Abstract
Scar formation is a potentially detrimental process of tissue restoration in adults, affecting organ form and function. During fetal development, cutaneous wounds heal without inflammation or scarring at early stages of development, but begin to heal with significant inflammation and scarring as the skin becomes more mature. One possible cell type that could regulate the change from scarless to fibrotic healing is the mast cell. We show here that dermal mast cells in scarless wounds generated at embryonic day 15 (E15) are fewer in number, less mature and do not degranulate in response to wounding as effectively as mast cells of fibrotic wounds made at embryonic day 18 (E18). Differences were also observed between cultured mast cells from E15 and E18 skin with regard to degranulation and preformed cytokine levels. Injection of mast cell lysates into E15 wounds disrupted scarless healing, suggesting that mast cells interfere with scarless repair. Finally, wounds produced at E18, which normally heal with a scar, healed with significantly smaller scars in mast cell-deficient KitW/W-v mice compared to Kit+/+ littermates. Together, these data suggest that mast cells enhance scar formation, and that these cells may mediate the transition from scarless to fibrotic healing during fetal development.
Introduction
Wound healing is an important sequence of events following tissue injury that can have detrimental outcomes, like fibrosis and scarring. While our understanding of the regulatory signals in adult wound healing and scar formation have improved, scarring still cannot be completely prevented in mature skin. Interestingly, scarless healing can occur in the skin at early stages of fetal development in many mammals (reviewed in Larson et al.; Wilgus, 2007). Cutaneous wounds generated early in development have very limited, if any, inflammation and heal scarlessly with regeneration of dermal appendages like hair follicles. In contrast, wounds generated late in fetal development have robust inflammation and heal with a scar (fibrotic healing). Previous studies have demonstrated that a limited inflammatory response is essential for scarless repair (Frantz et al., 1993; Haynes et al., 1994; Krummel et al., 1987; Liechty et al., 2000; Liechty et al., 1998; Martin et al., 2003; Wilgus et al., 2004). Many aspects of the inflammatory phase in fetal wounds have been studied, but surprisingly little is known about the response of mast cells to wounding at early stages of development.
Resident mast cells are known to rapidly degranulate after injury, releasing many pro-inflammatory and immunomodulatory proteins. Later in the healing process, mast cells migrate to the site of injury, dramatically increasing in number (Trautmann et al., 2000). Mast cells are thought to be important for wound healing by releasing many preformed mediators after injury, and upon stimulation, they produce even more mediators (reviewed in Artuc et al., 1999; Hebda et al., 1993). These molecules can influence important aspects of healing, such as inflammation, angiogenesis, and collagen production. Despite the growing understanding of the role of mast cells in adult wound healing, their role in the conversion from scarless to fibrotic healing has not been examined. In this study, a mouse model of fetal repair in which E15 wounds heal scarlessly and E18 wounds heal with a scar (Wilgus et al., 2004) is used to show that mast cells promote scarring in fetal wounds.
Results
Distinct features of resident mast cells in E15 and E18 fetal skin
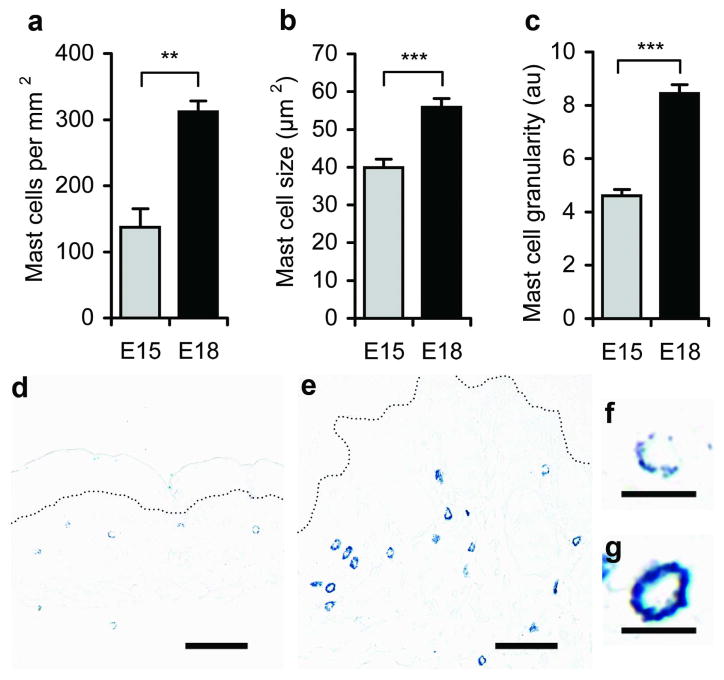
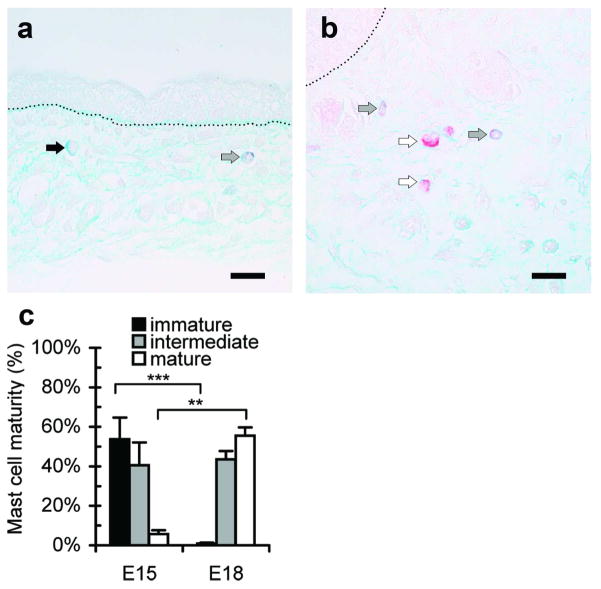
To determine differences in mast cells before and after the transition from scarless to fibrotic wound healing, mast cells were examined in unwounded dorsal skin from E15 and E18 fetuses. Marked differences were observed in the number, size, granularity and maturity of mast cells. Toluidine blue staining showed that E15 skin contained less than half the number of mast cells per mm2 as E18 skin (Figure 1a). Similar numbers of tryptase-positive mast cells were observed by immunohistochemistry, confirming reduced mast cell numbers in E15 skin (data not shown). Individual mast cells in E15 dermal tissue were significantly smaller in size (Figure 1b) and less granular (Figure 1c) than those of E18 skin, suggesting that the mast cells in E15 skin may be less mature. Alcian blue-safranin staining was then used as an indicator of mast cell maturity (Figure 2). Normal E15 and E18 fetal skin had similar percentages of mast cells at an intermediate stage of maturity (mixture of blue and red granules); however, there were strong differences in the percentage of mature (red granules) and immature (blue granules) mast cells. In E15 fetal skin, 54% of the mast cells were immature and only 6% of the mast cells were mature. Conversely, in E18 fetal skin, only 1% of the mast cells were immature, and 56% of the mast cells were of a mature phenotype.
