Abstract
Phthalate exposure has been associated with a wide range of adverse health outcomes in limited epidemiologic studies, and inflammation and oxidative stress have been hypothesized as potential mechanisms involved. In the present study we investigated associations between urinary concentrations of phthalate metabolites and potential blood markers of oxidative stress (bilirubin) and inflammation (alkaline phosphatase [ALP], absolute neutrophil count [ANC], ferritin [adjusted for iron status] and fibrinogen), using data from 10,026 participants in the National Health and Nutrition Examination Survey (NHANES) recruited between 1999 and 2006. After adjustment for covariates we found that bilirubin was inversely associated with several phthalate metabolites (all p-values < 0.0001), including the metabolites of di-2-ethylhexyl phthalate (DEHP) and dibutyl phthalate (DBP), in addition to mono-benzyl phthalate (MBzP) and mono-(3-carboxypropyl) phthalate (MCPP). Since bilirubin is a potent antioxidant these relationships suggest that phthalates may be associated with increased oxidative stress. Many of the same metabolites were also significantly and positively related with ANC, ALP and ferritin, suggesting phthalates may be associated with increased inflammation. These markers may be useful in other studies of low-dose exposure to environmental contaminants.
Introduction
Phthalate esters are chemicals used in a wide variety of consumer products and their urinary metabolites are detectable in the general population.1 High molecular weight phthalates, such as diethylhexyl phthalate (DEHP) and benzylbutyl phthalate (BBzP), are used in a variety of applications, but are primarily added to plastics such as polyvinyl chloride (PVC) to make them more flexible. Low molecular weight phthalates, such as dibutyl phthalate (DBP) and diethyl phthalate (DEP), are also used as plasticizers, and additionally as solvents in personal care products. Exposure has been linked to various health outcomes, including altered thyroid function, neurological development, asthma and allergic symptoms and reproductive health issues such as decreased sperm count and quality in men, and endometriosis, uterine leiomyomata and premature delivery in women.2–7 Although mechanisms for these and other health effects have not been firmly established, it has been hypothesized that phthalate induction of oxidative stress and inflammation may be involved.4,6 We recently reported several significant associations between urinary phthalate metabolites and gamma glutamyltransferase (GGT), a marker of oxidative stress, and C-reactive protein (CRP), a marker of inflammation.8 In the present analysis we continue our exploration by examining links between urinary phthalate metabolite levels and several other markers that have been indicated as representative of these health outcomes.
As a biomarker of oxidative stress, we examined serum bilirubin concentrations. Bilirubin is a potent antioxidant at physiologic concentrations, and reduced serum bilirubin has been proposed as a potential marker of oxidative stress.9 Furthermore bilirubin has been associated in some studies with other known biomarkers of oxidative stress, such as malondialdehyde (MDA) and GGT.10–11 Serum bilirubin has primarily been used as an oxidative stress marker in epidemiologic investigations of risk of coronary artery disease and metabolic disease.11–13 In our exploration of phthalates in relation to inflammatory markers, we examined serum ferritin, fibrinogen, absolute neutrophil count (ANC) and alkaline phosphatase (ALP). These parameters have been used to assess inflammation associated with cardiovascular disease and, occasionally, in relationship to environmental exposures.14–19
Although the markers evaluated in the present analysis may be related to other health indicators such as liver function, we suggest that phthalate exposure at the levels observed in this population are unlikely to lead to these pathological outcomes. Furthermore, though these markers may be analyzed less frequently as measures of oxidative stress and inflammation, we are unaware of evidence to suggest that they are less relevant to these outcomes than those used more commonly. Bilirubin, ALP, ANC and ferritin may be more readily available for use in large, population-based studies, particularly when data is examined retrospectively, and hence an understanding of their association with low-dose environmental exposures should be valuable.
Experimental Section
Study participants
The National Health and Nutrition Examination Survey (NHANES) is an ongoing cross-sectional study designed to estimate prevalence of exposures and disease in a sample representative of the general US population. For this analysis, data collected between 1999 and 2006 were combined.20 We included individuals who had measurements for one or more urinary phthalate metabolites, urinary creatinine and one or more of the outcomes of interest. We removed from the dataset 445 subjects who were pregnant as well as 5 who had levels of outcome measures greater than 5 times the standard deviation above the mean (n = 2 for ANC and n = 3 for fibrinogen). The final population used for analysis included 10,026 subjects. However, sample size varied by availability of phthalate metabolite, biomarker and covariate data.
Urinary phthalate metabolites
Urine samples were collected from subjects ages 6 and older at Mobile Examination Centers (MECs) and stored at −20° Celsius until analysis. Phthalate metabolites measured included mono-(2-ethylhexyl phthalate) (MEHP), mono-(2-ethyl-5- hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-n-butyl phthalate (MnBP), mono-isobutyl phthalate (MiBP), mono-benzyl phthalate (MBzP), mono-ethyl phthalate (MEP), mono-(3-carboxypropyl) phthalate (MCPP), mono-(carboxynonyl) phthalate (MCNP), mono-(carboxy-octyl) phthalate (MCOP), mono-cyclohexyl phthalate (MCHP), mono-isononyl phthalate (MiNP), mono-n-octyl phthalate (MnOP) and mono-n-methyl phthalate (MnMP). Measurements were performed by the Division of Environmental Health Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, as described elsewhere,1,21 using electrospray ionization (ESI) or atomic pressure chemical ionization (APCI) and tandem mass spectrometry.22–23 For descriptive statistics, levels were adjusted for urinary dilution by dividing phthalate level by creatinine concentration for a final measurement in μg/g creatinine. For regression models, unadjusted phthalate concentrations were used and creatinine was included as a covariate.24 The distributions of all phthalate measurements were lognormal and were ln-transformed for analysis. Phthalate metabolites with >75% of measurements below the limit of detection (LOD) were not included in our analysis. This excluded MCHP, MiNP, MnOP and MnMP. For other metabolites, measurements below the LOD were included and replaced by the LOD divided by the square root of two.25
In addition to the individual metabolite concentrations, we created several variables that combined metabolites of a parent phthalate diesters. A sum of the dibutyl phthalate (DBP) metabolites was created, DBPCOM, represented by MnBP alone in the 1999–2000 dataset and the combination of MiBP and MnBP in the 2001–2006 data sets. Also, as described in our previous papers, we created the variable MEHP% to compare the level of the putative toxic metabolite of di-2-ethylhexyl phthalate (DEHP), MEHP, to a sum of the major oxidized metabolites of DEHP (MEHP, MEOHP and MEHHP).26–27 It has been hypothesized that higher proportions of urinary MEHP compared to the other DEHP metabolites, as indicated by the MEHP% variable, may be associated with decreased metabolic efficiency and hence greater susceptibility to adverse health effects from DEHP exposure.26 To create MEHP%, we converted each DEHP metabolite into nanomoles using their molecular weights, dividing the molar mass of MEHP by the mass of the sum of all three metabolites, and then multiplying by 100. Though levels of MECPP, another oxidized metabolite of DEHP, were available in this dataset, we did not include them in the MEHP% variable because this analyte was measured only in the 2003–2006 datasets, and hence on a much smaller number of participants (n=4903). In addition, MECPP was highly correlated with both MEHHP (r=0.95, p < 0.0001) and MEOHP (r=0.96, p < 0.0001). Thus, its inclusion in the MEHP% variable would not be expected to impact results.
