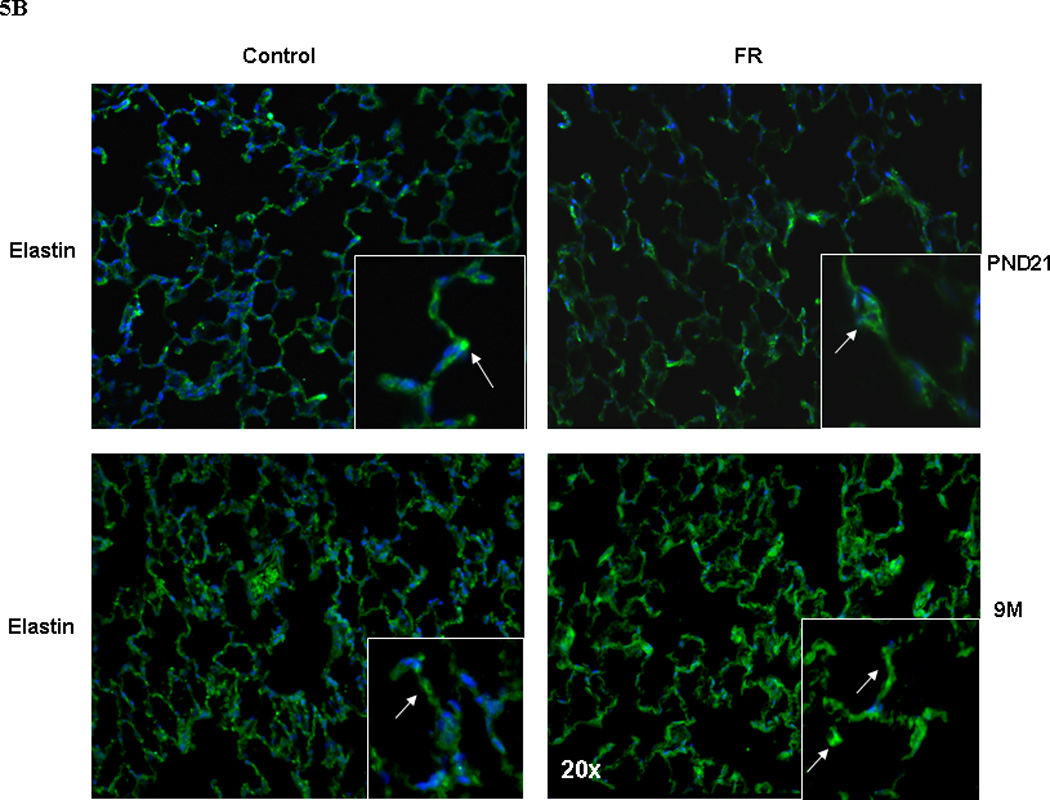
In response to MFR, pulmonary elastin (5A, shown in red) and α-SMA (5B, shown in green) protein levels were significantly increased at PND21 and 9M, shown by immuno-histochemistry staining (n=4. representative sections are shown).
Finally, to determine the effect of nutrient deprivation on elastin gene expression by alveolar interstitial fibroblasts, e19 fetal rat lung fibroblasts were cultured at 37° C for 24h in either DMEM + 10% fetal bovine serum, or in DMEM with reduced (5, 1, or 0 %) fetal bovine serum supplementation. Serum restriction increased elastin protein levels dose-dependently (Fig. 6). Similarly, serum restriction increased the transcription of tropoelastin and fibroblast growth factor receptors 3 and 4 (FGFR3 and FGFR4), the main receptors for elastin, significantly (Fig. 7). Interestingly, protein supplementation, i.e., supplementation with 5% fetal bovine albumin, blocked the serum deprivation-induced increases in the elastin and FGFR4 mRNA (Fig. 8A) protein levels (Fig. 8B).
Fig. 6.
