Abstract
Objective
To determine whether delivery room cardiopulmonary resuscitation (DR-CPR) independently predicts morbidities and neurodevelopmental impairment (NI) in extremely low birth weight (ELBW) infants.
Study design
Cohort study of infants born with birth weight (BW) 401-1000g and gestational age (GA) 23-30wks. DR-CPR was defined as chest compressions and/or drugs. Logistic regression was used to determine associations between DR-CPR and morbidities, mortality and NI at 18-24 months (Bayley II mental or psychomotor index < 70, cerebral palsy, blindness or deafness). Data are adjusted Odds Ratio (OR) with 95% confidence interval.
Results
Of 8685 infants, 1333(15%) received DR-CPR. DR-CPR infants had lower BW (708±141vs 764±146g, p<0.0001) and GA (25±2 vs 26±2 wks, p<0.0001). DR-CPR infants had more pneumothoraces (OR 1.28, 1.48-2.99), Grade 3-4 intraventricular hemorrhage (OR 1.47, 1.23-1.74), bronchopulmonary dysplasia (OR 1.34, 1.13-1.59), death by 12 hours (OR 3.69, 2.98-4.57) and by 120 days after birth (OR 2.22, 1.93-2.57). NI among survivors (OR 1.23, 1.02-1.49), and death or NI (OR 1.70, 1.46-1.99) were higher for DR-CPR infants. Only 14% of DR-CPR recipients with 5-minute Apgar score<2 survived without NI.
Conclusions
DR-CPR is a prognostic marker for higher mortality and NI for ELBW survivors. New DR-CPR strategies are needed for this population.
Keywords: cardiac compressions, epinephrine, neurodevelopmental outcomes
The birth of an extremely-low-birth-weight (ELBW ≤1000g) infant is associated with tremendous anxiety for families and physicians. Despite improvements in survival of infants at the limits of viability, ELBW survivors continue to be at risk for adverse neurodevelopmental sequelae.1,2 Failure to initiate effective respiration at birth is common among ELBW infants. The incidence of true cardiovascular collapse and need for cardiopulmonary resuscitation in the delivery-room (DR-CPR) is unknown and its use varies significantly across clinical centers.3,4 Although immaturity, lack of establishment of a functional residual capacity5 and impaired placental gas exchange with profound fetal acidemia are possible etiologies for DR-CPR,6 some reports describe cardiac compressions being given prior to establishment of effective ventilation.3,7-9 This raises concern as to whether DR-CPR was really needed as differences in the effectiveness of ventilation may explain some of the variation in use of DR-CPR for ELBW infants.
Although several neonatal morbidities are associated with adverse neurodevelopmental outcome following preterm birth,10,11 data are limited regarding neonatal morbidities and neurodevelopmental outcome of ELBW infants who undergo DR-CPR. The few published reports have low rates of follow-up.4 This analysis of ELBW infants born at centers participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) determined the frequency of DR-CPR and whether DR-CPR is an independent predictor of adverse neurodevelopmental outcome. Associations between DR-CPR and neonatal morbidities and mortality were examined. An a priori secondary objective was to explore the association of a 5 minute Apgar score < 2 versus ≥ 2 on neonatal morbidities, mortality and neurodevelopmental outcome in infants who received DR-CPR.
Methods
The study population included all inborn infants with birth weight (BW) 401-1000g and estimated gestational age (GA) of 23-30 weeks who were enrolled in the NRN Generic Database (GDB) from January 1996 to December 2002. During this time, the GDB included inborn and outborn babies < 1500g admitted to a participating center in the first 14 days of life. Infants with congenital anomalies and those who were not candidates for CPR and died without receiving resuscitation and mechanical ventilation were excluded. Perinatal data were collected prospectively by research personnel using standard registry forms. All definitions, protocols, and procedures were uniform throughout the study period. Complete antenatal steroids was defined as either 2 doses of betamethasone or 4 doses of dexamethasone given in the 7 days prior to delivery. Data regarding neonatal morbidities and mortality were collected through 120 days of life. As previously described,10 families are invited to participate in the NRN Follow-up Study that includes a comprehensive assessment at 18-22 months corrected age. This visit consists of a structured medical history, anthropometrics, and a neuromotor exam and the Bayley Scales of Infant Development II (BSID-II) performed by certified examiners. Details of the procedures have been previously published.10,12,13 The BSID-II was used to derive a Mental Developmental Index (MDI) and a Psychomotor Developmental Index (PDI). Cerebral palsy (CP) was defined as a nonprogressive central nervous system disorder characterized by abnormal muscle tone in at least one extremity and abnormal control of movement or posture. Moderate to severe CP included children who were non-ambulatory or required an assistive device for ambulation.