Blood biomarkers
Biomarkers assessed as dependent variables in our analysis were measured in serum or plasma using methods described in detail on the NHANES website.28 Methods and eligible samples differed slightly across study year. Bilirubin was measured in refrigerated serum using a diazo reaction with a rate of change measured photometrically by the Beckman Coulter LX20 analyzer (2001–2006) or the Hitachi 917 chemical analyzer (1999–2000). ALP concentration was measured in the same matrix and with the same machinery using an enzymatic reaction. Ferritin levels were assessed from frozen serum using the Bio-Rad laboratories QuantImune Ferritin IRMA kit in 1999–2002, and via the Roche Tina-quant method in 2003–2006 only. We adjusted ferritin measurements for iron status by dividing ferritin by the transferrin saturation, as has been done previously when utilizing ferritin as an inflammation marker.15 Transferrin measurements were made by dividing iron levels by unsaturated iron binding capacity (UIBC), the methods for which are described in detail elsewhere.28 Ferritin and transferrin levels were measured in all subjects in 1999–2002 data, but were only available with phthalate metabolite measures in women aged 12–59 from 2003–2006. Therefore there are fewer samples available for analysis for these outcome measures, and the population we examined for these variables was composed of more women than men for most phthalate metabolites, and only of women between 12 and 59 years of age for those phthalates measured only in the 2003–2006 datasets (MECPP, MCNP and MCOP). Fibrinogen was measured in plasma of men and women over age 40 between the years of 1999–2002 only, using the Clauss clotting method with detection by the STA-compact, an electromagnetic-mechanical clotting detection system. ANC was measured in whole blood upon receipt of sample in MECs. It was measured using the Beckman Coulter MAXM in the same way over all four study periods. Appropriate quality control methods were used for all analyses, as described on the NHANES website.28 We did not examine data on liver disease; rather, subjects with measurements for any outcome variable greater than 5 standard deviations from the mean were excluded from our analysis. All blood biomarker measures except for fibrinogen were log-normally distributed and ln-transformed for analysis.
Covariates
Multivariable linear regression models included covariates that were associated with exposure and outcome variables in crude analysis and/or changed effect estimates by greater than 10% upon inclusion in models. Demographic information was collected from participants using an in-home survey. From this data we included the variables age, sex, race and ethnicity, and poverty income ratio (PIR), an indicator of socioeconomic status. Alcohol use and education level were also considered but were not included because of high numbers of missing values (missing for alcohol use n=4674, missing for education n=4293); furthermore, there was a lack of association between these variables and urinary phthalate metabolites (p > 0.05). Body mass index (BMI) was included from physical examination data. Serum cotinine and urinary creatinine were included from other laboratory analyses, and both were ln-transformed in the statistical analysis. NHANES dataset (i.e., 2-year study periods) was considered as a covariate but did not markedly impact effect estimates and was not included. Lastly, we examined the effect of the time of day of sample collection (morning, afternoon or evening), as it has been suggested that both phthalate metabolite levels and the blood markers of interest exhibit some diurnal patterns.29–32 However, changes in effect estimates were small and this variable was omitted from final models.
Statistical analysis
Data analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC). NHANES utilizes a complex, multistage design, in which certain sub-populations are oversampled for improved accuracy of data within those groups. To adjust for this in the data analysis, samples are weighted for results that are generalizable to the US population. However, when a wide range of weights exists in the dataset and factors used in the formation of the weighting variables, such as age, sex, ethnicity and PIR, are included in the analysis as covariates, a weighted analysis can be inefficient.33 For this reason and for consistency with our previous findings, we performed both unweighted and weighted analyses, compared the two, and presented unweighted results when point estimates were similar.
In descriptive analyses we examined distributions of exposure and outcome variables with geometric means and selected percentiles in the total population and by the categorical covariates sex, age group and race and ethnicity. T-tests and one-way ANOVA were used to examine differences between groups. Crude associations between outcome measures and phthalate metabolites, as well as with the previously examined markers GGT and CRP, were explored using Pearson correlations of values that were ln-transformed, when appropriate. Multivariable linear regression models were created to examine adjusted relationships between individual phthalate metabolites and each blood marker of interest. The same covariates were added to all models for consistency and comparability, and included age (continuous), sex (dichotomous), race and ethnicity (categorical), ln-transformed serum cotinine, PIR (continuous), BMI (continuous) and ln-transformed urinary creatinine. We next examined several models including multiple phthalates for metabolites that were strongly correlated with one other. In secondary analyses we stratified our data by gender, race and ethnicity (Mexican American, other Hispanic, Non-Hispanic White, Non- Hispanic Black, other race/multi-racial) and age group (6–12, 13–19, 20–50 and > 50 years of age) to explore any differences and the potential for effect modification. We also examined associations across phthalate quintiles to assess non-linear relationships. Finally, using similar multivariable models, associations were assessed in pregnant women alone.
