Abstract
Cerebellar adaptive plasticity regulates posture and movement in response to changing conditions of sensory stimulation. Study of adaptive plasticity of cerebellar circuitry in vitro confines experimental interest to mechanisms with a time scale of minutes. However, cerebellar plasticity, measured behaviorally or electrophysiologically in vivo, occurs over a time scale of tens of minutes and hours. Here we investigate how optokinetically-evoked increases in climbing fiber activity influence expression of key subcellular signaling proteins that regulate the accumulation of GABAA receptors (GABAARs) in the cytoplasm of Purkinje cells and their insertion into the plasma membrane. We used long-term horizontal optokinetic stimulation (HOKS) to activate climbing fibers that project to the flocculus of mice. While long-term increases in climbing fiber activity in vivo do not alter the expression of any of the subunits of GABAARs expressed by Purkinje cells, they do influence other subcellular events such as transcription and interaction of signaling proteins. Specifically, increased climbing fiber activity evoked decreased expression of 14-3-3-θ, reduced serine phosphorylation of GABAAγ2 and reduced the interaction of 14-3-3-θ with Protein Kinase C-γ (PKC-γ). Knockdown of 14-3-3-θ in vivo reduced the serine phosphorylation of GABAAγ2. Conversely, treatment of cerebellar lysates with phorbol 12-myristate-13-acetate (PMA), a PKC activator, increased serine phosphorylation of GABAAγ2. Knockdown of 14-3-3-θ or PKC-γ in N2a cells in vitro reduced serine phosphorylation of GABAAγ2 and reduced its cell-surface expression. We interpret these data to mean that a prolonged increase in climbing fiber activity decreases the cell surface expression of GABAARs in Purkinje cells and thereby reduces their sensitivity to GABAergic inhibition. This provides a homeostatic mechanism by which Purkinje cells become less sensitive to stellate cell inhibition also evoked by climbing fiber activity.
Keywords: climbing fiber, optokinetic stimulation, flocculus, GABAAγ2, 14-3-3-θ, Protein Kinase C, serine phosphorylation
INTRODUCTION
The cerebellum adapts posture and movement to changing stimulus conditions. Such adaptation has been the focus of behavioral (Raymond et al., 1996; Nagao and Kitazawa, 2003) and chronic electrophysiological studies (Medina and Lisberger, 2008; Soetedjo et al., 2008; Svensson et al., 2010). Cerebellar adaptation has also been a topic of in vitro electrophysiological experiments in which the phenomenon of “Long-Term Depression” (LTD), a form of synaptic plasticity in which electrically-evoked conjunctive activity of parallel and climbing fibers reduces the sensitivity of AMPA receptors on Purkinje cell dendrites to parallel fiber release of glutamate (Sakurai, 1987; Crépel and Jaillard, 1991; Linden and Connor, 1993; Narasimhan and Linden, 1996; Ito, 2002; Mapelli and D'Angelo, 2007). Other forms of cerebellar plasticity include “Long-Term Potentiation” (LTP) of parallel fiber synapses (van Beugen et al., 2006) as well as “Rebound Potentiation” of Purkinje cell GABAARs evoked by electrical stimulation of climbing fibers (Kano et al., 1992; Kawaguchi and Hirano, 2007).
Cerebellar plasticity, measured behaviorally or electrophysiologically in vivo occurs over a time scale of hours. Cerebellar plasticity measured in vitro typically occurs over a time scale of minutes. In a broader context, in vitro LTD, LTP and RP may reflect fundamentally different mechanisms than are engaged during behavioral reflex adaptation. Indeed reflex adaptation is retained in mice in which LTD is blocked (Faulstich et al., 2006). Longer-term changes in cerebellar plasticity evoked by natural stimulation can reveal important transcriptional events and protein interactions not evident when experimental interests are focused on synaptic events of shorter duration.
In previous in vivo experiments we showed that long-term horizontal optokinetic stimulation (HOKS) evoked sustained increases in activity of visual climbing fibers that originate from the inferior olive and project to Purkinje cells in the cerebellar flocculus (Fig. 1B). While we have monitored the HOKS-evoked activity of individual olivary neurons for several minutes we have relied on other measures to confirm that this activity persists over hours and days. Binocular or monocular long-term HOKS evokes a 10-fold linear increase in corticotropin-releasing factor (CRF) transcripts as well as expression of CRF in inferior olivary neurons contralateral to the eye stimulated in the Posterior→Anterior (P→A) direction (Barmack and Young, 1990; Barmack and Errico, 1993). Monocular A→P HOKS evokes no change in expression of CRF in either ipsilateral or contralateral olivary neurons. Once HOKS is stopped, evoked increases in CRF transcripts decay with a time constant of ~4 h. Long-term HOKS also evokes significant changes in eye movements, evoking an ocular nystagmus that can be monitored chronically for tens of hours (Barmack and Nelson, 1987).
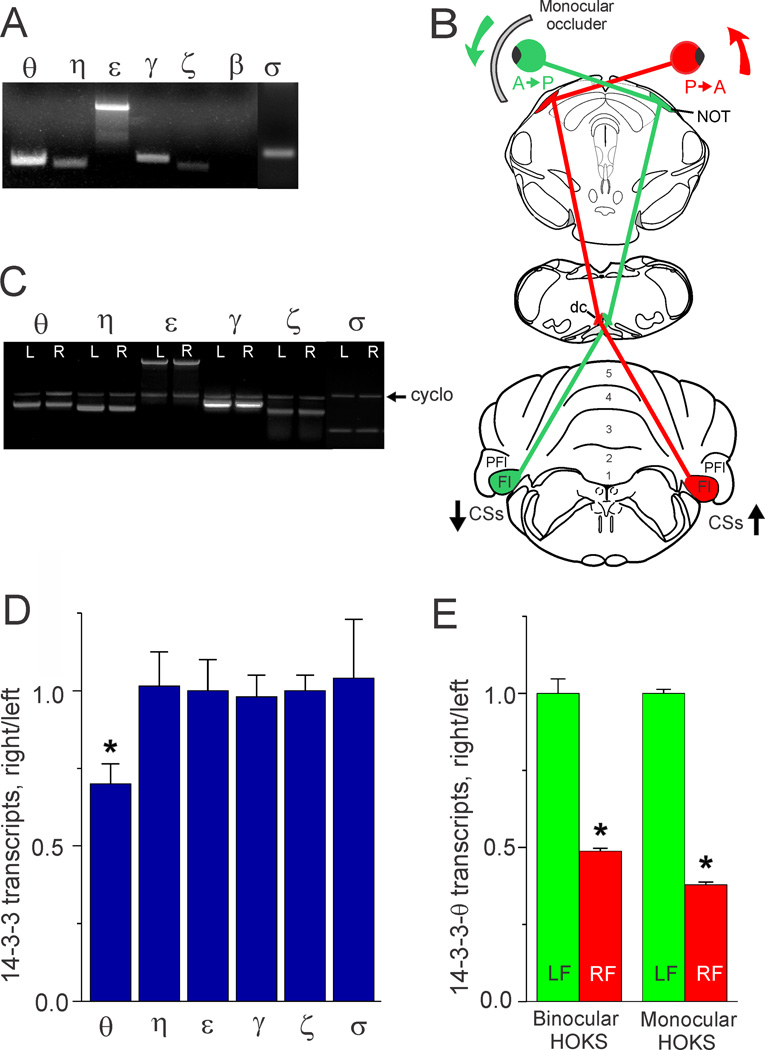
Figure 1. Detection of 14-3-3 protein isoform transcripts in mouse flocculus and decreased transcription of 14-3-3-θ mRNA evoked by increased climbing fiber activity.
A. In three mice we surveyed the mouse flocculus for seven 14-3-3 isoform mRNAs (θ, η, ε, γ, ζ, β and σ) using PCR. All seven isoforms were detected except β. B. We used HOKS to control climbing fiber activity. The cartoon shows that HOKS of the right eye in the P→A direction increased climbing fiber activity projecting to Purkinje cells in the right flocculus. HOKS of the left eye in the A→P direction decreases climbing fiber activity projecting to the left flocculus. Placement of a contact occluder in front of the left eye effectively created a monocular HOKS condition in which only the right eye was stimulated. C. In three mice we extracted mRNA from the left and right flocculi following 24 h of HOKS and analyzed these samples for transcription of six different 14-3-3 isoforms (θ, η, ε, γ, ζ and σ). The optical density of PCR bands was measured and normalized with respect to cyclophilin (cyclo). Density is expressed as a ratio of mRNA transcripts (right/left flocculus). The transcription of 14-3-3-θ, but not η, ε, γ, ζ, β and σ was reduced by increased climbing fiber input evoked HOKS in the right flocculus. D. The data obtained from the PCR bands in C are plotted in a histogram and show that 14-3-3-θ, but not η, ε, γ, ζ, β and σ transcripts were reduced during HOKS (ANOVA, p< 0.04, indicated by asterisk). E. In six mice we tested the efficacy of binocular vs monocular HOKS in evoking changes in 14-3-3-θ transcription. Each group of three mice received either binocular or monocular HOKS for 24 h. We detected 14-3-3-θ transcripts using real-time PCR. All transcripts were normalized with respect to β-actin. Monocular HOKS proved equally effective as binocular HOKS in evoking a relative decrease in 14-3-3-θ transcripts in the right flocculus (increased climbing fiber activity) (ANOVA, p< 0 .001, indicated by asterisk). Error bars indicate standard error of the mean. Abbreviations: CSs, complex spikes; dc, dorsal cap; Fl, flocculus; NOT, nucleus of the optic tract; PFl, paraflocculus.