DR-CPR was defined as chest compressions ± drugs. Neonatal morbidities included pulmonary hemorrhage, pneumothorax, patent ductus arteriosus (PDA) requiring medical or surgical therapy, severe intraventricular hemorrhage (Grade 3 or 4 IVH)14, cystic periventricular leukomalacia (PVL), early-onset sepsis (positive blood culture within 72 hours of birth and antibiotic therapy for five or more days or intent to treat in those who died before 5 days), late-onset sepsis (occurring after 3 days of age), necrotizing enterocolitis (NEC, Bell’s stage II or greater), bronchopulmonary dysplasia (BPD-defined as oxygen requirement at 36 weeks postmenstrual age), postnatal steroids for ventilator dependence due to BPD, death at < 12 hours (including deaths in the delivery room), 12-24 hours, 24-72 hours, 3-7 days, and in hospital death by ≤ 28 and ≤ 120 days of life. NDI was assessed at 18-22 months corrected age and was defined as any of MDI<70, PDI<70, moderate or severe cerebral palsy, blindness in both eyes or deafness requiring amplification in both ears. Post-discharge mortality was assessed at 18-22 months of age.
Student T-test, Chi-square analysis, and Fisher exact test were used to compare the demographic and outcome variables between infants with and without DR-CPR. Logistic regression models were developed to determine the independent effects of DR-CPR on neonatal morbidities, mortality and neurodevelopmental outcomes. To explore the prognostic implications of DR-CPR, only covariates antecedent to DR-CPR were included in the logistic models: estimated GA, BW, multiple birth, maternal hypertension, maternal hemorrhage, complete course of antenatal steroids within 7 days of birth, mode of delivery, sex, race, and center of birth. The results are expressed as adjusted odd ratios (ORs) and 95% confidence intervals (CI). Infants with and without follow-up were compared for demographic and neonatal morbidities using student t-tests and chi-square analysis. In order to assess the potential impact of a longer versus a shorter interval of DR-CPR, a similar set of analyses were performed among infants who received DR-CPR comparing infants with an Apgar score at 5 minutes of < 2 with those ≥ 2. A p value of <0.05 was considered significant. All data were analyzed by RTI International.
Results
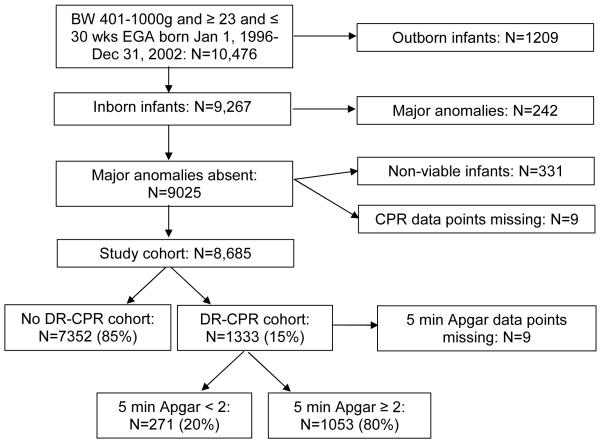
During the study period 10,476 infants 401-1000g BW and ≥ 23 and ≤ 30 weeks estimated GA were managed at 19 participating academic centers. After excluding outborn infants (n=1209), those with major congenital anomalies (n=242), infants who were not candidates for CPR and mechanical ventilation (n=331), and those missing data related to chest compressions or medications in the delivery- room (n=9), 8685 infants comprised the study cohort (Figure 1; available at www.jpeds.com). Among these infants, 1333 (15%) received DR-CPR and 7352 (85%) did not. Demographic factors among infants who did and did not receive DR-CPR are compared in Table I. No differences were found in proportions of multiple births between DR-CPR and No DR-CPR infants. Infants whose mothers had hypertension or had received antenatal steroids were less likely to receive DR-CPR. Antepartum hemorrhage, vaginal breech delivery, younger GA, lower BW, and male sex increased the likelihood of receiving DR-CPR. There were more black and fewer Hispanic infants in the DR-CPR group.
Figure 1.
Flow diagram of patient selection
Table 1.