Results
Population distributions by categorical covariate are presented in Table 1. Geometric means and selected percentiles of phthalate metabolite levels were presented previously and thus are not displayed here.8 Variation in exposure levels by categorical covariates was as also described in our previous analysis, where there were differences in various phthalate metabolites by gender, race and ethnicity and age, consistent with findings in an analysis of 1999–2000 NHANES data.1,8
Table 1.
NHANES 1999–2006 population distribution by categorical covariates.
| Variable | Category | N (%) |
|---|---|---|
| Sex | Male | 5102 (50.9) |
| Female | 4924 (49.1) | |
| Age group | 6–12 | 1605 (16.0) |
| 13–19 | 2866 (28.6) | |
| 20–50 | 3036 (30.3) | |
| >50 | 2519 (25.1) | |
| Race/ethnicity | Mexican American | 2641 (26.3) |
| Other Hispanic | 404 (4.01) | |
| Non-Hispanic White | 4020 (40.1) | |
| Non-Hispanic Black | 2575 (25.7) | |
| Other/Multi-racial | 386 (3.85) |
Table 2 displays distributions and sample size for each outcome variable. Several covariates were associated with these variables in preliminary analyses. There were significant sex differences for all outcome variables (p < 0.0001), with women having lower levels of bilirubin, ALP and adjusted ferritin, and higher levels of ANC and fibrinogen. There were no differences in bilirubin level by age group; however, ALP level showed a significantly decreasing trend with increasing age group (p for trend < 0.0001), and the other markers of inflammation showed significantly increasing trends (p for ANC = 0.062, p for adjusted ferritin < 0.0001, p for fibrinogen = 0.014). Both bilirubin and ALP showed a significant downward trend with increasing BMI category (p < 0.0001) while those for ANC, adjusted ferritin and fibrinogen were also significant but positive (p < 0.0001). Lastly, all outcome variables differed significantly by race/ethnicity (p < 0.0001).
Table 2.
Weighted distributions of biomarkers for oxidative stress and inflammation.
| Percentiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Years measured | N | Geometric Mean (SE) | 25th | 50th | 75th | 90th | 95th | Max. |
| Bilirubin (mg/dL) | 1999–2006 | 8053 | 0.66 (1.00) | 0.48 | 0.61 | 0.80 | 1.02 | 1.23 | 4.40 |
| Alkaline Phosphatase (U/L) | 1999–2006 | 8054 | 84.6 (1.01) | 56.4 | 69.8 | 86.7 | 114 | 163 | 728 |
| Ferritin (ng/mL) | 1999–2006c | 6031 | 49.7 (1.01) | 27.6 | 56.3 | 121 | 228 | 324 | 2310 |
| Adjusted Ferritina | 1999–2006c | 6007 | 2.63 (1.02) | 1.31 | 2.53 | 5.07 | 9.98 | 13.9 | 112 |
| ANC count (1000 cells/uL) | 1999–2006 | 9437 | 6.82 (1.00) | 5.69 | 6.91 | 8.33 | 9.95 | 11.2 | 31.5 |
| Fibrinogen (mg/dL) | 1999–2002d | 1871 | 378 (1.90)b | 313 | 360 | 411 | 473 | 513 | 775 |
Adjusted ferritin = ferritin/transferrin saturation.
Arithmetic mean and standard error.
Measured in all subjects 1999–2002 and only in women ages 12–59 in 2003–2006.
Measured only in men and women over age 40 from 1999–2002
Correlation analysis comparing markers used previously with those in this study showed that bilirubin was very weakly but statistically significantly associated with GGT (r = 0.05, p < 0.0001). CRP was weakly to moderately correlated with all markers of inflammation, including ALP (r = −0.075, p < 0.0001), ANC (r = 0.25, p < 0.0001), adjusted ferritin (r = 0.40, p < 0.0001) and fibrinogen (r = 0.54, p < 0.0001). Relationships between markers for inflammation in this study were mostly statistically significant, but the magnitudes of the correlations were weak. ALP was slightly and inversely correlated with ANC (r = −0.02, p = 0.08) as well as with adjusted ferritin (r = −0.09, p < 0.0001), and positively correlated with fibrinogen (r = 0.25, p < 0.0001). ANC showed slightly stronger positive correlations with adjusted ferritin (r = 0.10, p < 0.0001) and fibrinogen (r = 0.22, p < 0.0001), and, lastly, adjusted ferritin showed a weak positive correlation with fibrinogen (r = 0.18, p < 0.0001).
Crude analysis using correlations to examine the relationships between phthalate metabolites and biomarkers of oxidative stress/inflammation revealed many statistically significant relationships and were similar to adjusted results (data not shown). Multivariable linear regression results are displayed in Table 3. Because they were similar with and without sample weights, unweighted results are presented. The associations between phthalate metabolites and fibrinogen are not shown, as there were fewer subjects with data on this measure (n=1871) and none of the relationships were observed to be statistically significant. Also, results for unadjusted ferritin are not included as the relationships were primarily weakly inverse and did not approach statistical significance.
Table 3.
