Abstract
The biological actions of retinoids are mediated by nuclear retinoic acid receptors (RARs) and retinoid × receptors (RXRs). We have recently reported that decreased expression of RARα and RXRα has an important role in high glucose (HG)-induced cardiomyocyte apoptosis. However, the regulatory mechanisms of HG effects on RARα and RXRα remain unclear. Using neonatal cardiomyocytes, we found that ligand-induced promoter activity of RAR and RXR was significantly suppressed by HG. HG promoted protein destabilization and serine-phosphorylation of RARα and RXRα. Proteasome inhibitor MG132 blocked the inhibitory effect of HG on RARα and RXRα. Inhibition of intracellular reactive oxidative species (ROS) abolished the HG effect. In contrast, H2O2 stimulation suppressed the expression and ligand-induced promoter activity of RARα and RXRα. HG promoted phosphorylation of ERK1/2, JNK and p38 MAP kinases, which was abrogated by an ROS inhibitor. Inhibition of JNK, but not ERK and p38 activity, reversed HG effects on RARα and RXRα. Activation of JNK by over expressing MKK7 and MEKK1, resulted in significant downregulation of RARα and RXRα. Ligand-induced promoter activity of RARα and RXRα was also suppressed by overexpression of MEKK1. HG-induced cardiomyocyte apoptosis was potentiated by activation of JNK, and prevented by ATRA and inhibition of JNK. Silencing the expression of RARα and RXRα activated the JNK pathway. In conclusion, HG-induced oxidative stress and activation of the JNK pathway negatively regulated expression/activation of RAR and RXR. The impaired RAR/RXR signaling and oxidative stress/JNK pathway forms a vicious circle, which significantly contributes to hyperglycemia induced cardiomyocyte apoptosis.
Keywords: High Glucose, Retinoid Receptor, Cardiomyocytes, Oxidative Stress, JNK
INTRODUCTION
Diabetic cardiomyopathy is represented by myocardial dysfunction in the absence of coronary artery disease and hypertension (Ho et al., 1993). The pathophysiology of diabetic cardiomyopathy is incompletely understood. Metabolic perturbations such as hyperglycemia, hyperlipidemia, hyperinsulinemia, and changes in cardiac metabolism seem to be central to the pathogenesis of diabetic cardiomyopathy and to trigger a series of maladaptive stimuli that result in increased oxidative stress, interstitial fibrosis, cell death, and altered intracellular ion transients and calcium homeostasis (Murarka and Movahed, 2010). Oxidative stress may be a common denominator mediating these damaging effects. Vitamin A (retinol) and metabolites (retinoids) are a group of potent natural or synthetic molecules which exert a number of biological activities, including regulation of differentiation, proliferation, apoptosis and developmental changes. The pleiotropic activities of retinoids are mediated by two classes of nuclear receptors, retinoic acid receptors (RARα, β and γ), which respond to all-trans retinoic acid (ATRA); and retinoid × receptors (RXRα, β and γ), which are activated by the 9-cis-isomers of RA, exclusively. These receptors form RXR/RXR homodimers and RXR/RAR heterodimers, which directly activate gene transcription by binding to specific retinoic acid response elements (RAREs) in target gene promoter regions. RXR homodimers and RXR/RAR heterodimers bind to distinct RAREs, resulting in the activation of different signal transduction pathways. There is a correlation between retinoid signaling and development of diabetes. A vitamin A “relative deficiency” and impaired metabolic availability of vitamin A has been confirmed in poorly controlled type 1 diabetic children and animal models (Baena et al., 2002; Basu et al., 1989; Lu et al., 2000; Tuitoek et al., 1996). Activation of RAR/RXR by ATRA inhibits type 1diabetes by increasing immune tolerance, through suppression of IFNγ-producing T-cells and by promoting in vivo expansion of T regulatory cells (Van et al., 2009). RXR agonists function as insulin sensitizers and can decrease hyperglycemia, hypertriglyceridemia and hyperinsulinemia (Altucci et al., 2007; Van et al., 2009; Yamauchi et al., 2001). These studies suggest that changes in RA signaling via the extracellular/intracellular RA level or the expression/activation of RAR/RXR, closely correlate with the development of diabetes and insulin resistance.
The functional role of RAR and RXR in the pathogenesis of cardiac remodeling is largely unknown. We and others have demonstrated that activation of RAR and RXR suppresses myocardial cell hypertrophy, apoptosis and fibrosis in response to a variety of hypertrophic stimuli (Palm-Leis et al., 2004; Wang et al., 2002; Wu et al., 1996; Zhou et al., 1995), indicating that RAR/RXR mediated signaling has an important role in regulating the transition from adaptive cardiac hypertrophy to heart failure. Recently, we reported that high glucose (HG)-induced oxidative stress and apoptosis in both neonatal and adult cardiomyocytes, were prevented by activation of RAR and RXR mediated signaling. We also found that RARα and RXRα were major subtype receptors that were downregulated by HG in cardiomyocytes (Guleria et al., 2011). Silencing the expression of RARα and RXRα in cardiomyocytes promoted HG-induced cell apoptosis, indicating that decreased expression of RARα and RXRα has an important role in HG-induced cardiomyocyte apoptosis. However, the regulatory mechanisms of HG-induced impairment of RAR and RXR remain unclear.
Retinoid receptor transcriptional activity is regulated by factors both intrinsic and extrinsic to the receptor complex. In the absence of ligand, RA target genes are silenced due to the recruitment of histone deacetylase containing multicomponent complexes that are tethered through co-repressor proteins to the unliganded RAR/RXR heterodimer. Ligand binding causes conformational changes in the receptors that allow the release of co-repressors and bind to co-activator complexes. Co-activators form multiprotein complexes that possess intrinsic histone acetyltransferase activity, which is required for retinoid receptor transcriptional activation (Bastien and Rochette-Egly, 2004; Minucci and Ozato, 1996). Although ligand binding is thought to be the primary means of activation, the transcriptional activity of RAR and RXR is also modulated by protein kinase-mediated phosphorylation and degradation (Bastien and Rochette-Egly, 2004). Phosphorylation of RXRα at serine 260, a consensus MAP kinase site, results in attenuation of ligand-dependent transactivation by the vitamin D3 receptor/RXRα complex (Solomon et al., 1999). Stress-induced phosphorylation of RXRα, through MAPK kinase 4 (MKK4) and JNK, results in suppression of retinoid signaling in COS-7 cells (Adam-Stitah et al., 1999; Lee et al., 2000). JNK activation by oxidative stress suppresses retinoid signaling through proteasomal degradation of RARα in hepatic cells (Hoshikawa et al., 2011). Oxidative stress and activation of MAP kinases have been implicated in diabetes-induced cardiac remodeling (Giacco and Brownlee, 2010; Guleria et al., 2011; Liang et al., 2010; Tang et al., 2007; Thandavarayan et al., 2009; Zhou et al., 2011). However, the association between oxidative stress/MAP kinases and hyperglycemia mediated impairment of RAR/RXR signaling in cardiomyocytes remains unclear. We hypothesize that high glucose-induced oxidative stress and activation of MAP kinase pathways have an important role in the suppressed RAR/RXR signaling in response to high glucose stimulation, through phosphorylation and/or degradation mechanisms, in cardiomyocytes.
To more completely define the mechanism by which high glucose-induced repression of RAR and RXR, primary cultured neonatal cardiomyocytes were used in this study. The neonatal rat cardiomyocyte model is well established and permits the study of many of the morphological, biochemical and molecular characteristics of the heart. We have observed consistent findings in neonatal, adult cultured cardiomyocytes and in heart tissue, with regard to cell apoptosis and regulation of RAR and RXR in response to high glucose (in vitro) and in diabetic animals (Guleria et al., 2011). We found that high glucose not only downregulated the expression of RARα and RXRα, it also repressed ligand-induced transcriptional activity of these receptors. The activated proteasome system-mediated degradation and protein destabilization have an important role in HG-induced repression of RAR/RXR signaling. High glucose-induced oxidative stress and activation of the JNK pathway, negatively regulates the expression and transcriptional activation of RARα and RXRα, in cardiomyocytes.
MATERIALS AND METHODS
Antibodies and Reagents
Cell culture medium, antibiotics and fetal bovine serum (FBS) were obtained from Invitrogen (Baltimore, MD). RAR (α, β, γ), RXR (α, β, γ); total and phospho-ERK, JNK and p38 antibodies were from Cell signaling Technology (Danvers, MA); actin and histone antibodies were obtained from Santa Cruz (Delaware, CA). All-trans retinoic acid (ATRA, pan-RAR agonist), 9-cis RA (pan-RXR agonist), and other reagents were purchased from Sigma (St. Louis, MO). Am580 (selective RARα agonist) was from Biomol International (Plymouth Meeting, PA). LGD1069 (RXR selective agonist) was from LC Laboratories (Woburn, MA). DOSPER [1, 3-dioleoyloxy-2-(6-carboxyspermly)-propyl amide] was from Roche (Indianapolis, IN). NAC (N-acetyl cysteine), MG132, U0129, SB203580 and SP600125 were purchased from Calbiochem (Gibbstown, NJ).
Rat cardiomyocyte culture
Animal use was approved by the Institutional Animal Careand Use Committee of the Texas A&M Health Science Center and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Pub. No.85-23, 1996). Primary cultures of neonatal cardiomyocytes were prepared from ventricles of 1- to 2-day-old Sprague-Dawley rat pups, as previously described (Palm-Leis et al., 2004).
Cardiomyocyte apoptosis
Apoptotic cardiomyocytes were detected using the terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) assay (Choudhary et al., 2008a). TUNEL assay was performed using a commercial kit (Roche Applied Science, Indianapolis, IN), following the manufacturer’s instructions. Myocyte cytoplasm and nuclei were counterstained with phalloidin and DAPI, respectively. The number of positively stained cells (TUNEL assay) was counted from 20 fields per slide.
Real time RT-PCR
Gene expression of RARα and RXRα was determined by real time RT-PCR, as described previously (Choudhary et al., 2008b). PCR was performed using the Mx3005P Real-time PCR System (Stratagene, TX). The relative amount of mRNAs was calculated using the comparative CT method. GAPDH mRNA was used as an internal control for all experiments.
