Abstract
Objectives
To determine whether adhering to a healthy lifestyle in midlife may reduce the risk of dementia.
Design
Case-control study nested in a prospective cohort.
Setting
The Honolulu-Asia Aging Study on Oahu, Hawaii.
Participants
3468 Japanese American men (mean age 52, 1965–1968) examined for dementia after 25 years.
Measurements
Men at low risk were defined as those with the following midlife characteristics: nonsmoking, body mass index <25.0 kg/m2, physically active, and having a healthy diet (based on alcohol, dairy, meat, fish, fruit, vegetables, cereals, and monounsaturated-to-saturated fat). Logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (CI) for developing overall dementia, Alzheimer’s disease (AD), and vascular dementia (VaD), adjusting for potential confounders.
Results
Dementia was diagnosed in 6.4% of men (52.5% with AD, 35.0% with VaD). Examining the risk factors individually, BMI was most strongly associated with increased risk of overall dementia (OR, 1.87; 95% CI, 1.26–2.77; BMI >25.0 vs. <22.6 kg/m2). All of the individual risk factors except diet score were significantly associated with VaD, whereas none were significantly associated with AD alone. Men with all four low-risk characteristics (7.2% of cohort) had the lowest OR for overall dementia (OR, 0.36; 95% CI, 0.15–0.84), as compared to other men. There were no significant associations between the combined low-risk characteristics and the risk of AD alone.
Conclusion
Having a healthy lifestyle in midlife is associated with a lower risk of dementia in late life among Japanese American men.
Keywords: dementia, lifestyle, risk
INTRODUCTION
Confronting the growing burden of dementia requires effective treatment once symptoms develop, but also an understanding of the causes and predictors of dementia in order to develop preventive strategies. Treatments for dementia currently available provide only marginal improvements in cognition,1 underscoring the need for preventive measures.
Previous studies, including those from the Honolulu Heart Program cohort, have demonstrated that adherence to a healthy lifestyle may prevent the development of cardiovascular disease2, 3 and diabetes mellitus.4, 5 Several lifestyle factors have recently been associated with the development of dementia, including obesity in midlife,6 smoking,7, 8 and physical inactivity.9, 10 Lower risks of dementia have also been linked to moderate alcohol consumption and adherence to a diet pattern higher in fish, fruit, vegetables, ratio of monounsaturated to saturated fat, legumes, and cereals, and lower in meat and dairy.9 It remains unclear, however, how patterns of healthy behaviors may be associated with dementia.
We examined the associations between patterns of modifiable factors in midlife and the risk of dementia 25 years later in a nested case-control study of more than 3400 men.
METHODS
Study Population
Data were from the Honolulu-Asia Aging Study (HAAS), a community-based prospective cohort of 3734 Japanese American men followed since 1965 as part of the Honolulu Heart Program (HHP). Details of the study methods have been described previously.11 Briefly, the HHP included 8,006 men of Japanese ancestry born in 1900–1919 who were residing on Oahu, Hawaii, in 1965. The first examination for the HHP cohort took place from 1965–1968, at which time standardized physical and laboratory evaluations were performed. Information on baseline characteristics, including demographic, dietary, physical activity, and medical history were obtained through structured interview. The HAAS was established in 1991 as a continuation of the HHP to study aging-related conditions, with a special focus on brain diseases, and included 3734 men, ages 71–93 years (representing approximately 80% of surviving members of the original cohort).11 The Kuakini Medical Center Institutional Review Board reviewed and approved this study. All participants or their representatives provided written informed consent.
Of the 8006 men evaluated at the baseline HHP examination, those who did not participate in the HAAS were more likely in midlife to be obese (P<0.001), to smoke (P <0.001), to have a less healthy diet (less likely to have diet scores in the top 40%; P =0.01), and to have high cholesterol (P =0.01), diabetes, hypertension, and cardiovascular disease (P <0.001). They also drank more alcohol and had fewer years of education (P <0.001). There were no differences according to midlife physical activity or childhood years spend in Japan.