Percent change (95% confidence intervals) in markers of oxidative stress and inflammation associated with an interquartile range increase in urinary phthalate metabolite concentrations.a
| Parent compound | Phthalate metabolite | Bilirubin | Alkaline phosphatase | ||
|---|---|---|---|---|---|
| N | Percent change (95% CI) | N | Percent change (95% CI) | ||
| DEHP | MEHP | 7179 | −5.20 (−6.55, −3.63)** | 7180 | −4.03 (−5.78, −2.23)** |
| MEHHP | 5523 | −5.47 (−6.64, −4.13)** | 5524 | 2.37 (0.64, 4.14)* | |
| MEOHP | 5523 | −5.01 (−6.33, −3.82)** | 5524 | 3.16 (1.32, 4.94)* | |
| MECPP | 3613 | −4.35 (−5.90, −2.78)** | 3614 | 1.95 (−0.13, 4.24) | |
| MEHP%b | 5523 | 0.78 (−0.40, 2.04) | 5524 | −7.59 (−8.98, −6.18)** | |
| DBP | MnBP | 5523 | −4.38 (−5.85, −2.90)** | 5524 | 11.1 (8.92, 13.3)** |
| MiBP | 5523 | −4.06 (−5.64, −2.46)** | 5524 | 7.04 (4.75, 9.37)** | |
| DBPCOMc | 7179 | −3.72 (−5.20, −2.36)** | 7180 | 10.0 (8.03, 11.9)** | |
| BzBP | MBzP | 7179 | −3.94 (−5.37, −2.48)** | 7180 | 13.8 (11.7, 15.9)** |
| DEP | MEP | 7175 | 1.06 (−0.30, 2.42) | 7176 | −3.33 (−5.05, −1.84)** |
| DOP | MCPP | 5523 | −6.23 (−7.64, −4.94)** | 5524 | 11.1 (-.01, 13.3)** |
| DiDP | MCNP | 1775 | −0.93 (−3.49, 1.73) | 1776 | 4.31 (1.17, 7.37)* |
| DiNP | MCOP | 1775 | −2.14 (−4.50, 0.42) | 1776 | 2.33 (−0.67, 5.32) |
| Parent compound | Phthalate metabolite | Adjusted ferritin | Absolute neutrophil count | ||
|---|---|---|---|---|---|
| N | Percent change (95% CI) | N | Percent change (95% CI) | ||
| DEHP | MEHP | 5303 | 4.19 (0.14, 8.56)* | 8335 | 0.89 (−0.13, 1.93) |
| MEHHP | 3408 | 9.31 (5.12, 16.7)** | 6434 | 1.54 (0.60, 2.53)* | |
| MEOHP | 3408 | 6.92 (2.68, 11.3)* | 6434 | 1.36 (0.39, 2.36)* | |
| MECPP | 1188 | 7.53 (1.13, 14.1)* | 4202 | 0.89 (−0.27, 2.10) | |
| MEHP%b | 3408 | −4.45 (−7.78, −0.95)* | 6434 | −0.46 (−1.31, 0.40) | |
| DBP | MnBP | 3408 | 2.99 (−1.53, 7.71) | 6434 | 1.98 (0.84, 2.99)* |
| MiBP | 3408 | 3.20 (−1.97, 8.46) | 6434 | 2.18 (0.95, 3.37)* | |
| DBPCOMc | 5303 | 3.43 (−0.43, 7.43) | 8335 | 2.27 (1.32, 3.28)** | |
| BzBP | MBzP | 5303 | 3.40 (−0.35, 7.28) | 8335 | 1.69 (0.76, 2.71)* |
| DEP | MEP | 5299 | −5.99 (−9.31, −2.75)* | 8331 | −0.83 (−1.72, 0.06) |
| DOP | MCPP | 3408 | −0.48 (−4.68, 3.91) | 6434 | 1.38 (0.36, 2.50)* |
| DiDP | MCNP | 578 | 3.21 (−6.00, 13.2) | 2065 | 0.38 (−1.23, 2.00) |
| DiNP | MCOP | 578 | 2.18 (−6.14, 11.3) | 2065 | 1.09 (−0.50, 2.63) |
Calculated with coefficients from full multivariate regression models, adjusting for age, sex, race and ethnicity, ln-serum cotinine, PIR, BMI and ln-urinary creatinine.
Proportion of MEHP compared to sum MEHP, MEHHP and MEOHP.
Sum of MnBP and MiBP.
p < 0.05
p < 0.0001
In adjusted models, bilirubin was significantly and inversely associated with MEHP, MEHHP, MEOHP, MECPP, MnBP, MiBP, DBPCOM, MBzP and MCPP (p < 0.0001). A positive relationship with MEP was observed, though it was not statistically significant (p = 0.13). However, upon stratification by sex the association was statistically significant among females (p = 0.009) but not males. Other stratified results, by sex as well as race and age group, showed relationships consistent with those in unstratified models.
There were significant positive associations between ALP and MnBP, MiBP, DBPCOM, MBzP and MCPP (p < 0.0001). Slightly weaker but similarly positive relationships were observed with MEHHP, MEOHP and MCNP (p < 0.01), as was a statistically suggestive relationship with MECPP (p = 0.067). Unexpectedly, MEHP and MEHP% were inversely associated with ALP (p < 0.0001). In stratified analysis there were no differences in phthalate-ALP relationships by age group or sex, but interestingly relationships between ALP and MEHHP, MEOHP and MECPP were significant only in Mexican Americans (data not shown for stratified analyses).
Adjusted ferritin, though only available for a much smaller number of participants and higher proportion of females compared to males (60.7% females for MEHP model), also showed several significant relationships with phthalate metabolites. Levels were positively associated with MEHP, MEHHP and MEOHP and for MECPP in females alone (p-values < 0.05). Contrary to our hypothesis we observed significant inverse relationships with MEHP% and MEP. There were no major differences observed in stratified analyses.
Finally, ANC was positively associated with MEHHP, MEOHP, MnBP, MiBP, DBPCOM, MBzP and MCPP (p-values < 0.01). There were no differences in effect estimates by age group, race or gender stratification.
In a bivariate analysis the metabolites of DBP were highly correlated with MBzP (for MnBP and MBzP r = 0.55, p<0.0001, for MiBP and MBzP r = 0.54, p<0.0001). Thus, we examined effects of including these metabolites together as predictors. In models including MnBP and MBzP, as well as models with MiBP and MBzP, coefficients were nearly identical to those from models with only one phthalate metabolite predictor, with a slight attenuation of effect estimates in some models but no changes in overall conclusions (results not shown).