Demographic and maternal clinical characteristics of infants who received DR-CPR vs those with No DR-CPR
| DR-CPR (N = 1333) |
No DR-CPR (N = 7352) |
P-value | |
|---|---|---|---|
| % Multiple birth | 24% | 25% | 0.53 |
|
| |||
| % Maternal hypertension | 20% | 25% | < 0.0001 |
|
| |||
| % Antepartum hemorrhage | 21% | 16% | < 0.0001 |
| % Antenatal steroids* | 43% | 51% | <0.0001 |
|
| |||
| Mode of delivery | |||
| % Vaginal vertex | 31% | 34% | < 0.0001 |
| % Vaginal breech | 14% | 7% | |
| % Cesarean section | 55% | 59% | |
|
| |||
| Gestational age (wks) | 25 ± 2 | 26 ± 2 | <0.0001 |
|
| |||
| Birth weight (g) | 708 ± 141 | 764 ± 146 | <0.0001 |
|
| |||
| % Males | 52% | 49% | 0.01 |
|
| |||
| Race/ethnicity | |||
| % Black, not Hispanic | 46% | 43% | |
| % White, not Hispanic | 39% | 39% | 0.04 |
| % Hispanic | 12% | 15% | |
| % Other | 3% | 3% | |
Complete antenatal steroids within 7 d of delivery
By bivariate analysis, DR-CPR infants experienced more death within 12 hours of birth, had more early onset sepsis, more pneumothorax, pulmonary hemorrhage, Grade 3 or 4 IVH, BPD and use of postnatal steroids than infants who did not receive DR-CPR (Table II). No difference was found in proportions of infants with PDA, PVL, late-onset sepsis, or NEC. Table II includes the adjusted OR and 95% CI for each short term outcome for all infants receiving DR-CPR. Results of adjusted analyses indicated similar associations as unadjusted analyses except pulmonary hemorrhage did not reach significance after logistic modeling. Among DR-CPR infants who died during the initial hospitalization before 120 days, 38% died within 12 hours, 44% by 24 hours, 61% by 72 hours, and 88% by day 28.
Table 2.
Short-Term and long-term outcomes of infants who received DR-CPR vs those with No DR-CPR
| DR-CPR | No DR-CPR | Unadjusted P-value |
Adjusted OR† (95% CI) |
|||
|---|---|---|---|---|---|---|
| N* | % | N* | % | |||
| Short-term outcomes | ||||||
| Death < 12 h | 1333 | 16% | 7352 | 4% | <0.0001 | 3.69 (2.98 – 4.57) |
| % Early onset sepsis | 1118 | 4% | 7025 | 2% | <0.0001 | 2.10 (1.48 – 2.99) |
| % Pneumothorax | 1119 | 12% | 7027 | 9% | 0.0002 | 1.28 (1.04 – 1.58) |
| % Pulm hemorrhage | 1119 | 13% | 7027 | 9% | 0.0009 | 1.21 (0.98 – 1.48) |
| % PDA | 1116 | 46% | 7028 | 43% | 0.15 | 0.98 (0.85 – 1.12) |
| % Grade 3 or 4 IVH | 986 | 25% | 6139 | 16% | <0.0001 | 1.47 (1.23 – 1.74) |
| % PVL by 36 wk | 971 | 6% | 6434 | 5% | 0.06 | 1.13 (0.84 – 1.51) |
| % Late onset sepsis | 1116 | 38% | 7020 | 35% | 0.06 | 0.95 (0.83 – 1.09) |
| % NEC Stage 2 or 3 | 1117 | 10% | 7028 | 10% | 0.56 | 0.82 (0.66 – 1.02) |
| % Postnatal steroids | 1119 | 44% | 7023 | 35% | < 0.0001 | 1.20 (1.05 – 1.38) |
| % BPD (O2 at 36 wk) | 796 | 58% | 5858 | 46% | < 0.0001 | 1.34 (1.13 – 1.59) |
| Hospital death ≤ 120 d | 1333 | 42% | 7352 | 21% | < 0.0001 | 2.22 (1.93 – 2.57) |
| Long-term outcomes | ||||||
| Death by follow-up | 1332 | 44% | 7338 | 24% | <0.0001 | 2.06 (1.79 – 2.37) |
| NDI | 579 | 44% | 4389 | 35% | 0.0001 | 1.23 (1.02 – 1.49) |
| NDI or death** | 1167 | 72% | 6127 | 54% | <0.0001 | 1.70 (1.46 – 1.99) |
| MDI < 70 | 587 | 36% | 4420 | 29% | 0.0003 | 1.20 (0.98 – 1.45) |
| PDI < 70 | 574 | 29% | 4375 | 19% | <0.0001 | 1.59 (1.29 – 1.96) |
| Moderate or severe CP | 628 | 10% | 4716 | 6% | 0.0002 | 1.64 (1.22 – 2.20) |
| Blind in both eyes | 624 | 0.3% | 4717 | 0.8% | 0.22 | ‡ |
| Hearing aid in both ears | 627 | 3% | 4694 | 1% | 0.009 | 1.92 (1.12 –3.27) |
Number of infants evaluated for each outcome.
NDI or death outcome available for 84% of the cohort population. The mean (± sd) corrected age at follow-up was 20 (±2) months with a range of 16 to 37 months.