Figure 1. Characterization of mast cells in unwounded fetal skin.
Mast cell density (a), size (b), and granularity (c) as estimated by staining intensity were determined in toluidine blue-stained sections of unwounded E15 (
 ) or E18 (■) fetal skin. Bars represent the mean ± SEM (n=9 E15 and n=6 E18 skin samples; **p<0.01, ***p<0.001). Representative photomicrographs of toluidine blue-stained E15 (d) and E18 fetal skin (e) are shown. Scale bars = 50 μm and dotted lines mark the dermal-epidermal junction. Higher magnification photomicrographs show a representative mast cell from E15 (f) and E18 (g) skin. Scale bars = 10 μm.
) or E18 (■) fetal skin. Bars represent the mean ± SEM (n=9 E15 and n=6 E18 skin samples; **p<0.01, ***p<0.001). Representative photomicrographs of toluidine blue-stained E15 (d) and E18 fetal skin (e) are shown. Scale bars = 50 μm and dotted lines mark the dermal-epidermal junction. Higher magnification photomicrographs show a representative mast cell from E15 (f) and E18 (g) skin. Scale bars = 10 μm.
Figure 2. Analysis of mast cell maturity in fetal skin.
Mast cells in E15 or E18 skin were categorized as immature, intermediate, or mature based on staining with alcian blue-safranin. Representative photomicrographs of E15 (a) and E18 (b) skin are shown. Scale bars = 20 μm and dotted lines mark the dermal-epidermal junction. Cells with granules that stained blue were considered immature (■), cells with granules that stained red were considered mature (□), and cells with red and blue granule staining were considered intermediate (
 ). Arrows indicate representative mast cells from each category. The percentages of cells at each level of maturity are shown in (c). Bars represent mean ± SEM (n=8 E15 and n=6 E18 skin samples; **p<0.01, ***p<0.001).
). Arrows indicate representative mast cells from each category. The percentages of cells at each level of maturity are shown in (c). Bars represent mean ± SEM (n=8 E15 and n=6 E18 skin samples; **p<0.01, ***p<0.001).
Age-dependent differences in mast cells after injury
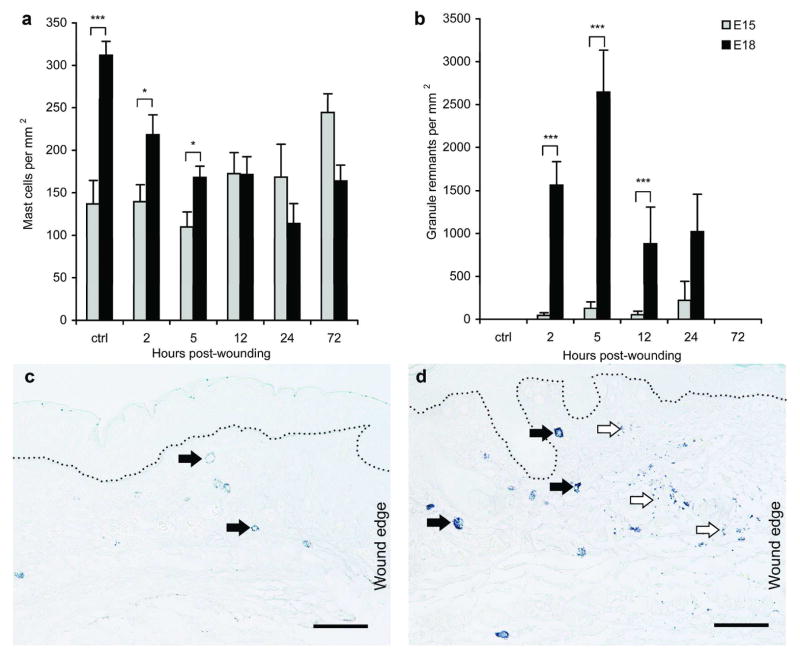
Next, changes in mast cell number and activation in response to wounding were examined. Fetal skin was harvested at times ranging from 2 hours to 72 hours post-wounding and stained with toluidine blue. The number of fully granulated mast cells decreased significantly in the dermis adjacent to wounds in E18 fetal skin at all timepoints after wounding compared to unwounded E18 skin. A decrease in mast cell density is commonly seen in adult wounds with toluidine blue staining, and is attributed to the degranulation of mast cells (el Sayed and Dyson, 1993). In contrast to E18 wounds, the number of mast cells in E15 wounds is initially stable over time and eventually increases after several days (Figure 3a). This would be expected based on the increase in mast cell density seen as fetal skin develops (Figure 1a).
Figure 3. Comparison of mast cell degranulation after wounding.
