Abstract
Background
Cannabis is commonly consumed by Ecstasy (3,4-methylenedioxymethamphetamine; MDMA) users, including as an intentional strategy to manipulate the drug experience. The most active psychoactive constituent in cannabis, Δ9-tetrahydrocannabinol (THC), and other drugs with partial or full agonist activity at the CB1 receptor, produces a reduction of body temperature in rodents. Reports show that administration of THC can attenuate temperature increases caused by MDMA in mice or rats however a recent study in humans shows that THC potentiates MDMA-induced temperature elevations. Relatively little scientific evidence on the thermoregulatory effects of THC in monkeys is available.
Methods
The body temperature of male rhesus macaques was recorded after challenge with THC (0.1–0.3 mg/kg, i.m.) or combined challenge of THC with the CB1 receptor antagonist SR141716 (Rimonabant; 0.3 mg/kg, i.m.) or combined challenge of THC (0.1, 0.3 m/gkg, i.m.) with 3,4-methylenedioxymethamphetamine (MDMA; 1.78 mg/kg p.o.) using minimally-invasive, implanted radiotelemetry techniques.
Results
THC reduced the body temperature of monkeys in a dose-dependent manner with the nadir observed 3–5 hrs post-injection, however an attenuation of normal circadian cooling was also produced overnight following dosing. Hypothermia induced by THC (0.3 mg/kg, i.m.) was prevented by Rimonabant (0.3 mg/kg, i.m.). Finally, 0.3 mg/kg THC (i.m.) attenuated the elevation of body temperature produced by MDMA for about 4 hours after oral dosing.
Conclusions
As with rodents THC produces a robust and lasting decrement in the body temperature of rhesus monkeys; this effect is mediated by the CB1 receptor. THC also protects against the immediate hyperthermic effects of MDMA in monkeys in a dose-dependent manner. Nevertheless, a paradoxical attenuation of circadian cooling overnight after the THC/MDMA combination cautions that longer term effects may be critical in assessing risks for the recreational user of cannabis in combination with MDMA.
Keywords: Ecstasy, hypothermia, endocannabinoid, thermoregulation, nonhuman primate, radiotelemetry
1. Introduction
Prior work has shown that 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) increases the body temperature of freely moving rhesus macaques following either intravenous or oral administration (Taffe et al., 2006, Crean et al., 2007) of doses (0.5–5.0 mg/kg) which overlap with those consumed by most human recreational users. These effects are present across a range of ambient temperatures (Von Huben et al., 2007) across which rat (Malberg and Seiden, 1998), but not human (Freedman et al., 2005), temperature responses change from hypothermia to hyperthermia. Given species differences in thermoregulation, it continues to be critical to determine the degree to which primate responses to MDMA differ from those of rats under conditions likely to contribute to, or protect against, medical emergency and even death in the human user (Patel et al., 2004, Schifano et al., 2010). One such condition that may be important for the human user is the concurrent use of cannabis and the resulting exposure to one of the more active constituents, Δ9-tetrahydrocannabinol (THC) (Mohamed et al., 2011). This is particularly critical since cannabis use may be an intentional strategy to modulate the Ecstasy experience:
“A male participant explained, “Marijuana helps keep high going, gives you another little spike, and then eases your comedown.”” (Levy et al., 2005).
It is therefore of interest to determine if THC affects physiological responses to MDMA in addition to the sought-after subjective effects.
A reduction in body temperature is a canonical feature of THC in rodents and indeed was one of the tetrad of tests indicative of cannabinoid action prior to the identification of the CB1 receptor (Razdan, 1986, Little et al., 1988). Cannabis smoking might therefore be inferred to oppose MDMA-induced temperature elevation in the human user and indeed, preclinical work shows that THC attenuates MDMA-induced increases of body temperature in rats (Morley et al., 2004) and mice (Tourino et al., 2010). Caution is warranted, however, since these effects may be as misleading as the above referenced sensitivity of MDMA-associated thermodysregulation to ambient temperature in rodents, but not human or nonhuman primates. Indeed, a recent report indicates that inhaled THC fails to attenuate body temperature increases caused by MDMA, and may even potentiate them, in humans (Dumont et al., 2009). Nevertheless, the THC-only condition failed to alter body temperature relative to the control condition in that study. This consideration raises the possibility that the dose employed was too low, perhaps because of an acquired tolerance in the highly drug-experienced subject population. These questions can be further addressed by using a nonhuman primate model within which it is possible to confirm that the THC doses used are capable themselves of disrupting thermoregulation.
Minimal evidence is available on the thermoregulatory properties of THC in macaque monkeys. An early study of the effects of large, oral THC doses (3,000–9,000 mg/kg) did not report significant amounts of hypothermia (Thompson et al., 1973). The methods are not well specified in that work and more recent studies in chair restrained rhesus monkeys report (rectal) hypothermia of about 1–2°C with maximum effect observed about 2–3 hours after intraperitoneal or intramuscular injection (Matsuzaki et al., 1987, McMahon et al., 2005). We have shown, however, that thermoregulatory responses to 3,4-methylenedioxymethamphetamine (MDMA) in freely moving monkeys (Crean et al., 2006, Taffe et al., 2006), can be qualitatively different from thermoregulatory responses in chaired monkeys (Bowyer et al., 2003). Therefore a clearer understanding of the thermoregulatory effects of THC in unrestrained rhesus monkeys is important to obtain.
A study was designed to investigate the thermoregulatory influence of THC in unrestrained rhesus macques using an established radiotelemetric paradigm. The goal was first to determine if THC reduces body temperature and if those effects depend on interaction with the endocannabinoid CB1 receptor. Next this model was used to determine if THC influences the MDMA-induced elevations (Taffe et al., 2006, Crean et al., 2007) in the body temperature of monkeys. Together these studies sought to determine if apparent disparities between prior reports in humans and rodent models can be attributed to Order differences.
