Abstract
Galectin-1 (Gal-1) has been shown to play a major role in tumor immune escape by inducing apoptosis of effector leukocytes and correlating with tumor aggressiveness and disease progression. Targeting the Gal-1 – Gal-1 ligand axis, thus, represents a promising cancer therapeutic approach. Here, to test the Gal-1-mediated tumor immune evasion hypothesis and demonstrate the importance of Gal-1-binding N-acetyllactosamines in controlling the fate and function of anti-tumor immune cells, we treated melanoma- or lymphoma-bearing mice with peracetylated 4-fluoro-glucosamine (4-F-GlcNAc), a metabolic inhibitor of N-acetyllactosamine biosynthesis, and analyzed tumor growth and immune profiles. We found that 4-F-GlcNAc spared Gal-1-mediated apoptosis of T and NK cells by decreasing their expression of Gal-1-binding determinants. 4-F-GlcNAc enhanced tumor lymphocytic infiltration and promoted elevations in tumor-specific cytotoxic T cells and IFN-γ levels, while lowering IL-10 production. Collectively, our data suggest that metabolic lowering of Gal-1-binding N-acetyllactosamines may attenuate tumor growth by boosting anti-tumor immune cell levels, representing a promising approach for cancer immunotherapy.
Introduction
Galectin-1 (Gal-1), a soluble β-galactoside-binding lectin, can function as a biological modifier of tumor growth and metastasis (Andre et al., 1999; Jung et al., 2007; Rabinovich and Ilarregui, 2009; Rabinovich et al., 2007). Gal-1 mediates autocrine and paracrine activities that favor invasiveness (Clausse et al., 1999; van den Brule et al., 2003; Woynarowska et al., 1996; Woynarowska et al., 1994), drives angiogenesis (Thijssen et al., 2006) and regulates anti-tumor immune responses (Ilarregui et al., 2009; Rubinstein et al., 2004; Stannard et al., 2010). Gal-1 influences the fate and function of effector leukocytes by inducing pro-apoptotic signals and skewing cancer microenvironments towards Th2 and regulatory cytokine profiles (Ilarregui et al., 2009; Juszczynski et al., 2007).
Considering recent studies targeting Gal-1 as anti-cancer therapeutic strategies (Ito et al., 2011; Ouyang et al., 2011; Rubinstein et al., 2004), we hypothesize that perturbing the synthesis of Gal-1 carbohydrate-binding determinants (Gal-1 ligands) might interfere with Gal-1-mediated effects that promote immune privilege of cancer. Gal-1 ligands are membrane proteins with one or more N-acetyllactosamine Type1 (Galβ1,3GlcNAc) or Type 2 (Galβ1,4GlcNAc) disaccharides on N-linked and O-linked glycans (Allen et al., 1998; Karmakar et al., 2008). Gal-1 ligands are expressed on activated lymphocytes and, upon their binding to Gal-1, transmit pro-apoptotic signals or promote regulatory cytokine production (Perillo et al., 1995; Toscano et al., 2007). Ligands identified to date include CD4, CD7, CD43 and CD45 among others (Pace et al., 1999). Because anti-tumor effector T cells express a high level of Gal-1 ligands, their viability and function is compromised in tumor microenvironments rich in Gal-1. In fact, when tumor-derived Gal-1 is abrogated, enhanced levels of IFN-γ-producing cells help prevent tumor progression (Ilarregui et al., 2009; Rubinstein et al., 2004); similarly, when Gal-1 ligands (e.g. CD43) are absent, tumor growth is impaired (Fuzii and Travassos, 2002).
Here, we show that limiting Gal-1-binding to N-acetyllactosamines on T cell membrane proteins with a well-characterized metabolic inhibitor of N-acetyllactosamines synthesis, 4-F-GlcNAc (Barthel et al., 2011; Descheny et al., 2006; Dimitroff et al., 2003b; Gainers et al., 2007; Woynarowska et al., 1996; Woynarowska et al., 1994), attenuated growth of B16 melanomas and EL-4 lymphomas through enhancement of anti-tumor immune responses. 4-F-GlcNAc treatment inhibited Gal-1-binding N-acetyllactosamine formation on effector T and NK cells, which prevented Gal-1-induced apoptosis and caused concomitant increases in melanoma-specific CTLs and IFN-γ levels. Overall, metabolic antagonism of Gal-1 ligand formation strengthens the pharmacologic concept that interfering with Gal-1 – Gal-1 ligand axis could result in an effective cancer immunotherapy.
Results
Gal-1 and Gal-1 ligands are major determinants of host immune response to melanoma
To validate the role of Gal-1 in tumor immune evasion, we engineered B16 melanoma cell lines that over-expressed Gal-1 (B16-Gal-1hi) or that were silenced for Gal-1 expression (B16-Gal-1KD). B16-Gal-1hi cells expressed 30% more Gal-1 than wild type (wt) cells, and were characterized by a Gal-1 molecule that is fused to a human immunoglobulin Fc domain to impart constitutively-active dimeric form (Gal-1hFc) (Cedeno-Laurent et al., 2010) (Figure 1a and S1a). Conversely, B16-Gal-1KD cells, produced by RNAi, exhibited 95% less Gal-1 (Figure 1a and S1a). Notably, all cell lines exhibited similar growth activity (Figure S1b).
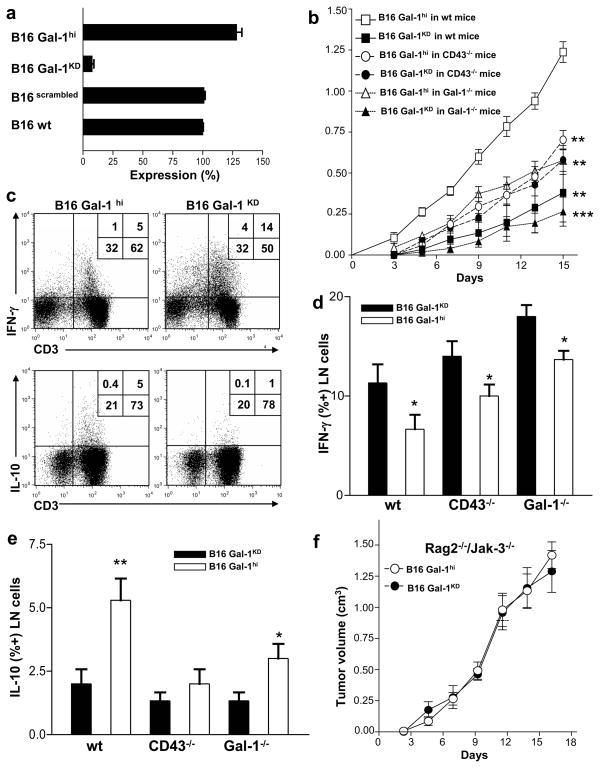
Figure 1. Gal-1 and Gal-1 ligands promote melanoma immune evasion.