The density of mast cells (a) and granule remnants (b) were determined in the dermis adjacent to wounds in E15 (
 ) or E18 (■) fetal skin using toluidine blue-stained sections. Values for unwounded control skin (ctrl) are included for reference. Bars represent the mean ± SEM (n=3–9 fetuses per group; *p<0.05, **p<0.01, ***p<0.001). Representative photomicrographs of toluidine blue-stained E15 (c) and E18 (d) skin harvested at 5 hours post-wounding are shown. Scale bars = 50 μm and dotted lines mark the dermal-epidermal junction. Arrows indicate mast cells (
) or E18 (■) fetal skin using toluidine blue-stained sections. Values for unwounded control skin (ctrl) are included for reference. Bars represent the mean ± SEM (n=3–9 fetuses per group; *p<0.05, **p<0.01, ***p<0.001). Representative photomicrographs of toluidine blue-stained E15 (c) and E18 (d) skin harvested at 5 hours post-wounding are shown. Scale bars = 50 μm and dotted lines mark the dermal-epidermal junction. Arrows indicate mast cells (
 ) and granule remnants (
) and granule remnants (
 ).
).
After mast cells degranulate, granule remnants can often be observed in the tissue (Kovanen, 1993). These granule remnants adjacent to the wound edge were counted to quantify degranulation (Figure 3b). Very few granule remnants were observed in E15 wounds at any timepoint, suggesting that mast cells were not activated in response to injury (Figure 3c). However, in E18 skin the number of granule remnants in the dermis increased at early timepoints post-wounding, indicating strong mast cell activation/degranulation (Figure 3d). This corresponded to the decrease in toluidine blue-stained mast cells seen in E18 skin (Figure 3a). Together, the differences in mast cell characteristics in unwounded skin and mast cell degranulation after injury support the hypothesis that mast cells contribute to the transition from scarless to fibrotic wound healing.
Phenotypic differences in cultured fetal mast cells
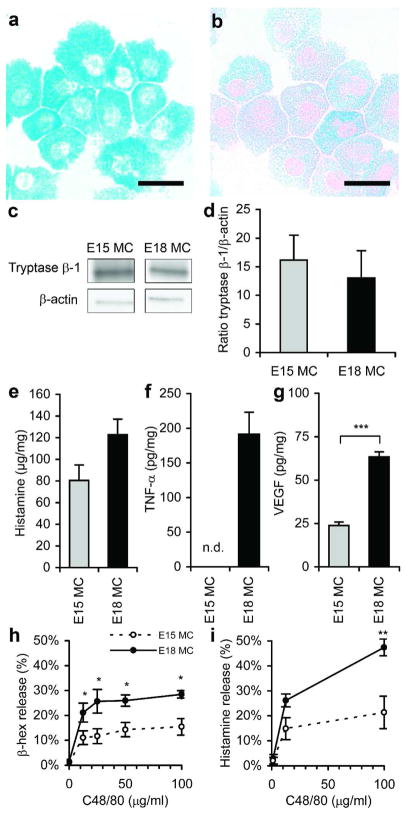
To further understand developmental changes in mast cells, in vitro experiments were performed using cultured mast cells from E15 skin (E15MC) and E18 skin (E18MC). Similar to what was seen in vivo (Figure 2), staining with alcian blue-safranin showed that E15MC had an immature phenotype (blue granules; Figure 4a), compared to E18MC (red or red and blue granules; Figure 4b). Tryptase β-1 was present in both E15MC and E18MC by Western blot (Figure 4c) and no differences in protein levels were detected by densitometry (Figure 4d). Histamine levels were higher in E18MC compared to E15MC, but this difference was not statistically significant (Figure 4e). However, TNF-α (tumor necrosis factor-α; Figure 4f) and VEGF (vascular endothelial growth factor; Figure 4g) levels were both higher in E18MC compared to E15MC. Responsiveness of the mast cells to C48/80 was determined by quantification of β-hexosaminidase (β-hex) and histamine release. Both β-hex and histamine release were significantly higher in E18MC compared to E15MC (Figure 4h–i), suggesting that E18MC are more sensitive to degranulation stimuli than E15MC. These in vitro findings support the differences seen in vivo demonstrating a more mature and responsive phenotype in E18 mast cells.
Figure 4. Differences in cultured E15MC and E18MC.
Cells from E15 or E18 skin were cultured for 14 days and mast cells were purified by centrifugation in 40% Percoll. Representative alcian blue-safranin-stained cytospins of E15MC (a) and E18MC (b) are shown. Scale bars = 20 μm. Tryptase β-1 was detected by Western blot (c) and the density of tryptase β-1/β-actin was determined (d). Histamine (e), TNF-α (f), and VEGF (g) content in cell lysates were determined by ELISA. TNF-α was not detected (n.d.) in E15MC samples. Values were normalized to total protein (mg). Degranulation in response to C48/80 at various doses was determined by β-hex (h) and histamine (i) release. Bars represent the mean ± SEM (n=3–8 per group from separate cultures; *p<0.05, **p<0.01, ***p<0.001).