2. Experimental Procedures
2.1 Animals
Ten adult male rhesus monkeys (Macaca mulatta) were used in this investigation. Animals were 5.5–6.5 years of age, weighed 11.5–14 kg at the start of the study and exhibited body condition scores (Clingerman and Summers, 2005) of 2.5–4.0 out of 5 at the nearest quarterly exam. Daily chow (LabDiet® 5038, PMI Nutrition International, Richmond, IN, USA; 3.22 kcal of metabolizable energy (ME) per gram) allocations were determined by a power function (Taffe, 2004) fit to data provided in a National Research Council recommendation (NRC/NAS, 2003) and modified individually by the veterinary weight management plan. The animals' normal diet was supplemented with fruit or vegetables seven days per week and water was available ad libitum in the home cage at all times. All animals were individually housed throughout the study. The lights were turned on at 06:30 and off at 18:30 for a 12 hr day/night cycle. Animals on this study had previously been immobilized with ketamine (5–20 mg/kg) no less than semiannually for purposes of routine care and some experimental procedures. Some of the animals had also participated in similar acute challenge studies involving dosing with test compounds no more frequently than twice per week. The United States National Institutes of Health guidelines for laboratory animal care (Clark et al., 1996) were followed and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute (La Jolla).
2.2 Apparatus
Radio telemetric transmitters (TA10TA-D70; Transoma / Data Sciences International, St. Paul, MN, USA) were implanted subcutaneously in the flank. The surgical protocol was adapted from the manufacturer’s surgical manual and implantation was conducted by, or under supervision of, the TSRI veterinary staff using sterile techniques under isoflurane anesthesia. Temperature recordings were obtained from the transmitters implanted in the monkeys via in-cage receivers (RMC-1; Transoma / Data Sciences International, St. Paul, MN, USA). Data were recorded on a 5 minute sample interval basis by the controlling computer and represented as a moving average of three samples (−5 min, current, + 5 min) for each 10 minutes. Ambient room temperature was also recorded by the system via a thermometer mounted near the top of the housing room.
2.3 Drug challenge studies
For these studies Δ9-tetrahydrocannabinol (0.1, 0.2, 0.3 mg/kg) and SR141716 (0.3 mg/kg) were administered intramuscularly (i.m.) in a vehicle of Emulphor:Ethanol:Saline in a ratio of 1:1:18 in a concentration of 5 mg/ml. (±)3,4-Methylenedioxymethamphetamine HCl (1.78 mg/kg) was administered per os (p.o.) in a flavored vehicle (Tang, Kool-Aid, etc) in a volume of 1–2 ml/kg. We have shown previously that this dose of oral MDMA elevates body temperature relative to the oral vehicle (Crean et al., 2007). The MDMA and THC were provided by the National Institute on Drug Abuse (Bethesda, MD, USA) and the SR141716 was purchased from a commercial vendor (Sigma/Aldrich). All challenges were administered in the middle of the light cycle, either at 1030 or 1300 hours, with active dose conditions separated by no less than 3 days (1 week for MDMA). A given subject was challenged at only a single time of day in this repeated-measures studies. The ambient room temperature averaged 23–25°C for these studies.
2.4 Data Analysis
Randomized block analysis of variance (ANOVA) was employed to evaluate acute treatment-related effects on temperature. In general two repeated measures factors were included to determine the effects of drug treatment condition and time relative to drug administration. The analyses were designed based on results from our prior studies. The first analysis was of 10-min samples, started 10 min prior to injection (referred to as “baseline”) and continued for 5 hours post-injection (sample “+300 min”) as in our prior study (Crean et al., 2006), based on the shortest interval between dosing and the beginning of the dark period. Additional analysis of the data was focused on hourly temperature average, expressed as a change from the pre-treatment hour average, for up to 18 hrs after dosing consistent with findings in our prior study of injected METH. This interval was selected based on the shortest interval between dosing and the beginning of the following light period. In addition, examination of the temperature data past this interval (i.e., into the next daytime interval) did not show any lasting effects of drug challenge. Post hoc testing used the Fisher’s LSD procedure including all possible comparisons. All statistical analyses were conducted using GB-STAT v7.0 for Windows (Dynamic Microsystems, Inc., Silver Spring MD) and the criterion for significance in all tests was p < 0.05.
3. Results
3.1 Hypothermia is induced by Δ9THC in unrestrained rhesus monkeys
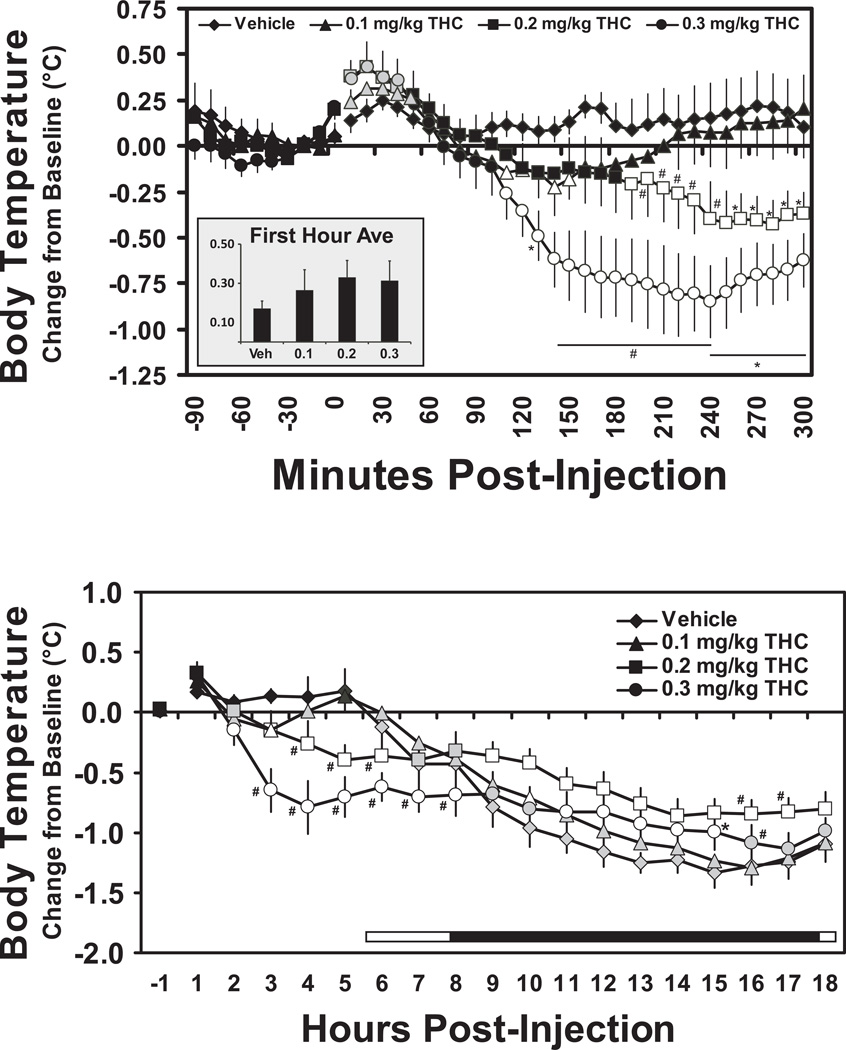
The administration of THC altered body temperature in the first 300 min after injection (Figure 1) as was confirmed by significant main effects of drug treatment condition [F3,27 = 10.62; p < 0.0001], time post- administration [F31,279 = 11.72; p < 0.0001] and an interaction of factors [F93,837 = 5.86; p < 0.0001]). The post hoc test confirmed that temperature was significantly higher than baseline within the first hour after the 0.1 mg/kg (10–50 min), 0.2 mg/kg (10–40 min) and 0.3 mg/kg (10–40 min) THC doses, but not after vehicle injection, and subsequently lower than baseline after the 0.1 mg/kg (110–150 min), 0.2 mg/kg (190–300 min) and 0.3 mg/kg (110–300 min) THC. The posthoc test also confirmed that temperature was lower than the respective time point after vehicle administration following 0.1 mg/kg (110–180 min), 0.2 mg/kg (100–300 min) and 0.3 mg/kg (110–300 min) THC. Dose dependency of the hypothermia was also confirmed, since body temperature was significantly lower than the 0.1 mg/kg challenge condition after 0.2 mg/kg (190–300 min) and 0.3 mg/kg (130–300 min) treatments. Furthermore, temperature was lower after 0.3 (140–240 min) mg/kg when compared with the same timepoints following 0.2 mg/kg.