(a) Gal-1 expression was quantified by qRT-PCR in B16 Gal-1hi or B16 Gal-1KD cells. (b) Tumor growth (mean±S.D) in mice inoculated with B16-Gal-1hi or B16-Gal-1KD cells (n=15) was assayed. Statistically significant differences compared with B16 Gal-1hi tumors grown in wt mice by Student’s paired t-test, **p<0.001 and ***p<0.0001. T cells from B16 Gal-1hi or B16 Gal-1KD-draining LNs were analyzed for IFN-γ and IL-10 by flow cytometry (c) and are graphically represented in (d) and (e). Statistically significant differences compared with B16-Gal-1KD tumors by Student’s paired t-test, *p<0.01, **p<0.001. (f) Tumor growth (mean±SD) was assayed in Rag2−/−/Jak3−/− mice (n=15) inoculated with B16 Gal-1hi or B16 Gal-1KD cells. Experiments were repeated 3-times.
Subcutaneous inoculation into C57/BL6 mice demonstrated B16-Gal-1hi tumor growth >1cm3 within the first 15 days post-injection, whereas mice injected with B16-Gal-1KD cells developed significantly smaller tumors (p<0.001) (Figure 1b). Importantly, B16-Gal-1KD and B16-Gal-1hi tumors conserved their phenotype 15 days post inoculation (Figure S1c). To determine the impact of Gal-1 ligands in controlling B16 melanoma growth, we performed identical studies in mice lacking CD43, a putative Gal-1 ligand. CD43, a leukocyte membrane protein that displays core 2 O-glycans upon T cell activation (Daniels et al., 2002), has been shown to favor Gal-1-mediated pro-apoptotic activity and promote Gal-1-driven tolerogenic features on antigen-presenting cells (Ilarregui et al., 2009; Pace et al., 1999). CD43−/− mice inoculated with B16-Gal-1hi cells grew smaller tumors compared to wt mice (p<0.001), but abrogation of tumor-derived Gal-1 did not show an additive tumor-suppressive effect, suggesting that CD43 is an important component of Gal-1-mediated immune escape (Figure 1b). Alternatively, Gal-1−/− mice inoculated with B16-Gal-1hi tumors also developed smaller tumors than wt mice, while those inoculated with B16-Gal-1KD exhibited negligible growth (p<0.0001) (Figure 1b), suggesting a role for host-derived Gal-1 in tumor immune evasion. In all, B16-Gal-1KD-draining lymph nodes (LN) exhibited higher levels of IFN-γ-producing cells, and lower levels of IL-10 than found in LN draining B16-Gal-1hi tumors (Figure 1c). These effects were more pronounced in CD43−/− mice and Gal-1−/− mice (Figure 1d and e), suggesting that Gal-1 ligand expression and host-derived Gal-1 are key components in the formation of immune-privileged sites in cancer. Notably, in Rag2- and Janus kinase (Jak)3-doubly deficient mice, which lack in B, T and NK cells (Barthel et al., 2009), tumors grew identically, independent of their Gal-1 level (Figure 1f). These data suggest as similarly shown (Banh et al., 2011), that Gal-1-mediated immune regulation plays a critical role in controlling tumor growth juxtaposed to other pro-tumorigenic activities, such as angiogenesis.
A peracetylated 4-fluorinated analog of glucosamine (4-F-GlcNAc) disrupts Gal-1 ligand synthesis sparing T cells from Gal-1-mediated cell death
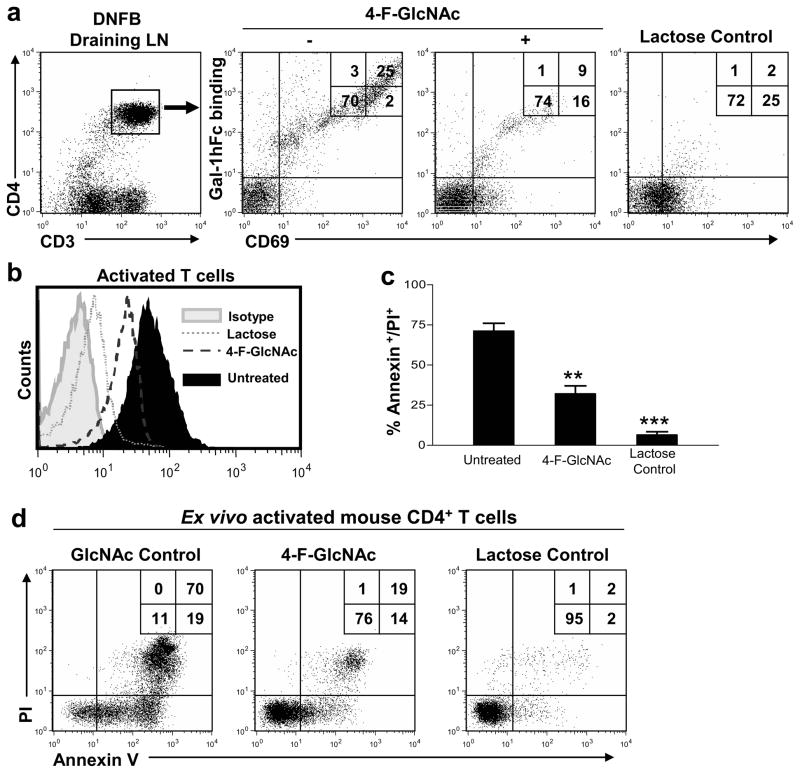
To determine whether lowering Gal-1 ligand expression could avert Gal-1-mediated effects and boost anti-tumor immunity, we utilized a DNFB-induced T cell activation model in mice treated with a fluorinated sugar analog of N-acetyllactosamine biosynthesis, peracetylated 4-fluorinated glucosamine (4-F-GlcNAc). 4-F-GlcNAc has been shown to inhibit N-acetyllactosamine and Gal-1-binding determinant formation on membrane proteins expressed by T cells (Barthel et al., 2011) and on ovarian and colon cancer cells (Descheny et al., 2006; Dimitroff et al., 2003a; Dimitroff et al., 2003b; Gainers et al., 2007; Woynarowska et al., 1996; Woynarowska et al., 1994). Stability analysis of 4-F-GlcNAc in mouse plasma showed that the parent compound, fully acetylated 4-F-GlcNAc, exhibited a half life of ~2.3h, sufficient for uptake by relevant immune cells (Figure S1d). Previous immunofluorescence analyses of DNFB-draining LNs showed that non-activated T cells did not bind Gal-1, whereas robust Gal-1hFc staining was evident on T cells expressing the early activation marker, CD69 (Cedeno-Laurent et al., 2010). In contrast, we found here that Gal-1hFc binding on activated T cells of 4-F-GlcNAc-treated mice was markedly reduced, while the number of CD69+ cells remained unchanged (Figure 2a). In all assays, use of lactose-containing buffers confirmed β-galactoside-dependent binding activity of Gal-1hFc. To assay whether inhibiting Gal-1-binding N-acetyllactosamines with 4-F-GlcNAc also prevented Gal-1-mediated apoptosis, T cells activated ex-vivo in the presence or absence of 4-F-GlcNAc were incubated with Gal-1hFc with or without competitive inhibitor, lactose. 4-F-GlcNAc-treated T cells exhibited a significant reduction in Gal-1-binding N-acetyllactosamines, and a marked resistance to Gal-1-mediated apoptosis (p<0.001) (Figures 2b–d).