Transfection
The replication-defective adenovirus-encoding constitutively active MKK7 (AdMKK7, Cell Biolab) and control virus (AdLacZ) were plaque purified, and amplified using HEK293 cells. The multiplicity of viral infection (MOI) for each virus was determined by dilution assay in HEK293 cells. Cardiomyocytes were infected with AdMKK7 or AdLacZ at a MOI of 25–50 plaque-forming units for 8 h, at 37 °C. Subsequently, cells were cultured in serum-free DMEM medium for an additional 24 h before treatment or analysis. The plasmid vector for the constitutively active form of MEKK1 (pCMV-MEKK1) was from Clontech. Cells were transfected with pCMV-empty vector and pCMV-MEKK1 vector (150–250 ng) for 6 h, using DOSPER Liposomal transfection reagent (3μg/ml, Roche). After transfection, cells were exposed to different treatments and a luciferase reporter assay was performed.
Luciferase reporter assay
The effect of HG on the transcriptional activity of RAR and RXR in cardiomyocytes was determined by transfection using RARE- and RXRE-containing luciferase reporter plasmids, pRAR-Luc and pRXR-Luc (Panomics). Transfection with pRAR-Luc, pRXR-Luc and control reporter vector was performed using DOSPER Liposomal transfection reagent. Briefly, neonatal cardiomyocytes were plated in 6-well plates 2 days before transfection. Cells were transfected with pRAR-Luc and pRXR-Luc at 500 ng per well, for 6 h, and then washed with media and treated with different reagents. Transfection efficiency was corrected by co-transfection of 200 ng of pRL-TK Vector (Promega, Madison, WI). After experimental treatments, cells were washed twice with PBS, lysed in passive lysis buffer (1X) provided in the dual luciferase kit (Promega) and assayed for luciferase activity, using the LB96V MicroLumat Plus luminometer (EG & G Berthold, TN), according to the manufacturer’s protocol. All transfections were performed in triplicate. The firefly luciferase activity was normalized by Renilla luciferase activity.
Nuclear expression of RAR and RXR
Nuclear proteins were extracted from cardiomyocytes, using NE-PER reagents (Thermo Scientific). The purity of nuclear protein extraction was determined before performing experiments. The same amount of cytoplasmic and nuclear proteins (40 μg) was separated by SDS–PAGE and transferred to a PVDF membrane for Western blotting using a specific HSP90 (heat shock protein 90) antibody, which provided detection in the cytoplasmic, but not nuclear extraction. The absence of expression of HSP90 in the nuclear proteins, demonstrates the absence of contamination of cytoplasmic protein in the nuclear extraction. Nuclear expression of RARα and RXRα was determined by Western blotting, using antibodies against RARα and RXRα. Membranes were reprobed with anti-histone antibody to verify equal loading.
Immunoprecipitation and Western blot analysis
Cardiomyocytes were lysed in buffer as previously described (Pan et al., 1999) and incubated with 1 μg/mL of the antibodies against RARα and RXRα, overnight at 4°C. Immunocomplexes were collected by incubating with 50 μL of protein A-Sepharose for 2 hours. After washing with lysis buffer, pellets were resuspended in sample buffer and subjected to SDS-PAGE. The membranes were immunolabeled overnight at 4°C with anti-phosphoserine antibody. Proteins were visualized by enhanced chemiluminescence (ECL) kit (Perkin Elmer Life Sciences, Boston, MA), according to the manufacturer’s instructions. The blots were stripped and reprobed with the same antibodies used for their immunoprecipitation, to ensure equal loading of the proteins. For Western blot, equal amounts of total extracted proteins (50 μg) were separated on SDS-PAGE (Palm-Leis et al., 2004), transferred to a PVDF membrane and probed with primary antibodies. Binding of primary antibody was detected with horse-radish peroxidase-conjugated, goat anti-mouse or goat anti-rabbit secondary antibody and visualized using an ECL detection kit.
Small Interfering RNA (siRNA) transfection of cardiomyocytes
Cardiomyocytes were transfected with Stealth/siRNA oligoribonucleotides (50 nM for RXRα and 100 nM for RARα, Invitrogen) using 3 μg/ml DOSPER (1,3-di-oleoxyloxy-2-(6-carboxyspermly)-propylamid, Roche) for 12 h, in OPTI-MEM I medium (Gibco, Invitrogen). Scrambled probe was used as a negative control. After washing, cells were maintained in DMEM medium with 5 % fetal bovine serum. The siRNA probes used as described previously (Guleria et al., 2011).
Statistical analysis
Data are expressed as the mean ± SEM. Statistical significance between experimental groups was determined using one-way ANOVA, combined with the Tukey-Kramer Multiple Comparisons test. P < 0.05 was considered statistically significant.
RESULTS
High glucose inhibits transcriptional activation of RARα and RXRα in cardiomyocytes
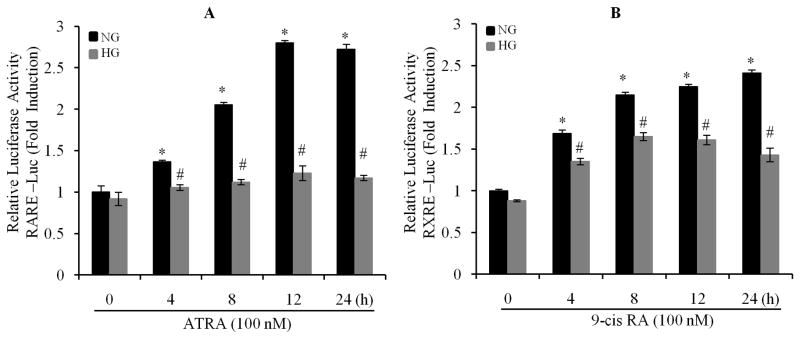
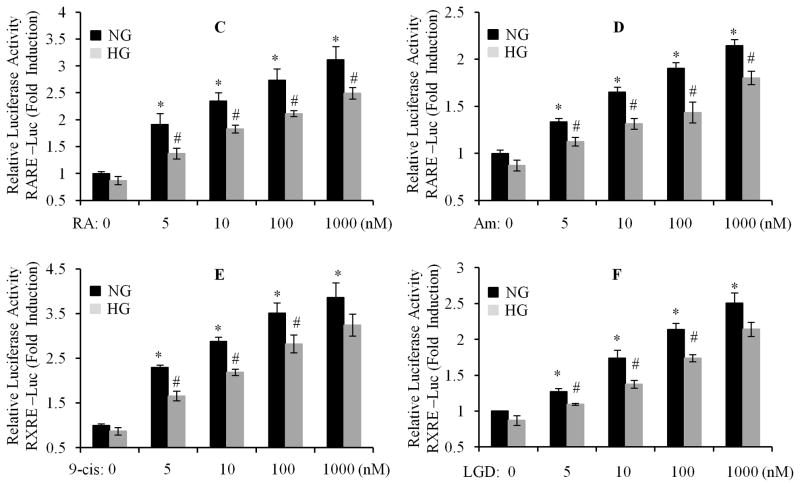
We have recently reported that the gene and protein expression of RARα and RXRα was downregulated in response to high glucose (HG) stimulation (Guleria et al., 2011). Thus, we hypothesized that RAR and RXR-mediated transcriptional activity may be also impaired by HG. After being transfected with RARE- and RXRE-containing luciferase constructs, cardiomyocytes were exposed to 100 nM of ATRA or 9-cis RA up to 24 h, in normal (NG) or HG medium. RARE- or RXRE-dependent luciferase activity was determined. The promoter activity of RAR and RXR was stimulated by ATRA and 9-cis RA, and peaked at 12 and 24 h, in normal glucose treated cells. However, ATRA and 9-cis RA-induced promoter activity was significantly suppressed in HG treated cells (Fig. 1A & B). We further determined the dose response. Cardiomyocytes were exposed to HG for 12 h, and then treated with different doses of ATRA (pan-RAR ligand), Am580 (RARα selective ligand), 9-cis RA and LGD1069 (RXR ligand). As shown in Fig. 1C to F, RARE- or RXRE-dependent luciferase activity was significantly increased following RAR or RXR ligand stimulation, in a dose-dependent manner, in normal glucose treated cells. High glucose significantly suppressed ligand-induced promoter activity. There was ~30% inhibition of the RARE and RXRE-dependent luciferase activity, compared to normal glucose treated groups. The doses of retinoids (5–1000 nM) we used were within the range of physiological levels observed in normal human plasma and rat tissue (Basualdo et al., 1997; Jacques et al., 1988; Krempf et al., 1991; Napoli, 1986). These results indicate that ligand-stimulated activation of RAR- and RXR-mediated signaling is significantly impaired by HG, and suggest that hyperglycemia-induced suppression of RA/RAR/RXR signaling may have an important role in the development of insulin resistance and metabolic perturbations observed in diabetic cardiomyopathy.
Fig. 1. High glucose downregulates the expression and transcriptional activation of RARα and RXRα in cardiomyocytes.
Neonatal rat cardiomyocytes were transfected with pRAR-Luc and pRXR-Luc (Materials and Methods section), exposed to normal (5.5 mM) or HG (25 mM), in the presence of 100 nm of ATRA (A) or 9-cis RA (B), up to 24 h, RARE and RXRE-dependent luciferase activity was measured and changes in firefly luciferase activity were calculated and plotted after normalization to Renilla luciferase activity of untreated cells. C to F. After transfection, cardiomyocytes were exposed to normal or HG for 12 h, and then treated with or without different doses of ATRA (RA, C), Am580 (Am, D), 9-cis RA (9-cis, E) and LGD1069 (LGD, F), for 12 h. RARE and RXRE-dependent luciferase activity was measured. Each value represents the mean ± SEM (n = 3). *, p<0.05, versus control (column 1 in each set); #, p<0.05, versus individual corresponding normal glucose (NG) treated group. Representative results from three independent experiments with similar results are shown.