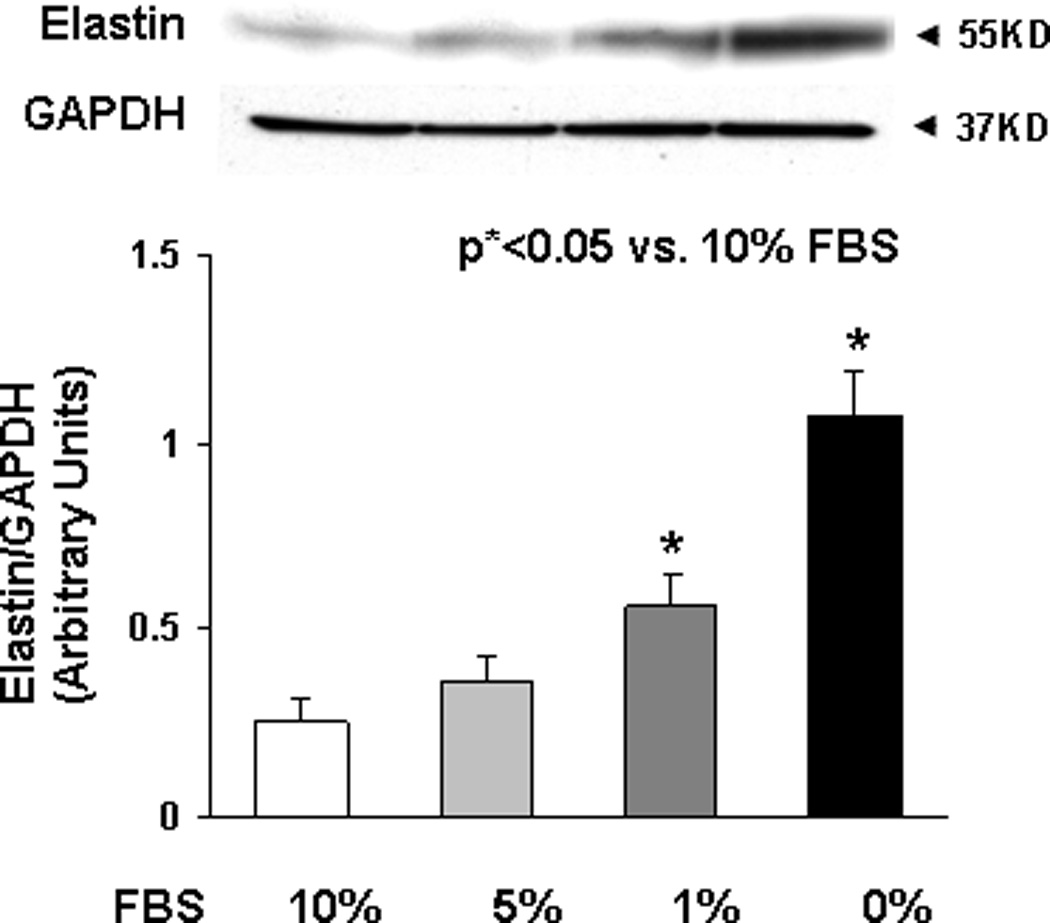
Elastin protein levels in lysates of embryonic day 19 cultured fetal rat lung fibroblasts for 24h in either DMEM + 10% fetal bovine serum, or in DMEM with reduced (5, 1, or 0 %) fetal bovine serum supplementation are shown. Serum restriction increased elastin protein levels dose-dependently, shown by Western blotting. (*p<0.05 vs. 10% FBS; n=3).
Fig. 7.