We have used long-term HOKS to detect activity-dependent changes in gene transcription in floccular Purkinje cells (Barmack and Qian, 2002; Barmack et al., 2004). The transcripts of one such gene, GABARAP, implicated in trafficking of GABAARs through its interaction with a serine phosphorylated GABAAγ2 subunit, were reduced by prolonged climbing fiber activity (Qian et al., 2011). Here we investigate another gene, 14-3-3-θ mRNA, whose transcripts are reduced by climbing fiber activity. It is a member of a family of cellular scaffolding proteins that are abundantly expressed in the brain and that regulate protein kinase activity (Fantl et al., 1994; Muslin et al., 1996; Fu et al., 2000). We demonstrate a regulatory influence of climbing fiber activity on 14-3-3-θ and we illustrate how 14-3-3-θ regulates the phosphorylation of GABAARs through its activation of an isoform of protein kinase C, PKC-γ. PKC-γ interacts specifically with the γ2 subunit of the GABAAR. When 14-3-3-θ is knocked down serine phosphorylation of GABAAγ2 is reduced. We infer that long-term increases in climbing fiber activity could induce a sequence of subcellular events that homeostatically regulate Purkinje cell surface expression of GABAARs.
EXPERIMENTAL PROCEDURES
Long-term HOKS
We used 51 C57BL/6J mice, weighing 16.0–29.0 g as subjects (Jackson Lab, Bar Harbor, ME). All animal procedures were approved in advance by the Institutional animal care and use committee of Oregon Health & Science University. Mice were partially restrained in plastic tubes. The head end of each tube was placed near the center of an optokinetic sphere. The sphere has a diameter of 46 cm. Sixteen equally spaced longitudinal meridians, fashioned with blue tape, lined the sphere’s interior white wall; each subtending 5 deg. The sphere rotated at a constant velocity of 6 deg/s in the P→A direction with respect to the right eye. Mice were removed from the sphere every 8 h, weighed, and given food and water for 20 min. The maximum duration of HOKS was 30 h. During monocular HOKS a stationary white opaque barrier obscured vision of the left eye by 190 deg horizontally and 160 deg vertically (Fig. 1B). When HOKS stopped, the mice were euthanized with an intraperitoneal injection of ketamine hydrochloride (70 mg/kg) and xylazine (6 mg/kg). The brain was removed and immediately cooled (4°C). The two flocculi were removed.
Real-Time Polymerase Chain Reaction (qPCR)
We used qPCR to compare levels of 14-3-3-θ transcripts in mouse flocculi after HOKS. Following 24h of HOKS, mice were anesthetized and euthanized. We used Trizol (Invitrogen, Carlsbad, CA) to extract total RNA from dissected flocculi. RNA samples were treated with RNase-free DNase I (Invitrogen) for 30 min at 37°C to eliminate DNA contamination. Reverse transcription was performed with SuperScript III reverse transcriptase (Invitrogen). Fluorescent signals were generated using Fast SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA). qPCR for 14-3-3-θ and β-actin were run in triplicate on a Bio-Rad CFX96 real time PCR system as follows: 20 s at 95°C and 40 cycles of 3 s at 95°C, 30 s at 60°C. Primer sequences were:
| 14-3-3-θ | forward, 5-CATCATGCAGTTGCTTAGAGACA-3, |
| reverse, 5-TTAGTTTTCGGCCCCCTCT-3. | |
| 14-3-3-ε | forward, 5-GATCGGGAGGATCTGGTGT-3, |
| reverse, 5-TCATTGATTCCACCATTTCG-3. | |
| PKC-γ | forward, 5-ACCAGGAGGAGGGCGAATAT-3, |
| reverse, 5-GGAGGAGGCTGCAGTTGTCA-3. | |
| PKC-δ | forward, 5-CAATAGCCGGGACACCATCT-3, |
| reverse, 5-AATCGGTGAGGCATGTCGAT-3. | |
| β-actin: | forward, 5-ACTGGGACGACATGGAGAAGA-3, |
| reverse, 5-GCCACACGCAGCTCATTGTA-3. |
β-actin served as an internal control. A melting curve analysis was performed at the end of the PCR cycles. Experimental controls included non-reverse-transcribed RNA samples. Data were analyzed by Bio-Rad CFX96 software to determine the threshold cycle (CT) for each reaction.
Floccular protein preparation, gel electrophoresis and immunoblotting
After dissection, the flocculi were immediately homogenized and sonicated with a 10× volume of Tris-buffered saline (TBS; 20 mM Tris-HCl and 150 mM NaCl, pH 7.5, containing 1% SDS or 1% IGEPAL CA-630). Protease inhibitors, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin and 10 µg/ml leupeptin were added to the buffer. All subsequent procedures were performed at 4°C. Homogenates were centrifuged at 14,000 rpm for 30 min. We measured protein concentration with a Bradford assay (Bio-Rad, Hercules, CA). Cerebellar lysate proteins were separated on a 12% SDS-PAGE gel. The gel was transferred onto a Polyvinylidene fluoride (PVDF) membrane and incubated with a primary antibody in TBS. A polyclonal antibody to β-actin was used to immunolabel β-actin as an equal loading control (Santa Cruz Biotech, Santa Cruz, CA). Antibodies were visualized with horseradish peroxidase-conjugated anti-goat IgG using enhanced chemiluminescence (GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
Protein "pull-down" assay using histidine-tagged 14-3-3-θ
A plasmid containing histidine-tagged 14-3-3-θ was transformed into E. coli competent cells (TOPO Expression kit, Invitrogen). Expressed 14-3-3-θ was purified using nickel-impregnated agarose beads (Ni-NTA, Qiagen Corp., Valencia, CA). The 14-3-3-θ beads were incubated with cerebellar lysates. The beads were poured into a column and washed with three column volumes of buffer containing a low imidazole concentration (20 mM imidazole in 50 mM Na2PO4, pH 8.0, 300 mM NaCl) to remove proteins that bind nonspecifically to the beads. Proteins pulled down by 14-3-3-θ were eluted from the beads with buffer containing 250 mM imidazole.
Immunoprecipitation
Cerebellar lysates were sonicated 3× times for 15 s in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EGTA, 0.25% IGEPAL, plus a complete protease inhibitor mixture tablet for each 15 ml of buffer) (Roche, Branchburg, NJ). The lysates were centrifuged at 14,000 rpm for 30 min at 4°C to remove insoluble debris. The supernatant was pre-cleared with protein A/G plus agarose beads (Santa Cruz Biotech) to remove proteins that bind nonspecifically to the beads. The beads were removed by centrifugation at 500 × g. The cleared lysates were incubated with an antibodies to: 14-3-3-θ (Cell Signaling Tech., Danvers, MA), GABAAγ2 (Santa Cruz Biotech) and PKC-γ (Epitomics, Burlingame, CA) and rocked overnight at 4°C. Agarose beads (A/G plus, Santa Cruz) were then added to the mixture for 1 h. The beads were removed by centrifugation at 500 × g and washed three times with lysis buffer. The immunoprecipitate was boiled for 10 min to dissociate the proteins immunoprecipitated from the beads. The dissociated proteins were subsequently analyzed on a 12% SDS-PAGE gel.
PMA activation of PKC in NIH/3T3 and N2a cells
Mouse NIH/3T3 or Neuro2a cells (N2a) (American Type Culture Collection, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) containing 10% fetal bovine serum (FBS). Cells were maintained in 5% CO2 at 37°C. The cells were removed by trypsinization and split 1:3 every three days. Cells were grown for three passages after being brought out of freeze-down in liquid N2 and then used through passage 12. Both untransfected and transfected NIH/3T3 or N2a cells were plated at 90% confluence and treated for 30 min with 200 nM PMA in Dimethyl sulfoxide (DMSO) or DMSO alone. After treatment, the cells were harvested and the protein extracts were prepared as described above. Cell viability assay was performed with cell titre blue reagent (Promega, Madison, WI) as previous described (Qian et al., 2008).
In vitro knockdown of 14-3-3-θ and PKC-γ by RNA interference
Three different siRNA sequences were designed to target the 14-3-3-θ mRNA (GeneBank accession number NM_011739) using the Block-iT RNAi designer (Invitrogen):
s321 siRNA (5’-CCACGGUCCUGGAAUUGUUGGAUAA-3’),
s357 siRNA (5’-CCAAUGCAACUAAUCCAGAGAGUAA-3’),
s479 siRNA (5’-GCCUACCAAGAGGCGUUUGAUAUAA-3’).
Four miRNAs were constructed by inserting oligo nucleotides targeting different portions of the PKC-γ mRNA (GeneBank accession number NM_011102) into pcDNA6.2-GW/EmGFP-miR vector using the BLOCK-iT Pol II miR RNAi kit (Invitrogen).
miR-PKC-γ-1: (5’-TGCTGAAAGCTACAGACTTGACATTGGTTTTGGCCACTGACTG ACC AATGTCATCTGTAGCTT-3’), 451–471,
miR-PKC-γ-2: (5’-TGCTGTATGGATCTCATCCGACGTAGGTTTTGGCCACTGACTG ACC TACGTCGTGAGATCCAT-3’), 761–780,
miR-PKC-γ-3 (5’-TGCTGTAACTTGTACCATCCATCCACGTTTTGGCCACTGACTGA CGT GGATGGGGTACAAGTT-3’), 1069–1086,
miR-PKC-γ-4 (5’-TGCTGTACATTGTAATATTCGCCCTCGTTTTGGCCACTGACTGA CGA GGGCGAATTACAATGT-3’), 1102–1121,
Mouse NIH/3T3 or N2a cells were grown in 6-well plates to 50–70% confluence in media lacking antibiotics. The cells were transfected using 100 pmol siRNA or 4 µg miRNA plasmid and Lipofectamine 2000 reagent (Invitrogen). For control transfections we used a mis-matched PKC miRNA plasmid or the transfection solution alone without the siRNA. The transfection efficiency of the siRNA was ~60% and was measured by fluorescence microscopy using a size-matched fluorescein-labeled dsRNA oligomer. The ~40% transfection efficiency of the miRNA plasmid was also measured using fluorescence microscopy.