Mechanism of glucose regulation of RARα and RXRα
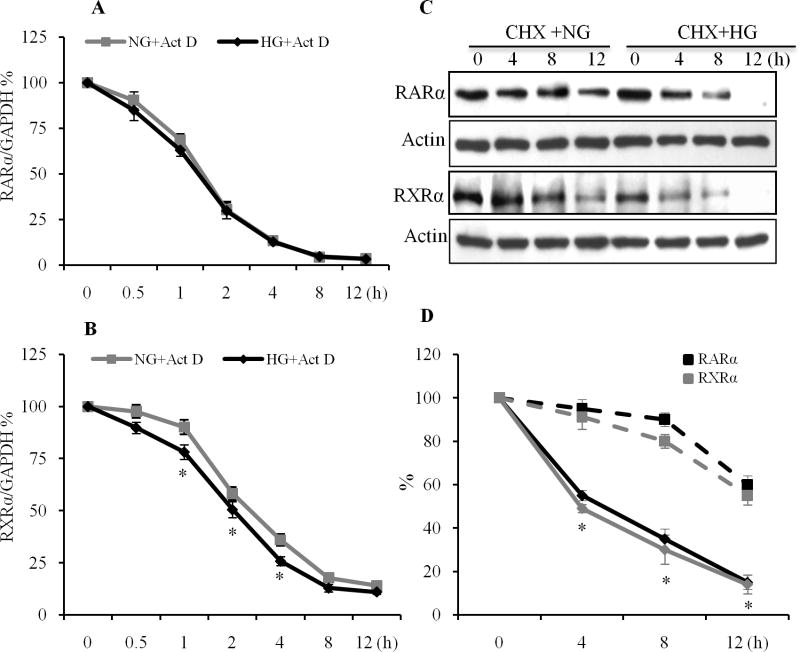
We have shown that HG regulates the RARα and RXRα at both the gene and protein levels (Guleria et al., 2011). To determine whether changes in RARα and RXRα mRNA levels with HG may be due to alterations in mRNA stability, cardiomyocytes were treated with the RNA synthesis inhibitor actinomycin D (Act D). Act D reduced the level of RARα mRNA to 50% after 1.5 h (Fig. 2A). The decline in RARα mRNA induced by Act D was similar in normal and HG-treated cells, indicating that mRNA destabilization was not involved in the reduction of RARα transcripts by HG. The decline in RXRα mRNA by Act D in HG-treated cells was faster than control cells. Act D reduced the level of RXRα mRNA to 50% after 2 h of treatment in HG stimulated cells, and 3 h in normal cells (Fig. 2B), indicating that mRNA destabilization may be involved in the reduction of RXRα transcripts by HG. We further determined whether the changes in protein levels of RARα and RXRα by HG were due to alterations in protein stability. As shown in Fig. 2C & D, the half-life of the RARα and RXRα protein was shorter in the HG-treated cells. RARα and RXRα protein levels decreased with an apparent half-life of 4 h in cells treated with HG; whereas in cells incubated with normal glucose, RARα and RXRα protein expression was stable, with a half-life at 8 to 12 h, following treatment with cycloheximide, suggesting that protein destabilization contributed to the reduced RARα and RXRα protein levels in HG stimulated cells.
Fig. 2. Effect of HG on RARα and RXRα mRNA and protein stability.
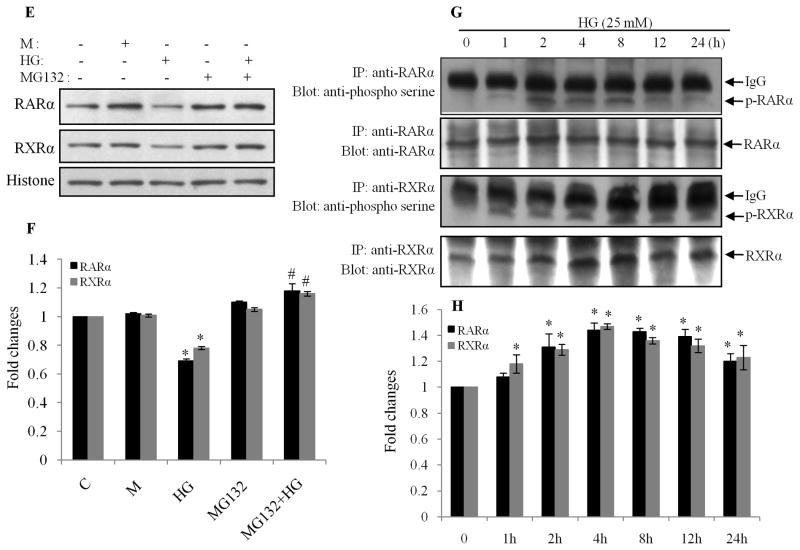
A & B. RARα and RXRα mRNA half-life was determined from real-time RT-PCR in cells incubated with normal (NG) or HG for 24 h. After this period (time 0), cells were incubated in the presence of actinomycin D (0.25 μg/ml, Act-D). At the time indicated, RARα and RXRα mRNA levels were determined by real-time RT-PCR. The data are expressed as percentages of the RARα and RXRα mRNA levels obtained at time 0. *, p<0.05, versus individual corresponding normal glucose (NG) treated group. C. RARα and RXRα protein half-life was determined by Western blot after the same treatments in the presence of cycloheximide (10μg/ml). Actin was used as a loading control. D. Intensity of the bands was analyzed by densitometry. Data are expressed as the mean ± SEM (n=3). Broken line: normal glucose treated cells; solid line: HG treated cells. *, p<0.05, versus normal glucose group. E. HG induces proteasomal degradation of RARα and RXRα. Cardiomyocytes were exposed to HG for 24 h, in the presence or absence of the proteasomal inhibitor MG132 (1 μM). Nuclear protein expression of RARα and RXRα was determined by Western blot. Histone levels were used as a loading control. F. Intensity of the bands (E) was analyzed by densitometry. Data are expressed as the mean ± SEM (n=3). *, p<0.05, versus control group; #, p<0.05, versus HG. G. Cell lysates were immunoprecipitated with antibodies to RARα and RXRα and immunoblotted with anti-phosphoserine antibody. Membranes were stripped and reprobed with anti-RARα and -RXRα antibodies. Data are representative of three separate experiments. H. Intensity of the bands (G) was analyzed by densitometry. Data are expressed as the mean ± SEM (n=3). *, p<0.05, versus non-treated group.
It has been shown that the ubiquitin-proteasome pathway is involved in regulation of RAR and RXR (Bastien and Rochette-Egly, 2004; Zhu et al., 1999). To determine whether an active proteasome pathway was involved in the HG effect on RARα and RXRα, cardiomyocytes were pretreated with the proteasome inhibitor MG132, and nuclear expression of RARα and RXRα determined. HG-induced downregulation of nuclear protein expression of RARα and RXRα was prevented by MG132 (Fig. 2E & F), suggesting that proteasome-mediated degradation contributes to the HG effects. Previous studies have suggested that phosphorylation of RAR and RXR at specific serine sites leads to degradation and transcriptional inhibition of RAR and RXR (Hoshikawa et al., 2011; Srinivas et al., 2005). Thus, we determined whether HG-induced degradation of RAR and RXR is regulated by phosphorylation. Cardiomyocytes were exposed to HG up to 24 h, and serine phosphorylation of RARα and RXRα was determined by immunoprecipitation and Western blot. As shown in Fig. 2G & H, the serine phosphorylation of RARα and RXRα was observed after 1 h of HG stimulation, peaked from 2 to 8 h and decreased after 24 h. The time phase of the phosphorylation is consistent with the decreased expression of RARα and RXRα, which is evident following 4 to 24 h of HG stimulation (Guleria et al., 2011), suggesting that HG-induced serine phosphorylation of RARα and RXRα may lead to degradation and inhibition of RAR and RXR-mediated signaling events.