Figure 1.
Mean (N=10, bars indicate SEM) change in body temperature in the initial 300 minutes (upper panel) and 18 hrs (lower panel) after administration of THC (0.1, 0.2 0.3 mg/kg, i.m.). Shaded symbols indicate a significant difference from the baseline (10 min prior to dosing in 300 min analysis; first hour post-dosing in the 18 hr analysis) at a given timepoint. Open symbols indicate significant differences from the baseline within condition and from the vehicle condition at the respective timepoint. A statistically reliable difference from the vehicle condition and 0.1 mg/kg THC conditions at a given timepoint is indicated by * and a difference from all other conditions by #. The bar in the lower panel indicates the dark period (18:30-06:30 in real time), including the 150 min overlap in which animals treated at 10:30 and 13:00 were offset.
Analysis of the hourly averages also confirmed significant effects of time post-administration [F17,153 = 49.93; p < 0.0001] and an interaction of factors [F51,459 = 7.90; p < 0.0001]. Although body temperature was only lowered in the first few hours after THC, but not the vehicle, the expected circadian decrement in temperature (Taffe, 2011) was observed after the lights were extinguished in all treatment conditions. The posthoc analysis confirmed that, in comparison with the first hour after injection, temperature was lower 6–18 hrs after vehicle, 2–4 and 6–18 hrs after 0.1 m/gkg, 2–18 hrs after both 0.2 and 0.3 mg/kg THC. The posthoc test also confirmed that temperature was significantly lower than the respective time point after vehicle treatment following 0.1 mg/kg (2 hrs), 0.2 mg/kg (3–6 hrs) and 0.3 mg/kg (3–8 hrs) of THC. The effect was dose dependent since temperature after 0.2 mg/kg differed from both 0.1 mg/kg (4–6 hrs) and 0.3 mg/kg (3–11 hrs) of THC. There was also an inversion of the thermoregulatory effect of THC overnight, i.e., temperature was higher than the respective vehicle time points following 0.2 mg/kg (9–18 hrs) and 0.3 mg/kg (11–15 hrs) doses of THC.
3.2 Hypothermia induced by Δ9THC is mediated by the CB1 receptor
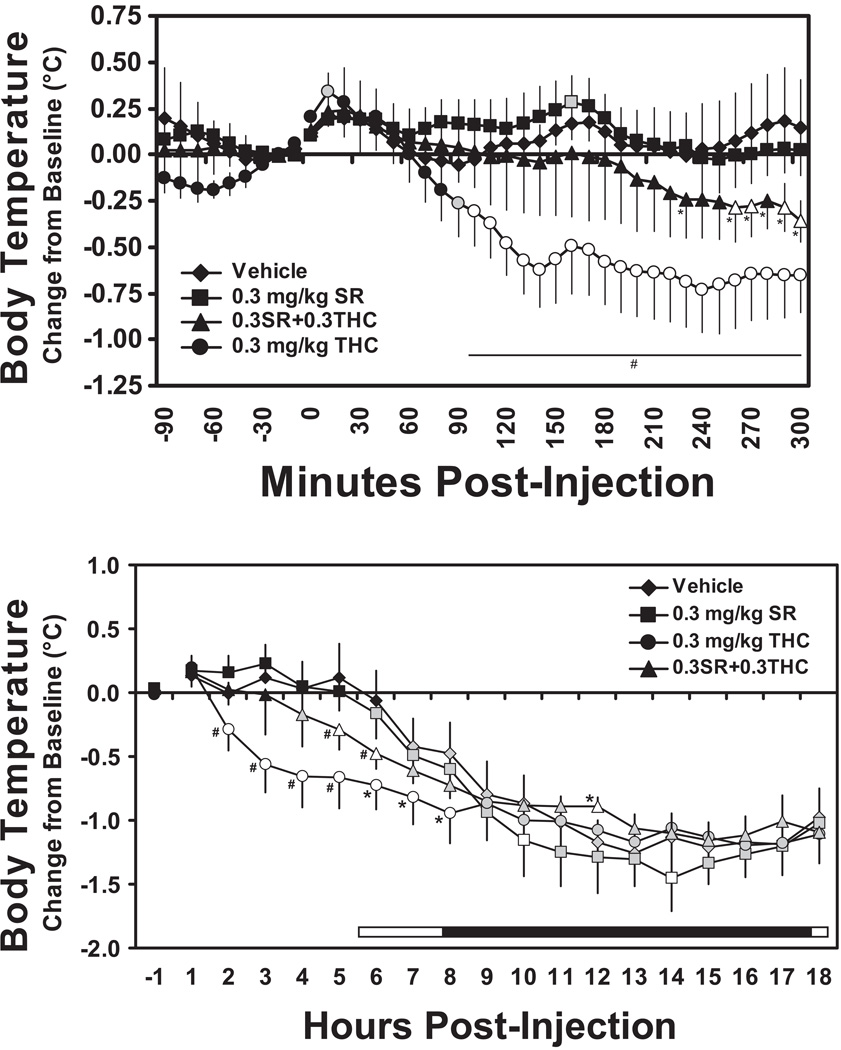
Additional study confirmed that these hypothermic effects of THC were CB1 mediated since the administration of the CB1 antagonist SR141716 in combination with THC attenuated the development of hypothermia (Figure 2). A subset (N=6) of the animals were available for challenge with 0.3 mg/kg SR or with 0.3 mg/kg SR and 0.3 mg/kg THC. These challenges were analyzed together with the vehicle and 0.3 mg/kg THC data for these individuals. The analysis of 10-min samples up to 300 min after injection confirmed significant effects of time post-administration [F31,155 = 5.07; p < 0.0001] and an interaction of factors [F93,465 = 3.05; p < 0.0001]. As confirmed by the posthoc test, temperature was altered following 0.3 mg/kg Δ9THC when compared with the pre-injection baseline (10, 90–300 min) and the respective timepoints after vehicle (100–300), 0.3 mg/kg SR (80–300) or the SR/ THC (90–300 min) combination. Temperature also significantly differed in the SR/THC combined condition when compared with the pre-injection baseline (260–270, 290–300) and the respective timepoints after vehicle (240–300) as well as the 0.3 mg/kg SR condition (60–70, 230 and 260–300 min).