Knockdown of 14-3-3-θ in vivo by microinjection of siRNA into cerebellum
We confirmed the efficacy of s479 siRNA (5’-GCCUACCAAGAGGCGUUUGAUAUAA-3’) in knocking down 14-3-3-θ using NIH/3T3 and Neuro2a cells. For in vivo knockdowns we mixed equal amounts (0.8 µg/µl) of s479 siRNA and transfection solution (PolyPlus Corp, Illkirch, France). Using a 1 µl syringe connected hydraulically with a micropipette we made multiple injections of 50–200 nl of the s470-transfection solution into folia 7–8 of the left vermis. We injected an equal volume of transfection solution without the siRNA into folia 7–8 of the right vermis. The microinjections were spaced ~0.7 mm on either side of the midline.
Diffusion of a microinjected siRNA across the midline into the site of the other microinjected siRNA would tend to minimize expected differences. We tested the extent of diffusion of microinjections by injecting 800 nl of a control fluorescein-labeled dsRNA oligomer into the posterior cerebellum. The diffusion of the microinjection was contained within a 1 mm sphere of tissue. Three days after the microinjection of 14-3-3-θ siRNA, mice were anesthetized and decapitated. The injected folia were removed to analyze 14-3-3-θ transcription and expression.
Identification of cell-surface proteins by biotinylation
Freshly harvested N2a cells were transferred to T75 flasks and grown to 50–70% confluence in media lacking antibiotics. 14-3-3-θ and PKC-γ were knocked down using siRNA or miRNA. After transfection, the cells were grown for 48 h and PMA was added for 30 min as described above. Cell-surface proteins were biotinylated with 250 µg/ml Sulfo-NHS-SS-Biotin (Thermo-Fisher, Rockford, IL) for 30 min at 4°C. The cells were harvested and sonicated in lysis buffer. The cleared lysate was applied to NeutrAvidin biotin-binding columns (Thermo-Fisher) according to the manufacturer’s instructions to separate the biotinylated membrane proteins from cytosolic proteins.
RESULTS
Identification of 14-3-3 isoforms in mouse flocculus
Previously we used the technique of “Differential Display” to show that enhanced climbing fiber activity evoked by binocular HOKS in the nodulus and flocculus of the rabbit decreased transcription of 14-3-3-θ in Purkinje cells ipsilateral to the eye stimulated in the P→A direction (Barmack and Qian, 2002). Since more complete sequence information was available for 14-3-3 proteins in mouse than in rabbit, we re-examined this observation using semi-quantitative PCR with selected primers for seven isoforms of 14-3-3 proteins. Six 14-3-3 protein isoforms (θ, η, ε, γ, ζ and σ) were transcribed in mouse cerebellum (Fig. 1A). We tested each of these isoforms to determine whether their transcript levels in the flocculus were influenced by optokinetically increased climbing fiber evoked by P→A HOKS of the ipsilateral eye for 24 h (Fig. 1B). Transcripts for 14-3-3-θ in the ipsilateral flocculus were the only isoforms that decreased with respect to the contralateral flocculus during binocular HOKS (ANOVA, p < 0.04, N=3, Fig. 1C, D). We attributed this relative decrease in 14-3-3-θ transcripts in the right flocculus to increased climbing fiber activation. However, since our measurements of 14-3-3-θ transcripts in the right flocculus were always expressed as a ratio of the transcripts with respect to the left flocculus, it was possible that this apparent decrease could be attributed to a relative increase in 14-3-3-θ transcripts in the left flocculus evoked by decreased climbing fiber activity. We tested this possibility in two groups of three mice each by comparing the effects of binocular and monocular HOKS. Monocular HOKS was achieved by stimulating only the right eye in the P→A direction for 24 h while blocking A→P stimulation of the contralateral eye with an ocular occluder (Fig. 1B). Monocular HOKS decreased the transcript levels of 14-3-3-θ in the right flocculus relative to the non-stimulated left flocculus by a factor of 2 (ANOVA, p < 0.001, N=6, Fig. 1E). These data indicate that the relative decrease in 14-3-3-θ transcripts ipsilateral to the eye stimulated in the P→A direction was, in fact, an absolute decrease and depended on increased climbing fiber activity.
14-3-3-θ interacts with PKC-γ
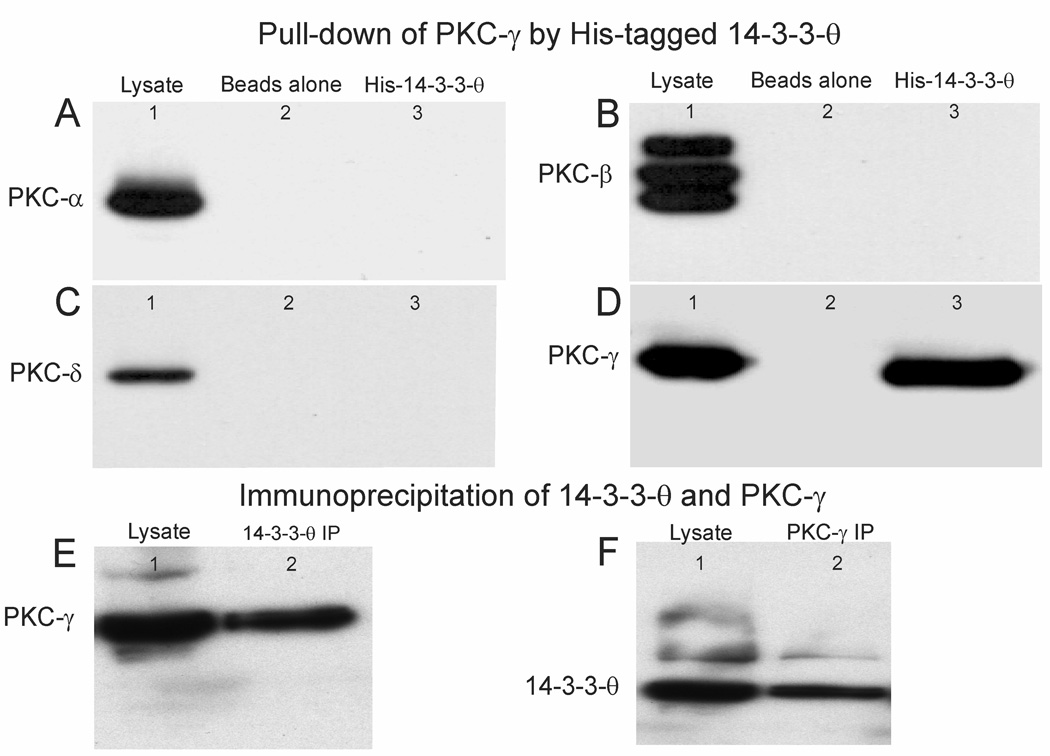
In the cell cycle, 14-3-3 proteins bind with and stimulate PKC-ε activity controlling the final step in cell division (Saurin et al., 2008). We explored possible interactions of 14-3-3-θ with PKC isoforms in the cerebellum using histidine-tagged 14-3-3-θ beads to pull down PKC isoforms from cerebellar lysates. We identified proteins that bound to 14-3-3-θ beads by immunoblotting with antibodies to PKC that are expressed in the cerebellum. These include: PKC-α, -β, -γ and -δ isoforms (Fig. 2A–D). 14-3-3-θ interacted with PKC-γ, but not other PKC isoforms (α, β and δ, Fig. 2D). The specificity of the interaction of 14-3-3-θ with PKC-γ was confirmed by immunoprecipitation with antibodies against 14-3-3-θ and PKC-γ. We used an antibody to 14-3-3-θ to immunoprecipitate 14-3-3-θ from a mouse cerebellar lysate. PKC-γ was co-immunoprecipitated along with 14-3-3-θ (Fig. 2E). We used an antibody to PKC-γ to immunoprecipitate PKC-γ from a lysate and it co-immunoprecipitated 14-3-3-θ (Fig. 2F). These data indicate that PKC-γ and 14-3-3-θ interact in cerebellar lysates.
Figure 2. 14-3-3-θ interacts with PKC-γ in cerebellar lysates.
Cerebellar lysates extracted from three mice were incubated with recombinant 14-3-3-θ probes attached to histidine beads. We tested recovered proteins with antibodies against: A, PKC-α, B, PKC-β, C, PKC-δ, D, PKC-γ. In A–D: Lane 1 shows cerebellar lysates that are immunolabeled with antibodies to the above PKC isoforms. Lane 2, shows absence of non-specific binding of cerebellar lysates to his-binding beads without 14-3-3-θ attached. Lane 3 shows specific binding of PKC isoforms to his-tagged 14-3-3-θ. PKC-γ was the only isoform pulled-down from cerebellar lysates with his-tagged 14-3-3-θ. E. The interaction of PKC-γ with 14-3-3-θ was confirmed by co-immunoprecipitation with 14-3-3-θ from cerebellar lysates with antibodies to 14-3-3-θ. Lane 1 shows immunolabeling of a cerebellar lysate by an antibody to PKC-γ. Lane 2 shows the immunoprecipitate of 14-3-3-θ immunolabeled by an antibody to PKC-γ. F. Lane 1, shows immunolabeling of a cerebellar lysate by an antibody to 14-3-3-θ. Lane 2, shows the immunoprecipitate of PKC-γ immunolabeled by an antibody to 14-3-3-θ.