Mast cell lysates disrupt scarless healing
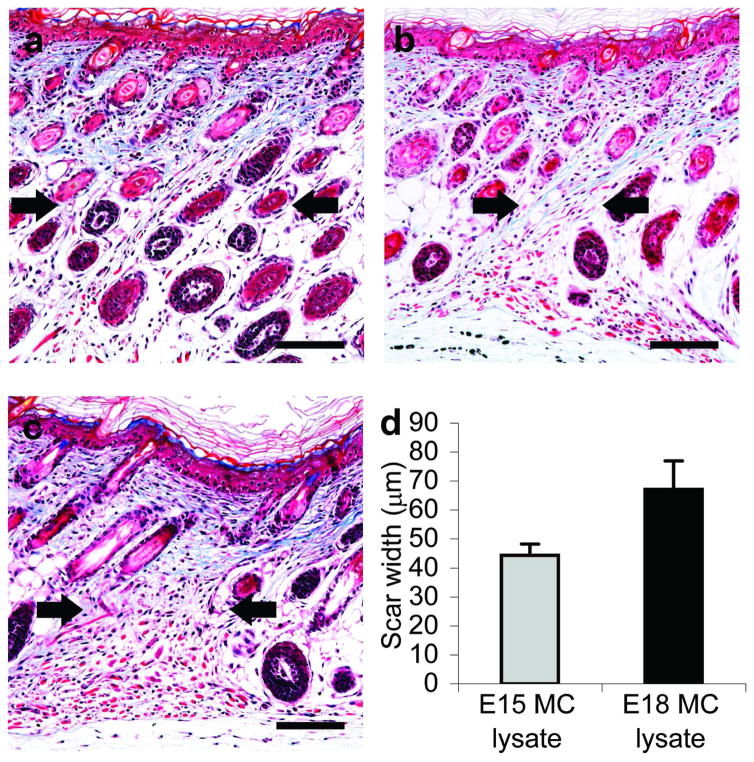
To determine if preformed mast cell mediators affect scarless fetal healing, E15MC or E18MC lysates were injected into E15 wounds, which normally heal without a scar. Examination of trichrome-stained tissue sections showed that scarless healing had occurred in all phosphate buffered saline (PBS)-injected control wounds at 10 days post-wounding (Figure 5a). Both lysate preparations disrupted scarless healing (Figure 5b–c), but only 26% (5/19) of wounds injected with E15MC lysate healed with a scar compared to 53% (19/36) of wounds injected with E18MC lysate. In addition, scars from wounds injected with E15MC lysate were 2/3 the width of those injected with E18MC lysate, although this difference was not statistically significant (Figure 5d). These results suggest that mast cell mediators augment scar formation.
Figure 5. Effects of mast cell lysate injection on scarless healing.
Wounds were generated at day E15 and injected with PBS (a) or lysate from E15MC (b) or E18MC (c). Samples were harvested at 10 days post-wounding. Representative photomicrographs of trichrome-stained wound sections are shown. Scale bars = 100 μm and arrows denote the wound/scar margins. The width of the scars was measured and the data are shown in (d). Bars represent mean ± SEM (n=5 E15MC scars and n=19 E18MC scars)
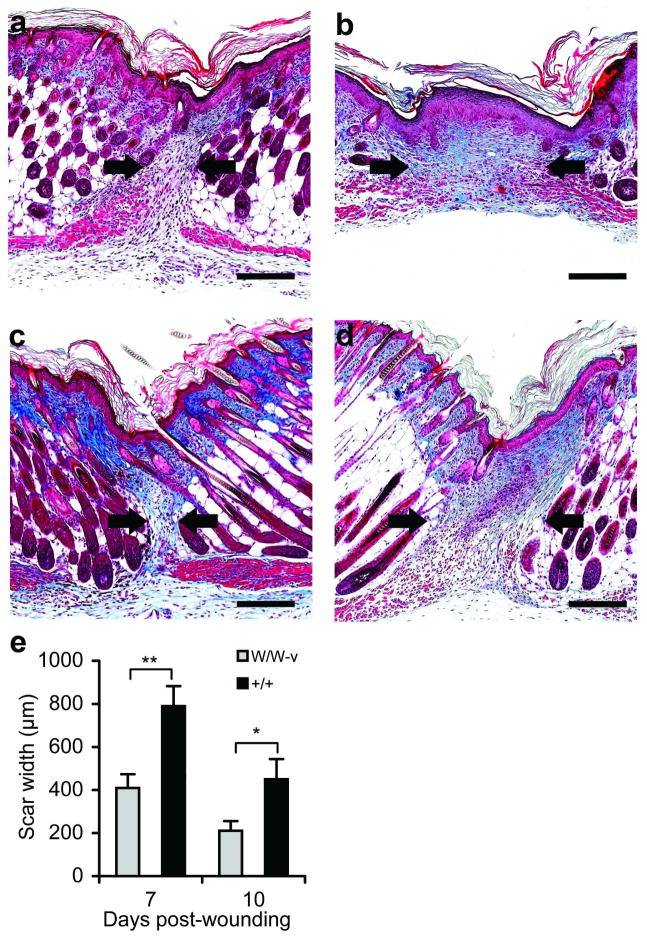
Reduced scarring in mast cell-deficient fetuses
To assess whether the absence of mast cells would reduce scar formation, mast cell-deficient (KitW/W-v) and wild-type (Kit+/+) fetuses were wounded at E18 and harvested at 7 or 10 days post-wounding (Figure 6a–d). Significant differences in scar width were observed in trichrome-stained sections (Figure 6e), with less scar tissue produced in the absence of mast cells. No differences were detected in the density of collagen types I and III or in collagen organization within the scars using immunohistochemistry and picrosirius red-staining (data not shown). Together with the results in Figure 5, these data provide strong evidence that mast cells contribute to the production of scar tissue in fetal wounds.
Figure 6. Analysis of scar formation in mast cell-deficient fetuses.
KitW/W-v and Kit+/+ littermates were wounded at E18 and wounds were harvested 7 (a, b) or 10 (c, d) days post-wounding. Representative photomicrographs of trichrome-stained scars from mast cell-deficient KitW/W-v mice (a, c) and normal Kit+/+ littermates (b, d) are shown. The scar margins are marked by arrows. Scale bars = 200 μm. Trichrome-stained sections were used to determine the size of the scars (e). Bars represent the mean scar width ± SEM (n=5–11 wounds per group; *p<0.05, **p<0.01).
Discussion
Evidence has been mounting in support of a role for mast cells in various phases of wound healing in adult skin, especially during the inflammatory phase. In contrast to mature skin, early embryonic wounds lack a pronounced inflammatory phase, and heal quickly and scarlessly (Colwell et al., 2003; Hantash et al., 2008; Larson et al.; Wilgus, 2007). At later stages of development, fetal skin heals similarly to adult skin. While many studies have shown that a vigorous inflammatory response is coincident with fibrotic healing in late gestation fetal wounds, it is still not clear what is responsible for the onset of inflammation and subsequent scarring in this model. Surprisingly, despite the known importance of resident mast cells in the initiation of acute inflammation and their suggested link to fibrosis, the role of mast cells in fetal wound healing has not been assessed.
