Abstract
Acute neuroinflammation reduces adult hippocampal neurogenesis but the role of chronic neuroinflammation, which may be more representative of ongoing processes in CNS disorders, remains relatively unknown. Interleukin-1β (IL-1β) is a pro-inflammatory cytokine that has been shown to acutely impair neurogenesis. To further investigate the relationship between sustained IL-1β expression and adult neurogenesis, a mouse model with an IL-1β excisionally activated transgene, IL-1βXAT, was utilized. Upon exposure to Cre recombinase, IL-1β overexpression in this model results in chronic neuroinflammation, which persists up to 12 months and causes glial activation, cellular recruitment, and deficits in learning and memory. We hypothesized that adult neurogenesis would be reduced by sustained hippocampal IL-1β overexpression and rescued by voluntary running, which has been shown to enhance neurogenesis. Hippocampal inflammation in the IL-1βXAT model severely impaired doublecortin (DCX) positive cells at 1 month and 3 months after IL-1β induction. Furthermore, BrdU labeling demonstrated a shift in cell lineage from neuronal to astroglial in the context of sustained hippocampal IL-1β overexpression. Deletion of the IL-1 receptor prevented the decrease in DCX+ cells. Voluntary running did not attenuate the effects of IL-1β expression demonstrated by DCX staining. These results suggest that chronic neuroinflammation severely impairs adult hippocampal neurogenesis and voluntary running is not beneficial as a therapy to rescue these effects.
Keywords: neuroinflammation, interleukin-1, adult, neurogenesis, running
Introduction
During development, neural precursor cells (NPCs) give rise to several cell types of the adult brain including neurons in a process known as neurogenesis. Following development, neurogenesis persists in two specific regions in the adult: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus. NPCs in the adult continue to give rise to new neurons but also have the ability to become glia under certain conditions. Adult hippocampal neurogenesis is thought to be important in learning and memory (Deng et al., 2010; Leuner et al., 2006) and is involved in injury models such as stroke, seizure, trauma, or radiation (Kaneko and Sawamoto, 2009; Monje, 2008). More recent evidence suggests that adult neurogenesis may be involved in other behaviors such as stress (Ben Menachem-Zidon et al., 2008; Goshen et al., 2008; Koo and Duman, 2008; Lagace et al., 2010) and depressive-like behavior (David et al., 2009; Sahay and Hen, 2007). Adult neurogenesis is also affected by a wide range of stimuli including environmental enrichment (Goshen et al., 2009), growth factors (Cao et al., 2004; Rossi et al., 2006; Sairanen et al., 2005; Trejo et al., 2008), and anti-depressants (David et al., 2009; Encinas et al., 2006; Malberg et al., 2000; Santarelli et al., 2003).
One important mediator of adult neurogenesis that is being closely examined is neuroinflammation. Neuroinflammation is the local CNS response to injury that involves phenotypic activation of glia, specifically microglia and astrocytes, recruitment of inflammatory cells, and the upregulation of chemokines, cytokines, and other inflammatory mediators. Neuroinflammation is a feature of stroke, brain trauma, epilepsy, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, motor neuron disease, and movement disorders (Lucas et al., 2006). In general, neuroinflammation is considered detrimental to adult hippocampal neurogenesis. For example, treatment with a non-steroidal anti-inflammatory drug partially rescues hippocampal neurogenesis after exposure to radiation (Monje et al., 2003). Previous work has focused on interleukin(IL)-6 and tumor necrosis factor alpha (TNFα) as potential candidates (Monje et al., 2003; Vallieres et al., 2002), but the exact mechanism through which neuroinflammation downregulates adult neurogenesis is unknown.
One potential candidate for effecting reduced neurogenesis is the pro-inflammatory cytokine interleukin-1 (IL-1). IL-1 is rapidly upregulated with injury and is implicated in the pathogenesis of Alzheimer’s disease, traumatic brain injury, epilepsy, stroke, and Parkinson’s disease (Allan et al., 2005). The IL-1 family consists of two agonists, cell-bound IL-1α and soluble IL-1β, that signal via the type 1 IL-1 receptor (IL-1R1). In the CNS, IL-1 can act on resident cells to induce expression of other cytokines and chemokines, activate glia, and recruit inflammatory cells (Allan et al., 2005). The IL-1 family is an ideal system to understand the effect of a pro-inflammatory cytokine on adult hippocampal neurogenesis due to a well characterized signaling cascade and a single functional receptor with a high density in the hippocampus (Cunningham et al., 1992). Several groups have shown that acute increases in IL-1β decrease adult hippocampal neurogenesis and blocking IL-1 signaling can abrogate this effect (Ben Menachem-Zidon et al., 2008; Goshen et al., 2008; Koo and Duman, 2008). These studies involved in vitro approaches and acute exposure paradigms, but the effect of chronic IL-1-dependent inflammation on adult neurogenesis in vivo is not as well understood.