Figure 2.
Mean (N=6, bars indicate SEM) change in body temperature in the initial 300 minutes (upper panel) and 18 hrs (lower panel) after administration of THC (0.3 mg/kg, i.m.), SR141716 (0.3 mg/kg, i.m.) or the combination. Shaded symbols indicate a significant difference from the baseline (10 min prior to dosing in 300 min analysis; first hour post-dosing in the 18 hr analysis) at a given timepoint. Open symbols indicate significant differences from the baseline within condition and from the vehicle condition at the respective timepoint. A statistically reliable difference from the vehicle condition and 0.3 mg/kg SR141716 conditions at a given timepoint is indicated by * and a difference from all other conditions by #. The bar in the lower panel indicates the dark period (18:30-06:30 in real time), including the 150 min overlap in which animals treated at 10:30 and 13:00 were offset.
Analysis of the hourly averages confirmed significant effects of time post-administration [F17,85 = 41.00; p < 0.0001] and an interaction of factors [F51,255 = 3.24; p < 0.0001]. The circadian decrement in temperature, and effect of THC, were again observed. The post hoc test confirmed that temperature was significantly lower than the first hour following vehicle (7–18 hrs), 0.3 mg/kg SR (6–18 hrs), 0.3 mg/kg THC (2–18 hrs) and for the SR / THC combination (4–18 hrs). The effect of 0.3 mg/kg SR was nearly indistinguishable from vehicle with temperature differing only 10 and 14 hrs post-injection. In contrast, body temperature was significantly different from vehicle in the 0.3 mg/kg THC (2–8 hrs) and combined challenge (5–6, 12 hrs) conditions. Finally, the SR countered the immediate hypothermic effects of THC since temperature differed significantly between the SR/THC and THC only conditions from 2–5 hrs after injection. This effect was partial because temperature was lower in the SR/THC conditions compared with the SR condition 5–6, 10–12 hours after injection.
3.3 MDMA-Induced Hyperthermia is attenuated by Δ9THC
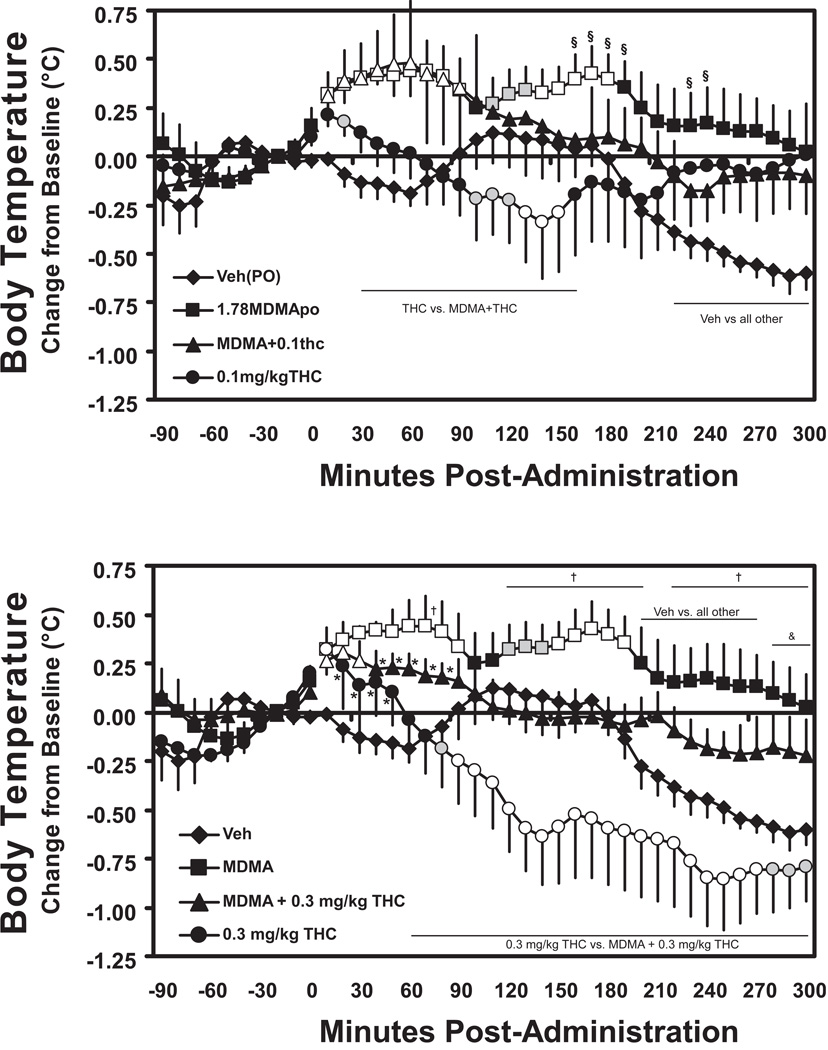
The final set of challenges compared the thermoregulatory effects of 1.78 mg/kg MDMA (p.o.) administered with or without 0.1 or 0.3 THC mg/kg in an available subset (N=5) of the animals participating in the prior challenges. These four new dosing conditions were compared with the original 0.1 and 0.3 mg/kg, i.m., THC data for each individual in the analysis. Overall, MDMA elevated body temperature, THC dose dependently reduced body temperature and either dose of THC was capable of attenuating the body temperature increase associated with MDMA. The overall ANOVA for the first 300 minutes in the 10 min sample dataset (Figure 3) confirmed significant main effects of treatment condition [F5,20 = 5.05; p < 0.005], time post-administration [F31,124 = 11.43; p < 0.0001] and an interaction of factors [F155,620 = 2.76; p < 0.0001].The post-hoc test confirmed that 1.78 MDMA, p.o, by itself significantly elevated temperature above the baseline value 10–90 and 120–190 minutes after consumption. Likewise, temperature was elevated relative to vehicle 10–90 and 150–300 minutes after oral MDMA consumption. Temperature was not elevated above the baseline in the oral vehicle condition, but was significantly lower 210–300 minutes after consumption. The hyperthermic effect of MDMA was attenuated by the co-administration of THC since temperature was only higher than baseline 10–90 minutes after