The present study compared mast cells at different stages of development and examined the function of these cells in scar formation using a mouse model of fetal wound healing. There were striking differences in resident mast cell populations in the skin at times that correspond with scarless (E15) and fibrotic (E18) repair. Unwounded E18 skin contained approximately twice the number of mast cells compared to E15 skin, and the mast cells present in E18 skin were larger, more granular and more mature. These results are similar to other published studies indicating an increase in mast cell number and maturity during embryonic development (Combs et al., 1965; Kasugai et al., 1993; Savall and Ferrer, 1981). When the mast cell response to injury was examined, mast cell degranulation was not observed in E15 wounds. In contrast, a pattern of mast cell degranulation similar to what has been reported for adult skin wounds (el Sayed and Dyson, 1993) was seen in E18 wounds. Interestingly, cultured E15MC also presented as less mature and less responsive to C48/80, a commonly used activator of degranulation, compared to E18MC. The increased capacity of mast cells in E18 skin to degranulate could be due to the fact that the cells are more mature and thus more responsive to stimuli than mast cells from E15 skin. It is also likely that signals stimulating mast cell degranulation may only be present in the skin at later stages of development. The marked increase in mast cell number, maturity and activation in E18 skin suggests a possible role for mast cells in the conversion from scarless to fibrotic healing in the fetus.
To further explore the role of mast cells in the switch to fibrotic healing, experiments were designed to test the effects of mast cells on scarless repair. In these studies, a single injection of lysate from either E15MC or E18MC into E15 wounds disrupted scarless healing, suggesting that mediators derived from mast cells augment scar formation in fetal wounds. While the exact mediator or combination of mediators responsible is not known, many molecules stored in mast cell granules could be contributing to scar formation. Several studies have indicated that mast cell proteases have fibrogenic effects. Tryptase has been shown to have chemotactic and mitogenic effects on dermal fibroblasts and also stimulates procollagen mRNA synthesis, contraction and differentiation into myofibroblasts (Albrecht et al., 2005; Gailit et al., 2001; Gruber et al., 1997). Chymase can cleave procollagen type I, which allows for collagen fibril formation (Kofford et al., 1997). Other mast cell mediators such as histamine can also increase collagen production in fibroblasts (Gailit et al., 2001; Hatamochi et al., 1985; Kupietzky and Levi-Schaffer, 1996). In addition, VEGF and IL-6, which are stored in mast cells, have been shown to promote scar formation in early gestation fetal wounds (Liechty et al., 2000; Wilgus et al., 2008). Interestingly, cultured E15MC and E18MC retained some of the features of mast cells in E15 and E18 fetal skin. E15MC were less mature by alcian blue-safranin staining and contained significantly less TNF-α and VEGF than E18MC. E15MC were also less responsive to C48/80. Despite these differences, both lysates had some effect when injected into E15 wounds. However, a higher percentage of the wounds injected with E18MC lysate healed with a frank scar and the size of the scars were 1/3 larger compared to wounds injected with E15MC lysate. Interestingly, both E15MC and E18MC contained tryptase, which could explain why the E15MC lysate also disrupted scarless healing to some extent. It should be noted that fetal skin would not likely encounter the mediators present within E15MC under normal circumstances since E15 mast cells do not degranulate in response to injury. In any case, these data show that exposure of fetal wounds to mast cell mediators can interfere with scarless repair.
In addition to artificially exposing E15 wounds to mast cell mediators, mast cell-deficient KitW/W-v mice were used to assess the contribution of mast cells to scar formation in E18 wounds. Mast cell-deficient KitW/W-v fetuses healed with significantly smaller scars compared to wild-type Kit+/+ mice. While there was less scarring in KitW/W-v mice, these wounds did not heal entirely without a scar, suggesting that mast cells play a part in scar formation but are not the only factor involved. For example, inherent differences in early gestation dermal fibroblasts are thought to direct scarless healing (Lorenz et al., 1995) and differences in the behavior of early and late gestation fibroblasts have been described (Brink et al., 2009; Carter et al., 2009; Hirt-Burri et al., 2008; Sandulache et al., 2007). Therefore, it is possible that even in the absence of mast cells, E18 KitW/W-v fetuses cannot achieve completely scarless healing because the fibroblasts have already lost the ability to mediate scarless repair at this late stage in development.
Several mast cell-deficient strains exist (Grimbaldeston et al., 2005; Kitamura et al., 2001), but KitW/W-v mice are the most commonly used strain and have been used to study adult wound healing. Using the Kitw/w-v strain, mast cells have been shown to impact neutrophil infiltration, suggesting that mast cells are heavily involved in the regulation of acute wound inflammation (Egozi et al., 2003; Weller et al., 2006). Another study suggested a role for mast cells in collagen remodeling (Iba et al., 2004), but to date, a comprehensive study examining scar formation in KitW/W-v mice has not been published. In addition to lacking mast cells, KitW/W-v mice have defects in melanogenesis, are sterile, and develop macrocytic anemia. While we do not believe these issues contributed significantly to the results seen in the fetal wound model described here, we cannot completely rule out this possibility. Other mast cell-deficient mouse models were considered for these experiments, but it has been reported that dermal mast cells are present in developing skin in these alternative strains, with the skin only becoming significantly mast-cell deficient after birth (Kitamura and Go, 1979; Kitamura et al., 2001; Yamazaki et al., 1994). For this reason, the KitW/W-v strain was the most suitable for these studies.
While the studies presented here are the first to describe a role for mast cells in scar formation in fetal wounds, previous studies have suggested that mast cells are involved in clinically significant abnormal scarring. Keloids and hypertrophic scars both contain high numbers of mast cells (Boyadjiev et al., 1995; Hakanson et al., 1969; Harunari et al., 2006; Kischer et al., 1978; Smith et al., 1987), and recently, the mast cell stabilizer ketotifen was shown to reduce contraction and scar tissue deposition in a hypertrophic scar model (Gallant-Behm et al., 2008). Mast cells are also thought to contribute significantly to fibrotic diseases in the skin and other organs, such as scleroderma and pulmonary fibrosis (Atkins and Clark, 1987; Gruber, 2003). Overall, the data presented here provide further evidence that mast cells could be a viable target for anti-fibrosis and anti-scarring therapies.