We conducted a nested case-control study using risk factor information collected from the HHP cohort in 1965–1968, with cases and controls defined in 1991–1993, the first time at which dementia case finding and standardized cognitive assessments were performed. We limited analyses to men with available Cognitive Abilities Screening Instrument (CASI) scores (n=3734) and excluded those with scores <74 who did not participate in the dementia evaluation phase (n=240). Comparing the 240 men with CASI scores <74 who did not undergo dementia evaluations with the 347 men with scores <74 who did have dementia evaluations, there were no significant differences according to baseline age, smoking status, BMI, or diet score. Those who did have dementia evaluations were less physically active in midlife (P =0.01). We further excluded those missing baseline exposure information – physical activity (n=18), alcohol consumption (n=2), BMI (n=1), diet (n=5) – leaving 3468 men available for analysis.
Outcomes
Case finding was conducted in 1991–1993 by a multi-step, standardized protocol, as described previously.11 Briefly, cognitive function of all participants was tested with the 100-point CASI, a combination of the Hasegawa Dementia Screening Scale, the Folstein Mini-Mental State Examination, and the Modified Mini-Mental State Test.12 The CASI score was used to identify a subgroup for further evaluation that included a neurological examination, neuropsychological testing, and informant interview about changes in cognitive function and behavior. Computed tomography or magnetic resonance brain imaging was performed and routine blood tests conducted for men suspected to have dementia.
Based on these data, a consensus diagnosis for dementia was given by the study neurologist and two physicians with expertise in dementia, according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised.13 Probable or possible Alzheimer’s disease (AD) was diagnosed according to criteria from the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association.14 Mixed dementia involving AD as the primary cause was considered as possible AD. In a previously examined subset of the HAAS cohort, 77% of men with the clinical diagnosis of probable pure AD demonstrated sufficient neuritic plaques to meet neuropathologic criteria for definite or probable AD.15 Probable or possible vascular dementia (VaD) was diagnosed using criteria of the California Alzheimer’s Disease Diagnostic and Treatment Centers.16 Mixed dementia involving VaD as the primary cause was considered as possible VaD. For this analysis, we included both probable and possible AD and VaD in the definition of cases.
Assessment of modifiable risk factors
Weight and height were measured for all participants during the 1965–1968 examination. We calculated BMI as the weight (kg) divided by the square of the height (m). BMI was examined as tertiles (15.5–22.6, >22.6–25.0, >25.0–39.9 kg/m2) and according to World Health Organization (WHO) categories (normal, <25.0; overweight, 25.0–29.9; obesity, ≥30.0 kg/m2). Smoking history was self-reported at baseline as never, former, or current. Physical activity was assessed in midlife as hours typically spent per day in no activity (eg, sleeping, lying), sedentary (eg, sitting, standing), slight (eg, walking), moderate (eg, gardening, carpentry), and heavy (eg, shoveling, digging) activity.
Dietary information was obtained from a standardized 24-hour dietary recall in midlife. Dieticians used appropriate food models and serving utensils to establish food consumption.17 The 24-hour dietary recall was validated against a full week dietary record for 329 of the 8006 men in the original cohort. Comparison between the two assessments showed no significant differences in mean intake of nine nutrients.18 Food and nutrient intakes were adjusted for total caloric intake using the residual method.19 Information on alcohol consumption was obtained from the questionnaire administered during the baseline examination interview, which included the usual frequency and amount of intake for wine, beer, and liquor (oz/mo).