Role of oxidative stress in regulation of expression/activation of RARα and RXRα
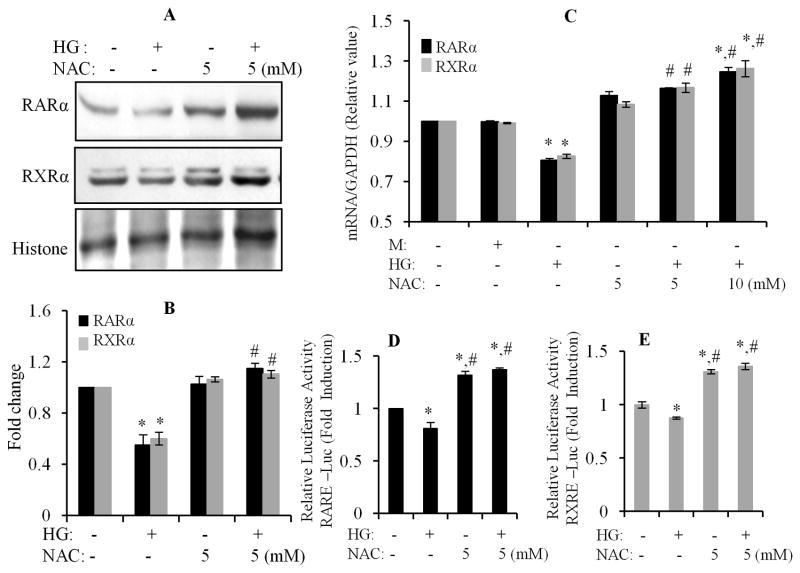
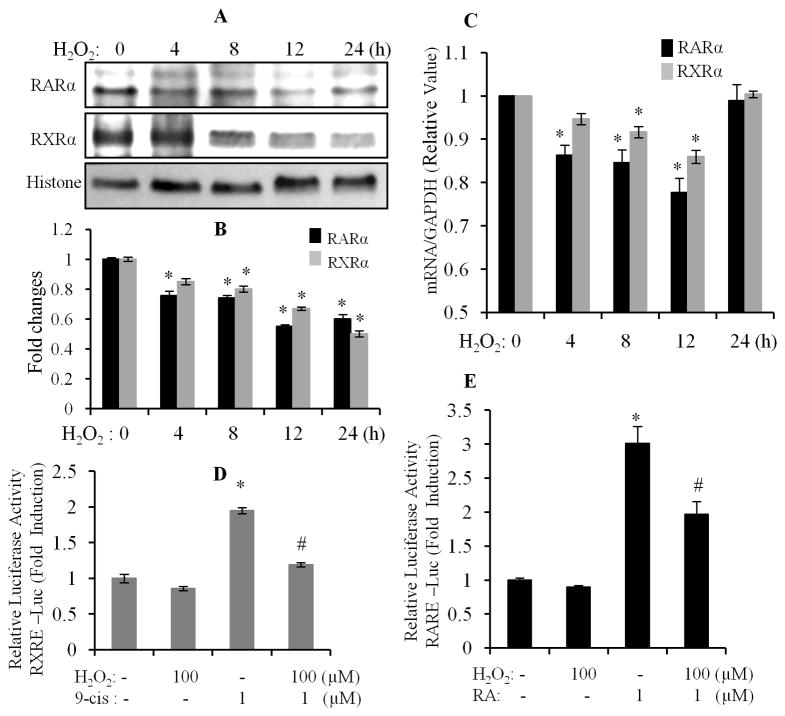
A previous study had shown that oxidative stress suppressed retinoid signaling through proteasomal degradation in HUH7 hepatocarcinoma cells (Hoshikawa et al., 2011). It is well known that increased production of reactive oxygen species (ROS) and an altered cellular redox state contribute to hyperglycemia induced cardiac remodeling (Cai et al., 2006; Fiordaliso et al., 2004). We also demonstrated that HG-promoted intracellular ROS generation, which has an important role in HG-induced apoptosis in cardiomyocytes (Guleria et al., 2011). Thus, we determined whether oxidative stress was involved in the HG effects on expression/activation of RARα and RXRα. Cardiomyocytes were pretreated with NAC (N-acetyl cysteine, an ROS scavenger), and exposed to HG for 12 h. Gene and nuclear protein expression of RARα and RXRα was determined. HG-induced decreases in protein (Fig. 3A & B) and gene expression (Fig. 3C) of RARα and RXRα was prevented by NAC treatment. NAC alone had no significant effect. We further determined the role of oxidative stress in regulating the transcriptional activity of RARα and RXRα. As shown in Fig. 3D & E, HG-induced inhibition of the promoter activity of RAR and RXR was reversed by NAC treatment. NAC also promoted RARE- or RXRE-dependent luciferase activity in normal glucose conditions. To further confirm the involvement of oxidative stress in HG effects, cardiomyocytes were exposed to H2O2 for different time periods and the protein/gene expression of RARα and RXRα were determined. A significant decrease in protein expression of RARα and RXRα was observed following 8 to 24 h of H2O2 treatment (Fig. 4A & B). A modest decreased gene expression of RARα and RXRα was observed from 4–12 h following H2O2 treatment, and returned to a normal level at 24 h (Fig. 4C). We further determined the effect of H2O2 on ligand-stimulated promoter activity of RAR and RXR. As shown in Fig. 4D & E, 9-cis RA and ATRA stimulated RXRE and RARE-dependent luciferase activity was significantly suppressed in H2O2 treated cells, compared to normal control. These data indicate that oxidative stress has an important role in HG-induced suppression of the RAR/RXR-mediated signaling in cardiomyocytes.
Fig. 3. Effect of oxidative stress on high glucose action on RARα and RXRα.
A & B. Cardiomyocytes were pretreated with NAC (5 mM) for 30 min and exposed to HG for 24 h. Nuclear protein expression of RARα and RXRα was determined by Western blot. Histone levels were used as a loading control. Results are representative of three independent experiments. The intensity of the bands was analyzed by densitometry (B). Data are expressed as the mean ± SEM (n=3). *, p < 0.05 versus control; #, p < 0.05 versus HG. C. Cardiomyocytes were pretreated with NAC (5 & 10 mM) for 30 min, and then exposed to HG for 12 h. Gene expression of RARα and RXRα was determined by real-time RT-PCR. Data were normalized to a housekeeping gene (GAPDH). Data (mean ± SEM; n=3) were expressed as a relative value compared to control. *, p<0.05, versus control; #, p<0.05, versus HG. D & E. Cardiomyocytes were transfected with pRAR-Luc and pRXR-Luc, for 6 h, and then subjected to similar treatment as noted in Fig. 3C. RARE and RXRE-dependent luciferase activity was measured as described in Fig. 1. Each value represents the mean ± SEM (n = 3). *, p<0.05, versus control; #, p<0.05, versus HG.
Fig. 4. H2O2 inhibits gene and protein expression of RARα and RXRα.
Cardiomyocytes were exposed to 100 μM of H2O2 for different time periods, and nuclear protein (A & B) and gene (C) expression of RARα and RXRα was determined. Each value represents the mean ± SEM (n = 3). *, p<0.05, versus control. D & E. Cardiomyocytes were transfected with pRXR-Luc and pRAR-Luc, for 6 h, and exposed to 1 μM of 9-cis RA (9-cis) and ATRA (RA), in the absence or presence of 100 μM of H2O2, for 12 h. RARE and RXRE-dependent luciferase activity was measured, as described in Fig. 1. Each value represents the mean ± SEM (n = 3). *, p<0.05, versus control; #, p<0.05, versus HG.
Role of the MAP kinase cascade in HG-induced downregulation of RAR and RXR
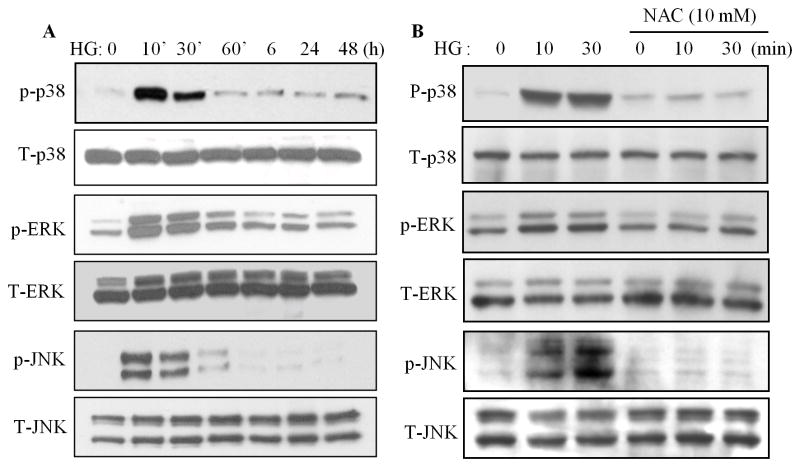
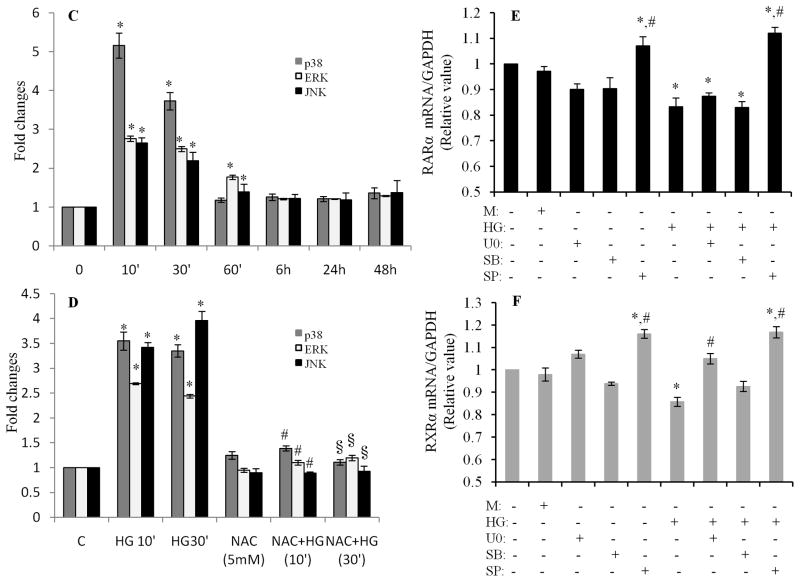
To elucidate the potential regulatory mechanisms involved in HG and oxidative stress-mediated impairment of RAR/RXR signaling, the role of the MAP kinase cascade including p38, ERK1/2 and JNK1/2 was determined. Exposure of cardiomyocytes to HG for 10 to 30 min significantly activated p38, ERK1/2 and JNK, without affecting respective total protein expression (Fig. 5A & C). HG-induced phosphorylation of p-38, ERK1/2 and JNK1/2 was significantly inhibited by NAC (Fig. 5B & D), indicating that oxidative stress is involved in HG-induced activation of the MAP kinase pathway. We next determined the role of MAP kinases in regulation of the gene expression of RARα and RXRα. Cardiomyocytes were pretreated with or without inhibitors for p38 (SB203580), ERK1/2(U0126) and JNK1/2 (SP600125) and exposed to HG for 12 h, gene expression of RARα and RXRα was determined. The JNK inhibitor SP600125 reversed the inhibitory effect of HG on gene expression of RARα and RXRα (Fig. 5E & F). The basal level of RARα and RXRα mRNA was also increased in SP600125 treated cells. The ERK1/2 inhibitor, U0126 had a modest effect on the decreased expression of RXRα; but, had no effect on the expression of RARα. No changes were observed in SB203580 treated cells. These results indicate that inhibition of the JNK pathway abrogated the inhibitory effect of HG on RARα and RXRα, in both normal and high glucose conditions.
Fig. 5. Role of MAP kinases in regulation of the HG action on RARα and RXRα.
A. High glucose induces phosphorylation of MAP kinases. Cardiomyocytes were exposed to HG for different time periods, and the phosphorylation of p38, ERK1/2 and JNK was determined by Western blotting. C. Intensity of the phosphorylation of p38, ERK and JNK (A) was analyzed by densitometry and standardized to the corresponding total proteins. Data are expressed as the mean ± SEM (n=3). *, p<0.05, versus non-treated group. B. Oxidative stress is involved in HG-induced phosphorylation of MAP kinases. Cardiomyocytes were pretreated with NAC for 30 min and exposed to HG for 10 and 30 min, and the phosphorylation of MAP kinases was determined and quantified (D) as noted in C. *, p<0.05, versus non-treated group; #, p<0.05, versus HG 10 min; §, p<0.05, versus HG 30 min. E & F. Role of MAP kinases in regulation of HG-induced downregulation of RARα and RXRα. Cardiomyocytes were pretreated with 10 μM of U0126, SB203580 or SP600125 for 30 min, and exposed to HG for 12 h. Gene expression of RARα (E) and RXRα (F) was determined. Data (mean ± SEM, n = 3) are expressed as a relative value compared to control. *, p<0.05, versus control; #, p<0.05, versus HG.