Materials and Methods
Animals
Mice were handled in accordance with the Ohio State University Institutional Animal Care and Use Committee guidelines. FVB mice (Taconic Farms, Hudson, NY) or male C57BL/6J-KitW-v/J and female WB/Rej KitW/J mice (Jackson Laboratories, Bar Harbor, ME) were time-mated and detection of a vaginal plug was designated E0. Because mast cell-deficient KitW/W-v mice are sterile, heterozygous WB/Rej Kitw/J females were mated with C57BL/6J-kitw-v/J males to generate mast cell-deficient WBB6F1-KitW/W-v (KitW/W-v) fetuses and normal WBB6F1-Kit+/+ (Kit+/+) littermates. Mast cell-deficiency in KitW/W-v skin was confirmed by toluidine blue staining (data not shown). FVB mice were used in all studies unless otherwise indicated.
Full-thickness dorsal fetal wounds were generated at E15 or E18 as described (Wilgus et al., 2008). After wounding, 1 μl of PBS or mast cell lysate (described below) containing 10% India ink was injected subcutaneously at the wound site. Wounded animals were euthanized at various timepoints post-surgery. Skin from age-matched unwounded animals served as controls. Samples were fixed in 10% formalin overnight and embedded in paraffin for histology.
Mast cell culture
Mast cells were isolated using published methods with slight modifications. E15 or E18 skin was digested by incubation in complete RPMI 1640 containing 1.5 mg/ml collagenase and 0.5 mg/ml hyaluronidase at 37°C for 1 hour (Sigma Aldrich, St. Louis, MO) (Benyon et al., 1987). Cells were strained through a nylon strainer and red blood cells were lysed with ACK buffer (Invitrogen, Carlsbad, CA). Cells were then cultured for 14 days as described (Yamada et al., 2003) in RPMI 1640 containing interleukin-3 and stem cell factor (10 ng/ml each; Peprotec, Rock Hill, NJ). Mast cells were purified using 40% isotonic Percoll (Yamada et al., 2003). Cells were used for cytospin preparations or resuspended at a concentration of 4×106 mast cells/ml in PBS and lysed via sonication (three rounds of ten one-second pulses). Lysates were centrifuged to remove cellular debris and aliquots were stored at −80°C until use. Lysates were prepared for injection by diluting with PBS and India ink (10%) to a concentration equivalent to 900 lysed mast cells/μl. This preparation (1 μl) was injected into fetal wounds as described above.
Protein analysis
For protein analysis, cultured mast cells were lysed by sonication in RIPA buffer containing protease inhibitor cocktail (Thermo Scientific, Rockford, IL). After centrifugation, total protein concentrations were determined using the BCA (bicinchoninic acid) protein assay (Thermo).
Tryptase β-1 and β-actin levels were determined by Western blot using standard methods. Total protein (5 μg) was separated on 12% SDS-polyacrylamide gels, and transferred onto PVDF membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were blocked and probed for mouse tryptase β-1 (R&D Systems, Minneapolis, MN). Membranes were stripped with Restore PLUS buffer (Thermo), blocked and probed for β-actin (Cell Signaling Technology, Danvers, MA). Proteins were visualized using Immun-Star WesternC chemiluminescence reagents and digital images were captured using the Chemidoc XRS imaging system (Bio-Rad). Densitometry was performed using Quantity One software (Bio-Rad) and data are presented as density of tryptase β-1/β-actin.
Histamine, TNF-α, and VEGF content was measured using commercially available kits according to manufacturer’s instructions. For each sample, 5 ng (histamine EIA; Oxford Biomedical Research, Oxford, MI) or 50 μg (TNF-α and VEGF ELISA; R&D Systems) of total protein was used for the assays.
Degranulation assays
Degranulation of cultured mast cells was assesed by measuring β-hex and histamine release. β-hex assays were performed as described previously (Ortega et al., 1989). A 20 μl volume containing 2×104 cultured mast cells and C48/80 at final concentrations ranging from 0–100 μg/ml in Tyrode’s buffer were used for each reaction. For histamine release 10-fold dilutions of supernatant and lysed pellet from the β-hex assay were analyzed using a histamine EIA (Oxford Biomedical Research). The percent release was calculated using the following formula:
Histochemical Stains
Toluidine blue, which stains mast cell granules blue (Smith and Atkinson, 1956), was used to identify dermal mast cells by staining paraffin sections with 0.2% toluidine blue (Sigma Aldrich) in 0.7N HCl overnight. Mast cells were counted under light microscopy using an Axioscope 40 microscope (Carl Ziess Inc., Thornwood, NY) in five high-power fields per sample of unwounded dorsal skin. Digital pictures were then taken using an AxioCam MRc 5 digital camera (Carl Ziess). Ten individual mast cells per sample were traced and the mean area and the integrated density of staining, as a measurement of granularity, were calculated for each mast cell. For wounds, mast cell numbers and extracellular granule remnants (Lindstedt et al., 2001) were counted in two fields of skin immediately adjacent to either side of the wound (total of four fields). To control for differences in dermal thickness between E15 and E18 skin, the area of the dermis for each field was determined using Axiovision software (Carl Zeiss). Mast cell or granule remnant densities (numbers/mm2) were then calculated.
Alcian blue-safranin was used to distinguish between immature and mature mast cells by staining paraffin sections or acetone-fixed cytospin preparations as described (Csaba, 1969; Kalesnikoff and Galli, 2011). Mast cells were categorized as immature if the granules stained blue, mature if the granules stained red or intermediate if they contained a mixture of blue and red granules (Combs et al., 1965).
Masson’s trichrome staining was used to visualize scar tissue as described (Wilgus et al., 2008; Wilgus et al., 2003), and scar widths were measured using Axiovision software (Carl Zeiss).