Figure 2. 4-F-GlcNAc-mediated reduction of Gal-1-binding N-acetyllactosamines prevents Gal-1-mediated T cell death.
(a) Gated CD4+ T cells from DNFB-draining LNs in mice treated with 4-F-GlcNAc or GlcNAc control were analyzed for Gal-1hFc binding and CD69 expression by flow cytometry. (b) Naïve CD4+ T cells were activated ex vivo for 48h +/− 15μM 4-F-GlcNAc. Cell binding to Gal-1hFc was analyzed in the presence or absence of lactose by flow cytometry. (c) Activated CD4+ T cells were incubated with 2.5μM Gal-1hFc for 24h, and cell death was evaluated by Annexin V staining/PI uptake and graphically represented from 3 experiments in (d). Statistically significance difference compared with untreated cells by Student’s paired t-test, **p<0.001 and ***p<0.0001.
4-F-GlcNAc treatment decreases melanoma growth and reduces Gal-1 binding to T and NK cells
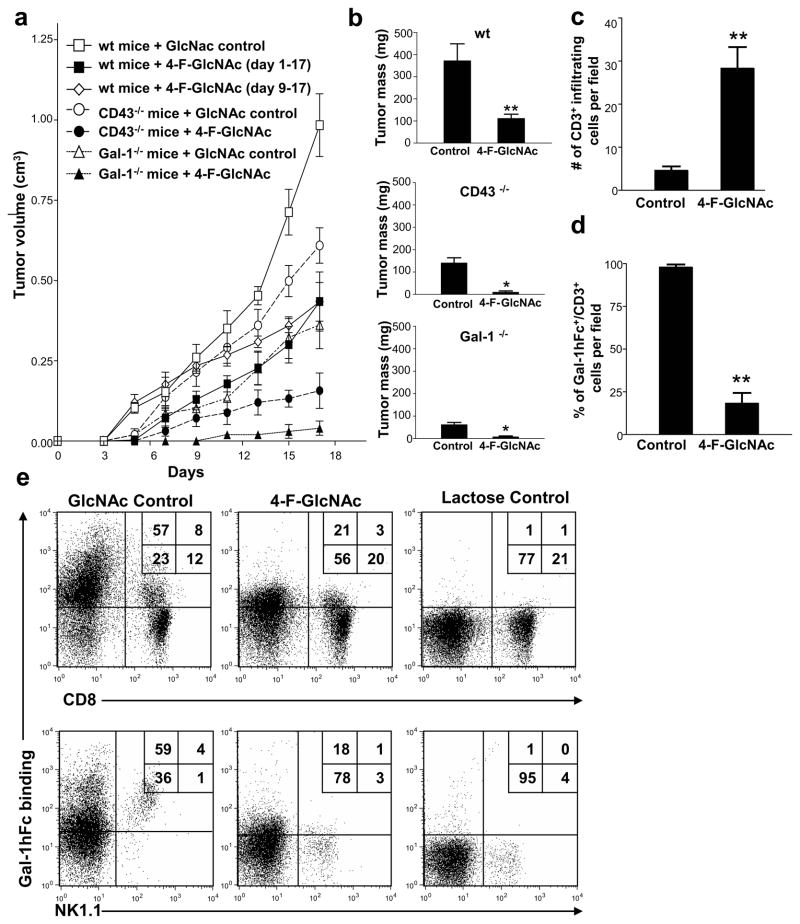
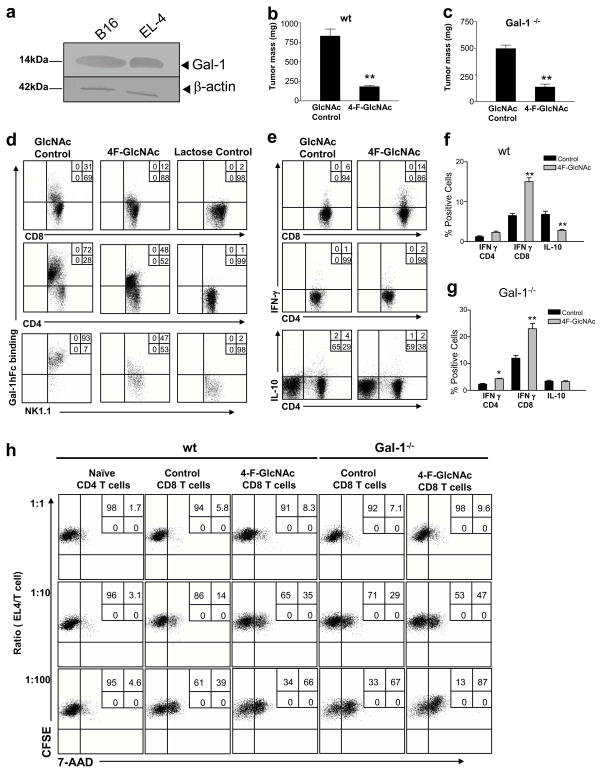
To ascertain whether 4-F-GlcNAc-dependent lowering of Gal-1-binding N-acetyllactosamines on activated T cells translated to enhanced anti-tumor immunity, mice were inoculated with B16 wt cells and treated with 4-F-GlcNAc or GlcNAc control for 17 days. Compared with control treatment, 4-F-GlcNAc significantly inhibited tumor growth (Figures 3a and b) (p<0.001). In established tumors, 4-F-GlcNAc treatment from days 9–17 also attenuated tumor growth (p<0.001) (Figure 3a). When 4-F-GlcNAc treatment was evaluated in CD43- or Gal-1-deficient mice bearing B16 tumors, tumor growth was almost completely prevented (p<0.01) (Figures 3a and b). To validate 4-F-GlcNAc-mediated enhancement of anti-tumor immune responses, we analyzed the presence of tumor-infiltrating T cells by immunostaining formalin-fixed paraffin-embedded tumors with Gal-1hFc and anti-CD3 mAb. Notably, control-treated mice showed minimal tumor-lymphocytic infiltration (Figures 3c and S1e) with 90.4% ± 3.1 of CD3+ cells expressing Gal-1 ligands (Figure 3d and S1e). Conversely, 4F-GlcNAc-treated mice exhibited 5.4-fold more tumor-infiltrating CD3+ cells than control-treated mice (Figures 3c and S1f) and most T cells did not bind to Gal-1hFc (18.3% ± 6.4) (Figures 3d and S1f). To validate modification of Gal-1-binding N-acetyllactosamine expression on anti-tumor immunocytes of 4-F-GlcNAc-treated mice, tumor-draining LN were harvested and flow cytometry analyses revealed significant inhibition of Gal-1 binding determinants on T and NK cells (*p<0.01 or **p<0.001) (Figures 3e and S2a), and that lack of membrane protein CD43 markedly lowered Gal-1-binding on both cell types (Figure S2a).