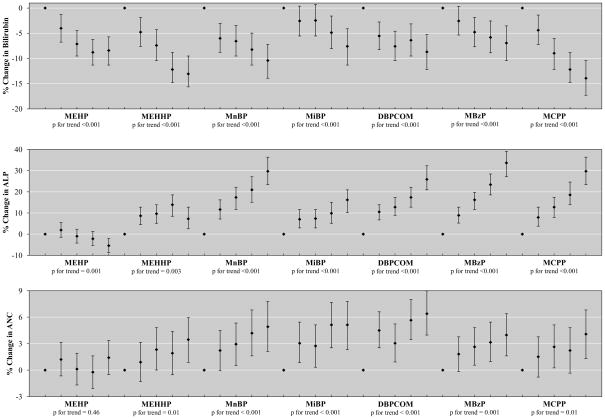
Figure 1 shows results from selected adjusted models of the phthalate quintiles of MEHP, MEHHP, MiBP, MnBP, DBPCOM, MBzP and MCPP relationships with bilirubin, ALP and ANC. Results for MEOHP and MECPP were very similar to those for MEHHP and are not shown. Relationships with other phthalate metabolites, as well as with fibrinogen, were not displayed to conserve space because they were not statistically significant. For models with fibrinogen this may have been due to insufficient sample size. These data showed primarily monotonic associations between exposure quintile and percent change in outcome variable, although for some relationships, such as with ALP and MEHHP, estimates plateaued prior to the highest quintile of exposure. Finally, no notable results were observed in our analysis limited to pregnant women (data not shown).
Figure 1.
Associationa between urinary phthalate metabolite quintiles and percent change (95% confidence interval) in: a) Bilirubin; b) Alkaline phosphatase; and c) Absolute Neutrophil Count.
aCalculated with coefficients from full multivariate regression models for phthalate quintile association with outcome measure, adjusting for age, sex, race and ethnicity, ln-serum cotinine, PIR, BMI and ln-urinary creatinine.
Discussion
The results from this study show strong and significant associations between urinary phthalate metabolites and blood biomarkers for oxidative stress and inflammation that have previously not been examined in this context. They indicate both that phthalate exposure is associated with significant physiological changes in the general US population, and that some of the markers examined may be useful in examining the oxidative stress and inflammatory effects of phthalate and potentially other low-dose environmental exposures in large human studies. Investigation of these relationships has the potential to improve our understanding of environmental contaminant contribution to human health and disease because of the critical roles oxidative stress and inflammation can play in disease etiology and progression.
Inflammation is a multi-stage multi-factorial response to infectious microorganisms, injury and toxicants that involves rapid-onset innate immune responses (characterized by cytokine release, acute phase proteins and leukocyte recruitment) and acquired immune responses (culminated by antibody response). Oxidative stress is commonly defined as a condition in which cellular protective antioxidant systems are overwhelmed by reactive oxidant chemicals, leading to oxidative damage of macromolecules such as DNA, proteins and lipids. Responses to inflammatory and pro-oxidant stimuli may overlap and interact, as well. Serum levels of CRP, an acute phase protein, and GGT, an enzyme involved in antioxidant defense response, have been widely applied as biomarkers of inflammation and oxidative stress, respectively, and were associated with various urinary phthalate metabolites in our previous report.8 In the present study, we report new results from an expanded analysis of relationships between urinary phthalate metabolites and additional potential markers of oxidative stress (bilirubin) and inflammation (ALP, ANC, ferritin and fibrinogen).
In limited human studies of phthalate exposure, there is considerable variation in results by phthalate or phthalate metabolite and oxidative stress exposure markers. Evidence for oxidative stress associations in humans have been observed with MEP and MEHP as indicated by a potential downstream effect (increased sperm DNA damage),6,34 with MEHP and MEHHP by increased MDA and 8-OHdG,35 and with MEHP and MEHP% by increased GGT as we reported recently.8 These associations with oxidative stress in humans are consistent with results from animal studies showing that exposure to the parent diesters DEHP and DBP stimulated reactive oxidant species generation in liver36 and testis,37–38 and increased MDA generation in liver.39 Likewise, DEHP stimulated 8-OHdG formation in rat liver.39–40
Here we used serum bilirubin level as a biomarker of oxidative stress. Bilirubin is a heme breakdown product with potent antioxidant properties that acts as a scavenger of reactive oxygen species (ROS).9 Upon reaction with ROS, bilirubin can be converted to biliverdin, which is then metabolized back to bilirubin by the enzyme biliverdin reductase. Alternatively, bilirubin can form oxidative metabolites that are excreted as biopyrrins into the urine.41 As a result of its antioxidant activity, decreased bilirubin, or increased biopyrrins, may serve as a useful biomarkers of increases in oxidative stress. Furthermore, its value as a marker has been evidenced by high correlations with other known markers of oxidative stress such as MDA (for total serum bilirubin < 16 mg/dL r = −0.74, p < 0.01).10 In the present study we observed a correlation in the opposite direction expected with the oxidative stress marker GGT, however it was very weak in magnitude (r = 0.05). Previously, bilirubin has been used as a marker of oxidative stress in prediction of cardiovascular disease.12–13,42 Here we observe a decrease in serum bilirubin, in association with all of the phthalate metabolites previously shown to be associated with oxidative stress, except for MEP. In regards to our earlier study where oxidative stress was indicated by an increase in GGT, we confirmed the relationship between MEHP and increased oxidative stress. However, we did not observe the inverse relationship with MEHP% and MEOHP, and we also observed many additional significant associations.8 The first inconsistency indicates a need for closer examination of the toxicological effects of the different DEHP metabolites, as their roles are unclear. The second suggests that bilirubin may be a more sensitive marker for detection of oxidative stress, or may be measuring different aspects of and/or effects beyond oxidative stress.