Definition of low-risk groups
We defined groups expected to be at low risk for dementia according to lifestyle characteristics that have been individually associated with dementia in previous studies. For BMI, men at decreased risk were defined as those who were not overweight or obese at midlife, with a BMI <25.0 kg/m2. Overweight and obesity in midlife have been associated with an increased risk of dementia.6
For cigarette smoking, men who were not currently smoking in midlife were considered as being at lower risk for dementia. Prior studies have demonstrated a higher risk of dementia among smokers.7
The low-risk group for physical activity was defined as men in the highest quartile of slight or moderate physical activity, corresponding to a mean (SD) of 7.2 (3.2) hours typically spent in slight activity per day or 4.4 (3.0) hours in moderate activity. Reduced risks of dementia have been associated with higher physical activity.10
We identified favorable dietary patterns similarly to previous studies of diet and dementia.9, 20 Diets low in meat and dairy, and high in fruit, vegetables, fish, cereals, monounsaturated-to-saturated fat ratio, and legumes, that include mild-moderate alcohol consumption, have been associated with a lower risk of dementia.9, 20 Moderate alcohol intake in midlife has also been associated with improved cognitive performance in late life, with a higher risk of cognitive impairment among nondrinkers and heavy drinkers.21 A composite diet score was created by assigning 1 (expected least healthy) to 5 (most healthy) points for each quintile of energy-adjusted intake for the following categories: 5 points for the highest quintile of intake for fruit, vegetables (not including salted Japanese vegetables), fish, ratio of monounsaturated to saturated fat, and cereals (including rice, noodles, breakfast cereal, and bread, whole and refined grains), and 5 points for the lowest quintile of intake for meat and dairy. Legumes were not included in the diet score, as measured legume intake in this cohort was mainly comprised of shoyu and miso, foods high in sodium content. Based on prior reports indicating a “U-shaped” relationship between alcohol consumption and cognitive decline,21 we assigned 1 point to nondrinkers and men with an average intake of >32 oz/mo (>2 drinks/d), 2 points to those with >0 to <4 oz/mo (up to ~0.25 drinks/d), 3 points to those with 4–<8 oz/mo (~0.25–0.50 drinks/d), 4 points to those with 8–<16 oz/mo (~0.50–1 drink/d), and 5 points to those with 16–32 oz/mo (~1–2 drinks/d). The diet score was calculated as the sum of points for each of these 8 intake categories, and men with the highest 40% of scores were defined as being at low-risk.2, 4
Covariates
Covariates assessed at the baseline examination included age (continuous), education (years of completed schooling), occupational complexity (6-level ordinal scale reflecting average educational attainment), number of childhood years spent in Japan (<5/≥5), elevated cholesterol (serum level ≥240 mg/dL), hypertension (systolic blood pressure [SBP] ≥140 mmHg, diastolic blood pressure [DBP] ≥90 mmHg, or history of antihypertensive medication use), diabetes (history of diabetes or use of insulin or oral medications for diabetes), and cardiovascular disease (history of stroke, angina, myocardial infarction, or other coronary heart disease). Genotyping for the presence of apolipoprotein E (APOE) e4 (1 or 2 alleles vs. none) was performed at Duke University, Durham, NC, using conventional methods.22 Information on cardiovascular disease history was obtained through participant interview and verified using medical record review.
Analysis
We used logistic regression to examine the association between midlife characteristics and risk of dementia, calculating multivariable-adjusted odds ratios (ORs) and corresponding 95% confidence intervals (CIs). We first evaluated each low-risk factor individually, adjusting for age, years of education, APOE e4 status, childhood years spent in Japan, occupational status, high cholesterol, and history of hypertension, diabetes, and cardiovascular disease, without adjustment for the other low-risk factors.
Low-risk groups were then examined with the risk factors considered together, comparing men in the low-risk group to all other men in the cohort,23 adjusting for age, years of education, APOE e4 status, childhood years spent in Japan, and the interaction between APOE e4 status and the low-risk subgroups. Secondary models additionally adjusted for occupational complexity and history of hypertension, diabetes, elevated cholesterol, and cardiovascular disease. The risk factors were evaluated together in combinations that resulted in the lowest ORs for dementia.
Analyses were also performed stratified by APOE e4 status (≥1/0 alleles). We used the likelihood ratio test (LRT) to assess for statistically significant effect modification, comparing age-adjusted models with and without interaction terms of interest. In subgroup analyses, we excluded men with a history of hypertension and those who were overweight or obese (BMI ≥25.0kg/m2) at baseline, because of potential unmeasured confounding in these groups. Two-sided P-values were reported in all analyses. P-values <0.05 were considered statistically significant. All data analyses were performed using SAS Software Version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
A total of 223 cases of dementia (including 117 AD, 78 VaD) were diagnosed 25 years following cohort entry. Other causes of dementia included Parkinson’s disease (n=13), progressive supranuclear palsy (n=4), subdural hematoma (n=2), vitamin B12 deficiency (n=1), and trauma (n=2), with the cause undetermined in 6 men. Considering each of the four factors of the low-risk profile individually, men with higher BMI, those who were less physically active, and those who smoked in midlife were more likely to have dementia in late life (Table 1a). Diet score alone was not a statistically significant predictor of dementia in this population.