Figure 3. 4-F-GlcNAc treatment blunts melanoma growth and interferes with Gal-1-binding to T and NK cells.
(a) Tumor growth was assayed in mice inoculated with B16 cells and treated with 4-F-GlcNAc or control, starting from days 1 or 9 (n=15/group). (b) Tumor masses (mean ± SD) were calculated on day 17 for each mouse group. (c) Melanoma-infiltrating T cells were quantified in 8 non-overlapping 20× fields from control or 4-F-GlcNAc-treated mice (n=4). (d) Percentage of melanoma-infiltrating T cells binding Gal-1hFc were quantified. (e) Cells from melanoma-draining LNs of 4-F-GlcNAc or control-treated mice were gated on TCR-β and analyzed for CD8 expression and Gal-1hFc binding or gated on TCR-β(−) cells and analyzed for NK1.1 expression and Gal-1hFc binding by flow cytometry. Statistically significant differences compared with control treatments on day 17 by Student’s paired t-test, *p<0.01 and **p<0.001. Data represents mean ± SD of 3 independent experiments.
4-F-GlcNAc inhibitory efficacy on melanoma growth is driven by higher levels of anti-melanoma CTLs and lower levels of IL-10
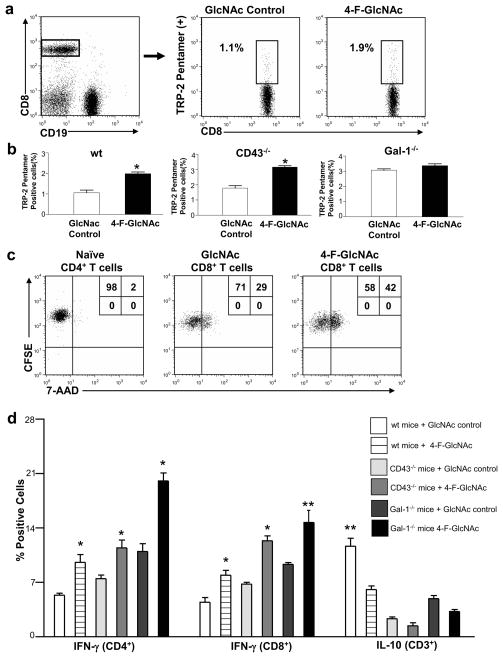
To show that tumor-specific CTL death was reduced in 4-F-GlcNAc-treated mice, we stained cells from tumor-draining LN with a pentamer recognizing the B16-melanoma epitope TRP-2 (180-188/H2Kb) TCR-specific CTLs (Wells et al., 2008). Indeed, we found that TRP-2-specific CTLs were elevated 2-fold compared with GlcNAc control treatment, and that tumor-bearing CD43−/− and Gal-1−/− mice expressed 3-fold greater levels of TRP-2 specific CTLs compared to control treated wt mice (p<0.01) (Figures 4a–b and S2b), fortifying Gal-1’s role in regulating anti-tumor CTL levels. Additionally, we sorted non-naïve (CD62L−) CTLs from tumor draining LN, and co-cultured them ex vivo at incremental ratios of CFSE-labeled B16 tumor cells. At 6h post-exposure, we found that CTLs from 4-F-GlcNAc-treated mice induced greater tumor cytotoxicity, as determined by 7-AAD uptake than controls (p<0.05) (Figure 4c and S2c). Notably, increased cytotoxicity paralleled the amount of CTLs present in the B16/T cell mixed culture, while no significant tumor cell death was observed in naïve T cell co-cultures (Figure 4c and S2c). These data suggested that attenuated B16 tumor growth rates in 4-F-GlcNAc-treated, Gal-1−/− and/or CD43−/− mice correlated with higher levels of anti-tumor CTLs.
Figure 4. 4-F-GlcNAc treatment increases melanoma-specific CTLs and enhances the level of anti-tumor effector molecule, IFN-γ.
(a) CD8+/CD19− T cells from melanoma-draining LNs of 4-F-GlcNAc- or control-treated mice on day 17 were stained with TRP-2 pentamer and (b) represented graphically from 3 different experiments. (c) CD62L− CTLs or naïve T cells from 4-F-GlcNAc-treated or control-treated melanoma-bearing mice were co-cultured with CFSE-labeled B16 cells; and after 6h, cells were stained with 7-AAD and assayed for CFSE+ cell viability. (d) CD4+ and CD8+ T cells from melanoma-draining LNs of 4-F-GlcNAc- or control-treated mice on day 17 were analyzed for IFN-γ and IL-10 and graphically represented from 3 different experiments. Statistically significant difference compared with GlcNAc control group by Student’s paired t-test, *p<0.01 or **p<0.001.
To exclude whether 4-F-GlcNAc-dependent decrease in N-acetyllactosamine synthesis could be altering tumor growth by immune-independent mechanisms, we performed identical experiments in Rag2−/−/Jak3−/− mice lacking B, T and NK cells, and found that B16 tumor growth rates were identical (Figure S2d), suggesting that altering Gal-1-binding N-acetyllactosamine synthesis on effector anti-tumor immunocytes with 4-F-GlcNAc directly translated into enhanced anti-tumor responses.