Climbing fiber activity decreases serine phosphorylation of GABAAγ2 in cerebellar lysates
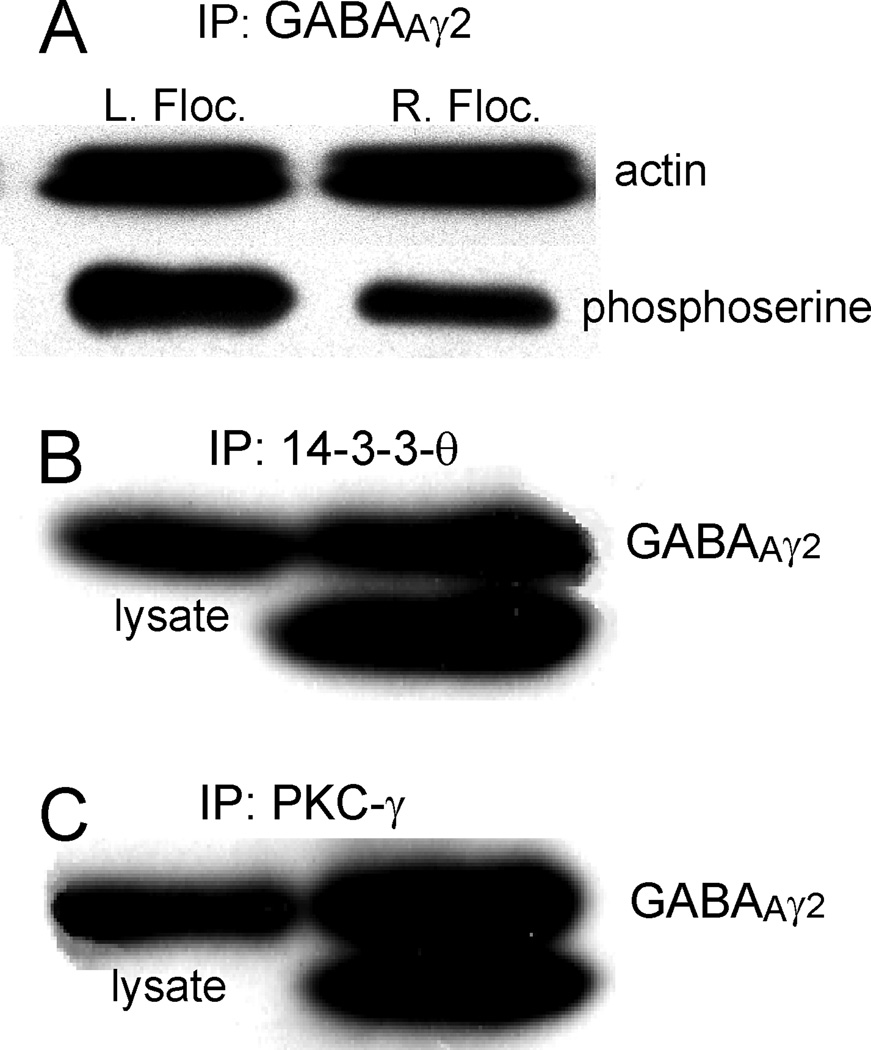
Increased climbing fiber activity reduces transcription and expression of GABAAR-associated protein (GABARAP) (Qian et al., 2011). GABARAP interacts preferentially with serine phosphorylated GABAAγ2. Serine dephosphorylation of GABAAγ2 in vitro reduces its association with GABARAP and may reduce its transport to the cell surface membrane. Could climbing fiber activity influence serine phosphorylation of GABAAγ2? We examined this question by immunoprecipitating GABAAγ2 from cerebellar lysates prepared from the left and right flocculus of three mice that received 24 h of HOKS. The right flocculus received increased climbing fiber activity. We probed both immunoprecipitates with an antibody to phosphoserine. The density of phosphoserine immunostaining was reduced in the right flocculus (Fig. 3A).
Figure 3. Increased climbing fiber activity decreases serine phosphorylation of GABAAγ2 and GABAAγ2 is co-immunoprecipitated with 14-3-3-θ and PKC-γ.
A. Proteins from the left and right flocculus of three mice were immunoprecipitated separately with an antibody to GABAAγ2 after 24h of HOKS in which the right flocculus received increased climbing fiber activity. The blots were immunoprobed with an antibody to phosphoserine and a control antibody to β-actin. Serine phosphorylation of the immunoprecipitate from the right flocculus was reduced by sustained climbing fiber activity. B, C. Proteins were immunoprecipitated by an antibody to PKC-γ (B) and by an antibody to 14-3-3-θ (C). The immunoprecipitates were immunoprobed with an antibody to GABAAγ2. The left lanes show cerebellar lysates immunolabeled by the antibody to GABAAγ2. The right lanes show immunoprecipitates immunolabeled by the antibody to GABAAγ2. The double bands in the immunoprecipitates are attributed to two different phosphorylated states.
GABAAγ2 is co-immunoprecipitated with 14-3-3-θ and PKC-γ
An antibody to 14-3-3-θ co-immunoprecipitates PKC-γ from cerebellar lysates and, conversely, an antibody to PKC-γ co-immunoprecipitates 14-3-3-θ (Fig. 2). These interactions suggest that the combined action of 14-3-3-θ and PKC-γ induce serine phosphorylation of GABAAγ2. We tested this idea directly by using antibodies against 14-3-3-θ and PKC-γ separately to immunoprecipitate proteins from cerebellar lysates. GABAAγ2 co-immunoprecipitated in two bands with each of the immunoprecipitates (Fig. 3B,C). Although GABAAγ2 has two isoforms that differ by ~8 amino acids, it is likely that the two bands that differ by ~2 kDa reflect a de-phosphorylated state of a fraction of the GABAAγ2 in the normal lysate, caused by the immunoprecipitation (Qi et al., 2007).
Localization of 14-3-3-θ, PKC-γ and GABAAγ2 to Purkinje cells
Having identified an interaction between 14-3-3-θ, PKC-γ, and GABAAγ2, we investigated the immunohistochemical distribution of each of these proteins, as well as another protein, calbindin, in the cerebellum. Not surprisingly all were expressed in Purkinje cells. These data were confirmed by qPCR performed using single cerebellar cell types isolated by laser capture microscopy. PKC-γ transcripts in Purkinje cells exceeded those in basket and stellate cells by a factor of 163 and exceeded those in granule cells by a factor of 187. Purkinje cell transcripts for 14-3-3-θ exceeded those in basket and stellate cells by a factor of 209 and those in granule cells by a factor of 208. The transcripts for GABAAγ2 were 15–30% higher in Purkinje cells relative to those in basket and stellate cells. Although we cannot exclude the possibility of residual expression of 14-3-3-θ and PKC-γ in cerebellar interneurons such expression is small with respect to that of Purkinje cells.
Efficacy of knocking down 14-3-3-θ in NIH/3T3 cells by RNA interference
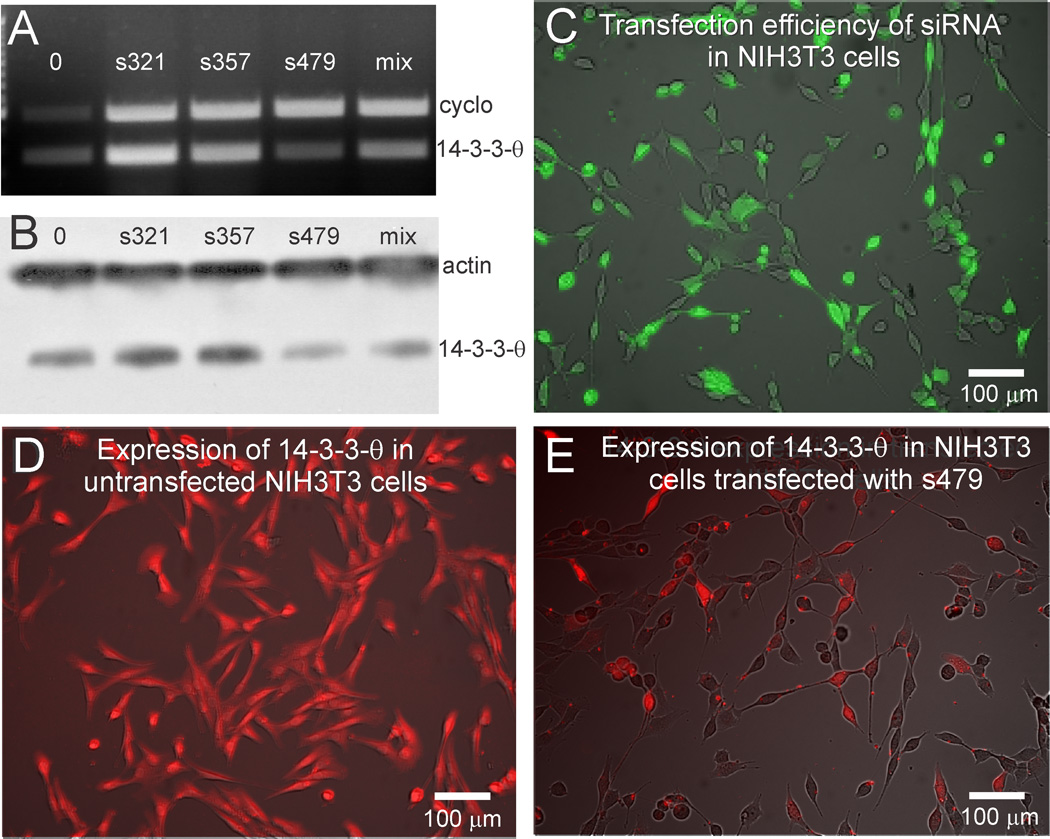
We elucidated several important cellular changes that are a consequence of prolonged increases in climbing fiber activity. However, an in vivo preparation raises serious challenges for addressing mechanistic questions at the cellular level. Consequently, we established an in vitro system using an NIH/3T3 cell line in which 14-3-3-θ and PKC-γ are endogenously expressed, to investigate interactions between 14-3-3-θ, PKC-γ and GABAAγ2. We used RNA interference to knockdown 14-3-3-θ, testing three 25 mer siRNAs (s321, s357 and s479) that complemented different 14-3-3-θ mRNA sequences. The relative efficacy of each of the three siRNAs in reducing 14-3-3-θ mRNA transcripts was measured by PCR (Fig. 4A). Expression of 14-3-3-θ was measured by Western blot (Fig. 4B). Of the three siRNAs, s479 was most effective in knocking down 14-3-3-θ mRNA transcripts and reducing expression of 14-3-3-θ. s479 was also more effective than an equal mixture of all three siRNAs. As a control for siRNA specificity, s479 failed to knockdown 14-3-3-ε (data not shown). We confirmed transfection efficacy in vitro using a control fluorescent oligonucleotide (Fig. 4C). We checked 14-3-3-θ expression in NIH/3T3 cells by immunolabeling untreated NIH/3T3 cells with an antibody to 14-3-3-θ (Fig. 4D). In NIH/3T3 cells transfected with s479, 14-3-3-θ was reduced relative to the untransfected control cells (Fig. 4E).