Table 1.
Risk factor distribution and odds ratios (OR) for dementia after 25 years (n=3,468)
| (a) Overall dementia (n=223 cases)
| |||
|---|---|---|---|
| Factor | Percentage of men | No. of cases | OR (95% CI)* |
| BMI tertile (kg/m2) | |||
| 15.5–22.6 | 33.6 | 67 | 1.00 |
| >22.6–25.0 | 33.3 | 76 | 1.82 (1.24–2.67) |
| >25.0–39.9 | 33.1 | 80 | 1.87 (1.26–2.77) |
| Midlife smoking | |||
| Never | 35.1 | 76 | 1.00 |
| Past | 28.6 | 64 | 1.17 (0.80–1.72) |
| Current | 36.4 | 83 | 1.48 (1.03–2.14) |
| Diet score† | |||
| Highest 40% | 38.4 | 85 | 1.00 |
| Lowest 60% | 61.6 | 138 | 1.20 (0.88–1.63) |
| Physical activity‡ | |||
| High | 42.5 | 84 | 1.00 |
| Low | 57.5 | 139 | 1.59 (1.15–2.18) |
| (b) Vascular dementia (n=78 cases)
| |||
|---|---|---|---|
| Factor | Percentage of men | No. of cases | OR (95% CI)* |
| BMI tertile (kg/m2) | |||
| 15.5–22.6 | 33.6 | 19 | 1.00 |
| >22.6–25.0 | 33.3 | 25 | 1.72 (0.90–3.29) |
| >25.0–39.9 | 33.1 | 34 | 2.29 (1.22–4.29) |
| Midlife smoking | |||
| Never | 35.1 | 21 | 1.00 |
| Past | 28.6 | 18 | 1.17 (0.61–2.27) |
| Current | 36.4 | 39 | 2.69 (1.50–4.81) |
| Diet score† | |||
| Highest 40% | 38.4 | 33 | 1.00 |
| Lowest 60% | 61.6 | 45 | 1.00 (0.62–1.62) |
| Physical activity‡ | |||
| High | 42.5 | 24 | 1.00 |
| Low | 57.5 | 54 | 1.80 (1.07–3.03) |
| (c) Alzheimer’s dementia (n=117 cases)
| |||
|---|---|---|---|
| Factor | Percentage of men | No. of cases | OR (95% CI)* |
| BMI tertile (kg/m2) | |||
| 15.5–22.6 | 33.6 | 42 | 1.00 |
| >22.6–25.0 | 33.3 | 38 | 1.57 (0.95–2.59) |
| >25.0–39.9 | 33.1 | 37 | 1.51 (0.90–2.54) |
| Midlife smoking | |||
| Never | 35.1 | 44 | 1.00 |
| Past | 28.6 | 38 | 1.15 (0.71–1.87) |
| Current | 36.4 | 35 | 0.97 (0.59–1.60) |
| Diet score† | |||
| Highest 40% | 38.4 | 42 | 1.00 |
| Lowest 60% | 61.6 | 75 | 1.36 (0.89–2.08) |
| Physical activity‡ | |||
| High | 42.5 | 48 | 1.00 |
| Low | 57.5 | 69 | 1.44 (0.94–2.21) |
Models adjust for age, years of education, APOE e4 status, childhood years spent in Japan, occupational status, high cholesterol, and history of hypertension, diabetes, and cardiovascular disease, but not for the other components of the low-risk profile.
We assigned 1–5 points for each quintile of intake of dairy, meat, cereals, fish, fruit, vegetables, and ratio of monounsaturated to saturated fat, with 5 points assigned to the healthiest category. For alcohol consumption, we assigned 1 point to nondrinkers and those with alcohol intakes of >2 drinks/d, 2 points to those with up to 0.25 drinks/d, 3 points to those with 0.25–0.50 drinks/d, 4 points to those with 0.50–1 drink/d, and 5 points to those with 1–2 drinks/d. A diet score was calculated for each participant as the sum of points for each of the eight food and nutrient categories, and we defined the low risk group as those in the highest two quintiles of scores.