Role of the JNK pathway in regulation of the expression/activation of RARα and RXRα
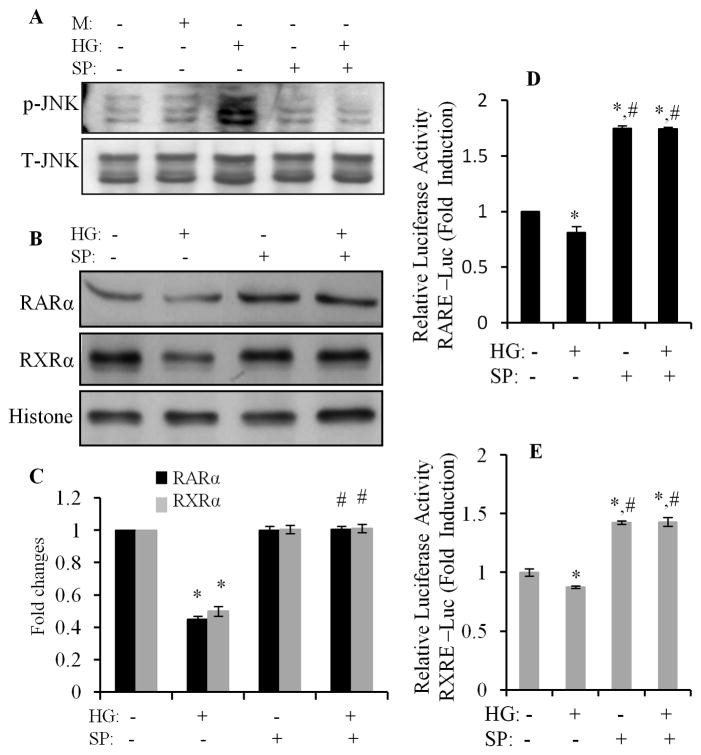
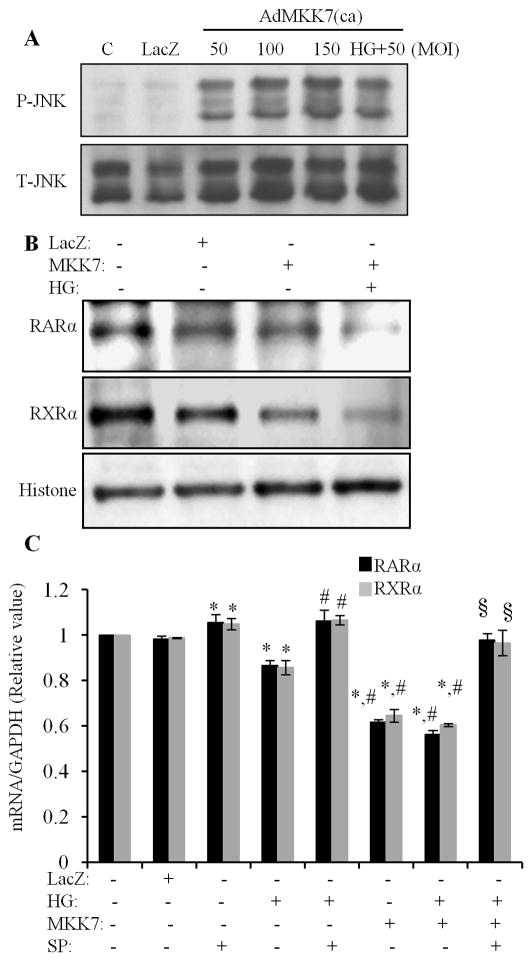
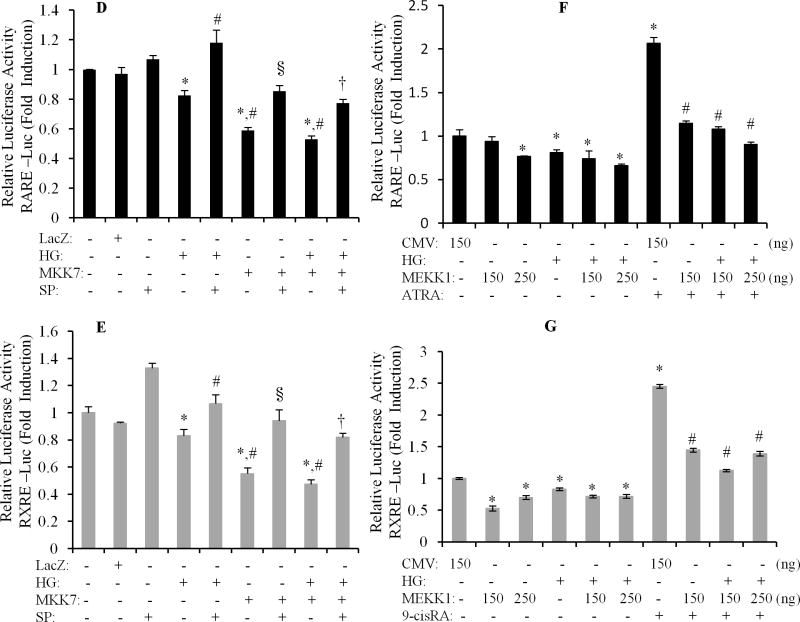
We further determined the role of JNK signaling in regulation of the HG effects on RARα and RXRα. Cardiomyocytes were pretreated with SP600125 for 1 h and exposed to HG for 12 h, and nuclear protein expression of RARα and RXRα was determined. The specificity of SP600125 on the activation of JNK was confirmed in Fig. 6A, as HG-induced phosphorylation of JNK was completely inhibited by SP600125. The downregulation of nuclear protein expression RARα and RXRα by HG was reversed by SP600125 (Fig. 6B & C). SP600125 also abrogated the inhibitory effect of HG on promoter activity of RAR and RXR (Fig. 6D & E). SP600125 also significantly increased the basal level of the promoter activity of RAR and RXR. To further confirm the role of JNK in HG effects on RARα and RXRα, we used adenovirus-mediated overexpression of constitutively active MKK7 (AdMKK7ca) and plasmid-mediated constitutively active MEKK1 (pCMV-MEKK1). MKK7 is an upstream kinase that directly activates JNK, and MEKK1 is an upstream kinase that directly activates MKK7. Overexpression of AdMKK7ca stimulated the phosphorylation of JNK (Fig. 7A) and decreased the protein and gene expression of RARα and RXRα (Fig. 7B & C); and significantly inhibited the promoter activity of RAR and RXR, in both normal and HG treated cells (Fig. 7D & E). Overexpression of pCMV-MEKK1 had a similar effect on the promoter activity of RARα and RXRα, as compared to AdMKK7ca. ATRA or 9-cis RA induced promoter activity of RAR and RXR was dramatically inhibited by overexpression of pCMV-MEKK1. These results indicate that JNK functions as upstream molecule, negatively regulating RAR and RXR-mediated signaling.
Fig. 6. Inhibition of JNK reverses the HG action on RARα and RXRα.
Cardiomyocytes were pretreated with SP600125 for 30 min, exposed to HG for 30 min (A) or 24 h (B & C), and the phosphorylation of JNK (A) and nuclear protein expression of RARα and RXRα (B & C) determined by Western blot. Total JNK and histone levels were used as a loading control. Data are expressed as the mean ± SEM (n=3). *, p < 0.05 versus control; #, p < 0.05 versus HG. D & E. After transfection with pRAR-Luc and pRXR-Luc, cardiomyocytes were pretreated with SP600125 for 30 min, exposed to HG for 12 h, and RARE and RXRE-dependent luciferase activity was measured as described in Fig. 1. *, p<0.05, versus control; #, p<0.05, versus HG.
Fig. 7. Activation of JNK inhibits the expression/activation of RARα and RXRα.
A. Cardiomyocytes were infected with AdMKK7 and AdLacZ, exposed to normal or HG for 30 min, and phosphorylation of JNK determined. Total JNK levels were used as loading control. B. Cardiomyocytes were infected with AdMKK7ca (MKK7, 50 MOI), exposed to HG for 24 h, nuclear protein expression of RARα and RXRα was determined. Histone levels were used as a loading control. C. Cardiomyocytes were infected with ADMKK7ca (MKK7), pretreated with or without SP600125 and exposed to HG for 12 h. Gene expression of RARα and RXRα was determined. The mRNA levels were normalized to GAPDH. Data (mean ± SEM, n = 3) are expressed as a relative value compared to control. *, p<0.05, versus control; #, p<0.05, versus HG; §, p<0.05, versus AdMKK7ca+HG group. D&E. After infected with AdMKK7ca, cardiomyocytes were transfected with pRAR-Luc and pRXR-Luc, pretreated with or without SP600125 and then exposed to HG for 12 h, and luciferase activity measured. Each value represents the mean ± SEM (n = 3). *, p<0.05, versus control; #, p<0.05, versus HG; §, p<0.05, versus AdMKK7ca; †, p<0.05, versus AdMKK7ca+HG. F &G. Cardiomyocytes were transfected with pCMV-empty vector (CMV) or pCMV-MEKK1 vector (MEKK1, 150 and 250 ng), pretreated with or without μM of ATRA (RA, F) and 9-cis RA (9-cis, G) and exposed to normal or HG for 12 h. RARE and RXRE-dependent luciferase activity was determined. *, p<0.05, versus control; #, p<0.05, versus RA or 9-cis group.