Adjusted ferritin has been used recently as a marker of inflammation in the study of childhood obesity.15 In that study, Skinner et al. introduced the ferritin/transferrin saturation ratio as a way to adjust ferritin values for whole-body iron status. Although serum ferritin has been used historically as an assessment of iron status and, therefore, anemia, ferritin is also an acute phase protein of the inflammatory response to infection and injury, similar to CRP.43 Despite the markedly smaller sample sizes for adjusted ferritin compared to other markers and measurement availability restricted to women of ages 12–59 years, we still observed significant positive associations with MEHP, MEHHP and MEOHP. Notably, adjusted ferritin was the only inflammatory marker to show a positive association with urinary levels of MEHP. The associations between MEHP and ferritin are supported by laboratory studies showing that MEHP stimulated release of inflammatory cytokines from human epithelial cells,44 human umbilical vein endothelial cells45 and rat alveolar macrophages.46 In addition to being an acute phase protein, ferritin synthesis is closely linked to heme oxygenase-1 expression, an enzyme in the antioxidant response pathway that initiates heme conversion to bilirubin.47 As such, the positive associations observed with adjusted ferritin are consistent with the inverse relationships observed with bilirubin as potential indicators of oxidative stress. Also, with this measure we observed an unexpected inverse relationship with MEP. As a recently introduced marker for inflammation, further studies are needed to interpret relationships between adjusted ferritin and environmental contaminants more completely.
Although elevated ALP levels are traditionally used as markers of liver damage and disease, recent reports that serum ALP was positively correlated with the inflammatory marker CRP in epidemiologic studies, particularly in women, suggest that ALP may serve as a marker of inflammation.50–53 In the present analysis, we observed strong positive relationships between ALP and the phthalate metabolites MEHHP, MEOHP, MnBP, MiBP, the combination of the DBP metabolites, MBzP, MCPP and MCNP. Our current findings include associations with more phthalate metabolites than what we previously reported with CRP, where we only noted significant relationships with MBzP and MiBP.8 We unexpectedly observed inverse associations of ALP with MEHP, the MEHP% variable and MEP. The relationships with MEHP and MEHP% were particularly surprising; because MEHP is the putative major toxic metabolite of DEHP, we anticipated that individuals with higher urinary concentrations of MEHP or higher MEHP% would exhibit higher indication of inflammation as reflected by the marker ALP due to either higher DEHP exposure or decreased ability to transform MEHP to its oxidized metabolites. The inverse relationships we observed could be a result of interferences due to the fact that ALP is also a marker for liver function and cytotoxicity. Furthermore, the lack of a clear biological explanation linking elevated serum ALP to inflammation leaves open the possibility that ALP may not serve as a reliable marker of inflammation in all circumstances. Low, null or inverse correlations observed in this study between ALP and other potential markers of inflammation, including CRP, ANC, adjusted ferritin and fibrinogen, may also add doubt to the validity of this measure.
ANC is a clinical measure of the immune status of an individual, commonly used to assess risk of infection after chemotherapy. Increased ANC has been used as an indicator of the acute phase response in studies of disease and, more recently, as a marker of inflammation in the study of environmental exposures.14,16,19 In the present study we observed significant positive relationships between ANC and MEHHP, MEOHP, MnBP, MiBP, DBPCOM, MBzP and MCPP, and no significant inverse relationships were found. However, inconsistent with previous studies, we did not observe a significant relationship with MEHP or MEP.
Lastly, despite a limited sample size, we investigated relationships between phthalate metabolites and fibrinogen, which has been used as a marker of inflammation in association with cardiovascular disease as well as more recently in studies of exposure to environmental tobacco smoke and other air pollutants.14,17–19,54 Though no significant relationships were observed here, the potential for use of fibrinogen as a biomarker of inflammation in response to phthalate exposure should be further explored with larger sample sizes, particularly as we observed this marker to be most highly correlated with the marker CRP and the other markers used in the present study.
The results from our analysis of phthalate metabolites with the inflammatory markers ALP and ANC were consistent with previous findings of significant positive associations between MnBP and MBzP with CRP.8 Furthermore, this analysis additionally indicated that the metabolites MiBP and MCPP may be associated with inflammation. However, whereas in the previous study we observed significant inverse relationships between more oxidized DEHP metabolites MEHHP and MEOHP and CRP, we observed positive relationships with all of the markers measured here. Again, this indicates a need for a better understanding of the toxicological effects of the DEHP metabolites, and more details on which facets of inflammation or oxidative stress are reflected in the various blood markers we examined.
There were several limitations to our study. First, as is always the case with cross-sectional data like NHANES, we are unable to establish a causative relationship between exposure to phthalates and adverse health outcomes. Second, since we made a large number of comparisons in our study, it is possible that some of our observations were due to chance. The strength and consistency of our results argue against chance findings. Lastly, because phthalate exposure is thought to be primarily dietary, and because compounds are metabolized relatively quickly, measurements taken from a single urine sample may not be representative of average exposure. However, several studies have indicated that a single measurement may represent long-term averages, though the temporal reliability varies by phthalate metabolite.30,55–56
Despite these limitations, this study also has many strengths. It offers a novel exploration of phthalate associations with oxidative stress and inflammation involving potentially under-utilized and powerful biomarkers of these pathways in a large representative population. Also, our findings were robust to secondary stratified and quintile analyses. Future exploration to more thoroughly investigate these associations should involve toxicological studies and also epidemiologic analyses using more robust study designs with longitudinal information and repeat urine samples for exposure assessment, as well as the examination of relationships in sensitive subgroups.
In conclusion, we observed that various urinary phthalate metabolites, particularly MBzP, MCPP and the metabolites of DEHP and DBP, are associated with increases in biomarkers of inflammation and oxidative stress. Not only does this have implications for the potential human health effects of phthalates, but our findings out also suggest that ALP, ANC and adjusted ferritin may be useful markers of inflammation, and that bilirubin may similarly be a useful marker of oxidative stress, in the study of health impacts of low-dose environmental chemical exposures.
Supplementary Material
Acknowledgments
Work supported by grants R01ES018872, P42ES017198, and P20ES018171 from the National Institute of Environmental Health Sciences (NIEHS), and RD83480001 from the US Environmental Protection Agency (USEPA).