High physical activity defined as the highest quartile of daily hours spent in slight or moderate activity.
The individual components of the low-risk profile were more strongly associated with VaD than overall dementia (Table 1b). Men who smoked in midlife had an OR for VaD of 2.69 (95% CI, 1.50–4.81), as compared to those who had never smoked. Men in the highest BMI tertile (>25.0 kg/m2) had an OR of 2.29 (95% CI, 1.22–4.29) for VaD, as compared to men in the lowest tertile (≤22.6 kg/m2). None of the individual components of the low-risk profile were statistically significantly associated with AD (Table 1c).
Considering combinations of the low-risk factors, having healthier lifestyle characteristics conferred progressively lower ORs for overall dementia (Table 2). Men who were not smoking in midlife, had a healthy diet, were not overweight or obese, and were more physically active comprised only 7.2% of the cohort and had the lowest risk of developing dementia, with an OR of 0.36 (95% CI, 0.15–0.84) as compared to all other men, adjusting for age, education, APOE e4 status, childhood years spent in Japan, and the interaction between the low-risk subgroup and APOE e4 status. Additionally adjusting for occupational complexity and history of hypertension, diabetes, cardiovascular disease and high cholesterol did not materially alter the results (OR, 0.37; 95% CI, 0.16–0.87, for men with all four healthy characteristics). There were no significant associations between the combined low-risk characteristics and the risk of AD alone, although analyses were limited by small numbers of cases.
Table 2.
Associations between modifiable risk factor combinations and overall dementia (n=3,468)
| Low-Risk Subgroup | % of men | No. of cases | Odds Ratio (95% CI) * |
|---|---|---|---|
| One low-risk factor† | 63.6 | 140 | 0.72 (0.50–1.03) |
| Nonsmoking | |||
| Two low-risk factors‡ | 25.7 | 53 | 0.66 (0.44–0.99) |
| Nonsmoking | |||
| Diet score in top 40% | |||
| Three low-risk factors§ | 17.0 | 33 | 0.57 (0.35–0.94) |
| Nonsmoking | |||
| Diet score in top 40% | |||
| BMI <25.0 kg/m2 | |||
| Four low-risk factors | 7.2 | 11 | 0.36 (0.15–0.84) |
| Nonsmoking | |||
| Diet score in top 40% | |||
| BMI <25.0 kg/m2 | |||
| High physical activity |
All models adjust for age, years of education, APOE e4 status, the interaction between APOE e4 status and the low-risk subgroup, and childhood years spent in Japan.
Model also adjusts for diet score, BMI, and physical activity.
Model also adjusts for BMI and physical activity.
Model also adjusts for physical activity.
In subgroup analyses, there were similar associations between lifestyle and the risk of dementia after excluding men with a history of hypertension in midlife (n=1177), although results did not consistently reach statistical significance, in part due to small case numbers. As compared to all other men, having the four components of the low-risk profile was associated with an OR of 0.38 (95% CI, 0.13–1.08) for dementia.
Results were similar after excluding men who were overweight or obese at baseline. Even among men with normal weight (BMI <25.0 kg/m2) in midlife, additionally being a nonsmoker, more physically active, and having diet scores in the highest 40% was associated with an OR for dementia of 0.37 (95% CI, 0.16–0.89), as compared to normal-weight men without these healthy behaviors.