The JNK pathway is involved in HG-induced cardiomyocyte apoptosis
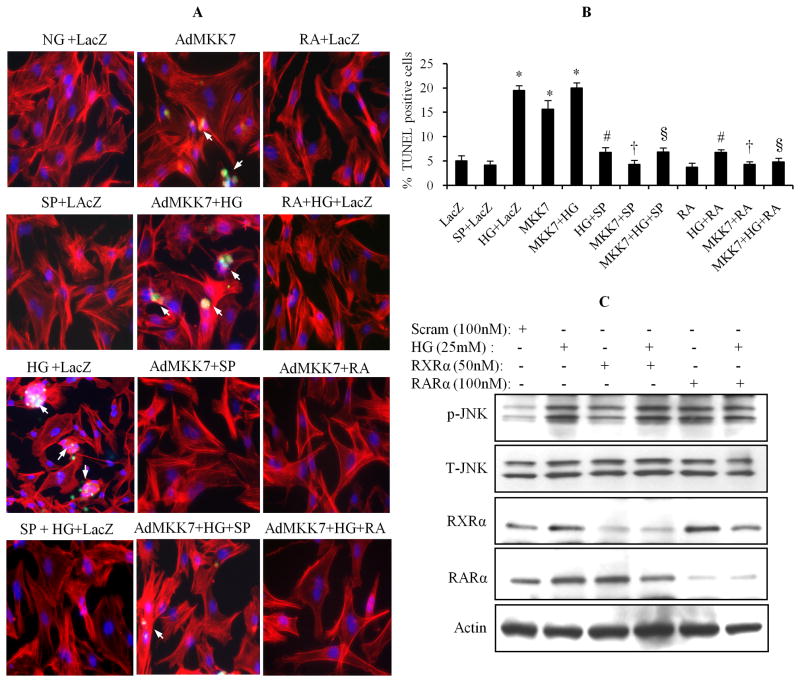
To determine the role of JNK in HG-induced cell apoptosis, cardiomyocytes were infected with AdMKK7ca, and then exposed to normal or HG, in the presence or absence of SP600125 and ATRA for 24 h. Apoptosis was determined by the TUNEL assay. As shown in Fig. 8A & B, the increased TUNEL positive cell population in HG-stimulated cells was prevented by SP600125. Activation of JNK by overexpression of AdMKK7ca caused an increased number of the TUNEL positive cell population, at a comparable level of HG stimulation, indicating that JNK activation has an important role in regulation of cell apoptosis. Activation of RAR/RXR-mediated signaling by ATRA prevented both HG and AdMKK7ca-induced cell apoptosis. We have shown that silencing the expression of RARα and RXRα promoted HG-induced cell apoptosis (Guleria et al., 2011). Thus, we hypothesize that HG-induced suppression of the RAR/RXR signaling can activate the JNK pathway, leading to cell apoptosis. To address our question, the expression of RARα and RXRα in cardiomyocytes was silenced by siRNA, the phosphorylation of JNK was determined. As shown in Fig. 8C, silencing RARα and RXRα induced phosphorylation of JNK. These data suggested that HG-induced impairment of RAR/RXR signaling directly associated to increased intracellular oxidative stress and activation of JNK pathway, and contributed to HG-induced cardiomyocyte apoptosis.
Fig. 8. JNK pathway is involved in HG-induced apoptosis.
A & B. After infecting with AdLacZ or AdMKK7ca, cardiomyocytes were pretreated with or without SP600125(10 μM), and exposed to normal or HG, in the presence or absence of ATRA (1 μM), for 24. Apoptosis was determined by TUNEL assay. TUNEL positive cell counting was expressed as the mean ± SEM. *, p < 0.001 versus control; #, p < 0.001 versus HG; †, p < 0.001 versus AdMKK7ca; §, p < 0.001 versus AdMKK7ca+HG. C. Scrambled or RARα and RXRα siRNA transfected cells were exposed to HG for 30 min, and phosphorylation of JNK was determined by Western blot. Blots were reprobed for total JNK, RARα and RXRα. Actin was used as a loading control.
DISCUSSION
We have recently reported that downregulated RAR/RXR signaling contributed to high glucose-induced cardiomyocyte apoptosis and oxidative stress (Guleria et al., 2011). Thus, understanding the mechanisms of how high glucose affects RAR/RXR-mediated signaling may have important clinical relevance in addressing the pathophysiology of diabetes-induced cardiac remodeling. In the present study, we found that ligand stimulated transcriptional activity of RAR and RXR was significantly suppressed under high glucose conditions. High glucose promoted serine-phosphorylation of RARα and RXRα, which may associated with protein destabilization and proteasomal degradation of RARα and RXRα in response to high glucose stimulation. High glucose-induced oxidative stress and activation of the JNK pathway suppressed the expression and transcriptional activation of RARα and RXRα. Inhibition of ROS and the JNK pathway prevented the high glucose effect on RARα and RXRα. Silencing RARα and RXRα promoted phosphorylation of JNK and activation of JNK resulted in cell apoptosis. These data suggest that high glucose-induced oxidative stress and activation of the JNK pathway caused repression of RAR/RXR signaling. The impaired RAR/RXR signaling further accelerated the generation of ROS and activation of the JNK pathway, leading to cardiomyocyte apoptosis (Fig. 9).
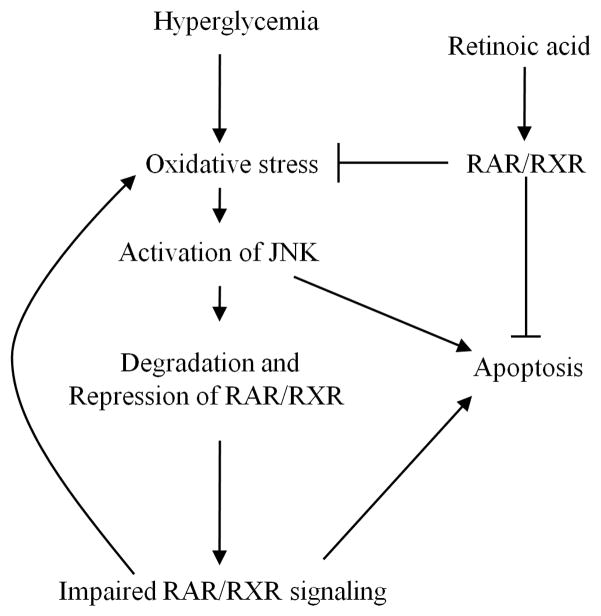
Fig 9. Proposed interaction between RAR/RXR and oxidative stress/JNK signaling.
The relationship between high glucose, oxidative stress and impaired RAR/RXR signaling are proposed. Retinoic acid, through activation of RAR/RXR-mediated signaling, inhibits intracellular oxidative stress and the JNK pathway, protecting cardiomyocytes from HG-induced apoptosis. High glucose promotes oxidative stress, which leads to activation of the JNK pathway and degradation of RAR/RXR, resulting in repression of RAR/RXR signaling. Impaired RAR/RXR signaling further potentiates intracellular oxidative stress and activation of JNK-mediated apoptotic signaling.
There are limited studies on the link between cardiac function and the expression of nuclear receptor RARs and RXRs. It has been reported that decreased expression of RXR is involved in the altered myocardial metabolic phenotype in severe heart failure, and that the downregulation of RXR may be responsible for the impairment in free fatty acid oxidative pathways in the failing heart (Feingold et al., 2004; Osorio et al., 2002). However, the interaction between RAR/RXR and the development of diabetic cardiac remodeling remains unknown. We have recently reported that high glucose downregulated the nuclear expression of RARα and RXRα in cultured cardiomyocytes and in diabetic rat hearts (Guleria et al., 2011). Silencing the expression of RARα and RXRα promoted cardiomyocyte apoptosis and gene expression of the RAS (renin-angiotensin system) components angiotensinogen and renin. Activation or upregulation of RAR and RXR by respective selective ligands, attenuated high glucose-induced cell apoptosis, intracellular ROS generation and the expression of RAS components. These data suggested that downregulated expression of RARα and RXRα contributed to hyperglycemia-induced cardiomyocyte apoptosis, through upregulation and activation of the intracellular ROS-mediated signaling and the renin-angiotensin system. We further confirmed the action of high glucose on RAR and RXR signaling, and demonstrated that high glucose not only affected the gene and protein expression of RARα and RXRα; but, also repressed physiological doses of RA-induced transcriptional activity of RAR and RXR. We observed that a 10–50 fold higher dose of RA was required under high glucose conditions, in order to maintain an equal level of transcriptional activation of RAR and RXR as induced under normal conditions. Since we didn’t measure the intracellular and nuclear level of RA, we couldn’t rule out the possibility that high glucose may affect the transfer of RA into nuclei for initiating transcriptional activity of RAR/RXR. In correlation with the in vivo situation, the tissue RA level is maintained through a cascade of metabolic reactions. A number of key enzymes and proteins involved in retinoid metabolism, such as serum retinol binding protein (RBP), cellular retinol-binding proteins (CRBPs), retinol dehydrogenases (RoDHs), retinal dehydrogenases (RalDHs) and cellular retinoic acid binding proteins (CRABPs) have been identified and functionally characterized (Napoli, 1996). Previous studies have shown that vitamin A metabolic availability is impaired in diabetes mellitus (Baena et al., 2002; Basu et al., 1989; Lu et al., 2000; Tuitoek et al., 1996); and some key enzymes and proteins like RBP4, CRBP-I and CRABP2 have been linked to insulin resistance and lipid metabolism (Salazar et al., 2007; Yang et al., 2005; Zizola et al., 2010). Our data provide further evidence that high glucose targets the receptor level, resulting in impairment of cellular functions mediated by RAR/RXR signaling. Understanding the role of the retinoid metabolic cascade in diabetes-induced cardiac injury will have clinical significance in the prevention and treatment of diabetic related complications.