To study the effect of chronic neuroinflammation on adult neurogenesis, we utilized a recently developed model of sustained interleukin-1β (IL-1β) expression, the IL-1βXAT mouse model, which mimics chronic neuroinflammation observed in neurodegenerative diseases. We have previously shown that IL-1β overexpression in our model results in chronic neuroinflammation which persists up to 12 months and causes glial activation, cellular recruitment, and deficits in learning and memory (Hein et al., 2010; Moore et al., 2009; Shaftel et al., 2007a; Shaftel et al., 2007b). We wanted to determine whether sustained IL-1β expression in the adult hippocampus of IL-1βXAT mice would decrease neurogenesis. Additionally, we also evaluated whether voluntary running would be beneficial in the context of ongoing neuroinflammation, as studies have shown that such a stimulus can increase adult hippocampal neurogenesis in conditions that adversely affect it (Naylor et al., 2008; Wu et al., 2007). These results may have important implications for therapies in chronic neuroinflammatory diseases where adult neurogenesis is adversely affected.
Methods
Animals
All protocols were approved by the Institutional Animal Care and Use Committee at the University of Rochester. Wild-type and IL-1βXAT C57BL/6 mice aged 8–12 weeks were used in these studies. The development and characterization of the IL-1βXAT mouse has been described previously (Shaftel et al., 2007b). Briefly, IL-1βXAT mice contain a ssIL-1β transgene driven by a GFAP promoter that is transcriptionally silent due to an upstream loxP flanked transcriptional stop. ssIL-1β encodes the signal sequence from the human IL-1 receptor antagonist (hIL-1Ra) fused to human IL-1β (hIL-1β) cDNA, producing a mature, secreted hIL-1β that does not require caspase-1 cleavage (Wingren et al., 1996). Expression of ssIL-1β is achieved by injection of a feline immunodeficiency virus (FIV) expressing Cre recombinase, which allows temporal and spatial control of IL-1β expression. IL-1βXAT animals were crossed to IL-1r1−/− mice (stock no. 2724, The Jackson Laboratory) to block the activity of IL-1β signaling. Genotyping of animals was performed using appropriate primers (Shaftel et al., 2007b) from tail snips digested with PrepGem (Zygem, Solana Beach, CA).
Feline immunodeficiency viruses
FIV(Cre), FIV(GFP) (System Biosciences, Mountain View, CA), and FIV(lacZ) (Invitrogen, Carlsbad, CA) were utilized. FIV(Cre) was created and packaged as previously described (Lai et al., 2006). FIV(Cre) encodes for Cre recombinase, a nuclear localization sequence, and V5 epitope tag under a cytomegalovirus promoter. All viruses were packaged with a final titer of ~107 infectious viral particles/mL.
Stereotactic injections
Animals received intrahippocampal viral injections while under isoflurane anesthesia (1.75% isoflurane in 30/70% oxygen/nitrogen gas) using a Kopf stereotactic apparatus. Mice were secured using ear bars and a head holder. Ophthalmic ointment was applied to prevent drying of the eyes. Betadine was used to disinfect the scalp prior to incision with a scalpel. A 0.5 mm burr hole was drilled 1.8 mm caudal and 1.8 mm lateral from Bregma. 1.5 μL of FIV was injected 1.8 mm deep from the dura surface. In order to determine the cell fate of SGZ neural progenitors, a subset of animals received additional injections at 0.3 mm caudal and 1.0 mm lateral from Bregma and 2 mm deep from the dura of 12 μg i.c.v. 5-bromo-2′-deoxyuridine (BrdU) 24 h or 4 weeks prior to hippocampal viral injections via a 0.5 mm burr hole (adapted from Kokoeva et al., 2007). For both types of injections, a 33 gauge needle affixed to a 10 μL syringe (Hamilton, Reno, NV) was utilized. The needle was lowered over 2 minutes and injections proceeded at a rate of 150 nL/min using a Micro-1 microsyringe pump controller (World Precision Instruments, Sarasota, FL). The needle was left in place for 5 minutes to allow for adequate diffusion before being retracted over 2 minutes. The burr hole was filled with bone wax (Ethicon, Somerville, NJ) and the incision closed with 5-0 suture Dermalon (Covidien Mansfield, MA). Betadine and topical lidocaine were applied to the top of the suture to prevent infection and for analgesia, respectively. Mice were allowed to recover in a heated recovery area before being placed in their home cage. Animals were sacrificed at either 2 weeks, 1 month, or 3 months post viral injection.