Covariates included in the logistic regression included estimated GA, BW, multiple birth, maternal hypertension, maternal hemorrhage, complete antenatal steroids within 7 days of birth, mode of delivery, sex, race, and center of birth
There were too few cases of blindness in both eyes to perform logistic modeling
DR-CPR recipients had higher rates of death by 18-22 month follow-up, more NDI, and thus more composite NDI or death according to bivariate analysis (Table II). The individual components of NDI such as MDI < 70, PDI < 70, moderate or severe cerebral palsy and hearing aid in both ears were higher in DR-CPR infants although there was no difference in blindness. DR-CPR infants also had lower mean ± sd MDI (76±18 (n=587) vs 80±18 (n=4420), p<0.0001) and PDI (80±20 (n=574) vs 84±18 (n=4375), p<0.0001) scores. Adjusted analyses for neurodevelopmental outcomes were similar to bivariate analyses (Table II) except for MDI < 70 which no longer reached significance. DR-CPR increased the risk of death by 18-22 months follow-up, NDI at 18-22 months, NDI or Death, PDI < 70, moderate or severe CP and need for hearing aid in both ears. There were too few cases of blindness in both eyes to perform logistic modeling for this variable.
Outcome data for death or NDI were available for 84% of study patients. Infants lost to follow-up were of higher GA (26.2±1.8 vs 26.0±1.7 wks, p=0.0003) and BW (797±138 vs 786±134 g, p=0.0091) compared with those who completed follow-up. Infants lost to follow-up had similar rates of DR-CPR compared with those who completed the follow-up visit (11% vs 12%, p=0.26). Those lost to follow-up had less PDA (39 vs 44%, p=0.0002) and IVH Grade 3 or 4 (8 vs 12%, p<0.0001) and were more likely to have been discharged prior to 120 days in the hospital (84 vs 82%, p=0.047) but had similar rates of pneumothorax, PVL, NEC, BPD and use of postnatal steroids.
Of the previously described 1333 DR-CPR infants, 9 were missing 5 minute Apgar score data. This left a cohort of 1324 DR-CPR infants for further analysis of associations between low 5 minute Apgar score and outcomes: 271 (20%) infants with 5 minute Apgar < 2 and 1053 (80%) with 5 minute Apgar ≥ 2. Perinatal and demographic factors for DR-CPR infants with 5 minute Apgar < 2 and 5 minute Apgar ≥ 2 infants are compared in Table III. DR-CPR infants with 5 minute Apgar < 2 were less likely to be exposed to maternal hypertension and antenatal steroids than those with 5 minute Apgar ≥ 2, but more likely to be of younger GA, lower BW and male sex. Racial distribution between groups did not differ.
Table 3.
Demographic characteristics for all infants who received DR-CPR and had 5 minute Apgar < 2 versus ≥ 2
| 5 min Apgar < 2 (N = 271) |
5 min Apgar ≥ 2 (N = 1053) |
P-value | |
|---|---|---|---|
| % Multiple birth | 23% | 24% | 0.93 |
|
| |||
| % Maternal hypertension | 13% | 21% | 0.0015 |
|
| |||
| % Antepartum hemorrhage | 24% | 20% | 0.11 |
|
| |||
| % Antenatal steroids* | 35% | 46% | 0.0025 |
|
| |||
| Mode of delivery | |||
| % vaginal vertex | 34% | 30% | 0.10 |
| % vaginal breech | 17% | 13% | |
| % cesarean section | 50% | 57% | |
|
| |||
| Gestational age (wks) | 24.9 ± 1.6 | 25.3 ± 1.6 | 0.0028 |
|
| |||
| Birth weight (g) | 692 ± 142 | 713 ± 140 | 0.03 |
|
| |||
| % Small for gestational age | 15% | 15% | 1.00 |
|
| |||
| % Males | 59% | 51% | 0.02 |
|
| |||
| Race/Ethnicity | |||
| % Black, not Hispanic | 43% | 47% | |
| % White, not Hispanic | 42% | 39% | 0.61 |
| % Hispanic | 12% | 12% | |
| % Other | 4% | 3% | |
Complete antenatal steroids within 7 d of delivery.
DR-CPR infants with 5 minute Apgar < 2 were more likely to die before 12 hours of life or by hospital day 120 in both unadjusted and adjusted analyses (Table IV). They were also more likely to suffer Grade 3 or 4 IVH and develop BPD compared with those with higher 5 minute Apgar scores. There were no significant differences in early-onset sepsis, pneumothorax, pulmonary hemorrhage, PDA, PVL, late-onset sepsis, NEC or use of postnatal steroids with either unadjusted or adjusted analyses.
Table 4.