The transcriptional activation of RAR and RXR is regulated by multiple mechanisms, including co-activator, co-repressor, ubiquitin-proteasome system and phosphorylation (Bastien and Rochette-Egly, 2004). We have observed that protein destabilization contributed to HG-induced decreases in the expression of RARα and RXRα; and that an activated proteasome system may be an initiating factor leading to protein destabilization. However, the mechanisms whereby HG promotes proteasome-mediated degradation remain unclear. As with most nuclear hormone receptors, retinoid receptors exhibit a modular structure composed of 6 conserved regions designated A–F. The N-terminal A/B region harbors a ligand-independent transcriptional activation function (AF-1). This domain contains several consensus phosphorylation sites (Rochette-Egly, 2003) for proline-dependent kinases, which include cyclin-dependent kinases (CDKs) and MAP kinases (Bour et al., 2005; Bour et al., 2007; Gianni et al., 2002; Rochette- Egly et al., 1997). The ligand binding domain (LBD) and ligand-dependent transcriptional activation/repression domain AF-2, located in region E, also contains consensus phosphorylation sites, which can be phosphorylated by PKA, ERK, JNK and p38 MAP kinases (Adam-Stitah et al., 1999; Bruck et al., 2009; Matsushima-Nishiwaki et al., 2001; Rochette-Egly et al., 1995; Srinivas et al., 2005). The phosphorylation of RAR and RXR are further linked to their degradation or increased/decreased transcriptional activation. These studies suggest that RAR and RXR are substrates for these protein kinases. It is well accepted that oxidative stress induced by hyperglycemia could be a major factor affecting the different pathways leading to diabetes complications (Baynes, 1991; Giacco and Brownlee, 2010). Importantly, acute glucose fluctuations exhibit a more specific triggering effect on oxidative stress, than chronic sustained hyperglycemia (Choi et al., 2008; Monnier et al., 2006). We have reported that hyperglycemia-induced intracellular ROS generation has an important role in cardiomyocyte apoptosis (Guleria et al., 2011). Inhibition of ROS generation prevented the downregulated expression and transcriptional activation of RARα and RXRα in response to high glucose stimulation. On the other hand, H2O2 stimulation significantly downregulated the expression of RARα and RXRα, and suppressed ligand-induced RAR and RXR promoter activity in cardiomyocytes, indicating that oxidative stress has a major role in hyperglycemia-induced repression of RAR/RXR signaling.
There are accumulating data which implicate the role of oxidative stress in the activation of MAP kinases (Aikawa et al., 1997; Clerk et al., 1998; Turner et al., 1998). This is in agreement with our finding that high glucose-induced phosphorylation of ERK1/2, p38 and JNK1/2 was inhibited by NAC, a direct scavenger of ROS. Thus, it is possible that high glucose-induced activation of MAP kinases may be involved in the repression of RAR and RXR. Using specific inhibitors for ERK1/2, p38 and JNK, we found that JNK is the major kinase involved in high glucose-mediated repression of RAR and RXR. Inhibition of the phosphorylation of JNK blocked the high glucose effects on the expression/transcriptional activation of RARα and RXRα. Activation of JNK by overexpressing its upstream activator MKK7 and MEKK1, significantly inhibited the expression of RARα and RXRα, under normal and high glucose conditions. More importantly, ATRA and 9-cis RA-induced promoter activity of RAR and RXR was also repressed by activation of JNK. These results indicate that RAR and RXR are downstream substrates of JNK. It has been reported that JNK promotes phosphorylation of RAR and RXR, which further leads to proteasomal degradation and transcriptional inhibition of RAR and RXR (Adam-Stitah et al., 1999; Bruck et al., 2005; Srinivas et al., 2005). This is consistent with our findings, that high glucose-induced downregulation of RARα and RXRα was reversed by the proteasome inhibitor MG132. On the basis of these findings, we confirmed the hypothesis that high glucose-induced oxidative stress and activation of JNK, leads to degradation of RAR and RXR and repression of ligand-induced transcriptional activation of the receptors, which subsequently contributes to retinoid receptor dysfunction in high glucose stimulated cardiomyocytes. Phosphorylation of RAR and RXR by JNK may be involved in the degradation of RAR and RXR. We observed that HG induced serine phosphorylation of RARα and RXRα in cardiomyocytes; however, the role of JNK in the phosphorylation of RARα and RXRα and the relationship between phosphorylation and proteasome-mediated degradation of RARα and RXRα remains to be determined.
Activation of JNK has been implicated in the development of insulin resistance and type 2 diabetes (Hirosumi et al., 2002; Sabio et al., 2010; Vallerie and Hotamisligil, 2010). JNK signaling also has an important role in hyperglycemia-induced cardiomyocyte apoptosis (Gurusamy et al., 2004; Liang et al., 2010). This is consistent with our study that JNK activation promoted cardiomyocyte apoptosis, and inhibition of JNK protected cardiomyocytes from high glucose-induced apoptosis. We further demonstrated that activation of JNK-induced cell apoptosis was abolished by RA, indicating that activation of RAR/RXR signaling protects cardiomyocytes from high glucose-induced apoptosis, through inhibiting the activation of JNK. We have shown previously that silencing RARα and RXRα in cardiomyocytes promotes cell apoptosis (Guleria et al., 2011). Here, we further confirmed that silencing RARα and RXRα activated the JNK pathway. Thus, it is likely that high glucose-induced repression of RAR/RXR signaling can potentiate the activation of the JNK pathway and lead to apoptosis.
In summary, high glucose induced oxidative stress and activation of JNK signaling suppressed the expression and transcriptional activation of RARα and RXRα. Activation of RARα and RXRα protects cardiomyocytes through inhibition of JNK signaling. The impaired RAR/RXR signaling and oxidative stress/JNK activation provides a putative mechanism for the development of diabetic cardiomyopathy.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas.
References
- Adam-Stitah S, Penna L, Chambon P, Rochette-Egly C. Hyperphosphorylation of the retinoid × receptor alpha by activated c-Jun NH2-terminal kinases. J Biol Chem. 1999;274(27):18932–18941. doi: 10.1074/jbc.274.27.18932. [DOI] [PubMed] [Google Scholar]
- Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest. 1997;100(7):1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6(10):793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- Baena RM, Campoy C, Bayes R, Blanca E, Fernandez JM, Molina-Font JA. Vitamin A, retinol binding protein and lipids in type 1 diabetes mellitus. Eur J Clin Nutr. 2002;56(1):44–50. doi: 10.1038/sj.ejcn.1601279. [DOI] [PubMed] [Google Scholar]
- Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Basu TK, Tze WJ, Leichter J. Serum vitamin A and retinol-binding protein in patients with insulin-dependent diabetes mellitus. Am J Clin Nutr. 1989;50(2):329–331. doi: 10.1093/ajcn/50.2.329. [DOI] [PubMed] [Google Scholar]
- Basualdo CG, Wein EE, Basu TK. Vitamin A (retinol) status of first nation adults with non-insulin-dependent diabetes mellitus. J Am Coll Nutr. 1997;16(1):39–45. doi: 10.1080/07315724.1997.10718647. [DOI] [PubMed] [Google Scholar]
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Bour G, Gaillard E, Bruck N, Lalevee S, Plassat JL, Busso D, Samama JP, Rochette-Egly C. Cyclin H binding to the RARalpha activation function (AF)-2 domain directs phosphorylation of the AF-1 domain by cyclin-dependent kinase 7. Proc Natl Acad Sci U S A. 2005;102(46):16608–16613. doi: 10.1073/pnas.0505556102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour G, Lalevee S, Rochette-Egly C. Protein kinases and the proteasome join in the combinatorial control of transcription by nuclear retinoic acid receptors. Trends Cell Biol. 2007;17(6):302–309. doi: 10.1016/j.tcb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Bruck N, Bastien J, Bour G, Tarrade A, Plassat JL, Bauer A, Adam-Stitah S, Rochette-Egly C. Phosphorylation of the retinoid × receptor at the omega loop, modulates the expression of retinoic-acid-target genes with a promoter context specificity. Cell Signal. 2005;17(10):1229–1239. doi: 10.1016/j.cellsig.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Bruck N, Vitoux D, Ferry C, Duong V, Bauer A, de The H, Rochette-Egly C. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARalpha to target promoters. EMBO J. 2009;28(1):34–47. doi: 10.1038/emboj.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48(8):1688–1697. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Choi SW, Benzie IF, Ma SW, Strain JJ, Hannigan BM. Acute hyperglycemia and oxidative stress: direct cause and effect? Free Radic Biol Med. 2008;44(7):1217–1231. doi: 10.1016/j.freeradbiomed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Choudhary R, Baker KM, Pan J. All-trans retinoic acid prevents angiotensin II- and mechanical stretch-induced reactive oxygen species generation and cardiomyocyte apoptosis. J Cell Physiol. 2008a;215 (1):172–181. doi: 10.1002/jcp.21297. [DOI] [PubMed] [Google Scholar]
- Choudhary R, Palm-Leis A, Scott RC, 3rd, Guleria RS, Rachut E, Baker KM, Pan J. All-trans retinoic acid prevents development of cardiac remodeling in aortic banded rats by inhibiting the renin-angiotensin system. Am J Physiol Heart Circ Physiol. 2008b;294(2):H633–644. doi: 10.1152/ajpheart.01301.2007. [DOI] [PubMed] [Google Scholar]