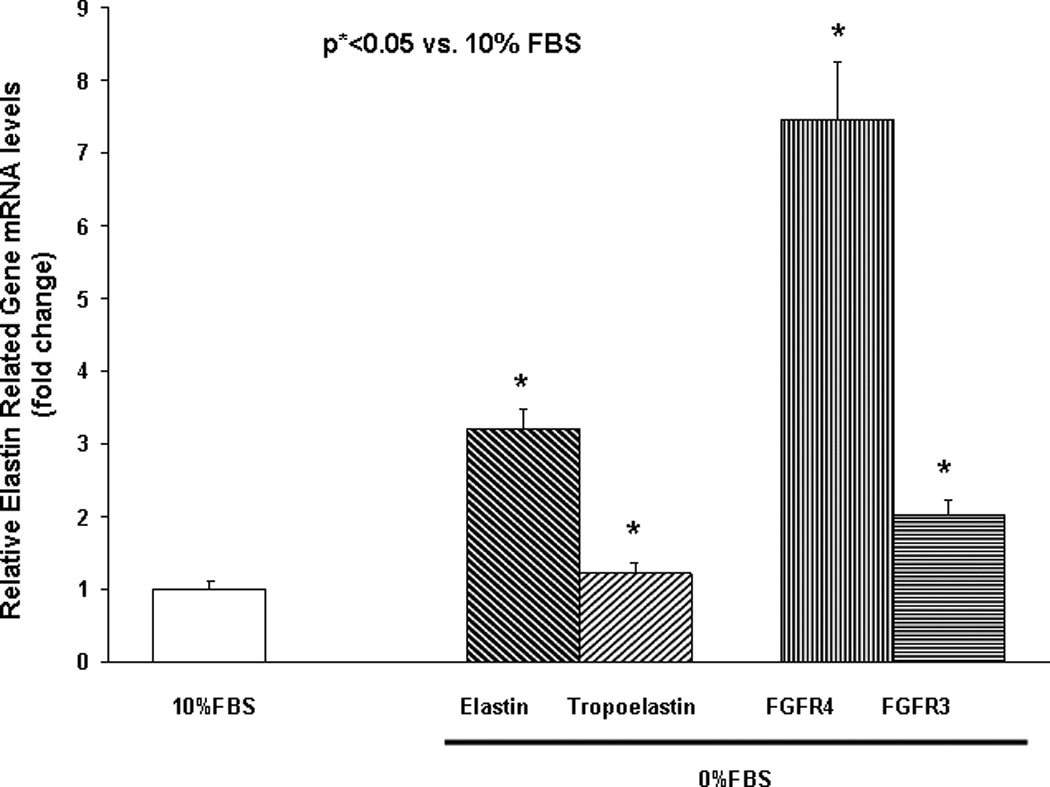
mRNA expression of elastin, tropoelastin, and main receptors for elastin [Fibroblast Growth Factor Receptors 3 and 4 (FGFR3 and FGFR4)] by cultured embryonic day 19 fetal rat lung fibroblasts following 24h in DMEM with or without + 10% fetal bovine serum supplementation (determined by Real-Time PCR, *p<0.05 vs. 10% FBS; n=3).
Fig. 8.
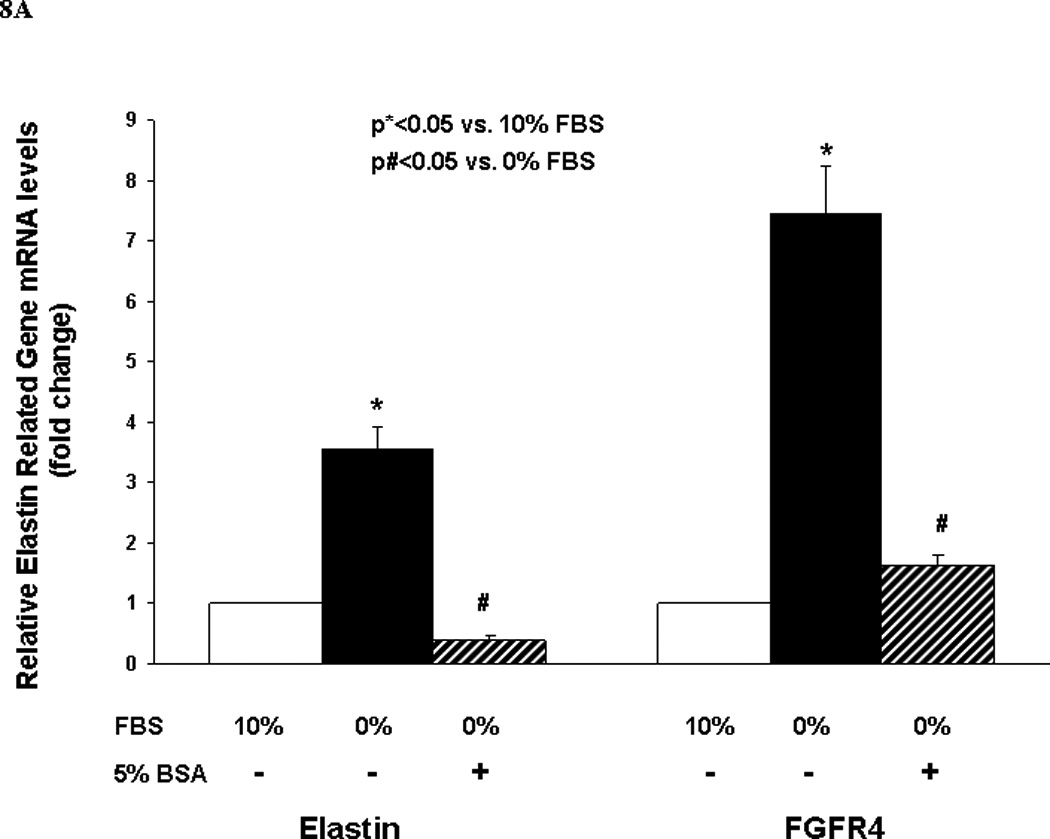
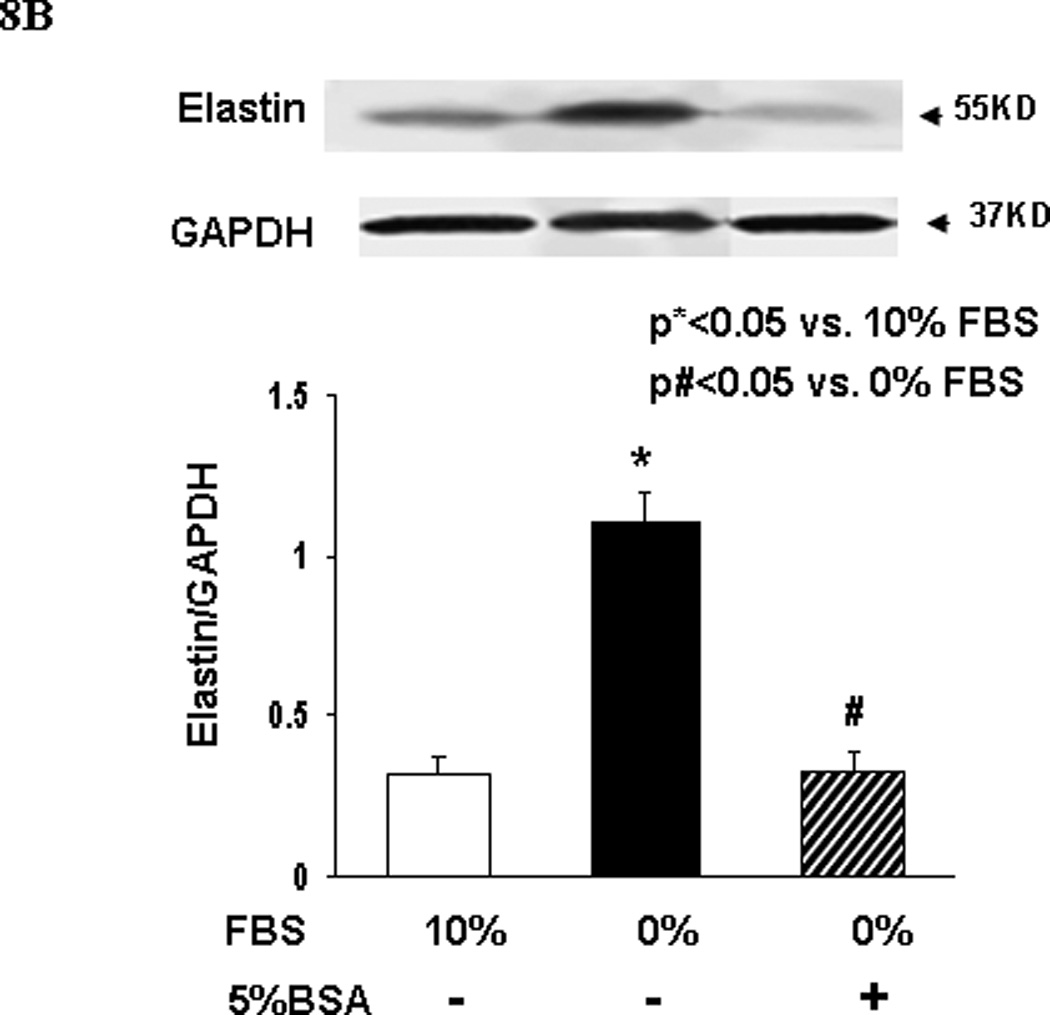
A and B. The elastin and FGFR4 mRNA (Fig. 8A) and protein levels (Fig. 8B) expressed by embryonic day 19 fetal rat lung fibroblasts following 24h culture in DMEM with or without + 5% fetal bovine serum supplementation are shown. Fetal bovine serum supplementation blocked the serum deprivation-induced increases in elastin and FGFR4 mRNA (Fig. 8A) and elastin protein levels (Fig. 8B) (determined by Real-Time PCR, *p<0.05 vs. 10% FBS; n=3).
DISCUSSION
In a well established model of fetal programming, we examined the effect of MFR during pregnancy on offspring lung alveolar extracellular matrix deposition and pulmonary function. Consistent with the previous description of the model utilized in this study,15 MFR altered lung function in the offspring. Measurement of pulmonary mechanics at PND21 and 9M showed that, compared to controls, in