Short-term and long-term outcomes for all infants who received DR-CPR and had 5 minute Apgar < 2 versus ≥ 2
| 5 min Apgar < 2 |
5 min Apgar ≥ 2 |
Unadjusted P-value |
Adjusted OR† (95% CI) |
|||
|---|---|---|---|---|---|---|
| N* | % | N* | % | |||
| Short-Term Outcomes | ||||||
| Death < 12 h | 271 | 44% | 1053 | 8% | <0.0001 | 8.01 (5.64 – 11.38) |
| % Pneumothorax | 152 | 16% | 964 | 12% | 0.11 | 1.34 (0.82 – 2.18) |
| % Pulm hemorrhage | 152 | 15% | 964 | 12% | 0.35 | 1.21 (0.74 – 1.99) |
| % PDA | 151 | 50% | 962 | 45% | 0.33 | 1.10 (0.76 – 1.59) |
| % Grade 3 or 4 IVH | 134 | 34% | 850 | 23% | 0.0096 | 1.57 (1.04 – 2.39) |
| % PVL by 36 wks | 131 | 10% | 838 | 6% | 0.08 | 1.81 (0.94 – 3.46) |
| % Early-onset sepsis | 151 | 7% | 964 | 4% | 0.08 | 1.89 (0.92 – 3.85) |
| % Late-onset sepsis | 150 | 39% | 963 | 38% | 0.83 | 1.12 (0.78 – 1.61) |
| % NEC Stage 2 or 3 | 152 | 7% | 962 | 10% | 0.30 | 0.69 (0.36 – 1.33) |
| % Postnatal steroids | 152 | 46% | 964 | 44% | 0.66 | 1.03 (0.72 – 1.48) |
| % BPD (O2 at 36 wks) | 101 | 73% | 694 | 56% | 0.001 | 1.79 (1.07 – 2.99) |
| Hospital death ≤ 120 d | 271 | 64% | 1053 | 36% | < 0.0001 | 3.01 (2.19 – 4.15) |
| Long-term outcomes | ||||||
| Death by follow-up | 271 | 66% | 1052 | 38% | <0.0001 | 2.88 (2.09 – 3.96) |
| NDI | 71 | 52% | 507 | 43% | 0.16 | 1.67 (0.97 – 2.86) |
| NDI or death** | 249 | 86% | 909 | 68% | <0.0001 | 2.94 (1.93 – 4.49) |
| MDI < 70 | 72 | 44% | 514 | 35% | 0.15 | 1.61 (0.95 – 2.74) |
| PDI < 70 | 68 | 37% | 505 | 28% | 0.16 | 1.59 (0.90 – 2.78) |
| MDI (Mean ± sd) | 72 | 73±19 | 514 | 76±18 | 0.21 | |
| PDI (Mean ± sd) | 68 | 77±20 | 505 | 80±20 | 0.25 | |
| Moderate or severe CP | 77 | 13% | 550 | 10% | 0.42 | ‡ |
| Blind in both eyes | 77 | 0.0% | 546 | 0.4% | 0.59 | ‡ |
| Hearing aid both ears | 77 | 4% | 549 | 3% | 0.48 | ‡ |
Number of infants evaluated for each outcome.
NDI or death outcome available for 87% of the cohort.
Covariates: GA, BW, maternal hypertension, complete course of antenatal steroids, male sex, and center. The model for PVL did not include center.
Too few cases of CP, blindness, and need for hearing aids to perform logistic modeling.
Outcomes at 18-22 months for infants with 5 minute Apgar < 2 versus ≥ 2 are also shown in Table IV. Mortality was higher in DR-CPR infants with 5 minute Apgar < 2 by 18-22 month follow-up compared with those with 5 minute Apgar ≥ 2; however, there were no differences in NDI or the individual components of NDI. The combined NDI or death outcome was available for 87% of DR-CPR recipients and was greater in the low 5 minute Apgar group. DR-CPR infants lost to follow-up had similar proportions of 5 minute Apgar < 2 (13 versus 12%, p>0.99) compared with those who presented for follow-up. Odds ratios and 95% CI for long-term outcomes for DR-CPR infants with 5 minute Apgar < 2 following logistic regression are included in Table IV. Similar to the bivariate analysis, increased risk of death by time of follow-up and the combined NDI or death outcome remained significant and NDI, MDI < 70 and PDI < 70 did not.
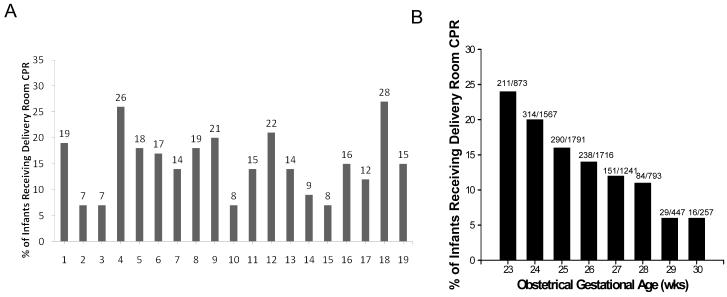
Rates of DR-CPR differed among network centers and ranged from 7% to 28%; the percentage of infants receiving DR-CPR increased with decreasing GA (Figure 2; available at www.jpeds.com).