We and others have shown that Gal-1 not only promotes apoptosis on effector immune cells, but can also promote IL-10 cytokine secretion (Cedeno-Laurent et al., 2010; Ilarregui et al., 2009; Juszczynski et al., 2007; Motran et al., 2008; van der Leij et al., 2007). Therefore, we hypothesized that by abrogating Gal-1-binding N-acetyllactosamine formation on effector T cells could prevent Gal-1-mediated IL-10 production and perhaps increase levels of IFN-γ, a key determinant in T cell-directed anti-melanoma responses (Kortylewski et al., 2004; Rubinstein et al., 2004). Accordingly, 17 days post-injection, we analyzed T cell cytokine profiles of B16 tumor-draining LN in 4-F-GlcNAc-and control-treated mice. Compared with control animals, 4-F-GlcNAc-treated mice exhibited significantly higher levels of IFN-γ+ T cells (p<0.01) (Figures 4d and S2e and f). Similar elevations in IFN-γ were observed in CD43- and Gal-1-deficient mice treated with 4-F-GlcNAc, even though baseline levels of IFN-γ in control groups were higher than those in wt mice (Figures 4d and S2e and f). In contrast, IL-10+ T cells were significantly diminished in mice treated with 4-F-GlcNAc (p<0.001) (Figures 4d and S2g). Of note, the number of IL-10+ cells from control treated CD43- and Gal-1-deficient mice were markedly decreased compared with wt mice (Figures 4d and S2g), confirming the role of both molecules in Gal-1-mediated induction of IL-10 synthesis. In addition, to rule out the possibility that 4-F-GlcNAc had a cytotoxic effect on regulatory T cells, which produce IL-10, we analyzed the expression of the transcription factor FoxP3 in CD4+ T cells from tumor draining LNs of 4-F-GlcNAc-treated mice and found no significant changes in their level (Figure S2h).
4-F-GlcNAc treatment similarly inhibits lymphoma growth by increasing IFN-γ+ CTLs and lowering IL-10+ T cells
To strengthen observations of 4-F-GlcNAc efficacy on effector anti-tumor immune cells and corresponding anti-B16 melanoma activity, we performed identical experiments in mice bearing Gal-1-expressing EL-4 lymphomas (Figure 5a). As expected, there was a dramatic reduction in EL-4 tumor size in 4-F-GlcNAc-treated (wt and Gal-1−/−) mice compared with those in GlcNAc-treated mice (p<0.001) (Figures 5b and c). We found that T and NK cells from tumor-draining LN showed diminished expression of Gal-1-binding N-acetyllactosamines after 4-F-GlcNAc treatment (Figure 5d), with concomitant increased levels of IFN-γ levels compared to control-treated mice (Figures 5e and f). Conversely, IL-10+ T cells were significantly diminished in 4-F-GlcNAc-treated wt and in Gal-1−/− mice (p<0.001) (Figures 5e and f). Accordingly, we observed enhanced tumor cytolytic activity in co-cultures of EL-4 tumor cells and CTLs cells from tumor-draining LN of wt or Gal-1−/− mice treated with 4-F-GlcNAc (Figures 5g). These data corroborated 4-F-GlcNAc inhibitory effects on B16 tumor growth and stimulatory effects on effector anti-tumor immunocyte levels.
Figure 5. 4-F-GlcNAc enhances anti-tumor immunity against EL-4 lymphomas.
(a) Gal-1 expression on B16 and EL-4 cells was confirmed by immunoblotting. Tumor masses (mean ± SD) from wt (b) or Gal-1−/− (c) mice treated with 4-F-GlcNAc or control (n=6/group) is represented graphically. (d) Cells from EL-4-draining LNs were stained for CD4, CD8, NK1.1 and Gal-1 ligands. (e) Cells from EL-4-draining LNs cells were stained for CD4, CD8, IFN-γ and IL-10 and graphically represented in (f) and (g). (h) Sorted CD62L− CTLs from 4-F-GlcNAc-treated or GlcNAc control-treated mice bearing EL-4 tumors (n=5/group) were co-cultured with CFSE-labeled EL-4 cells; and after 6hr, cells were stained with 7-AAD and assayed for CFSE+ cell viability. Statistically significant difference compared with GlcNAc controls by Student’s paired t-test, *p<0.01, **p<0.001. Experiments were repeated 3-times.
Discussion
Emerging evidence suggests that Gal-1 aids in establishing immune privilege in cancer (Rubinstein et al., 2004). Gal-1 has been shown to hamper the vitality of effector T cells and production of anti-tumor cytokine, IFN-γ, while inducing the expression of IL-10+ regulatory T cells (Juszczynski et al., 2007; Kortylewski et al., 2004; Rubinstein et al., 2004). To analyze the dependence of Gal-1-binding N-acetyllactosamines in Gal-1-mediated tumor immune evasion, we used a fluoro-glucosamine inhibitor of N-acetyllactosamine formation, 4-F-GlcNAc. This inhibitor prevents N-acetyllactosamine synthesis on N- and O-glycans in glyco-metabolically active immune cells and prevents consequent lectin-binding activity with relatively high specificity for Gal-1-binding determinants (Barthel et al., 2011; Descheny et al., 2006; Dimitroff et al., 2003a; Dimitroff et al., 2003b; Gainers et al., 2007; Woynarowska et al., 1996; Woynarowska et al., 1994). Importantly, in this report, we show that diminution of Gal-1 binding on activated T cells via 4-F-GlcNAc treatment can alleviate Gal-1-mediated T cell apoptotic activity. This observation indicated that effector anti-tumor T cells in 4-F-GlcNAc-treated mice could expand without restriction from a Gal-1 checkpoint, favoring effective anti-tumor immune activity.
To evaluate 4-F-GlcNAc-mediated anti-tumor efficacy in mice bearing syngeneic tumors whose growth and progression is dependent on effector T cell suppression controlled in part by Gal-1 (Rubinstein et al., 2004), we treated tumor-bearing mice with N-acetyllactosamine-lowering doses of 4-F-GlcNAc and used mice deficient in candidate Gal-1 ligand, CD43. We found that tumor growth was significantly reduced and paralleled concomitant lower levels of Gal-1-binding N-acetyllactosamine on effector T cells and NK cells, elevations in IFN-γ+ and melanoma-specific CTLs, and reductions in IL-10+ T cells. Furthermore, increased tumor cytolytic activity was observed on tumor cells co-cultured with non-naïve CTLs from 4-F-GlcNAc-treated tumor-bearing mice. Additionally, we found that established tumors were also sensitive to 4-F-GlcNAc treatment, suggesting that anti-tumor immunity can be stimulated even after effector T cell subset differentiation has been imprinted in tumor-draining LN. Since Gal-1 has been shown to stimulate tumor angiogenesis and enhance metastatic potential (Thijssen et al., 2010; Thijssen et al., 2008; Thijssen et al., 2006), inhibition of Gal-1-binding N-acetyllactosamine synthesis on tumor endothelial cells could be a reason for suppressed tumor growth. However, 4-F-GlcNAc was ineffective at slowing tumor growth in immunodeficient mice, implying that 4-F-GlcNAc-dependent immune cell protection may be more influential than on Gal-1-binding N-acetyllactosamine-dependent tumor angiogenesis. This interpretation is supported by similar observations recently made on a lung carcinoma mouse model (Banh et al., 2011). Our collective results suggest that 1.) Gal-1-binding N-acetyllactosamines are targetable moieties on effector Th cells, CTLs and NK cells, 2.) CD43 is one of the membrane proteins transmitting Gal-1-mediated immunoregulation and 3.) 4-F-GlcNAc treatment can interfere with N-acetyllactosamine formation to prevent Gal-1-mediated apoptosis and raise the level of anti-tumor immune cells.