the MFR offspring there were no significant differences in pulmonary resistance at baseline at either PND21 or 9M. In contrast, compliance was significantly decreased in the MFR group at 9M. In response to a methacholine challenge, pulmonary resistance increased significantly, whereas compliance decreased significantly in the MFR group at both time-points examined. Fetal rat lung fibroblasts, when subjected to nutrtional stress under in vitro conditions, increased elastin expression, which was blocked by protein supplementation. These changes are consistent with the finding of altered alveolar extracellular matrix deposition in the MFR offspring lung, potentially explaining the altered lung structure and function observed in the IUGR offspring, with relative protein deficiency as the principal underlying cause.
In a recently published study of the uterine artery ligation model of IUGR, Joss-Moore et al also found changes in lung elastin expression and deposition between birth and post-natal day 21.29 However, in that study elastin was found to decrease, accompanied by increased lung compliance. Such differences between the effects of MFR and uterine artery ligation are likely due to the cell-specific effects of these different perturbations. Once the underlying molecular mechanism is determined, the reason for these seemingly disparate results will become apparent. Furthermore, it is important to point out that though a number of investigators have used protein restriction during pregnancy to study IUGR, to our knowledge, none of these studies have assessed offspring lung structure and function in detail, precluding the comparison of our findings with those of others.30, 31
Elastin is known to play a critical role in alveolarization during lung development. Cross-linking of the soluble monomer tropoelastin (elastin) to numerous other microfibril-forming minor glycoprotein components such as fibrillins, fibulins, and other matrix-associated glycoproteins confers the elastic properties of elastin-containing tissues. Proper elastin depostion in the lung is important in respiratory physiology, accounting for the orderly elastic recoil of the lung during passive expiration. At the alveolar level, elastic interdependence is mediated by optimal elastin expression, correct cross-linking, and the proper orientation of the elastin and collagen fibers. In the absence of optimal elastin deposition, and a correctly cross-linked and oriented elastin-containing matrix, there is failure of alveolarization and the correct establishment of elastic interdependence.