Immunohistochemistry
Animals were deeply anesthestized using a mixture of ketamine and xylazine (50 mg ketamine/10 mg xylazine) before transcardial perfusion via the left ventricle. A perfusate containing 0.15 M phosphate buffer with 0.5% w/v sodium nitrite and 2 IU/mL heparin was flushed through each animal to dilate blood vessels and prevent clotting. This was followed by perfusion with approximately 50 mL of freshly-made, ice-cold 4% paraformaldehyde (PFA) in 0.15 M phosphate buffer, pH 7.2. Whole brains were immersion fixed for 2 h at 4°C, dehydrated overnight in 30% sucrose in 0.15 M phosphate buffer, and snap frozen using dry ice and isopentane. Our 3 month time point utilized tissue from a previous behavioral study (Hein et al., 2011) in which half-brains were collected differently. For these half-brains, a similar protocol was followed except only the sodium nitrite/heparin perfusate was used, and half-brains were immersion fixed for 24 h at 4°C before being processed similarly. Brains were sectioned coronally at 30 μm on a sliding microtome. Staining of free-floating sections was performed using rat anti-mouse major histocompability complex class II (MHC-II) antibody (BD Pharmingen #556999) diluted 1:5000, goat anti-doublecortin (DCX) antibody (Santa Cruz #sc-8066) diluted 1:1000, rat anti-bromodeoxyuridine (BrdU) (Abcam #ab6326) diluted 1:300, biotinylated mouse anti-NeuN (Chemicon #MAB377B) diluted 1:500, rabbit anti-glial fibrillary acidic protein (GFAP) (Dako #Z0334) diluted 1:500, or rabbit anti-ionized calcium binding adapter protein 1 (Iba-1) (Wako #016-20001) diluted 1:2000. Sections were then visualized using Elite Vectastain ABC and 3,3-diaminobenzidine kits (Vector #PK-6100 and SK-4100) or Alexa Fluor conjugated secondary antibodies (Invitrogen) and coverslipped with DPX (Electron Microscopy Sciences #13512) or Prolong Gold (Invitrogen #P36930), respectively. Hoechst 33258 (Invitrogen #H3569) staining was used to visualize nuclei for fluorescent images. DCX antigen retrieval was performed by microwaving for 15 min in 0.1 M Na Citrate pH 9 or incubating 20 min at room temperature in L.A.B. solution (Polysciences, Warrington, PA). For BrdU, incubating in 4 N HCl for 30 min at room temperature was necessary for antigen retrieval. Images were captured on a Zeiss Axioplan IIi microscope or Olympus FV1000 laser scanning confocal microscope.
Voluntary running
For the voluntary running experiment, Fast Trac Blue with Mouse Igloos (Bioserv, Frenchtown, NJ #K3251, K3328) were used for running wheels. Animals were pair-housed in standard cages with free access to a functional (n = 6 females and 2 males) or non-functional running wheel (n = 4 females and 2 males). They were habituated to the running wheels for 2 weeks before FIV(Cre) stereotactic injections into the hippocampus and were sacrificed a month later and processed as described above. One animal in the control group was removed as an outlier based on quantification of DCX+ cells that was greater than two standard deviations from the mean.
Analysis
Ipsilateral, FIV(Cre)-injected regions were compared to the corresponding contralateral, non-injected side when applicable. For DCX counts, both hippocampi were quantified from a representative section around the injection site. Quantification was done at 40X on a Zeiss Axioplan IIi while blinded to the identity of each animal. For BrdU analysis, individual BrdU+ cells were identified at 60× and a z-stack of images was taken on an Olympus FV1000 laser scanning confocal microscope. Colocalization with NeuN or GFAP was determined using Olympus Fluoview Ver. 2.0c by scanning through each stack to ensure proper double labeling within each plane. Statistical significance (p < 0.05) was determined by Student’s t-test or analysis of variance (ANOVA) followed by a Bonferroni post-hoc test.
Software
Statistical analysis as well as graphs were made using Prism 5 (GraphPad, San Diego, CA). Resizing of images was done using Photoshop (Adobe) or GNU Image Manipulation Program Ver 2.4.7 (gimp.org). Layout was done using vector graphics editor Inkscape Ver 0.48.0 (inkscape.org).
Results
1. Sustained hippocampal IL-1β expression causes focal inflammation and reduces adult neurogenesis
One month after FIV(Cre) administration, we confirmed the presence of inflammatory cells in unilaterally FIV(Cre)-injected IL-1βXAT mice. We found robust expression of MHC-II staining in the ipsilateral, FIV(Cre) injected hippocampus and did not observe any MHC-II+ cells in the contralateral, uninjected hippocampus (Fig. 1). Focal staining indicated that IL-1β-dependent inflammation was confined to the FIV(Cre) injected area. We did not detect enhanced MHC-II staining in WT animals that received FIV(Cre) in either the ipsilateral or contralateral hippocampi. This lack of MHC-II expression indicated that FIV(Cre) alone was not immunogenic at 1 month after injection. We observed similar results at 2 weeks and 3 months after injection (data not shown). These findings support our previously published results showing that FIV(Cre) induction of IL-1β expression in the IL-1βXAT model induces focal and persistent neuroinflammation within the hippocampus (Hein et al., 2010; Moore et al., 2009; Shaftel et al., 2007a; Shaftel et al., 2007b).