co-administration with 0.1 mg/kg THC and 10–30 min when 0.3 mg/kg THC was co-administered. Similarly, the combination of 0.3 mg/kg THC with MDMA produced lower temperature than MDMA alone (70, 120–200, 220–300 min post-administration). The post hoc test also confirmed that significant hypothermic effects of THC were present in this smaller subset of the original sample because temperature was significantly lower compared with the pre-treatment baseline after 0.1 (130–150 min) or 0.3 (80–300 min) mg/kg THC. Likewise THC also reduced temperature significantly below the vehicle condition 100–150 min after administration of 0.1 mg/kg and 10–80 and 200–300 min after administration of 0.3 mg/kg. The co-administration of MDMA with THC attenuated this hypothermic effect as the post hoc test confirmed significant differences between the combined and THC-alone conditions for the 0.1 mg/kg (30–160, 200 min) and 0.3 mg/kg (60–300 min) conditions.
Figure 3.
Mean (N=5, bars indicate SEM) change in body temperature associated with 1.78 mg/kg MDMA (p.o.) in combination with 0.3 mg/kg THC (i.m.) in the initial 300 minutes after administration. The Vehicle and MDMA conditions have been duplicated on upper and lower panels to depict the relative effect of the two THC doses more clearly. Shaded symbols indicate a significant difference from the baseline (10 min prior to dosing) at a given timepoint. Open symbols indicate significant differences from the baseline within condition and from the vehicle condition at the respective timepoint. A statistically reliable difference from the vehicle condition at a given timepoint is indicated by *, between vehicle and both MDMA conditions by &, between MDMA and the MDMA+THC conditions by †, and a difference from MDMA+THC conditions is indicated by §.
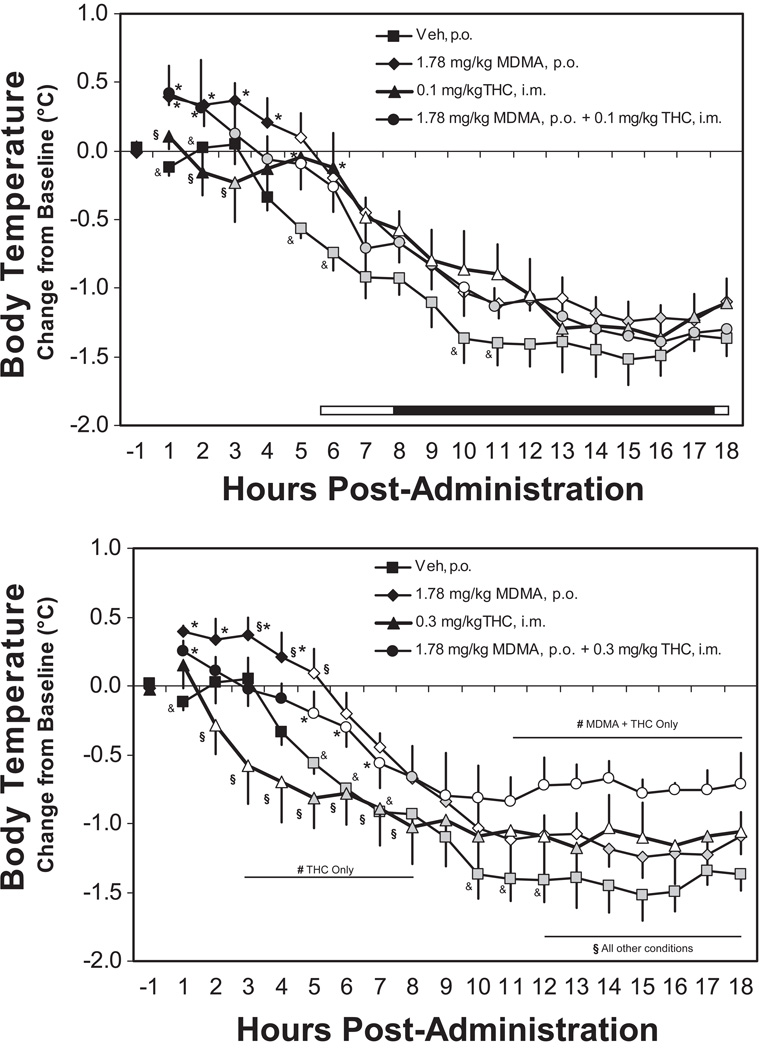
Figure 4 depicts the change in temperature observed in the 18 hours after dosing and, in general, MDMA elevated temperature for up to 13 hours after dosing, the higher dose of THC reduced temperature in the hours after dosing and both doses of THC resulted in blunted circadian cooling overnight. The administration of MDMA and 0.3 mg/kg THC produced opposing effects in the initial hours after dosing but combined to produce additional attenuation of the overnight cooling effect. Analysis of the post-challenge temperature changes confirmed a significant main effect of time post-administration [F17,68 = 48.09; p < 0.0001] and the interaction of time with drug treatment condition [F85,340 = 3.15; p < 0.0001]. The post-hoc test confirmed that temperature differed significantly from the vehicle treatment condition after MDMA (1–7, 10–13 hrs), 0.1 mg/kg THC (5–12 hrs), 0.3 mg/kg THC (2–4, 11–12, 14–16, 18 hrs), MDMA+0.1 mg/kg THC (1–2, 5–6, 10, 12 hrs) and MDMA+0.3 mg/kg THC (1, 5–7, 9–18 hrs). The influence of combining THC with MDMA depended on the dose. The post hoc test confirmed that temperature differed significantly between MDMA and MDMA+0.3 mg/kg THC (3–5, 12–18 hrs) but not between MDMA and the MDMA+0.1 mg/kg THC conditions. Conversely, temperature was higher after MDMA+0.1 mg/kg THC compared with 0.1 THC alone (1–3 hrs) and after MDMA+0.3 mg/kg THC compared with 0.3 mg/kg THC alone (2–8, 12–18 hrs). The effect of THC was dose related since the two MDMA+THC combination conditions differed from each other overnight (11–18 hrs) and the two THC-alone conditions differed from 3–8 hrs after injection.