In the lung, normal deposition and arrangement of elastin fibers is particularly important in the formation and maintenance of alveolar secondary crests. The necessity for optimal elastin expression for proper alveolar formation is highlighted by numerous examples of failed/abnormal alveoralization in mice that are deficient in many components of elastin formation. For example, elastin-null mice die soon after birth from cardiorespiratory failure in association with the lack of lung septation to form alveoli, defective airway branching, and smooth muscle overgrowth of pulmonary arteries.32,33 In these mice, strikingly, alveolar secondary crests fail to form.33 Null mutation of lysyl oxidase, an enzyme necessary for elastin cross-linking, prevents correct elastin cross-linking, and hence incomplete alveolarization in affected mice.34 In mice with the PDGF-A null mutation, alveolar myofibroblasts fail to differentiate and produce elastin; hence, alveolar secondary crests also fail to form.35 Similarly, in mice with the Fgfr3/Fgfr4 double null mutation, excessive dysmorphic elastin is laid down, which also disrupts the formation of alveolar secondary crests.36 And lastly, mutations in several other genes known to affect elastin synthesis and assembly, such as fibulin-5, fibrillin-1, and lysyl oxidase-like 1 also result in offspring that exhibit abnormal lung development, which in some cases results in pulmonary pathology resembling emphysema in mice surviving beyond the perinatal period.34,37–39 Therefore, with the increased expressoin of elastin and other genes (such as Lox 1 and 2, fibrillin 1, and fibulin 5) needed for normal elastogenesis, it is not surprising that MFR lungs are abnormal structurally and functionally.
Factors such as hypoxia, undernutrition, and elevated corticosteroid levels have previously been shown to affect elastin synthesis in the IUGR lung. However, Cook et al, using an in vivo late-gestational sheep IUGR model secondary to uteroplacental embolization, did not find abnormal elastin synthesis and deposition, suggesting that hypoxemia and undernutrition associated with IUGR do not affect elastin synthesis and deposition, leading them to conclude that the alterations in lung structure following IUGR must be due to causes other than hypoxemia and undernutrition.40 The differences in the experimental models in the two studies can account for these differences in the findings between this study and the study by Cook et al.40 It is also important to note that in the current model we also saw evidence of increased corticosterone levels in the MFR offspring (data not shown), suggesting that any or all of the previously outlined factors (hypoxemia, undernutrition, increased corticosterone levels) might contribute to abnormal elastin deposition in the lung extracellular matrix of IUGR offspring. However, since alveolar interstitial fibroblasts subjected to nutrient stress showed increased elastin expression, which was blocked by protein supplementation, this suggests that nutrient deficiency, specifically protein deficieny, alters lung programming resulting in increased elastin synthesis and deposition in the long run.
Overall, we conclude that in addition to our previously reported abnormal structural and molecular findings in the MFR offpring lung,15 there is altered lung extracellular matrix deposition, accompanying altered lung function in the MFR offspring. It will be of interest to determine the specific amino acid deficiency that leads to increased elastin expression, and if supplementation of that specific amino acid (s), in the face of general MFR, will prevent the lung molecular, structural, and functional changes in the MFR offspring observed in the current study.
Acknowledgments
Grant Support: This study was supported in part by grants from the NIH (HL075405, HD51857, HD058948 and HL55268) and the TRDRP (14RT-0073, 15IT-0250, and 17RT-0170). Ahmet Karadag, MD, was supported by a grant from The Scientific and Technological Research Council of Turkey.