Figure 1. Sustained IL-1β overexpression causes focal activation of MHC-II+ cells in the inflamed hippocampus.
Staining for MHC-II in the IL-1βXAT model 1 month post-injection of FIV(Cre). Scale bars = 50μm.
To understand the effect of sustained IL-1β on adult neurogenesis, we stained tissues for DCX, a marker of migrating neuroblasts. One month after intrahippocampal injection, we found a significant interaction effect between viral injection and genotype using a 2-way ANOVA (F (2,24) = 14.74, p = 0.0004, Fig. 2). There was a significant decrease (94%) in DCX+ cells in the FIV(Cre)-injected hippocampus of IL-1βXAT animals relative to the contralateral side (p < 0.001). Thus, the decrease in DCX+ cells mirrored the pattern of neuroinflammation. Interestingly, WT animals (n = 14) also showed a decrease in DCX+ cells on the FIV(Cre)-injected hippocampus at one month, though the decrease was much smaller (25%, p < 0.05). To determine if this effect persisted beyond 1 month, we looked at DCX+ cells after 3 months exposure to IL-1β in a separate experiment where mice received bilateral injections of the same virus. A 1-way ANOVA revealed a significant main effect between groups (F (2,24) = 10.40, p = 0.0006, Fig. 2C). We found a significant reduction (87%) in DCX+ cells 3 months after IL-1βXAT animals received FIV(Cre) (n = 8) when compared to IL-1βXAT that received FIV(GFP) (n = 10), a viral control (p < 0.001). We could not detect a significant difference between WT+FIV(Cre) (n = 9) and IL-1βXAT+FIV(GFP) suggesting that neither the vector nor the transgene alone are capable of eliciting a strong reduction of DCX+ cells. These results indicate that the effect of sustained IL-1β expression on DCX+ cell populations persists for at least 3 months.
Figure 2. Chronic hippocampal IL-1β overexpression ablates adult neurogenesis.
(A) Doublecortin staining (green) and Hoechst-stained nuclei (blue) of hippocampal sections from adult IL-1βXAT and control WT mice one month after unilateral FIV(Cre) injection into the dentate gyrus. Scale bars = 50μm. (B,C) Quantification of DCX+ cells in inflamed hippocampi revealed significant decreases at 1 month and 3 months post-induction. n = 11–15 animals/group, 2-way ANOVA for 1 month, and n = 8–9 animals/group, 1-way ANOVA for 3 months. No significant difference was seen between IL-1βXAT + FIV(GFP) and WT + FIV(Cre) groups (C). Data represent means ± SEM. * = p<0.05, ** = p<0.01, *** = p<0.001.
We previously showed that microglia, astrocytes, neutrophils, T cells, and antigen presenting cells (APCs) are activated in or recruited to the hippocampus of our IL-1βXAT model (Shaftel et al., 2007a; Shaftel et al., 2007b). In order to examine the spatial relationship between DCX+ cells and inflammatory cells in the hippocampi of IL-1βXAT mice that had been injected with FIV(Cre), we stained for DCX along with the following markers: Iba-1 (microglia), GFAP (astrocytes), 7/4 (neutrophils), CD3 (T cells), and MHC-II (APCs). Iba-1 staining reaffirmed that IL-1β caused focal inflammation on the ipsilateral, FIV(Cre)-injected hippocampus as demonstrated by the increased numbers of activated microglia with thicker processes (Fig. 3). We did not observe these changes in the non-injected, contralateral side where microglia maintained a ramified, quiescent morphology. Microglia were not clustered around DCX+ cells regardless of the inflammatory environment suggesting no clear association of microglia with DCX+ cells. When we looked at other markers, we found robust recruitment of peripheral inflammatory cells (neutrophils, T cells, APCs) as well as astrogliosis but did not find clear evidence that any of these cell types directly contacted or surrounded DCX+ cells (data not shown). It should be noted that DCX+ cells were relatively rare in inflamed hippocampi and tended to occur in the ventral portions of the hippocampus.
Figure 3. Staining for activated Iba-1+ microglia as well as DCX+ cells in the presence of sustained IL-1β expression.
FIV(Cre)-injected hippocampus of IL-1βXAT showed robust microglial activation as shown by increased numbers of Iba-1+ cells with thicker processes 1 month after injection. No direct association with DCX+ cells was seen. Iba-1 = red, DCX = green, Hoechst-stained nuclei = blue. Scale bars = 5μm.