Figure 4. RNA interference knocks down 14-3-3-θ in NIH/3T3 cells.
Three siRNAs were tested for their potency in reducing in vitro transcription and expression of 14-3-3-θ. A. PCR-amplified 14-3-3-θ transcripts and cyclophilin (cyclo) transcripts in mouse brain and in NIH/3T3 cells are shown. Transcripts in Lane 0 are from a mouse brain lysate. Transcripts from NIH/3T3 cells treated with three different siRNAs are shown in the other lanes. Treatment with s479 produced maximal reduction of 14-3-3-θ. B. Western blots were immunoprobed with antibodies to 14-3-3-θ, with β-actin serving as a loading control. Maximal reduction in the expression of 14-3-3-θ is also shown with s479. C. Transfection efficiency was determined using a control fluorescent oligonucleotide. D. Expression of 14-3-3-θ was tested in untransfected NIH/3T3 cells. All cells were immunolabeled by the 14-3-3-θ antibody. E. Transfection of NIH/3T3 cells with s479 reduced expression of 14-3-3-θ.
Reduced expression of 14-3-3-θ in vivo reduces GABAAγ2 serine phosphorylation in vivo
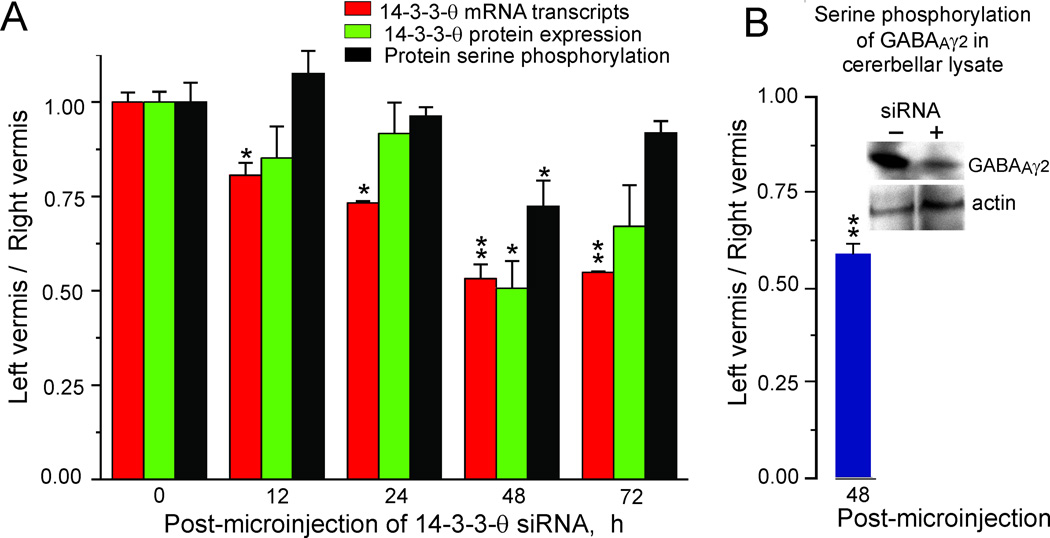
If 14-3-3-θ is essential for serine phosphorylation of GABAAγ2 in vivo, then reducing its expression should reduce GABAAγ2 serine phosphorylation. We used siRNA interference to knock down the expression of 14-3-3-θ in a total of 30 mice. We microinjected ~ 200–600 nl of 14-3-3-θ siRNA (s479) in a transfection vehicle (PolyPlus Corp) into the right vermis (folia 7–8). We injected the left vermis with a control solution containing only the transfection vehicle. We chose the vermis for these injections rather than the flocculus because we could more easily visualize and control the positioning of the microinjection pipette and the ejection of the solution. After the microinjections were completed we measured 14-3-3-θ mRNA transcripts and proteins at intervals of 0, 12, 24, 48 and 72 h. Microinjection of the 14-3-3-θ siRNA knocked down 14-3-3-θ mRNA transcripts (Fig. 5A, red bars) and 14-3-3-θ expression (Fig. 5A, green bars) detected with PCR and Western blotting. These effects were evident 12 h post-microinjection and reached a maximum after 48 h. 14-3-3-θ mRNA transcripts and 14-3-3-θ protein were reduced by >50%. Serine phosphorylation had a similar time course reaching maximal 25% reduction at 48 h (Fig. 5A, black bars). Partial recovery of transcription, expression and serine phosphorylation was observed after 72 h.
Figure 5. Knockdown of 14-3-3-θ in vivo by siRNA microinjection decreases 14-3-3-θ mRNA transcription, protein expression, protein serine phosphorylation and GABAAγ2 serine phosphorylation.
A. 14-3-3-θ siRNA was micro-injected into the left cerebellar vermis (folia 7–8) in 30 anesthetized mice. Vehicle alone was injected into the right folia 7–8. We removed the left and right folia 7–8 at the indicated times and prepared samples for measuring 14-3-3-θ transcripts (red bars), protein expression (green bars) and protein serine phosphorylation (black bars) in groups of three mice. Protein expression and protein serine phosphorylation were measured from separate blots of the same samples. We normalized the measurement of 14-3-3-θ mRNA transcripts using cyclophilin as an equal loading control. We measured 14-3-3-θ expression using β-actin as a loading control, normalized with respect to samples taken at time 0. We measured protein serine phosphorylation in dot blots of cerebellar lysates with a phosphoserine antibody. The error bars indicate one standard error of the mean. We used a single factor ANOVA to test significance. A single asterisk indicates p < 0.01. Double asterisks indicate p < 0.001. B. In three mice we measured serine phosphorylation of GABAAγ2 in cerebellar lysates 48 h after microinjecting knocking down 14-3-3-θ as described above. The insert shows a Western blot probed a phosphoserine antibody for a single mouse. Knocking down 14-3-3-θ reduced serine phosphorylation of GABAAγ2. The error bar indicates one standard error of the mean. The double asterisks indicate that a t-test showed statistical significance at p < 0.001.
If 14-3-3-θ was responsible for the serine phosphorylation of GABAAγ2 then, knocking down 14-3-3-θ mRNA should reduce serine phosphorylation of GABAAγ2. We examined this possibility in three mice that received microinjections 14-3-3-θ siRNA (s479) in the right vermis and control microinjections in the left vermis. We immunoprecipitated GABAAγ2 from the cerebellar lysates and immunoprobed them with an anti-phosphoserine antibody 48 h after the microinjections. Post-microinjection, serine phosphorylation of GABAAγ2 was reduced by 40% (Fig. 5B). We conclude that 14-3-3-θ is at least partially responsible for the serine phosphorylation of GABAAγ2.