References
- 1.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meeker JD. Exposure to environmental endocrine disruption compounds and men’s health. Maturitas. 2010;66(3):236–241. doi: 10.1016/j.maturitas.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA. Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environ Health Perspect. 2010;118(6):825–832. doi: 10.1289/ehp.0901543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Téllez-Rojo MM. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 2009;117(10):1587–1592. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108(2):177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, Calafat AM. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod. 2007;22(3):688–695. doi: 10.1093/humrep/del428. [DOI] [PubMed] [Google Scholar]
- 7.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monester and oxidative metabolites. Epidemiology. 2006;17(6):682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson KK, Loch-Caruso R, Meeker JD. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999–2006. Environ Res. 2011;111(5):718–726. doi: 10.1016/j.envres.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 10.Shekeeb Sahab M, Kumar P, Sharma N, Narang A, Parasad R. Evaluation of oxidant and antioxidant status in term neonates: a plausible protective role of bilirubin. Mol Cell Biochem. 2008;317(1–2):51–59. doi: 10.1007/s11010-008-9807-4. [DOI] [PubMed] [Google Scholar]
- 11.Giral P, Ratziu V, Couvert P, Carrie A, Kontush A, Girerd X, Chapman MJ. Plasma bilirubin and gamma-glutamyltransferase activity are inversely related in dyslipidemic patients with metabolic syndrome: relevance to oxidative stress. Atherosclerosis. 2010;210(2):607–613. doi: 10.1016/j.atherosclerosis.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with risk of coronary artery disease. Clin Chem. 1994;40(1):18–23. [PubMed] [Google Scholar]
- 13.Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Aterioscler Thromb Vasc Biol. 1996;16(2):250–255. doi: 10.1161/01.atv.16.2.250. [DOI] [PubMed] [Google Scholar]
- 14.Frost-Pineda K, Liang Q, Liu J, Rimmer L, Jin Y, Feng S, Kapur S, Mendes P, Roethig H, Sarkar M. Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine Tob Res. 2011;13(3):182–193. doi: 10.1093/ntr/ntq235. [DOI] [PubMed] [Google Scholar]
- 15.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125(4):e801–e809. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang YJ, Hong YC, Oh SY, Park MS, Kim H, Leem JH, Ha EH. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ Res. 2009;109(6):797–801. doi: 10.1016/j.envres.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Venn A, Britton J. Exposure to secondhand smoke and biomarkers of cardiovascular disease risk in never-smoking adults. Circulation. 2007;115(8):990–995. doi: 10.1161/CIRCULATIONAHA.106.648469. [DOI] [PubMed] [Google Scholar]
- 18.Menzies D, Nair A, Williamson PA, Schembri S, Al-Kahiralla MZ, Barnes M. Respiratory symptoms, pulmonary function, and markers of inflammation among bar workers before and after a legislative ban on smoking in public places. JAMA. 2006;296(14):1742–1748. doi: 10.1001/jama.296.14.1742. [DOI] [PubMed] [Google Scholar]
- 19.Liao D, Heiss G, Chincilli VM, Duan Y, Folsom AR, Lin HM, Salomaa V. Association of criteria pollutants with plasma hemostatic/inflammatory markers: a population-based study. J Expo Anal Environ Epidemiol. 2005;15(4):319–328. doi: 10.1038/sj.jea.7500408. [DOI] [PubMed] [Google Scholar]
- 20.NCHS (National Center for Health and Statistics) [(accessed October 31, 2011)];Introduction to NHANES. http http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/overviewbrochure_0708.pdf.
- 21.Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, Needham LL, Brock JW. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biom Life Sci. 2003;789(2):393–404. doi: 10.1016/s1570-0232(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 22.NCHS (National Center for Health and Statistics) [(accessed July 12, 2010).];Documentation, codebook, and frequencies for urinary phthalates, phytoestrogens and PAHs 2001 to 2002. http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/phpypa_b.pdf.
- 23.NCHS (National Center for Health and Statistics) [(accessed July 12, 2010).];Data documentation, codebook, and frequencies for urinary phthalates. 2005–2006 http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/PHTHTE_D.htm.
- 24.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung RW, Reed L. Estiamtion of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 26.Hauser R. Urinary phthalate metabolites and semen quality: a review of a potential biomarker of susceptibility. Int J Androl. 2008;31(2):112–117. doi: 10.1111/j.1365-2605.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- 27.Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115(7):1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NCHS (National Center for Health and Statistics) [(accessed April 24, 2011).];NHANES 2005–2006 Laboratory Files. http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/lab05_06.htm.