2. The fate of neural progenitors is shifted towards an astrocytic phenotype in the presence of sustained IL-1β expression
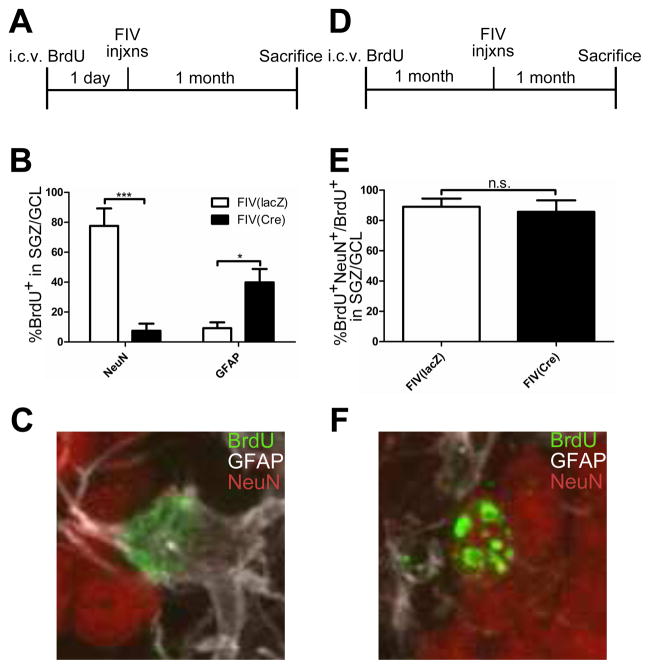
To understand how sustained IL-1β affected cell fate, we carried out BrdU injections in a separate set of experiments. Traditionally, intraperitoneal BrdU injections have been administered to label dividing neural progenitors in the CNS. This paradigm was not feasible using our IL-1βXAT model due to the large recruitment of peripheral inflammatory cells that were also labeled by systemic BrdU administration. Instead, we injected BrdU directly into the ventricles of the brain prior to hippocampal FIV injections in order to fate map dividing neural progenitors and their offspring in the CNS without the interference of peripherally derived cells (adapted from Kokoeva et al., 2007). We used IL-1βXAT animals in these experiments and injected FIV(Cre) in one hemisphere and FIV(lacZ) into the other, providing an internal control within each animal. In the first experiment, we labeled dividing cells in IL-1βXAT mice (n = 5) with BrdU 1 day before FIV administration and sacrificed them a month later (Fig. 4A). A 2-way ANOVA revealed a significant interaction effect between viral injection and cell marker (F (1,12) = 39.95, p < 0.0001). We observed a significant reduction in the percentage of BrdU+ cells that colocalized with NeuN in the FIV(Cre) side compared to the FIV(lacZ) side suggesting that cells labeled 29 days previously were not developing into mature neurons (−70%, p < 0.01, Fig. 4B,F). In contrast, we found that a larger percentage of BrdU+ cells developed into astrocytes as shown by GFAP reactivity (32%, p < 0.05, Fig. 4B). Thicker processes and hypertrophic cell bodies seen with GFAP staining confirmed that the BrdU+ cells had developed into reactive astrocytes due to ongoing inflammation (Fig. 4C). When IL-1βXAT mice were injected with BrdU 1 month before FIV injections, no significant difference in the percentage of BrdU+ cells that colocalized with NeuN was observed between FIV(Cre) and FIV(lacZ) hippocampi (n = 4, Fig. 4D,E). Taken together, these results suggest that IL-1β overexpression for 1 month skews the fates of immature neural progenitors (BrdU-labeled 24 h prior) to a glial fate and away from a neuronal lineage but does not adversely affect recently differentiated neurons over the time period studied.
Figure 4. Newborn cells in the SGZ fail to develop into neurons and instead become astroglia in the context of IL-1β overexpression.
Experimental design for pulse-labeling with intracerebroventricular BrdU (A,D). IL-1βXAT mice received two intrahippocampal injections, one on each side: FIV(Cre) and FIV(lacZ). Quantification of BrdU+ cells labeled 29 days and 8 weeks previously that colocalize with neuronal (NeuN) and astroglial (GFAP) markers (B,E). Representative immunofluorescent images of BrdU+GFAP+ cells and BrdU+NeuN+ cells (C,F). Images taken at 60X. n = 4 animals per time point, 2-way ANOVA or Student’s t-test. Data represent means ± SEM. * = p<0.05, *** = p<0.001.