Knockdown of 14-3-3-θ and PKC-γ in N2a cells decreases serine phosphorylation and cell surface expression of GABAAγ2
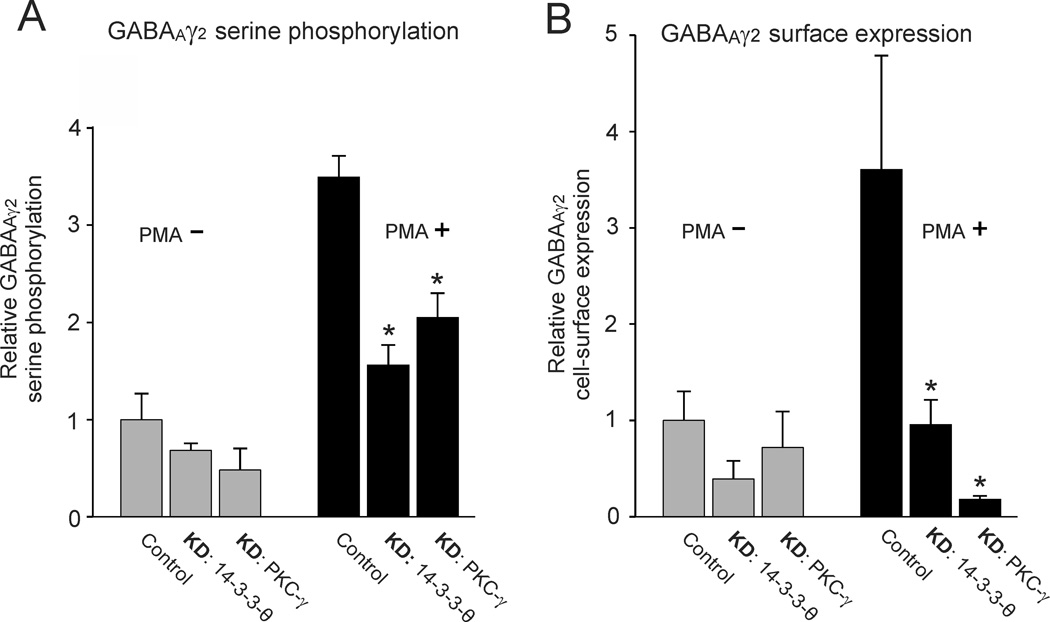
Interpretation of the effects of 14-3-3-θ on serine phosphorylation of GABAAγ2 in vivo is limited by the complexity of cerebellar circuitry. Returning to an in vitro preparation, we examined how changes in 14-3-3-θ and PKC-γ expression modulated GABAAγ2 serine phosphorylation in a single neuronal cell type, N2a cells, by directly manipulating 14-3-3-θ and PKC-γ expression. The N2a cell line endogenously expresses several GABAAR subunits, α1, α4, α6, β1-3 and γ2 as well as both 14-3-3-θ and PKC-γ. We normalized all measurements of serine phosphorylation relative to control cells, not treated with PMA. When either 14-3-3-θ or PKC-γ was knocked down in the presence of the PKC activator PMA, we observed significant reductions in serine phosphorylation of GABAAγ2 (Fig. 6A, black bars; asterisks indicate p < 0.001). In untreated controls, knockdowns of 14-3-3-θ and PKC-γ caused only a nominal decrease in serine phosphorylation of GABAAγ2 (Fig. 6A gray bars).
Figure 6. Knockdown of 14-3-3-θ and PKC-γ in N2a cells reduces serine phosphorylation of GABAAγ2 and its cell-surface expression.
14-3-3-θ and PKC-γ were knocked down in N2a cells in vitro by siRNA or miRNA transfection as described in PROCEDURES. The N2a cells were either treated with 200 nM PMA to activate PKC (PMA+, black bars) or were left without PMA treatment (PMA, gray bars). A. PMA treatment increased serine phosphorylation of N2a cells (p < 0.005). Knockdown of either 14-3-3-θ or PKC-γ reduced serine phosphorylation of GABAAγ2 (p < 0.050, indicated by asterisk). Equal loading was checked by Coomasie staining of duplicate gels. B. PMA treatment increased cell-surface expression of GABAAγ2 in N2a cells with respect to control N2a cells not treated with PMA (p < 0.005). Knockdown of either 14-3-3-θ or PKC-γ reduced cell surface expression of GABAAγ2 (p < 0.050, indicated by asterisk). The cell-surface expression of GABAAγ2 was measured by selective biotinylation of membrane proteins. Measurements were normalized with respect to control cells treated with vehicle alone. Equal loading was checked by Coomasie staining of duplicate gels. The error bars indicate one standard error of the mean.
Reduced serine phosphorylation of GABAAγ2 might impede its insertion into the cell surface membrane. We tested whether knockdowns of either 14-3-3-θ or PKC-γ reduced the cell surface expression of GABAAγ2 using an assay that preferentially biotinylates cell surface proteins (see METHODS). Knockdowns of either 14-3-3-θ or PKC-γ reduced cell-surface expression of GABAAγ2 in PMA treated N2a cells (Fig. 6B, black bars; p < 0.001). In untreated N2a cells knockdowns of either 14-3-3-θ or PKC-γ were ineffective in reducing cell surface expression of GABAAγ2 (Fig. 6B, gray bars). These results confirm that GABAAγ2 serine phosphorylation and cell-surface expression are dependent on both 14-3-3-θ and PKC-γ.
DISCUSSION
Climbing fiber activity decreases 14-3-3-θ expression and serine phosphorylation of GABAAγ2 in Purkinje cells
Long-term HOKS evoked increased climbing fiber activity in vivo. This increased activity was correlated with reduced expression of 14-3-3-θ expression and decreased GABAAγ2 serine phosphorylation. Complementary in vitro experiments demonstrated that 14-3-3-θ specifically associated with PKC-γ. When either 14-3-3-θ or PKC-γ was knocked-down, serine phosphorylation of GABAAγ2 in the presence of PMA was reduced. These data suggest that PKC-dependent phosphorylation of GABAAγ2 is regulated by 14-3-3-θ and that serine phosphorylation is necessary for its cell surface expression (Fig. 6).
GABAAγ2 has multiple PKC phosphorylation sites (Song and Messing, 2005). Three of these sites, S322, S327 and S343, are specifically targeted by PKC-γ. Since PKC phosphorylation depends on specific recognition sequences in the target protein, it is likely that the observed change in the overall serine phosphorylation levels of GABAAγ2 is driven by a similar pattern of phosphorylated serines. These specific serine residues can be determined by site-directed mutagenesis and mass spectroscopy in future experiments.
GABAAγ2 is not the only GABAAR subunit whose cell surface expression in N2a cells was knocked down after transfection with 14-3-3-θ siRNA or PKC-γ miRNA. We have observed similar decreases in GABAAα1 and GABAAβ2/3 as well, but not GABAAα4 or GABAAα6 (unpublished data). GABAAα1, GABAAβ2/3 and GABAAγ2 are expressed in Purkinje cells (Laurie et al., 1992).
Specificity of 14-3-3-θ interaction with PKC-γ and GABAAγ2
Phosphorylation of GABAARs is important for their cell surface expression. We entertained the idea that GABAARs could be phosphorylated by many different serine/threonine kinases, such as PKC, PKA, CaMK II and by a number of tyrosine kinases. Phosphorylation of GABAAγ2L (S343) by PKC facilitates the postsynaptic clustering of GABAARs (Meier and Grantyn, 2004). Phosphorylation of β subunits of GABAARs (GABAAβ1:S409, GABAAβ3:S408/S409) by both PKA and PKC increases their interaction with Activating Protein 2 and allows for GABAAR subtype-specific modulation of GABAAR endocytosis (Luscher et al., 2011).
14-3-3 proteins interact with a variety of target proteins involved in signal transduction, cell cycle regulation and apoptosis (Aitken, 1996; Fu et al., 2000; van Hemert et al., 2001). Protein kinase C, a major serine/threonine kinase family is one of the proteins with which 14-3-3-θ interacts. 14-3-3 proteins also bind to PKC-γ, -ε, -θ, -ζ and -μ (Meller et al., 1996; Yaffe et al., 1997; Hausser et al., 1999; Van Der Hoeven et al., 2000). Association with 14-3-3 protein alters the target protein’s interactions with other partners or changes the target protein’s subcellular localization (Muslin and Xing, 2000).
14-3-3-θ interacts with PKC-γ and/or GABAAγ2. Other target proteins that interact with 14-3-3 proteins contain a phosphoserine or phosphothreonine at their 14-3-3 protein binding site (Tzivion and Avruch, 2002). These binding sites contain a consensus sequence --- RXX(pS/pT)XP (Yaffe et al., 1997) --- present in PKC-γ in two residues; 597–602 and 686–691. However, this sequence is not present in GABAAγ2. This suggests that while interaction between 14-3-3-θ and PKC-γ is phosphorylation dependent, the interaction between 14-3-3-θ and GABAAγ2 is not.
CaMKII phosphorylates GABAAR β subunits (β1: S384/409, β2: S410, β3: S383/409) as well as GABAAγ2 (S343/S348/T350). Phosphorylation by CaMKII alters both the expression of the GABAAR at the cell surface and its function (Houston et al., 2007). However, we found no evidence for a modulation of CaMKII by HOKS-induced climbing fiber activity. We focused on serine phosphorylation of GABAAγ2 by PKC because HOKS-induced climbing fiber activity did not alter the phosphorylation of threonine or tyrosine residues.