Figure 2.
Proportion of ELBW infants receiving DR-CPR by A, center and B, gestational age. Note: There were center differences (p<0.0001) and gestational age differences (p<0.0001) in the proportion of infants who needed DR-CPR.
Discussion
Although DR-CPR is a prognostic marker for mortality in ELBW infants, over half of DR-CPR recipients survived (56%). Unfortunately, DR-CPR survivors had worse short-term complications such as pneumothorax, severe IVH, and BPD. Those who survived until the 18-22 month follow-up visit had a heavier burden of psychomotor dysfunction, CP, bilateral deafness and composite neurodevelopmental impairment compared with infants who did not receive DR-CPR. Only 28% of infants who received DR-CPR were alive and unimpaired at 18-22 month follow-up. Loss to follow-up appears unlikely to bias the results given similar rates of DR-CPR between those who did and did not complete follow-up, the respectable follow-up rates (>80%) and the fact that those lost to follow-up were more mature and of higher BW.
Low 5 minute Apgar scores were used as a proxy to assess outcomes for infants receiving brief versus more prolonged DR-CPR. Impairment-free survival decreased to 14% in those with 5 minute Apgar scores < 2 and these infants had much higher odds of dying in the first 12 hours of life as well as by time of follow-up. Surprisingly, 5 minute Apgar score < 2 was not associated with a higher burden of short and long term morbidities for DR-CPR recipients with the exception of increased BPD. Thus, it appears that the initial length of the intensive resuscitative efforts (at least up to 5 minutes) may help prognosticate regarding mortality but not morbidities.
Initial reports regarding the efficacy of CPR in the delivery-room for ELBW infants were not encouraging with a high burden of mortality and very few reported intact survivors,7,16-20 but the total number of infants reported was small. This was in stark contrast to a subsequent single center experience where 79% of the 19 DR-CPR ELBW infants were reported to be alive and well at discharge.21 Ten month outcomes were available for 66% of survivors and were reported to be normal. This difference prompted the investigators to propose that intact survival was possible for DR-CPR ELBW infants. The Vermont Oxford Network subsequently reported on a large multi-center experience detailing short-term outcomes of survival, severe IVH (Grade 3 or 4), and survival without severe IVH for VLBW infants who did or did not receive DR-CPR.3 Of 27,707 501 to 1500g infants, 6% received DR-CPR. Similar to our findings, DR-CPR infants were less likely to survive (63% vs 89%), had more severe IVH (16% vs 5%), and were less likely to survive without severe IVH (53% vs 85%) than those who did not receive DR-CPR. The investigators concluded that, because more than one-half survived and one-half of survivors were free of IVH, DR-CPR was a reasonable intervention for VLBW infants. No information was available regarding whether DR-CPR was administered according to guidelines and no long-term outcomes were available. Subsequent reports of DR-CPR for preterm infants suffer from similar shortcomings9,22-24 with limited insight into the need for DR-CPR, low rates of follow-up for long-term outcomes and small numbers of reported babies, making the data difficult to interpret. In addition, the length of follow-up is not consistent among all reports. The corrected age at time of follow-up varies from 4-60 months and results are not stratified by birth weight or gestational age, making it impossible to determine the outcome of VLBW or ELBW infants separately. These are major shortcomings to the above studies as noted in a recent systematic review.4
The current study using the NRN GDB and 18-22 month follow-up data provides a large number of infants from multiple geographically and ethnically diverse clinical centers, uniform definitions of neonatal morbidities, greater detail regarding in-hospital morbidities, and a consistent follow-up examination at 18-22 months for infants ≤ 1000g with a uniform battery of tests. To our knowledge, this cohort with > 80% follow-up is the first of its kind for infants receiving DR-CPR. In addition, those that were lost to follow-up had similar rates of DR-CPR compared with those who received follow-up and were not different in short-term outcomes other than lower rates of PDA and IVH. The examination of those with continued Apgar score < 2 at 5 minutes offers insight into a group of ELBW infants who received more prolonged CPR. The study is limited by its retrospective design, the inability to determine the effect of differences in obstetrical or pediatric practice on DR-CPR, the inability to glean the exact necessity for the DR-CPR or other measures of the quality of delivery room resuscitations as well as variable criteria for determining who to resuscitate at the edge of viability.