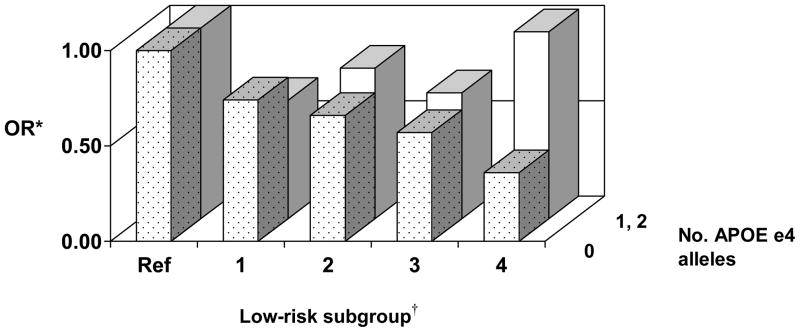
Among the 3380 men with available information on APOE e4 status, 30.3% of the 109 men with AD had at least one e4 allele, as compared to 16.9% of the 77 men with VaD, and 18.2% of those without either dementia type. In analyses stratified by APOE e4 status, the elements of the low-risk profile were associated with a decreased OR for dementia among men without the e4 allele (n=2753), but not among men with 1 or 2 alleles (n=627; Figure 1), with evidence for effect modification (LRT, P <0.001). Among men without the e4 allele, the OR for those with all four low-risk factors was 0.36 (95% CI, 0.15–0.84), as compared to other men. For men with 1 or 2 APOE e4 alleles, the OR for those with all four low-risk factors was 0.98 (95% CI, 0.35–2.69).
Figure 1. Modifiable risk factor combinations and odds ratios (OR) for dementia according to APOE e4 status.
* Model adjusts for age, years of education, and childhood years spent in Japan. Model for low-risk subgroup 2 also adjusts for diet score, BMI, and physical activity. Model for subgroup 3 also adjusts for BMI and physical activity. Model for subgroup 4 also adjusts for physical activity.
†Low-risk subgroup: 1= nonsmoking; 2= nonsmoking and diet scores in the highest 40%; 3= nonsmoking, diet scores in the highest 40%, and BMI <25.0 kg/m2; 4= nonsmoking, diet scores in the highest 40%, BMI <25.0 kg/m2, and high physical activity. Ref= referent group.
DISCUSSION
In this nested case-control study, men with a healthy lifestyle in midlife were less likely to have dementia after 25 years. Having more elements of the healthy profile provided incremental reduction in dementia risk. Even among men who were not hypertensive or overweight in midlife, those with a healthy lifestyle were similarly less likely to have dementia in late life. Furthermore, having a healthier lifestyle appeared particularly important in reducing the risk of dementia for men without the APOE e4 allele. Previous studies have similarly shown stronger associations between lifestyle factors and dementia risk among persons without the APOE e4 allele.8, 10
Previous studies of lifestyle and dementia have generally addressed risk factors individually, including obesity,6 smoking,7 physical inactivity,10 and diet.9 In a cohort from northern Manhattan, adherence to a Mediterranean-type diet and higher physical activity were each independently and in concert associated with reduced risk for AD.9 As lifestyle risk factors are often correlated, addressing them in combination accounts for the clustering of these behaviors within individuals and allows for evaluations of behavior patterns.2, 4
Although our study has several strengths, including its longitudinal design with prospective collection of information on risk factors and multiple potential confounders, a large number of participants, and confirmation of endpoints, our findings should be interpreted in the context of certain limitations. The cohort included only Japanese American men residing on Oahu, which thereby limits generalization of our results. However, limiting the study demographic also reduces potential confounding due to ethnicity and geography.
Secondly, risk factors were assessed 25 years prior to cognitive function, and more recently validated measures of physical activity and diet were not used. However, evaluating risk factors in midlife may be more reflective of relevant long-term exposures, rather than examining exposures in late-life. Furthermore, we expect that any potential risk factor misclassification would have been random with respect to outcome assessment and led to underestimation of associations. We also did not aim to identify the “Mediterranean-type” diet described in previous studies of diet and dementia risk9 due to differences in study methods as well as differences in population and cultural patterns.
Lastly, we identified prevalent, rather than incident, cases of dementia. However, while baseline cognition may be correlated with cognition in late life, such correlation would be expected to lead to underestimates of associations. In addition, although dementia may have initially developed years earlier, it is unlikely that participants were demented at the time of risk factor assessment in midlife. Furthermore, while our study included only men who survived the 25 years following risk factor evaluation, we expect that censoring prior to case-finding should lead to underestimates of the association between lifestyle and risk of dementia.