3. Deletion of the IL-1 receptor inhibits the detrimental effect of IL-1β
We have previously shown that the inflammation seen in the IL-1βXAT model is dependent on IL-1R1 (Shaftel et al., 2007b). We stained sections from these unilaterally FIV(Cre)-injected IL-1βXAT animals that were wild-type, heterozygous, or knocked out for IL-1R1 to determine whether DCX+ cells were affected by the absence of IL-1R1 in the context of sustained IL-1β overexpression (n = 3). A 2-way ANOVA revealed a significant interaction between viral injection and genotype (F (2,12) = 5.013, p < 0.05, Fig. 5). No significant change in DCX+ cells was seen between the FIV(Cre)-injected hippocampi and the contralateral, uninjected hippocampi of IL-1βXAT, IL-1R1−/− animals showing that the decrease in DCX+ cells was dependent on the presence of IL-1R1. Significant decreases were found in DCX+ cell numbers in IL-1βXAT mice that retained at least one wild-type allele for IL-1R1 suggesting that only homozygous knockouts were protected from IL-1β (n = 3/group; −79%, p < 0.001 for IL-1R1+/+; −72%, p < 0.01 for IL-1R1+/−). Interestingly, the presence or absence of IL-1R1 did not significantly alter the number of DCX+ cells in the non-inflamed, contralateral hippocampi suggesting that basal signaling through IL-1R1 did not have an impact on adult neurogenesis.
Figure 5. The negative effect of IL-1β on DCX+ cells is dependent on the type 1 IL-1 receptor.
Quantification of DCX+ cells from FIV(Cre)-injected IL-1βXAT animals 2 weeks post-injection that are wild-type (+/+), heterozygote (+/−), or knockouts (−/−) for the type 1 IL-1 receptor, n = 3 animals/group, 2-way ANOVA. Data represent means ± SEM. ** = p<0.01, *** = p<0.001.
4. Voluntary running does not increase DCX+ cells in the inflamed hippocampus
Voluntary running, a form of environmental enrichment, has been shown by others to increase adult neurogenesis including the number of DCX+ cells (Naylor et al., 2008; van Praag et al., 1999a; van Praag et al., 1999b). To investigate whether voluntary running would be beneficial to neurogenesis in a sustained inflammatory environment, IL-1βXAT mice were split into two groups with access to a functional or non-functional running wheel. Animals were pair-housed with free access to their functional/nonfunctional wheel for the entire 6 weeks. Mice were habituated to their environment for 2 weeks before unilateral FIV(Cre) injections and were sacrificed a month later (Fig. 6A). Quantification of DCX+ cells demonstrated that IL-1β essentially eliminated this population regardless of physical activity (Fig. 6B). A 2-way ANOVA demonstrated a significant interaction effect between viral injection and group (F (1,22) = 7.122, p = 0.014). A post-hoc Bonferroni test revealed no significant difference between the numbers of DCX+ cells in FIV(Cre)-injected hippocampi in either the runners or controls (p > 0.05). The contralateral, uninjected side demonstrated a significant increase in DCX+ cells in the running group by 41% compared to controls suggesting that running did increase neurogenesis in the absence of inflammation (p < 0.001). These results indicate that voluntary running was able to increase neurogenesis under basal conditions but could not restore neurogenesis in the inflamed hippocampus.
Figure 6. Voluntary running does not alleviate the detrimental effect of IL-1β on DCX+ cells in the SGZ.
Experimental design for IL-1βXAT animals that were given access to non-functional (controls) or functional running wheels for 6 weeks prior to sacrifice (A). Quantification of DCX staining demonstrated that voluntary running increased cell numbers only on the contralateral, uninjected hippocampi (B). n = 6–8 animals/group, 2-way ANOVA. Data are means ± SEM. ** = p<0.01.
Discussion
Our data agree with previous evidence that inflammation is detrimental to hippocampal neurogenesis in the adult brain. We found that focal, sustained hippocampal inflammation causes severe depletion of developing neuroblasts and skews the fate of neural progenitors in the SGZ away from a neuronal lineage and toward an astroglial fate in the adult brain. Interestingly, if offspring from neural progenitors are allowed to mature, they are resistant to the effects of sustained expression of IL-1β. This resistance suggests that the blockade caused by IL-1β occurs prior to the commitment of neural progenitors to a neuronal fate. In contrast, Mathieu and colleagues saw a decrease in BrdU-labeled cells that developed into neurons but no difference in astroglial differentiation after 14 days using an adenovirus expressing human IL-1β in rats (Mathieu et al., 2010). Belarbi and colleagues recently showed no alteration in total numbers of BrdU labeled neurons in rats pretreated with 28 days of intraventricular infusion of lipopolysaccharide (Belarbi et al., 2011). Differences between our models of neuroinflammation as well as timing may account for these conflicting results.