Statistics
Prism (Graphpad, La Jolla, CA) was used for statistical analysis. Student’s two-tailed t-test or two-factor ANOVA with Bonferroni’s post-hoc tests were used. P-values less than 0.05 were considered statistically significant.
Acknowledgments
These studies were supported by funds from the OSU Department of Pathology. The laboratory is also supported by NIH grant CA127109 (TAW).
Abbreviations
- E15
embryonic day 15
- E18
embryonic day 18
- PBS
phosphate buffered saline
- E15MC
cultured mast cells from E15 skin
- E18MC
cultured mast cells from E18 skin
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- β-hex
β-hexosaminidase
- n.d.
not detected
Footnotes
Conflict of interest
The Authors have no conflicts of interest to disclose.
References
- Albrecht M, Frungieri MB, Kunz L, Ramsch R, Meineke V, Kohn FM, et al. Divergent effects of the major mast cell products histamine, tryptase and TNF-alpha on human fibroblast behaviour. Cell Mol Life Sci. 2005;62:2867–2876. doi: 10.1007/s00018-005-5289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artuc M, Hermes B, Steckelings UM, Grutzkau A, Henz BM. Mast cells and their mediators in cutaneous wound healing--active participants or innocent bystanders? Exp Dermatol. 1999;8:1–16. doi: 10.1111/j.1600-0625.1999.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Atkins FM, Clark RA. Mast cells and fibrosis. Arch Dermatol. 1987;123:191–193. [PubMed] [Google Scholar]
- Benyon RC, Lowman MA, Church MK. Human skin mast cells: their dispersion, purification, and secretory characterization. J Immunol. 1987;138:861–867. [PubMed] [Google Scholar]
- Boyadjiev C, Popchristova E, Mazgalova J. Histomorphologic changes in keloids treated with Kenacort. J Trauma. 1995;38:299–302. doi: 10.1097/00005373-199502000-00030. [DOI] [PubMed] [Google Scholar]
- Brink HE, Bernstein J, Nicoll SB. Fetal dermal fibroblasts exhibit enhanced growth and collagen production in two- and three-dimensional culture in comparison to adult fibroblasts. J Tissue Eng Regen Med. 2009;3:623–633. doi: 10.1002/term.204. [DOI] [PubMed] [Google Scholar]
- Carter R, Jain K, Sykes V, Lanning D. Differential expression of procollagen genes between mid- and late-gestational fetal fibroblasts. J Surg Res. 2009;156:90–94. doi: 10.1016/j.jss.2009.03.056. [DOI] [PubMed] [Google Scholar]
- Colwell AS, Longaker MT, Lorenz HP. Fetal wound healing. Front Biosci. 2003;8:s1240–1248. doi: 10.2741/1183. [DOI] [PubMed] [Google Scholar]
- Combs JW, Lagunoff D, Benditt EP. Differentiation and proliferation of embryonic mast cells of the rat. J Cell Biol. 1965;25:577–592. doi: 10.1083/jcb.25.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaba G. Mechanism of the formation of mast-cell granules. II. Cell-free model. Acta Biol Acad Sci Hung. 1969;20:205–210. [PubMed] [Google Scholar]
- Egozi EI, Ferreira AM, Burns AL, Gamelli RL, Dipietro LA. Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair Regen. 2003;11:46–54. doi: 10.1046/j.1524-475x.2003.11108.x. [DOI] [PubMed] [Google Scholar]
- el Sayed SO, Dyson M. Responses of dermal mast cells to injury. J Anat. 1993;182 (Pt 3):369–376. [PMC free article] [PubMed] [Google Scholar]
- Frantz FW, Bettinger DA, Haynes JH, Johnson DE, Harvey KM, Dalton HP, et al. Biology of fetal repair: the presence of bacteria in fetal wounds induces an adult-like healing response. J Pediatr Surg. 1993;28:428–433. doi: 10.1016/0022-3468(93)90243-e. discussion 433–424. [DOI] [PubMed] [Google Scholar]
- Gailit J, Marchese MJ, Kew RR, Gruber BL. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J Invest Dermatol. 2001;117:1113–1119. doi: 10.1046/j.1523-1747.2001.15211.x. [DOI] [PubMed] [Google Scholar]
- Gallant-Behm CL, Hildebrand KA, Hart DA. The mast cell stabilizer ketotifen prevents development of excessive skin wound contraction and fibrosis in red Duroc pigs. Wound Repair Regen. 2008;16:226–233. doi: 10.1111/j.1524-475X.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber BL. Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2003;5:147–153. doi: 10.1007/s11926-003-0043-3. [DOI] [PubMed] [Google Scholar]
- Gruber BL, Kew RR, Jelaska A, Marchese MJ, Garlick J, Ren S, et al. Human mast cells activate fibroblasts: tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol. 1997;158:2310–2317. [PubMed] [Google Scholar]
- Hakanson R, Owman C, Sjoberg NO, Sporrong B. Direct histochemical demonstration of histamine in cutaneous mast cells: urticaria pigmentosa and keloids. Experientia. 1969;25:854–855. doi: 10.1007/BF01897918. [DOI] [PubMed] [Google Scholar]
- Hantash BM, Zhao L, Knowles JA, Lorenz HP. Adult and fetal wound healing. Front Biosci. 2008;13:51–61. doi: 10.2741/2559. [DOI] [PubMed] [Google Scholar]
- Harunari N, Zhu KQ, Armendariz RT, Deubner H, Muangman P, Carrougher GJ, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 2006;32:669–677. doi: 10.1016/j.burns.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatamochi A, Fujiwara K, Ueki H. Effects of histamine on collagen synthesis by cultured fibroblasts derived from guinea pig skin. Arch Dermatol Res. 1985;277:60–64. doi: 10.1007/BF00406482. [DOI] [PubMed] [Google Scholar]
- Haynes JH, Johnson DE, Mast BA, Diegelmann RF, Salzberg DA, Cohen IK, et al. Platelet-derived growth factor induces fetal wound fibrosis. J Pediatr Surg. 1994;29:1405–1408. doi: 10.1016/0022-3468(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Hebda PA, Collins MA, Tharp MD. Mast cell and myofibroblast in wound healing. Dermatol Clin. 1993;11:685–696. [PubMed] [Google Scholar]