References
- 1.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 3.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20:63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Winick M, Noble A. Cellular response in rats during malnutrition at various ages. J Nutr. 1996;89:300–306. doi: 10.1093/jn/89.3.300. [DOI] [PubMed] [Google Scholar]
- 5.Sahebjami H, MacGee J. Effects of starvation on lung mechanics and biochemistry in young and old rats. J Appl Physiol. 1985;58:778–784. doi: 10.1152/jappl.1985.58.3.778. [DOI] [PubMed] [Google Scholar]
- 6.Cock ML, Albuquerque CA, Joyce BJ, Hooper SB, Harding R. Effects of intrauterine growth restriction on lung liquid dynamics and lung development in fetal sheep. Am J Obstet Gynecol. 2001;184:209–216. doi: 10.1067/mob.2001.108858. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Lechner AJ. Surfactant content and type II cell development in fetal guinea pig lungs during prenatal starvation. Pediatr Res. 1991;29:288–291. doi: 10.1203/00006450-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Chen CM, Wang LF, Su B. Effects of maternal undernutrition during late gestation on the lung surfactant system and morphometry in rats. Pediatr Res. 2004;56:329–335. doi: 10.1203/01.PDR.0000134254.83113.8E. [DOI] [PubMed] [Google Scholar]
- 9.Faridy EE. Effect of maternal malnutrition on surface activity of fetal lungs in rats. J Appl Physiol. 1975;39:535–540. doi: 10.1152/jappl.1975.39.4.535. [DOI] [PubMed] [Google Scholar]
- 10.Curle DC, Adamson IY. Retarded development of neonatal rat lung by maternal malnutrition. J Histochem Cytochem. 1978;26:401–408. doi: 10.1177/26.5.659840. [DOI] [PubMed] [Google Scholar]
- 11.Lipsett J, Tamblyn M, Madigan K, Roberts P, Cool JC, Runciman SI, McMillen IC, Robinson J, Owens JA. Restricted fetal growth and lung development: a morphometric analysis of pulmonary structure. Pediatr Pulmonol. 2006;41:1138–1145. doi: 10.1002/ppul.20480. [DOI] [PubMed] [Google Scholar]
- 12.Maritz GS, Cock ML, Louey S, Suzuki K, Harding R. Fetal growth restriction has long-term effects on postnatal lung structure in sheep. Pediatr Res. 2004;55:287–295. doi: 10.1203/01.PDR.0000106314.99930.65. [DOI] [PubMed] [Google Scholar]
- 13.Wignarajah D, Cock ML, Pinkerton KE, Harding R. Influence of intrauterine growth restriction on airway development in fetal and postnatal sheep. Pediatr Res. 2002;51:681–688. doi: 10.1203/00006450-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Das RM. The effects of intermittent starvation on lung development in suckling rats. Am J Pathol. 1984;117:326–332. [PMC free article] [PubMed] [Google Scholar]
- 15.Karadag A, Sakurai R, Wang Y, Guo P, Desai M, Ross MG, Torday JS, Rehan VK. Effect of maternal food restriction on fetal rat lung lipid differentiation program. Pediatr Pulmonol. 2009;44:635–644. doi: 10.1002/ppul.21030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindahl P, Karlsson L, Hellström M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- 17.Desai R, Wigglesworth JS, Aber V. Assessment of elastin maturation by radioimmunoassay of desmosine in the developing human lung. Early Hum Dev. 1988;16:61–71. doi: 10.1016/0378-3782(88)90087-4. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda Y, Ferrans VJ, Crystal RG. Development of elastic fibers of nuchal ligament, aorta, and lung of fetal and postnatal sheep: an ultrastructural and electron microscopic immunohistochemical study. Am J Anat. 1984;170:597–629. doi: 10.1002/aja.1001700407. [DOI] [PubMed] [Google Scholar]
- 19.Berk JL, Massoomi N, Hatch C, Goldstein RH. Hypoxia downregulates tropoelastin gene expression in rat lung fibroblasts by pretranslational mechanisms. Am J Physiol. 1999;277:L566–L572. doi: 10.1152/ajplung.1999.277.3.L566. [DOI] [PubMed] [Google Scholar]