This report suggests that there are antenatal risk factors that increase the need for DR-CPR for ELBW infants such as antepartum hemorrhage, lack of antenatal steroids, breech position, lower gestational age and lower birth weight, and presence of early-onset sepsis. With this in mind, it is critical that when such risk factors are present, appropriately trained personnel and resources are present at delivery in the event that CPR is needed. Indeed, one might speculate that if a team of well-trained providers were to be present at deliveries of ELBW infants and able to provide adequate positive pressure ventilation when such risk factors are present, the need for DR-CPR might be reduced. The wide center to center variation in rates of DR-CPR from as low as 7% to as high as 28% is striking. Variations in obstetrical practice may have an impact on DR-CPR but as the NRN GDB does not collect center level obstetrical practice data, this study cannot adequately address this. Similarly, there may be center differences in the pediatric approach as to whether DR-CPR is an “experimental” procedure for this vulnerable population at the extreme of prematurity (e.g. <25 weeks) and whether CPR should be routinely offered versus solely optimizing airway management. Differences in ELBW population may exist from center to center including rates of delivery of ELBWs at the lowest gestations, differences in allowing families to forego resuscitation at the edge of viability, and adequacy of resuscitation training. Given the strong association between decreasing GA and increased use of DR-CPR, centers may need to examine who they are sending to stabilize ELBW infants at birth. Focused team training and education regarding neonatal resuscitation that closely looks at actual competency of resuscitation skills is a strong consideration.25,26 High-fidelity simulation models of ELBW infants could be developed to enhance such training.
It is unknown whether the increased rates of pneumothoraces in DR-CPR infants were caused by the intensive resuscitation or led to the need for the intensive resuscitation. Certainly, during DR-CPR, pneumothorax should be considered as a possible reason for lack of response to ventilation. The increased risk for Grade 3-4 IVH in DR-CPR infants is not surprising given that blood pressure swings are associated with IVH.15 Infants receiving DR-CPR may go from very low blood pressure during asphyxia induced bradycardia to rebound hypertension following epinephrine administration. The small number of infants who developed PVL did not allow sufficient power to evaluate DR-CPR as a risk factor for PVL. Increased rates of BPD and the need for postnatal steroids may be exacerbated by barotrauma from aggressive positive pressure ventilation and significant oxygen exposures during the resuscitation.27
This report suggests DR-CPR is a prognostic marker for adverse neurodevelopmental outcome in ELBW infants. Its occurrence should prompt appropriate parental counseling and neurodevelopmental follow-up. The significant mortality and morbidity in ELBW DR-CPR recipients suggests that improved CPR techniques potentially tailored for the ELBW infant, enhanced resuscitation education and educational tools such as high-fidelity ELBW simulators and better post-resuscitation cardiovascular and neuro-protective strategies should all be investigated for this vulnerable population.
Acknowledgments
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Supported by the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), which provided grants for the Neonatal Research Network’s Generic Database Study and Follow-up Study. The authors declare no conflicts of interest.
Abbreviations
- BW
birth weight
- CP
cerebral palsy
- CI
confidence intervals
- DR-CPR
delivery room cardiopulmonary resuscitation
- ELBW
extremely low birth weight
- GA
gestational age
- GDB
generic database
- IVH
intraventricular hemorrhage
- MDI
Mental Developmental Index
- NICHD
National Institute of Child Health and Human Development
- NRN
neonatal research network
- NDI
neurodevelopmental impairment
- OR
odds ratios
- PDI
Psychomotor Developmental Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity--moving beyond gestational age. N Engl J Med. 2008;358:1672–81. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gargus RA, Vohr BR, Tyson JE, High P, Higgins RD, Wrage LA, et al. Unimpaired outcomes for extremely low birth weight infants at 18 to 22 months. Pediatrics. 2009;124:112–21. doi: 10.1542/peds.2008-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finer NN, Horbar JD, Carpenter JH. Cardiopulmonary resuscitation in the very low birth weight infant: the Vermont Oxford Network experience. Pediatrics. 1999;104:428–34. doi: 10.1542/peds.104.3.428. [DOI] [PubMed] [Google Scholar]