Figure 4.
Mean (N=5, bars indicate SEM) change in body temperature associated with 1.78 mg/kg MDMA (p.o.) in combination with 0.1 and 0.3 mg/kg THC i.m. in the 18 hrs after administration. The Vehicle and MDMA conditions have been duplicated on upper and lower panels to depict the relative effect of the two THC doses more clearly. Shaded symbols indicate a significant difference from the first hour post-dosing at a given timepoint. Open symbols indicate significant differences from the first hour within condition and from the vehicle condition at the respective timepoint. A difference between 0.1 and 0.3 mg/kg THC conditions (either alone or in combination with MDMA) is indicated by #. All other statistical conventions are as in Figure 3. The bar in the upper panel (omitted in the lower panel for clarity) indicates the dark period (18:30-06:30 in real time), including the 150 min overlap in which animals treated at 10:30 and 13:00 were offset.
4. Discussion
The major outcome of the investigation was that THC was capable of reversing the MDMA-induced hyperthermia in a dose-dependent manner, although this effect was only seen in the daylight hours after administration. A paradoxical attenuation of circadian cooling during the dark cycle was induced by THC and this response was exaggerated by the co-administration of MDMA. This outcome suggests that the findings in humans (Dumont et al., 2011) may be attributable to THC dose or tolerance but likely do not reflect a difference between rodent and primate Orders. This study also confirmed that the primary psychoactive ingredient in cannabis, Δ9-tetrahydrocannabinol (THC), reduces the body temperature of freely moving rhesus macaques. This confirms and extends two prior reports of rectal temperature change in chaired macaque monkeys (Matsuzaki et al., 1987, McMahon et al., 2005). The effect of THC was specific since the magnitude of change was determined by the dose administered and the hypothermia was attenuated by the co-administration of the CB1 receptor antagonist SR141716/Rimonabant. As in our prior investigation (Crean et al., 2007), the oral administration of a moderate, human typical dose of 3,4-methylenedioxymethamphetamine (MDMA) increased the temperature of monkeys. This was evidenced by an immediate elevation relative to the baseline and the vehicle condition, as well as a prolongation of higher temperatures into the late part of the daylight phase when temperature declined in the vehicle condition (Figures 3,4) similar to a prior report using this model (Taffe et al., 2006).
One important aspect of the present study was the selection of THC doses below those which produce substantial sedation. Doses that are higher have been shown to produce behavioral disruption, sedation, task refusal and other non-cognitive effects (Winsauer et al., 1999, McMahon et al., 2005) and we have observed similar dose thresholds in cognitive studies not reported here. While monkeys were visibly intoxicated (drooping eyelids, slowed reaction to conspecifics and investigative staff) within about 15–20 minutes of injection (a time course consistent with “behavioral depression” reported by Matsuzaki et al, 1987) of the THC doses used for this study, they were still responsive to stimuli. It is therefore likely that these conditions approximate the self-selected dose level of human users and are not in the range leading to substantial sedation or immobility. This is an important demonstration since the doses of THC which are reported to reduce body temperature in the rat produce hypolocomotion and/or catalepsy (Whitlow et al., 2002, Smirnov and Kiyatkin, 2008) and are above those (~0.5–1.0 mg/kg, i.p.) shown to produce cognitive disruption (Han and Robinson, 2001, Egerton et al., 2005). The present dose ranges are consistent with prior observations in monkeys. One study reported temperature reductions for up to six hours after administration of 0.4–4.0 mg/kg THC, i.p. (Matsuzaki et al., 1987); the other presented hypothermic effects of 0.32–3.2 mg/kg THC, i.m., but only sampled rectal temperature from 135 to 240 minutes post-injection (McMahon et al., 2005). The data from the present study show a fuller time course after intramuscular injection, extending to 18 hours post-injection and thereby identifying a disruption of normal circadian cooling in the nighttime hours; such data would not be possible using a chair restrained preparation. The radiotelemetric methods employed also show the temperature effects in the first several hours after dosing uncontaminated by chair restraint of the animals although in this case it appears that such methodological differences do not play a major role for the effects observed from about 1–6 hrs after dosing. These less-invasive techniques also permitted the identification of a moderate hyperthermic effect in the first hour after administration (see inset of Figure 1), consistent with low-dose effects sometimes reported for rodents (Johansson et al., 1975, Fennessy and Taylor, 1978).
One minor methodological caveat lies in the offset of the dosing time by 150 min across the group which obviously obscures the precise registration of drug effects with circadian cycle. Although we selected the midday interval because it affords several hours of temperature stability (Taffe et al., 2006) and minimized extraneous activity on challenge days for this study, there are still general vivarium events (e.g., staff work cycle) that may affect activity and / or temperature of the monkeys. A mixed dosing time (between animals) was therefore done intentionally to ensure that effects of the typical daily vivarium routine would not be inferred as a drug effect. The fact that within-subjects effects of drug challenges in these data are statistically reliable and different from the vehicle condition shows that the drug effects generalize across variations induced by the temporal offset. Similarly, the comparison of the present results with the prior studies (Matsuzaki et al., 1987, McMahon et al., 2005) in which the restraint interval and route of drug administration differed and in which the age, sex and prior drug-exposure history likely differed, shows the THC-induced hypothermia is a robust phenomenon in rhesus monkeys.