- Hirt-Burri N, Scaletta C, Gerber S, Pioletti DP, Applegate LA. Wound-healing gene family expression differences between fetal and foreskin cells used for bioengineered skin substitutes. Artif Organs. 2008;32:509–518. doi: 10.1111/j.1525-1594.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- Iba Y, Shibata A, Kato M, Masukawa T. Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int Immunopharmacol. 2004;4:1873–1880. doi: 10.1016/j.intimp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. Antiinflammatory and immunosuppressive functions of mast cells. Methods Mol Biol. 2011;677:207–220. doi: 10.1007/978-1-60761-869-0_15. [DOI] [PubMed] [Google Scholar]
- Kasugai T, Oguri K, Jippo-Kanemoto T, Morimoto M, Yamatodani A, Yoshida K, et al. Deficient differentiation of mast cells in the skin of mi/mi mice. Usefulness of in situ hybridization for evaluation of mast cell phenotype. Am J Pathol. 1993;143:1337–1347. [PMC free article] [PubMed] [Google Scholar]
- Kischer CW, Bunce H, 3rd, Shetlah MR. Mast cell analyses in hypertrophic scars, hypertrophic scars treated with pressure and mature scars. J Invest Dermatol. 1978;70:355–357. doi: 10.1111/1523-1747.ep12543553. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979;53:492–497. [PubMed] [Google Scholar]
- Kitamura Y, Morii E, Ogihara H, Jippo T, Ito A. Mutant mice: a useful tool for studying the development of mast cells. Int Arch Allergy Immunol. 2001;124:16–19. doi: 10.1159/000053657. [DOI] [PubMed] [Google Scholar]
- Kofford MW, Schwartz LB, Schechter NM, Yager DR, Diegelmann RF, Graham MF. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem. 1997;272:7127–7131. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- Kovanen PT. The mast cell--a potential link between inflammation and cellular cholesterol deposition in atherogenesis. Eur Heart J. 1993;14(Suppl K):105–117. [PubMed] [Google Scholar]
- Krummel TM, Nelson JM, Diegelmann RF, Lindblad WJ, Salzberg AM, Greenfield LJ, et al. Fetal response to injury in the rabbit. J Pediatr Surg. 1987;22:640–644. doi: 10.1016/s0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- Kupietzky A, Levi-Schaffer F. The role of mast cell-derived histamine in the closure of an in vitro wound. Inflamm Res. 1996;45:176–180. doi: 10.1007/BF02285158. [DOI] [PubMed] [Google Scholar]
- Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000;12:671–676. doi: 10.1006/cyto.1999.0598. [DOI] [PubMed] [Google Scholar]
- Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77:80–84. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- Lindstedt KA, Wang Y, Shiota N, Saarinen J, Hyytiainen M, Kokkonen JO, et al. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001;15:1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- Lorenz HP, Lin RY, Longaker MT, Whitby DJ, Adzick NS. The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg. 1995;96:1251–1259. doi: 10.1097/00006534-199511000-00002. discussion 1260–1251. [DOI] [PubMed] [Google Scholar]
- Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, et al. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Ortega E, Hazan B, Zor U, Pecht I. Mast cell stimulation by monoclonal antibodies specific for the Fc epsilon receptor yields distinct responses of arachidonic acid and leukotriene C4 secretion. Eur J Immunol. 1989;19:2251–2256. doi: 10.1002/eji.1830191211. [DOI] [PubMed] [Google Scholar]
- Sandulache VC, Parekh A, Dohar JE, Hebda PA. Fetal dermal fibroblasts retain a hyperactive migratory and contractile phenotype under 2-and 3-dimensional constraints compared to normal adult fibroblasts. Tissue Eng. 2007;13:2791–2801. doi: 10.1089/ten.2006.0412. [DOI] [PubMed] [Google Scholar]
- Savall R, Ferrer I. Mast cells in the skin of rats during development. Med Cutan Ibero Lat Am. 1981;9:345–350. [PubMed] [Google Scholar]
- Smith CJ, Smith JC, Finn MC. The possible role of mast cells (allergy) in the production of keloid and hypertrophic scarring. J Burn Care Rehabil. 1987;8:126–131. doi: 10.1097/00004630-198703000-00008. [DOI] [PubMed] [Google Scholar]
- Smith EW, Atkinson WB. Simple procedure for identification and rapid counting of mast cells in tissue sections. Science. 1956;123:941–942. doi: 10.1126/science.123.3204.941. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Toksoy A, Engelhardt E, Brocker EB, Gillitzer R. Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J Pathol. 2000;190:100–106. doi: 10.1002/(SICI)1096-9896(200001)190:1<100::AID-PATH496>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366–2368. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- Wilgus TA. Regenerative healing in fetal skin: a review of the literature. Ostomy Wound Manage. 2007;53:16–31. quiz 32–13. [PubMed] [Google Scholar]
- Wilgus TA, Bergdall VK, Tober KL, Hill KJ, Mitra S, Flavahan NA, et al. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am J Pathol. 2004;165:753–761. doi: 10.1016/S0002-9440(10)63338-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008;88:579–590. doi: 10.1038/labinvest.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen. 2003;11:25–34. doi: 10.1046/j.1524-475x.2003.11106.x. [DOI] [PubMed] [Google Scholar]
- Yamada N, Matsushima H, Tagaya Y, Shimada S, Katz SI. Generation of a large number of connective tissue type mast cells by culture of murine fetal skin cells. J Invest Dermatol. 2003;121:1425–1432. doi: 10.1046/j.1523-1747.2003.12613.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Tsujimura T, Morii E, Isozaki K, Onoue H, Nomura S, et al. C-kit gene is expressed by skin mast cells in embryos but not in puppies of Wsh/Wsh mice: age-dependent abolishment of c-kit gene expression. Blood. 1994;83:3509–3516. [PubMed] [Google Scholar]