- Clerk A, Fuller SJ, Michael A, Sugden PH. Stimulation of “stress-regulated” mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J Biol Chem. 1998;273(13):7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- Feingold K, Kim MS, Shigenaga J, Moser A, Grunfeld C. Altered expression of nuclear hormone receptors and coactivators in mouse heart during the acute-phase response. Am J Physiol Endocrinol Metab. 2004;286(2):E201–207. doi: 10.1152/ajpendo.00205.2003. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Bianchi R, Staszewsky L, Cuccovillo I, Doni M, Laragione T, Salio M, Savino C, Melucci S, Santangelo F, Scanziani E, Masson S, Ghezzi P, Latini R. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol. 2004;37(5):959–968. doi: 10.1016/j.yjmcc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni M, Bauer A, Garattini E, Chambon P, Rochette-Egly C. Phosphorylation by p38MAPK and recruitment of SUG-1 are required for RA-induced RAR gamma degradation and transactivation. Embo J. 2002;21(14):3760–3769. doi: 10.1093/emboj/cdf374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria RS, Choudhary R, Tanaka T, Baker KM, Pan J. Retinoic acid receptor-mediated signaling protects cardiomyocytes from hyperglycemia induced apoptosis: role of the renin-angiotensin system. J Cell Physiol. 2011;226(5):1292–1307. doi: 10.1002/jcp.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurusamy N, Watanabe K, Ma M, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Dominant negative 14-3-3 promotes cardiomyocyte apoptosis in early stage of type I diabetes mellitus through activation of JNK. Biochem Biophys Res Commun. 2004;320(3):773–780. doi: 10.1016/j.bbrc.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- Hoshikawa Y, Kanki K, Ashla AA, Arakaki Y, Azumi J, Yasui T, Tezuka Y, Matsumi Y, Tsuchiya H, Kurimasa A, Hisatome I, Hirano T, Fujimoto J, Kagechika H, Shomori K, Ito H, Shiota G. c-Jun N-terminal kinase activation by oxidative stress suppresses retinoid signaling through proteasomal degradation of RARalpha protein in hepatic cells. Cancer Sci. 2011;102:934–941. doi: 10.1111/j.1349-7006.2011.01889.x. [DOI] [PubMed] [Google Scholar]
- Jacques PF, Hartz SC, Chylack LT, Jr, McGandy RB, Sadowski JA. Nutritional status in persons with and without senile cataract: blood vitamin and mineral levels. Am J Clin Nutr. 1988;48(1):152–158. doi: 10.1093/ajcn/48.1.152. [DOI] [PubMed] [Google Scholar]
- Krempf M, Ranganathan S, Ritz P, Morin M, Charbonnel B. Plasma vitamin A and E in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) adult diabetic patients. Int J Vitam Nutr Res. 1991;61(1):38–42. [PubMed] [Google Scholar]
- Lee HY, Suh YA, Robinson MJ, Clifford JL, Hong WK, Woodgett JR, Cobb MH, Mangelsdorf DJ, Kurie JM. Stress pathway activation induces phosphorylation of retinoid × receptor. J Biol Chem. 2000;275(41):32193–32199. doi: 10.1074/jbc.M005490200. [DOI] [PubMed] [Google Scholar]
- Liang JL, Xiao DZ, Liu XY, Lin QX, Shan ZX, Zhu JN, Lin SG, Yu XY. High glucose induces apoptosis in AC16 human cardiomyocytes via macrophage migration inhibitory factor and c-Jun N-terminal kinase. Clin Exp Pharmacol Physiol. 2010;37(10):969–973. doi: 10.1111/j.1440-1681.2010.05420.x. [DOI] [PubMed] [Google Scholar]
- Lu J, Dixon WT, Tsin AT, Basu TK. The metabolic availability of vitamin A is decreased at the onset of diabetes in BB rats. J Nutr. 2000;130(8):1958–1962. doi: 10.1093/jn/130.8.1958. [DOI] [PubMed] [Google Scholar]
- Matsushima-Nishiwaki R, Okuno M, Adachi S, Sano T, Akita K, Moriwaki H, Friedman SL, Kojima S. Phosphorylation of retinoid × receptor alpha at serine 260 impairs its metabolism and function in human hepatocellular carcinoma. Cancer Res. 2001;61(20):7675–7682. [PubMed] [Google Scholar]
- Minucci S, Ozato K. Retinoid receptors in transcriptional regulation. Curr Opin Genet Dev. 1996;6(5):567–574. doi: 10.1016/s0959-437x(96)80085-2. [DOI] [PubMed] [Google Scholar]
- Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- Murarka S, Movahed MR. Diabetic cardiomyopathy. J Card Fail. 2010;16(12):971–979. doi: 10.1016/j.cardfail.2010.07.249. [DOI] [PubMed] [Google Scholar]
- Napoli JL. Quantification of physiological levels of retinoic acid. Methods Enzymol. 1986;123:112–124. doi: 10.1016/s0076-6879(86)23015-3. [DOI] [PubMed] [Google Scholar]
- Napoli JL. Biochemical pathways of retinoid transport, metabolism, and signal transduction. Clin Immunol Immunopathol. 1996;80(3 Pt 2):S52–62. doi: 10.1006/clin.1996.0142. [DOI] [PubMed] [Google Scholar]
- Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid × receptor-alpha in pacing-induced heart failure. Circulation. 2002;106(5):606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- Palm-Leis A, Singh US, Herbelin BS, Olsovsky GD, Baker KM, Pan J. Mitogen-activated protein kinases and mitogen-activated protein kinase phosphatases mediate the inhibitory effects of all-trans retinoic acid on the hypertrophic growth of cardiomyocytes. J Biol Chem. 2004;279(52):54905–54917. doi: 10.1074/jbc.M407383200. [DOI] [PubMed] [Google Scholar]
- Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, Takahashi T, Kato T, Ogawa S. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1999;84(10):1127–1136. doi: 10.1161/01.res.84.10.1127. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15(4):355–366. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C, Adam S, Rossignol M, Egly JM, Chambon P. Stimulation of RAR alpha activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell. 1997;90(1):97–107. doi: 10.1016/s0092-8674(00)80317-7. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C, Oulad-Abdelghani M, Staub A, Pfister V, Scheuer I, Chambon P, Gaub MP. Phosphorylation of the retinoic acid receptor-alpha by protein kinase A. Mol Endocrinol. 1995;9(7):860–871. doi: 10.1210/mend.9.7.7476969. [DOI] [PubMed] [Google Scholar]
- Sabio G, Kennedy NJ, Cavanagh-Kyros J, Jung DY, Ko HJ, Ong H, Barrett T, Kim JK, Davis RJ. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol Cell Biol. 2010;30(1):106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar J, Guardiola M, Ferre R, Coll B, Alonso-Villaverde C, Winklhofer-Roob BM, Rock E, Fernandez-Ballart JD, Civeira F, Pocovi M, Masana L, Ribalta J. Association of a polymorphism in the promoter of the cellular retinoic acid-binding protein II gene (CRABP2) with increased circulating low-density lipoprotein cholesterol. Clin Chem Lab Med. 2007;45(5):615–620. doi: 10.1515/CCLM.2007.131. [DOI] [PubMed] [Google Scholar]
- Solomon C, White JH, Kremer R. Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid × receptor alpha. J Clin Invest. 1999;103(12):1729–1735. doi: 10.1172/JCI6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas H, Juroske DM, Kalyankrishna S, Cody DD, Price RE, Xu XC, Narayanan R, Weigel NL, Kurie JM. c-Jun N-terminal kinase contributes to aberrant retinoid signaling in lung cancer cells by phosphorylating and inducing proteasomal degradation of retinoic acid receptor alpha. Mol Cell Biol. 2005;25(3):1054–1069. doi: 10.1128/MCB.25.3.1054-1069.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Zhang W, Lin H, Jiang H, Dai H, Zhang Y. High glucose promotes the production of collagen types I and III by cardiac fibroblasts through a pathway dependent on extracellular-signal-regulated kinase 1/2. Mol Cell Biochem. 2007;301(1–2):109–114. doi: 10.1007/s11010-006-9401-6. [DOI] [PubMed] [Google Scholar]
- Thandavarayan RA, Watanabe K, Ma M, Gurusamy N, Veeraveedu PT, Konishi T, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Dominant-negative p38alpha mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am J Physiol Heart Circ Physiol. 2009;297(3):H911–919. doi: 10.1152/ajpheart.00124.2009. [DOI] [PubMed] [Google Scholar]
- Tuitoek PJ, Ziari S, Tsin AT, Rajotte RV, Suh M, Basu TK. Streptozotocin-induced diabetes in rats is associated with impaired metabolic availability of vitamin A (retinol) Br J Nutr. 1996;75(4):615–622. doi: 10.1079/bjn19960164. [DOI] [PubMed] [Google Scholar]
- Turner NA, Xia F, Azhar G, Zhang X, Liu L, Wei JY. Oxidative stress induces DNA fragmentation and caspase activation via the c-Jun NH2-terminal kinase pathway in H9c2 cardiac muscle cells. J Mol Cell Cardiol. 1998;30(9):1789–1801. doi: 10.1006/jmcc.1998.0743. [DOI] [PubMed] [Google Scholar]
- Vallerie SN, Hotamisligil GS. The role of JNK proteins in metabolism. Sci Transl Med. 2010;2(60):60rv65. doi: 10.1126/scitranslmed.3001007. [DOI] [PubMed] [Google Scholar]
- Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 2009;58(1):146–155. doi: 10.2337/db08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Zhu YC, Yao T. Effects of all-trans retinoic acid on angiotensin II-induced myocyte hypertrophy. J Appl Physiol. 2002;92(5):2162–2168. doi: 10.1152/japplphysiol.01192.2001. [DOI] [PubMed] [Google Scholar]
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97(7):1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Waki H, Kamon J, Murakami K, Motojima K, Komeda K, Miki H, Kubota N, Terauchi Y, Tsuchida A, Tsuboyama-Kasaoka N, Yamauchi N, Ide T, Hori W, Kato S, Fukayama M, Akanuma Y, Ezaki O, Itai A, Nagai R, Kimura S, Tobe K, Kagechika H, Shudo K, Kadowaki T. Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J Clin Invest. 2001;108(7):1001–1013. doi: 10.1172/JCI12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Zhou H, Li YJ, Wang M, Zhang LH, Guo BY, Zhao ZS, Meng FL, Deng YG, Wang RY. Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol Sin. 2011;32(8):999–1008. doi: 10.1038/aps.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MD, Sucov HM, Evans RM, Chien KR. Retinoid-dependent pathways suppress myocardial cell hypertrophy. Proc Natl Acad Sci U S A. 1995;92(16):7391–7395. doi: 10.1073/pnas.92.16.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Gianni M, Kopf E, Honore N, Chelbi-Alix M, Koken M, Quignon F, Rochette-Egly C, de The H. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RARalpha) and oncogenic RARalpha fusion proteins. Proc Natl Acad Sci U S A. 1999;96(26):14807–14812. doi: 10.1073/pnas.96.26.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizola CF, Frey SK, Jitngarmkusol S, Kadereit B, Yan N, Vogel S. Cellular retinol-binding protein type I (CRBP-I) regulates adipogenesis. Mol Cell Biol. 2010;30(14):3412–3420. doi: 10.1128/MCB.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]