This study also demonstrated, with antagonist co-administration, that the hypothermia relies on the CB1 receptor. Although one prior report (McMahon et al., 2005), showed a similar attenuation of THC-induced hypothermia with SR141716/Rimonabant, that investigation did not provide the time course of effect. In addition, that prior study found only partial blockade of the hypothermic effects of 0.32–3.2 mg/kg THC associated with 0.32 mg/kg Rimonabant and no additional effect of 1.0 mg/kg Rimonabant when administered with 1.0 mg/kg THC. Here we demonstrate that 0.3 mg/kg Rimonabant fully blocks the hypothermic effects of 0.3 mg/kg THC for the first ~4 hrs since temperature was lower than the vehicle condition in the 5th and 6th hours after the combined challenge. The reasons for differences of these results with the McMahon et al (2005) study are unknown but it may be related to effects of the chair restraint procedure. It is also very likely that the specific time course of effects with either preparation would depend on the respective doses of THC and Rimonabant that were administered, however, for the present purposes it is clear that the hypothermia reported did depend on activity of THC at the CB1 receptor.
Although there are some differences, the general consistency of our THC data with that of prior studies using the chair-restraint method further reinforce a conclusion that temperature responses to drugs in the monkey are more consistent across the body in comparison to rat or other smaller experimental species. For example, cutaneous temperature can be cooled or unaffected at the same time that core temperature is increased by MDMA in rabbit or rat (Pedersen and Blessing, 2001, Mechan et al., 2002). In contrast, mean temperature elevations induced by MDMA in humans are of the same magnitude measured at the skin or intragastrically by telemetry and in monkeys measured by subcutaneous telemetry or rectal thermometer (Freedman et al., 2005, Crean et al., 2007); these issues have been discussed at greater length in our prior studies (Taffe et al., 2006, Von Huben et al., 2007). Additional work has shown that hyperthermia induced by methamphetamine is similar in monkeys when telemetry probes are implanted subcutaneously or intraperitoneally (Taffe, 2011). The present THC data, in consideration with prior work (Matsuzaki et al., 1987, McMahon et al., 2005), emphasize the consistency of body temperature responses regardless of the measurement location (there may still be relevant issues related to restraint, pre-study baselines, activity levels, etc) within monkeys.
This study then went on to demonstrate that the combined administration of THC with MDMA results in an attenuation of the hyperthermic response to MDMA that has been demonstrated experimentally in human and nonhuman primates (Freedman et al., 2005, Taffe et al., 2006, Banks et al., 2007, Crean et al., 2007). Cannabis use is common in the Ecstasy using population (Grov et al., 2009, Wu et al., 2009, Wu et al., 2010) and there is some evidence that it is used as an intentional “come-down” strategy to modulate the subjective experience (Levy et al., 2005). This would be a potential concern for cases of medical emergency involving MDMA-induced hyperthermia based on one report in which inhaled THC potentiated MDMA-induced hyperthermia in humans (Dumont et al., 2011). Nevertheless the present results were similar to the effects of combined THC and MDMA in rats (Morley et al., 2004, Shen et al., 2011) and mice (Tourino et al., 2010) in which THC opposes MDMA-induced hyperthermia. One important consideration is that that low doses of THC may increase body temperature of rats (Perron et al., 2001) and there was some evidence of temperature elevation relative to the vehicle condition in the first hour after THC administration in the present study (Figure 1, inset). It remains possible that the Dumont et al (2011) study exposed humans to much lower doses than used here, although this seems unlikely to be the explanation because 0.1 mg/kg THC appears to be the threshold for any detectable effect in monkeys in our studies, both telemetric and behavioral. It is rather more likely that the drug-experienced human subjects exhibited some degree of tolerance to the hypothermic effects of THC. Studies involving chronic dosing in animal models such as the one used in this study might further explicate the seeming disconnect between the finding in humans (Dumont et al., 2011) and the present investigation.
Highlights.
Δ9-tetrahydrocannabinol (THC) reduces the temperature of freely moving macaques
Effective at doses (0.1–0.3 mg/kg, i.m.) below those which sedate
THC hypothermia is reversed by the CB1 antagonist SR141716/Rimonabant
MDMA-induced hyperthermia is attenuated by THC
Acknowledgements
The author is grateful for expert technical assistance provided by Sophia A. Vandewater, Christopher L. Lay and Stefani N. Von Huben. Research funds were provided by USPHS Grants DA018418, DA024105 and DA024194. This is publication #21449 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug metabolism and disposition: the biological fate of chemicals. 2007;35:1840–1845. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Young JF, Slikker W, Itzak Y, Mayorga AJ, Newport GD, Ali SF, Frederick DL, Paule MG. Plasma levels of parent compound and metabolites after doses of either d-fenfluramine or d-3,4-methylenedioxymethamphetamine (MDMA) that produce long-term serotonergic alterations. Neurotoxicology. 2003;24:379–390. doi: 10.1016/S0161-813X(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggarda J-AD, Vandenbergh JG, White WJ, Williams-Blangero S, VandeBerg JL. Guide for the Care and Use of Laboratory Animals. Washington D.C.: Institute of Laboratory Animal Resources, National Research Council; 1996. p. 125. [Google Scholar]
- Clingerman KJ, Summers L. Development of a body condition scoring system for nonhuman primates using Macaca mulatta as a model. Lab Anim (NY) 2005;34:31–36. doi: 10.1038/laban0505-31. [DOI] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Taffe MA. Oral administration of (+/−)3,4-methylenedioxymethamphetamine and (+)methamphetamine alters temperature and activity in rhesus macaques. Pharmacol Biochem Behav. 2007;87:11–19. doi: 10.1016/j.pbb.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA. Effects of (+/−)3,4-methylenedioxymethamphetamine, (+/−)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience. 2006;142:515–525. doi: 10.1016/j.neuroscience.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont GJ, Kramers C, Sweep FC, Touw DJ, van Hasselt JG, de Kam M, van Gerven JM, Buitelaar JK, Verkes RJ. Cannabis coadministration potentiates the effects of "ecstasy" on heart rate and temperature in humans. Clin Pharmacol Ther. 2009;86:160–166. doi: 10.1038/clpt.2009.62. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, van Hasselt JG, de Kam M, van Gerven JM, Touw DJ, Buitelaar JK, Verkes RJ. Acute psychomotor, memory and subjective effects of MDMA and THC co-administration over time in healthy volunteers. J Psychopharmacol. 2011;25:478–489. doi: 10.1177/0269881110376687. [DOI] [PubMed] [Google Scholar]