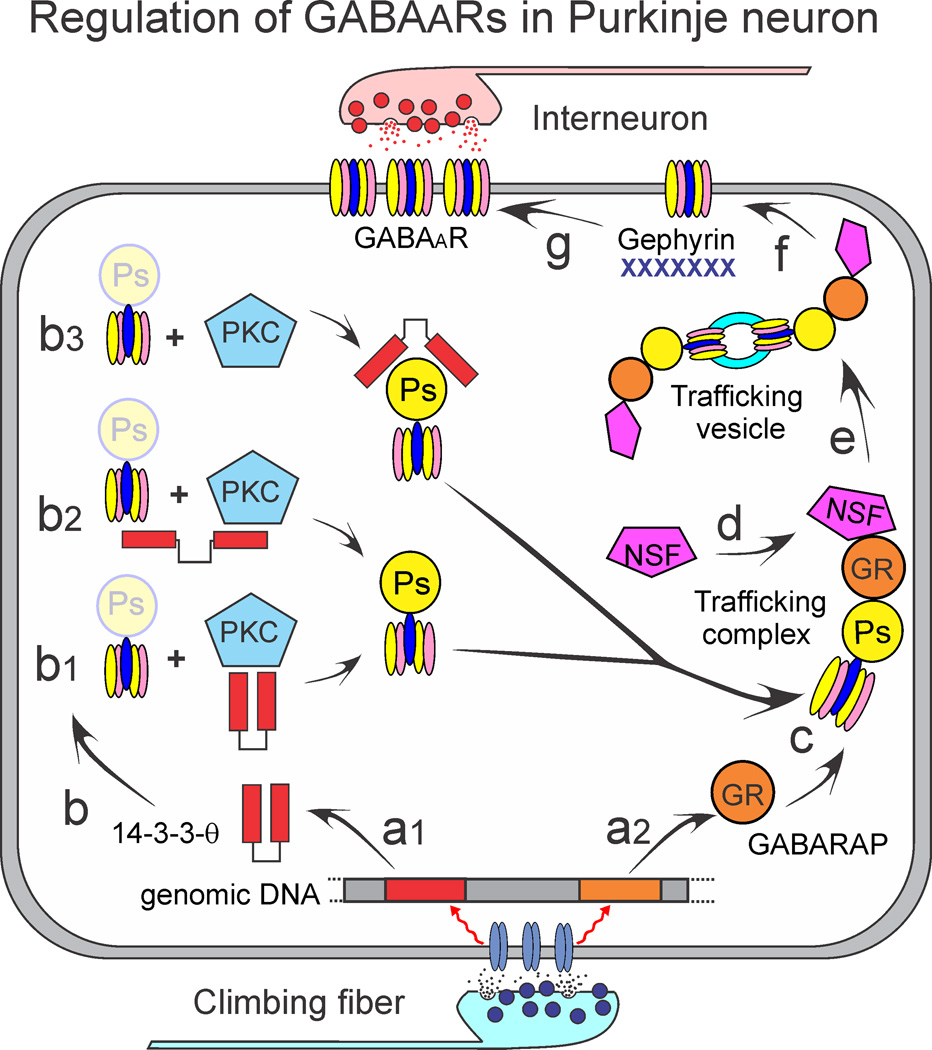
Both 14-3-3-θ and PKC-γ may be required for the serine phosphorylation of GABAAγ2. However, our data do not demonstrate how the combined action of 14-3-3-θ and PKC-γ mechanistically leads to the observed changes in GABAAγ2 serine phosphorylation. We summarize the possibilities diagrammatically in Figure 7. Following synaptic depolarization transcription of 14-3-3-θ and GABARAP are reduced (Fig. 7a1,2). Three possible interactions between 14-3-3-θ and GABAAγ2 could occur. First, 14-3-3-θ may directly stimulate PKC-γ activity (Fig. 7b1), as has been reported for other PKC and 14-3-3 protein interactions (Meller et al., 1996; Kostelecky et al., 2009). Second, 14-3-3-θ may act as a scaffolding protein by binding both PKC-γ and GABAAγ2 concurrently (Fig. 7b2). This would increase the rate of phosphorylation by PKC-γ by tethering it to its substrate. Third, 14-3-3-θ may bind to GABAAγ2 independently of its interaction with PKC-γ (Fig. 7b3), thereby preventing de-phosphorylation or degradation of GABAAγ2 (Chen and Wagner, 1994; Cotelle et al., 2000; Henriksson et al., 2002; Sun et al., 2007). This allows maintenance of higher cytoplasmic concentrations of serine phosphorylated GABAAγ2. GABARAP and serine phosphorylated GABAAγ2 form a complex with N-ethylmaleimide-sensitive factor (NSF), a protein critical for intracellular membrane trafficking (Kittler et al., 2001; Luscher et al., 2011) (Fig. 7c,d). Once assembled into a trafficking vesicle (Fig. 7e), the complex is inserted into the membrane. GABAARs can diffuse within the membrane. However, the presence of gephyrin limits this diffusion and creates clusters of GABAARs. If gephyrin is reduced by RNA interference, cell surface concentrations of GABAARs remain the same, but clustering is reduced (Jacob et al., 2005). Changes in protein serine phosphorylation directly affect the interaction of GABAARs with GABARAP as well as with β-tubulin and gephyrin (Qian et al., 2011).
Figure 7. Regulation of GABAARs in Purkinje neurons.
The sequence of events that begins with climbing fiber evoked synaptic activity and ends with a change in the cell surface expression of GABAARs is summarized. a) Transcription of 14-3-3-θ and GABARAP are reduced. 14-3-3-θ interacts with GABAAγ2 in one of three ways: b1) 14-3-3-θ may directly stimulate PKC-γ activity, b2) 14-3-3-θ may act as a scaffolding protein by binding both PKC-γ and GABAAγ2, b3) 14-3-3-θ may bind to GABAAγ2 independently of its interaction with PKC-γ and prevent de-phosphorylation or degradation of GABAAγ2. c, d) GABARAP and serine phosphorylated GABAAγ2 form a complex with N-ethylmaleimide-sensitive factor (NSF), e) the trafficking complex forms a vesicle, f) Once assembled, the complex is inserted into the plasma membrane. g) GABAARs diffusion within the membrane is limited by gephyrin, forming clusters of GABAARs.
Functional role of climbing fiber regulation of 14-3-3-θ and PKC-γ in regulation of GABAARs
We have shown that HOKS-evoked increases in climbing fiber activity reduces the intracellular concentration of 14-3-3-θ, reduces the activity of PKC-γ. It also reduces serine phosphorylation and GABARAP expression. Knockdown of 14-3-3-θ and PKC-γ in vitro reduces cell surface expression of GABAARs in N2a cells.
Physiologically, the initial effect of climbing fiber-evoked activity is a complete depolarization of Purkinje cell dendrites and soma leading to the generation of a “complex spike.” The complex spike has a relatively long duration (5–8 ms) and a low discharge frequency (1–3 imp/s). It is followed immediately by stellate cell inhibition of Purkinje cell “simple spikes.” Stellate cells discharge in phase with climbing fibers and are activated by glutamate spillover from climbing fiber terminals (Szapiro and Barbour, 2007; Barmack and Yakhnitsa, 2008). Although it is widely assumed that SSs are regulated independently in the cerebellum through the action of mossy fibers on granule cells (Ghez and Thach, 2000; Apps and Garwicz, 2005; Bloedel and Bracha, 2009), no known mossy fiber pathways activated by HOKS project to the flocculus (Voogd and Barmack, 2005). Furthermore in other cerebellar systems such as the uvula-nodulus where significant primary afferent vestibular mossy fibers project, these afferents discharge 180 deg out of phase with respect to SSs during vestibular stimulation (Barmack and Shojaku, 1995; Barmack and Yakhnitsa, 2003).
At first glance, it seems improbable that climbing fibers excite Purkinje cells and at the same time reduce Purkinje cell GABAA synapses, rendering the Purkinje cell even more excitable. This would consitute an apparently unstable positive feedback loop in which an excitatory input renders a cell even more excitable. In fact, the opposite regulation, a negative feedback loop, is demonstrated by cortical neurons in vitro. Decreased neuronal activity caused by incubation with TTX leads to decreased surface expression of GABAARs (Saliba et al., 2007).
If feedback loops are examined in the context of cerebellar microcircuitry, a long-term increase in “complex spikes” would evoke a decrease “simple spikes” through stellate cell inhibition. A consequent reduction in GABAARs could render the Purkinje cell less sensitive to stellate cell inhibition and reestablish an optimal operating point at a higher discharge rate for SSs. In this context, reduced cell surface expression of GABAARs could provide a homeostatic mechanism with a time course of tens of minutes by which SSs recover from climbing fiber-initiated stellate cell inhibition.
Presently, we do not know whether the observed influence of climbing fiber activity on the regulation of GABAAR trafficking and insertion into the Purkinje cell membrane is caused by the large climbing fiber-evoked EPSP or the subsequent stellate cell-evoked IPSP or to a combination of the two. A solution to this problem will require long-term independent control of both excitatory and inhibitory inputs to Purkinje cells.
HIGHLIGHTS.
Climbing fibers activate signaling proteins that regulate the GABAA receptors in Purkinje cells. >Climbing fiber activity decreases 14-3-3-θ and serine phosphorylation of GABAAγ2. >Climbing fiber activity also decreases interaction of 14-3-3-θ with PKCγ. >Knockdown 14-3-3-θ in vivo reduces serine phosphorylation of GABAAγ2. >Knockdown of 14-3-3-θ or PKC-γ in N2a cells In vitro reduces serine phosphorylation and cell surface expression of GABAAγ2.