Because tumor endothelial and stromal cells have been shown also to express Gal-1, it is possible that host-derived Gal-1 might contribute to facilitate tumor immune evasion (Thijssen et al., 2008; Thijssen et al., 2006). To this end, we investigated tumor growth in Gal-1−/− mice. We show that tumor growth was attenuated in Gal-1−/− mice and, compared with wt mice, displayed higher levels of IFN-γ, melanoma-specific CTLs, and suppressed expression of IL-10+ T cells. Of note, when 4-F-GlcNAc treatment was included in tumor-bearing Gal-1−/− mice, the tumors were even smaller and effector T cell populations were higher, suggesting that to effectively interfere with the Gal-1 – Gal-1 ligand axis, Gal-1 from the host and the tumor or all prospective Gal-1 ligands need to be targeted to achieve maximal anti-tumor immune efficacy.
Because N-acetyllactosamines are key moieties for sialyl Lewis X and platelet (P)-/leukocyte (L)- and endothelial (E)-selectin ligand formation, 4-F-GlcNAc treatment can also interfere with the capacity of T cells to traffic to inflamed sites. Indeed, 4-F-GlcNAc can alleviate T cell recruitment to inflamed skin by interfering with E-selectin ligand synthesis (Descheny et al., 2006; Dimitroff et al., 2003a; Dimitroff et al., 2003b; Gainers et al., 2007). In studies performed here, while we demonstrate that Gal-1-binding N-acetyllactosamines are significantly lowered on effector T cells following 4-F-GlcNAc treatment, this inhibitory effect did not interfere with the ability of these cells to traffic to tumors. Unlike inflammation in the skin, which requires E- and P-selectin-binding activity on inflammatory leukocytes (Catalina et al., 1999; Hirata et al., 2002; Staite et al., 1996), the tumor vasculature, particularly in melanomas, does not express E-selectin or P-selectin (Weishaupt et al., 2007). That is, 4-F-GlcNAc-treated tumor-infiltrating T cells could mobilize to melanomas independent of E- and P-selectin-dependent binding activity and elicit anti-tumor activity. Furthermore, recent data from our laboratory indicate that E-selectin-binding carbohydrate determinants, which include N-acetyllactosamines displayed by both membrane glycoproteins and glycolipids, are more difficult to eliminate by 4-F-GlcNAc treatment than Gal-1-binding N-acetyllactosamines, which are found only on membrane glycoproteins (Barthel et al., 2011). This is likely due to several observations showing that 4-F-GlcNAc treatment does not interfere with E-selectin-binding determinants and N-acetyllactosamine formation on glycolipids (Barthel et al., 2011; Descheny et al., 2006; Dimitroff et al., 2003a; Dimitroff et al., 2003b; Gainers et al., 2007). In addition, we have found that 4-F-GlcNAc treatment does not greatly affect L-selectin ligand expression on high endothelial venules and alter the distribution of pattern of naïve lymphocytes to secondary lymphoid organs (Dimitroff et al., 2003b). Thus, endothelial cells do not appear to be particularly sensitive to glyco-metabolic modulation in vivo.
Interestingly, while N-acetyllactosamines can serve as binding-partners for other galectins, tumor growth in both murine tumor models used in this report shows a high dependency on Gal-1 expression. Nevertheless, data regarding pro-tumorigenic activity of other pro-apoptotic β-galactoside-binding lectins, such as Gal-9, remain controversial. While Gal-9 has been shown to enhance anti-tumor immunity via dendritic cell and monocyte stimulation during early stages of anti-tumor immune responses (Nagahara et al., 2008), recent reports suggest that, at later phases, Gal-9 induces T cell exhaustion through TIM-3 signaling, favoring the progression of solid and hematopoietic malignant tumors (Sakuishi et al., ; Zhou et al.).
In summary, this report highlights the critical role of Gal-1 in melanoma immunity and established for the first time that boosting anti-tumor immune responses via modifications of Gal-1-binding N-acetyllactosamines can be an effective mode of cancer treatment.
Materials and Methods
Mice, Cell lines, Antibodies and Chemicals
C57BL/6 wild type (wt) mice and Gal-1−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 CD43−/− mice were kindly provided by Dr. Hermann J. Ziltener (University of British Columbia, British Columbia, Canada). Immunodeficient mice lacking B, T and NK cells that deficient in Rag2 and Jak3 genes (Rag2−/−/Jak3−/−) were generated as described (Barthel et al., 2009). J558L plasmacytoma cells and EL-4 cells were purchased from American Type Culture Collection (ATCC, Inc., Manassas, VA). All mouse experiments were approved by IACUC of Harvard Center for Comparative Medicine. B16F10 melanoma cells were a kind gift from Dr. Hans Widlund (Brigham and Women’s Hospital). Antibodies included: anti-mouse/human Gal-1 (N16) and anti-mouse β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); anti-goat IgG (Southern biotech, Inc., Birmingham, AL); anti-human Fc (Jackson Immunoresearch, Inc., West Grove, PA); anti-mouse CD4, CD3, CD8, CD16, CD19, CD69, NK1.1, IL-4, IL-10, IL-17, IFN-γ, Annexin-V, and propidium iodide (PI) (Biolegend, Inc., San Diego, CA); TRP-2 pentamer (Proimmune, Springfield, VA), 2-Acetamido-1,3,6-tri-O-acetyl-4-deoxy-4-fluoro-D-glucopyranose, 4-F-GlcNAc, was prepared as described (Sharma et al., 1990; Woynarowska et al., 1996; Woynarowska et al., 1994). N-acetylglucosamine was purchased from Sigma Chemical, Inc. (St. Louis, MO).