- 20.Willet KE, McMenamin P, Pinkerton KE, Ikegami M, Jobe AH, Gurrin L, Sly PD. Lung morphometry and collagen and elastin content: changes during normal development and after prenatal hormone exposure in sheep. Pediatr Res. 1999;45:615–625. doi: 10.1203/00006450-199905010-00002. [DOI] [PubMed] [Google Scholar]
- 21.Pierce RA, Mariencheck WI, Sandefur S, Crouch EC, Parks WC. Glucocorticoids upregulate tropoelastin expression during late stages of fetal lung development. Am J Physiol. 1995;268:L491–L500. doi: 10.1152/ajplung.1995.268.3.L491. [DOI] [PubMed] [Google Scholar]
- 22.Foster JA, Curtiss SW. The regulation of lung elastin synthesis. Am J Physiol. 1990;259:L13–L23. doi: 10.1152/ajplung.1990.259.2.L13. [DOI] [PubMed] [Google Scholar]
- 23.Foster JA, Rich CB, Miller M, Benedict MR, Richman RA, Florini JR. Effect of age and IGF-I administration on elastin gene expression in rat aorta. J Gerontol. 1990;45:B113–B118. doi: 10.1093/geronj/45.4.b113. [DOI] [PubMed] [Google Scholar]
- 24.McGowan SE, Doro MM, Jackson SK. Endogenous retinoids increase perinatal elastin gene expression in rat lung fibroblasts and fetal explants. Am J Physiol. 1997;273:L410–L416. doi: 10.1152/ajplung.1997.273.2.L410. [DOI] [PubMed] [Google Scholar]
- 25.Kalenga M, Eeckhout Y. Effects of protein deprivation from the neonatal period on lung collagen and elastin in the rat. Pediatr Res. 1989;26:125–127. doi: 10.1203/00006450-198908000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 27.Novak DA, Desai M, Ross MG. Gestational programming of offspring obesity/hypertension. J Matern Fetal Neonatal Med. 2006;19:591–599. doi: 10.1080/14767050600708233. [DOI] [PubMed] [Google Scholar]
- 28.Torday JS, Rehan VK. Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin. Am J Physiol Lung Cell Mol Physiol. 2002;283:L130–L135. doi: 10.1152/ajplung.00380.2001. [DOI] [PubMed] [Google Scholar]
- 29.Joss-Moore LA, Wang Y, Yu X, Campbell MS, Callaway CW, McKnight RA, Wint A, Dahl MJ, Dull RO, Albertine KH, Lane RH. IUGR decreases elastin mRNA expression in the developing rat lung and alters elastin content and lung compliance in the mature rat lung. Physiol Genomics. 2011;43:499–505. doi: 10.1152/physiolgenomics.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 31.Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23:320–326. doi: 10.1165/ajrcmb.23.3.3906. 2000. [DOI] [PubMed] [Google Scholar]
- 32.Mäki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167:927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Törnell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- 35.Ito S, Bartolák-Suki E, Shipley JM, Parameswaran H, Majumdar A, Suki B. Early emphysema in the tight skin and pallid mice: roles of microfibril-associated glycoproteins, collagen, and mechanical forces. Am J Respir Cell Mol Biol. 2006;34:688–694. doi: 10.1165/rcmb.2006-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 38.Cock ML, Joyce BJ, Hooper SB, Wallace MJ, Gagnon R, Brace RA, Louey S, Harding R. Pulmonary elastin synthesis and deposition in developing and mature sheep: effects of intrauterine growth restriction. Exp Lung Res. 2004;30:405–418. doi: 10.1080/01902140490451244. [DOI] [PubMed] [Google Scholar]
- 39.Desai M, Crowther NJ, Lucas A, Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br J Nutr. 1996;76:591–603. doi: 10.1079/bjn19960065. [DOI] [PubMed] [Google Scholar]
- 40.Burdge GC, Delange E, Dubois L, Dunn RL, Hanson MA, Jackson AA, Calder PC. Effect of reduced maternal protein intake in pregnancy in the rat on the fatty acid composition of brain, liver, plasma, heart and lung phospholipids of the offspring after weaning. Br J Nutr. 2003;90:345–352. doi: 10.1079/bjn2003909. [DOI] [PubMed] [Google Scholar]