Our study does not address how sustained IL-1β affects other cell populations. For example, the effect of sustained IL-1β on NPCs in the SGZ of the hippocampus is unknown. Common markers for NPCs, such as nestin and GFAP, are upregulated in reactive astrocytes due to inflammation and distinguishing between NPCs and reactive astrocytes becomes difficult. Pulse labeling with BrdU should label dividing NPCs prior to the onset of inflammation but would not indicate their presence or absence a month or more later. Other more specific methods of examining NPC survival will be needed for further analysis.
This work shows that inflammation inhibits neurogenesis but the mechanism remains unclear. The absence of DCX+ cells is confined to the inflamed hippocampus illustrating a change in the microenvironment. One potential alteration may be disruption of nutrients being supplied by the microvasculature or exposure to CSF. For example, vascular endothelial growth factor has been shown to increase adult hippocampal neurogenesis (Cao et al., 2004). Another possibility is a cell-mediated disruption of DCX+ cells. We did not observe any direct association of DCX+ cells with microglia, astrocytes, T cells, neutrophils, or MHC-II+ cells. This finding contrasts with results from Sierra and colleagues who recently demonstrated that microglia phagocytose apoptotic neural progenitors as part of normal clearance (Sierra et al., 2010). Unfortunately, the late time points in the current study are insufficient to draw any clear conclusions about whether clearance by microglia is occurring. In addition, at the time points examined in this study, surviving DCX+ cells were rare and found distal to the FIV(Cre) injection site in sections less populated with inflammatory cells. Given the complexity of the inflammatory environment, multiple factors may contribute to the inflammatory induced suppression of neurogenesis.
Our findings also show that voluntary exercise increases neurogenesis only in the absence of neuroinflammation. The mechanism of exercise-induced increase in neurogenesis is unknown, but our results suggest that it does not act or is insufficient in the presence of inflammation. This contrasts with a recent study demonstrating forced running prevented reduction in BrdU-labeled DCX+ cells in the SGZ following repetitive i.p. lipopolysaccharide injections in adult mice (Wu et al., 2007). Some of the differences between our study and the study by Wu and colleagues may be related to distinctions in central versus peripheral inflammatory models as well as duration of inflammation. It is interesting that we see a beneficial effect of voluntary running in a non-inflamed region of the brain in animals that have focal inflammation. Differences between animals regarding access to the running wheel, distance traveled, and running speed may have existed. We purposely housed only two animals per running wheel to allow each animal as much access as possible and to avoid social isolation that can downregulate neurogenesis. We visually observed running by all animals but did not monitor distance traveled or running speed which may be possible confounders. We also controlled for the presence of the running wheel as a source of environmental enrichment. Due to the pronounced negative effect of sustained IL-1β on the DCX+ cell population, a floor effect is likely to have occurred where the sustained inflammation was so severe that it could not be overcome by exercise. Interestingly, in a model of peripheral E. coli infection, running was shown to reduce IL-1β in aged rats (Barrientos et al., 2011). Because we saw no improvement in DCX+ cell survival due to exercise in the presence of human IL-1β, we did not examine any molecular changes in endogenous markers such as levels of caspase-1, IL-1Ra, murine IL-1β, etc. Further studies examining levels of endogenous cytokines could be utilized to address this issue.
Our results about the negative effect of increased IL-1β on DCX+ neuroblasts in the SGZ are consistent with previous reports of decreased adult hippocampal neurogenesis due to IL-1β caused by stress or i.c.v. infusion (Goshen et al., 2008; Koo and Duman, 2008). Our model differs by the injection of virus directly into the hippocampus that may contribute to decreased neurogenesis. Indeed, WT animals that received a similar viral injection exhibited a modest decrease in DCX+ cells. However, the effect with virus that activated IL-1β expression was much greater. We were able to prevent this outcome by disrupting IL-1β signaling using animals deficient in IL-1R1; similarly, other groups have shown that IL-1β is less harmful in IL-1R1 knockouts or in the presence of exogenous IL-1Ra (Goshen et al., 2008; Koo and Duman, 2008). In addition, IL-1β may be responsible for the decrease in neurogenesis seen with aging (Kuzumaki et al., 2010) since this can be alleviated by pharmacologic inhibition of caspase-1, which is necessary for IL-1β production (Gemma et al., 2007). Overall, these results support IL-1β’s negative role in regulating adult hippocampal neurogenesis.