- 29.Lazo M, Selvin E, Clark JM. Brief communication: clinical implications of short-term variability in liver function test results. Ann Intern Med. 2008;148(5):348–352. doi: 10.7326/0003-4819-148-5-200803040-00005. [DOI] [PubMed] [Google Scholar]
- 30.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112(17):1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bremner WF, Sothern RB, Kanabrocki EL, Ryan M, McCormick JB, Dawson S, Connors ES, Rothschild R, Third JL, Vahed S, Nemchausky BM, Shirazi P, Olwin JH. Relation between circadian patterns in levels of circulating lipoprotein(a), fibrinogen, platelets, and related lipid variables in men. Am Heart J. 2000;139(1 Pt 1):164–173. doi: 10.1016/s0002-8703(00)90324-7. [DOI] [PubMed] [Google Scholar]
- 32.Bridges AB, Fisher TC, Scott N, McLaren M, Belch JJ. Circadian rhythm of white blood cell aggregation and free radical status in healthy volunteers. Free Radic Res Commun. 1992;16(2):89–97. doi: 10.3109/10715769209049162. [DOI] [PubMed] [Google Scholar]
- 33.Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81(9):1166–1173. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pant N, Shukla M, Kumar Patel D, Shukla Y, Mathur N, Kumar Gupta Y, Saxena DK. Correlation of phthalate exposures with semen quality. Toxicol Appl Pharmacol. 2008;231(1):112–116. doi: 10.1016/j.taap.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Hong YC, Park EY, Park MS, Ko JA, Oh SY, Kim H, Lee KH, Leem JH, Ha EH. Community level exposure to chemicals and oxidatve stress in adult population. Toxicol Lett. 2009;184(2):139–144. doi: 10.1016/j.toxlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Rusyn I, Kadiiska MB, Dikalova A, Kono H, Yin M, Tsuchiya K, Mason RP, Peters JM, Gonzalez FJ, Segal BH, Holland SM, Thurman RG. Phthalates rapidly increase production of reactive oxygen species in vivo: role of Kupffer cells. Mol Pharmacol. 2001;59(4):744–750. doi: 10.1124/mol.59.4.744. [DOI] [PubMed] [Google Scholar]
- 37.Zhou D, Wang H, Zhang J, Gao X, Zhao W, Zheng Y. Di-n-butyl phthalate (DBP) exposure induces oxidative damage in testes of adult rats. Syst Biol Reprod Med. 2010;56(6):413–419. doi: 10.3109/19396368.2010.509902. [DOI] [PubMed] [Google Scholar]
- 38.Kasahara E, Sata EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, Tokuda M, Nakano Y, Inoue M. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl) phthalate. Biochem J. 2002;365(Pt 3):849–856. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seo KW, Kim KB, Kim YJ, Choi JY, Lee KT, Choi KS. Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem Toxicol. 2004;42(1):107–114. doi: 10.1016/j.fct.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Ito Y, Yamanoshita O, Asaeda N, Tagawa Y, Lee CH, Aoyama T, Ichihara G, Furuhashi K, Kamijima M, Gonzalez FJ, Nakajima T. Di(2-ethylhexyl) phthalate induces hepatic tumorigenesis through a peroxisome proliferator-activated receptor alpha-independent pathway. J Occup Health. 2007;49(3):172–182. doi: 10.1539/joh.49.172. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi T, Shioji I, Sugimoto A, Komoda Y, Nakajima H. Chemical structure of a new family of bile pigments from human urine. J Biochem. 1994;116:298–303. doi: 10.1093/oxfordjournals.jbchem.a124523. [DOI] [PubMed] [Google Scholar]
- 42.Huang SS, Huang PH, Leu HB, Wu TC, Lin SJ, Chen JW. Serum bilirubin predicts long-term clinical outcomes in patients with cardiac syndrome X. Heart. 2010;96(15):1227–1237. doi: 10.1136/hrt.2009.192393. [DOI] [PubMed] [Google Scholar]
- 43.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response—lessons from malaria and human immunodeficiency virus. Ann Clin Biochem. 2008;45(Pt 1):18–32. doi: 10.1258/acb.2007.007167. [DOI] [PubMed] [Google Scholar]
- 44.Jepsen KF, Abildtrup A, Larsen ST. Monophthalates promote IL-6 and IL-8 production in the human epithelial cell line A549. Toxicol In Vitro. 2004;18(3):265–269. doi: 10.1016/j.tiv.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Rael LT, Bar-Or R, Ambruso DR, Mains CW, Slone DS, Craun ML, Bar-Or D. Phthalate esters used as plasticizers in packed red blood cell storage bags may lead to progressive toxin exposure and the release of pro-inflammatory cytokines. Oxid Med Cell Longev. 2009;2(3):166–171. doi: 10.4161/oxim.2.3.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakkestad KE, Holme JA, Paulsen RE, Schwarze PE, Becher R. Mono(2-ethylhexyl) phthalate induces both pro- and anti-inflammatory responses in rat alveolar macrophages through crosstalk between p38, the lipoxygenase pathway and PPARalpha. Inhal Toxicol. 2010;22(2):140–150. doi: 10.3109/08958370903019885. [DOI] [PubMed] [Google Scholar]
- 47.Tell G, Gustincich S. Redox state, oxidatve stress, and molecular mechanisms of protective and toxic effects on bilirubin on cells. Curr Pharm Des. 2009;15(25):2908–2914. doi: 10.2174/138161209789058174. [DOI] [PubMed] [Google Scholar]
- 48.Webber M, Krishnan A, Thomas NG, Cheung BM. Association between serum alkaline phosphatase and C-reactive protein in the United States National Health and Nutrition Examination Survey 2005–2006. Clin Chem Lab Med. 2010;48(2):167–173. doi: 10.1515/CCLM.2010.052. [DOI] [PubMed] [Google Scholar]
- 49.Nayeem F, Anderson KE, Nagamani M, Grady JJ, Lu LJ. Alkaline phosphatase and percentage body fat predict circulating C-reactive protein in premenopausal women. Biomarkers. 2010;15(8):663–670. doi: 10.3109/1354750X.2010.509811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung BM, Ong KL, Cheung RV, Wong LY, Wat NM, Tam S, Leung GM, Cheng CH, Woo J, Janus ED, Lau CP, Lam TH, Lam KS. Association between plasma alkaline phosphatase and C-reactive protein in Hong-Kong Chinese. Clin Chem Lab Med. 2008;46(4):523–527. doi: 10.1515/CCLM.2008.111. [DOI] [PubMed] [Google Scholar]
- 51.Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, Levy Y, Brook GJ, Aronson D. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Throb Vasc Biol. 2005;25(1):193–197. doi: 10.1161/01.ATV.0000148324.63685.6a. [DOI] [PubMed] [Google Scholar]
- 52.Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PloS One. 2010;5(1):e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300(11):1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 54.Thompson AM, Zanobetti A, Silverman F, Schwartz J, Coull B, Urch B, Speck M, Brook JR, Manno M, Gold DR. Baseline repeated measures from controlled human exposure studies: associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ Health Perspect. 2010;118(1):120–124. doi: 10.1289/ehp.0900550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106(2):257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki Y, Niwa M, Yoshinaga J, Watanabe C, Mizumoto Y, Serizawa S, Shiraishi H. Exposure assessment of phthalate esters in Japanese pregnant women by using urinary metabolite analysis. Environ Health Prev Med. 2009;14(3):180–187. doi: 10.1007/s12199-009-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.