- 4.Shah PS. Extensive cardiopulmonary resuscitation for VLBW and ELBW infants: a systematic review and meta-analyses. J Perinatol. 2009;29:655–61. doi: 10.1038/jp.2009.71. [DOI] [PubMed] [Google Scholar]
- 5.Vyas H, Field D, Milner AD, Hopkin IE. Determinants of the first inspiratory volume and functional residual capacity at birth. Pediatr Pulmonol. 1986;2:189–93. doi: 10.1002/ppul.1950020403. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists Umbilical cord blood gas and acid-base analysis. ACOG Committee Opinion. 2006;(No 348):1–4. [Google Scholar]
- 7.Perlman JM, Risser R. Cardiopulmonary resuscitation in the delivery room. Associated clinical events. Arch Pediatr Adolesc Med. 1995;149:20–25. doi: 10.1001/archpedi.1995.02170130022005. [DOI] [PubMed] [Google Scholar]
- 8.Jankov RP, Asztalos EV, Skidmore MB. Favourable neurological outcomes following delivery room cardiopulmonary resuscitation of infants < or = 750 g at birth. J Paediatr Child Health. 2000;36:19–22. doi: 10.1046/j.1440-1754.2000.00434.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Torres AM, Garcia-Alix A, Cabanas F, Elorza MD, Madero R, Perez J, et al. Impact of cardiopulmonary resuscitation on extremely low birth weight infants. An Pediatr (Barc) 2007;66:38–44. doi: 10.1157/13097357. [DOI] [PubMed] [Google Scholar]
- 10.Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116:635–43. doi: 10.1542/peds.2004-2247. [DOI] [PubMed] [Google Scholar]
- 11.Shankaran S, Johnson Y, Langer JC, Vohr BR, Fanaroff AA, Wright LL, et al. Outcome of extremely-low-birth-weight infants at highest risk: gestational age < or =24 weeks, birth weight < or =750 g, and 1-minute Apgar < or =3. Am J Obstet Gynecol. 2004;191:1084–91. doi: 10.1016/j.ajog.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics. 2000;105:1216–26. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 13.Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113:781–89. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 14.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 15.Bada HS. Prevention of Intracranial Hemorrhage. NeoReviews. 2000;1:e48–e53. [Google Scholar]
- 16.Jain L, Ferre C, Vidyasagar D, Nath S, Sheftel D. Cardiopulmonary resuscitation of apparently stillborn infants: survival and long-term outcome. J Pediatr. 1991;118:778–82. doi: 10.1016/s0022-3476(05)80046-0. [DOI] [PubMed] [Google Scholar]
- 17.Sood S, Giacoia GP. Cardiopulmonary resuscitation in very low birthweight infants. Am J Perinatol. 1992;9:130–33. doi: 10.1055/s-2007-994686. [DOI] [PubMed] [Google Scholar]
- 18.Davis DJ. How aggressive should delivery room cardiopulmonary resuscitation be for extremely low birth weight neonates? Pediatrics. 1993;92:447–50. [PubMed] [Google Scholar]
- 19.Sims DG, Heal CA, Bart.le SM. Use of adrenaline and atropine in neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed. 1994;70:F3–F9. doi: 10.1136/fn.70.1.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Donnell AI, Gray PH, Rogers YM. Mortality and neurodevelopmental outcome for infants receiving adrenaline in neonatal resuscitation. J Paediatr Child Health. 1998;34:551–56. doi: 10.1046/j.1440-1754.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- 21.Finer NN, Tarin T, Vaucher YE, Barrington K, Bejar R. Intact survival in extremely low birth weight infants after delivery room resuscitation. Pediatrics. 1999;104:e40. doi: 10.1542/peds.104.4.e40. [DOI] [PubMed] [Google Scholar]
- 22.Haddad B, Mercer BM, Livingston J, Talati A, Sibai BM. Outcome after successful resuscitation of babies born with Apgar scores of 0 at both 1 and 5 minutes. Am J Obstet Gynecol. 2000;182:1210–14. doi: 10.1067/mob.2000.104951. [DOI] [PubMed] [Google Scholar]
- 23.Deulofeut R, Sola A, Lee B, Rogido M. Delivery room cardiopulmonary resuscitation of the very preterm infant is associated with adverse short- and long-term outcomes. An Pediatr (Barc) 2007;66:31–37. doi: 10.1157/13097355. [DOI] [PubMed] [Google Scholar]
- 24.Shah PS, Shah P, Tai KFY. Chest compression and/or epinephrine at birth for preterm infants < 32 weeks gestational age: matched cohort study of neonatal outcomes. J Perinatol. 2009;29:693–97. doi: 10.1038/jp.2009.70. [DOI] [PubMed] [Google Scholar]
- 25.Thomas EJ, Williams AL, Reichman EF, Lasky RE, Crandell S, Taggart WR. Team training in the Neonatal Resuscitation Program for interns: teamwork and quality of resuscitations. Pediatrics. 2010;125:539–546. doi: 10.1542/peds.2009-1635. [DOI] [PubMed] [Google Scholar]
- 26.Wayne DB, Didwania A, Feinglass J, Fudala MJ, Barsuk JH, McGaghie WC. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56–61. doi: 10.1378/chest.07-0131. [DOI] [PubMed] [Google Scholar]
- 27.Jobe AH, Hillman N, Polglase G, Kramer BW, Kallapur S, Pillow J. Injury and inflammation from resuscitation of the preterm infant. Neonatology. 2008;94:190–6. doi: 10.1159/000143721. [DOI] [PubMed] [Google Scholar]