Production of B16 Melanoma Gal-1hi Cells
B16 melanoma cells were transfected with a previously reported plasmid encoding Gal-1 fused in frame to the Fc portion of human IgG (Gal-1hFc)(Cedeno-Laurent et al., 2010). Gal-1hi cells were selected in Zeocin, cloned and analyzed for Gal-1 expression by western blot and real time RT-PCR using the primers: Gal-1 F: 5′ TCTCAAACCTGGGGAATGTC 3′, R: 5′ GCGAGGATTGAAGTGTAGGC 3′ and β-actin F: 5′ CATCGTACTCCTGCTTGCTG 3′, R 5′ AGCGCAAGTAC TCTGTGTGG. Samples were analyzed in triplicate and normalized to β-actin expression
Production of B16 Melanoma Gal-1KD Cells by RNA Silencing
The pLKO.1 vector encoding a short-hairpin RNA (shRNA) sequence against mouse Gal-1 (NM_008495) or encoding a non-silencing, scrambled shRNA control sequence was obtained from the RNAi Consortium (TRC) of the Broad Institute (MIT, Cambridge, MA) or from Addgene (Cambridge, MA), respectively. B16 melanoma cells were transduced with shRNA-expressing lentivirus and then further selected. The target 21mer sequence of mouse Gal-1 recognized by the shRNA was: 5′-CCTACACTTCAATCCTCGCTT-3′. Gal-1 knockdown was confirmed by WB and by quantitative RT-PCR (qRT-PCR) analysis with the following primer sequences: F, 5′-TCTCAAACCTGGGGAATGTC-3′, R, 5′-GCGAGGATTGAAGTGTAGGC-3′.
Production of Gal-1hFc
Gal-1hFc and non-β-galactoside-binding mutant (dmGal-1hFc) containing the non-Fc receptor FcγRI-binding variant (pFUSE-hIgG1e3-Fc2; InvivoGen, Inc.) were prepared as described (Cedeno-Laurent et al., 2010).
Gal-1 Ligand Expression Analysis
Cells were analyzed for Gal-1 ligand expression using Gal-1hFc or dmGal-1hFc as previously described (Cedeno-Laurent et al., 2010). Experiments were performed in triplicate.
Treatment of Mice bearing Tumors with 4-F-GlcNAc
B16 melanoma cell variants were injected subcutaneously (2×105) into the right flank of 6-week old C57BL/6 wt, CD43−/−, Gal-1−/− or Rag2−/−/Jak3−/− mice (n=15 for each group, 5 mice per experiment). Tumor development was monitored as described (Rubinstein et al., 2004). Intraperitoneal injections of 4-F-GlcNAc or GlcNAc control (Sigma Chemicals; St. Louis, MO) (each at 100mg/kg) were performed every other day starting on day 1 or 9 post-injection. Tumor-draining lymph nodes were retrieved for FACS analysis.
Western Blotting
Gal-1 and β-actin was immunoblotted as previously described (Cedeno-Laurent et al., 2010).
In vivo T cell Activation
Abdominal skin of C57/B6 mice was painted on day 0 and day 1 with 25μL of 0.5% 2,4-dinitrofluorobenzene (DNFB) (Sigma) in a 4:1 acetone:olive oil vehicle. Mice were treated with 100mg/kg 4-F-GlcNAc or GlcNAc control on days 1, 3 and 5 post-sensitization and LN were harvested on day 6 for analysis by flow cytometry.
Pentamer Staining
Tumor-draining LN were harvested on day 17 and minced, and single cell suspensions were blocked with anti-mouse CD16 (FcRγ) (1:100) for 10 minutes on ice, and immediately stained with 1 test PE-conjugated TRP-2 pentamers, for 10 minutes at room temperature. Cells were further stained and negatively gated on CD19 and CD8 mAbs.
Tumor cytotoxicity assays
CD62L−/CD8+ T cells from tumor-draining LN were isolated from 4-F-GlcNAc-treated or control-treated mice 17 days post-tumor inoculation by immunomagnetic bead negative selection for CD62L and positive selection for CD8+ T cells (Miltenyi Biotec, Auburn, CA). Sorted CD62L−/CD8+ T cells or naïve CD4+ T cells were plated at incremental ratios with B16 or EL-4 tumor cells labeled with CFSE in 96-well round bottom plates. After 6h incubation, cells were stained with 7-Amino-Actinomycin D (7-AAD) for 10 minutes and analyzed by flow cytometry.
Statistical Analyses
Statistical significance was ascertained between 2 groups using a Student’s paired t-test.
Supplementary Material
Acknowledgments
F.C.L designed the research, performed the experiments, analyzed the data and wrote the paper. M.O. designed the research, performed the experiments, and analyzed the data. S.R.B. designed the research, performed the experiments and analyzed the data. D.H. performed the experiments and analyzed the data. K.L.M. provided reagents. J.G.S. performed the experiments and analyzed the data. X.H. performed the experiments and analyzed the data. M.H.F. performed the experiments and analyzed the data. G.F.M. performed the experiments and analyzed the data. T.S. performed the experiments and analyzed the data. Q.Z. performed the experiments and analyzed the data. C.J.D. conceived the study, designed the research, analyzed the data, wrote the paper and supervised all experimentation. This work was supported by an NIH/NCI RO1 grant CA118124 to C. Dimitroff and an NIH/NCCAM RO1 grant AT004268 to C. Dimitroff.