Acknowledgement
This research was supported by NEI: EY18561 and by NIDCD: DC006668.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aitken A. 14-3-3 and its possible role in co-ordinating multiple signalling pathways. Trends Cell Biol. 1996;6:341–347. doi: 10.1016/0962-8924(96)10029-5. [DOI] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Bilderback TR, Liu H, Qian Z, Yakhnitsa V. Activity-dependent expression of Acyl-coenzyme A-binding protein in retinal Muller glial cells evoked by optokinetic stimulation. J Neurosci. 2004;24:1023–1033. doi: 10.1523/JNEUROSCI.3936-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH, Errico P. Optokinetically-evoked expression of corticotropin-releasing factor in inferior olivary neurons of rabbits. J Neurosci. 1993;13:4647–4659. doi: 10.1523/JNEUROSCI.13-11-04647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH, Nelson BJ. Influence of long-term optokinetic stimulation on eye movements of the rabbit. Brain Res. 1987;437:111–120. doi: 10.1016/0006-8993(87)91532-0. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Qian Z. Activity-dependent expression of calbindin in rabbit floccular Purkinje cells modulated by optokinetic stimulation. Neuroscience. 2002;113:235–250. doi: 10.1016/s0306-4522(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Shojaku H. Vestibular and visual signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol. 1995;74:2573–2589. doi: 10.1152/jn.1995.74.6.2573. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Cerebellar climbing fibers modulate simple spikes in cerebellar Purkinje cells. J Neurosci. 2003;23:7904–7916. doi: 10.1523/JNEUROSCI.23-21-07904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci. 2008;28:1140–1152. doi: 10.1523/JNEUROSCI.3942-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH, Young WSI. Optokinetic stimulation increases corticotropin-releasing factor mRNA in inferior olivary neurons of rabbits. J Neurosci. 1990;10:631–640. doi: 10.1523/JNEUROSCI.10-02-00631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloedel JR, Bracha V. Cerebellar Functions. In: Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedic Reference of Neuroscience. vol. 1. Heidelberg: Springer Verlag; 2009. pp. 667–671. [Google Scholar]
- Chen F, Wagner PD. 14-3-3 proteins bind to histone and affect both histone phosphorylation and dephosphorylation. FEBS Lett. 1994;347:128–132. doi: 10.1016/0014-5793(94)00520-6. [DOI] [PubMed] [Google Scholar]
- Cotelle V, Meek SE, Provan F, Milne FC, Morrice N, Mackintosh C. 14-3-3s regulate global cleavage of their diverse binding partners in sugar-starved Arabidopsis cells. EMBO J. 2000;19:2869–2876. doi: 10.1093/emboj/19.12.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crépel F, Jaillard D. Pairing of pre- and postsynaptic activities in cerebellar Purkinje cells induces long-term changes in synaptic efficacy in vitro. J Physiol (Lond) 1991;432:123–141. doi: 10.1113/jphysiol.1991.sp018380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl WJ, Muslin AJ, Kikuchi A, Martin JA, MacNicol AM, Gross RW, Williams LT. Activation of Raf-1 by 14-3-3 proteins. Nature. 1994;371:612–614. doi: 10.1038/371612a0. [DOI] [PubMed] [Google Scholar]
- Faulstich M, Van Alphen AM, Luo C, du LS, De Zeeuw CI. Oculomotor plasticity during vestibular compensation does not depend on cerebellar LTD. J Neurophysiol. 2006;96:1187–1195. doi: 10.1152/jn.00045.2006. [DOI] [PubMed] [Google Scholar]
- Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- Ghez C, Thach WT. The Cerebellum. In: Kandel ER, Schwartz J, Jessel TM, editors. Principles of Neuroscience. New York: Elsevier; 2000. pp. 832–852. [Google Scholar]
- Hausser A, Storz P, Link G, Stoll H, Liu YC, Altman A, Pfizenmaier K, Johannes FJ. Protein kinase C mu is negatively regulated by 14-3-3 signal transduction proteins. J Biol Chem. 1999;274:9258–9264. doi: 10.1074/jbc.274.14.9258. [DOI] [PubMed] [Google Scholar]
- Henriksson ML, Francis MS, Peden A, Aili M, Stefansson K, Palmer R, Aitken A, Hallberg B. A nonphosphorylated 14-3-3 binding motif on exoenzyme S that is functional in vivo. Eur J Biochem. 2002;269:4921–4929. doi: 10.1046/j.1432-1033.2002.03191.x. [DOI] [PubMed] [Google Scholar]
- Houston CM, Lee HH, Hosie AM, Moss SJ, Smart TG. Identification of the sites for CaMK-II-dependent phosphorylation of GABA(A) receptors. J Biol Chem. 2007;282:17855–17865. doi: 10.1074/jbc.M611533200. [DOI] [PubMed] [Google Scholar]
- Ito M. The molecular organization of cerebellar long-term depression. Nat Rev Neurosci. 2002;3:896–902. doi: 10.1038/nrn962. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Rexhausen U, Dreessen J, Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T. Sustained structural change of GABA(A) receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J Neurosci. 2007;27:6788–6799. doi: 10.1523/JNEUROSCI.1981-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ. The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors. Mol Cell Neurosci. 2001;18:13–25. doi: 10.1006/mcne.2001.1005. [DOI] [PubMed] [Google Scholar]
- Kostelecky B, Saurin AT, Purkiss A, Parker PJ, McDonald NQ. Recognition of an intra-chain tandem 14-3-3 binding site within PKCepsilon. EMBO Rep. 2009;10:983–989. doi: 10.1038/embor.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ, Connor JA. Cellular mechanisms of long-term depression in the cerebellum. Curr Opin Neurobiol. 1993;3:401–406. doi: 10.1016/0959-4388(93)90133-j. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli J, D'Angelo E. The spatial organization of long-term synaptic plasticity at the input stage of cerebellum. J Neurosci. 2007;27:1285–1296. doi: 10.1523/JNEUROSCI.4873-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–1192. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J, Grantyn R. Preferential accumulation of GABAA receptor gamma 2L, not gamma 2S, cytoplasmic loops at rat spinal cord inhibitory synapses. J Physiol. 2004;559:355–365. doi: 10.1113/jphysiol.2004.066233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller N, Liu YC, Collins TL, Bonnefoy-Berard N, Baier G, Isakov N, Altman A. Direct interaction between protein kinase C theta (PKC theta) and 14-3-3 tau in T cells: -14-3-3 overexpression results in inhibition of PKC theta translocation and function. Mol Cell Biol. 1996;16:5782–5791. doi: 10.1128/mcb.16.10.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Muslin AJ, Xing H. 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell Signal. 2000;12:703–709. doi: 10.1016/s0898-6568(00)00131-5. [DOI] [PubMed] [Google Scholar]
- Nagao S, Kitazawa H. Effects of reversible shutdown of the monkey flocculus on the retention of adaptation of the horizontal vestibulo-ocular reflex. Neuroscience. 2003;118:563–570. doi: 10.1016/s0306-4522(02)00991-0. [DOI] [PubMed] [Google Scholar]
- Narasimhan K, Linden DJ. Defining a minimal computational unit for cerebellar long-term depression. Neuron. 1996;17:333–341. doi: 10.1016/s0896-6273(00)80164-6. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, Zhang C, Shokat KM, Messing RO. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Qian Z, Bilderback TR, Barmack NH. Acyl coenzyme A-binding protein (ACBP) is phosphorylated and secreted by retinal Muller astrocytes following protein kinase C activation. J Neurochem. 2008;105:1287–1299. doi: 10.1111/j.1471-4159.2008.05229.x. [DOI] [PubMed] [Google Scholar]
- Qian Z, Yakhnitsa V, Barmack NH. Climbing fiber-evoked Purkinje cell discharge reduces expression of GABA(A) receptor-associated protein and decreases its interaction with GABA(A) receptors. J Neurochem. 2011;117:197–208. doi: 10.1111/j.1471-4159.2010.07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- Sakurai M. Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J Physiol (Lond) 1987;394:463–480. doi: 10.1113/jphysiol.1987.sp016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RS, Michels G, Jacob TC, Pangalos MN, Moss SJ. Activity-dependent ubiquitination of GABA(A) receptors regulates their accumulation at synaptic sites. J Neurosci. 2007;27:13341–13351. doi: 10.1523/JNEUROSCI.3277-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin AT, Durgan J, Cameron AJ, Faisal A, Marber MS, Parker PJ. The regulated assembly of a PKCepsilon complex controls the completion of cytokinesis. Nat Cell Biol. 2008;10:891–901. doi: 10.1038/ncb1749. [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol. 2008;100:1949–1966. doi: 10.1152/jn.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Messing RO. Protein kinase C regulation of GABAA receptors. Cell Mol Life Sci. 2005;62:119–127. doi: 10.1007/s00018-004-4339-x. [DOI] [PubMed] [Google Scholar]
- Sun L, Stoecklin G, Van WS, Hinkovska-Galcheva V, Guo RF, Anderson P, Shanley TP. Tristetraprolin (TTP)-14-3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-alpha mRNA. J Biol Chem. 2007;282:3766–3777. doi: 10.1074/jbc.M607347200. [DOI] [PubMed] [Google Scholar]
- Svensson P, Jirenhed DA, Bengtsson F, Hesslow G. Effect of conditioned stimulus parameters on timing of conditioned Purkinje cell responses. J Neurophysiol. 2010;103:1329–1336. doi: 10.1152/jn.00524.2009. [DOI] [PubMed] [Google Scholar]
- Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10:735–742. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- Tzivion G, Avruch J. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem. 2002;277:3061–3064. doi: 10.1074/jbc.R100059200. [DOI] [PubMed] [Google Scholar]
- van Beugen BJ, Nagaraja RY, Hansel C. Climbing fiber-evoked endocannabinoid signaling heterosynaptically suppresses presynaptic cerebellar long-term potentiation. J Neurosci. 2006;26:8289–8294. doi: 10.1523/JNEUROSCI.0805-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Hoeven PC, Van Der Wal JC, Ruurs P, Van Dijk MC, Van Blitterswijk J. 14-3-3 isotypes facilitate coupling of protein kinase C-zeta to Raf-1: negative regulation by 14-3-3 phosphorylation. Biochem J. 2000;345:297–306. doi: 10.1042/0264-6021:3450297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert MJ, Steensma HY, van Heusden GP. 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays. 2001;23:936–946. doi: 10.1002/bies.1134. [DOI] [PubMed] [Google Scholar]
- Voogd J, Barmack NH. Oculomotor cerebellum. Prog Brain Res. 2005;151:231–268. doi: 10.1016/S0079-6123(05)51008-2. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]