In conclusion, our findings suggest that having a healthier lifestyle in midlife is associated with a lower risk of dementia and underscore the importance of adherence to healthy behaviors in disease prevention. Future emphasis on promoting healthy behaviors in midlife, while challenging, may prove valuable in reducing the public health burden of dementia.
Acknowledgments
We are indebted to the participants in the Honolulu-Asia Aging Study for their outstanding commitment and cooperation and to the entire Honolulu-Asia Aging Study staff for their expert and unfailing assistance.
Supported by a contract (N01-AG-4-2149) and grant (1-R01-AG17155-01A1) from the National Institute on Aging, a contract (N01-HC-05102) from the National Heart, Lung, and Blood Institute, and by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs. The information contained in this article does not necessarily reflect the position or the policy of the United States Government, and no official endorsement should be inferred.
Sponsor’s Role: The study’s sponsors did not influence the design, methods, participant recruitment, data collection, analysis or preparation of the manuscript.
Footnotes
Conflict of Interest: The authors have no relevant conflicts of interest.
Presented at the International Conference on Alzheimer’s Disease, July, 2010.
Author Contributions: Dr. Gelber was responsible for the study’s concept and design, analysis and interpretation of the data, and preparation of the manuscript. Drs. Petrovitch, Masaki, Abbott, and Ross were responsible for interpretation of the data and preparation of the manuscript. Dr. Launer participated in preparation of the manuscript. Dr. White was responsible for acquisition of study participants and data, interpretation of the data, and preparation of the manuscript.
References
- 1.Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: Evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ, Hu FB, Manson JE, et al. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RJ, Burchfiel CM, Benfante R, et al. Lifestyle and biologic factors associated with atherosclerotic disease in middle-aged men. 20-year findings from the Honolulu Heart Program. Arch Intern Med. 1995;155:686–694. [PubMed] [Google Scholar]
- 4.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 5.Burchfiel CM, Sharp DS, Curb JD, et al. Physical activity and incidence of diabetes: The Honolulu Heart Program. Am J Epidemiol. 1995;141:360–368. doi: 10.1093/aje/141.4.360. [DOI] [PubMed] [Google Scholar]
- 6.Whitmer RA, Gunderson EP, Barrett-Connor E, et al. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyas SL, White LR, Petrovitch H, et al. Mid-life smoking and late-life dementia: The Honolulu-Asia Aging Study. Neurobiol Aging. 2003;24:589–596. doi: 10.1016/s0197-4580(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 8.Reitz C, den Heijer T, van Duijn C, et al. Relation between smoking and risk of dementia and Alzheimer disease: The Rotterdam Study. Neurology. 2007;69:998–1005. doi: 10.1212/01.wnl.0000271395.29695.9a. [DOI] [PubMed] [Google Scholar]
- 9.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbott RD, White LR, Ross GW, et al. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 11.White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
- 12.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1987. revised. [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Petrovitch H, White LR, Ross GW, et al. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57:226–234. doi: 10.1212/wnl.57.2.226. [DOI] [PubMed] [Google Scholar]
- 16.Chui HC, Victoroff JI, Margolin D, et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 17.Hankin JH, Nomura A, Rhoads GG. Dietary patterns among men of Japanese ancestry in Hawaii. Cancer Res. 1975;35:3259–3264. [PubMed] [Google Scholar]
- 18.McGee D, Rhoads G, Hankin J, et al. Within-person variability of nutrient intake in a group of Hawaiian men of Japanese ancestry. Am J Clin Nutr. 1982;36:657–663. doi: 10.1093/ajcn/36.4.657. [DOI] [PubMed] [Google Scholar]
- 19.Willett W, Stampfer MJ. Total energy intake: Implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 20.Feart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galanis DJ, Joseph C, Masaki KH, et al. A longitudinal study of drinking and cognitive performance in elderly Japanese American men: The Honolulu-Asia Aging Study. Am J Public Health. 2000;90:1254–1259. doi: 10.2105/ajph.90.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 23.Wacholder S, Benichou J, Heineman EF, et al. Attributable risk: Advantages of a broad definition of exposure. Am J Epidemiol. 1994;140:303–309. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]