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Allen HJ, Ahmed H, Matta KL. Binding of synthetic sulfated ligands by human splenic galectin 1, a beta-galactoside-binding lectin. Glycoconjugate journal. 1998;15:691–695. doi: 10.1023/a:1006988515346. [DOI] [PubMed] [Google Scholar]
- Andre S, Kojima S, Yamazaki N, et al. Galectins-1 and -3 and their ligands in tumor biology. Non-uniform properties in cell-surface presentation and modulation of adhesion to matrix glycoproteins for various tumor cell lines, in biodistribution of free and liposome-bound galectins and in their expression by breast and colorectal carcinomas with/without metastatic propensity. J Cancer Res Clin Oncol. 1999;125:461–474. doi: 10.1007/s004320050303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banh A, Zhang J, Cao H, et al. Tumor Galectin-1 Mediates Tumor Growth and Metastasis through Regulation of T-Cell Apoptosis. Cancer research. 2011;71:4423–4431. doi: 10.1158/0008-5472.CAN-10-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel SR, Antonopoulos A, Cedeno-Laurent F, et al. Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. The Journal of biological chemistry. 2011;286:21717–21731. doi: 10.1074/jbc.M110.194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel SR, Wiese GK, Cho J, et al. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19491–19496. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]
- Cedeno-Laurent F, Barthel SR, Opperman MJ, et al. Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation. J Immunol. 2010;185:4659–4672. doi: 10.4049/jimmunol.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausse N, van den Brule F, Waltregny D, et al. Galectin-1 expression in prostate tumor-associated capillary endothelial cells is increased by prostate carcinoma cells and modulates heterotypic cell-cell adhesion. Angiogenesis. 1999;3:317–325. doi: 10.1023/a:1026584523789. [DOI] [PubMed] [Google Scholar]
- Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- Descheny L, Gainers ME, Walcheck B, et al. Ameliorating skin-homing receptors on malignant T cells with a fluorosugar analog of N-acetylglucosamine: P-selectin ligand is a more sensitive target than E-selectin ligand. The Journal of investigative dermatology. 2006;126:2065–2073. doi: 10.1038/sj.jid.5700364. [DOI] [PubMed] [Google Scholar]
- Dimitroff CJ, Bernacki RJ, Sackstein R. Glycosylation-dependent inhibition of cutaneous lymphocyte-associated antigen expression: implications in modulating lymphocyte migration to skin. Blood. 2003a;101:602–610. doi: 10.1182/blood-2002-06-1736. [DOI] [PubMed] [Google Scholar]
- Dimitroff CJ, Kupper TS, Sackstein R. Prevention of leukocyte migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen. The Journal of clinical investigation. 2003b;112:1008–1018. doi: 10.1172/JCI19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzii HT, Travassos LR. Transient resistance to B16F10 melanoma growth and metastasis in CD43−/− mice. Melanoma research. 2002;12:9–16. doi: 10.1097/00008390-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Gainers ME, Descheny L, Barthel SR, et al. Skin-homing receptors on effector leukocytes are differentially sensitive to glyco-metabolic antagonism in allergic contact dermatitis. J Immunol. 2007;179:8509–8518. doi: 10.4049/jimmunol.179.12.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Furie BC, Furie B. P-, E-, and L-selectin mediate migration of activated CD8+ T lymphocytes into inflamed skin. J Immunol. 2002;169:4307–4313. doi: 10.4049/jimmunol.169.8.4307. [DOI] [PubMed] [Google Scholar]
- Ilarregui JM, Croci DO, Bianco GA, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nature immunology. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- Ito K, Scott SA, Cutler S, et al. Thiodigalactoside inhibits murine cancers by concurrently blocking effects of galectin-1 on immune dysregulation, angiogenesis and protection against oxidative stress. Angiogenesis. 2011 doi: 10.1007/s10456-011-9213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung EJ, Moon HG, Cho BI, et al. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. International journal of cancer. 2007;120:2331–2338. doi: 10.1002/ijc.22434. [DOI] [PubMed] [Google Scholar]
- Juszczynski P, Ouyang J, Monti S, et al. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13134–13139. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S, Stowell SR, Cummings RD, et al. Galectin-1 signaling in leukocytes requires expression of complex-type N-glycans. Glycobiology. 2008;18:770–778. doi: 10.1093/glycob/cwn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Komyod W, Kauffmann ME, et al. Interferon-gamma-mediated growth regulation of melanoma cells: involvement of STAT1-dependent and STAT1-independent signals. The Journal of investigative dermatology. 2004;122:414–422. doi: 10.1046/j.0022-202X.2004.22237.x. [DOI] [PubMed] [Google Scholar]
- Motran CC, Molinder KM, Liu SD, et al. Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. European journal of immunology. 2008;38:3015–3027. doi: 10.1002/eji.200838295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara K, Arikawa T, Oomizu S, et al. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J Immunol. 2008;181:7660–7669. doi: 10.4049/jimmunol.181.11.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Juszczynski P, Rodig SJ, et al. Viral induction and targeted inhibition of galectin-1 in EBV+ posttransplant lymphoproliferative disorders. Blood. 2011;117:4315–4322. doi: 10.1182/blood-2010-11-320481. [DOI] [PubMed] [Google Scholar]
- Pace KE, Lee C, Stewart PL, et al. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
- Perillo NL, Pace KE, Seilhamer JJ, et al. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Ilarregui JM. Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol Rev. 2009;230:144–159. doi: 10.1111/j.1600-065X.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Liu FT, Hirashima M, et al. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand J Immunol. 2007;66:143–158. doi: 10.1111/j.1365-3083.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- Rubinstein N, Alvarez M, Zwirner NW, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Bernacki RJ, Paul B, et al. Fluorinated carbohydrates as potential plasma membrane modifiers. Synthesis of 4- and 6-fluoro derivatives of 2-acetamido-2-deoxy-D-hexopyranoses. Carbohydrate research. 1990;198:205–221. doi: 10.1016/0008-6215(90)84293-4. [DOI] [PubMed] [Google Scholar]
- Staite ND, Justen JM, Sly LM, et al. Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood. 1996;88:2973–2979. [PubMed] [Google Scholar]
- Stannard KA, Collins PM, Ito K, et al. Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model. Cancer Lett. 2010;299:95–110. doi: 10.1016/j.canlet.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Barkan B, Shoji H, et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer research. 2010;70:6216–6224. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Hulsmans S, Griffioen AW. The galectin profile of the endothelium: altered expression and localization in activated and tumor endothelial cells. The American journal of pathology. 2008;172:545–553. doi: 10.2353/ajpath.2008.070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen VL, Postel R, Brandwijk RJ, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano MA, Bianco GA, Ilarregui JM, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- van den Brule F, Califice S, Garnier F, et al. Galectin-1 accumulation in the ovary carcinoma peritumoral stroma is induced by ovary carcinoma cells and affects both cancer cell proliferation and adhesion to laminin-1 and fibronectin. Laboratory investigation; a journal of technical methods and pathology. 2003;83:377–386. doi: 10.1097/01.lab.0000059949.01480.40. [DOI] [PubMed] [Google Scholar]
- van der Leij J, van den Berg A, Harms G, et al. Strongly enhanced IL-10 production using stable galectin-1 homodimers. Mol Immunol. 2007;44:506–513. doi: 10.1016/j.molimm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Weishaupt C, Munoz KN, Buzney E, et al. T-cell distribution and adhesion receptor expression in metastatic melanoma. Clin Cancer Res. 2007;13:2549–2556. doi: 10.1158/1078-0432.CCR-06-2450. [DOI] [PubMed] [Google Scholar]
- Wells JW, Cowled CJ, Farzaneh F, et al. Combined triggering of dendritic cell receptors results in synergistic activation and potent cytotoxic immunity. J Immunol. 2008;181:3422–3431. doi: 10.4049/jimmunol.181.5.3422. [DOI] [PubMed] [Google Scholar]
- Woynarowska B, Dimitroff CJ, Sharma M, et al. Inhibition of human HT-29 colon carcinoma cell adhesion by a 4-fluoro-glucosamine analogue. Glycoconj J. 1996;13:663–674. doi: 10.1007/BF00731455. [DOI] [PubMed] [Google Scholar]
- Woynarowska B, Skrincosky DM, Haag A, et al. Inhibition of lectin-mediated ovarian tumor cell adhesion by sugar analogs. The Journal of biological chemistry. 1994;269:22797–22803. [PubMed] [Google Scholar]
- Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.