- Egerton A, Brett RR, Pratt JA. Acute delta9-tetrahydrocannabinol-induced deficits in reversal learning: neural correlates of affective inflexibility. Neuropsychopharmacology. 2005;30:1895–1905. doi: 10.1038/sj.npp.1300715. [DOI] [PubMed] [Google Scholar]
- Fennessy MR, Taylor DA. Antagonism of the effects on thermoregulation of delta9-tetrahydrocannabinol by clomipramine in the rat. Br J Pharmacol. 1978;63:267–273. doi: 10.1111/j.1476-5381.1978.tb09756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR, Johanson CE, Tancer ME. Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2005;183:248–256. doi: 10.1007/s00213-005-0149-6. [DOI] [PubMed] [Google Scholar]
- Grov C, Kelly BC, Parsons JT. Polydrug use among club-going young adults recruited through time-space sampling. Subst Use Misuse. 2009;44:848–864. doi: 10.1080/10826080802484702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CJ, Robinson JK. Cannabinoid modulation of time estimation in the rat. Behav Neurosci. 2001;115:243–246. doi: 10.1037/0735-7044.115.1.243. [DOI] [PubMed] [Google Scholar]
- Johansson JO, Jarbe TU, Henriksson BG. Acute and subchronic influences of tetrahydrocannabinols on water and food intake, body weight, and temperature in rats. T-I-T journal of life sciences. 1975;5:17–27. [PubMed] [Google Scholar]
- Levy KB, O'Grady KE, Wish ED, Arria AM. An in-depth qualitative examination of the ecstasy experience: results of a focus group with ecstasy-using college students. Subst Use Misuse. 2005;40:1427–1441. doi: 10.1081/JA-200066810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247:1046–1051. [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Casella GA, Ratner M. delta 9-Tetrahydrocannabinol: EEG changes, bradycardia and hypothermia in the rhesus monkey. Brain Res Bull. 1987;19:223–229. doi: 10.1016/0361-9230(87)90087-6. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Amin MR, France CP. SR 141716A differentially attenuates the behavioral effects of delta9-THC in rhesus monkeys. Behav Pharmacol. 2005;16:363–372. doi: 10.1097/00008877-200509000-00008. [DOI] [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O'Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, 'ecstasy') to rats. Br J Pharmacol. 2002;135:170–180. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed WM, Hamida SB, Cassel JC, de Vasconcelos AP, Jones BC. MDMA: Interactions with other psychoactive drugs. Pharmacol Biochem Behav. 2011;99:759–774. doi: 10.1016/j.pbb.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Morley KC, Li KM, Hunt GE, Mallet PE, McGregor IS. Cannabinoids prevent the acute hyperthermia and partially protect against the 5-HT depleting effects of MDMA ("Ecstasy") in rats. Neuropharmacology. 2004;46:954–965. doi: 10.1016/j.neuropharm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- NRC/NAS. Nutrient Requirements of Nonhuman Primates: Second Revised Edition. Washington D.C.: National Research Council of The National Academy of Sciences; 2003. [Google Scholar]
- Patel MM, Wright DW, Ratcliff JJ, Miller MA. Shedding new light on the "safe" club drug: methylenedioxymethamphetamine (ecstasy)-related fatalities. Acad Emerg Med. 2004;11:208–210. [PubMed] [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron RR, Tyson RL, Sutherland GR. Delta9 -tetrahydrocannabinol increases brain temperature and inverts circadian rhythms. Neuroreport. 2001;12:3791–3794. doi: 10.1097/00001756-200112040-00038. [DOI] [PubMed] [Google Scholar]
- Razdan RK. Structure-activity relationships in cannabinoids. Pharmacol Rev. 1986;38:75–149. [PubMed] [Google Scholar]
- Schifano F, Corkery J, Naidoo V, Oyefeso A, Ghodse H. Overview of amphetamine-type stimulant mortality data--UK, 1997–2007. Neuropsychobiology. 2010;61:122–130. doi: 10.1159/000279302. [DOI] [PubMed] [Google Scholar]
- Shen EY, Ali SF, Meyer JS. Chronic administration of THC prevents the behavioral effects of intermittent adolescent MDMA administration and attenuates MDMA-induced hyperthermia and neurotoxicity in rats. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov MS, Kiyatkin EA. Behavioral and temperature effects of delta 9-tetrahydrocannabinol in human-relevant doses in rats. Brain Res. 2008;1228:145–160. doi: 10.1016/j.brainres.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA. Effects of parametric feeding manipulations on behavioral performance in macaques. Physiol Behav. 2004;81:59–70. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA. A comparison of intraperitoneal and subcutaneous temperature in freely moving rhesus macaques. Physiol Behav. 2011;103:440–444. doi: 10.1016/j.physbeh.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN. Hyperthermia induced by 3,4-methylenedioxymethamphetamine in unrestrained rhesus monkeys. Drug Alcohol Depend. 2006;82:276–281. doi: 10.1016/j.drugalcdep.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol Appl Pharmacol. 1973;25:363–372. doi: 10.1016/0041-008x(73)90310-4. [DOI] [PubMed] [Google Scholar]
- Tourino C, Zimmer A, Valverde O. THC Prevents MDMA Neurotoxicity in Mice. PLoS One. 2010;5:e9143. doi: 10.1371/journal.pone.0009143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia induced by (+/−)3,4-methylenedioxymethamphetamine in rhesus macaques. Neuropsychopharmacology. 2007;32:673–681. doi: 10.1038/sj.npp.1301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Freedland CS, Porrino LJ. Metabolic mapping of the time-dependent effects of delta 9-tetrahydrocannabinol administration in the rat. Psychopharmacology (Berl) 2002;161:129–136. doi: 10.1007/s00213-002-1001-x. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Wu LT, Parrott AC, Ringwalt CL, Yang C, Blazer DG. The variety of ecstasy/MDMA users: results from the National Epidemiologic Survey on alcohol and related conditions. Am J Addict. 2009;18:452–461. doi: 10.3109/10550490903206049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Liu X, Fan B. Factors associated with initiation of ecstasy use among US adolescents: findings from a national survey. Drug Alcohol Depend. 2010;106:193–198. doi: 10.1016/j.drugalcdep.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]