While our results show that sustained hippocampal IL-1β expression drastically reduces adult neurogenesis in the SGZ, the mechanism remains unclear. One possibility is that IL-1β acts directly on NPCs. While some observers have not been able to detect any effect of IL-1β on NPCs in vitro (Mathieu et al., 2010; Monje et al., 2003), Koo and Duman recently showed that NPCs in the hippocampus express IL-1R1 in vivo, and IL-1β decreases neurogenesis by causing cell cycle arrest that is dependent on NF-κB activation in vitro (Koo and Duman, 2008). Therefore, IL-1β may prevent differentiation to mature neurons without causing cell death of NPCs. Future experiments will have to address the survival of NPCs during sustained IL-1β expression and whether IL-1β is acting directly on them. While hIL-1β initiates and maintains sustained inflammation, we know that TNF-α, IL-6, mIL-1β, and mIL-1α are all elevated in our model (Hein et al., 2010; Shaftel et al., 2007b). Both TNF-α and IL-6 are believed to be detrimental to adult hippocampal neurogenesis (Iosif et al., 2006; Monje et al., 2003; Vallieres et al., 2002). Given the complexity of inflammation in vivo, it is likely that multiple factors contribute to the effect that we see in our IL-1βXAT model. By understanding the effect of sustained inflammation on adult neurogenesis, more appropriate therapies can be developed for inflammatory CNS disorders that alter the neurogenic regions of the brain.
Highlights.
Sustained IL-1β expression reduces adult hippocampal neurogenesis and shifts cellular fate towards astroglia; this reduction is not alleviated by voluntary running.
Acknowledgments
We thank Jen-nie Miller and Dr. Stephanos Kyrkanides for providing the feline immunodeficiency viral vectors. Lee Trojanczyk, Jack Walter, Mallory Olschowka, and Renee Johnson assisted with tissue processing. Dr. Linda Callahan helped with the confocal images. MDW is a student in the Department of Neurobiology & Anatomy and Medical Scientist Training Program at the University of Rochester SMD. Supported by NIH Research Award AG030149 and NIGMS T32 GM07356.
Footnotes
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belarbi K, Arellano C, Ferguson R, Jopson T, Rosi S. Chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.07.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Wada E, Carter DB, Tracey DE, Battey JF, De Souza EB. In situ histochemical localization of type I interleukin-1 receptor messenger RNA in the central nervous system, pituitary, and adrenal gland of the mouse. J Neurosci. 1992;12:1101–1114. doi: 10.1523/JNEUROSCI.12-03-01101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur J Neurosci. 2007;26:2795–2803. doi: 10.1111/j.1460-9568.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- Goshen I, Avital A, Kreisel T, Licht T, Segal M, Yirmiya R. Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J Neurosci. 2009;29:3395–3403. doi: 10.1523/JNEUROSCI.5352-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O’Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Zarcone TJ, Parfitt DB, Matousek SB, Carbonari DM, Olschowka JA, O’Banion MK. Behavioral, Structural and Molecular Changes following Long-term Hippocampal IL-1beta Overexpression in Transgenic Mice. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Sawamoto K. Adult neurogenesis and its alteration under pathological conditions. Neurosci Res. 2009;63:155–164. doi: 10.1016/j.neures.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64:721–728. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, Decarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YC, Shaftel SS, Miller JN, Tallents RH, Chang Y, Pinkert CA, Olschowka JA, Dickerson IM, Puzas JE, O’Banion MK, Kyrkanides S. Intraarticular induction of interleukin-1beta expression in the adult mouse, with resultant temporomandibular joint pathologic changes, dysfunction, and pain. Arthritis Rheum. 2006;54:1184–1197. doi: 10.1002/art.21771. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu P, Battista D, Depino A, Roca V, Graciarena M, Pitossi F. The more you have, the less you get: the functional role of inflammation on neuronal differentiation of endogenous and transplanted neural stem cells in the adult brain. J Neurochem. 2010;112:1368–1385. doi: 10.1111/j.1471-4159.2009.06548.x. [DOI] [PubMed] [Google Scholar]
- Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev. 2008;14:238–242. doi: 10.1002/ddrr.26. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moore AH, Wu M, Shaftel SS, Graham KA, O’Banion MK. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AS, Bull C, Nilsson MK, Zhu C, Bjork-Eriksson T, Eriksson PS, Blomgren K, Kuhn HG. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105:14632–14637. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007a;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O’Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007b;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Wingren AG, Bjorkdahl O, Labuda T, Bjork L, Andersson U, Gullberg U, Hedlund G, Sjogren HO, Kalland T, Widegren B, Dohlsten M. Fusion of a signal sequence to the interleukin-1 beta gene directs the protein from cytoplasmic accumulation to extracellular release. Cell Immunol. 1996;169:226–237. doi: 10.1006/cimm.1996.0113. [DOI] [PubMed] [Google Scholar]
- Wu CW, Chen YC, Yu L, Chen HI, Jen CJ, Huang AM, Tsai HJ, Chang YT, Kuo YM. Treadmill exercise counteracts the suppressive effects of peripheral lipopolysaccharide on hippocampal neurogenesis and learning and memory. J Neurochem. 2007;103:2471–2481. doi: 10.1111/j.1471-4159.2007.04987.x. [DOI] [PubMed] [Google Scholar]